Abstract
Botulinum neurotoxin serotype A (BoNTA) causes a life-threatening neuroparalytic disease known as botulism that could afflict large, unprotected populations if the toxin were employed in an act of bioterrorism. Current post-exposure therapy is limited to symptomatic treatment or passive immunization that is effective for treating infant botulism at a cost of US $45,300 per treatment regimen. Antibodies can neutralize the extracellular but not the intracellular BoNTA. Moreover, antibody production, storage, and administration in a mass casualty scenario pose logistical challenges. Alternatively, small-molecule inhibitors of BoNTA endopeptidase (BoNTAe) are sought to antagonize the extracellular or intracellular toxin. While several such molecules reportedly demonstrated efficacy in protecting cells against BoNTA, there is scant information to show that small molecules can significantly protect mammals against BoNTA. Herein we report the development of effective small-molecules BoNTAe inhibitors with promising in vivo pharmacokinetics. One such molecule has an in vivo half-life of 6.5 hours and is devoid of obvious sign of toxicity. Pre-treatment with this molecule at 2 mg/kg protected 100% and 70% of treated mice against BoNTA at 5 times of its median-lethal dose during the periods of 2 and 4 half-lives of the inhibitor, respectively. In contrast, 40% and 0% of untreated mice survived during the respective periods. Similar levels of protection were also observed with two other small molecules. These results demonstrate that small molecules can significantly protect mice against BoNTA and support the pursuit of small-molecule antagonists as a cost-effective alternative or as an adjunct to passive immunity for treating botulism.
Introduction
Seven distinct serotypes (A to G) of the spore-forming Clostridium botulinum have been characterized based upon production of structurally and functionally unique botulinum neurotoxins (BoNTs) [1]. Such toxins can cause a life-threatening neuroparalytic disease known as botulism [1] by inhibiting normal release of the neurotransmitter acetylcholine at peripheral neuromuscular junctions and thereby causing prolonged flaccid paralysis, serious medical sequelae, or death [1]. Despite its toxicity, the purified and diluted BoNT serotype A (BoNTA) can be harnessed to treat cholinergic nerve and muscle dysfunctions, as well as for cosmetic treatment of facial wrinkles [2], [3]. Even in carefully controlled clinical scenarios, however, overdoses of BoNTA can occur and result in systemic botulism [4]; such incidents may rise as the number of therapeutic indications increases [5]. Mishaps also may occur involving the use of unregulated or counterfeit formulations of BoNTA at unknown concentrations [6]. Moreover, due to its long in vivo half-life (t1/2 >31 days [7]), BoNTA is a recognized biological weapon that has been sought or stockpiled by both small terrorist cells and large industrial countries [8], [9]. Recently, it has been projected that botulism could afflict a large number of unprotected civilians if a food supply, for example the milk production and distribution chain [10], were intentionally contaminated by the toxin in an act of bioterrorism. There is an urgent need for small-molecule BoNTA inhibitors as effective and safe post-exposure treatment for BoNTA intoxication to respond to food poisoning, accidental clinical overdoses, and mass-casualty situations.
Current post-exposure therapy is limited to symptomatic treatment or passive immunization that is effective for treating infant botulism [11] at a cost of US $45,300 per treatment regimen [12]. Antibodies can neutralize the extracellular but not the intracellular BoNTA. Moreover, antibody production, storage, and administration in a mass casualty scenario pose logistical challenges. To antagonize the extracellular or intracellular BoNTA, small molecules [13]–[20] have been developed to inhibit BoNTA endopeptidase (BoNTAe) – the catalytic domain of BoNTA that specifically cleaves a critical component of the neurosecretory apparatus required for acetylcholine release [21]. While several such molecules have demonstrated efficacy in protecting cells against BoNTA [13], [15], [20], there is scant information to show that small molecules can significantly protect mammals against BoNTA, although an in vivo study of small-molecule BoNTAe inhibitors has been reported [22].
Herein, we report the development of effective small-molecule BoNTAe inhibitors with in vivo half-live of 4–6 hours. These inhibitors showed 100% and 70% of protection of mice against BoNTA at 5 times of its median-lethal dose during the periods of 2 and 4 half-lives of the inhibitors at an inhibitor concentration of 2 mg/kg, respectively. We also discuss the prospect of small-molecule inhibitors as a cost-effective alternative or as an adjunct to passive immunity for treating botulism.
Results
Design and Synthesis
We previously reported a serotype-specific, small-molecule BoNTAe inhibitor, H3H (structure shown in Figure 1), which has a K i app value of 3.8±0.77 µM and was resulted from our lead identification and optimization as summarized in Figure 1 [14], [23]. One drawback of H3H is insolubility in water. In optimizing H3H for water solubility and higher potency in inhibiting BoNTAe, we encountered problems in derivatizing H3H caused by chemical instability under acidic conditions (pH<2.0) that was presumably due to the proton at position 3 of the indole ring. These problems hampered the structural modifications of H3H guided by insights from computer simulations or the crystal structures of inhibitor-bound BoNTAe complexes.
Figure 1. The development process of H3H, F4H and F3A as small-molecule BoNTAe inhibitors.
Recognizing the synthesis step as the rate-determining step of the optimization, we set out to first establish a facile synthetic scheme that can lead to a group of inhibitor analogues and then use computer simulations of the inhibitor-bound BoNTAe complexes to prioritize the syntheses of the analogues. This was different from what we did earlier, namely, first finding alternative analogues on the basis of computer simulations and then determining whether the alternatives were synthetically accessible.
Accordingly, we developed a simple synthetic scheme shown Figure 2 that begins with a known intermediate used for the synthesis of H3H [14]. The new scheme, which readily leads to a handful of new analogues of H3H by varying substituents R1, R2, and R3, enabled us to address the problems of water solubility and chemical instability of H3H by introducing hydrophilic groups and replacing the position-3 proton of the indole ring with a fluorine atom [24], respectively. Preliminary multiple molecular dynamics simulations (10 1-ns-long simulations) suggested that two of such analogues, F3A and F4H (structures shown in Figure 1), might be able to interact favourably with the active site of BoNTAe. The simulation results were later supported by the extended multiple molecular dynamics simulations (10 10-ns-long simulations) described below.
Figure 2. Synthetic scheme for F4H and F3A.
Therefore, we made F4H and F3A with relative ease according to the scheme shown in Figure 2. Gratifyingly, we found that both F4H and F3A are water soluble at concentrations up to 5.0 mM and stable under acidic conditions.
Computer Simulation
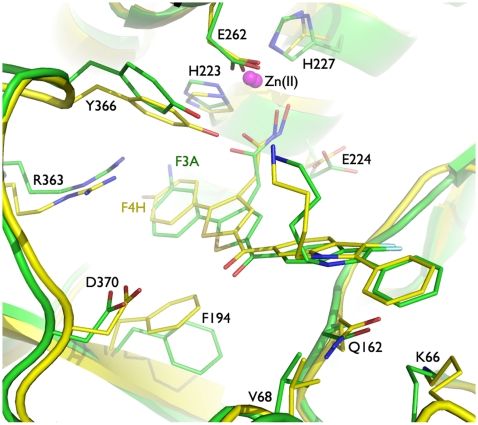
Subsequent extended multiple molecular dynamics simulations (10 10-ns-long simulations) of BoNTAe in complex with F4H or F3A suggested that both inhibitors have (1) the hydroxamate coordinating the zinc ion embedded in the active site, (2) the hydroxamate forming a hydrogen bond to Glu224, (3) the cation-pi interaction of the thiophene-substituted phenyl group with Arg363, (4) the pi-pi interactions of the thiophene-substituted phenyl group with Phe194 and Tyr366, (5) the interaction of the ketone oxygen atom with Asp370 that is bridged by at least one water molecule, and (6) the cation-pi and pi-pi interactions of the indole-substituted phenyl group with Lys66 and Gln162, respectively (Figure 3). The main differences between the two inhibitor complexes are that (1) the thiophene-substituted phenyl group has stronger pi-pi interactions (judged by distance) with Tyr366 and Phe194 in F4H•BoNTAe than in F3A•BoNTAe, (2) Tyr366 forms a hydrogen bond with the carbonyl oxygen atom of the hydroxamate in F4H•BoNTAe but not in F3A•BoNTAe, and (3) the interaction between the ketone oxygen atom and Asp370 is bridged by one or two water molecules in F4H•BoNTAe or F3A•BoNTAe, respectively. The coordinates of the simulation-generated F4H•BoNTAe and F3A•BoNTAe complexes are available in Datasets S1 and S2, respectively.
Figure 3. Overlay of simulation-generated models of F4H•BoNTAe (yellow) and F3A•BoNTAe (green).
For clarity the water molecules that bridge the interaction between Asp370 and the ketone oxygen atom are not displayed, but these water molecules along with other active-site water molecules are included in the coordinates of Datasets S1 and S2.
Biological Evaluation
High performance liquid chromatography (HPLC)-based BoNTAe inhibition assays [25] showed that F4H is as potent as H3H in inhibiting BoNTAe, and F3A is less potent than H3H (Table 1). Furthermore, H3H, F4H, and F3A showed no acute toxicity to mice. We therefore performed in vivo pharmacokinetic studies on all three inhibitors. Interestingly, the exposures of F4H and F3A to mice are nearly the same but slightly less than that of H3H, as measured by the area under the time-concentration curve (AUC), even though the maximum concentration (Cmax) and the concentration 24 hours after one dose of a test compound (C24) for each inhibitor are different (Table 1). The nearly identical half-lives (t1/2≈6 hours) of F4H and F3A are longer than that of H3H (t1/2≈4 hours). In this context, we further evaluated all three inhibitors using a standardized mouse model of botulism [26] to determine if they can protect mice against either extracellular or intracellular BoNTA during the period of 8 half-lives of the test inhibitor in a single-dose experiment.
Table 1. In Vitro Inhibition of BoNTAe and in Vivo Pharmacokinetic Data for H3H, F4H, and F3A.
| Inhibitor | % BoNTAe inhibition1 | Cmax (ng/mL) | C24 (ng/mL) | AUClast (hr•ng/mL) | T1/2 (hr) |
| H3H | 78±4 | 497.4 | 3.0 | 1547.3 | 4.35 |
| F4H | 82±6 | 738.4 | <0.5 | 1386.4 | 6.50 |
| F3A | 47±1 | 256.0 | 7.0 | 1385.9 | 6.25 |
The inhibition assays were conducted at an inhibitor concentration of 20 µM.
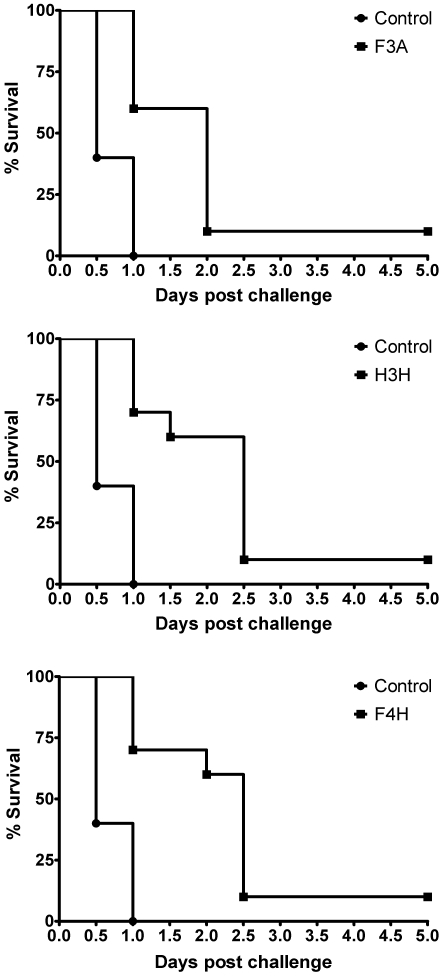
Groups of Balb/c mice were given one 2-mg/kg intraperitoneal injection of H3H, F4H, F3A, or dimethyl sulfoxide as a control and, after 30 minutes, each mouse was challenged intraperitoneally with BoNTA at 5 times of its median-lethal dose. All mice were examined twice daily for survival, behaviour, motor activity, breath, and extraocular symptoms of botulism. Each of the three inhibitors significantly (p<0.05) increased survival at different time intervals (Figure 4). Importantly, all mice treated with any of the three inhibitors survived during the 12-hour period (∼2t1/2 for F4H) after the BoNTA challenge. During this period, the inhibitors are expected to work optimally according to the time course of the inhibitor concentration in mouse plasma. In contrast, 60% of the untreated mice died during the 12-hour period. Consistently, all untreated mice died 24 hours (∼4t1/2 for F4H) after the challenge, whereas 70% and 60% of the F4H-treated mice survived 24 hours and 48 hours (∼8t1/2 for F4H) after the challenge, respectively (Figure 4). Furthermore, 10% of the mice treated with any of the three inhibitors survived without symptoms of botulism until they were euthanized on day 5 (Figure 4).
Figure 4. The survival curves of mice treated with placebo or a BoNTAe inhibitor.
F3A: top, H3H: middle, and F4H: bottom.
Discussion
Small-molecule BoNTAe inhibitors have been pursued actively by different research groups [13]–[20], but concern remains with regard to the feasibility of the small-molecule therapy for botulism, primarily because (1) BoNTA has a long in vivo half-life (t1/2 >31 days [7]), (2) small-molecule BoNTAe inhibitors with low nanomolar potencies are difficult to obtain [19], and (3) there has been only one article to date reporting an in vivo study of small-molecule BoNTAe inhibitors [22]. The work described above offers the following insights into the prospect of the small-molecule botulism therapy, although additional studies are needed to determine if the observed protection of mice against BoNTA by the pre-treatment of F4H, H3H, or F3A involves inhibition and clearance of extracellular toxin depots, uptake by intoxicated neurons, or both routes.
F4H, H3H, and F3A have in vivo half-lives of 4–6 hours, and all mice treated with any of the three inhibitors survived during the 12-hour period (∼2t1/2 for F4H) after the BoNTA challenge. It is therefore plausible that the problem with a long in vivo half-life of BoNTA can be mitigated by treating with an F4H-like compound one dose per day for multiple days. This treatment could be shortened if the compound were used in combination with long-lasting antibodies [27] that are effective to neutralize the extracellular toxin.
F4H showed 82±6% inhibition of BoNTAe at the inhibitor concentration of 20 µM. However, with one 2-mg/kg intraperitoneal injection, F4H showed 100, 70, and 60% protection of mice against BoNTA during the 12-, 24-, and 48-hour periods after the toxin challenge, respectively. This suggests that small-molecule BoNTAe inhibitors with low nanomolar potencies might not be necessary; inhibitors with low micromolar or high nanomolar potencies may suffice.
All three different inhibitors protected 100% of treated mice during the 12-hour period (∼2t1/2 for F4H) and 10% of the mice during the standard 5-day observation period, with a single intraperitoneal injection of the inhibitor against a supralethal BoNTA challenge. Furthermore, 90% of the F3A-treated mice, 40% of the H3H-treated mice, and 40% of the F4H-treated mice died 48 hours after the toxin challenge, respectively (Figure 4). The in vivo potencies appeared to be consistent with the in vitro potencies of the three inhibitors (Table 1). These results support the hypothesis that protection of mice against BoNTA can be achieved by treatment with a small-molecule BoNTAe inhibitor and are incentive to improve BoNTAe inhibitor structures and dosing regimen to optimize in vivo efficacies.
In summary, the present work demonstrates that small-molecule inhibitors can significantly protect mice against BoNTA and encourages the pursuit of small-molecule BoNTAe inhibitors for alternative or complementary treatment of botulism.
Materials and Methods
The animal experiments were performed with an approved protocol by the Institutional Animal Care and Use Committee at the Walter Reed Army of Institute of Research (IACUC number: B02-08) that is in compliance with the Animal Welfare Act and other United States federal statutes and regulations involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition.
Reagents
Hexanes (Hex), ethyl acetate (EtOAc), and trifluoroacetic acid (TFA) were purchased from Fisher Scientific (Pittsburgh, PA). BSA, HEPES buffer, and zinc chloride were purchased from Sigma-Aldrich (St. Louis, MO). Dithiothreitol was obtained from BioRad (Hercules, CA). All commercially available reagents were used as received. Recombinant BoNTAe was provided by Dr. Leonard Smith of the United States Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD.
Chemical Synthesis
General Description
The 1H NMR (400 MHz) and 13C NMR (100 MHz) spectra were recorded on a Mercury 400 spectrometer from Varian (Palo Alto, CA). Chemical shifts are reported in ppm using either tetramethylsilane or the solvent peak as an internal standard. Data are reported as follows: chemical shift, multiplicity (s = singlet, brs = broad singlet, d = doublet, t = triplet, brt = broad triplet, q = quartet, m = multiplet), coupling constant, and integration. Low-resolution mass spectra were recorded using either Hewlet Packard 5973 Mass Spectrometer with SIS Direct Insertion Probe (Palo Alto, CA) or Waters ZQ/EMD 1000 Mass Spectrometer (Milford, MA). High-resolution mass spectra were obtained on a Bruker BioTOF II ESI. IR spectra were obtained on a ThermoNicolet Avatar 370 FT-IR (Waltham, MA) using KBr pellet. Medium pressure liquid chromatography (MPLC) was performed with Biotage SP-1 (Charlottesville, VA) using silica gel (EM Science, 230–400 mesh). HPLC was carried out on a 5-µm C18 column (analytical: 4.60×250 mm, HyperClone; semi-preparative: 21.2×250 mm, Gemini) from Phenomenex (Torrance, CA) eluting with linear gradient of 80% of solution A (1000 mL of H2O and 1 mL of TFA) to 100% of solution B (100 mL of H2O, 900 mL of MeCN and 1 mL of TFA) over 20 minutes at a flow rate of 1.0 mL/min (analytical) or over a specified amount of time at a flow rate of 10 mL/min (semi-preparative) with UV detection at 254 nm on a Beckman Coulter System Gold HPLC system (166P detector and 125P solvent module) from Beckman Coulter (Brea, CA). KCN is highly toxic and must be handled with extreme care by trained personnel.
Methyl 2-(2-bromo-5-(1-(4-(1,3-dioxoisoindolin-2-yl)butyl)-3-fluoro-2-phenyl-1H-indole-6-carbonyl)thiophen-3-yl)acetate (2)
To a solution of methyl 2-(2-bromo-5-(1-(4-(1,3-dioxoisoindolin-2-yl)butyl)-2-phenyl-1H-indole-6-carbonyl)-thiophen-3-yl)acetate (1 in Figure 2) [14] (156 mg, 0.24 mmol) in 3 mL CH2Cl2 was added 1-fluoropyridinium triflate (78 mg, 0.28 mmol), and then the mixture was stirred at room temperature for 6 days. The resulting mixture was diluted with 40 mL Et2O, washed with brine (2×10 mL), dried over MgSO4, filtered, and then concentrated in vacuo. MPLC purification (Hex∶EtOAc/9∶1) gave 2 (66 mg, 41%) as a yellow solid foam. 1H NMR (CDCl3) δ 7.94 (s, 1H), 7.78−7.75 (m, 2H), 7.71−7.63 (m, 4 H), 7.57 (s, 1H), 7.50−7.39 (m, 5H), 4.25 (t, J = 7.2 Hz, 2H), 3.71 (s, 3H), 3.69 (s, 2H), 3.49 (t, J = 6.6 Hz, 2H), 1.67−1.60 (m, 2H), and 1.50−1.43 (m, 2H) (see Figure S1 for proton NMR spectrum of 2); 13C NMR (CDCl3) δ 187.42, 170.49, 168.57, 143.91, 141.87 (1 J CF = 244.0 Hz), 136.06, 135.98, 135.02, 134.26, 132.30 (3 J CF = 7.0 Hz), 132.08, 131.54, 129.95, 129.17, 128.44 (3 J CF = 3.0 Hz), 127.60 (2 J CF = 21.0 Hz), 123.48, 122.10, 122.20, 120.17 (2 J CF = 16.0 Hz), 117.04 (3 J CF = 3.0 Hz), 112.66, 52.61 (q, J = 10.7 Hz), 43.64, 37.23, 35.07, 27.32, and 25.76; IR cm−1 2921.2, 1707.6, and 1393.0; LRMS-EI m/z 672 and 674 (12% each, [M+]), 160 (100%, [CH2NPhth+]); HRMS-ESI calculated for C34H26BrFN2O5SNa+ [M+Na+] 695.0622, found 695.0619.
Methyl 2-(5-(1-(4-(1,3-dioxoisoindolin-2-yl)butyl)-3-fluoro-2-phenyl-1H-indole-6-carbonyl)-2-(4-hydroxyphenyl)thiophen-3-yl) (3x)
A mixture of 2 (42 mg, 0.062 mmol), Pd(PPh3)4 (8 mg, 0.007 mmol), CsF (28 mg, 0.18 mmol), 4-hydroxyphenylboronic acid (13 mg, 0.094 mmol), and H2O (200 µL) in 1,2-dimethoxyethane (8 mL) was degassed with N2 for 10 minutes and then refluxed for 6 hours. The resulting suspension was poured into H2O (10 mL) and then extracted with 70 mL Et2O. The organic layer was washed with brine (2×10 mL), dried over MgSO4, and then concentrated in vacuo. MPLC purification (Hex∶EtOAc/5∶1) of the residue gave 3x as a yellow solid foam (34 mg, 79%). 1H NMR (CDCl3) δ 7.99 (s, 1H), 7.78−7.66 (m, 7H), 7.50−7.38 (m, 7H), 6.94 (d, J = 8.4 Hz, 2H), 6.27 (s, 1H), 4.26 (t, J = 7.0 Hz, 2H), 3.69 (m, 5H), 3.48 (t, J = 6.8 Hz, 2H), 1.68−1.59 (m, 2H), and 1.50−1.43 (m, 2H) (see Figure S2 for proton NMR spectrum of 3x); 13C NMR (CDCl3) δ 188.36, 171.87, 168.57, 157.05, 149.86, 141.93 (1 J CF = 244.6 Hz), 141.28, 137.87, 137.80, 134.24, 132.38, 132.35, 132.10, 131.03, 130.98 (3 J CF = 3.0 Hz), 129.98, 129.16, 128.56 (3 J CF = 3.0 Hz), 127.28 (2 J CF = 15.3 Hz), 125.25, 123.49, 121.42, 119.98 (2 J CF = 15.3 Hz), 116.93, 116.17, 112.64, 52.53 (q, J = 9.9 Hz), 43.61, 37.27, 34.49, 27.35, and 25.77; IR cm−1 3391.2, 2929.4, 2851.8, 1711.7, and 1442.0; LRMS-EI m/z 687 (100%, [M+]), 439 (65%); HRMS-ESI calculated for C40H31FN2O6SNa+ [M+Na+] 709.1779, found 709.1787.
2-(5-(1-(4-Aminobutyl)-3-fluoro-2-phenyl-1H-indole-6-carbonyl)-2-(4-hydroxyphenyl)thiophen-3-yl)-N-hydroxyacetamide(F4H)
To a stirred solution of 3x (34 mg, 0.049 mmol) in THF/MeOH (3 mL/5 mL), 1 mL of 50% aqueous NH2OH was added, followed by a catalytic amount (two crystals) of KCN. The resulting mixture was stirred for 23 hours at room temperature, and then filtered through a short Celite column. HPLC purification of the filtrate gave F4H•TFA as a yellow amorphous solid (20 mg, 60%). The semi-preparative and analytical HPLC retention times of F4H•TFA are 14.00 and 14.57 minutes, respectively (see Figure S3 for chromatograms of F4H•TFA before and after the HPLC purification). 1H NMR (CD3OD) δ 8.11 (s, 1H), 7.78 (s, 1H), 7.73 (d, J = 8.4 Hz, 1H), 7.66 (dd, J = 1.2, 8.4 Hz, 1H), 7.59−7.58 (m, 4H), 7.54−7.50 (m, 1H), 7.39 (d, J = 8.6 Hz, 2H), 6.92 (d, J = 8.6 Hz, 2H), 4.36 (t, J = 7.2 Hz, 2H), 3.51 (s, 2H), 2.79 (t, J = 7.2 Hz, 2H), 1.76−1.69 (m, 2H), and 1.50−1.43 (m, 2H) (see Figure S4 for proton NMR spectrum of F4H); 13C NMR (CD3OD) δ 188.80, 169.26, 160.90 (q, CF3 CO2H, 2 J CF = 36.6 Hz), 158.74, 150.11, 141.75 (1 J CF = 243.1 Hz), 140.72, 137.85, 132.22, 132.15 (3 J CF = 5.3 Hz), 130.73, 130.53, 129.85, 129.02, 128.96, 128.45 (3 J CF = 3.8 Hz), 127.30 (2 J CF = 20.6 Hz), 123.86, 120.58, 119.64 (2 J CF = 16.0 Hz), 116.65, 115.71, 113.10, 43.29, 39.18, 32.29, 27.11, and 24.70; IR cm−1 3438.5, 3227.7, 1677.0, 1608.9, 1551.6, 1428.1, 1250.1, 1202.9, 1138.2; LRMS-EI m/z 558 (48%, [M+]), 309 (36%); HRMS-ESI calculated for C31H29FN3O4S+ [M+H+] 558.1857, found 558.1901.
Methyl 2-(2-(3-aminophenyl)-5-(1-(4-(1,3-dioxoisoindolin-2-yl)butyl)-3-fluoro-2-phenyl-1H-indole-6-carbonyl)thiophen-3-yl)acetate (3y)
A mixture of 2 (20 mg, 0.03 mmol), Pd(PPh3)4 (7 mg, 0.006 mmol), CsF (13 mg, 0.09 mmol), 3-aminophenylboronic acid (6 mg, 0.04 mmol), and H2O (60 µL) in 1,2-dimethoxyethane (4 mL) was degassed with N2 for 10 minutes and then refluxed until all the starting ester had been consumed (3 hours). The resulting black suspension was poured into H2O (10 mL) and then extracted with 40 mL Et2O. The organic layer was washed with brine (2×20 mL), dried over MgSO4, and then concentrated in vacuo. MPLC purification (Hex∶EtOAc/5∶1) of the residue gave 3y as a yellow solid foam (15 mg, 74%). 1H NMR (CDCl3) δ 7.99 (s, 1H), 7.78−7.76 (m, 2H), 7.71−7.67 (m, 5H), 7.51−7.37 (m, 5H), 7.24 (t, J = 7.6 Hz, 1H), 6.88 (d, J = 7.2 Hz, 1H), 6.83 (s, 1H), 6.73 (d, J = 7.2 Hz, 1H), 4.26 (t, J = 7.0 Hz, 2H), 3.72 (s, 2H), 3.69 (s, 3H), 3.48 (t, J = 6.8 Hz, 2H), 1.66−1.58 (m, 2H), and 1.50−1.45 (m, 2H) (see Figure S5 for proton NMR spectrum of 3y); 13C NMR (CDCl3) δ 188.12, 171.73, 168.51, 149.72, 147.07, 141.92 (1 J CF = 245.0 Hz), 141.74, 137.49, 134.19, 134.09, 132.46, 132.36 (3 J CF = 6.0 Hz), 132.13, 130.36, 129.96, 129.15, 129.05, 128.58 (3 J CF = 3.0 Hz), 127.21 (2 J CF = 10.7 Hz), 123.46, 121.39, 119.94 (3 J CF = 6.1 Hz), 119.71, 116.89, 115.80 (2 J CF = 15.3 Hz), 112.59, 52.41, 43.61, 37.25, 34.50, 27.36, and 25.77; IR cm−1 3456.5, 3366.6, 2945.7, 1711.7, 1601.4 and 1393.0; LRMS-EI m/z 686 (100%, [M+]); HRMS-ESI calculated for C40H33FN3O5S+ [M+H+] 686.2119, found 686.2128.
2-(5-(1-(4-Aminobutyl)-3-fluoro-2-phenyl-1H-indole-6-carbonyl)-2-(3-aminophenyl)thiophen-3-yl)-N-hydroxyacetamide (F3A)
To a stirred solution of 3y (15 mg, 0.022 mmol) in THF/MeOH (0.5 mL/0.5 mL), 0.5 mL of 50% aqueous NH2OH was added, followed by a catalytic amount (two crystals) of KCN. The resulting mixture was stirred for 16 hours at room temperature, and then filtered through a short Celite column. HPLC purification (eluting time: 20 minutes) of the filtrate gave F3A•2TFA as a yellow solid foam (12 mg, 71%). The semi-preparative and analytical HPLC retention times of F3A•2TFA are 13.12 and 12.25 minutes, respectively (see Figure S6 for chromatograms of F3A•2TFA before and after the HPLC purification). 1H NMR (CD3OD) δ 8.12 (s, 1H), 7.82 (s, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.69 (d, J = 8.4 Hz, 1H), 7.66−7.51 (m, 8H), 7.44−7.42 (m, 1H), 4.37 (t, J = 6.8 Hz, 2H), 3.54 (s, 2H), 2.78 (t, J = 7.2 Hz, 2H), 1.76−1.68 (m, 2H), and 1.49−1.41 (m, 2H) (see Figure S7 for proton NMR spectrum of F3A); 13C NMR (CD3OD) δ 188.54, 168.75, 160.22 (q, CF3 CO2H, 2 J CF = 39.7 Hz), 146.93, 142.57, 141.73 (1 J CF = 243.1 Hz), 137.34, 134.89, 134.07, 132.54, 132.20 (3 J CF = 5.4 Hz), 131.88, 130.72, 129.86, 129.08, 128.99, 128.38 (3 J CF = 3.1 Hz), 128.29, 127.57 (2 J CF = 20.5 Hz), 122.62 (2 J CF = 32.0 Hz), 120.69, 119.83 (2 J CF = 15.3 Hz), 116.65, 116.23 (q, CF3CO2H, 1 J CF = 287.4 Hz), 113.08, 43.28, 39.15, 32.28, 27.06, and 24.67; IR cm−1 3432.0, 2925.3, 1679.0, 1200.9, 1135.5; LRMS-EI m/z 557 (60%, [M+]), 309 (62%); HRMS-ESI calculated for C31H30FN4O3S+ [M+H+] 557.2017, found 557.2040.
in Vitro Evaluation
Assays of the BoNTAe activity were done at 37°C and contained 0.5 mM substrate, 0.5–1.5 µg/mL recombinant BoNTAe, 40 mM HEPES, 1 mM dithiothreitol, 25 µM ZnCl2, 0.5 mg/mL BSA, and 0.05% tween at pH 7.3. Substrate for BoNTAe was an SNAP-25 fragment containing residues 187–203 with N- and C-termini acylated and amidated, respectively [28]. Inhibitors were dissolved in dimethyl sulfoxide at 10 times the final assay concentration, then diluted into the assay mixture containing substrate, followed by addition of the endopeptidase (i.e., inhibitor and endopeptidase were not preincubated). Assay times and endopeptidase concentrations were adjusted so that less than 10% of the substrate was hydrolyzed. Assays were stopped by acidification with TFA and analyzed by reverse-phase HPLC as described previously [25].
in Vivo Evaluation
Pharmacokinetics Study
The in vivo pharmacokinetic parameters were determined by dosing 6 Balb/c mice intraperitoneally with a test inhibitor at 2 mg/kg at which concentration no obvious sign of toxicity was observed. Blood was collected by cardiac puncture at 0.5, 1, 2, 4, 8, and 24 hours and the plasma was separated and kept frozen at −80°C until processing. Each experiment was repeated three times. The plasma was thawed and extracted with two volumes of ice-cold acetonitrile to precipitate plasma proteins and release the inhibitor. The organic phase was analyzed by liquid chromatography mass spectrometry and the concentration of the inhibitor was determined based on a standard curve run in parallel. The stability of the inhibitor in acetonitrile was determined prior to analyzing pharmacokinetic samples. Pharmacokinetic values were determined with WinNonLin software from Pharcite based on the plasma concentration curve.
Protection Study
The protection studies were carried out by using a standardized mouse model of botulism [26]. Briefly, groups of Balb/c mice were given a single 2-mg/kg intraperitoneal injection of H3H, F4H, F3A or dimethyl sulfoxide as a control and, after 30 minutes, each mouse was challenged intraperitoneally with BoNTA at 5 times of its median-lethal dose. Dimethyl sulfoxide was used as a carrier vehicle because H3H is water insoluble. All mice were examined twice daily for survival, behaviour, motor activity, breath, and extraocular symptoms of botulism. The numbers of mice in the treated and control groups were 10 and 5, respectively. Survival curves were constructed based on the number of survivors and statistically analyzed using GraphPad Prism 5.0 (Graphpad Software, Inc.).
Computer Simulations
Model Preparation
The atomic charges of F4H and F3A were obtained according to the RESP procedure [29] with ab initio calculations at the HF/6-31G*//HF/6-31G* level using the Gaussian 98 program [30]. The starting structure of inhibitor•BoNTAe was generated by (1) manually docking the inhibitor into the BoNTAe active site and (2) replacing the active-site zinc ion with the tetrahedral zinc ion using the cationic dummy atom approach [23], [31]–[33]. In the manual docking, the hydroxamate group was placed near the tetrahedral zinc ion, the thiophene-substituted phenyl group was placed near Arg363, and the ammonium group was placed near Glu64. The BoNTAe structure used for the docking was taken from the crystal structure of an inhibitor-bound BoNTAe (Protein Data Bank Code: 3BOO [34]) whose conformations of missing residues 62–67 were taken from the crystal structure of a BoNTAe mutant in complex with SNAP-25 (Protein Data Bank Code: 1XTG [35]). For BoNTAe, His223 and His227 were treated as HIN (histidinate) [32], [36], [37]; His39, His230, and His269 were treated as HID; all other His residues were treated as HIP; Glu261 and Glu351 were treated as GLH [32], [36], [37]. A total of 111 crystallographically determined water molecules (named HOH) located inside the enzyme were included for simulations. The topology and coordinate files of the water-containing inhibitor•BoNTAe complex were generated by the PREP, LINK, EDIT, and PARM modules of the AMBER 5.0 program [38]. The complex was refined by energy minimization using a dielectric constant of 1.0 and 100 cycles of steepest-descent minimization followed by 100 cycles of conjugate-gradient minimization. The refined complex was solvated with 13,617 and 13,540 TIP3P water molecules (named WAT) [39] for F4H and F3A, leading to a system of 48,096 and 47,866 atoms, respectively. The WAT molecules were obtained from solvating the complex using a pre-equilibrated box of 216,000 TIP3P molecules, whose hydrogen atom charge was set to 0.4170, where any water molecule was removed if it had an oxygen atom closer than 2.2 Å to any solute atom or a hydrogen atom closer than 2.0 Å to any solute atom, or if it was located further than 9.0 Å along the x-, y-, or z-axis from any solute atom.
Multiple Molecular Dynamics Simulations
The solvated complex system was energy-minimized for 100 cycles of steepest-descent minimization followed by 100 cycles of conjugate-gradient minimization to remove close van der Waals contacts in the system, then heated from 0 to 300 K at a rate of 10 K/ps under constant temperature and volume, and finally simulated independently with a unique seed number for initial velocities at 300 K under constant temperature and pressure using the PMEMD module of the AMBER 8.0 program [40] with the AMBER force field (ff99SB) [41], [42]. All simulations used (1) a dielectric constant of 1.0, (2) the Berendsen coupling algorithm [43], (3) a periodic boundary condition at a constant temperature of 300 K and a constant pressure of 1 atm with isotropic molecule-based scaling, (4) the Particle Mesh Ewald method to calculate long-range electrostatic interactions [44], (5) a time step of 1.0 fs, (6) the SHAKE-bond-length constraints applied to all the bonds involving the H atom, (7) saving the image closest to the middle of the “primary box” to the restart and trajectory files, (8) formatted restart file, and (9) default values of all other inputs of the PMEMD module. Ten different molecular dynamics simulations (each lasted 10 ns) were carried out for the BoNTAe in complex with F4H or F3A on a cluster of Apple Mac Pros with 80 Intel Xeon cores (3.0 GHz).
Simulation Analysis
For each of the 10 simulations of F4H•BoNTAe or F3A•BoNTAe, 100 instantaneous conformations were saved at 10-ps intervals during the last 1-ns period. A total of 1,000 instantaneous conformations of F4H•BoNTAe or F3A•BoNTAe from the 10 simulations were subjected to a cluster analysis using the averagelinkage algorithm (epsilon = 2.0 Å and RMS on alpha-carbon atoms) [45] implemented in the PTRAJ module of the AMBER 10 program [40]. Only one cluster of the BoNTAe conformations was identified. All 1,000 instantaneous conformations of F4H•BoNTAe or F3A•BoNTAe were subjected to a second-round cluster analysis using the averagelinkage algorithm (epsilon = 1.5 Å and RMS on all atoms of F4H or F3A) [45]. This analysis identified 7 and 4 clusters for the F4H and F3A conformations, respectively. The numbers of the F4H conformations in Clusters 1–7 are 200, 100, 423, 27, 150, 30, and 70, respectively; the numbers of the F3A conformations in Clusters 1–4 are 600, 299, 1, and 100, respectively. The representative conformations of F4H•BoNTAe and F3A•BoNTAe from their most populated clusters overlay reasonably well (see Figure 3) and are considered as plausible complex structures in water. The coordinates of the representative conformations are available from Datasets S1 and S2. The coordinates of other conformations are available upon request.
Supporting Information
Proton NMR spectrum of 2.
(0.79 MB PDF)
Proton NMR spectrum of 3x.
(0.56 MB PDF)
Chromatograms of F4H⋅TFA before and after the HPLC purification.
(0.17 MB PDF)
Proton NMR spectrum of F4H.
(0.54 MB PDF)
Proton NMR spectrum of 3y.
(0.50 MB PDF)
Chromatograms of F3A⋅2TFA before and after the HPLC purification.
(0.17 MB PDF)
Proton NMR spectrum of F3A.
(0.43 MB PDF)
Coordinates of simulation-generated model of F4H⋅BoNTAe.
(0.46 MB TXT)
Coordinates of simulation-generated model of F3A⋅BoNTAe.
(0.47 MB TXT)
Acknowledgments
We thank Christine M. McGuire of the Mayo Clinic for preparing intermediates in the synthesis of F3A. We are grateful to Dr. Victor Melendez of the Walter Reed Army Institute of Research for his guidance and insight into the pharmacokinetic experiments.
Footnotes
Competing Interests: Y-PP, JD, SW, JGP, JJS, and CBM are inventors of a filed provisional patent application titled “Small-Molecule Botulinum Toxin Inhibitors” that covers the inhibitors described in this paper, but this does not alter the adherence to all the PLoS ONE policies on sharing data and materials.
Funding: This work was supported by the United States Army Medical Research and Materiel Command (W81XWH-04-2-0001 and W81XWH-08-1-0154), the United States Army Research Office (W911NF-09-1-0095), the United States Defense Threat Reduction Agency (3.10023_07_RD_B and 3.10014_08_WR_B), and the University of Minnesota Supercomputing Institute. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the United States Army, Navy or the Department of Defense.
References
- 1.Shapiro RL, Hatheway C, Swerdlow DL. Botulism in the United States - a clinical and epidemiologic review. Ann Intern Med. 1998;129:221–228. doi: 10.7326/0003-4819-129-3-199808010-00011. [DOI] [PubMed] [Google Scholar]
- 2.Kessler KR, Benecke R. Botulinum toxin—from poison to remedy. Neurotoxicology. 1997;18:761–770. [PubMed] [Google Scholar]
- 3.Springen K, Raymond J, Skipp C, Scelfo J, SS. The Botox boom. Newsweek. 2002. pp. 50–58.
- 4.Crowner BE, Brunstrom JE, Racette BA. Iatrogenic botulism due to therapeutic botulinum toxin A injection in a pediatric patient. Clin Neuropharmacol. 2007;30:310–313. doi: 10.1097/WNF.0b013e31804b1a0d. [DOI] [PubMed] [Google Scholar]
- 5.Kuehn BM. FDA requires black box warnings on labeling for botulinum toxin products. JAMA. 2009;301:2316. doi: 10.1001/jama.2009.780. [DOI] [PubMed] [Google Scholar]
- 6.Liang BA. Fade to black: importation and counterfeit drugs. Am J Law Med. 2006;32:279–323. doi: 10.1177/009885880603200207. [DOI] [PubMed] [Google Scholar]
- 7.Foran PG, Mohammed N, Lisk GO, Nagwaney S, Lawrence GW, et al. Evaluation of the therapeutic usefulness of botulinum neurotoxin B, C1, E, and F compared with the long lasting type A. Basis for distinct durations of inhibition of exocytosis in central neurons. J Biol Chem. 2003;278:1363–1371. doi: 10.1074/jbc.M209821200. [DOI] [PubMed] [Google Scholar]
- 8.Arnon SS, Schechter R, Inglesby TV, Henderson DA, Bartlett JG, et al. Botulinum toxin as a biological weapon: medical and public health management. JAMA. 2001;285:1059–1070. doi: 10.1001/jama.285.8.1059. [DOI] [PubMed] [Google Scholar]
- 9.Franz DR, Jahrling PB, Friedlander AM, McClain DJ, Hoover DL, et al. Clinical recognition and management of patients exposed to biological warfare agents. JAMA. 1997;278:399–411. doi: 10.1001/jama.278.5.399. [DOI] [PubMed] [Google Scholar]
- 10.Wein LM, Liu Y. Analyzing a bioterror attack on the food supply: the case of botulinum toxin in milk. Proc Natl Acad Sci USA. 2005;102:9984–9989. doi: 10.1073/pnas.0408526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnon SS. Creation and development of the public service orphan drug Human Botulism Immune Globulin. Pediatrics. 2007;119:785–789. doi: 10.1542/peds.2006-0646. [DOI] [PubMed] [Google Scholar]
- 12.Larsen JC. U.S. Army botulinum neurotoxin (BoNT) medical therapeutics research program: past accomplishments and future directions. Drug Develop Res. 2009;70:266–278. [Google Scholar]
- 13.Boldt GE, Eubanks LM, Janda KD. Identification of a botulinum neurotoxin A protease inhibitor displaying efficacy in a cellular model. Chem Commun (Camb) 2006. pp. 3063–3065. [DOI] [PubMed]
- 14.Tang J, Park JG, Millard CB, Schmidt JJ, Pang Y-P. Computer-aided lead optimization: improved small-molecule inhibitor of the zinc endopeptidase of botulinum neurotoxin serotype A. PLoS ONE. 2007;2:e761. doi: 10.1371/journal.pone.0000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnett JC, Ruthel G, Stegmann CM, Panchal RG, Nguyen TL, et al. Inhibition of metalloprotease botulinum serotype A from a pseudo-peptide binding mode to a small molecule that is active in primary neurons. J Biol Chem. 2007;282:5004–5014. doi: 10.1074/jbc.M608166200. [DOI] [PubMed] [Google Scholar]
- 16.Johnson SL, Chen LH, Harbach R, Sabet M, Savinov A, et al. Rhodanine derivatives as selective protease inhibitors against bacterial toxins. Chem Biol Drug Des. 2008;71:131–139. doi: 10.1111/j.1747-0285.2007.00617.x. [DOI] [PubMed] [Google Scholar]
- 17.Moe ST, Thompson AB, Smith GM, Fredenburg RA, Stein RL, et al. Botulinum neurotoxin serotype A inhibitors: Small-molecule mercaptoacetamide analogs. Bioorg Med Chem. 2009;17:3072–3079. doi: 10.1016/j.bmc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roxas-Duncan V, Enyedy I, Montgomery VA, Eccard VS, Carrington MA, et al. Identification and biochemical characterization of small-molecule inhibitors of Clostridium botulinum neurotoxin serotype A. Antimicrob Agents Ch. 2009;53:3478–3486. doi: 10.1128/AAC.00141-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang Y-P, Vummenthala A, Mishra RK, Park JG, Wang SH, et al. Potent new small-molecule inhibitor of botulinum neurotoxin serotype A endopeptidase developed by synthesis-based computer-aided molecular design. PLoS ONE. 2009;4:e7730. doi: 10.1371/journal.pone.0007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai S, Lindo P, Park JB, Vasa K, Singh BR. The identification and biochemical characterization of drug-like compounds that inhibit botulinum neurotoxin serotype A endopeptidase activity. Toxicon. 2010;55:818–826. doi: 10.1016/j.toxicon.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson LL. The origin, structure, and pharmacological activity of botulinum toxin. Pharmacol Rev. 1981;33:155–188. [PubMed] [Google Scholar]
- 22.Eubanks LM, Hixon MS, Jin W, Hong S, Clancy CM, et al. An in vitro and in vivo disconnect uncovered through high-throughput identification of botulinum neurotoxin A antagonists. Proc Natl Acad Sci USA. 2007;104:2602–2607. doi: 10.1073/pnas.0611213104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JG, Sill PC, Makiyi EF, Garcia-Sosa AT, Millard CB, et al. Serotype-selective, small-molecule inhibitors of the zinc endopeptidase of botulinum neurotoxin serotype A. Bioorg Med Chem. 2006;14:395–408. doi: 10.1016/j.bmc.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 24.Muller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt JJ, Bostian KA. Endoproteinase activity of type A botulinum neurotoxin: substrate requirements and activation by serum albumin. J Protein Chem. 1997;16:19–26. doi: 10.1023/a:1026386710428. [DOI] [PubMed] [Google Scholar]
- 26.Hatheway CH, Snyder JD, Seals JE, Edell TA, Lewis GE., Jr Antitoxin levels in botulism patients treated with trivalent equine botulism antitoxin to toxin types A, B, and E. J Infect Dis. 1984;150:407–412. doi: 10.1093/infdis/150.3.407. [DOI] [PubMed] [Google Scholar]
- 27.Sarvas H, Seppala I, Kurikka S, Siegberg R, Makela O. Half-life of the maternal IgG1 allotype in infants. J Clin Immunol. 1993;13:145–151. doi: 10.1007/BF00919271. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt JJ, Stafford RG, Bostian KA. Type A botulinum neurotoxin proteolytic activity - development of competitive inhibitors and implications for substrate specificity at the s-1' binding subsite. FEBS Lett. 1998;435:61–64. doi: 10.1016/s0014-5793(98)01041-2. [DOI] [PubMed] [Google Scholar]
- 29.Cieplak P, Cornell WD, Bayly C, Kollman PA. Application of the multimolecule and multiconformational resp methodology to biopolymers: charge derivation for DNA, RNA, and proteins. J Comput Chem. 1995;16:1357–1377. [Google Scholar]
- 30.Frisch MJ, Trucks GW, Schlegel HB, Gill PMW, Johnson BG, et al. GAUSSIAN 98, Revision A.7. 1999. Gaussian, Inc Pittsburgh, PA.
- 31.Pang Y-P. Novel zinc protein molecular dynamics simulations: steps toward antiangiogenesis for cancer treatment. J Mol Model. 1999;5:196–202. [Google Scholar]
- 32.Pang Y-P, Xu K, El Yazal J, Prendergast FG. Successful molecular dynamics simulation of the zinc-bound farnesyltransferase using the cationic dummy atom approach. Protein Sci. 2000;9:1857–1865. [PMC free article] [PubMed] [Google Scholar]
- 33.Pang Y-P. Successful molecular dynamics simulation of two zinc complexes bridged by a hydroxide in phosphotriesterase using the cationic dummy atom method. Proteins. 2001;45:183–189. doi: 10.1002/prot.1138. [DOI] [PubMed] [Google Scholar]
- 34.Silvaggi NR, Wilson D, Tzipori S, Allen KN. Catalytic features of the botulinum neurotoxin A light chain revealed by high resolution structure of an inhibitory peptide complex. Biochemistry. 2008;47:5736–5745. doi: 10.1021/bi8001067. [DOI] [PubMed] [Google Scholar]
- 35.Breidenbach MA, Brunger AT. Substrate recognition strategy for botulinum neurotoxin serotype A. Nature. 2004;432:925–929. doi: 10.1038/nature03123. [DOI] [PubMed] [Google Scholar]
- 36.El Yazal J, Pang Y-P. Ab initio calculations of proton dissociation energies of zinc ligands: hypothesis of imidazolate as zinc ligand in proteins. J Phys Chem B. 1999;103:8773–8779. [Google Scholar]
- 37.El Yazal J, Roe RR, Pang Y-P. Zinc's affect on proton transfer between imidazole and acetate predicted by ab initio calculations. J Phys Chem B. 2000;104:6662–6667. [Google Scholar]
- 38.Pearlman DA, Case DA, Caldwell JW, Ross WS, Cheatham TE, et al. AMBER, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput Phys Commun. 1995;91:1–41. [Google Scholar]
- 39.Jorgensen WL, Chandreskhar J, Madura JD, Impey RW, Klein ML. Comparison of simple potential functions for simulating liquid water. J Chem Phys. 1982;79:926–935. [Google Scholar]
- 40.Case DA, Cheatham TE, 3rd, Darden T, Gohlke H, Luo R, et al. The Amber biomolecular simulation programs. J Comput Chem. 2005;26:1668–1688. doi: 10.1002/jcc.20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, et al. Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins. 2006;65:712–725. doi: 10.1002/prot.21123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickstrom L, Okur A, Simmerling C. Evaluating the performance of the ff99SB force field based on NMR scalar coupling data. Biophys J. 2009;97:853–856. doi: 10.1016/j.bpj.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berendsen HJC, Postma JPM, van Gunsteren WF, Di Nola A, Haak JR. Molecular dynamics with coupling to an external bath. J Chem Phys. 1984;81:3684–3690. [Google Scholar]
- 44.Darden TA, York DM, Pedersen LG. Particle Mesh Ewald: An N log(N) method for Ewald sums in large systems. J Chem Phys. 1993;98:10089–10092. [Google Scholar]
- 45.Shao J, Tanner SW, Thompson N, Cheatham TE., III Clustering molecular dynamics trajectories: 1. characterizing the performance of different clustering algorithms. J Chem Theory Comput. 2007;3:2312–2334. doi: 10.1021/ct700119m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proton NMR spectrum of 2.
(0.79 MB PDF)
Proton NMR spectrum of 3x.
(0.56 MB PDF)
Chromatograms of F4H⋅TFA before and after the HPLC purification.
(0.17 MB PDF)
Proton NMR spectrum of F4H.
(0.54 MB PDF)
Proton NMR spectrum of 3y.
(0.50 MB PDF)
Chromatograms of F3A⋅2TFA before and after the HPLC purification.
(0.17 MB PDF)
Proton NMR spectrum of F3A.
(0.43 MB PDF)
Coordinates of simulation-generated model of F4H⋅BoNTAe.
(0.46 MB TXT)
Coordinates of simulation-generated model of F3A⋅BoNTAe.
(0.47 MB TXT)