Abstract
Background and Aims
Further cases of novel influenza A (H1N1) outbreak are expected in the coming months. Vaccination has been proven to be essential to control a pandemic of influenza; therefore, considerable efforts and resources have been devoted to develop a vaccine against the influenza A (H1N1) virus. With the current availability of the vaccine, it will be important to immunize as many people as possible. However, previous data with seasonal influenza vaccines have shown that there are multiple barriers related to perceptions and attitudes of the population that influence vaccine use. The aim of the study was to evaluate the acceptance of a newly developed vaccine against pandemic (H1N1) 2009 influenza A among healthcare workers (HCW) in Mexico.
Methods
We conducted a cross-sectional study among HCW in three hospitals in the two largest cities in Mexico—Mexico City and Guadalajara—between June and September 2009.
Results
A total of 1097 HCW participated in the survey. Overall, 80% (n = 880) intended to accept the H1N1 pandemic vaccine and 71.6% (n = 786) reported they would recommend the vaccine to their patients. Doctors were more likely to accept and recommend the vaccine than nurses. HCWs who intend to be immunized will be more likely to do so if they know that the vaccine is safe and effective.
Conclusions
Knowledge of the willingness to accept the vaccine can be used to plan strategies that will effectively respond to the needs of the population studied, reducing the health and economic impact of novel influenza A (H1N1) virus.
Keywords: Vaccine acceptance, Influenza A (H1N1) virus, Health care workers
Introduction
Influenza vaccines are among the most effective strategies for protecting individuals from illness during influenza epidemics. Previous experience with the seasonal influenza vaccine has shown that there are several barriers related to individual perceptions and attitudes that influence vaccine use (1). Recent studies have reported an increase in rates of vaccine refusal—particularly for pediatric vaccines (2,3). Several authors studied the acceptance of the seasonal influenza vaccine among health care workers (HCW), finding that the rates are universally low (4).
HCW are considered to be at high risk for seasonal and pandemic influenza due to their exposure to the virus by direct patient contact or contact with infectious substances. More importantly, HCW can act as extremely efficient transmitters of viruses to others in medical care settings. For these reasons, most countries have also prioritized HCWs for receiving pandemic influenza vaccine (5). The Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (6) (ACIP) has included health care workers among one of the initial target groups for a vaccination against novel influenza A (H1N1) virus.
Since the novel influenza A (H1N1) virus first emerged in Mexico in April 2009, Mexico has had a central role in implementing control measures to prevent the spread of the disease. Mexico's early and intense involvement in this pandemic may have impacted HCW vaccine attitudes and behaviors. Given that the effectiveness of influenza immunization program depends on broad acceptance of vaccination among high-risk groups, we conducted a cross-sectional study among HCW in the two largest cities in Mexico—Mexico City and Guadalajara—to evaluate the factors that influence the acceptance (for self-administration and for recommendation to their patients) of a newly developed vaccine against pandemic (H1N1) 2009 influenza among this high-risk and high-priority population.
Patients and Methods
Study Design and Sampling
We used a cross-sectional survey between June and September 2009 to assess the determinants of vaccine acceptance and willingness to recommend to their patients a new vaccine against the novel influenza A (H1N1) 2009 virus among HCW in Mexico. Three referral hospitals in the two largest cities in Mexico (Mexico City and Guadalajara) were selected to be included in the study: Hospital General de Mexico and Hospital General Dr. Manuel Gea Gonzalez in Mexico City and Hospital Civil Antiguo in Guadalajara. All three hospitals are affiliated with the Ministry of Health and provide general and specialty medical care to low socioeconomic status and uninsured populations.
All HCW (general and specialist physicians, medical residents, nurses and nurse practitioners) >18 years of age affiliated with one of the participating facilities were eligible to participate in this study. There were a total of 8245 eligible HCWs at the three institutions.
We calculated the sample size using Open Epi (Open Source Epidemiologic Statistics for Public Health, v. 2.3., Atlanta, GA). A stratified random sample of HCWs from all work shifts and all departments was selected from each of the three hospitals. Because no published data are available on acceptance rates of immunization for influenza among HCW in Mexico, a conservative 50% rate was hypothesized to estimate the sample size. Sample size was calculated for a type I error of 5%. The crude sample size was increased to account for an expected respondent's rate of 70%. This procedure yielded a total sample size of 1685.
Tools and Data Collection
Data were collected using a self-administered, paper-based questionnaire. In each of the three settings, researchers trained nurses and social workers in the study procedures for administering questionnaires to study participants.
The survey included 40 closed-ended questions. The majority of the questions were based upon a 5-point Likert scale. These included (separately for HCW and their patients): HCW perception of the possibility for them and for their patients to acquire the influenza A (H1N1) virus infection during a 1-year period given they were unimmunized (impossible to very likely), how serious they thought the disease would be for themselves and for their patients (not at all serious to very serious), how protective a newly developed vaccine against the influenza A (H1N1) virus would be for them and for their patients (not protective at all to very protective) and how safe they thought the vaccine would be for them and for their patients. The same scale was also used for various questions regarding their level of agreement to a series of immunization beliefs (strongly disagree to strongly agree), their level of trust towards available sources of information regarding the vaccine, and the perceived quality of such sources (extremely poor source to excellent source). Sources included the government, health care providers, the media and pharmaceutical companies. Furthermore, two questions regarding the HCW willingness to personally accept vaccination and recommend vaccination to others were included with yes, no or don't know answers. Finally, the survey had questions about frequency of previous vaccine use against seasonal influenza among HCW as well as demographic characteristics of the population. These included age, professional experience, and field of medical specialty (internal medicine, pediatrics, infectious diseases, epidemiology, surgery, anesthesiology or other). Survey completion took ~20–25 min and was performed during the regular working hours of the participants.
Ethical Considerations
The study was reviewed and approved by the Research Ethics Committees in each the participating hospitals as well as by the Institutional Review Board of Emory University.
Statistical Analysis
The primary outcomes in the study were willingness of the HCW to receive the immunization with the newly developed vaccine against influenza A (H1N1) virus (acceptors), and willingness to recommend the vaccine to the patients under their care (recommenders). Results were analyzed using acceptor/nonacceptor and recommender/nonrecommender categories.
Descriptive analyses were conducted for the demographic characteristics. HCWs were divided into two categories for analysis. The first category, ‘physicians,’ included general physicians, medical residents and specialists, whereas ‘nurses’ included nurses and nurse practitioners. Chi-squared tests, Fisher exact tests for proportions (when required), and t-tests were used to assess differences in demographic characteristics between acceptor/nonacceptor and recommender/nonrecommender status.
Logistic regression was used to identify associations between vaccine acceptance and the perceptions and beliefs of HCWs. Wald χ2 tests and t-tests were used to analyze categorical and continuous variables, respectively. Bivariate logistic regression was used to determine unadjusted odds ratios between HCW perceptions and beliefs and vaccine acceptance. All statistical analyses were conducted using SAS 9.2 software (SAS Institute, 2008, Cary, NC).
Results
Of the 1685 health care workers included in the survey, 1097 responded resulting in a 65.1% response rate. Seven pregnant participants were excluded from the analysis because they were part of another priority group for vaccination due to their pregnancy. The mean age of the respondents was 39.6 (SD 10.3 years) years and the mean years of professional experience were 16.4 (SD 9.7 years). The majority of respondents were nurses (60%, n = 625).
Overall, 80% (n = 880) of the HCW intended to accept the H1N1 pandemic vaccine and 71.6% (n = 786) reported they would recommend the vaccine to their patients. Doctors were more likely than nurses to accept the vaccine (83.8 vs. 78.4%) and recommend the vaccine (80.4 vs. 67.8%). Recommenders were older and had more years of professional experience than those who did not intent to recommend the vaccine (Table 1). There was no significant difference in age, years of professional experience, and proportion of doctors between acceptors and nonacceptors.
Table 1.
Characteristics of the survey respondents from three different hospitals in Mexico
| Characteristic | Total population | Acceptors | Nonacceptors | Missing | Recommenders | Nonrecommenders | Missing | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 1097 | n = 880 | n =203 | n = 14 | n = 786 | n = 245 | n = 66 | ||||||||||
| Means | ±SD | Means | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | p valueb | Mean | ±SD | Mean | ±SD | p valueb | |
| Age (years) | 39.6 | 10.3 | 39.5 | 10.4 | 39.7 | 9.3 | 42.5 | 13.9 | 0.81 | 40.1 | 10.6 | 37.1 | 9.0 | 40.8 | 11.0 | <0.01 |
| Professional | 16.4 | 9.7 | 16.4 | 9.9 | 16.4 | 8.9 | 17.6 | 11.3 | 0.95 | 16.9 | 10.0 | 14.5 | 8.7 | 17.2 | 9.1 | <0.001 |
| Experience (years) |
||||||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Profession | ||||||||||||||||
| Doctor | 438 | 39.7 | 367 | 83.8 | 69 | 15.8 | 2 | 0.5 | 352 | 80.4 | 72 | 16.4 | 14 | 3.1 | ||
| Nurses | 625 | 56.0 | 490 | 78.4 | 124 | 19.8 | 11 | 1.8 | 0.07 | 424 | 67.8 | 165 | 26.4 | 36 | 5.8 | <0.01 |
| Missing | 34 | 4.3 | 23 | 67.6 | 10 | 29.4 | 1 | 2.9 | 10 | 29.4 | 8 | 23.5 | 16 | 47.1 | ||
| Degree | ||||||||||||||||
| General physician |
25 | 2.3 | 18 | 72.0 | 6 | 24.0 | 1 | 4.0 | 19 | 76.0 | 4 | 16.0 | 2 | 8.0 | ||
| Specialized physician |
278 | 25.3 | 233 | 83.8 | 44 | 15.8 | 1 | 0.4 | 230 | 82.7 | 38 | 13.7 | 10 | 3.6 | ||
| Resident | 136 | 12.3 | 116 | 85.3 | 19 | 14.0 | 1 | 0.7 | 103 | 76.3 | 30 | 22.2 | 2 | 1.5 | ||
| Nurse | 602 | 55.9 | 479 | 79.6 | 123 | 20.4 | 0 | 417 | 68.0 | 161 | 26.3 | 35 | 5.7 | |||
| Nurse practitioner |
23 | 1.1 | 11 | 47.8 | 1 | 4.3 | 11 | 47.8 | 0.20 | 7 | 58.3 | 4 | 33.3 | 1 | 8.3 | <0.01 |
| Missing | 33 | 3.1 | 23 | 2,6 | 10 | 4.9 | 0 | 10 | 29.4 | 8 | 23.5 | 16 | 47.1 | |||
| Hospital | ||||||||||||||||
| HGMc | 437 | 39.8 | 33 | 76.2 | 103 | 23.6 | 1 | 0.2 | 310 | 70.9 | 117 | 26.8 | 10 | 2.3 | ||
| HCGd | 258 | 23.4 | 214 | 82.9 | 41 | 15.9 | 3 | 1.2 | 191 | 74.3 | 57 | 22.2 | 9 | 3.5 | ||
| HGMGGe | 402 | 36.6 | 333 | 82.8 | 59 | 14.7 | 10 | 2.5 | <0.01 | 285 | 70.9 | 70 | 17.4 | 47 | 11.7 | 0.04 |
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
χ2 test.
two-tailed t-test.
Hospital General de Mexico.
Hospital Civil de Guadalajara.
Hospital Genrral Dr. Manuel Gea Gonález.
HCWs were more likely to accept the vaccine if they had colleagues who had acquired a 2009 (H1N1) infection (OR = 1.79, 95% CI 1.21–2.65), perceived they were likely to acquire 2009 (H1N1) virus infection, perceived the 2009 (H1N1) influenza A to cause severe disease (OR = 1.69, 95% CI 1.23–2.31), considered the 2009 influenza A (H1N1) vaccine to be safe (OR = 6.51 95% CI 4.63–9.16) and efficacious (OR = 5.65 95% CI 4.03–7.93), had been immunized against seasonal influenza in the preceding 5 years (OR = 3.43, 95% CI 2.49–4.72), considered themselves to be a part of a high-risk group for acquiring influenza A 2009 (H1N1) infection (OR = 1.88, 95% CI 1.33–2.67), and if they considered themselves to be at high risk of transmitting the infection (OR = 1.85, 95% CI 1.34–2.55). Likelihood of recommending the vaccine was associated with perceptions of susceptibility to and severity of 2009 influenza A (H1N1) (OR = 2.57, 95% CI 1.90–3.46), vaccine efficacy (OR = 9.07, 95% CI 6.53–12.59) and safety (OR = 10.30, 95% CI 7.37–14.41), whether they considered themselves to be a part of a high-risk group for acquiring the infection (OR = 2.32, 95% CI 1.67–3.22) with or transmitting the pandemic 2009 (H1N1) virus (OR = 1.99, 95% CI 1.47–2.69), and whether they intended to receive the vaccine (OR = 2.03, 95% CI 1.44–2.86) (Table 2).
Table 2.
Association between the health care workers' perceptions and likelihood of accepting and recommending a pandemic influenza A (H1N1) vaccine
| Acceptors | Odds ratio (95% CI) |
Recommenders | ||
|---|---|---|---|---|
| p value | Odds ratio (95% CI) |
p value | ||
| Acquired influenza A (H1N1) infection during the recent outbreak |
0.69 (0.18–2.57) |
0.58 | 1.54 (0.33–7.08) |
0.58 |
| In contact with patients with influenza A (H1N1) infection |
1.19 (0.86–1.64) |
0.27 | 1.19 (0.88–1.61) |
0.25 |
| Had colleagues treating patients with influenza A (H1N1) virus who acquired the disease |
1.79 (1.21–2.65) |
<0.01 | 1.12 (0.80–1.57) |
0.50 |
| Knew someone outside the hospital who acquired influenza A (H1N1) virus infection |
1.29 (0.82–2.03) |
0.26 | 1.45 (0.94–2.25) |
0.08 |
| Likelihood to get novel influenza A (H1N1) virus |
1.96 (1.41–2.74) |
<0.01 | 2.39 (1.78–3.20) |
<0.01 |
| Severity of the disease for a health care worker |
1.69 (1.23–2.31) |
<0.01 | 2.57 (1.90–3.46) |
<0.01 |
| Protectiveness of a vaccine against novel influenza A (H1N1) virus |
5.65 (4.03–7.93) |
<0.01 | 9.07 (6.53–12.59) |
<0.01 |
| Safety of a vaccine against novel influenza A (H1N1) virus |
6.51 (4.63–9.16) |
<0.01 | 10.30 (7.37–14.41) |
<0.01 |
| Had been immunized vaccinated against seasonal influenza within the past 5 years |
3.43 (2.49–4.72) |
<0.01 | 1.23 (0.91–1.66) |
0.17 |
| Consider themselves as part of a high risk group for developing influenza A (H1N1) disease |
1.88 (1.33–2.67) |
<0.01 | 2.32 (1.67–3.22) |
<0.01 |
| Consider themselves as part of a high risk group for transmitting influenza A (H1N1) disease |
1.85 (1.34–2.55) |
<0.01 | 1.99 (1.47–2.69) |
<0.01 |
| Were unable to attend the clinic because of too much work |
1.03 (0.72–1.49) |
0.84 | 1.007 (0.72–1.40) |
0.97 |
| The times at the clinic were unsuitable for them |
0.68 (0.49–0.96) |
0.03 | 0.98 (0.71–1.35) |
0.93 |
| Accepting the vaccine for themselves |
2.03 (1.44–2.86) |
<0.01 |
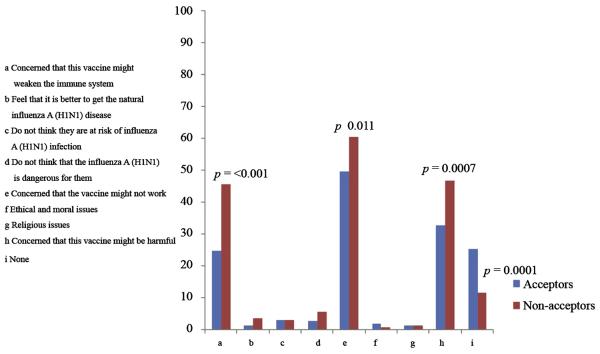
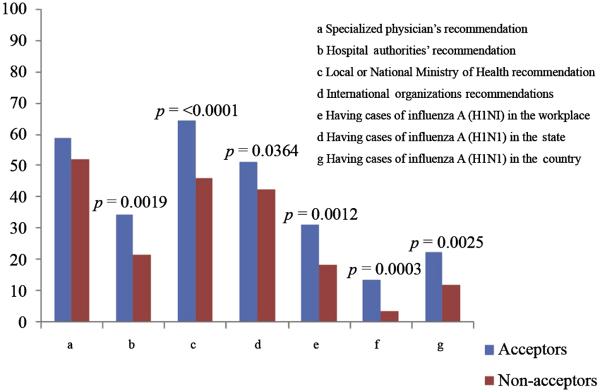
The most common reasons for rejecting the vaccine included concerns that the vaccine may not work, that the vaccine might be harmful, and that the vaccine may weaken the immune system (Figure 1.) These concerns were also held by some HCWs who planned on accepting the vaccine; however, the concerns were less frequent among acceptors than refusers. The three most frequent sources that would influence acceptance of the vaccine by recommending it were local or national ministries of health (64.67%), specialized physicians (58.93%) and international organizations (51.47%); however, these sources and all sources considered were less likely to influence acceptors than nonacceptors (Figure2).
Figure 1.
Respondents' reasons to reject the pandemic influenza A (H1N1) vaccine in three Mexican hospitals
Figure 2.
What would influence respondents to accept the influenza A (H1N1) pandemic vaccine.
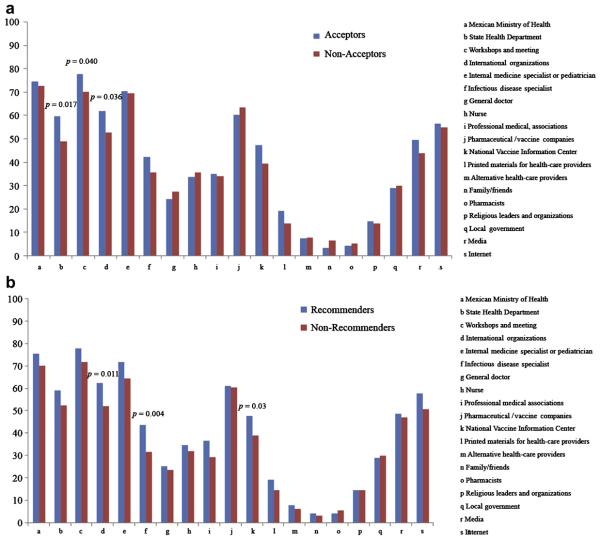
We assessed the level of trust in the various sources of information about novel influenza A (H1N1) virus immunization. Only trust in the information given by family and/or friends was significantly different between acceptors and nonacceptors (Figure 3a). Recommenders were more likely to trust information provided in hospital meetings, by an internist, a pediatrician, or a specialist in infectious diseases, and available on the internet compared to nonrecommenders (Figure 3b).
Figure 3.
Level of trust according to different sources of information regarding the vaccine against novel influenza A (H1N1) virus among HCW who are willing to accept or recommend the vaccine.
Discussion
Overall willingness to accept the 2009 influenza A (H1N1) vaccine among Mexican HCW was 80%, and 71.6% were likely to recommend it to their patients. The main motivators for acceptance of the vaccine were considerations of safety and effectiveness of the vaccine. Moreover, HCWs who accepted the vaccine were more likely to have had received the seasonal flu vaccine during the past 5 years. Acceptors of the vaccine were also less likely than the nonacceptors to have concerns that the vaccine may be harmful, that it may not work, or that it may weaken the immune system.
In our study the most frequent reasons that the HCWs (both acceptors and nonacceptors) said would influence them to accept the 2009 influenza A (H1N1) pandemic vaccine included recommendations from local or national ministries of health, specialized physicians, or international organizations. Similarly, a substantial proportion of HCWs considered government entities and international organizations as trusted sources of H1N1 vaccine information; and slightly more than half thought the media and internet were trusted sources. These findings are reassuring because they indicate that the domestic and international public health authorities may influence the vaccine acceptance behavior of Mexican HCWs. Moreover, the findings highlight the importance of specialized (infectious diseases) physicians in influencing the vaccine acceptance decisions of their colleagues
Our study took place during a unique opportunity in the epicenter of the 2009 influenza A (H1N1) outbreak, potentially impacting vaccine attitudes and likelihood to accept and recommend the vaccine. In spite of the high acceptance rate we found, it was notable that 20% of HCWs were not willing to take the vaccine and that being in contact with patients with the disease was not significantly associated with the willingness to accept the vaccine.
The rate of the willingness of HCWs to take the novel influenza A (H1N1) vaccine has varied greatly among different populations. In a study conducted in Hong Kong, overall acceptance rate was 47.9% (7). Our study did not assess the attitude of the HCW towards a vaccine in a prepandemic phase; however, we had records of the number of HCWs who were immunized against traditional seasonal flu in the past 5 years, which ranged from 10–26% in Mexico City and from 12–48% in Guadalajara.
There were limitations to our study. Although the overall response rate was very good, there is the potential for nonresponder or selection bias. The questionnaire was reasonably brief and, consequently, some information could not be collected. For example, we were unable to evaluate differences between the groups (acceptors vs. nonacceptors and recommenders vs. nonrecommenders) according to gender of the HCWs because this information was not obtained in the questionnaire. The majority of responders were most likely females because the vast majority of nursing positions in Mexico are held by women. The study was intentionally targeted to HCWs at a time and place that coincided with the epicenter of a major pandemic, which may (word missing, bias?) the generalizability of the study. Moreover, all the settings were referral centers for patients with severe influenza A (H1N1) disease. Therefore, our findings may be different in settings that did not treat these kinds of patients.
In conclusion, we found a high acceptance of an intention to recommend a vaccine against the pandemic influenza A (H1N1) virus among HCWs in three hospitals in Mexico. Historically, developing countries have been disproportionately affected by influenza pandemics. Understanding the prevalence of intention to accept and recommend a vaccine as well as vaccine attitudes that are associated with these behaviors will be helpful to policymakers to plan strategies that will effectively respond to the needs of high-priority populations, thus reducing the health and economic impact of H1N1 (swine) influenza. Improving the identification of the determinants of vaccine acceptance may allow development and evaluation of vaccine delivery strategies specifically targeted to high-risk groups who have refused immunization.
Acknowledgments
We would like to show our appreciation to the team of pollsters: Nurses Macrina Salgado Brito, Dolores Sanz Romero, Yolanda Cruz Zarate, Edith Castro Serralde, Maria Araceli Salazar Pereira, Maria Luisa Cruz Sandoval, Eva Ramirez Rojas and Adriana Hernandez Prado.
This study was supported in part by the NIH/FIC (Emory AITRP D43 TW0142).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ritvo P, Irvine J, Klar N, et al. A Canadian national survey of attitudes and knowledge regarding preventive vaccines. J Immune Based Ther Vaccines. 2003;1:3. doi: 10.1186/1476-8518-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Omer SB, Salmon DA, Orenstein WA, et al. Vaccine refusal, mandatory immunization, and the risks of vaccine-preventable diseases. N Engl J Med. 2009;360:1981–1988. doi: 10.1056/NEJMsa0806477. [DOI] [PubMed] [Google Scholar]
- 3.Salmon DA, Moulton LH, Omer SB, et al. Factors associated with refusal of childhood vaccines among parents of school-aged children: a case-control study. Arch Pediatr Adolesc Med. 2005;159:470–476. doi: 10.1001/archpedi.159.5.470. [DOI] [PubMed] [Google Scholar]
- 4.Hollmeyer HG, Hayden F, Poland G, et al. Influenza vaccination of health care workers in hospitals—a review of studies on attitudes and predictors. Vaccine. 2009;27:3935–3944. doi: 10.1016/j.vaccine.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 5.Uscher-Pines L, Omer SB, Barnett DJ, et al. Priority setting for pandemic influenza: an analysis of national preparedness plans. PLoS Med. 2006;3:e436. doi: 10.1371/journal.pmed.0030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.CDC Use of Influenza A (H1N1) 2009 Monovalent Vaccine Recommendations of the Advisory Committee on Immunization Practices (ACIP) 2009. MMWR. 2009;58:1–8. [PubMed] [Google Scholar]
- 7.Chor JS, Ngai KL, Goggins WB, et al. Willingness of Hong Kong healthcare workers to accept pre-pandemic influenza vaccination at different WHO alert levels: two questionnaire surveys. BMJ. 2009;25:339. doi: 10.1136/bmj.b3391. [DOI] [PMC free article] [PubMed] [Google Scholar]