Abstract
Objective
To determine if mesenchymal stem cells (MSC) derived from human fetal pancreatic tissue (pMSC) would engraft and differentiate in sheep pancreas following transplantation in utero.
Methods
A three-step culture system was established for generating human fetal pMSC. Sheep fetuses were transplanted during the fetal transplant receptivity period with human pMSC and evaluated for in situ and functional engraftment in their pancreas, liver and bone marrow.
Results
Isolation and expansion of adherent cells from the human fetal pancreas yielded a cell population with morphologic and phenotypic characteristics similar to MSC derived from bone marrow. This putative stem cell population could undergo multilineage differentiation in vitro. Three to 27 months after fetal transplantation, the pancreatic engraftment frequency (chimeric index) was 79% while functional engraftment was noted in 50% of transplanted sheep. Hepatic and marrow engraftment and expression was noted as well.
Conclusion
We have established a procedure for isolation of human fetal pMSC that display characteristics similar to bone marrow derived MSC. In vivo results suggest the pMSC engraft, differentiate and secrete human insulin from the sheep pancreas.
Keywords: pancreatic mesenchymal stem cells, in utero stem cell transplantation, Type 1 diabetes
Introduction
Type 1 diabetes is a chronic autoimmune disease characterized by organ specific inflammation with subsequent death of insulin producing beta-cells in the islets of Langerhans (1, 2). Markers include the development of multiple autoantibodies in individuals with susceptible major histocompatibility genotypes (3). Such patients develop close to complete insulin deficiency with impaired glucose homeostasis.
Exogenous delivery systems used as therapy in such patients have proved inadequate in preventing the devastating clinical consequences of impaired glucose homeostasis (4, 5) Cure of, or amelioration of disease, requires a system that will permit in vivo homeostatic control of circulating glucose concentrations by means of real time adjustment in insulin secretion. Present research is testing the feasibility of biologic systems/techniques to generate an adequate functioning beta-cell mass (6-9). All systems will likely require glucocorticoid free immunosuppression to prevent graft rejection or reoccurrence of active autoimmune insulitis.
Our laboratory has been interested in in utero transplantation in a large animal (sheep) for its feasibility in expanding understanding of basic and applied biologic systems / problems (10). This model has proved useful in refining our knowledge of immune tolerance to self, transplantation tolerance, stem cell compartments, stem cell physiology and cell/tissue transplantation (11-15). The theoretical basis rests in the well-recognized propensity of the fetus for transplantation tolerance (16, 17). For example, following transplantation at the appropriate gestational age, fetal sheep will characteristically permit stem cells (SC) to engraft and express donor derived cells in adult sheep and/or secondary recipients (18, 19). This gestational phase or period of transplant receptivity occurs when ontogenic programming of self-tolerance occurs. The period immediately follows demarcation of the thymus into cortex/medulla and is characterized by active immune cell maturation in the thymus, ending shortly after significant lymphocyte infiltration of the developing spleen (11, 20). We believe this fetal tolerance phase in a large animal provides a powerful tool to use in the investigation of biologic systems and may allow alternative solutions to a number of clinical problems.
To this end we began a series of studies aimed at determining the feasibility of this system to permit xenoengraftment and expression of functioning human pancreatic islets. We chose first to isolate human fetal pancreas derived mesenchymal stem cells (pMSC) using a three-step culture system. Putative pMSC gene transcription profiles, surface antigen expression and multilineage differentiation were assessed. Sheep fetuses were transplanted during the fetal immune tolerance induction phase with intra-peritoneal injections of pMSC populations. Human cell activity in the pancreas was identified by polymerase chain reaction (PCR) and immunohistochemistry up to 27 months following transplantation. Circulating human C-peptide (indicator of insulin synthesis) was detected in 62.5% of chimeric sheep.
These preliminary studies suggest that MSC will home, xenoengraft and differentiate into insulin secreting cells following in utero transplantation in sheep. Thus, this biologic system may offer an alternative approach to differentiate human stem cells into functioning human islets of Langerhans.
Materials and Methods
Isolation, culture and in vitro expansion of human fetal pMSC
Ten 20 week-old human fetal pancreatic tissue samples obtained from Advanced Bioscience Resources Inc, (Alameda, CA, USA) with their institutional approval and donor consent, were digested with 1mg/mL collagenase I and V (Sigma-Aldrich, St. Louis, MO, USA) in order to obtain single cell suspension. Cells from seven fetal pancreatic samples (0.5-1 × 10 6 cells/sample) were cultured in 75 cm2 uncoated plastic culture flasks (Corning Incorporated, Corning, NY, USA) in humidified incubators at 37°C, and 5% CO2 in initiation medium composed of RPMI Medium 1640 (Invitrogen, Grand Island, NY, USA), 10 mmol/L HEPES, 1 mmol/L sodium pyruvate, 71.5 mmol/L β-mercaptoethanol (all from Sigma-Aldrich), supplemented with 2% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, USA) for 48 hours (21, 22). Adherent cells were further cultured in restrictive medium which was the same as the initiation medium but with 0.5% FBS. Half of the culture medium was replaced weekly. After 4 weeks, the medium was supplemented with 10% FBS, 20 ng/mL basic fibroblast growth factor (bFGF) (Invitrogen) and 20 ng/mL epidermal growth factor (EGF) (Sigma). Human pMSCs were plated in triplicate at densities of 500-1000 cells/cm2 and split 1:3 upon reaching 80% confluency and passaged at least 7 times prior to being used for any of the in vivo or in vitro studies. Cell growth was monitored by calculating population doubling with the formula log N/log 2; where N is the cell number at confluence divided by the initial number of cells. Growth kinetics were estimated by plotting the cumulative cell number against time (23-25).
Flow cytometric analyses of pancreatic cells and cultured MSC
Freshly digested pancreatic cells from 3 different tissue samples and single cell suspension of pMSC (derived from 3 different tissue samples) digested with EDTA/0.5% trypsin (Invitrogen) were resuspended in PBS/10% FBS and aliquots of 0.5-1 × 105 cells were incubated with mouse anti-human FITC-, PE-, and RPE-conjugated antibodies and 7-amino-actinomycin D (Sigma) for three color flow cytometry with FACScan (BD Biosciences, San Jose, CA, USA) after gating on viable cells as described (26). The following antibodies were used: CD29 (Biosource/Invitrogen), CD105 (Caltag/Invitrogen), and CD2, CD15, CD19, CD20, CD31, CD34, CD36, CD38, CD44, CD45, CD54, CD56, CD71, CD73, CD79a, CD90, HLA-DR, HLA-ABC, and control isotype antibodies (BD Biosciences, San Jose, CA), CD49d, CD50, CD117 and CD36/Glyco-A (Immunotech/Beckman Coulter, Carlsbad, CA, USA), and CD113/1(AC133) (Miltenyi Biotec, Auburn, CA, USA) and CD7 (Pharmingen/BD Biosciences).
MSC in vitro differentiation assays
Osteogenic differentiation of pMSC was induced with NH OsteoDiff Medium (Miltenyi) according to the manufacturer’s protocol. Alizarin red and von Kossa (Sigma) staining detected calcium deposition. Adipogenic differentiation was induced with NH AdipoDiff Medium (Miltenyi). Oil Red O staining (Sigma) staining identified neutral lipids. Chondrogenic differentiation of the pelleted pMSC in a 15 ml tube was induced with NH ChondroDiff Medium (Miltenyi). Chondrocyte nodules were paraffin embedded and stained for glycosaminoglycans with Alcian green (Sigma). Each experiment was done 3 times. Photographs were taken on an Olympus IX71 microscope (Olympus, Melville, NY, USA) (26-29).
Polymerase chain reaction (PCR) and reverse transcription-PCR (RT-PCR) analyses
DNA was purified using DNeasy Tissue Kit (Qiagen, Valencia, CA, USA). mRNA was isolated using μMACS mRNA Isolation Kit (Miltenyi). cDNA was synthesized using SuperScript First-Strand Synthesis System for RT-PCR kit (Invitrogen). All procedures were carried out according to manufacturers’ protocols. PCR and RT-PCR were performed on a GeneAmp PCR System 9700 (PE Applied Biosystems, Foster City, CA, USA) with Platinum Taq SuperMix kit (Invitrogen) according to manufacturer’s directions. Three-step amplification (94°C for 15s, 56-60°C for 15s, and 72°C for 15s) was carried out after an initial incubation at 94°C for 4 min. Primers with the expected product size, annealing temperature and number of cycles were as follows: beta actin forward: 5′-GTC CTC TCC CAA GTC CAC AC-3′, reverse: 5′-GGG AGA CCA AAA GCC TTC AT-3′(200 bp, 56°C, 50 cycles); Gapdh forward: 5′-AGT CCC TGC CAC ACT CAG TC-3′, reverse: 5′-GCA CAG GGT ACT TTA TTG ATG G-3′(131 bp, 56°C, 50 cycles); c-Met forward: 5′-CAA TGT GAG ATG TCT CCA GC-3′, reverse: 5′-CCT TGT AGA TTG CAG GCA GA-3′ (559 bp; 60°C, 45 cycles); insulin forward: 5′-TGT AGA AGA AGC CTC GTT CC-3′, reverse 5′-GAG GCC ATC AAG CAC ATC AC-3′ (183 bp, 56°C, 45 cycles); nanog forward: 5′-CTT CTG CTG AGA TGC CTC AC-3′, reverse: 5′-GCT GAG GTT CAG GAT GTT GG 3′ (284 bp, 56°C, 45 cycles); nestin forward: 5′-GCC CTG ACC ACT CCA GTT TA-3′, reverse: 5′-GGA GTC CTG GAT TTC CTT CC-3′(200 bp, 56°C, 35 cycles); Pax6 forward: 5′-TCA CAG CGG AGT GAA TCA GC-3′, reverse: 5′-TAT CGT TGG TAC AGA CCC CCT C-3′ (377 bp, 58°C, 45 cycles); Pdx1 forward: 5′-GCA GGA ACC ACG ATG AGA GG-3′, reverse: 5′-CCA AGG TGG AGT GCT GTA GG-3′ (338bp, 56°C, 35 cycles). Primers were synthesized by Integrated DNA Technologies (IDT) (Coralville, IA). Beta actin amplified both human and sheep DNA, while the other primers were human-specific (30).
Sheep xenotransplantation
Fourteen sheep fetuses at 60 days gestation were transplanted by intra-peritoneal injections with 1-2 × 106 pMSC (passage no. 7) following the procedure described previously (18, 19). Transplanted lambs were born at term 3 months later and age-matched non-transplanted sheep were used as controls to evaluate human cell activity. All animals were cared for and all procedures were executed in compliance with the Institutional Animal Care and Use Committee at the University of Nevada Reno and the “Principles of laboratory animal care” (NIH publication #85-23, revised 1985).
Sheep sampling
Sheep blood samples were collected in Vacutainer blood collection tubes in the mornings prior to feeding the animals. Tubes were centrifuged briefly at low speed and the separated serum layer was transferred into fresh tubes and archived at −20°C until used. The animals were euthanized at indicated time points to obtain pancreatic, liver and bone marrow tissue samples, which were collected in RNAlater (Ambion, Austin, TX) and preserved. Pancreatic and liver tissue samples collected in buffered formalin (Astral Diagnostics, West Deptford, NJ) were fixed overnight and placed in tissue cassettes (Fisher, Fair Lawn, NJ) that were embedded in paraffin wax. Paraffin blocks were stored at room temperature until used.
ELISA
Human C-peptide ELISA kits were obtained from Alpha Diagnostic International (San Antonio, TX). Human albumin kits were obtained from Cygnus Technologies (Southport, NC). Assays were carried out according to manufacturer’s protocols. All ELISA assays were performed on fasting sheep.
Real- time quantitative PCR (QPCR)
DNA was prepared from pancreatic tissue as described above. Primers and probe were designed for human alpha satellite DNA as follows: forward primer: 5′- CTT CTT CAG GAT GTT TGC AT TT-3′; reverse primer: 5′- GGC CAC AAA GCG GTC TTA AT -3′; and dual-labelled internal hybridization TaqMan probe: 5′- TGG AGC AGT TTG GAA ACA CA-3′, which was labelled with 6- FAM at the 5′ end and black hole quencher 1 at the 3′ end. Real-time PCR was performed on an ABI PRISM 7000 Sequence Detection System (SDS; Applied Biosystems, FosterCity, CA) using ABI PRISM 7000 SDS Software (Applied Biosystems). The thermal cycler was programmed for 2 min at 50.0°C (to prevent reamplification of carry-over-PCR product), 10 min at 95.0°C followed by 50 cycles at 95.0°C and 60.0°C for 15 s and 1 min respectively. Reagents used included TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) in 50 μL final volume containing 500 ng DNA, and 1 μL each primer and probe from a 40 pmol/ μL stock, in 96-well PCR plates (Applied Biosystems, Foster City, CA) sealed with ThermalSeal RT (Excel Scientific Inc, Wrightwood, CA). Standard curves obtained from 10- fold serially diluted human genomic DNA in water yielded 90.5% amplification efficiency. The percentage of human DNA in chimeric sheep samples were determined by the ABI PRISM 7000 SDS Software based on a calibration curve generated by standards with serial dilutions of human DNA in sheep DNA (500 ng total DNA per well). This method was validated by testing the accuracy of different human samples. Also, ten different control sheep samples tested negative by this method.
Immunohistochemical analysis of paraffin embedded tissues for human cells
Tissue samples from transplanted animals and non-transplanted age-matched controls were fixed in 10% formalin for 24 hours followed by embedding in paraffin. The embedded tissue sections were cut into 4μm thick sections and applied to poly-L-lysine-coated slides that were baked at 60°C for 45 minutes, and then stored at room temperature until use. The slides were deparaffinated by two 5 minutes incubations in xylene followed by rehydration through a graded ethanol series (100%, 95%, 70%, and 50%) to deionized water for 5 min each. Target retrieval was carried out in pre-heated 10 mM sodium citrate buffer (pH 6.1) in the microwave for 2 minutes. The slides were allowed to cool for 20 minutes at RT and were then washed with Tris Buffered Saline washing buffer (TBS). Non-specific protein binding was blocked with a 15-minute incubation in 5% nonfat dry milk solution in TBS.
Detection of human insulin expressing cells was performed by using human-specific insulin primary antibody at 1:3000 dilution (clone MAB 1, Millipore, Temecula, CA, USA), while human albumin (hepatocytes) was detected with human-specific albumin antibody at 1:100 dilution (clone HSA-11, Sigma). Slides with the primary antibody were incubated overnight at 4°C than rinsed in 0.4% Tween 20 (Sigma) in TBS (3×5 min) and TBS (2×5 min). Alkaline phosphatase conjugated host-matched secondary antibodies (JacksonImmuno, West Grove, PA, USA), at a dilution of 1:100 after the initial dilution of 1:1 in glycerol (Sigma) for long term storage of the antibody at −20°C, was applied and incubated for 1 hour at RT, than the slides were rinsed in TBS (3×5 min). Ready to use alkaline phosphatase substrate Permanent Red (Dakocytomation, Carpinteria, CA) was applied for 10-15 minutes, than the slides were rinsed in deionized water.
For further detection of human hepatocyte-like cells after target retrieval, endogenous peroxidase activity was blocked with a 15 min incubation in 1 % H2O2 (Sigma) solution in methanol (Sigma). Non-specific protein binding was blocked with 15 min incubation in a 5% nonfat dry milk solution made in 10 mM phosphated-buffered saline pH 7.2 (PBS). Primary anti-human hepatocyte (Hep-Par1) antibody at a dilution of 1:100 (clone OCH1E5, DAKO Cytomation, Carpinteria, CA) was incubated 1 hr at RT followed by three washes. Host-matched secondary antibodies that were HRP conjugated (JacksonImmuno) were used at a dilution of 1:100 after the addition of 1:1 glycerol (Sigma) and incubated overnight at 4°C. After three 5 min washes, slides were developed with 3,3′ Diaminobenzidine (DAB) substrate chromogen system (DAKO Cytomation) which stained positive cells a brown color. In all cases the slides were counter-stained in Mayer’s Hematoxylin solution (Sigma) and blued with 0.5% ammonia water (Sigma) then washed in deionized water. Slides were air-dried, and mounted and cover-slipped in Permount (Fisher) then dried for 24 hours before viewing. Images were acquired using an Olympus BX60 microscope (Olympus, Melville, NY) with an Olympus UPlanFI 20 × 0.50 numeric aperture objective lens, an Olympus DP70 camera, and Olympus DP Controller 2.1.1.183 software. All images were processed globally using Adobe Photoshop CS (Adobe Systems) to adjust brightness, contrast and size. No specific region of any image was individually enhanced.
Results
Expansion and characterization of pMSCs
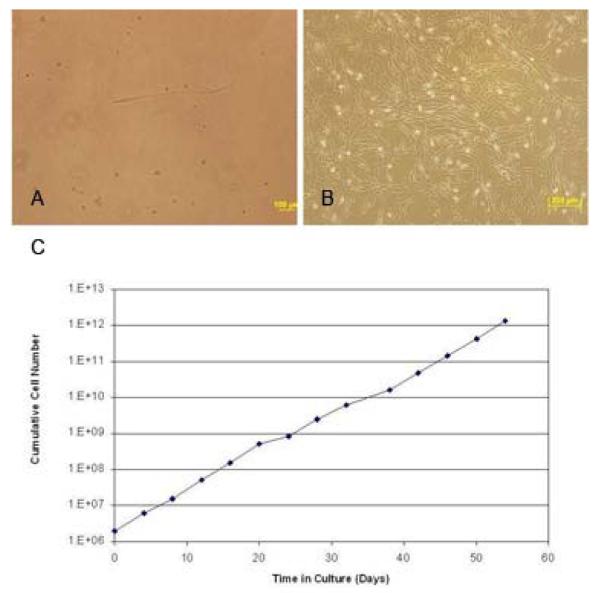
Fresh human fetal pancreas was collagenase digested and the resultant single cell suspensions were cultured on uncoated plastic in medium containing 2% FBS. Adherent cells were then placed in restrictive medium (0.5% FBS) for 4 weeks acquiring characteristic spindle-shaped fibroblastic morphology (Figure 1 A). In the third phase, FBS was increased to 10% and bFGF and EGF growth factors were added to favour SC expansion (31). The rapidly proliferating cells expanded 3- to 5-fold as the cells grew to confluency over about 2 weeks (Figure 1 B). From the 10 input pancreatic tissue samples we successfully derived pMSC from 6 (one sample was lost due to contamination and 3 were used for flow cytometric analysis). Further, the putative pMSC maintained proliferative capacity despite repeated freeze-thaw cycles (followed up to over 50 days). Population doubling varied from 1.58 to 3 days (with an average of 2.73). The growth rate of the putative pMSC at passage 14 is similar to growth rates of MSC obtained from other sources (24, 32) (Figure 1 C).
Figure 1. Generation of human pancreas derived MSC by in vitro three-step culture system.
(A) Culture of human fetal pancreatic cells in restrictive medium (RPMI 1640 with 0.5% FBS) eliminated the contaminating cells and allowed survival of the pMSC which acquired characteristic spindle-shaped morphology (original magnification × 40).
(B) Expansion medium (RPMI 1640 with 10% FBS and growth factors) induced rapid proliferation of the pancreas-derived mesenchymal stem cells (pMSC). Cells are shown at passage 7 (original magnification, × 10). (C) pMSC growth rate in culture. The x-axis represents the number of incubation days and the y-axis represents cumulative cell numbers.
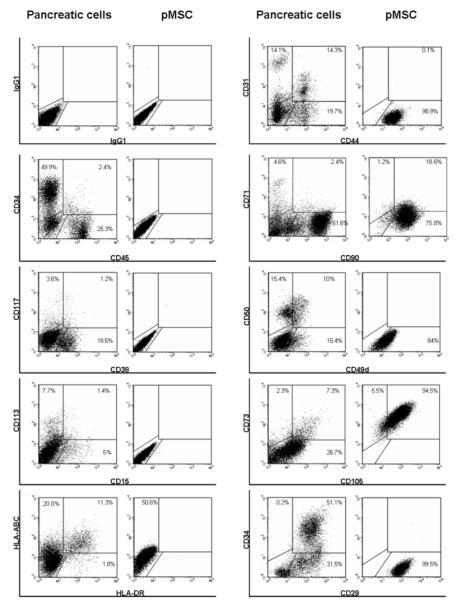
Surface antigen expression profiles comparing the input to cultured cell populations are presented in Figure 2. The input population expressed CD34+/CD45- (endothelial cell phenotype) (33); CD45+ (hematopoietic phenotype) and 2.4 % were CD45+/CD34+ (hematopoietic stem cell phenotype). By contrast, cultured cells exhibited the mesenchymal markers: CD29, CD44, CD49d, CD73 (SH3) and CD105 (SH2). CD31 (PECAM adhesion molecule) characteristic of endothelial cells was not expressed after culture. The human leukocyte antigen HLA class I molecule was expressed by both input and cultured populations while, HLA class II was not seen following culture (Figure 2).
Figure 2. Flow cytometric characterization of the human pancreas derived MSC obtained in the three-step culture system.
Epitope profile of the input pancreatic cells and pancreas-derived mesenchymal stem cells obtained in our three-step culture system was determined by flow-cytometry. The culture-derived pMSCs did not express CD34, CD38, CD45 and CD133 hematopoietic markers. However, they did express specific mesenchymal markers such as CD29, CD44, CD73, CD90, and CD105.
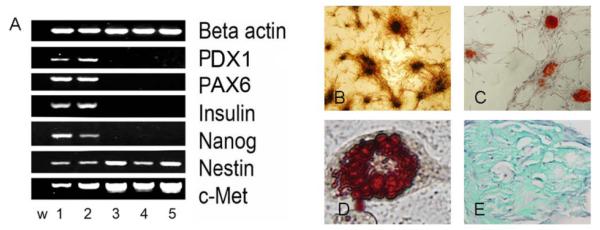
Transcription factor profiles comparing the input population to the cultured cell population is presented in figure 3a. The transcription factor Pdx1 is involved in early stages of pancreatic development and also determinant of β-cell fate and Pax6 is necessary at later stages of pancreatic development. Neither of these factors were expressed in the cultured cell population (34). Similarly, expression of the differentiated pancreatic tissue marker, insulin and the embryonic stem marker (ESC), nanog was seen only in the input population. The cultured population did express c-Met and nestin characteristic of pancreatic stem, progenitor and beta-cells (35). Figure 3B-E demonstrate the ability of pMSC to differentiate into bone, fat and chondrocytes following culture in differentiation inducing media. In summary, growth kinetics, phenotype, transcription factor profiles and differentiation all support the isolation of a MSC cell type.
Figure 3. Characterization of the human pancreas derived MSC obtained in the three-step culture system.
(A) Gene expression profile of the pMSC determined at passage 7 by RT-PCR. First lane (w) is water. Lanes 1 and 2 represent cDNA obtained from collagenase digested 20 week-old human fetal pancreas no. 11492 and 17492. Lanes 3, 4 and 5 represent cDNA obtained from pMSCs derived from human fetal pancreas no: 11492, 17492 and 17498 after culture. Beta actin was used as an internal standard to normalize the amount of cDNA.
(B) In-vitro differentiation of pMSC into osteocytes. MSC were cultured and differentiated as described in methods. Mineralized bone nodules detected by von Kossa staining appear brown to black (original magnification × 10).
(C) In-vitro differentiation of pMSC into osteocytes detected by Alizarin red staining of the mineral deposits that appears bright red (original magnification × 10).
(D) In-vitro differentiation of pMSC into adipocytes. MSC were cultured and differentiated as described in methods. Adipocytes stained with Oil Red O for neutral lipids appear red (original magnification × 40).
(E) In-vitro differentiation of pMSC into chondrocytes. MSC were cultured and differentiated as described in methods. Chondrogenic differentiation was detected by Alcian green staining of the glucosaminoglycans that appear green (original magnification × 40).
In vivo engraftment/differentiation of the pMSC
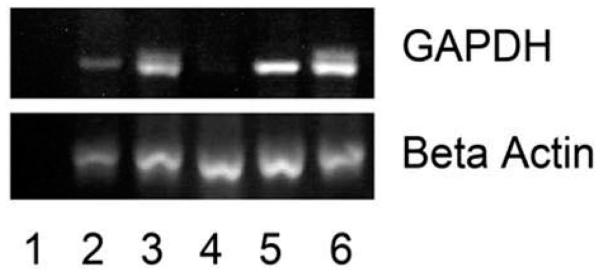
We then transplanted the putative pMSCs in utero to evaluate their in vivo engraftment potential. In a preliminary study, four animals were analyzed just after birth (3 months post transplantation) for pancreatic engraftment. The engraftment incidence (frequency) was 75% (figure 4). Furthermore, the pancreas of sheep # 2361 exhibited clusters of human cells producing and/or secreting human insulin (figure 5).
Figure 4. Sheep pancreatic tissue tested for chimerism by identification of the human GAPDH gene 3 months after transplantation.
Lane 1 represents water; lane 2, 3, 4 and 5 represent animal no 2350; 2355, 2357 and 2361. Lane 6 represents human pancreatic tissue and was used as positive control. Beta actin was used as an internal control. Three out of four transplanted sheep pancreas tested positive for human DNA at 3 months after transplantation.
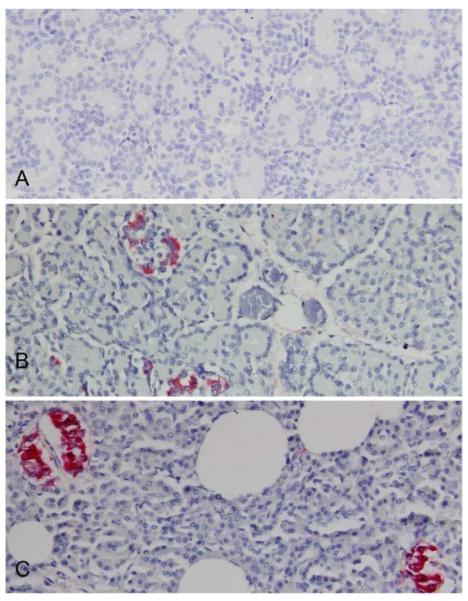
Figure 5. Localization of human insulin within a chimeric sheep pancreas three months after transplantation with human fetal pancreatic mesenchymal stem cells.
Human insulin containing cells were identified by immunohistochemistry using monoclonal anti-human insulin antibody (clone MAB 1). (B) Chimeric sheep no 2361 sacrificed one day after birth display cell clusters with human insulin identified by red coloration.
(A) Age matched non-transplanted negative control animal tissue section stained with the same antibody is negative.
(B) Chimeric sheep no 2361 sacrificed day one after birth displays cell clusters with human insulin secretion identified by red coloration
(C) Human positive control pancreas stained for insulin (red coloration). Original magnifications × 20.
In the second study, ten animals transplanted with human fetal pMSC were evaluated for long-term engraftment (7, 25 and 27 months) by QPCR. Real time PCR samples (4 from each pancreas) were run in triplicate with 500 ng DNA per reaction. Quantitation of the human DNA in each sheep pancreas was based on a standard curve. The detection limit was above 0.0001% of human DNA. The chimeric incidence (frequency) was 80% (i.e. positive in 1-3 of the 4 different tissue samples tested: Table 1).
Table 1. Real time quantitative PCR (QPCR) detection of human DNA in sheep pancreas following fetal transplantation with human fetal pancreatic mesenchymal stem cells.
The pancreas was harvested after animals were euthanized at indicated times. Four samples were tested from each animal with human-specific primers and probes. Eight of ten transplanted sheep exhibited pancreatic chimerism in at least one of four samples.
| Animal no. | When harvested (months after transplant) |
% Human DNA | |||
|---|---|---|---|---|---|
| 2349 | 27 | ND; | 0.0001; | 0.0004; | 0.002 |
| 2351 | 27 | ND; | ND; | ND; | ND |
| 2352 | 27 | ND; | ND; | ND; | 0.0008 |
| 2353 | 25 | ND; | ND; | ND; | ND |
| 2354 | 7 | ND; | 0.0009; | 0.0001; | ND |
| 2356 | 27 | ND; | 0.0001; | ND; | 0.0002 |
| 2358 | 25 | ND; | ND; | 0.002; | 0.0002 |
| 2359 | 27 | 0.004; | ND; | ND; | ND |
| 2360 | 27 | 0.001; | ND; | ND; | ND |
| 2362 | 27 | ND; | 0.002; | ND; | ND |
ND: Not detected, Limit of detection: ≥ 0.0001
We assessed functionality of the engrafted pMSC by assaying serum from the ten transplanted sheep for human C-peptide at varying intervals after birth up to 27 months (Table 2). Neither the control (n=5) nor the non-chimeric sheep (n=2) expressed circulating human C-peptide, while five of eight chimeric sheep repeatedly expressed detectable levels of circulating human C-peptide. Further confirmation is provided in Figure 6, where we note in situ production of human insulin at 27 months.
Table 2. Human C-peptide concentrations in sheep serum following fetal transplantation with human fetal pancreatic mesenchymal stem cells.
Five of ten transplanted sheep had detectable levels of human C-peptide at varying intervals post-transplantation. All samples were obtained after fasting for 24 hr.
| Animal no. |
When sampled (months post-transplant) |
Human C-peptide (ng/mL) |
CV% |
|---|---|---|---|
| 2349 | 7 | 0.43 | ± 5% |
| 25 | 2.73 | ± 10% | |
| 27 | 0.75 | ± 8% | |
| 2351 | 7 | ND | |
| 25 | ND | ||
| 27 | ND | ||
| 2352 | 7 | 0.47 | ± 1% |
| 25 | 0.36 | ± 9% | |
| 27 | 0.32 | ± 1% | |
| 2353 | 7 | ND | |
| 25 | ND | ||
| 2354 | 7 | ND | |
| 2356 | 7 | 1.86 | ± 14% |
| 25 | 1.46 | ± 6% | |
| 27 | 0.83 | ± 4% | |
| 2358 | 7 | ND | |
| 25 | ND | ||
| 2359 | 7 | ND | |
| 25 | ND | ||
| 27 | ND | ||
| 2360 | 7 | 1.21 | ± 1% |
| 25 | 4.49 | ± 2% | |
| 27 | ND | ||
| 2362 | 7 | 0.76 | ± 6% |
| 25 | 0.56 | ± 5% | |
| 27 | ND |
Human C-peptide assay (ELISA) standards ranged from 0.5-20 ng/mL with a detection limit of 0.30 ng/mL. Error is reported as the coefficient of variation (%). All control (non-transplanted, n=5) sheep failed to express human C-peptide. ND: not detected.
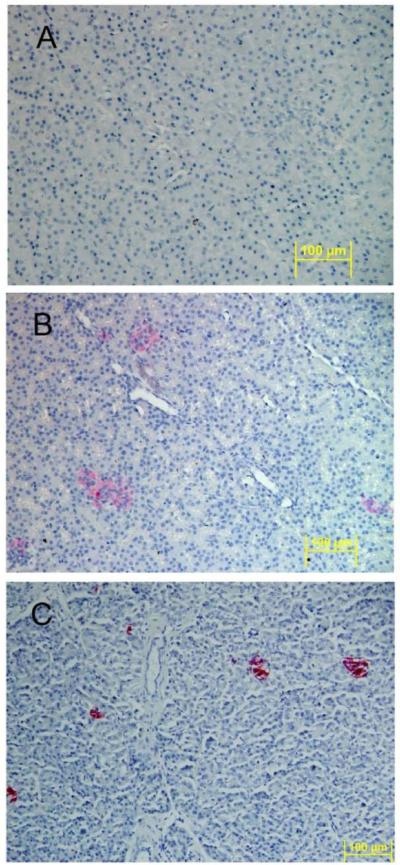
Figure 6. Localization of human insulin within a chimeric sheep pancreas 27 months after transplantation with human fetal pancreatic mesenchymal stem cells.
Human insulin containing cells were identified by immunohistochemistry using human-specific insulin antibody (clone MAB 1).
(A) Age matched non-transplanted negative control sheep pancreas section stained with anti-human insulin is negative for red coloration.
(B) Chimeric sheep sacrificed at 27 months after transplantation stained with anti-human insulin shows cell clusters with red coloration.
(C) Human pancreas stained with anti-human insulin demonstrates cell cluster with red coloration (positive control). Original magnifications × 20.
Discussion
Successful treatment of type 1 diabetes must overcome two problems: the first is the ability to engraft or expand an adequate islet cell mass and the second is the attenuation of likely immune reactivity either due to histocompatibility discrepancies (via allogeneic transplantation) or autoimmunity should autologous replacement methods be found more effective. With the continual refinement of safe and effective methods of immunosuppression, it appears unlikely that immune reactivity will be the major impediment to successful treatment (36).
Human fetal pMSC were chosen as we expected them to generate islet activity due in part to fetal SCs increased expansion and differentiation potential over their adult counterparts (7) and the fact that they exhibit similar telomerase activity to ESC but pose a much with lower risk of malignant transformation (26, 27). We isolated a population of pMSC from human fetal pancreas using established procedures that rely on the MSC adherence to plastic, resistance to trypsinization during passaging, expression of specific cell surface markers, and multi-lineage differentiation. Our three-step culture system was initiated with a heterogeneous pancreatic cell population that consisted of endothelial cells, mesenchymal cells and a small proportion of hemopoietic SC that were lost following culture of the adherent population in restrictive medium.
The putative pMSC obtained after culture exhibited morphologic and phenotypic characteristics similar to MSC derived from bone marrow (Figures 1-3; 23, 27, 37-39). Previous studies have shown that fetal MSC express HLA class I but not HLA class II antigens (37, 40). Accordingly, HLA class II expression in the pMSC population was lost after culture. Transcription factor profiles presented in figure 3A evaluated gene expression pertinent to the pancreas or to MSC. Pancreatic MSC are reported to express both nestin and Pdx1 markers (22, 40). Our MSC population expressed nestin but not Pax6 or Pdx1 (early pancreatic development marker) suggesting that this population is not committed to the pancreatic lineage (Figure 3A). Our pMSC population did not express the ESC marker, nanog, either (37). The presence of the hepatocyte growth factor receptor (c-Met; Figure 3A) was noted in both the pancreatic tissue and the pMSC population. c-Met is reported to be expressed in early pancreatic progenitors, β-cells, HSC and BM derived MSC (41-45). The pMSCs demonstrated multi-lineage differentiation into osteocytes, adipocytes and chondrocytes (Figure 3B-E). These results lead us to believe that our three-step culture system yielded a relatively pure, but not clonal, pMSC population that might be suitable for transplantation.
We then transplanted the human pMSC population into fetal sheep during the transplant receptivity / tolerance phase of gestation. The engraftment frequency (chimeric index) was 79% with functional engraftment at 50% (62.5% of chimeric sheep). This was seen without manipulations designed to improve graft expression (10, 15). Serum C-peptide concentrations in some sheep approached basal levels seen in humans 1.86-4.49 ng/mL (46). In situ staining for human insulin was noted up to 27 months following transplantation (Figures 5, 6). This supports the capability of our non-injury xenograft model to allow/promote engraftment and differentiation of human pMSC into human insulin secreting structures that remain functional years after transplantation. This same SC population was also noted to engraft in fetal liver, express human albumin and differentiate into the hematopoietic lineage in some animals (data not shown).
While it is well established that MSC differentiate into multiple lineages in vitro, an alternate explanation namely cell fusion between donor and recipient cells has been proposed to explain multi-lineage differentiation in vivo. A series of bone marrow transplant studies using different gene markers identified fusion of donor MSC with multiple lineages in vivo rather than differentiation as the mechanism underlying multi-lineage donor expression (47). Our and others formal investigation of this issue using xenograft systems (including human MSC transplantation in utero) failed to demonstrate significant cell fusion to explain multi-lineage differentiation, including hepatocyte differentiation (48-49). It should be noted that neither study specifically investigated chimeric pancreas. While the anti-human insulin antibody is human specific (Millipore product information), we cannot confirm that these cell clusters are uniformly human and contain requisite islet cell components. Studies are ongoing in our laboratory to investigate these possibilities. In addition, while we demonstrate human insulin in situ and in the circulation, we did not determine if circulating insulin is derived from the pancreatic circulation. This leaves open the possibility that ectopic production of some or all of the observed human insulin is occurring in these sheep.
Our preliminary observations support the possibility that human pMSC will engraft and differentiate into functioning islets. Reports demonstrating pluripotency of stem/progenitor cells, including differentiation into islet phenotypes in vitro and in vivo are supportive of this conclusion (41, 50, 51). Further investigation is required to prove that the full complement of cell phenotypes and requisite human endocrine islet function is possible using our technique.
In summary, we isolated a mesenchymal stem cell population from human fetal pancreas. Following transplantation of these pMSC in utero, evidence of pancreatic endocrine engraftment, differentiation and islet cell function is presented. These findings support further feasibility studies on mesenchymal stem cells’ potential to differentiate into functioning human islets in vivo.
Acknowledgments
NIH grants # HL52955 and HL49042 provided support for this work.
The contents of this publication do not represent the views of the Department of Veterans Affairs or the United States Government.
This report is the result of work partially supported by VA Sierra Nevada Health Care System staff resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosure No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Eisenbarth GS. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 2.Mathis D, Vence L, Benoist C. β-cell death during progression to diabetes. Nature. 2001;414:792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- 3.Knip M, Siljander H. Autoimmune mechanisms in type 1 diabetes. Autoimmunity Reviews. 2008;7:550–557. doi: 10.1016/j.autrev.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Inzucchi SE, Sherwin RS. Type 1 diabetes mellitus. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed. Saunders Elsevier; Philadelphia: 2008. chapter 247. [Google Scholar]
- 5.Alemzadeh R, Wyatt DT. Diabetes mellitus in children. In: Kliegman RMBR, Jenson HB, Stanton BF, editors. Nelson Textbook of Pediatrics. 18th Ed Saunders Elsevier; Philadelphia: 2007. [Google Scholar]
- 6.Davani B, Ikonomou L, Raaka BM, et al. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215–3222. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- 7.Kayali AG, Flores LE, Lopez AD, et al. Limited capacity of human adult islets expanded in vitro to redifferentiate into insulin-producing beta-cells. Diabetes. 2007;56:703–708. doi: 10.2337/db06-1545. [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Weir S, Weir GC. New sources of pancreatic β-cells. Nat Biotechnol. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 9.Heimberg H. Boosting β-cell numbers. N Engl J Med. 2008;359:2723–2724. doi: 10.1056/NEJMcibr0807675. [DOI] [PubMed] [Google Scholar]
- 10.Pixley JS, Mackintosh FR, Zanjani ED. Experimental and clinical basis of intrauterine stem cell transplantation. Rev Clin Exp Hematol. 1999;8:11–32. [Google Scholar]
- 11.Skopal-Chase JL, Pixley JS, Torabi A, et al. Immune ontogeny and engraftment receptivity in the sheep fetus. Fetal Diagnosis and Therapy. 2009;25:102–110. doi: 10.1159/000203399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran ND, Porada CD, Almeida-Porada G, Glimp HA, Anderson WF, Zanjani ED. Induction of stable prenatal tolerance to β-galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood. 2001;97:3417–3423. doi: 10.1182/blood.v97.11.3417. [DOI] [PubMed] [Google Scholar]
- 13.Zanjani ED, Almeida-Porada G, Livingston AG, Zeng H, Ogawa M. Reversible expression of CD34 by adult human bone marrow long-term engrafting hematopoietic stem cells. Experimental Hematology. 2003;31:406–412. doi: 10.1016/s0301-472x(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 14.Zanjani ED. The human sheep xenograft model for the study of the in vivo potential of human HSC and in utero gene transfer. Stem Cells. 2000;18:151. doi: 10.1634/stemcells.18-2-151. [DOI] [PubMed] [Google Scholar]
- 15.Almeida-Porada G, Porada C, Gupta N, Torabi A, Thain D, Zanjani ED. The human-sheep chimeras as a model for human stem cell mobilization and evaluation of hematopoietic grafts’ potential. Exp Hematol. 2007;35:1594–1600. doi: 10.1016/j.exphem.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science. 1945;102:400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- 17.Ohki H, Martin C, Corbel C, Coltey M, Le Douarin NM. Tolerance induced by thymic epithelial grafts in birds. Science. 1987;237:1032–1035. doi: 10.1126/science.3616623. [DOI] [PubMed] [Google Scholar]
- 18.Flake AW, Harrison MR, Adzick NS, Zanjani ED. Transplantation of fetal hematopoietic stem cells in utero: the creation of hematopoietic chimeras. Science. 1986;233:776–778. doi: 10.1126/science.2874611. [DOI] [PubMed] [Google Scholar]
- 19.Zanjani ED, Flake AW, Rice H, Hedrick M, Tavassoli M. Long-term repopulating ability of xenogeneic transplanted human fetal liver hematopoietic stem cells in sheep. J Clin Invest. 1994;93:1051–1055. doi: 10.1172/JCI117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz RH. Immunologic tolerance. In: Paul WE, editor. Fundamental Immunology. 6th Ed Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 898–942. [Google Scholar]
- 21.Huang H, Tang X. Phenotypic determination and characterization of nestin-positive precursors derived from human fetal pancreas. Lab Invest. 2003;83:539–547. doi: 10.1097/01.lab.0000062890.40534.1c. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Hong TP, Hu J, Liu Y, Wu Y, Li L. Nestin-positive progenitor cells isolated from human fetal pancreas have phenotypic markers identical to mesenchymal stem cells. World J Gastroenterol. 2005;11:2906–2911. doi: 10.3748/wjg.v11.i19.2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003;33:919–926. doi: 10.1016/j.bone.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 25.Guillot PV, Gotherstrom C, Chan J, Kurataa H, Fiska MN. Human first-trimester fetal MSC express pluripotency markers and grow faster and have longer telomeres than adult MSC. Stem Cells. 2007;25:646–654. doi: 10.1634/stemcells.2006-0208. [DOI] [PubMed] [Google Scholar]
- 26.Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914–1922. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 27.Martin DR, Cox NR, Hathcock TL, Niemeyer GP, Baker HJ. Isolation and characterization of multipotential mesenchymal stem cells from feline bone marrow. Exp Hematol. 2002;30:879–886. doi: 10.1016/s0301-472x(02)00864-0. [DOI] [PubMed] [Google Scholar]
- 28.Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringden O. Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 2003;57:11–20. doi: 10.1046/j.1365-3083.2003.01176.x. [DOI] [PubMed] [Google Scholar]
- 29.Chang W, Tu C, Bajra R, et al. Calcium sensing in cultured chondrogenic RCJ3.1C5.18 cells. Endocrinology. 1999;140:1911–1919. doi: 10.1210/endo.140.4.6639. [DOI] [PubMed] [Google Scholar]
- 30.Narayan AD, Chase JL, Lewis RL, et al. Human embryonic stem cell-derived hematopoietic cells are capable of engrafting primary as well as secondary fetal sheep recipients. Blood. 2006;107:2180–2183. doi: 10.1182/blood-2005-05-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou C, Suen PM, Zhang Y, et al. Isolation and in vitro characterization of pancreatic progenitor cells from the islets of diabetic monkey models. Int J Biochem Cell Biol. 2006;38:973–984. doi: 10.1016/j.biocel.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 32.Banfi A, Muraglia A, Dozin B, Mastrogiacomo M, Cancedda R, Quarto R. Proliferation kinetics and differentiation potential of ex vivo expanded human bone marrow stromal cells: Implications for their use in cell therapy. Exp Hematol. 2000;28:707–715. doi: 10.1016/s0301-472x(00)00160-0. [DOI] [PubMed] [Google Scholar]
- 33.Ozdogu H, Sozer O, Boga C, Kozanoglu I, Maytalman E, Guzey M. Flow cytometric evaluation of circulating endothelial cells: A new protocol for identifying endothelial cells at several stages of differentiation. Am J Hematol. 2007;82:706–711. doi: 10.1002/ajh.20904. [DOI] [PubMed] [Google Scholar]
- 34.Soria B. In-vitro differentiation of pancreatic beta-cells. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 35.Wiese C, Rolletschek A, Kania G, et al. Nestin expression - a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–2522. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huurman VA, Hilbrands R, Pinkse GG, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS ONE. 2008;3:e2435. doi: 10.1371/journal.pone.0002435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Armstrong MA, Li G. Mesenchymal stem cells in immunoregulation. Immunol Cell Biol. 2006;84:413–421. doi: 10.1111/j.1440-1711.2006.01458.x. [DOI] [PubMed] [Google Scholar]
- 38.Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016–3020. doi: 10.3748/wjg.v10.i20.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. PNAS. 2000;97:3213–3218. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith FO, Lampkin BC, Versteeg C, et al. Expression of lymphoid-associated cell surface antigens by childhood acute myeloid leukemia cells lacks prognostic significance. Blood. 1992;79:2415–2422. [PubMed] [Google Scholar]
- 41.Bodnar CA, Sen A, Kallos MS, Behie LA, Petropavlovskaia M, Rosenberg L. Characterization of human islet-like structures generated from pancreatic precursor cells in culture. Biotechnol Bioeng. 2006;93:980–988. doi: 10.1002/bit.20801. [DOI] [PubMed] [Google Scholar]
- 42.Calvo EL, Boucher C, Pelletier G, Morisset J. Ontogeny of hepatocyte growth factor and c-met/hgf receptor in rat pancreas. Biochem Biophys Res Commun. 1996;229:257–263. doi: 10.1006/bbrc.1996.1789. [DOI] [PubMed] [Google Scholar]
- 43.Otonkoski T, Cirulli V, Beattie M, et al. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic beta-cell growth. Endocrinology. 1996;137:3131–3139. doi: 10.1210/endo.137.7.8770939. [DOI] [PubMed] [Google Scholar]
- 44.Wojakowski W, Tendera M, Zebzda A, et al. Mobilization of CD34+, CD117+, CXCR4+, c-met+ stem cells is correlated with left ventricular ejection fraction and plasma NT-proBNP levels in patients with acute myocardial infarction. Eur Heart J. 2006;27:283–289. doi: 10.1093/eurheartj/ehi628. [DOI] [PubMed] [Google Scholar]
- 45.Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional Expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- 46.Pozzan R, Dimetz T, Gazzola HM, Gomes MB. The C-peptide response to a standard mixed meal in a group of Brazilian type 1 diabetic patients. Brazilian Journal of Medical and Biological Research. 1997;30:1169–1174. doi: 10.1590/s0100-879x1997001000005. [DOI] [PubMed] [Google Scholar]
- 47.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 48.Sato Y, Araki H, Kato J, et al. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756–763. doi: 10.1182/blood-2005-02-0572. [DOI] [PubMed] [Google Scholar]
- 49.Colletti EJ, Airey JA, Liu W, et al. Generation of tissue-specific cells from MSC does not require fusion or donor-to host mitochondrial/membrane transfer. Stem Cell Research. 2009;2:125–138. doi: 10.1016/j.scr.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 51.Ianus A, Holz GG, Theise ND, Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest. 2003;111:843–850. doi: 10.1172/JCI16502. [DOI] [PMC free article] [PubMed] [Google Scholar]