Abstract
Prognosis after stroke in children is difficult given the paucity of literature regarding motor and cognitive recovery. Spatial neglect has been described in children after stroke, yet little evidence exists to guide clinicians and parents regarding its resolution. Wallerian degeneration on magnetic resonance imaging (MRI) suggests poor recovery in neonates and adults. We report near complete resolution of spatial neglect in 4 weeks and significant improvement in hemiplegia in a 9-year-old boy with a right anterior cerebral artery and middle cerebral artery infarction, despite Wallerian degeneration apparent on diffusion-weighted imaging. Serial assessment of neglect documenting the rapid course of recovery is the unique feature of this case and may help serve as a guide to pediatricians and neurologists in assessment of young patients and counseling of parents. The lack of published outcome data suggests a need for larger studies about the recovery of spatial neglect and other cognitive symptoms following pediatric stroke.
Keywords: spatial neglect, Wallerian degeneration, stroke recovery
Unilateral spatial neglect is often defined as the inability to attend or respond to space contralateral to brain damage that is not attributable to a primary sensory or motor deficit. The neuroanatomical correlates of neglect following right or left hemisphere damage have been well described in adults.2–5 Despite numerous reports of unilateral spatial neglect in children6–10 and a recent prospective study design,7 its neuroanatomical correlates, severity, and recovery characteristics remain poorly defined. Much of this poor characterization stems from different neglect tests, single-center studies, and inadequate location and lesion volume analysis. Few reports have focused on the recovery from spatial neglect in children. One notes lack of complete recovery at 6 months,11 while still others mention improvement7,8 but do not describe the time course of recovery. We report on a child with spatial neglect after large right middle cerebral artery and anterior cerebral artery stroke. Our patient was formally tested 8 and 32 days poststroke onset. Initial testing showed severe neglect and follow-up testing showed near complete resolution of neglect. Wallerian degeneration was observed on the magnetic resonance imaging (MRI) 8 days poststroke, yet our patient regained significant motor function.12 This case highlights several features important for prognosis and the time course of recovery from acute stroke. This patient is part of a consent-based neurovascular database approved by our institutional review board. Our institutional review board does not review single case reports.
Case Report
A 9-year-old boy with reactive airway disease had shortness of breath not relieved by albuterol 5 days prior to outside hospital admission. Just prior to admission, he developed altered mental status, intermittently pounding the top of his head and gritting his teeth. On admission, he was diagnosed with pneumonia with bilateral pleural effusions requiring a chest tube. When his mental status improved, he was completely unable to move his left side. Magnetic resonance imaging showed an acute right anterior cerebral artery, right middle cerebral artery, and left pulvinar stroke, at which time he was transferred to our institution. Images show a 155 cm3 stroke (33% of the right hemisphere), which includes right caudate, putamen, and Brodmann areas 4, 5, 6, 7, 22, 23, 31, 39, 40–42 (Figure 1, top panels) determined using maps as previously described2 as well as the left pulvinar.
Figure 1.
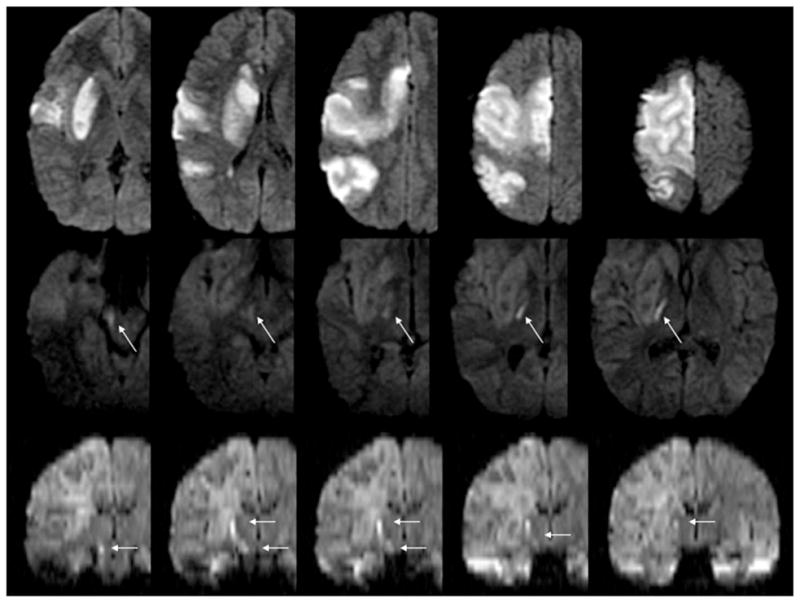
MRI in the acute and subacute time period. Diffusion-weighted imaging performed immediately after left-sided weakness was recognized shows an acute stroke, infarct volume of 155 cm3 (upper panels); MRI performed 11 days postsymptom recognition shows Wallerian degeneration (arrows) on axial diffusion-weighted images (middle panels) and coronal reconstruction diffusion-weighted images (arrows) in lower panels.
Cerebral digital subtraction angiography was performed immediately due to concerns of arterial dissection. The angiogram revealed clots in the right middle cerebral artery (M1 segment), the right anterior cerebral artery (A2 segment and pericallosal artery), and the left posterior cerebral artery (P1 segment), but no arterial dissection, suggesting thromboembolic etiology and possible hypercoagulable state.
Hypercoaguable workup revealed antiphospholipid syndrome with prolonged partial thromboplastin time and dilute Russell viper venom time, as well as decreased protein C activity (63%), and a decreased antithrombin III activity (59%). Normal studies included factor V Leiden, prothrombin 20210A mutation, anticardiolipin antibodies, factor VIII, homocysteine, rheumatologic screen, antinuclear antibody, C-reactive protein, and erythrocyte sedimentation rate. A transesophageal echocardiogram showed a small patent foramen ovale with no functional shunt by bubble study, making the left heart the likely source of emboli. Electrocardiogram revealed intermittent atrial fibrillation without reported palpitations.
Eight days after admission, he underwent formal neglect testing. He did not have perseverative symptoms, tactile or visual extinction, but had severe unilateral spatial neglect (Table 1, Figure 2). The same day, he was discharged to rehabilitation with a flaccid left hemiplegia. Wallerian degeneration was apparent on diffusion-weighted MRI 11 days poststroke, extending from the right internal capsule through the pons (Figure 1 middle and bottom panels).12 At this time, he had only trace movement of his left side. Assessment of spatial neglect at discharge, 32 days poststroke revealed near complete resolution (Table 1, Figure 2). He was able to walk with assistance but had little use of his left hand.
Table 1.
Percentage of Errors on Neglect Testing After Stroke
| Task and % Error | Day 8 | Day 32 |
|---|---|---|
| Copy scene | 25% | 5% |
| Clock drawing | 44% | 0% |
| Line cancellation | 10% | 0% |
| Circle cancellation left side | 47% | 0% |
| Circle cancellation right side | 7% | 0% |
Figure 2.
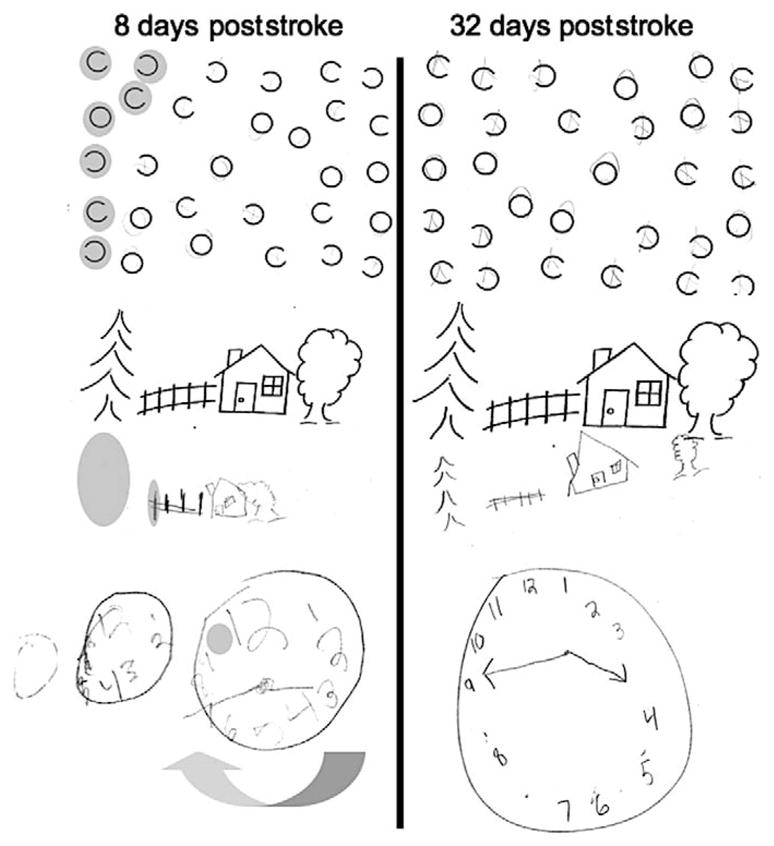
Neglect testing. Cancellation: Our patient was asked to circle the circles and put an X on anything else. He neglected the left portion of the page. Copy scene: Errors are shown in gray. Clock copy: Three attempts to draw a clock from memory showing 3:45, the first 2 “weren’t right.” Errors were scored for distortion and omission. At follow-up, only distortion was present.
Six months’ poststroke, he had moderate left hemiparesis (4/5 strength in his upper and lower extremities), hyperreflexia, and an upgoing toe. He was unable to straighten his wrist but could squeeze his fingers and abduct his thumb. Functionally, he needed help dressing, and his gait showed mild circumduction. He was back in regular classes at school and doing well while continuing outpatient rehabilitation.
Subsequent holter monitor tracings have shown sustained arrhythmias with asymptomatic junctional escape rhythms and supraventricular ectopic beats. He has been maintained on warfarin given that he has both cardiac arrhythmia and antiphospholipid syndrome as ongoing risk factors for stroke recurrence.
Discussion
This case demonstrates near complete resolution of spatial neglect in a school-age child approximately 30 days post-stroke. Motor recovery was dramatic though not complete, from hemiplegia at 8 days poststroke to ambulatory with only moderate motor deficits at 6 months poststroke. This recovery occurred despite extensive right hemisphere stroke and evidence of Wallerian degeneration on brain MRI.
In children, even with late-onset seizures and subsequent hemispherectomy, spatial neglect13 and language14 can recover, illustrating the remarkable plasticity of the young brain. Traditionally, focus has been placed on language recovery following brain damage with less emphasis placed on spatial perception.8 Thus, despite multiple descriptions in children, spatial and motor neglect remains poorly characterized. In adults, damage to Brodmann area 22 (superior temporal gyrus), 39 and 40 (temporal parietal junction), 44 (inferior frontal lobule), and underlying white matter have been associated with spatial neglect.2–5 Neglect is associated with larger strokes. Caudate and insula are often damaged with this type of stroke and have therefore been implicated in producing neglect,5 but in patients with only deep gray matter stroke, the presence of cortical hypoperfusion in specific regions such as the superior temporal gyrus was the only predictor of spatial neglect.2 Some of the recovery from spatial neglect could have been due to resolution of poststroke edema in areas associated with neglect,2–5 but the strongest predictors of spatial neglect (Brodmann areas 22, 39, and 40)5 were severely damaged in this case. It is also possible given hemispherectomy recovery in children, that the time course for recovery from spatial neglect in children is measured in weeks.
Strong correlation exists between the observance of Wallerian degeneration and long-term morbidity in adults.15 Furthermore, motor outcome correlates more closely with the finding of Wallerian degeneration than with the volume of lesion in children.16 Despite evidence of Wallerian degeneration on MRI, children may get back more function following the same insult as compared with adults.17 Our patient had significant recovery of motor function, but his recovery was not complete. His left hand dysfunction is particularly likely to cause long-term impairment.
Spatial neglect and motor deficits may improve quickly in children even after there is significant damage to areas associated with severe neglect in adults,9,10,13 yet in others, deficits persist for years.8 Neglect should be assessed using multiple tasks due to the multifaceted disorder this “catch-all term” describes.4 Authors have suggested standardized pediatric neglect tasks (teddy bear cancellation), for children under 6,8 but many adult neglect tasks have been successfully used in children.13 Similarly, Wallerian degeneration is associated with poor outcome,12 yet our patient has made significant improvements in motor function. Future studies will hopefully incorporate information such as time from brain injury to testing, as well as lesion size and location, and presence of Wallerian degeneration. These parameters should assist physicians and families as they discuss the potential for recovery.
Acknowledgments
LCJ was supported by NINDS K23NS062110, JTK was supported in part by NINDS RO1NS47691 to Argye E. Hillis. We would like to thank Argye E. Hillis for helpful comments on earlier versions of this work.
Footnotes
The authors have no conflicts of interest tp disclose with regard to this article.
For reprints and permissions queries, please visit SAGE’s Web site at http://www.sagepub.com/journalsPermissions.nav
Contributor Information
Jonathan T. Kleinman, Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Philippe Gailloud, Department of Radiology, Division of Neuroradiology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Lori C. Jordan, Department of Neurology, Division of Pediatric Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
References
- 1.Heilman K, Watson R, Valenstein E. Localization of lesions in neglect and related disorders. In: Kertesz A, editor. Localization and Neuroimaging in Neuropsychology. San Diego, CA: Academic Press; 1994. pp. 495–524. [Google Scholar]
- 2.Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits E, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25(12):3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8(11):1603–1610. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman JT, Newhart M, Davis C, Heidler-Gary J, Gottesman RF, Hillis AE. Right hemispatial neglect: frequency and characterization following acute left hemisphere stroke. Brain Cogn. 2007;64(1):50–59. doi: 10.1016/j.bandc.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karnath H-O, Fruhmann Berger M, Küker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cortex. 2004;14(10):1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- 6.Trauner DA. Hemispatial neglect in young children with early unilateral brain damage. Dev Med Child Neurol. 2003;45(3):160–166. doi: 10.1017/s0012162203000318. [DOI] [PubMed] [Google Scholar]
- 7.Laurent-Vannier A, Chevignard M, Pradat-Diehl P, Abada G, De Agostini M. Assessment of unilateral spatial neglect in children using the teddy bear cancellation test. Dev Med Child Neurol. 2006;48(2):120–125. doi: 10.1017/S0012162206000260. [DOI] [PubMed] [Google Scholar]
- 8.Laurent-Vannier A, Pradat-Diehl P, Chevignard M, Abada GS, De Agostini M. Spatial and motor neglect in children. Neurology. 2003;60(2):202–207. doi: 10.1212/01.wnl.0000048201.26494.0b. [DOI] [PubMed] [Google Scholar]
- 9.Ferro JM, Martins IP, Távora L. Neglect in children. Ann Neurol. 1984;15(3):281–284. doi: 10.1002/ana.410150314. [DOI] [PubMed] [Google Scholar]
- 10.Thompson NM, Ewing-Cobbs L, Fletcher JM, Miner ME, Levin HS. Left unilateral neglect in a preschool child. Dev Med Child Neurol. 1991;33(7):636–644. doi: 10.1111/j.1469-8749.1991.tb14935.x. [DOI] [PubMed] [Google Scholar]
- 11.Billingsley RL, Lang FF, Slopis JM, Schrimsher GW, Ater JL, Moore BD., 3rd Visual-spatial neglect in a child following subcortical tumor resection. Dev Med Child Neurol. 2002;44(3):191–200. doi: 10.1017/s001216220100192x. [DOI] [PubMed] [Google Scholar]
- 12.Kirton A, Shroff M, Visvanathan T, deVeber G. Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke. 2007;38(3):974–980. doi: 10.1161/01.STR.0000258101.67119.72. [DOI] [PubMed] [Google Scholar]
- 13.Marsh EB, Newhart M, Kleinman JT, et al. Hemispherectomy sustained before adulthood does not cause persistent hemispatial neglect. Cortex. 2009;45(5):677–685. doi: 10.1016/j.cortex.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Boatman D, Freeman JM, Vining EP, et al. Language recovery after left hemispherectomy in children with late-onset seizures. Ann Neurol. 1999;46(4):579–586. doi: 10.1002/1531-8249(199910)46:4<579::aid-ana5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Sawlani V, Gupta RK, Singh MK, Kohli A. MRI demonstration of Wallerian degeneration in various intracranial lesions and its critical implications. J Neurol Sci. 1997;146(2):103–108. doi: 10.1016/s0022-510x(96)00299-7. [DOI] [PubMed] [Google Scholar]
- 16.Staudt M, Niemann G, Grodd W, Krägeloh-Mann I. The pyramidal tract in congenital hemiparesis: relationship between morphology and function in periventricular lesions. Neuropediatrics. 2000;31(5):257–264. doi: 10.1055/s-2000-9239. [DOI] [PubMed] [Google Scholar]
- 17.Mazumadar A, Mukherjee P, Miller JH, Malde H, McKinstry RC. Diffusion-weighted imaging of acute cor ticospinal tract injury preceding Wallerian degeneration in the maturing human brain. AJNR Am J Neuroradiol. 2003;24(6):1057–1066. [PMC free article] [PubMed] [Google Scholar]


