Abstract
Background
Aortic arch plaques are a risk factor for ischemic stroke. Although the stroke mechanism is conceivably thromboembolic, no randomized studies have evaluated the efficacy of antithrombotic therapies in preventing recurrent events.
Methods and Results
The relationship between arch plaques and recurrent events was studied in 516 patients with ischemic stroke, double–blindly randomized to treatment with warfarin or aspirin as part of the Patent Foramen Ovale in Cryptogenic Stroke Study (PICSS), based on the Warfarin-Aspirin Recurrent Stroke Study (WARSS). Plaque thickness and morphology was evaluated by transesophageal echocardiography. End-points were recurrent ischemic stroke or death over a 2-year follow-up. Large plaques (≥4mm) were present in 19.6% of patients, large complex plaques (those with ulcerations or mobile components) in 8.5 %. During follow-up, large plaques were associated with a significantly increased risk of events (adjusted Hazard Ratio 2.12, 95% Confidence Interval 1.04-4.32), especially those with complex morphology (HR 2.55, CI 1.10-5.89). The risk was highest among cryptogenic stroke patients, both for large plaques (HR 6.42, CI 1.62-25.46) and large-complex plaques (HR 9.50, CI 1.92-47.10). Event rates were similar in the warfarin and aspirin groups in the overall study population (16.4% vs. 15.8%; p=0.43).
Conclusions
In patients with stroke, and especially cryptogenic stroke, large aortic plaques remain associated with an increased risk of recurrent stroke and death at two years despite treatment with warfarin or aspirin. Complex plaque morphology confers a slight additional increase in risk.
Keywords: aorta, atherosclerosis, stroke, echocardiography
The presence of atherosclerotic plaques in the aortic arch is a risk factor for ischemic stroke. The association between atherosclerosis in the aortic arch and stroke risk was initially established in autopsy studies1, and subsequently confirmed by in vivo studies that used transesophageal echocardiography (TEE) to identify the plaques and evaluate the associated risk of stroke using a case-control2-4 or a prospective5-9 design. Large plaques (≥ 4mm) have been proven to confer a sharply increased stroke risk5, 6. Complex morphologic features of the plaque, such as ulcerations or superimposed thrombi, have also been shown to contribute to the increased risk7, 10, 11, whereas the presence of calcification appears to decrease it12. However, the optimal medical treatment to decrease the risk of recurrent embolic events in patients with large aortic plaques has not yet been established. Since the stroke mechanism in patients with large plaques has been often considered to be thromboembolic in nature, systemic anticoagulation and antiplatelet agents have been proposed as possible preventative options. The actual role of these treatments, and their efficacy in patients with different types of plaques, has not yet been established. Anticoagulation has been occasionally advocated in plaques with superimposed mobile components suggestive of thrombus13. However, the role of anticoagulation in the far larger subset of patients with large but non-mobile plaques, as well as the role of the more frequently used antiplatelet treatment, has not been elucidated in randomized clinical studies to date. In the present study, we sought to define the adverse event rate in stroke patients with large aortic plaques, double-blindly randomly assigned to warfarin or aspirin treatment as part of the National Institute of Neurological Disorders and Stroke (NINDS)-funded Patent Foramen Ovale in Cryptogenic Stroke Study (PICSS), based on the study population of the Warfarin-Aspirin Recurrent Stroke Study (WARSS: NIH R01-NS-28371, JP Mohr, PI).
Methods
Patient Recruitment
PICSS relied on the WARSS for patient recruitment and follow-up. WARSS was a 48-center double-blind study that randomized 2,206 stroke patients to either warfarin or aspirin and followed them for stroke recurrence or death over a 24-month period14. Patient recruitment started in June 1993 and follow-up was completed in June 2000. At each center, cryptogenic stroke patients in WARSS were solicited to undergo TEE for the purposes of PICSS. PICSS also included all WARSS patients that underwent TEE for clinical purposes. Details on the PICSS protocol have been published previously15. All protocols for WARSS and PICSS were approved by the Institutional Review Board at each participating center and informed consent obtained from each participant.
Eligibility
Patients aged 30-85 deemed safe to undergo warfarin therapy were eligible. Eligible patients were those who experienced ischemic stroke within the previous 30 days, and rated ≥3 (no or moderate residual disability) on the Glasgow Outcome Scale16. Patients were ineligible if they had baseline International Normalized Ratio (INR) above normal range (>1.4), stroke related to a procedure or attributable to a cardioembolic source, or were scheduled to undergo surgery for high-grade carotid stenosis. Patients with contraindications to TEE were also excluded.
Stroke Subtyping
All baseline strokes were subtyped by a local neurology PI based on a pre-defined criteria modeled after the NINDS Stroke Data Bank and Trial of Organon in Acute Stroke Therapy (TOAST)17. Cryptogenic strokes typically had no definite source of the stroke despite a thorough diagnostic evaluation.
Medications and Blinding
Medications used were aspirin 325 mg tablets taken once daily, and warfarin in 2 mg scored tablets taken daily, adjusted to achieve and maintain INR 1.4 - 2.8. Patients were randomized to active aspirin or warfarin, and an identical placebo. All patients followed the same schedule of clinic contacts for blood draws for INR, medication monitoring, and warfarin (or warfarin-dummy) dose adjustment. All participants other than the principal investigator statistician were blinded.
Follow-up
All patients were followed for two years, operationalized as 24 ± 1 months (maximum 761 days). Follow-up was made on a monthly basis by phone or in-person to assess compliance and to regulate INRs. Quarterly and annual in-person follow-ups for detailed examination were also made.
TEE Protocol
All patients underwent TEE guided by a pre-defined PICSS protocol using either a biplane or multiplane probe, and the videotapes were sent to the core laboratory at Columbia for central analysis15. The TEE protocol emphasized delineation of embolic sources, including identification and characterization of aortic arch plaques.
Analysis of Tapes
For the purpose of the present report, all videotapes were reviewed by a single experienced echocardiographer (MDT) blinded to patient clinical characteristics and treatment status. Aortic plaques were defined as previously published18. The aortic arch was defined as the portion of aorta comprised between the curve at the end of the ascending portion and the takeoff of the left subclavian artery. A plaque was defined as a discrete protrusion of the intimal surface of the vessel at least 2 mm in thickness, different in appearance and echogenicity from the adjacent intact intimal surface. The presence and location of any plaque was recorded. In cases of multiple plaques, the most advanced lesion was considered. Plaque thickness was measured as continuous variable. Plaques were then classified into small (<4 mm) or large (≥ 4 mm; figure 1A). The presence of ulcerations or mobile components (figure 1B) was also recorded. An ulceration was defined as a discrete indentation of the luminal surface of the plaque with base width and maximum depth of at least 2 mm each. Plaques with ulceration and/or mobile components were defined as complex plaques, according to a previously published definition4.
Figure 1.
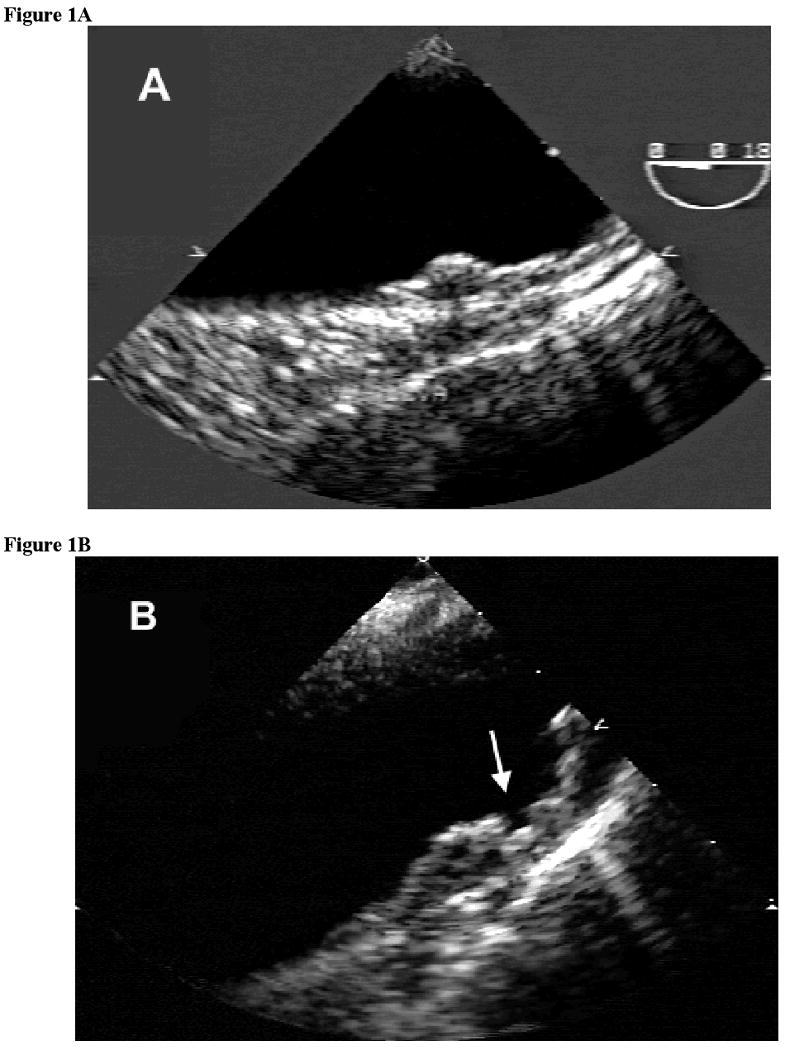
Examples of large aortic arch plaques by transesophageal echocardiography (TEE). (A) Non-complex plaque (B) Complex plaque with ulceration of the luminal surface (arrow)
Assessment of End-points
The primary end-point was recurrent ischemic stroke or death from any cause15. Clinical evidence of a recurrent ischemic stroke was a new lesion on CT or MRI or, when new lesions were absent, clinical syndrome consistent with stroke of more than 24 hours duration. All clinical and radiological events were adjudicated independently by five treatment-blinded neurologist adjudicators. Data from all hemorrhages were submitted to a treatment-blinded adjudicator who classified them as major or minor. Major hemorrhages were defined as intracranial, intraspinal, intracerebral, subarachnoid, subdural or epidural hemorrhage, or any other bleeding requiring transfusion. All other hemorrhagic events were considered as minor.
Statistical Analysis
The statistical power of the study was calculated with the software PASS (version 2002) with procedure for Cox proportional hazards model. With the 84 observed events in 516 patients, the overall event rate is assumed to be 16.3%. For continuous measurement of plaque thickness, the observed standard deviation was 1.893, and the R-squared value with other covariates was 0.09. With these values and at a two-sided 0.05 significance level, the present study had 80% power to detect a minimum hazard ratio of 1.18 per millimeter increase in plaque thickness.
For the cryptogenic stroke subgroup, the study had 80% power to detect a minimum hazard ratio of 1.35 per millimeter increase in plaque thickness, with the sample size 214, observed 12.5% event rates, the 1.893 standard deviation and the 0.09 R-squared value with other covariates., using two-sided 0.05 significance level.
Differences between proportions were assessed by the chi-square test, differences between mean values by unpaired Student's t-test. Unadjusted hazard ratios for the association between the various definitions of plaque (small, large, complex or non-complex) and recurrent ischemic stroke and death were calculated Cox proportional hazards models were used to assess the risk of stroke/death associated with aortic arch plaques. Hazard ratios and 95% confidence intervals for aortic plaques and stroke/death were calculated. Adjusted hazard ratios were obtained using stepwise models including the variables listed in Table 1, with entry and removal threshold set at p= 0.2. Multigroup comparisons were used to assess the risk associated with various plaque characteristics.
Table 1.
Socio-demographic variables, stroke characteristics and risk factors by arch plaque presence/thickness.
| Entire Group (n=516) | No Plaque (n=179) | Small Plaque (n=236) | Large Plaque (n=101) | P Value | |
|---|---|---|---|---|---|
| SOCIO-DEMOGRAPHIC | |||||
| Age, years | 59.5±12.1 | 51.7±12 | 62.7±10 | 66.1±9 | <0.0001 |
| Women, n (%) | 225/516 (43.6) | 78/179 (43.6) | 99/236 (42.0) | 48/101 (47.5) | 0.64 |
| Race-Ethnicity (White), n (%) | 228/516 (44.2) | 77/179 (43.0) | 101/236 (42.8) | 50/101 (49.5) | 0.49 |
| Married, n (%) | 282/515 (54.8) | 94/179 (52.5) | 131/235 (55.7) | 57/101 (56.4) | 0.75 |
| College Educated, n (%) | 143/509 (28.1) | 58/177 (32.8) | 64/233 (27.5) | 21/99 (21.2) | 0.12 |
| Medicaid, n (%) | 151/511 (29.6) | 60/178 (33.7) | 64/234 (27.4) | 27/99 (27.3) | 0.32 |
| STROKE CHARACTERISTICS | |||||
| Cryptogenic, n (%) | 214/516 (41.5) | 85/179 (47.5) | 92/236 (39.0) | 37/101 (36.6) | 0.12 |
| Glasgow score<5, n (%)* | 176/516 (34.1) | 49/179 (27.4) | 84/236 (35.6) | 43/101 (42.6) | 0.03 |
| Barthel score<95, n (%)** | 141/516 (27.3) | 33/179 (18.4) | 72/236 (30.5) | 36/101 (35.6) | 0.003 |
| RISK FACTORS | |||||
| Hypertension, n (%) | 324/509 (63.7) | 104/176 (59.1) | 141/233 (60.5) | 79/100 (79.0) | 0.002 |
| Diabetes, n (%) | 154/515 (29.9) | 39/179 (21.8) | 68/235 (28.9) | 47/101 (46.5) | <0.0001 |
| Sedentary Lifestyle, n (%) | 172/511 (33.7) | 45/177 (25.4) | 77/234 (32.9) | 50/100 (50.0) | 0.0002 |
| Heart Disease, n (%)§ | 100/516 (19.4) | 27/179 (15.1) | 42/236 (17.8) | 31/101 (30.7) | 0.005 |
| Prior Stroke, n (%) | 72/478 (15.1) | 15/162 (9.3) | 40/222 (18.0) | 17/94 (18.1) | 0.04 |
| Current Smoker, n (%) | 147/513 (28.7) | 55/178 (30.9) | 65/235 (27.7) | 27/100 (27.0) | 0.71 |
| Alcohol consumption, n (%)§§ | 264/515 (51.3) | 103/179 (57.5) | 118/236 (50.0) | 43/100 (43.0) | 0.06 |
| High Cholesterol, n (%) | 209/515 (40.6) | 79/178 (44.4) | 96/236 (40.7) | 34/101 (33.7) | 0.25 |
| Obesity, n (%) | 256/512 (50.0) | 103/178 (57.9) | 107/233 (45.9) | 46/101 (45.5) | 0.03 |
| Body Mass Index, kg/m2 | 28.3±5.5 | 29.5±6.3 | 27.9±5.1 | 27.3±4.6 | 0.0009 |
Denominators in the table may differ from the column totals due to missing values in the data collection.
Glasgow outcome score <5 indicates any degree of residual disability, either at work or in social life, because of physical or mental deficit.
Barthel score < 95 indicates less than complete independence in performing activities of daily life
History of myocardial infarction or angina, valvular heart disease, congestive heart failure, atrial fibrillation or other cardiac arrhythmias
Consumption of at least one alcoholic beverage per week
Two-year event rates (stroke and death) were calculated in patients with no/small plaques or with large plaques, and with different plaque complexity. Kaplan-Meier event-free curves were constructed in patients with different plaque definitions. The difference between groups was evaluated by means of the log-rank test.
In the warfarin group, a subanalysis was performed to evaluate the effect of the level of INR achieved on the risk of recurrent stroke/death. The average level of INR during the follow-up was used for this analysis.
SAS statistical package (version 9.1.3) was used in the analyses, performed by one investigator (ZJ). A two-tailed P-value of 0.05 or less was considered significant for all analyses.
Results
Baseline TEE Findings
Of 630 patients, TEE studies were available for analysis in 627. Adequate visualization of the aortic arch was obtained in 516 (82%) of them, who therefore constitute the study population for the present report. Compared with the study population of WARSS, patients included in the present study were younger (by an average 3.5 years), less often white (44.2% vs. 56.8%) and, because of the requirement of the PICSS protocol, had more often cryptogenic strokes (41.5% vs. 26.1%). Gender distribution, educational level, prevalence of cardiovascular risk factors, index stroke severity and residual disability were similar between the two study groups.
Aortic arch plaques were present in 337 of 516 patients (65.3%), large plaques (≥ 4mm) in 101 (19.6%). Complex plaque features (ulcerations, mobile components) were present in 46 patients (8.9%). Plaques both large and complex were present in 44 patients (8.5%). Patient characteristics by aortic plaque presence and thickness are shown in Table 1. The frequency of large plaques was not significantly different between patients with cryptogenic stroke (37/214, or 17.3%) and patients with stroke of known cause (64/302, or 21.2%; p=0.27).
Laboratory Tests
The mean INR in patients treated with warfarin was 1.95 ± 0.46 (median 1.96). Mean time interval between blood draws was 28.1 ± 13.4 days.
End-points
Of the 516 patients, 10 (1.9%) withdrew consent or were lost to follow-up at a mean 13.2 ± 10.5 months after randomization.
A total of 84 end-points (16.3%) occurred during follow-up, including 61 strokes and 23 deaths.
Primary Events in Relation to Aortic Arch Plaque Status
Two-year incidence of recurrent stroke or death progressively increased with arch plaque size, from 10.1% in patients with no plaque to 16.5% in patients with small plaque to 26.7% in those with large plaque (Table 2). The same trend was observed in cryptogenic stroke patients, with incidence rates of 4.7%, 15.2% and 24.3%, respectively. In the entire group, there was a significant difference in the time to recurrent stroke or death between patients with no arch plaque, small plaque, large complex plaque or large non-complex plaque (p=0.003). Kaplan-Meier curves are shown as Figure 2. Large complex plaques had similar 2-year event rate as large non-complex plaques, but most of the events occurred at an earlier time. It should be noted that patients with large or large/complex plaques had a worse risk profile than patients with small or no plaque (Table 1).
Table 2.
Rates of recurrent stroke/death by aortic arch plaque status in the entire study group and in stroke diagnostic subtypes
| Entire Study Group | Strokes of Known Cause | Cryptogenic Strokes | |
|---|---|---|---|
| Events/Total (%) | Events/Total (%) | Events/Total (%) | |
| Overall | 84/516 (16.3) | 57/302 (18.9) | 27/214 (12.6) |
| No Plaques | 18/179 (10.1) | 14/94 (14.9) | 4/85 (4.7) |
| Small Plaques (< 4 mm) | 39/236 (16.5) | 25/144 (17.4) | 14/92 (15.2) |
| Large Plaques (≥ 4 mm) | 27/101 (26.7) | 18/64 (28.1) | 9/37 (24.3) |
| Complex Plaques | 12/44 (27.3) | 8/29 (27.6) | 4/15 (26.7) |
| Non-Complex Plaques | 15/57 (26.3) | 10/35 (28.6) | 5/22 (22.7) |
Figure 2.
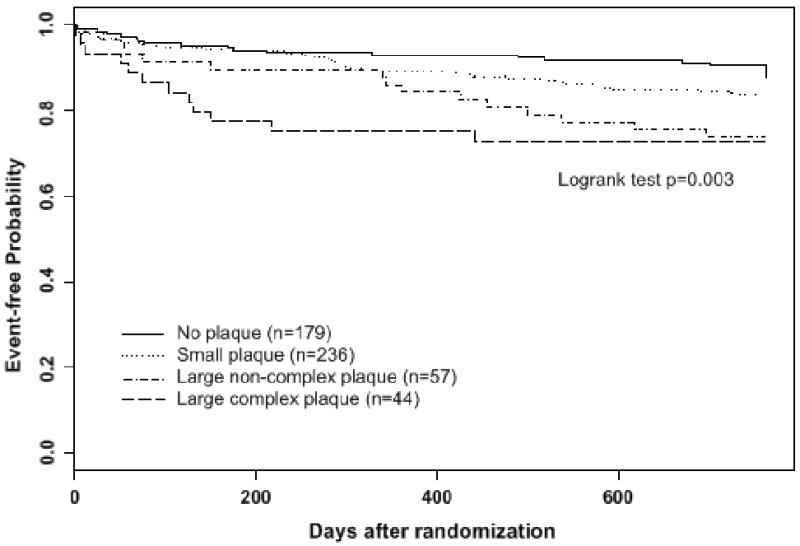
Kaplan-Meier curves of cumulative risk of recurrent stroke or death stratified by baseline arch plaque thickness and complexity
In multivariate analysis adjusted for race-ethnicity, Glasgow score, prior stroke, diabetes, heart disease and sedentary lifestyle, each millimeter increase in plaque thickness was associated with a significant increase in risk of events in the overall study group (Hazard Ratio 1.13, 95% Confidence Interval 1.01 to 1.28) and even more in cryptogenic stroke patients (HR 1.38, 95% CI 1.12 to 1.68). Despite this association, the covariate-adjusted multi-group comparison for plaque size (no vs. small vs. large plaque) was not statistically significant (p=0.11), and neither was the comparison for plaque size/complexity (no vs. small vs. large noncomplex vs. large complex plaque; p=0.17). Significant findings were however obseved in comparisons between specific plaque types. Compared with no plaque, large plaques were associated with increased risk in the overall group (HR 2.12, 95% CI 1.04-4.32), especially those with complex morphology (HR 2.55, CI 1.10-5.89). The risk was particularly high in patients with cryptogenic stroke (HR 6.42, CI 1.62-25.46 for large plaques; HR 9.50, CI 1.92-47.10 for large/complex plaques). No significant increase in risk was observed for any type of aortic plaque in patients with stroke of known cause.
Treatment with Warfarin or Aspirin
No significant differences between the two treatment arms were observed with regard to demographics, clinical characteristics, and cardiovascular risk factors. Frequencies of small, large and large/complex plaques were also not significantly different between the treatment groups. In patients treated with warfarin, the mean INR was not significantly different among those with no, small or large arch plaques (1.95±0.45, 1.92±0.47 and 2.00±0.45, respectively; p=0.34).
Two-year incidence of recurrent stroke/death was similar between warfarin (43/256, or 16.4%) and aspirin treated groups (41/260, or 15.8%; p=0.43). These events rates were similar to those observed in the overall WARSS population (17.8% and 16.0%, respectively). No interaction was observed between warfarin treatment and large plaques on the risk of events (HR 0.42, 95% CI 0.12 to 1.47).
In the warfarin group, the effect of the average INR achieved during the follow-up on the risk of recurrent stroke and death was evaluated. In a model including the various definitions of arch plaques as well as significant stroke risk factors, a significant protective effect was observed for a level of INR ≥1.5, both in the entire study group (adjusted HR 0.37, 95% CI 0.18 to 0.77) and in the cryptogenic subgroup (adjusted HR 0.14, 95% CI 0.03 to 0.60).
Hemorrhagic Events
The incidence of major hemorrhagic events was low and similar between the warfarin and aspirin groups (0.88/100 patient-years vs. 2.16/100 patient-years; p=0.13). Minor hemorrhagic events were more frequent in the warfarin group (26.76/100 patient years vs. 10.0/100 patient years; p<0.001).
Discussion
Our study is the first to report on the incidence of recurrent stroke and death in stroke patients with aortic arch plaques randomized to warfarin or aspirin treatment. The presence of large plaques in the proximal segment of the aorta is an established risk factor for ischemic stroke, linked to a 2.5-fold to 9-fold increase in stroke risk in case-control studies4, with an even greater risk in case of complex plaque morphology10, and with an over 4-fold increase in stroke risk in prospective studies 5, 7. In patients with a prior stroke, the risk of recurrent stroke associated with large or complex aortic plaques has been quite consistent among different studies, with hazard ratios ranging from 2.48, 9 to 3.8 6. Large aortic arch plaques have a definite embolic potential, demonstrated by the frequent microembolic signals observed by transcranial Doppler 19. The stroke mechanism in patients with large or complex aortic plaques is believed to be predominantly thromboembolic. The frequency of thromboembolic events in patients with severe arch plaques has been reported to be as high as 33% at one year5, compared with only 0.7% for atheroembolism13. Superimposed thrombus was found in 17 of 120 plaques (14%) in an autopsy study20. Because of this thromboembolic propensity, anticoagulation or antiplatelet treatment appear as reasonable preventive treatments for reducing the risk of embolic events. However, the available data regarding the effect of oral anticoagulation or antiplatelet therapy are sparse and inconclusive, and the information has been derived from observational studies not designed to test treatment efficacy. The type of treatment was often left to the discretion of the treating physician5-9, making the results difficult to interpret. Most studies included subjects with cardioembolic stroke mechanism, who therefore required anticoagulation because of it. In the present study, patients with aortic plaques were double-blindly randomized to treatment with warfarin or aspirin. Patients with cardioembolic stroke were excluded, as were those with high degree carotid stenosis, whose inclusion might have also affected the interpretability of the results. Over a follow-up of two years, we observed that large plaques (≥ 4mm) remained associated with a doubling of the risk of recurrent stroke and death in the overall study cohort, despite medical therapy and after adjustment for other pertinent covariates. The risk was exclusively seen in cryptogenic stroke patients, suggesting that aortic plaques may indeed have played an important role in the stroke mechanism when no other cause was present. Moreover, the highest risk estimates were obtained in the presence of complex plaque morphology both in the overall study group (hazard ratio 2.55) and in cryptogenic stroke patients (hazard ratio 9.50), further suggesting a possible direct role of the plaque in the embolic mechanism. This possibility was also corroborated by the earlier occurrence of outcome events observed for complex plaques (Figure 2). In cryptogenic stroke patients, the two-year event rate on medical treatment was relatively low (4.7%; Table 2), but the presence of aortic plaques increased it to the same level seen in patients with other causes of stroke. This observation suggests that aortic plaques are a strong risk factor for recurrent events in cryptogenic stroke patients. Plaque thickness ≥ 4 mm was a useful criterion for risk stratification, as previously reported. The presence of ulcerations or mobile components added to the prediction of risk; in particular, our data suggest that ulcerations of the plaque surface should be actively sought, because of the associated increase in risk and of their relatively frequent detection on large plaques (8.9% in our study, versus only 0.6% for mobile forms). It should be noted that, given the small numbers of events within individual classes and the consequent wide confidence intervals of the estimated hazard ratios, the plaque size-complexity analyses in our study should be regarded as exploratory, especially in the cryptogenic subgroup.
Medical treatment with warfarin or aspirin did not affect the statistical significance of the association between severe aortic plaques and the risk of stroke and death. In fact, the hazard ratios we observed were only slightly lower than those previously reported in the literature from studies in which the treatment was not randomized6, 8, 9, or even not prescribed to some of the study patients6.
The level of anticoagulation achieved in the warfarin group in our study was determined by the protocol requirements of the parent study (WARSS)14, with a lower target INR range (1.4 to 2.8) than that usually recommended for cardioembolic sources of stroke (INR 2 to 3). However, the mean INR achieved in our study was 1.95, and a protective effect of warfarin on the risk of stroke and death was observed starting at INR of 1.5, as was the case in the parent WARSS study14. Therefore, the level of anticoagulation achieved in our patients appears adequate.
Our study has preventive implications, suggesting that treatment with warfarin or aspirin is not sufficient to significantly affect the risk associated with large aortic plaques. For warfarin treatment, it is possible that further selection of suitable patients, beyond the assessment of plaque thickness, may be needed. We recently reported that patients presenting with acute ischemic stroke and large aortic plaques show an activation of coagulation parameters that is not observed in matching controls, and that the coexistence of large aortic plaques and hypercoagulability at presentation is associated with an increased risk of recurrent stroke and death21. The assessment of coagulation parameters may identify a subgroup of patients with large arch plaques that may benefit the most from treatment with warfarin.
Statin treatment holds promises in patients with large plaques, although the only study to date that reported a favorable effect of statins while showing no benefit from aspirin or warfarin treatment was retrospective, and with no treatment randomization22. Statin treatment is recommended in the current AHA/ASA guidelines for the prevention of recurrent stroke 23, but more as a general recommendation to be applied to all patients with evidence of atherosclerosis than as a specific recommendation in patients with aortic plaques. High-dose statin treatment decreased the risk of recurrent stroke in the Stroke Prevention by Aggressive Reduction of Cholesterol Levels (SPARCL) study24, but no separate data were available for patients with aortic atherosclerosis. More research is necessary to clarify the role of statins in reducing the risk of embolic events in stroke patients with large aortic plaques.
Our study has some limitations. Since the enrollment was performed at a time when statins were not routinely prescribed after stroke, the effect of statin treatment on our results cannot be evaluated. On the other hand, the effect of warfarin and aspirin treatment on the risk of stroke and death was assessed without the confounding effect of statin treatment. Although the level of anticoagulation achieved in the warfarin group appears adequate, the possibility that a higher level of anticoagulation might have significantly affected the results cannot be excluded.
In summary, our study shows that among patients with ischemic stroke treated with warfarin or aspirin, large aortic plaques, and especially those with complex morphology, remain associated with a significant increase in risk of recurrent stroke and death, which is observed exclusively in patients with an initial cryptogenic stroke. Further studies are needed to evaluate whether patient selection or different treatment strategies may be associated with more effective prevention of recurrent events.
Acknowledgments
The Authors wish to thank J.P. Thompson, PhD, and Bruce Levin, PhD, for their expert statistical suggestions.
Funding Sources: This study was supported by NIH – National Institutes of Neurological Disorders and Stroke R01-NS-32525 (SH), and R01-NS-28371 (JPM).
Footnotes
Conflict of Interest Disclosures: None.
Study Participants: National Institute of Neurological Disorders and Stroke (NINDS):J.R.Marler, Program Director
Data Monitoring Center members:R.M.Lazar,D.E.Gohs,M.Clavijo,K.Slane,D.Balbuena,D.Martino,C.Inguanzo,J.Pittman,R.R.Sciacca,K.Evans,K.Lord,B.Jaffe,J.Kim,L.Lynn,J.Ruzicka,P.Chugh,A.Zidel,B.Fields,M.Coleman,R.King,J.G.Mohr,I.Carretero,O.Mendoza,A.Barlow
Statistical Analysis Center members:J.L.P.Thompson,B.Levin,W.Ma,T.Costigan,A.Murphy,X.Chen,E.Etienne,R.Hilbawi,K.Sridharan,D.Burroughs,G.Kanu,R.Okunieff,D.Xu,K.Chin
Performance and Safety Monitoring Board members:D.G.Sherman(Chair),M.L.Dyken,A.Lowe,I.Meissner,D.W.Taylor
Adjudication Committee:H.J.M.Barnett,C.M.Fisher,J.C.Gautier,P.Sandercock,J.P.Whisnant
Neuroradiologist Adjudicator:S.K.Hilal(dec),J.Pile-Spellman
Hemorrhage Adjudicator:A.G.G.Turpie
| 82-Columbia-Presbyterian Med.Ctr. | R.Sacco,S.Homma, M. Di Tullio, |
| R.Marshall,M.Elkind,C.Stapf,H.Mast, M.Clavijo | |
| 53-Long Island-Jewish Med.Ctr. | R.Libman,S.Roth,R.Gonzaga-Camfield |
| 47-Georgetown University | M.Yaseen,D.Lu,J.Burfoot,E.Green |
| 41-Univ.Illinois Med.Ctr. | C.Helgason,S.Devries,J.Hoff,T.Gnutek |
| 38-Univ.Iowa Hospitals&Clinics | H.P.AdamsJr,B.Bendixen,B.Vandenberg,A.Tanna,L.Vining |
| 30-Johns Hopkins-Bayview Med.Ctr. | C.Johnson,E.Shapiro,C.Early,J.Alt |
| 29-U.of Texas Medical School (Houston) | J.Grotta,F.Thandrayen,D.Vital |
| 23-Buffalo General Hospital | P.Pullicino,Z.Hajduczek,M.Hens,N.Meiler,A.Martinez |
| 21-Cleveland Clinic Foundation | C.Sila,B.Stewart,B.Dyko,N.Rudd |
| 21-Massachusetts General Hospital | J.Kistler,M.Picard,K.Furie,F.Buonanno,L.Oertel |
| 19-Montefiore Med.Ctr. | D.M.Rosenbaum,M.Nanna,E.Klonowski,S.Rybak,J.Nonan |
| 17-Henry Ford Hospital | P.Mitsias,S.Smith,K.Sawaya,P.Marchese,J.Reuther |
| 17-University of Miami Sch.of Med. | R.Kelley,M.Bilsker,A.Forteza,J.Arias |
| 15-Lankenau Med. Research Center | M.Alter,A.Sokil,G.Friday,M.Lloyd,T.Listner,A.Smith |
| 15-Stanford Stroke Center | G.W.Albers,I.Schnittger,N.Hock,S.Kemp |
| 14-Mt.Sinai School of Medicine | S.Tuhrim,M.Goldman,S.Augustine |
| 13-Vanderbilt Med.Ctr. | H.Kirschner,B.F.Byrd,A.Nelson,S.O'Connell,K.Heyden,D. Klein |
| 12-Univ of Kentucky Med.Ctr. | R.Dempsey,P.Sapin,L.Pettigrew,B.Stidham,I.Lamb |
| 12-Pennsylvania Hospital | D.Jamieson,S.Mandal,C.Gonnella,M.Hellstern |
| 11-New England Med.Ctr. | M.Pessin,S.Schwartz,L.Caplan,L.Barron |
| 11-Rochester General Hospital | J.Hollander,L.von Doenhoff,C.Weber |
| 9-Indiana University Med.Ctr. | J.Biller,D.Segar,L.Chadwick |
| 8-Cleveland Clinic-Florida | B.Dandapani,H.Bush,V.Salanga,P.Parks,M.Piccirillo |
| 8-New York University-NY,VA | H.Weinreb,A.Gindea,K.Siller,L.Chin,G.Allen |
| 8-Wayne State University | S.Chaturvedi,S.Levine,L.Femino,E.St Pierre,L.Quinones, F.Mada |
| 6-Hennepin County Med.Ctr. | D.Anderson,R.Asinger,D.Brauer,D.Radtke |
| 6-Univ.Southern California | M.Fisher,P.A.N.Chandraratna,G.Fischberg,A.Scicli,A. Mohammadi |
| 5-Albert Einstein(PA) Med.Ctr. | J.Dissin,S.Sillman,L.Jacobs,C.Borschell |
| 5-Metrohealth Med.Ctr. | J.Schmidley,R.Finkelhor,M.Winkelman,A.Liskay |
| 4-Boston University Med.Ctr. | C.Kase,R.Davidoff,E.Licata-Gehr,N.Allen |
| 4-Marshfield Clinic | P.Karanjia,D.Horton,S.Lobner,L.Stephani |
| 4-Univ.Michigan Med.Ctr. | M.Chimowitz,W.Armstrong,Z.Noorani |
| 4-U. Calif.San Diego | C.Jackson,D.Blanchard,N.Kelly,J.Werner |
| 3-St.Paul-Ramsey Med.Ctr. | M.Ramirez-Lassepas,J.T.Suh,C.Espinosa |
| 3-Yale U.School of Medicine | L.Brass,C.C.Jaffe,A.Lovejoy,B.Kennedy |
| 3-Syracuse VA-Med.Ctr. | A.Culebras,R.Carleson,M.Benedict,D.Pastor,T.Dean |
| 2-Beth-Israel Hospital,Boston | C.Mayman,W.Manning,S.Warach,L.R.Caplan,M.Tijerina |
| 2-Little Rock,AR.VA-Med.Ctr. | M.Chesser,B.Boop,S.Nazarian,L.Kennedy |
| 2-University of South Alabama | J.Rothrock,R.Zweifler,S.Cunningham,R.Yunker |
| 1-Maimonides Med.Ctr. | A.Miller,A.Greengart,K.Chin,T.LaRocca |
| 1-U.Tennessee,Memphis | K.Gaines,S.Gubin,B.O'Brien,C.Bonds,J.Shaw,A.Payne |
| 1-University of Vermont | R.Battle,R.Hamill,P.Krusinski,M.Fitzpatrick |
Reference List
- 1.Amarenco P, Duyckaerts C, Tzourio C, Henin D, Bousser MG, Hauw JJ. The prevalence of ulcerated plaques in the aortic arch in patients with stroke. N Engl J Med. 1992;326:221–225. doi: 10.1056/NEJM199201233260402. [DOI] [PubMed] [Google Scholar]
- 2.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 3.Jones EF, Kalman JM, Calafiore P, Tonkin AM, Donnan GA. Proximal aortic atheroma. An independent risk factor for cerebral ischemia. Stroke. 1995;26:218–224. [PubMed] [Google Scholar]
- 4.Di Tullio MR, Sacco RL, Gersony D, Nayak H, Weslow RG, Kargman DE, Homma S. Aortic atheromas and acute ischemic stroke: a transesophageal echocardiographic study in an ethnically mixed population. Neurology. 1996;46:1560–1566. doi: 10.1212/wnl.46.6.1560. [DOI] [PubMed] [Google Scholar]
- 5.Tunick PA, Rosenzweig BP, Katz ES, Freedberg RS, Perez JL, Kronzon I. High risk for vascular events in patients with protruding aortic atheromas: a prospective study. J Am Coll Cardiol. 1994;23:1085–1090. doi: 10.1016/0735-1097(94)90595-9. [DOI] [PubMed] [Google Scholar]
- 6.Atherosclerotic disease of the aortic arch as a risk factor for recurrent ischemic stroke. The French Study of Aortic Plaques in Stroke Group. N Engl J Med. 1996;334:1216–1221. doi: 10.1056/NEJM199605093341902. [DOI] [PubMed] [Google Scholar]
- 7.Mitusch R, Doherty C, Wucherpfennig H, Memmesheimer C, Tepe C, Stierle U, Kessler C, Sheikhzadeh A. Vascular events during follow-up in patients with aortic arch atherosclerosis. Stroke. 1997;28:36–39. doi: 10.1161/01.str.28.1.36. [DOI] [PubMed] [Google Scholar]
- 8.Fujimoto S, Yasaka M, Otsubo R, Oe H, Nagatsuka K, Minematsu K. Aortic arch atherosclerotic lesions and the recurrence of ischemic stroke. Stroke. 2004;35:1426–1429. doi: 10.1161/01.STR.0000127788.32550.d4. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Yasaka M, Nagano K, Otsubo R, Oe H, Naritomi H. Moderate atheroma of the aortic arch and the risk of stroke. Cerebrovasc Dis. 2006;21:26–31. doi: 10.1159/000089590. [DOI] [PubMed] [Google Scholar]
- 10.Di Tullio MR, Sacco RL, Savoia MT, Sciacca RR, Homma S. Aortic atheroma morphology and the risk of ischemic stroke in a multiethnic population. Am Heart J. 2000;139:329–336. doi: 10.1067/mhj.2000.101225. [DOI] [PubMed] [Google Scholar]
- 11.Stone DA, Hawke MW, LaMonte M, Kittner SJ, Acosta J, Corretti M, Sample C, Price TR, Plotnick GD. Ulcerated atherosclerotic plaques in the thoracic aorta are associated with cryptogenic stroke: a multiplane transesophageal echocardiographic study. Am Heart J. 1995;130:105–108. doi: 10.1016/0002-8703(95)90243-0. [DOI] [PubMed] [Google Scholar]
- 12.Cohen A, Tzourio C, Bertrand B, Chauvel C, Bousser MG, Amarenco P. Aortic plaque morphology and vascular events: a follow-up study in patients with ischemic stroke. FAPS Investigators. French Study of Aortic Plaques in Stroke. Circulation. 1997;96:3838–3841. doi: 10.1161/01.cir.96.11.3838. [DOI] [PubMed] [Google Scholar]
- 13.Transesophageal echocardiographic correlates of thromboembolism in high-risk patients with nonvalvular atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators Committee on Echocardiography. Ann Intern Med. 1998;128:639–647. doi: 10.7326/0003-4819-128-8-199804150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jr, Jackson CM, Pullicino P. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 15.Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Effect of medical treatment in stroke patients with patent foramen ovale: patent foramen ovale in Cryptogenic Stroke Study. Circulation. 2002;105:2625–2631. doi: 10.1161/01.cir.0000017498.88393.44. [DOI] [PubMed] [Google Scholar]
- 16.Anderson SI, Housley AM, Jones PA, Slattery J, Miller JD. Glasgow Outcome Scale: an inter-rater reliability study. Brain Inj. 1993;7:309–317. doi: 10.3109/02699059309034957. [DOI] [PubMed] [Google Scholar]
- 17.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., III Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 18.Di Tullio MR, Sacco RL, Homma S. Ultrasound examination of the aortic arch in stroke. In: Welch M, Caplan LR, Reis D, Siesjo B, Weir B, editors. Primer on cerebrovascular diseases. Academic Press; 1997. pp. 628–34. [Google Scholar]
- 19.Rundek T, Di Tullio MR, Sciacca RR, Titova IV, Mohr JP, Homma S, Sacco RL. Association between large aortic arch atheromas and high-intensity transient signals in elderly stroke patients. Stroke. 1999;30:2683–2686. doi: 10.1161/01.str.30.12.2683. [DOI] [PubMed] [Google Scholar]
- 20.Khatibzadeh M, Mitusch R, Stierle U, Gromoll B, Sheikhzadeh A. Aortic atherosclerotic plaques as a source of systemic embolism. J Am Coll Cardiol. 1996;27:664–669. doi: 10.1016/0735-1097(95)00526-9. [DOI] [PubMed] [Google Scholar]
- 21.Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability and stroke: the Aortic Plaques and Risk of Ischemic Stroke (APRIS) Study. J Am Coll Cardiol. 2008;52:855–861. doi: 10.1016/j.jacc.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tunick PA, Nayar AC, Goodkin GM, Mirchandani S, Francescone S, Rosenzweig BP, Freedberg RS, Katz ES, Applebaum RM, Kronzon I. Effect of treatment on the incidence of stroke and other emboli in 519 patients with severe thoracic aortic plaque. Am J Cardiol. 2002;90:1320–1325. doi: 10.1016/s0002-9149(02)02870-9. [DOI] [PubMed] [Google Scholar]
- 23.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the American Heart Association/American Stroke Association Council on Stroke: co-sponsored by the Council on Cardiovascular Radiology and Intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–e449. [PubMed] [Google Scholar]
- 24.Amarenco P, Bogousslavsky J, Callahan A, III, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]


