SUMMARY
Gastroenteropathy manifesting in upper gastrointestinal symptoms, delayed gastric emptying, constipation, diarrhea and fecal incontinence occurs frequently in patients with diabetes mellitus and represents a significant health care burden. Current treatments are largely symptomatic and ineffective. Better understanding of the cellular and molecular pathogenesis of these disorders is required for the development of more effective therapies. Recent advances in our understanding of the inherent, high-level complexities of the control systems that execute and regulate gastrointestinal motility, together with the utilization of new experimental models and sophisticated physiological, morphological and molecular techniques have lead to the realization that diabetic gastroenteropathies cannot be ascribed to any singular defect or dysfunction. In fact, these disorders are multifactorial and involve a spectrum of metabolic and dystrophic changes that can potentially affect all key components of motor control including the systemic autonomic and enteric nervous systems, interstitial cells of Cajal and smooth muscle cells. Candidate pathomechanisms are also varied and include imbalance between pro- and anti-oxidative factors, altered trophic stimuli to mature cells and their progenitors, and, possibly, autoimmune factors. The goal of this paper is to review the cellular changes underlying diabetic gastroenteropathies and their potential causes, with particular focus on functional interactions between various cell types. It is proposed that diabetic gastroenteropathies should be considered a form of gastrointestinal neuromuscular dystrophy rather than a “functional” disorder. Future research should identify ways to block cytotoxic factors, support the regeneration of damaged cells and translate the experimental findings into new treatment modalities.
Keywords: Diabetes mellitus, hyperglycemia, growth factors, autoimmunity, gastrointestinal motility, slow wave, smooth muscle, myopathy, enteric nervous system, neuropathy, interstitial cells of Cajal
Diabetic gastroenteropathy: definitions and epidemiology
Gastrointestinal neuromuscular dysfunction and consequent symptoms cause considerable morbidity in patients with diabetes mellitus. The term diabetic gastroenteropathy encompasses all gastrointestinal complications of diabetes, which may manifest in dysphagia, heartburn, abdominal pain or discomfort, early satiety, nausea, vomiting, postprandial fullness and bloating; as well as in constipation, diarrhea, and fecal incontinence.1-3 The dominant symptoms vary among patients,3 but in most cases they are chronic or frequently recurrent.1 Gastroparesis is a specific form of neuromuscular dysfunction and is defined as slow gastric emptying (particularly of solids) in the absence of mechanical obstruction of the stomach. It is typically associated with early satiety, nausea, vomiting and bloating, which are reported by 5-12% of patients with diabetes.4 The correlation between symptom severity and degree of neuromuscular dysfunction in diabetes is variable.5 For example, otherwise silent gastroparesis may only manifest in poor glycemic control including unexplained episodes of hyper- or hypoglycemia, which may result from dyssynchrony of insulin administration and emptying of nutrients from the stomach into the small intestine.1,3,6,7 Conversely, sensory abnormalities may cause symptoms in patients without detectable motor dysfunction.3,6,8.
The true prevalence of diabetic gastroenteropathy remains unclear as the reported data vary widely depending on the targeted population (general community vs. patients seen at academic medical centers), the make-up of the sample (type 1 vs. type 2 patients, glucose control, etc.) and methodology. For example, in diabetic outpatients seen at tertiary referral centers symptom prevalence is typically high (76-83%)1,9 and gastroparesis demonstrable by diagnostic test is also common (up to 30-56%).10 In contrast, symptom prevalence in unselected patients with diabetes from the general community is much lower (6-27%).11 In general, symptoms tend to occur more frequently in patients with other complications of diabetes, poor glycemic control, and in women.9,12
Gastroparesis is usually considered the most significant manifestation of diabetic gastroenteropathy.7 Diabetic gastroparesis represents ~30% of all causes of gastroparesis13 and is more common in women than men9,13 Although it does not appear to increase mortality after adjustment for other disorders,14 gastroparesis can cause severe symptoms, nutritional insufficiency, electrolyte imbalance and impaired glycemic control4,7 and represents a major health care burden due to costly care and frequent hospitalizations.3 Diabetic gastroparesis also has a significant adverse effect on the patients’ quality of life,15 which is of prime concern in a population that typically also suffers from other devastating complications of diabetes.1,2 Therefore, it is most unsatisfactory that current therapeutic approaches are rather empiric, symptomatic, and clearly inefficient.1,3,7,16
The lack of fully validated, adequate treatment options mainly reflects our poor understanding of the pathogenetic mechanisms underlying diabetic gastroenteropathies. These disorders are broadly regarded as consequences of autonomic dysfunction particularly affecting vagal control.3,17 However, the pathomechanism of diabetic gastroenteropathy is multifactorial2,3,16,18,19 and involves changes in most cell types contributing to the regulation of neuromuscular functions of the gastrointestinal tract including enteric neurons (particularly those producing the inhibitory neurotransmitter nitric oxide (NO)),20 sympathetic input to the myenteric plexus,21 viscerosensory pathways,6,8 mucosal endocrine cells,22,23 smooth muscle cells24-26 and interstitial cells of Cajal (ICC).19,27 The purpose of this review is to provide an integrated view of the cellular pathologies, their functional implications and likely causes in diabetic gastroenteropathies, and, particularly, in gastroparesis. It is proposed that diabetic gastroenteropathies should be considered and treated as a form of gastrointestinal neuromuscular dystrophy affecting multiple cell types across the tunica muscularis rather than a disorder that could be managed solely by approaches targeting individual pathophysiological changes.
Pathophysiological features and therapeutic considerations
All aspects of gastrointestinal motility have been implicated in the pathogenesis of diabetic gastroenteropathies.1,2,7,16,18 However, the correlation between individual symptoms and pathophysiologic changes is rather poor and there is high variability both between and within individuals 16,18.
Esophageal dysfunction
Dysphagia, regurgitation and heartburn, symptoms of esophageal and lower esophageal sphincter dysfunction, are typically mentioned as symptoms of diabetic gastroenteropathy but not all epidemiological studies have supported their significance.2,18 Esophageal transit may be delayed but does not relate closely to delayed gastric emptying.18 Basal lower esophageal sphincter tone is diminished and this may be the cause of the higher prevalence of gastroesophageal reflux disease in diabetes.18,28 However, there is only weak association between impaired esophageal motility and symptoms18 and it is possible that the latter reflect abnormalities arising elsewhere in the upper gastrointestinal tract.29
Gastric dysfunction
Gastric symptoms and motor abnormalities are common in patients with both type 1 and type 2 diabetes.28,30 The motor abnormalities are heterogeneous and include both rapid and delayed gastric emptying (gastroparesis), impaired fundic relaxation and consequent abnormal intragastric meal distribution, reduced antral tone and motility, slow wave dysrhythmias, intense and prolonged pyloric contractions (“pylorospasm”) and impaired antropyloroduodenal coordination.18,28 Accelerated gastric emptying has most frequently been reported in “early” type 2 diabetes, i.e., in patients with relatively short duration (<2 years) of the disease and no evidence of autonomic neuropathy.30 Published results are contradictory as some described rapid solid emptying whereas others found accelerated emptying of liquids.30 Rapid gastric emptying is not restricted to type 2 diabetes. For example, a subset of patients with long-standing type 1 diabetes but without gastrointestinal symptoms was also found to have greatly accelerated solid emptying.5 The significance of rapid emptying is that it may represent a risk factor for the development of hyperglycemia and poor glucose control.30 From the human literature it is not clear whether rapid emptying progresses to normal or delayed emptying. Recent longitudinal studies in nonobese diabetic (NOD) mice treated with subtherapeutic doses of insulin to prevent excessive ketosis and death demonstrated a progression from rapid to normal emptying before the development of gastroparesis in a subset of the animals.31,32 This biphasic time course does not appear to be restricted to diabetic gastropathy since we found a very similar progression of gastric emptying half-times in mice exposed to chronic caloric restriction.33 Thus, acceleration during early stages of gastroparesis may be a general phenomenon and hold important clues about the development of the tissue-level changes.
Proximal gastric function has mainly been studied in functional dyspepsia with conflicting results. In general, dyspeptic symptoms and symptoms arising from rapid gastric emptying such as abdominal pain, diarrhea, dizziness and sweating after a meal, are typically attributed to impaired fundic receptive relaxation and accommodation.16,34 In type 1 diabetic patients, reduced accommodation to a liquid meal and lower fasting tone were described.35,36 Recently similar observations were reported in a mixed population of type 1 and type 2 patients with gastroparesis and symptoms refractory to prokinetic therapy except that fasting tone was normal.8 Impaired accommodation in symptomatic diabetic patients may lead to reduced retention of liquid meal in the proximal stomach throughout gastric emptying.37 In patients without demonstrable changes in gastric motility, dyspeptic symptoms may also arise from increased perception of gastric distention.6,16 Indeed, significantly lower sensory thresholds for discomfort have recently been described in diabetic patients with gastroparesis refractory to prokinetic therapy in both fasting and postprandial states.8 Thus, dyspeptic symptoms in diabetes may develop from a combination of upper gastric dysfunction and visceral hypersensitivity.8
Gastroparesis in diabetes may manifest in delayed emptying of both digestible and indigestible solids and nutrient liquids.7,38 Delayed gastric emptying of both liquids and solids has been demonstrated in diabetic rodents including streptozotocin (STZ)-diabetic rats39 and mice40,41, spontaneously diabetic NOD mice,31,40,42,43 a type 1 model; and in leptin receptor-deficient db/db mice, a model of type 2 diabetes.44 Delayed gastric emptying of gavaged barium was reported in conscious, restrained Chinese hamsters after 2-9 months of spontaneous diabetes and ketonuria by X-ray series.45 Gastroparesis may lead to disordered glycemic control, impaired nutrient and drug absorption, as well as symptoms such as fullness, upper abdominal pain and reduced hunger.16 However, the correlation with individual symptoms is poor and there is high variability between and within individuals.3,16,18 Delayed emptying of solids is more common than slow liquid emptying.3 Emptying of liquids depends mainly on the fundic “pressure pump” mechanism controlled by pyloric opening.46 Abnormally intense and prolonged pyloric contractions (“pylorospasm”) or loss of normal pyloric relaxation has been demonstrated both in patients and mouse models of diabetes.16,40,47 In contrast, gastric emptying of solids is more closely related to phasic antral motility.48 Thus, tests of solid emptying also provide more information about functions that are frequently impaired in diabetes. Delayed emptying of solids is caused primarily by antral hypomotility, which is frequently associated with dilation of the distal stomach.37,49 Reduced smooth muscle contractions may arise from abnormal smooth muscle function (myopathy),16,26 electrical slow wave dysrhythmias causing inadequate or abnormal pacing,7,50 or impaired electromechanical coupling, which may occur when slow wave frequency is abnormally rapid and plateaus are too short to permit sufficient Ca2+ influx.51 Gastric dysrhythmias including bradygastrias (slower-than-normal rhythm), tachygastrias (faster-than-normal rhythm) and mixed arrhythmias are commonly found in diabetic patients.7 Since the orderly orad-to-aborad propagation of peristaltic “rings” of contraction depend on a similar, organized spread of electrical slow waves,52,53 dysrhythmias developing along the propagation pathway will disrupt the normal pattern of slow wave propagation and peristalsis. Disrupted slow wave propagation may contribute to impaired gastric function both after meals and during phase III of the interdigestive migrating motor complex, which may lead to bezoar formation.18,28 Gastric myoelectric dysrhythmias accurately predict delayed gastric emptying.54 Conversely, normal electrical rhythmicity does not guarantee normal emptying and thus electrogastrography has not achieved widespread acceptance as a diagnostic tool.18,28 Abnormal slow wave activity has also been reported in STZ-diabetic rats55,56 and in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with type 2-like diabetes.57 In diabetic NOD mice we have shown focal losses of electrical slow waves.42 Acute hyperglycemia can also cause various dysrhythmias (particularly, tachygastrias and irregular rhythms) in normal volunteers,58 type I diabetic patients59 and in OLETF rats57. Elevated blood glucose has also been shown to cause reversible changes in fundic relaxation and vagal efferent function and lead to antral hypomotility, reduced phase III activity, increased pyloric contractions and delayed emptying of the stomach.3,7,18,58,59 Many of these effects are likely mediated by prostaglandins58 acting via EP3 prostanoid receptors.60 Hyperglycemia may also alter mitochondrial Ca2+ uptake and thereby affect electrical pacemaking.61 However, the magnitude of hyperglycemia-induced changes in gastric emptying are rather small and have not been detected in all studies.2 Readjustment of hypoglycemic treatment in type 2 diabetic patients also failed to improve delayed gastric emptying measured one week after therapy readjustment62. Thus, while hyperglycemia should be considered a potentially exacerbating factor in diabetic gastropathy, gastric dysfunction in diabetes cannot be attributed solely to acute hyperglycemic episodes.2
Small intestinal and colorectal dysfunction
Small intestinal and colorectal dysfunctions are common in diabetes, particularly in patients with gastroparesis.18 However, available data are contradictory as small intestinal transit may be slow, rapid or unchanged.18 In insulin-treated NOD mice, the transit in the small intestine of orally administered charcoal suspension was accelerated63 and rapid transit was also reported in STZ-diabetic mice.41 In contrast, in db/db mice, irregular small intestinal contractile activity was associated with prolonged whole-gut transit.44 Similar findings were described in diabetic Chinese hamsters by barium X-ray series.45 At a tertiary center, up to 60% of diabetic patients reported constipation, 22% had diarrhea and 20% had fecal incontinence.1 Factors that contribute to altered colonic motility are unclear but may include delayed transit, impaired gastrocolic reflex, abnormal internal anal sphincter tone and impaired rectal compliance and sensation.18 In both NOD mice and OLETF rats, constipation was associated with reduced neuromuscular neurotransmission in the distal colon.64,65 Interestingly, in the NOD model, distal colonic hypofunction was accompanied by a paradoxical increase in spontaneous spike complex activity in the proximal colon,65 which occurred in the absence of reduced inhibitory responses to electrical stimulation.65 Similar findings were reported in STZ-diabetic rats.66,67 The mechanism of the proximal colonic hypermotility remains unclear. It may reflect some form of functional compensation68 for distal colonic hypofunction and consequent accumulation of fecal material, loss of ICC and consequent depolarization67 or a response to small intestinal bacterial overgrowth.18
Why are current therapies inadequate?
Current options to treat gastroparesis, the most challenging manifestation of diabetic gastroenteropathies, focus on preventing flare-ups (e.g., by improving blood glucose control and thus avoiding hyperglycemic episodes or by eliminating indigestible solids and large meals), symptom management (with anti-emetics, non-pacing gastric electrical stimulation, psychological intervention, acupuncture and pain control), countering pathophysiological abnormalities (see below) and “bypassing” the diseased organ (by jejunostomy feeding or parenteral nutrition and, in desperate cases, by gastrectomy) (see refs.3,4,28,69 for reviews). Not surprisingly, treatments designed to fight specific pathophysiological features include the most widely used modalities. In this group belong pharmacological treatments with prokinetics (metoclopramide, erythromycin, domperidone and others), the mainstay for gastroparesis treatment, and high-energy, long-pulse electrical stimulation designed to entrain and pace electrical slow waves. These treatments stimulate gastrointestinal muscle contraction and, thereby, rely on improving residual function. Some drugs may improve fundic accommodation and dyspeptic symptoms.28 Endoscopic injection of botulinum toxin into the pylorus is meant to reduce pylorospasm. Although many patients have been successfully treated with various combinations of these therapies, many are also refractory to conventional treatments and management of advanced gastroparesis and end-stage gastric failure is unresolved. Thus, there is consensus that diabetic gastroenteropathies in general and gastroparesis in particular still represent a major therapeutic challenge.3,7,16 This is perhaps not surprising since, as described above, pathophysiological changes in diabetic gastroenteropathies are complex, variable among patients and experimental models, do not always correlate with symptoms, and have so far defied simple mechanistic explanations. It has been argued that the development of better therapies will require a better understanding of gastrointestinal motor physiology and pathophysiology. Below we review the control systems that regulate gastrointestinal motility and present a view that, due to the inherent complexities of these control mechanisms, it may be difficult to effectively treat diabetic gastroenteropathies solely by targeting specific pathophysiological mechanisms.
Integrative control of gastrointestinal motility predicts complex pathogenesis of diabetic dysmotilities
Gastrointestinal motility subserves complex and fundamental physiological processes such as ingestion and digestion of food, assimilation of its nutrients and waste removal, which are all critical for life. In order to properly execute these functions, the gastrointestinal motor apparatus has developed an immensely complex repertoire of mechanisms that ensures proper accommodation of meals, mixing of food with digestive juices, reduction of particle size and precisely timed transport of luminal contents between gastrointestinal organs that are highly specialized in terms of their digestive and absorptive capabilities. All these functions involve, and require the integrity of, several “key players”. Smooth muscle cells perform mechanical work and thus are a sine qua non for motility. The systemic autonomic and enteric nervous systems (ENS) convey sensory information, exert efferent control, generate reflexes and stereotyped motor patterns. Finally, interstitial cells of Cajal (ICC), which are the primary electrical pacemakers for rhythmic contractile activity, set smooth muscle membrane potential and serve as a bidirectional interface between nerves and smooth muscle.27,53,70-74 These cell types also receive and integrate information provided by a wide variety of signals including mechanical stimuli, luminal and absorbed nutrients, paracrine and endocrine signals, as well as inflammatory and immune mediators. Through their distinct but overlapping functions they constitute a highly complex and redundant control system.53,70,75 Below we provide a brief outline of an integrative model for the control of gastrointestinal motility; for additional details the reader is referred to recent reviews on this subject.27,53,60,71,72,74
Under physiological conditions, smooth muscle contraction, the elementary event central to all motor functions, largely depends on Ca2+ entry via voltage-sensitive Ca2+ channels.71 Therefore, the effects of all main control mechanisms are ultimately channeled through the regulation of smooth muscle membrane potential. Smooth muscle membrane depolarization above the threshold for Ca2+ entry can be brought about by myogenic, ICC-mediated and neuronal mechanisms and the efficacy of excitation-contraction coupling can be further regulated by altering the smooth muscle’s responsiveness to input signals71,76. Stretch of the smooth muscle cells per se causes depolarization via regulation of mechanosensitive ion channels including Cav1.2, the major source of voltage-dependent Ca2+ influx into smooth muscle cells; Nav1.5, a tetrodotoxin-insensitive Na+ channel; K2P2.1, K2P10.1 and KCa1.1 potassium channels and various nonselective cation channels.77 The smooth musculature is organized in layers of smooth muscle cells that are interconnected into larger functional units by gap junctions. Thus, depolarization at one point will spread to neighboring cells and activate entire bundles.
A second layer of regulation is provided by ICC, an evolutionarily preserved,78 heterogenous group of mesenchymal cells identifiable by ultrastructural features79 and the dependence on stem cell factor (Kit ligand; Kitl) signaling via Kit, a receptor tyrosine kinase.80,81 ICC occur throughout the digestive tract from the esophagus to the internal anal sphincter.82 They have been classified according to morphological criteria, e.g., their localization within the muscular layers (submuscular, intramuscular, myenteric, subserosal) and their basic morphology (stellate vs. bipolar).79,81 Although the division of labor between ICC classes is not absolute,52 ICC can also be classified by their primary function as some are mainly involved in electrical rhythm generation (e.g., multipolar ICC in the myenteric region of phasic muscles),60 whereas others (e.g., spindle-shaped, intramuscular ICC) mainly contribute to regulation of contractile activity by generating tone,83,84 by mediating certain types of neuroeffector inputs to the smooth muscle and pacemaker ICC60,85-87 and by serving as mechanotransducers77,88,89. ICC regulate smooth muscle membrane potential via electrical coupling60,79 and the hyperpolarizing gaseous mediator carbon monoxide.27
The third level of regulation is provided by the ENS and, indirectly, the systemic autonomic nervous system. The latter exerts efferent control via the ENS by two main pathways: by parasympathetic input from the vagal nuclei and the sacral spinal cord and by sympathetic postganglionic nerves from the prevertebral ganglia. There is also direct postganglionic sympathetic innervation of certain smooth muscle cells and blood vessels within the tunica muscularis externa. Both the vagus and sympathetic nerves carry afferent axons as well. The ENS is an enormously complex system in its own right: it contains approximately 108 neurons forming interconnected ganglia and also enteric glial cells. Processes of these cells form plexuses in the myenteric region and in the submucosa. ENS neurons can be classified according to their location, neurochemistry, shape, proportions, connections and function.73 For example, they include primary afferent neurons, excitatory and inhibitory neurons to the circular and longitudinal muscle layers, ascending and descending interneurons, secretomotor and vasomotor and intestinofugal neurons.73 ENS neurons are organized into functional circuits that execute reflex responses, some of which involve even intestinofugal neurons and the prevertebral ganglia.72 Along the entire length of the gut, ENS pathways form continuous overlapping networks.73 Besides (relatively) simple reflexes, the ENS may also have “hard-wired” circuits similar to central pattern generators that may be responsible for organized, repetitive behaviors such as neural peristalsis and migrating motor complexes (see below).74 Neural control is exerted by action potential-driven neurotransmitter release and, depending on the neurotransmitters involved, can be excitatory (e.g., acetylcholine, serotonin, substance P (SP)) or inhibitory (e.g., NO, vasoactive intestinal polypeptide (VIP), ATP).73
Depending on the neurotransmitters and the anatomical region, neural control of smooth muscle function is either direct or indirect via spindle-shaped intramuscular ICC and related ICC in the deep muscular plexus region of the small intestines.85 ICC may transmit the effects of neural inputs to the smooth muscle cells by electrical coupling or paracrine mediators90. Intramuscular ICC mediate some components of both excitatory and inhibitory neuromuscular neurotransmission. Evidence for these functions comes from analysis of postjunctional responses in the fundus, lower esophageal and pyloric sphincters and colon of mice and rats with hypomorphic mutations in Kit (W/Wv mice and Ws/Ws rats) or Kitl (Sl/Sld mice) and consequent reduced populations of intramuscular ICC85,91. Similar findings were also obtained in the small intestines of mice where ICC networks had been depleted by injections of neutralizing anti-Kit antibodies.92 A role for ICC in enteric neuromuscular neurotransmission is further supported by the presence of proteins involved in neurovesicle docking to presynaptic membranes in nerve fibers in close apposition to ICC and the expression of postsynaptic density proteins by ICC, which together form specialized junctions closely resembling synapses.85 ICC involved in neurotransmission also express several genes related to neurotransmitter-mediated signal transduction93 including those related to nitrergic signaling.94,95 Functional innervation of deep muscular plexus ICC is evidenced by their internalization of neurally-released, receptor-bound SP96 and translocation of protein kinase C(PKC)-ε in response to muscarinic activation.97 ICC mediate cholinergic and nitrergic responses, at least partially, but in the colon and stomach, purinergic inhibition and noncholinergic (peptidergic) excitation are rather preserved in their absence90,91. Other investigators failed to find evidence of impaired nitrergic neuromuscular neurotransmission in the lower esophageal, pyloric or internal anal sphincters or the whole stomach of W/Wv mice83,84,98,99 or in Ws/Ws fundus.100 Instead, these studies suggested a role for intramuscular ICC in maintaining smooth muscle tone or regulating excitability. These discrepancies remain to be resolved.
In the murine stomach, intramuscular ICC also participate in the chronotropic regulation of electrical slow waves by neural mechanisms.86 Specifically, they mediate cholinergic inputs to pacemaker ICC that reside in the myenteric region whereas they do not seem to be required for tachykininergic control of slow wave frequency.87 Moreover, intramuscular ICC may help maintain the proximal-to-distal slow-wave frequency gradient by contributing to the metabolism of acetylcholine87 and thereby preventing excessive chronotropic stimulation in the antrum during vagal stimulation.
Interaction between neural control and ICC has also been demonstrated in the context of afferent signaling. Similarly to smooth muscle cells, ICC can also sense and respond to mechanical stimuli, although these are the least well understood functions of ICC. For example, ICC have been shown to mediate the effects of distention to local enteric98 and vagal neuronal circuits.88,101 The latter function depends on a mutual trophic support between ICC and certain vagal nerve terminals: intramuscular ICC are required for the development and, perhaps, maintenance of vagal intramuscular arrays88,101 while survival of the ICC innervated by vagal afferent nerves depends on the integrity of the vagal fibers.102 However, ICC can also transduce passive stretch into excitatory input to pacemaker ICC in the stomach without the involvement of enteric neuronal activity, possibly by releasing prostaglandins.89 The mechanisms underlying mechanosensory functions of ICC are not clear but likely involve expression of mechanosensitive ion channels (e.g., Cav1.2 and Nav1.5)77.
Phasic contractile activity is critical for mixing the food with digestive juices, trituration and aborad propulsion of contents. This function is also ensured by multiple control mechanisms. The most robust pacemaker mechanism for rhythmic contractions is provided by relatively monotonous oscillations in membrane potential termed electrical slow waves, which are omnipresent in phasic muscles of the gastrointestinal tract. Although electrical slow waves can be recorded from the smooth muscle, this activity ultimately originates from ICC60. The elementary event underlying slow wave activity is the so-called unitary potential; a small, randomly occurring depolarization reflecting release of small quanta of Ca2+ from the smooth sarco-endoplasmic reticulum via inositol 1,4,5-trisphosphate-receptor (IP3R) channels and subsequent activation of pacemaker conductances52,60,103. The molecular identity of the pacemaker channel is unclear; both nonselective cation channels (NSCC) and Cl− channels have been proposed for this role52,60,104. The intracellular Ca2+ signals indirectly govern the openings of the NSCC by stimulating the influx of Ca2+ into energized mitochondria61. Since mitochondria are not in equilibrium with the cytoplasm, they take up more Ca2+ than the amount released through the IP3R, causing a net decrease in [Ca2+] in the vicinity of the pacemaker ion channels, which respond by increasing their open probability60,61. Increases in intracellular [Ca2+] inhibit the pacemaker NSCC by a calmodulin-dependent mechanism.105 The unitary potentials reflect ion fluxes in confined subcellular spaces called pacemaker units bounded by sarco-endoplasmic reticulum, mitochondria and the cell membrane106. The generation of slow waves requires the synchronization of many such pacemaker units, which is achieved by the summation of unitary potentials, subsequent activation of voltage-sensitive, dihydropyridine-insensitive Ca2+ currents, which lead to the rapid upstroke of slow waves and the recruitment of additional IP3Rs by Ca2+-induced Ca2+ release60,103. Full development of slow waves most likely requires the cooperation of several other ion channels.
An important property of the voltage-sensitive, dihydropyridine-resistant Ca2+ currents is that their activation by depolarization of electrically coupled, neighboring ICC (or electrical stimulation) can phase-advance slow waves. Thus, these voltage-dependent channels are ultimately responsible for slow wave propagation by sequentially triggering the pacemaker mechanisms in the network of electrically coupled ICC, which are all capable of producing spontaneous, rhythmic activity.107 This mechanism also explains how slow waves can propagate in a regenerative fashion, i.e., without decrement, throughout large gastrointestinal organs.53
In pacemaker ICC networks occupying any small piece of phasic muscle tissue, slow waves are initiated at random places and spread in all directions with similar velocity.53,103,108 However, in whole organs or larger pieces of excised tissue, they originate from a dominant pacemaker site. In the stomach, this pacemaker site is usually located in the orad corpus along the greater curvature.109 Slow waves spread from this site both circumferentially, across the thickness of the muscle layers, and in the aborad direction using the aforementioned regenerative mechanisms. Since regenerative propagation depends on phase-advancement of slow waves generated by downstream pacemaker cells, the direction is determined by intrinsic frequencies of pacemaker ICC in the pathway of propagation. In other words, anally directed slow wave propagation is ensured by the orad-to-aborad (fast-to-slow) gradient in the intrinsic frequencies of pacemaker ICC.110 A similar frequency gradient and voltage-dependent entrainment also appears to be responsible for the active spread of slow waves across the thickness of the circular muscle layer via ICC that lie within intermuscular septa111. The orderly propagation of electrical activity down the length of the tubular gastrointestinal tract also requires anisotropic slow wave propagation velocities that allow the rapid circumferential spread of slow waves before their wavefront can advance aborally. It has been proposed that the faster circumferential entrainment of pacemaker ICC in the stomach may be due to the rapid propagation of electrical signals along the low-resistance pathway provided by intramuscular ICC that are embedded within, and run parallel to, the circular smooth muscle cells52,107. An alternative hypothesis is that circumferential propagation may not be regenerated at every point during the organoaxial spread after the initial, omnidirectional propagation from the dominant pacemaker site. Rather, the initial ring of activation that develops in the orad corpus would spread aborally as a near-simultaneous, circumferential wavefront utilizing the myenteric ICC network and the orad-to-aborad frequency gradient.53
Slow waves regulate phasic contractile activity by periodically bringing the smooth muscle membrane potential close to the activation threshold for voltage-dependent Ca2+ entry into smooth muscle cells which, if sufficiently large, result in mechanically productive contractions60,71. Electromechanical coupling depends on the magnitude and duration of suprathreshold depolarization and the presence or absence of the slow-wave-associated, regenerative Ca2+ action potentials. Besides the slow wave depolarizations, depolarization from muscle distension, net neuronal excitation or smooth muscle sensitization by humoral factors can trigger slow wave-associated contractions.53 Thus, slow waves only determine the maximum achievable frequency of phasic contractions and not necessarily their actual rate. Slow wave-driven phasic, propagating motor patterns underlie propulsive behavior in the distal stomach and duodenum e.g. postprandially, when the increased electromechanical coupling is triggered by the presence of the food. A similar phenomenon also occurs in intact, conscious animals during fasting, when tonic excitation from the ENS causes increased electromechanical coupling and slow wave-driven contractions in a section of the gut and this increased contractile activity migrates slowly aborally as a “migrating myoelectric complex” (MMC).112 Although MMC is influenced by humoral and vagal input, it is likely orchestrated by a neural pattern generator.73
Ca2+ action potentials can also occur and propagate independently of slow wave activity. In isolated tubular and flat sheet segments of the feline duodenum, this activity was found to be associated with peristaltic movements, which propagated both orally and aborally.113 These electrical potentials most likely reflected migrating ENS excitation that was strong enough to depolarize smooth muscle cells and trigger action potentials without the help of slow waves.53 Similar mechanisms likely underlie mass movements in the colon.114
In mutant rodents deficient in pacemaker ICC slow waves are absent from the tissues.115,116 Survival of these animals depends on a compensatory activity resembling Ca2+ action potentials as it can be inhibited by L-type Ca2+ channel blockers, which do not affect slow waves. This activity most likely originates from the smooth muscles70,91,117; it is rather irregular, has a low amplitude, shows poor tendencies to propagate aborally118,119 and only has a weak net propulsive effect.118 Nevertheless, in response to significant in vitro distension, these slow wave-deficient tissues develop rhythmic peristalsis likely from the cooperation between this latent smooth muscle-derived pacemaking and ENS activity.70 The residual myogenic electrical rhythm also appears to be sufficient to support MMC activity.120 Interestingly, the overall frequency of this “surrogate” rhythmicity is not significantly different from that of normal electrical slow waves.119 However, it is important to note that this compensatory activity have only been observed in full form in constitutive mutant animals and comparable loss of pacemaker ICC induced pharmacologically in postnatal mice invariably results in more dramatic loss-of-function.80 Thus, adaptation of the smooth muscle cells and unmasking or developing their latent pacemaker activity may require the acquisition of new capabilities during intrauterine development.75
The foregoing have important implications for the expected behavior of the gastrointestinal neuromuscular apparatus under pathological conditions. Firstly, the inherent complexity affords a significant functional reserve to the system; i.e., it ensures that dysfunction of any individual component does not necessarily result in the failure of the entire control apparatus. Thus, the relationship between injury and loss-of-function cannot be expected to be linear: within the range of compensation, damage to any particular component is likely to result in dysfunction that is less than predicted; whereas beyond a certain limit, injuries may become catastrophic, similarly to how heart or kidney disease can become decompensated. The limits of functional compensation may be influenced by transient or environmental factors such as acute changes in blood glucose levels,18 infections,7 stress121 and other psychological factors,122 which can precipitate bouts of symptoms or, when reduced, bring about improvement. This concept is consistent with clinical observations that even severe episodes of symptoms from diabetic gastroparesis can remit or even disappear altogether,24 even though the condition of the tissue is unlikely to change rapidly enough to account for the change in the clinical manifestation. The tendency for gastroparesis to wax and wane may also make it hard to distinguish effective treatment from spontaneous recovery and failed treatment from relapse. Another consequence of redundancy and overlapping functions is that damages to individual components of the control system do not necessarily lead to distinct dysfunctions. Thus, the detection of a particular abnormality (e.g., delayed gastric emptying) cannot predict whether its main underlying cause is myopathy, neuropathy, ICC defect or various combinations thereof. Finally, a highly complex regulatory system is also likely to display greatly variable or even seemingly inconsistent pathophysiological changes depending on the relative involvement of various components of the control apparatus. For example, impaired ICC function in the fundus may cause accelerated gastric emptying, whereas a similar change in the antrum may cause delayed emptying; and when they occur together, they may make the functional outcome difficult to predict. Thus, the best therapeutic approach may not be to stimulate fundic relaxation or antral contractions, respectively, but, rather, to improve ICC function, which may take care of both problems.
In summary, the cooperation between the smooth muscle, ICC and extrinsic and intrinsic neural control provides for an intricate and highly redundant control system that has great functional reserve. However, it must be emphasized that while loss or dysfunction of any component may be sufficiently compensated by other mechanisms, the compensated system will have reduced functionality and, perhaps more importantly, reduced functional reserves to counter any new challenge. In the next section we review changes to key regulatory cell types that could reduce the functional capacity of the gastrointestinal motor apparatus in diabetic gastroenteropathies.
Cellular dystrophy and degeneration in diabetic gastropathy
Extrinsic nerves
The first mechanistic investigations into diabetic gastrointestinal dysfunction suggested a major role for irreversible autonomic neuropathy affecting primarily the vagus nerve. Studies on the thoracic vagus of diabetic patients mainly showed Wallerian degeneration and, in some cases, segmental demyelination.123 However, most of the myelinated fibers in the cervical vagus do not go to abdominal viscera so the relevance of these findings to gastroenteropathies is uncertain at best.24,123 In the wall of the esophagus, there was swelling, irregularity of caliber and disruption of parasympathetic fibres both within the myenteric plexus and in the extrinsic trunks.123 Subsequent studies focusing on unmyelinated fibers of the vagus yielded inconsistent results. For example, in a single patient that underwent gastrectomy for intractable gastroparesis, Guy et al. described a severe reduction in the density and diameter of unmyelinated axons, thickened basal lamina of Schwann cells, dense collection of collagen fibrils and abnormal endoneurial capillaries.17 However, others failed to detect similar changes in larger series.24,124,125 Vagal unmyelinated fibers were reduced in size and number and contained axonal glycogenosomes and axoplasm sequestrations in spontaneously diabetic BioBreeding (BB) rats.126 Guo et al. detected increased apoptosis in the sensory nodose ganglion of STZ rats127. In the same model, Tay and Wong described two phases of degeneration in the dorsal motor nucleus of the vagus nerve.128 After 3-7 days, degenerating dendrites had electron-dense cytoplasm with dilated rough endoplasmic reticulum and swollen mitochondria, while degenerating axon terminals showed clustering of small spherical agranular vesicles and swollen mitochondria. Later changes included macrophage infiltration and, after 9-12 months, a second wave of degeneration similar to the first wave occurred. However, STZ has been found to be able to elicit significant neuropathologic changes independent of diabetes and by directly inducing the formation of reactive oxygen species (ROS).129 STZ can also directly affect renal, hepatic and muscle tissues.129 Thus, and especially in view of the rapid development of the neuronal degeneration, findings in STZ-diabetic animals must be interpreted with caution. In summary, degenerative changes in parts of the vagus nerve that innervate abdominal viscera may occur in both patients and animals with diabetes but their true significance is still unclear.
Diabetic autonomic neuropathy also affects the prevertebral superior mesenteric and celiac sympathetic ganglia. In both patients and animal models, markedly enlarged distal axons and nerve terminals have been described130,131 and termed as neuroaxonal dystrophy.130 In postmortem samples from diabetic patients, enlarged cell bodies containing vacuoles and multilamellar bodies were also found.131 Neuroaxonal dystrophy may represent aberrant intraganglionic sprouting and occurs without cell death.132,133 This pathological change was only found in insulinopenic models such as STZ-diabetic mice and rats, NOD mice and BB/Worcester (BB/W) rats but not in the type 2 models db/db mice, BBZDR/W and Zucker Diabetic Fatty rats, suggesting that low insulin, C-peptide or insulin-like growth factor-I (IGF-I) may play a role in their pathogenesis.21,132,134,135 In a recent study, significant loss of tyrosine hydroxylase-positive intramural fibers was reported in a patient with severe, poorly controlled, long-standing diabetes and intractable symptoms requiring the implantation of gastric stimulator and a more modest decline in another gastroparetic patient with well-controlled, short-term diabetes (Figure 1).25 Degeneration of intramural adrenergic fibers was also reported in the jejunum of a patient with long-standing, complicated diabetes136 and in the ileum of STZ-diabetic rats.137 While these are certainly compelling results, the clinical relevance of sympathetic injury is not entirely clear.16 Nevertheless, these changes may very well contribute to overall reduced functional capacity of the affected organs.
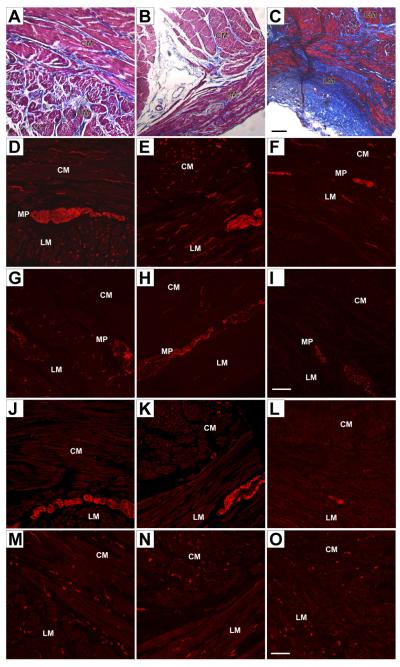
Figure 1. Cellular changes in the stomach of patients with diabetic gastroparesis and refractory symptoms.
Full thickness specimens from the anterior wall of the mid-corpus above the incisura. Left panels, control nondiabetic obese patient undergoing gastric bypass surgery. Middle panels: 37-year-old white female patient with a history of well-controlled type 1 diabetes for about 3 years (case 1). Right panels: 32-year-old white female patient with a history of poorly controlled type 1 diabetes for about a decade (case 2). CM, circular muscle; MP, myenteric plexus; LM, longitudinal muscle. (A–C) Masson’s trichrome staining (connective tissue: blue; nuclei: dark red/purple; cytoplasm: red/pink). The control (A) and sections from case 1 (B) showed no increase in fibrosis, whereas sections from case 2 (C) showed an increase in fibrosis in both muscle layers and along the myenteric plexus. Scale bar 200 μm. (D–F). PGP 9.5 immunoreactivity. PGP9.5 immunoreactivity as a marker for neuronal structures was normal in control (D) and in case 1 (E). There was a decrease in PGP 9.5 immunoreactivity in both the muscle layers and the myenteric plexus in sections from case 2 (F) suggesting a loss of neuronal structures. (G–I). Tyrosine hydroxylase immunoreactivity. Tyrosine hydroxylase immunoreactivity was normal in the control (G). Sections from case 1 (H) showing normal tyrosine hydroxylase immunoreactivity around the myenteric plexus with a slight decrease in tyrosine hydroxylase immunoreactivity in the muscle layers. Sections from case 2 (I) showed a marked loss of tyrosine hydroxylase immunoreactivity in all regions suggesting a loss of extrinsic nerve fibers. Scale bar 100 μm. (J–L) Immunoreactivity for nNOS. Normal immunoreactivity for nNOS in control (J) and case 1 (K). nNOS immunoreactivity was markedly decreased in case 2 (L). Scale bar 100 μm. (M–O) Kit expression as a marker for interstitial cells of Cajal. Control (M) and case 1 (N) showed normal c-Kit immunoreactivity while in case 2 (O) there was a loss of Kit immunoreactivity suggesting a decreased number of ICC. Scale bar 100 μm. Panels reprinted from Ref. 25 under terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0)
Although pain is a significant symptom of diabetic gastropathy, very little information is available about gastric spinal nerves. In one study, increased apoptosis was reported in T10-L2 dorsal root ganglia from STZ-diabetic rats127 suggesting a role for degenerative changes in gastroparesis-associated pain. However, this finding remains to be confirmed.
ENS
Diabetes-associated abnormalities in the ENS have recently been reviewed.20 In this section we mainly focus on dystrophic changes, particularly those affecting nitrergic innervation, which seems to be most consistently observed ENS injury in diabetes.
Early studies in STZ-diabetic rats suggested that diabetes-associated changes in the ENS depended on the neurotransmitter and also on the region of the gut.138,139 For example, while production of the inhibitory neurotransmitters NO, VIP and galanin tend to decrease in diabetes,20 neuropeptide Y is either increased or unchanged20,138. Cholinergic innervation is also increased or unchanged.20,137 Serotonin content may decrease in the proximal gastrointestinal tract and increase in the colon.20,137,138 Reports on changes in SP are inconsistent.20,136,138,140 Calcitonin gene-related peptide was mainly found to be reduced in diabetes,20,139 although not in all parts of the gastrointestinal tract.139 This rather complex picture is further complicated by time-dependent changes.20,138,139. Most authors interpret these findings as an indication of an altered balance between inhibitory efferent neurotransmission (which is generally reduced in diabetes) and excitatory mechanisms (which either increase or do not change).20,47
Since neurons and nerve fibers are frequently identified and quantified on the basis of their neurochemical coding, changes in a particular neuron or nerve class may reflect altered neurotransmitter synthesis and release, abnormal neuron/nerve fiber number, or both. To detect true neuronal or fiber degeneration, immunostaining for a general neural marker, such as protein gene product (PGP) 9.5 (also known as ubiquitin carboxy-terminal hydrolase L1 (Uchl1)), general histological staining or electron microscopy is required. In untreated, spontaneously diabetic Chinese hamsters, gastrointestinal hypomotility was associated with a reduction of myenteric ganglia in the proximal third of the intestines.141 Ultrastructural analysis indicated degeneration of distal, unmyelinated axons with glycogen particles, aggregates of neurofilaments, dense residual bodies, swelling and frequent lamellar inclusion bodies.45 In STZ-diabetic rats, reduced density of PGP 9.5 positive nerve fibers was noted in the circular and longitudinal muscle layers of the proximal colon, while myenteric ganglia were preserved.67 Electron microscopy revealed swollen mitochondria and reduced synapse-like junctions with ICC. In gastrectomy specimens from patients with long-standing type 1 diabetes and end-stage gastric failure, myenteric neurons showed vacuolation and basophilic inclusions and the axons appeared ballooned.142 Reduced density of PGP 9.5 positive intramural nerve fibers was described in the jejunum of a patient with poorly controlled type 1 diabetes complicated by nephropathy, neuropathy and severe gastroparesis.136 This change was accompanied by a decrease in nerve fibers positive for neuronal NO synthase (nNOS), VIP, pituitary adenylate cyclase-activating polypeptide and tyrosine hydroxylase. SP immunoreactivity was increased. PGP 9.5 positive fibers were also reduced in the longitudinal muscle of the appendix in six type 1 diabetic patients143 and in the stomach of a patient with severe, poorly controlled, long-standing diabetes, intractable gastroparesis and malnutrition (Figure 1).25 In both studies, nNOS immunoreactivity was also reduced. In contrast, Yoshida et al. detected no abnormalities in enteric nerves or neurons in 13 autopsy and 3 antrectomy specimens from patients with long-standing diabetes.125 Similarly, no significant changes were detected in myenteric ganglia or intramural fibers in gastrectomy samples from four long-standing type 1 diabetic patients with severe, intractable gastroparesis and smooth muscle degeneration24 and in a patient whose gastroparesis developed on the background of a well-controlled, relatively short-term diabetes.25 Nakahara et al. also failed to detect loss of intramural nerve fibers or myenteric neurons in the colons of seven type 2 diabetic patients.144 No reduction in general neuronal markers (PGP 9.5, synaptophysin, microtubule-associated protein-2) was observed in several animal models including BB/W rats, STZ-diabetic and NOD mice by Western immunoblotting, oligonucleotide microarrays or quantitative real-time polymerase chain reaction (qRT-PCR) (Figure 2).26,40,145 In another study, NOD mice were reported to have reduced nerve fibers and myenteric ganglia but this conclusion was based on comparing diabetic NOD mice with BALB/c controls, while nondiabetic and diabetic NOD mice were not different.146 Clearly, this observation reflected strain differences rather than effects of diabetes.
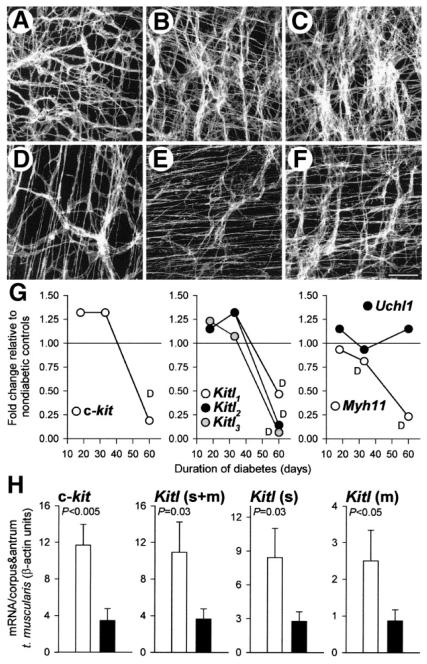
Figure 2. Reduced expression of Kit (c-kit), stem cell factor (Kitl), and smooth-muscle myosin in the stomachs of diabetic NOD mice.
(A–F) Representative confocal images of Kit immunofluorescence in the greater curvature of full-thickness whole mounts of gastric tunica muscularis tissues of nondiabetic NOD mice (A–C) and their long-term diabetic littermates (D–F). (A and D) Midcorpus; (B and E) orad antrum; (C and F) distal antrum. Scale bar in F is 50 μm and applies to all panels. Note reduction of Kit+ ICC networks in the diabetic mice. (G) Affymetrix Mouse Genome 430 2.0 GeneChip analysis of c-kit, Kitl, Myh11, and Uchl1 gene expression in NOD mice with short- (18 days), medium- (33 days), and long-term (60 days) diabetes. Fold changes in mean expression levels in groups of diabetic mice relative to groups of their nondiabetic littermates are shown. Values labeled D are significantly decreased by Affymetrix GeneChip Operating Software analysis (all others, “no change”). (H) Quantitative RT-PCR analysis of c-kit and Kitl expression in gastric corpus+antrum tunica muscularis tissues of nondiabetic NOD mice (open bars) and their long-term diabetic littermates (closed bars). Expression of membrane-bound Kitl (Kitl(m)) was calculated as the difference between total Kitl (Kitl(s+m)) and soluble Kitl (Kitl(s)) in each mouse. P values are from Mann–Whitney rank sum tests. Note that the reduction in c-kit was mirrored by a similar change in the expression of Kitl isoforms. Reprinted from Ref. 26 with permission from the American Gastroenterological Association Institute.
Reduced expression or activity of nNOS is perhaps the most consistent change affecting the ENS in diabetes although results showing no change25,66,67 or even increased expression39,147 have also been reported. Reduced nNOS mRNA and protein expression, as well as reduced numbers of nNOS-expressing neurons were observed in BB/W rats,145,148 STZ-diabetic rats,149,150 STZ-diabetic40,41 and NOD mice31,40. In one study where no decrease in nNOS expression was reported, female (but not male) STZ-diabetic mice with delayed gastric emptying had reduced levels of the active dimeric form of nNOSα.39 Reduced nNOS-expressing nerve fibers were also reported in the stomach, jejunum and appendix of long-term type 1 diabetic patients25,136,143 and in the stomach of type 2 patients.140 Decreased nitrergic neurotransmission upsets the balance between inhibitory and excitatory efferent control and leads to reduced nonadrenergic-noncholinergic relaxation responses in the gastrointestinal muscles.40,47,145,148 Thus, reduced nNOS expression or activity may underlie impaired fundic and pyloric relaxation, delayed gastric emptying and other dysmotilities seen in diabetes (see above). However, trials with pharmacological agents that increase NO levels or effects (e.g., nitroglycerin and sildenafil) have been disappointing due likely to the simultaneous, opposing effects of NO on gastric emptying via stimulating proximal gastric relaxation (which encourages storage) and pyloric relaxation (which increases emptying).151 This is a good example of how complex physiological mechanisms underlying organ-level functions can defeat pharmacological interventions targeting one particular mechanism or another.
As mentioned above, reduced NO levels could reflect impaired nNOS gene expression,40,145 posttranslational changes (e.g., nNOS dimerization)39 or axonal transport47 without a loss of neurons or could be the consequence of degenerative changes in the ENS.25,136,143 Clearly, responsiveness to pharmacological interventions targeting nNOS-expressing neurons would be expected to be quite different under these two circumstances. Indeed, while insulin treatment initiated 8 weeks after the induction of diabetes with STZ was able to restore nNOS mRNA, axonal protein levels and pyloric relaxation,40,47 the same treatment started 12 weeks after STZ failed to do so47 and the cause of this failure was a progressive apoptosis of nitrergic neurons after ~12 weeks of diabetes.47 These findings suggest that after a certain period of poorly controlled diabetes, reversible functional abnormalities may give rise to neurodegenerative changes that may be harder to treat by conventional pharmacological means.
Smooth muscle
Diabetic stomachs tend to have reduced ability to distend in response to meal or stretch16 suggesting changes in the physical properties of the smooth musculature. Dystrophic changes in gastric smooth muscles were first described in detail in two patients with end-stage gastric failure and severe weight loss arising from long-standing, complicated diabetes requiring gastrectomy.142 Light microscopy indicated the presence of scattered, homogenous, round eosinophilic bodies (“M” bodies) and subtotal smooth muscle cell atrophy with intercellular collagen accumulation. By electron microscopy, the “M” bodies were transformed smooth muscle cells with no nucleus or nuclear envelope and contained electron-dense granules resembling clumped heterochromatin. Many smooth muscle cells contained autophagosomes or signs of “controlled death”.142 Very similar findings were reported later in gastrectomy samples from four patients with intractable gastroparesis and severe weight loss.24 The eosinophilic bodies, which seem to be specific for diabetes,142 were also detected in the esophagus, stomach, small and large intestine, bladder and prostate of diabetic patients with autonomic neuropathy.131 Recently, Pasricha et al. reported diffuse smooth muscle atrophy and fibrosis in one of two patients requiring the implantation of gastric electrical stimulator for therapy-resistant diabetic gastroparesis (Figure 1).25 Although both patients had severe refractory symptoms and malnutrition, only the patient with poorly controlled, long-standing diabetes had smooth muscle involvement. In contrast, no obvious smooth muscle pathology was detected by more conventional histological techniques in a larger series of (mainly postmortem) samples from patients with long-standing diabetes including five patients with gastroparesis.125
Smooth muscle involvement has also been detected in several animal models of diabetes. In untreated, spontaneously diabetic Chinese hamsters, distension and reduced tone of the small intestines was accompanied by a reduction in the thickness of the tunica muscularis, infiltration of fibrous connective tissue, degeneration of smooth muscle cells and morphological changes suggestive of early stages of apoptosis.141 In NOD mice we reported decreased dry mass of gastric corpus+antrum tunica muscularis after ~60 days of untreated diabetes, which was preceded by a significant decline in smooth muscle myosin heavy chain 11 (Myh11) expression detected by hybridization to oligonucleotide microarrays (Affymetrix Mouse Genome 430.2 GeneChips) and by qRT-PCR.26 Production of Kitl by the smooth muscles was also significantly reduced (Figure 2). Decreased Kitl expression in the small intestine and colon and reduction in the amplitude of spontaneous contractions of the small intestine were also described in 12-week-old db/db mice, a type 2 model.44 Animal models also permitted the detection of more subtle signs of smooth muscle impairment. For example, In BB/W rats, gastric circular muscle contractions elicited by muscarinic (carbachol) or tachykininergic (SP) stimulation were reduced despite normal neural release of acetylcholine and normal voltage-dependent Ca2+ entry.152 Induction of IP3 release and PKC translocation by carbachol were also diminished. Furthermore, in both STZ-diabetic rats and db/db mice, there was an early and specific reduction in the muscarinic control of contraction of antral smooth muscles at a post-synaptic level, which was associated with an alteration of the GTP-binding proteins coupled to muscarinic receptors.153 Thus, from the human and animal studies it appears that the smooth muscle involvement is likely an important factor in proximal diabetic gastroenteropathy but dramatic atrophy and fibrosis detectable by histology may only occur in advanced, poorly controlled (or, in animals, untreated) cases. Massive fibrosis may signal end-stage gastric failure and may in part be the cause of therapy resistance. However, these profound changes are likely to be preceded by a more subtle deterioration in intracellular pathways mediating neurotransmitter responses and a decrease in contractile protein expression. These changes could interfere with proper contractile responses and may eventually lead to a catastrophic loss of function.
In contrast to the dystrophic changes in the proximal gut, Forrest et al. detected smooth muscle hypertrophy in the colon of STZ-diabetic mice and this change was accompanied by increased force of spontaneous and induced contractions.66,67 We observed a similar hypertrophy in the colon of diabetic NOD mice that accompanied constipation and accumulation of fecal material in the proximal colon.65 Nowak et al. noted a 39% increase in intestinal smooth muscle mass in STZ-diabetic rats.154 The diabetic intestine was also significantly longer. Similarly to the increased contractility discussed earlier, the mechanism of the smooth muscle hypertrophy in the distal gut of diabetic rodents remains unclear. It may reflect differences in how different parts of the gastrointestinal tract react to the metabolic changes associated with diabetes67 or functional compensation for the distal colonic hypofunction and constipation similarly to how partial obstruction leads to hyperplasia and hypertrophy aborad to the affected segment.68 Alternatively, since reduced innervation was found in both the STZ rats and the NOD mice,65,67 it may have reflected denervation-induced smooth muscle hypertrophy (discussed in ref.24). The exact mechanism remains to be elucidated.
ICC
The role of ICC in diabetic gastroenteropathies was only recognized relatively recently.42,136 Since then, several studies have reported depletion of ICC networks in both patients and various animal models. The pathophysiological significance of ICC loss in diabetes has been recently reviewed.19 In this section we discuss available data on ICC involvement in diabetic gastroenteropathy and its animal models including new findings.
In our studies, untreated NOD mice, a well-established model of human type 1 diabetes mellitus, developed constipation and extended gastrointestinal tracts distal to the gastric corpus 6-8 weeks after the onset of diabetes.42 Gastric motor dysfunction manifested in delayed emptying of solids, electrical dysrhythmias and reduced postjunctional electrical responses. In two studies, 20 of 21 long-term (~60-day) diabetic NOD mice had clearly reduced Kit+ ICC networks by immunohistochemistry in their corpus and antrum relative to the corresponding regions in their age-matched, nondiabetic littermates (Figure 2).26,42 ICC depletion was more frequently focal than diffuse and could be detected both in the myenteric region and within the muscle layers. The focal lesions were variable in size and locations but were more frequent in the distal stomach. The decrease in ICC number was also verified by electron microscopy.42 Remaining ICC only had minor abnormalities and we found no signs of apoptosis, necrosis or mononuclear infiltration. Microarrays and qRT-PCR indicated a significant decrease in the expression of both Kitl and Kit ~60 days after the onset of diabetes and these changes occurred after the aforementioned decline in Myh11 expression.26 In the fundus ICC were not depleted although the typical close associations between intramuscular ICC and enteric nerve terminals were missing due to the accumulation of extracellular matrix. We also detected an invasion of myenteric ICC deep into the fundus, which normally lacks this class.42 ICC were reduced in foci across the thickness of the entire colon by both Kit immunohistochemistry and electron microscopy.65 The latter technique revealed no obvious degenerative or inflammatory changes. In the distal colon, ICC loss was associated with impaired excitatory and inhibitory neuromuscular neurotransmission. However, in the proximal colon paradoxically augmented phasic activity and hyperexcitability were detected, which were interpreted as (possibly myogenic) compensatory changes that developed in response to the distal hypomotility and severe constipation. In another study, NOD mice were kept on subtherapeutic insulin treatments to prevent severe hyperglycemia (>500 mg/dL) and ketosis without normalizing blood glucose levels.31 Under these conditions, 20% of the diabetic mice developed delayed gastric emptying of solids within 8 weeks of diabetes. Kit protein expression measured by Western immunoblotting in the gastric corpus was significantly reduced in the gastroparetic mice but not in their age-matched diabetic controls without delayed emptying. Yamamoto et al. observed delayed gastric emptying of solid food, increased whole-gut transit time of gavaged dye and small and irregular small intestinal contractile activity in 12-week-old db/db mice.44 These functional changes were accompanied by a decreased density of ICC in the antrum, proximal and distal small intestine and the midcolon. All ICC classes were diffusely affected. The authors found no evidence of apoptosis. Others detected reduced ICC by electron microscopy in the gastric antrum of 12-week STZ-diabetic rats with significant gastric dysrhythmias.56 The remaining ICC had reduced gap junctions and organelles, swollen mitochondria, dilated endoplasmic reticula, vacuoles, myelin figures and broad perinuclear spaces and were separated from other cells by wide extracellular spaces. In another study in the stomach of STZ-diabetic rats, a significant reduction in the density of intramuscular ICC was observed but myenteric ICC remained unaffected. The surviving ICC showed several ultrastructural abnormalities including disrupted lamellar bodies, swollen mitochondria, vacuolation and loss of connections with nerves. The latter also occurred in the fundus.155 In the colon of the same model, ICC were significantly reduced within the muscle layers and on the submucosal surface of the circular muscle but not in the myenteric region.67 Electron microscopy indicated swollen mitochondria, lamellar bodies, depletion of organelles, loss of synapse-like connections with enteric nerves and transformation into fibroblast-like cells. It is remarkable that marked degenerative changes were only found in the residual ICC in the STZ-diabetic rats but not in the spontaneously diabetic mice.42 While species differences may also account for these differences, a direct cytotoxic effect of STZ should also be considered.129
In the first report on ICC in human diabetes, He et al. described ICC loss in a jejunal biopsy taken from a patient with 15-year, poorly controlled type 1 diabetes complicated by nephropathy, neuropathy and severe gastroparesis.136 ICC were completely missing from the inner two-thirds of the circular layer and severely reduced in the myenteric region and the outer third of the circular muscle. Forster et al.156 examined gastric wall biopsies taken from the greater curvature of the gastric antrum during surgeries for the placement of stimulating electrode in patients refractory to conventional medical therapy. Profound loss of ICC was found in 4 of 9 biopsies from diabetic patients and modest depletion was noted in one. Patients with severe ICC loss had more tachygastria, higher symptom scores and showed less improvement to electrical stimulation than patients with normal ICC. More recently the authors reported that 9 of 23 diabetic patients with refractory gastroparesis had no ICC in their antrum biopsies; ICC loss also occurred in the corpus but was less frequent.157 Loss of ICC correlated with electrical dysrhythmias but not with the degree of gastroparesis as assessed by symptom scores.158 However, since patients with 20-100% residual ICC were all scored as normal, the reference group may have contained some patients with abnormally reduced ICC populations. In antral tissues from 42 diabetic patients who underwent gastrectomy for gastric cancer, Iwasaki et al.140 detected a significant reduction in intramuscular ICC in the circular muscle layer in 8 patients with severe diabetes defined by HbA1c levels exceeding 8%. No ICC loss was found in the myenteric region or in patients with milder forms of the disease. The mean ICC number did not correlate with the duration of diabetes, presence of other complications, drugs or insulin use. Recently a 30-40% reduction in intramuscular ICC was described in the anterior surface of the mid-corpus of a malnourished patient with therapy-resistant gastroparesis and long-standing, poorly controlled diabetes (Figure 1).25 Similar changes were not detected in another patient whose gastroparesis was associated with short and well-controlled diabetes. In a study by Nakahara et al., seven colon cancer patients with type 2 diabetes were found to have significantly reduced ICC (to ~40% of controls).144 The ICC loss did not correlate with constipation. In a recent study, Miller et al. investigated whether appendix specimens routinely obtained during abdominal surgeries to prevent potential future complications could be used as investigational tools or indicators of ICC status elsewhere in the gastrointestinal tract.143 In normal, noninflamed appendices, ICC were detected in the circular and longitudinal muscle layers. In six type 1 diabetic patients, the volume of ICC in the circular muscle layer was significantly reduced but no difference was found in the longitudinal muscle. While this result indicates that diabetes also affects ICC in the appendix, establishing the utility of the appendix as a model or indicator needs further studies.
In summary, both the animal and patient data strongly support the notion that loss of ICC is common in gastrointestinal complications of diabetes – in fact, one of the most consistently observed changes in both type 1 and type 2 diabetes. ICC depletion probably does not occur in isolation and may be accompanied by dystrophy of the extrinsic and enteric nerves and smooth muscle, as illustrated in Figures 1-2.25,26 The degree of cellular dystrophy and tissue remodeling seems to be related to the severity and duration of diabetes25,140 and, not surprisingly, is the most severe in advanced forms of gastroenteropathies.25,136 This relationship is also evident from animal studies. For example, while ~95% of untreated, long-term diabetic NOD mice showed loss of ICC26,42, only 20% of animals from the same strain had reduced Kit protein when treated with subtherapeutic doses of insulin31. Dramatic changes in cellular content of diabetic gastrointestinal tissues are likely to be preceded by more subtle abnormalities that can be detected at the cellular-molecular level. Such changes have been best explored in smooth muscle cells, which are more accessible for cell biological studies than ICC or enteric neurons.152,153 The significance of these early abnormalities is that in combination with other factors (e.g., acute hyperglycemia, infections, stress and psychological factors7,18,121,122) they may lead to quite severe clinical forms of dysmotilities.25
Mechanisms of cellular degeneration or injury in diabetic gastroenteropathies
In diabetic gastroenteropathies, cellular injury or depletion could potentially arise from autoimmune attack, hyperglycemia and associated oxidative damage, impaired trophic support from reduced growth factor signaling, or combinations thereof (Figure 3).
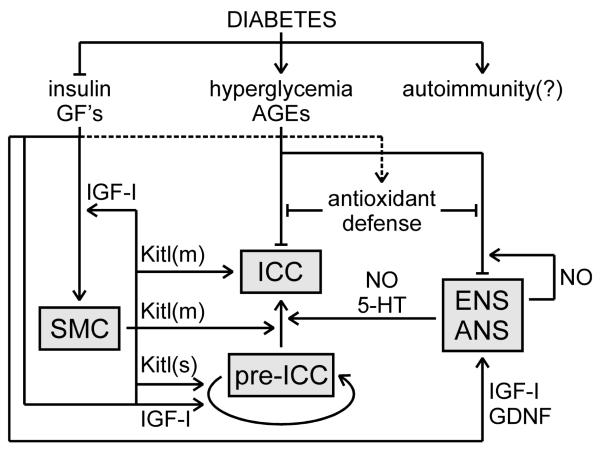
Figure 3. Hypothetical model for the cellular pathogenesis of diabetic gastroenteropathies.
Cellular degeneration in diabetic tissues arises from the combined actions of oxidative stress and reduced trophic signals. Autoimmunity may contribute but its role remains to be established. Hyperglycemia and advanced glycation end-products (AGEs) cause oxidative stress when they overwhelm antioxidant defenses. Nitric oxide (NO)-producing neurons of the enteric and systematic autonomic nervous system (ENS, ANS) may be particularly susceptible to oxidative stress due to the combination of superoxide and NO into peroxynitrite, an aggressive reactive nitrogen species. The ENS provides some trophic support to interstitial cells of Cajal (ICC), which may be reduced in diabetes (5-HT, serotonin). Reduced trophic support contributes to dysfunction and dystrophy of smooth muscle cells (SMC), the ENS and ANS. SMC also produce several growth factors including insulin-like growth factor-I (IGF-I) and both membrane-bound and soluble stem cell factor (Kitl(m) and Kitl(s), respectively). Self-renewal of ICC progenitors (pre-ICC) is stimulated by Kitl(s) and IGF-I. IGF-I is also required for ICC differentiation and maintenance but those effects are indirect via Kitl(m). Arrows indicate stimulation, blocked lines, inhibition. Dotted line indicates putative effect.
Autoimmunity
Type 1 diabetes results from autoimmune destruction of pancreatic β cells and the same autoimmune process may also inflict “collateral damage” to cells of the gastrointestinal neuromuscular apparatus, particularly neurons.159 Recently there has been an upsurge of interest in the role of autoimmune processes in the pathogenesis of gastrointestinal neuromuscular disorders160,161 and it is now well established that autoantibodies play a key role in paraneoplastic dysmotilites and they are also likely to contribute to the development of several “idiopathic” gastrointestinal dysmotilites.162 However, there is only scant evidence supporting autoimmune pathogenesis of diabetic gastroenteropathies. Lymphocytic infiltration of the myenteric region has been described in the esophagus of both type 1 and type 2 diabetic patients123 and infiltrations by lymphocytes, macrophages and occasional plasma cells were reported in autonomic nerve bundles and ganglia in five patients with longstanding diabetes complicated by diarrhea and gastroparesis.131 However, gastrectomy specimens from patients with severe, intractable diabetic gastroparesis failed to reveal inflammatory or other cell infiltrates.24,142 In animal models, lymphocytic aggregates were described in the proximal third of the small intestines of spontaneously diabetic Chinese hamsters141 but no such changes were found in NOD mice42 or STZ diabetic rats.67 Inflammatory infiltrates have been described in the intestines of diabetes-prone BB rats but they turned out to be a feature of the strain and unrelated to the presence or absence of diabetes.163 Circulating, complement-fixing autoantibodies against autonomic nerves are frequently found in type 1 diabetes and more rarely in type 2 diabetes, which is regarded as a metabolic and not autoimmune disease.159 However, the relevance of these findings to gastrointestinal cellular injury remains unproven.159 Loss of ICC caused by anti-Kit autoantibodies has been found in a patient with paraneoplastic gastroparesis and intestinal pseudo-obstruction164 but a similar autoantibody has not been reported in diabetes. So far only a single study identified functional autoantibodies in type 1 diabetic patients with gastrointestinal symptoms (mainly abdominal bloating and fullness).165 In physiological assays, these IgG antibodies disrupted MMC in mice both in vitro and in vivo after passive transfer. Pharmacological studies indicated that the antibody activated L-type Ca2+ channels of intestinal smooth muscle cells at the dihydropyridine binding site. In the absence of follow-up studies, the significance of this finding remains unclear.
Hyperglycemia, oxidative and nitrosative stress
Chronic complications of diabetes are generally attributed to recurring episodes of hyperglycemia and resultant oxidative injury arising from an imbalance between pro-and anti-oxidative factors.166 Hyperglycemia similar to that seen in poorly controlled diabetic patients has been shown to inhibit neurite growth in superior cervical ganglia in vitro and cause neurite degeneration (reduced caliber, beading, retraction of growth cone) and apoptosis.167 Hyperglycemia-induced apoptosis of dorsal root ganglion neurons and Schwann cells characterized by mitochondrial swelling, disruption of the internal cristae and activation of caspase-3 has also been shown in vitro, in STZ-diabetic rats and in rats made acutely hyperglycemic with infused glucose.168 Importantly, 20 mM glucose also elicited apoptosis in primary cultures of fetal murine enteric neurons and this effect was mediated by reduced phosphorylation of PKB (Akt) and enhanced nuclear translocation of forkhead box O3a (FOXO3a) transcription factor.41
The effects of hyperglycemia on apoptosis may be mediated by multiple mechanisms including increased formation of advanced glycation end-products (AGE), glucose-induced activation of PKC and nuclear factor κB (NFκB) and increased glucose flux through the aldose reductase pathway. In endothelial cells and neurons, oxidative stress due to hyperglycemia has been attributed to the increased production of ROS by the mitochondrial electron transfer chain.168,169 However, there also is evidence that AGEs rather than hyperglycemia per se are the prerequisite of ROS formation and apoptosis. For example, as discussed above, insulin treatment of STZ-diabetic rats initiated after a certain period was unable to prevent apoptosis of nitrergic neurons despite normalizing blood glucose levels47 and this was due to the continuing accumulation of AGEs, consequent accumulation of ROS and caspase-3-dependent apoptosis.150,170 Myenteric neurons express receptors for AGEs (RAGE), which mediate the apoptotic effects of AGEs.171 Some effects of diabetes on the ENS could also be alleviated by the combined administration of the anti-oxidants alpha-lipoic acid and evening primrose oil.172
Different cells have different sensitivity to oxidative stress. For example, nitrergic neurons are particularly sensitive due to their very expression of NO, which, when reacting with superoxide, produces peroxynitrite, a highly aggressive reactive nitrogen species.170 In contrast, some populations of sympathetic neurons have reduced susceptibility to oxidative stress and this may be related to their gene expression profile.130 In organotypic cultures of intact murine tunica muscularis tissues, ICC were resistant to prolonged hyperglycemia (up to 55 mmol/L for >2 months).173 One possible explanation for this finding is that ICC may be resistant to hyperglycemia-induced apoptosis. Indeed, no apoptotic ICC were reported in diabetic NOD42 or db/db mice44 or in STZ-diabetic rats.67 In a microarray study we found small intestinal ICC to overexpress superoxide dismutase and several anti-apoptotic genes including Bcl-2, which can also protect cells by altering the intracellular redox potential and preventing oxidant-induced damage. This is likely critical for the survival of ICC, which are characterized by an abundance of mitochondria and depend on oxidative metabolism for generating electrical slow waves.93 While these observations are certainly in agreement with earlier reports that ICC, instead of undergoing apoptosis, transform into fibroblast-like cells,174 recent reports indicate that ICC are also not immune to apoptotic cell death175,176 so the resistance of normal ICC to hyperglycemia may involve other mechanisms as well. In a recent paper Choi et al. offered an alternative explanation by showing that maintenance of ICC in diabetic NOD mice was dependent on the diabetes-induced upregulation in tissue macrophages of heme oxygenase-1 (HO-1), an enzyme that generates the cytoprotective gaseous mediator carbon monoxide (CO).31 In mice that could not sustain elevated levels of HO-1, oxidative stress was increased and Kit expression declined dramatically. This finding implies that diabetes may increase the susceptibility of cells to oxidative damage by interfering with anti-oxidant defenses. The mechanisms responsible for this loss-of-protection are unclear but may be related to the endocrine abnormalities central to diabetes (Figure 3).
Trophic factors
Although loss of trophic support has received less attention than oxidative stress as a mediator of diabetes-associated enteric dysfunction, there also is strong evidence supporting a role for various growth factors as pathogenetic factors and potential therapeutic agents. Particular important in this regard are insulin, which is reduced or ineffective depending on the type of diabetes, and IGF-I, which is reduced in both type 1 and type 2 diabetes.177 For example, insulin has been shown to completely restore reduced expression and axonal transport of nNOS in diabetic rodents, at least when applied sufficiently early.40,47 Insulin also has anti-apoptotic effects, which are mediated by NFκB-dependent survival genes such as tumor necrosis factor receptor-associated factor 2 and manganese superoxide dismutase178 although these effects may be difficult to harness due to insulin’s strong hypoglycemic action. Neuroaxonal dystrophy in certain sympathetic ganglia was only detected in diabetes models with low circulating levels of insulin and IGF-I21,132,134 and in STZ-diabetic rats, two months of IGF-I treatment nearly completely reversed bilateral neuroaxonal dystrophy in the superior mesenteric ganglion and ileal mesenteric nerves without altering the severity of diabetes.135 Hyperglycemia-induced apoptosis and neurite degeneration could also be prevented by in vitro application of growth factors with anti-apoptotic potency such as IGF-I167,168 or glial-derived neurotrophic factor (GDNF).41 GDNF’s effects were mediated by increased Akt phosphorylation and reduced nuclear translocation of FOXO3a. Akt also plays a key role in IGF-I’s anti-apoptotic actions.179 Moreover, STZ-diabetes failed to induce myenteric neuronal apoptosis in the ileum of transgenic mice overexpressing GDNF in glial cells.41
We explored the role of insulin and IGF-I in the maintenance of ICC populations in organotypic cultures of intact murine gastric corpus+antrum muscles and in diabetic NOD mice.26,173,180 Maintenance of ICC depend on continuing Kit signaling50,181 supported by Kitl production by the smooth muscle cells.26,182 In explant cultures, ICC are supported by intrinsic reserves but beyond a certain time, these tissue reserves become exhausted and ICC are depleted. We found that loss of Kit expression, ICC and slow waves could be completely prevented by the application of either insulin or IGF-I.173 The pro-survival effects of insulin and IGF-I on mature ICC were probably indirect because gastric ICC do not express insulin or IGF-I receptors.26,180 The membrane-bound isoform of Kitl, a critical developmental, growth and survival factor for ICC, appeared to mediate the effects of insulin and IGF-I as evidenced by the decline of its expression in diabetic stomachs (Figure 2) and its rescue in vitro by insulin and IGF-I treatments that also prevented ICC loss.26 The reduction in Kitl mRNA in diabetic mouse stomachs mirrored the decrease in smooth muscle mass26 but our recent data indicate that loss of Kitl production does not necessarily require smooth muscle dystrophy as Kitl expression by smooth muscle cells is also regulated acutely by IGF-I signaling involving primarily the Akt-glycogen synthase kinase 3β pathway (unpublished). In intact tissues, a significant proportion of tissue IGF-I is actually produced by the smooth muscle cells,183 which explains why ICC can survive for several weeks even in the absence of exogenous support.26,50,173 Recently we further studied the significance of local IGF-I signaling in the maintenance of gastric ICC by exposing mice to chronic moderate caloric restriction (~20% below ad-libitum intake), which invariably reduces IGF-I levels.184 In these studies we detected a profound decline in gastric expression of IGF-I mRNA and a 47% decrease in antral ICC volumes, which occurred in the presence of normoglycemia and without any increase in oxidative stress. These findings strongly support the notion that reduced IGF-I in diabetes is an independent factor of ICC loss.
In most tissues, maintenance of cell mass also involves regeneration from local precursors. Recently we have identified ICC progenitors in postnatal murine gastric muscles.180 These cells considerably differ from ICC by their morphology, cell surface markers (including expression of IGF-I receptors) and factor requirements. For example, their proliferation can be sustained with soluble Kitl, which has very little, if any, effect on mature ICC and by the direct action of IGF-I (Figure 3). IGF-I is also required for their differentiation into ICC although most of the latter effects are indirect via membrane-bound Kitl since the differentiating progenitors gradually lose expression of both insulin and IGF-I receptors.180 The behavior of these cells in diabetes and other disorders affecting ICC remains to be investigated. ICC mass is also regulated by other factors including serotonin,185 interleukin-9186 and NO.187 Since NO is reduced in diabetes, it may contribute to the reduced ICC mass in this disorder (Figure 3). However, a recent study indicated that a decline in Kit protein did not necessarily accompany reduced nNOS expression in diabetic NOD mice31 and other studies did not support a role for the ENS in the development of ICC.182 Reduced extrinsic innervation in diabetes may also contribute to the depletion of ICC.102
In summary, diabetes leads to cellular injury in gastrointestinal tissues by multiple, interconnected mechanisms that involve increased oxidative and nitrosative stress, reduced expression of trophic factors and, possibly, autoimmunity. Key components of the gastrointestinal tissues regulate each other by producing growth, survival or differentiation factors. Certain intracellular compounds (e.g., NO) may increase the susceptibility of cells to injury. Cell mass in gastrointestinal muscles is also influenced by turnover from intrinsic progenitors, which may also be targets of the metabolic and endocrine abnormalities associated with diabetes.
Concluding remarks
It is becoming increasingly clear that the complex pathophysiology underlying diabetic gastroenteropathies does not simply reflect impaired tissue function but also profound changes in the cellular composition of the gastrointestinal tunica muscularis and its extrinsic nerve supply. Thus, gastroenteropathies should be considered and treated as a form of gastrointestinal neuromuscular dystrophy rather than a purely functional disorder. Diabetes leads to dystrophy and degeneration of neurons, smooth muscle cells and ICC via an imbalance between pro- and anti-oxidative factors, altered trophic stimuli to mature cells and their precursors, and, possibly, autoimmune factors (Figure 3). Cellular dystrophy and dysfunction affecting one cell type can also deprive other cells of trophic input and the sum of these changes can lead to greater functional injury than would be predicted from the individual abnormalities. The tissue-level changes reduce functional reserves and, if sufficiently severe or in response to other precipitating factors, manifest in organ-level dysfunction. These changes occur with time and, more importantly, after repeated insults. At the earliest stage, cellular injury likely manifests exclusively at the level of impaired gene expression and intracellular signaling and correcting metabolic and endocrine abnormalities, together with alleviating oxidative stress, should permit cellular repair and prevent further progression. Sustained endocrine and metabolic imbalance eventually precipitates cell dystrophy and depletion but opportunities for restoring tissue integrity from local stem/progenitor cells or by axon regeneration probably persist for extended periods of time. Supporting endogenous defense mechanisms and replacing missing trophic factors would still be expected to lead to recovery from this stage. Major cell loss and remodeling with an accumulation of extracellular matrix only occur after repeated insults. At this point, endogenous progenitor cells may also be affected and if so, tissue regeneration from intrinsic resources may not be possible. Thus, blocking cytotoxic factors and supporting cell regeneration at early stages could significantly improve the success rate of treatment modalities that target specific functions and could eliminate the need for more desperate measures. More research is needed to identify the extent and nature of cellular injury and relate the findings to the stage of diabetic gastroenteropathy to assist in earlier detection of the injury and to allow intervention at a time consistent with better outcomes.
Acknowledgements and disclosures
This review and work in the authors’ laboratory have been supported by grant R01 DK58185 from the United States Public Health Service, National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
The authors have no competing financial interests.
REFERENCES
- 1.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–84. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 2.Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47–56. [PubMed] [Google Scholar]
- 3.Parkman HP, Hasler WL, Fisher RS. American gastroenterological association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 4.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–9. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 5.Nowak TV, Johnson CP, Kalbfleisch JH, Roza AM, Wood CM, Weisbruch JP, et al. Highly variable gastric emptying in patients with insulin dependent diabetes mellitus. Gut. 1995;37:23–9. doi: 10.1136/gut.37.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enck P, Frieling T. Pathophysiology of diabetic gastroparesis. Diabetes. 1997;46(Suppl 2):S77–81. doi: 10.2337/diab.46.2.s77. [DOI] [PubMed] [Google Scholar]
- 7.Koch KL. Diabetic gastropathy: Gastric neuromuscular dysfunction in diabetes mellitus: A review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–75. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 8.Kumar A, Attaluri A, Hashmi S, Schulze KS, Rao SS. Visceral hypersensitivity and impaired accommodation in refractory diabetic gastroparesis. Neurogastroenterol Motil. 2008;20:635–42. doi: 10.1111/j.1365-2982.2008.01081.x. [DOI] [PubMed] [Google Scholar]
- 9.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, et al. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–9. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 11.Maleki D, Locke GR, 3rd, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, et al. Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–16. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 12.Schvarcz E, Palmer M, Ingberg CM, Aman J, Berne C. Increased prevalence of upper gastrointestinal symptoms in long-term type 1 diabetes mellitus. Diabet Med. 1996;13:478–81. doi: 10.1002/(SICI)1096-9136(199605)13:5<478::AID-DIA104>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Soykan I, Sivri B, Sarosiek I, Kiernan B, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 14.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–6. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 16.Vittal H, Farrugia G, Gomez G, Pasricha PJ. Mechanisms of disease: The pathological basis of gastroparesis--a review of experimental and clinical studies. Nat Clin Pract Gastroenterol Hepatol. 2007;4:336–46. doi: 10.1038/ncpgasthep0838. [DOI] [PubMed] [Google Scholar]
- 17.Guy RJ, Dawson JL, Garrett JR, Laws JW, Thomas PK, Sharma AK, et al. Diabetic gastroparesis from autonomic neuropathy: Surgical considerations and changes in vagus nerve morphology. J Neurol Neurosurg Psychiatry. 1984;47:686–91. doi: 10.1136/jnnp.47.7.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips LK, Rayner CK, Jones KL, Horowitz M. An update on autonomic neuropathy affecting the gastrointestinal tract. Curr Diab Rep. 2006;6:417–23. doi: 10.1007/s11892-006-0073-0. [DOI] [PubMed] [Google Scholar]
- 19.Ordog T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8–18. doi: 10.1111/j.1365-2982.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekharan B, Srinivasan S. Diabetes and the enteric nervous system. Neurogastroenterol Motil. 2007;19:951–60. doi: 10.1111/j.1365-2982.2007.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt RE, Dorsey DA, Beaudet LN, Frederick KE, Parvin CA, Plurad SB, et al. Non-obese diabetic mice rapidly develop dramatic sympathetic neuritic dystrophy: A new experimental model of diabetic autonomic neuropathy. Am J Pathol. 2003;163:2077–91. doi: 10.1016/S0002-9440(10)63565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spangeus A, Kand M, El-Salhy M. Gastrointestinal endocrine cells in an animal model for human type 2 diabetes. Dig Dis Sci. 1999;44:979–85. doi: 10.1023/a:1026664715280. [DOI] [PubMed] [Google Scholar]
- 23.El-Salhy M, Sitohy B. Abnormal gastrointestinal endocrine cells in patients with diabetes type 1: Relationship to gastric emptying and myoelectrical activity. Scand J Gastroenterol. 2001;36:1162–9. doi: 10.1080/00365520152584789. [DOI] [PubMed] [Google Scholar]
- 24.Ejskjaer NT, Bradley JL, Buxton-Thomas MS, Edmonds ME, Howard ER, Purewal T, et al. Novel surgical treatment and gastric pathology in diabetic gastroparesis. Diabet Med. 1999;16:488–95. doi: 10.1046/j.1464-5491.1999.00086.x. [DOI] [PubMed] [Google Scholar]
- 25.Pasricha PJ, Pehlivanov ND, Gomez G, Vittal H, Lurken MS, Farrugia G. Changes in the gastric enteric nervous system and muscle: A case report on two patients with diabetic gastroparesis. BMC Gastroenterol. 2008;8:21. doi: 10.1186/1471-230X-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horvath VJ, Vittal H, Lorincz A, Chen H, Almeida-Porada G, Redelman D, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–70. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 27.Farrugia G. Interstitial cells of Cajal in health and disease. Neurogastroenterol Motil. 2008;20(Suppl 1):54–63. doi: 10.1111/j.1365-2982.2008.01109.x. [DOI] [PubMed] [Google Scholar]
- 28.Hasler WL. Type 1 diabetes and gastroparesis: Diagnosis and treatment. Curr Gastroenterol Rep. 2007;9:261–9. doi: 10.1007/s11894-007-0029-9. [DOI] [PubMed] [Google Scholar]
- 29.Sifrim D, Mittal R, Fass R, Smout A, Castell D, Tack J, et al. Review article: Acidity and volume of the refluxate in the genesis of gastro-oesophageal reflux disease symptoms. Aliment Pharmacol Ther. 2007;25:1003–17. doi: 10.1111/j.1365-2036.2007.03281.x. [DOI] [PubMed] [Google Scholar]
- 30.Intagliata N, Koch KL. Gastroparesis in type 2 diabetes mellitus: Prevalence, etiology, diagnosis, and treatment. Curr Gastroenterol Rep. 2007;9:270–9. doi: 10.1007/s11894-007-0030-3. [DOI] [PubMed] [Google Scholar]
- 31.Choi KM, Gibbons SJ, Nguyen TV, Stoltz GJ, Lurken MS, Ordog T, et al. Heme oxygenase-1 protects interstitial cells of Cajal from oxidative stress and reverses diabetic gastroparesis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi KM, Zhu J, Stoltz GJ, Vernino S, Camilleri M, Szurszewski JH, et al. Determination of gastric emptying in nonobese diabetic mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1039–45. doi: 10.1152/ajpgi.00317.2007. [DOI] [PubMed] [Google Scholar]
- 33.Bardsley M, Lorincz A, Popko L, Izbeki F, Farrugia G, Ördög T. Gastric emptying in mouse models of eating disorders. Gastroenterology. 2008;134(4 Suppl 1):A-315. [Google Scholar]
- 34.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 35.Samsom M, Roelofs JM, Akkermans LM, van Berge Henegouwen GP, Smout AJ. Proximal gastric motor activity in response to a liquid meal in type i diabetes mellitus with autonomic neuropathy. Dig Dis Sci. 1998;43:491–6. doi: 10.1023/a:1018894520557. [DOI] [PubMed] [Google Scholar]
- 36.Undeland KA, Hausken T, Aanderud S, Berstad A. Lower postprandial gastric volume response in diabetic patients with vagal neuropathy. Neurogastroenterol Motil. 1997;9:19–24. doi: 10.1046/j.1365-2982.1997.d01-3.x. [DOI] [PubMed] [Google Scholar]
- 37.Troncon LE, Rosa-e-Silva L, Oliveira RB, Iazigi N, Gallo L, Jr., Foss MC. Abnormal intragastric distribution of a liquid nutrient meal in patients with diabetes mellitus. Dig Dis Sci. 1998;43:1421–9. doi: 10.1023/a:1018834025351. [DOI] [PubMed] [Google Scholar]
- 38.Kong MF, Horowitz M. Gastric emptying in diabetes mellitus: Relationship to blood-glucose control. Clin Geriatr Med. 1999;15:321–38. [PubMed] [Google Scholar]
- 39.Gangula PR, Maner WL, Micci MA, Garfield RE, Pasricha PJ. Diabetes induces sex-dependent changes in neuronal nitric oxide synthase dimerization and function in the rat gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2007;292:G725–33. doi: 10.1152/ajpgi.00406.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Watkins CC, Sawa A, Jaffrey S, Blackshaw S, Barrow RK, Snyder SH, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–84. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anitha M, Gondha C, Sutliff R, Parsadanian A, Mwangi S, Sitaraman SV, et al. Gdnf rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J Clin Invest. 2006;116:344–56. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ordog T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–9. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 43.Song GQ, Chen JD. Synchronized gastric electrical stimulation improves delayed gastric emptying in nonobese mice with diabetic gastroparesis. J Appl Physiol. 2007;103:1560–4. doi: 10.1152/japplphysiol.00319.2007. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto T, Watabe K, Nakahara M, Ogiyama H, Kiyohara T, Tsutsui S, et al. Disturbed gastrointestinal motility and decreased interstitial cells of Cajal in diabetic db/db mice. J Gastroenterol Hepatol. 2008;23:660–7. doi: 10.1111/j.1440-1746.2008.05326.x. [DOI] [PubMed] [Google Scholar]
- 45.Diani AR, Grogan DM, Yates ME, Risinger DL, Gerritsen GC. Radiologic abnormalities and autonomic neuropathology in the digestive tract of the ketonuric diabetic chinese hamster. Diabetologia. 1979;17:33–40. doi: 10.1007/BF01222975. [DOI] [PubMed] [Google Scholar]
- 46.Indireshkumar K, Brasseur JG, Faas H, Hebbard GS, Kunz P, Dent J, et al. Relative contributions of “Pressure pump” And “Peristaltic pump” To gastric emptying. Am J Physiol Gastrointest Liver Physiol. 2000;278:G604–16. doi: 10.1152/ajpgi.2000.278.4.G604. [DOI] [PubMed] [Google Scholar]
- 47.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–62. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 48.Camilleri M, Malagelada JR, Brown ML, Becker G, Zinsmeister AR. Relation between antral motility and gastric emptying of solids and liquids in humans. Am J Physiol. 1985;249:G580–5. doi: 10.1152/ajpgi.1985.249.5.G580. [DOI] [PubMed] [Google Scholar]
- 49.Camilleri M, Brown ML, Malagelada JR. Relationship between impaired gastric emptying and abnormal gastrointestinal motility. Gastroenterology. 1986;91:94–9. doi: 10.1016/0016-5085(86)90444-0. [DOI] [PubMed] [Google Scholar]
- 50.Ordog T, Ward SM, Sanders KM. Interstitial cells of Cajal generate electrical slow waves in the murine stomach. J Physiol. 1999;518:257–69. doi: 10.1111/j.1469-7793.1999.0257r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Telander RL, Morgan KG, Kreulen DL, Schmalz PF, Kelly KA, Szurszewski JH. Human gastric atony with tachygastria and gastric retention. Gastroenterology. 1978;75:497–501. [PubMed] [Google Scholar]
- 52.Hirst GD, Edwards FR. Electrical events underlying organized myogenic contractions of the guinea pig stomach. J Physiol. 2006;576:659–65. doi: 10.1113/jphysiol.2006.116491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huizinga JD, Lammers WJ. Gut peristalsis is governed by a multitude of cooperating mechanisms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1–8. doi: 10.1152/ajpgi.90380.2008. [DOI] [PubMed] [Google Scholar]
- 54.Chen JD, Lin Z, Pan J, McCallum RW. Abnormal gastric myoelectrical activity and delayed gastric emptying in patients with symptoms suggestive of gastroparesis. Dig Dis Sci. 1996;41:1538–45. doi: 10.1007/BF02087897. [DOI] [PubMed] [Google Scholar]
- 55.Xue L, Suzuki H. Electrical responses of gastric smooth muscles in streptozotocin-induced diabetic rats. Am J Physiol. 1997;272:G77–83. doi: 10.1152/ajpgi.1997.272.1.G77. [DOI] [PubMed] [Google Scholar]
- 56.Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of Cajal. World J Gastroenterol. 2004;10:1227–30. doi: 10.3748/wjg.v10.i8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takano H, Imaeda K, Koshita M, Xue L, Nakamura H, Kawase Y, et al. Alteration of the properties of gastric smooth muscle in the genetically hyperglycemic OLETF rat. J Auton Nerv Syst. 1998;70:180–8. doi: 10.1016/s0165-1838(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 58.Hasler WL, Soudah HC, Dulai G, Owyang C. Mediation of hyperglycemia-evoked gastric slow-wave dysrhythmias by endogenous prostaglandins. Gastroenterology. 1995;108:727–36. doi: 10.1016/0016-5085(95)90445-x. [DOI] [PubMed] [Google Scholar]
- 59.Jebbink RJ, Samsom M, Bruijs PP, Bravenboer B, Akkermans LM, Vanberge-Henegouwen GP, et al. Hyperglycemia induces abnormalities of gastric myoelectrical activity in patients with type I diabetes mellitus. Gastroenterology. 1994;107:1390–7. doi: 10.1016/0016-5085(94)90541-x. [DOI] [PubMed] [Google Scholar]
- 60.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–43. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 61.Ward SM, Ordog T, Koh SD, Baker SA, Jun JY, Amberg G, et al. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000;525(Pt 2):355–61. doi: 10.1111/j.1469-7793.2000.t01-1-00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holzapfel A, Festa A, Stacher-Janotta G, Bergmann H, Shnawa N, Brannath W, et al. Gastric emptying in type II (non-insulin-dependent) diabetes mellitus before and after therapy readjustment: No influence of actual blood glucose concentration. Diabetologia. 1999;42:1410–2. doi: 10.1007/s001250051311. [DOI] [PubMed] [Google Scholar]
- 63.El-Salhy M. Gastrointestinal transit in nonobese diabetic mouse: An animal model of human diabetes type 1. J Diabetes Complications. 2001;15:277–84. doi: 10.1016/s1056-8727(01)00158-1. [DOI] [PubMed] [Google Scholar]
- 64.Imaeda K, Takano H, Koshita M, Yamamoto Y, Joh T, Suzuki H. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1–11. doi: 10.1540/jsmr.34.1. [DOI] [PubMed] [Google Scholar]
- 65.Ordog T, Irwin N, Takayama I, Ward SM, Sanders KM. Depletion of interstitial cells of Cajal and electrical abnormalities in a murine model of diabetic colon dysfunction. Neurogastroenterol Motil. 2004;16:664. [Google Scholar]
- 66.Forrest A, Parsons M. The enhanced spontaneous activity of the diabetic colon is not the consequence of impaired inhibitory control mechanisms. Auton Autacoid Pharmacol. 2003;23:149–58. doi: 10.1046/j.1474-8673.2003.00290.x. [DOI] [PubMed] [Google Scholar]
- 67.Forrest A, Huizinga JD, Wang XY, Liu LW, Parsons M. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315–26. doi: 10.1152/ajpgi.00196.2007. [DOI] [PubMed] [Google Scholar]
- 68.Chen J, Chen H, Sanders KM, Perrino BA. Regulation of Srf/Carg-dependent gene transcription during chronic partial obstruction of murine small intestine. Neurogastroenterol Motil. 2008;20:829–42. doi: 10.1111/j.1365-2982.2008.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Abell TL, Bernstein RK, Cutts T, Farrugia G, Forster J, Hasler WL, et al. Treatment of gastroparesis: A multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–83. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 70.Huizinga JD. Gastrointestinal peristalsis: Joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239–47. doi: 10.1002/(SICI)1097-0029(19991115)47:4<239::AID-JEMT3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 71.Sanders KM. Regulation of smooth muscle excitation and contraction. Neurogastroenterol Motil. 2008;20(Suppl 1):39–53. doi: 10.1111/j.1365-2982.2008.01108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furness JB. The enteric nervous system: Normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl 1):32–8. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 73.Costa M, Brookes SJ, Hennig GW. Anatomy and physiology of the enteric nervous system. Gut. 2000;47(Suppl 4):iv15–9. doi: 10.1136/gut.47.suppl_4.iv15. discussion iv26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wood JD. Enteric nervous system: Reflexes, pattern generators and motility. Curr Opin Gastroenterol. 2008;24:149–58. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]
- 75.Ordog T. Do we need to revise the role of interstitial cells of Cajal in gastrointestinal motility? Am J Physiol Gastrointest Liver Physiol. 2008;294:G368–71. doi: 10.1152/ajpgi.00530.2007. [DOI] [PubMed] [Google Scholar]
- 76.Sergeant GP, Large RJ, Beckett EA, McGeough CM, Ward SM, Horowitz B. Microarray comparison of normal and W/Wv mice in the gastric fundus indicates a supersensitive phenotype. Physiol Genomics. 2002;11:1–9. doi: 10.1152/physiolgenomics.00052.2002. [DOI] [PubMed] [Google Scholar]
- 77.Kraichely RE, Farrugia G. Mechanosensitive ion channels in interstitial cells of Cajal and smooth muscle of the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:245–52. doi: 10.1111/j.1365-2982.2006.00880.x. [DOI] [PubMed] [Google Scholar]
- 78.Rich A, Leddon SA, Hess SL, Gibbons SJ, Miller S, Xu X, et al. Kit-like immunoreactivity in the zebrafish gastrointestinal tract reveals putative ICC. Dev Dyn. 2007;236:903–11. doi: 10.1002/dvdy.21086. [DOI] [PubMed] [Google Scholar]
- 79.Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–8. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maeda H, Yamagata A, Nishikawa S, Yoshinaga K, Kobayashi S, Nishi K. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–75. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- 81.Sanders KM, Ordog T, Koh SD, Torihashi S, Ward SM. Development and plasticity of interstitial cells of Cajal. Neurogastroenterol Motil. 1999;11:311–38. doi: 10.1046/j.1365-2982.1999.00164.x. [DOI] [PubMed] [Google Scholar]
- 82.Streutker CJ, Huizinga JD, Driman DK, Riddell RH. Interstitial cells of Cajal in health and disease. Part I: Normal ICC structure and function with associated motility disorders. Histopathology. 2007;50:176–89. doi: 10.1111/j.1365-2559.2006.02493.x. [DOI] [PubMed] [Google Scholar]
- 83.Sivarao DV, Mashimo HL, Thatte HS, Goyal RK. Lower esophageal sphincter is achalasic in nNOS(-/-) and hypotensive in W/W(v) mutant mice. Gastroenterology. 2001;121:34–42. doi: 10.1053/gast.2001.25541. [DOI] [PubMed] [Google Scholar]
- 84.Sivarao DV, Mashimo H, Goyal RK. Pyloric sphincter dysfunction in nNOS-/- and W/Wv mutant mice: Animal models of gastroparesis and duodenogastric reflux. Gastroenterology. 2008;135:1258–66. doi: 10.1053/j.gastro.2008.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ward SM, Sanders KM. Involvement of intramuscular interstitial cells of Cajal in neuroeffector transmission in the gastrointestinal tract. J Physiol. 2006;576:675–82. doi: 10.1113/jphysiol.2006.117390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beckett EA, McGeough CA, Sanders KM, Ward SM. Pacing of interstitial cells of Cajal in the murine gastric antrum: Neurally mediated and direct stimulation. J Physiol. 2003;553:545–59. doi: 10.1113/jphysiol.2003.050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forrest AS, Ordog T, Sanders KM. Neural regulation of slow-wave frequency in the murine gastric antrum. Am J Physiol Gastrointest Liver Physiol. 2006;290:G486–95. doi: 10.1152/ajpgi.00349.2005. [DOI] [PubMed] [Google Scholar]
- 88.Fox EA, Phillips RJ, Byerly MS, Baronowsky EA, Chi MM, Powley TL. Selective loss of vagal intramuscular mechanoreceptors in mice mutant for steel factor, the c-kit receptor ligand. Anat Embryol (Berl) 2002;205:325–42. doi: 10.1007/s00429-002-0261-x. [DOI] [PubMed] [Google Scholar]
- 89.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci U S A. 2005;102:14913–8. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sanders KM, Ward SM. Kit mutants and gastrointestinal physiology. J Physiol. 2007;578:33–42. doi: 10.1113/jphysiol.2006.122473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alberti E, Mikkelsen HB, Wang XY, Diaz M, Larsen JO, Huizinga JD, et al. Pacemaker activity and inhibitory neurotransmission in the colon of Ws/Ws mutant rats. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1499–510. doi: 10.1152/ajpgi.00136.2006. [DOI] [PubMed] [Google Scholar]
- 92.Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006;573:147–59. doi: 10.1113/jphysiol.2006.105189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen H, Ordog T, Chen J, Young DL, Bardsley MR, Redelman D, et al. Differential gene expression in functional classes of interstitial cells of Cajal in murine small intestine. Physiol Genomics. 2007;31:492–509. doi: 10.1152/physiolgenomics.00113.2007. [DOI] [PubMed] [Google Scholar]
- 94.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience. 2008;152:437–48. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 95.Iino S, Horiguchi K, Nojyo Y, Ward SM, Sanders KM. Interstitial cells of Cajal contain signalling molecules for transduction of nitrergic stimulation in guinea pig caecum. Neurogastroenterol Motil. 2009 doi: 10.1111/j.1365-2982.2008.01236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen H, Redelman D, Ro S, Ward SM, Ordog T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007;292:C497–507. doi: 10.1152/ajpcell.00147.2006. [DOI] [PubMed] [Google Scholar]
- 97.Wang XY, Ward SM, Gerthoffer WT, Sanders KM. PKC-epsilon translocation in enteric neurons and interstitial cells of Cajal in response to muscarinic stimulation. Am J Physiol Gastrointest Liver Physiol. 2003;285:G593–601. doi: 10.1152/ajpgi.00421.2002. [DOI] [PubMed] [Google Scholar]
- 98.de Lorijn F, de Jonge WJ, Wedel T, Vanderwinden JM, Benninga MA, Boeckxstaens GE. Interstitial cells of Cajal are involved in the afferent limb of the rectoanal inhibitory reflex. Gut. 2005;54:1107–13. doi: 10.1136/gut.2004.051045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dixit D, Zarate N, Liu LW, Boreham DR, Huizinga JD. Interstitial cells of Cajal and adaptive relaxation in the mouse stomach. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1129–36. doi: 10.1152/ajpgi.00518.2005. [DOI] [PubMed] [Google Scholar]
- 100.Huizinga JD, Liu LW, Fitzpatrick A, White E, Gill S, Wang XY, et al. Deficiency of intramuscular ICC increases fundic muscle excitability but does not impede nitrergic innervation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G589–94. doi: 10.1152/ajpgi.00130.2007. [DOI] [PubMed] [Google Scholar]
- 101.Fox EA, Phillips RJ, Martinson FA, Baronowsky EA, Powley TL. C-kit mutant mice have a selective loss of vagal intramuscular mechanoreceptors in the forestomach. Anat Embryol (Berl) 2001;204:11–26. doi: 10.1007/s004290100184. [DOI] [PubMed] [Google Scholar]
- 102.Huizinga JD, Reed DE, Berezin I, Wang XY, Valdez DT, Liu LW, et al. Survival dependency of intramuscular ICC on vagal afferent nerves in the cat esophagus. Am J Physiol Regul Integr Comp Physiol. 2008;294:R302–10. doi: 10.1152/ajpregu.00398.2007. [DOI] [PubMed] [Google Scholar]
- 103.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, et al. The mechanism and spread of pacemaker activity through myenteric interstitial cells of Cajal in human small intestine. Gastroenterology. 2007;132:1852–65. doi: 10.1053/j.gastro.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 104.Wang B, Kunze WA, Zhu Y, Huizinga JD. In situ recording from gut pacemaker cells. Pflugers Arch. 2008;457:243–51. doi: 10.1007/s00424-008-0513-6. [DOI] [PubMed] [Google Scholar]
- 105.Takeda Y, Koh SD, Sanders KM, Ward SM. Differential expression of ionic conductances in interstitial cells of Cajal in the murine gastric antrum. J Physiol. 2008;586:859–73. doi: 10.1113/jphysiol.2007.140293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sanders KM, Ordog T, Koh SD, Ward SM. A novel pacemaker mechanism drives gastrointestinal rhythmicity. News Physiol Sci. 2000;15:291–8. doi: 10.1152/physiologyonline.2000.15.6.291. [DOI] [PubMed] [Google Scholar]
- 107.Bayguinov O, Ward SM, Kenyon JL, Sanders KM. Voltage-gated Ca2+ currents are necessary for slow-wave propagation in the canine gastric antrum. Am J Physiol Cell Physiol. 2007;293:C1645–59. doi: 10.1152/ajpcell.00165.2007. [DOI] [PubMed] [Google Scholar]
- 108.Park KJ, Hennig GW, Lee HT, Spencer NJ, Ward SM, Smith TK, et al. Spatial and temporal mapping of pacemaker activity in interstitial cells of Cajal in mouse ileum in situ. Am J Physiol Cell Physiol. 2006;290:C1411–27. doi: 10.1152/ajpcell.00447.2005. [DOI] [PubMed] [Google Scholar]
- 109.Kelly KA, Code CF. Canine gastric pacemaker. Am J Physiol. 1971;220:112–8. doi: 10.1152/ajplegacy.1971.220.1.112. [DOI] [PubMed] [Google Scholar]
- 110.Ordog T, Baldo M, Danko R, Sanders KM. Plasticity of electrical pacemaking by interstitial cells of Cajal and gastric dysrhythmias in W/Wv mutant mice. Gastroenterology. 2002;123:2028–40. doi: 10.1053/gast.2002.37056. [DOI] [PubMed] [Google Scholar]
- 111.Lee HT, Hennig GW, Fleming NW, Keef KD, Spencer NJ, Ward SM, et al. Septal interstitial cells of Cajal conduct pacemaker activity to excite muscle bundles in human jejunum. Gastroenterology. 2007;133:907–17. doi: 10.1053/j.gastro.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szurszewski JH. A migrating electric complex of canine small intestine. Am J Physiol. 1969;217:1757–63. doi: 10.1152/ajplegacy.1969.217.6.1757. [DOI] [PubMed] [Google Scholar]
- 113.Lammers WJ, Stephen B, Slack JR. Similarities and differences in the propagation of slow waves and peristaltic waves. Am J Physiol Gastrointest Liver Physiol. 2002;283:G778–86. doi: 10.1152/ajpgi.00390.2001. [DOI] [PubMed] [Google Scholar]
- 114.Li M, Johnson CP, Adams MB, Sarna SK. Cholinergic and nitrergic regulation of in vivo giant migrating contractions in rat colon. Am J Physiol Gastrointest Liver Physiol. 2002;283:G544–52. doi: 10.1152/ajpgi.00114.2001. [DOI] [PubMed] [Google Scholar]
- 115.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 116.Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994;480(Pt 1):91–7. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ward SM, Burns AJ, Torihashi S, Harney SC, Sanders KM. Impaired development of interstitial cells and intestinal electrical rhythmicity in steel mutants. Am J Physiol. 1995;269:C1577–85. doi: 10.1152/ajpcell.1995.269.6.C1577. [DOI] [PubMed] [Google Scholar]
- 118.Der-Silaphet T, Malysz J, Hagel S, Arsenault A Larry, Huizinga JD. Interstitial cells of Cajal direct normal propulsive contractile activity in the mouse small intestine. Gastroenterology. 1998;114:724–36. doi: 10.1016/s0016-5085(98)70586-4. [DOI] [PubMed] [Google Scholar]
- 119.Hou X, Yin J, Liu J, Pasricha PJ, Chen JD. In vivo gastric and intestinal slow waves in W/Wv mice. Dig Dis Sci. 2005;50:1335–41. doi: 10.1007/s10620-005-2783-6. [DOI] [PubMed] [Google Scholar]
- 120.Spencer NJ, Sanders KM, Smith TK. Migrating motor complexes do not require electrical slow waves in the mouse small intestine. J Physiol. 2003;553:881–93. doi: 10.1113/jphysiol.2003.049700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: Lack of association with neuropathy. Am J Gastroenterol. 1989;84:868–72. [PubMed] [Google Scholar]
- 123.Smith B. Neuropathology of the oesophagus in diabetes mellitus. J Neurol Neurosurg Psychiatry. 1974;37:1151–4. doi: 10.1136/jnnp.37.10.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hensley GT, Soergel KH. Neuropathologic findings in diabetic diarrhea. Arch Pathol. 1968;85:587–97. [PubMed] [Google Scholar]
- 125.Yoshida MM, Schuffler MD, Sumi SM. There are no morphologic abnormalities of the gastric wall or abdominal vagus in patients with diabetic gastroparesis. Gastroenterology. 1988;94:907–14. doi: 10.1016/0016-5085(88)90546-x. [DOI] [PubMed] [Google Scholar]
- 126.Yagihashi S, Sima AA. Diabetic autonomic neuropathy in bb rat. Ultrastructural and morphometric changes in parasympathetic nerves. Diabetes. 1986;35:733–43. doi: 10.2337/diab.35.7.733. [DOI] [PubMed] [Google Scholar]
- 127.Guo C, Quobatari A, Shangguan Y, Hong S, Wiley JW. Diabetic autonomic neuropathy: Evidence for apoptosis in situ in the rat. Neurogastroenterol Motil. 2004;16:335–45. doi: 10.1111/j.1365-2982.2004.00524.x. [DOI] [PubMed] [Google Scholar]
- 128.Tay SS, Wong WC. Short- and long-term effects of streptozotocin-induced diabetes on the dorsal motor nucleus of the vagus nerve in the rat. Acta Anat (Basel) 1994;150:274–81. doi: 10.1159/000147630. [DOI] [PubMed] [Google Scholar]
- 129.Pabbidi RM, Cao DS, Parihar A, Pauza ME, Premkumar LS. Direct role of streptozotocin in inducing thermal hyperalgesia by enhanced expression of transient receptor potential vanilloid 1 in sensory neurons. Mol Pharmacol. 2008;73:995–1004. doi: 10.1124/mol.107.041707. [DOI] [PubMed] [Google Scholar]
- 130.Carroll SL, Byer SJ, Dorsey DA, Watson MA, Schmidt RE. Ganglion-specific patterns of diabetes-modulated gene expression are established in prevertebral and paravertebral sympathetic ganglia prior to the development of neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:1144–54. doi: 10.1093/jnen/63.11.1144. [DOI] [PubMed] [Google Scholar]
- 131.Duchen LW, Anjorin A, Watkins PJ, Mackay JD. Pathology of autonomic neuropathy in diabetes mellitus. Ann Intern Med. 1980;92:301–3. doi: 10.7326/0003-4819-92-2-301. [DOI] [PubMed] [Google Scholar]
- 132.Schmidt RE, Dorsey DA, Beaudet LN, Parvin CA, Zhang W, Sima AA. Experimental rat models of types 1 and 2 diabetes differ in sympathetic neuroaxonal dystrophy. J Neuropathol Exp Neurol. 2004;63:450–60. doi: 10.1093/jnen/63.5.450. [DOI] [PubMed] [Google Scholar]
- 133.Schmidt RE. Neuronal preservation in the sympathetic ganglia of rats with chronic streptozotocin-induced diabetes. Brain Res. 2001;921:256–9. doi: 10.1016/s0006-8993(01)03155-9. [DOI] [PubMed] [Google Scholar]
- 134.Schmidt RE, Dorsey DA, Beaudet LN, Peterson RG. Analysis of the zucker diabetic fatty (ZDF) type 2 diabetic rat model suggests a neurotrophic role for insulin/IGF-I in diabetic autonomic neuropathy. Am J Pathol. 2003;163:21–8. doi: 10.1016/S0002-9440(10)63626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schmidt RE, Dorsey DA, Beaudet LN, Plurad SB, Parvin CA, Miller MS. Insulin-like growth factor i reverses experimental diabetic autonomic neuropathy. Am J Pathol. 1999;155:1651–60. doi: 10.1016/S0002-9440(10)65480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–34. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 137.Lincoln J, Bokor JT, Crowe R, Griffith SG, Haven AJ, Burnstock G. Myenteric plexus in streptozotocin-treated rats. Neurochemical and histochemical evidence for diabetic neuropathy in the gut. Gastroenterology. 1984;86:654–61. [PubMed] [Google Scholar]
- 138.Belai A, Lincoln J, Milner P, Burnstock G. Progressive changes in adrenergic, serotonergic, and peptidergic nerves in proximal colon of streptozotocin-diabetic rats. Gastroenterology. 1988;95:1234–41. doi: 10.1016/0016-5085(88)90356-3. [DOI] [PubMed] [Google Scholar]
- 139.Belai A, Lincoln J, Milner P, Burnstock G. Differential effect of streptozotocin-induced diabetes on the innervation of the ileum and distal colon. Gastroenterology. 1991;100:1024–32. doi: 10.1016/0016-5085(91)90278-s. [DOI] [PubMed] [Google Scholar]
- 140.Iwasaki H, Kajimura M, Osawa S, Kanaoka S, Furuta T, Ikuma M, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–87. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 141.Diani AR, Gerritsen GC, Stromsta S, Murray P. A study of the morphological changes in the small intestine of the spontaneously diabetic chinese hamster. Diabetologia. 1976;12:101–9. doi: 10.1007/BF00428973. [DOI] [PubMed] [Google Scholar]
- 142.Moscoso GJ, Driver M, Guy RJ. A form of necrobiosis and atrophy of smooth muscle in diabetic gastric autonomic neuropathy. Pathol Res Pract. 1986;181:188–94. doi: 10.1016/S0344-0338(86)80009-7. [DOI] [PubMed] [Google Scholar]
- 143.Miller SM, Narasimhan RA, Schmalz PF, Soffer EE, Walsh RM, Krishnamurthi V, et al. Distribution of interstitial cells of Cajal and nitrergic neurons in normal and diabetic human appendix. Neurogastroenterol Motil. 2008;20:349–57. doi: 10.1111/j.1365-2982.2007.01040.x. [DOI] [PubMed] [Google Scholar]
- 144.Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, et al. Deficiency of kit-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–70. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 145.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–44. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 146.Spangeus A, el-Salhy M. Myenteric plexus in the gastrointestinal tract of non-obese diabetic mice. Histol Histopathol. 1998;13:989–94. doi: 10.14670/HH-13.989. [DOI] [PubMed] [Google Scholar]
- 147.Adeghate E, al-Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K, et al. Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci. 2003;60:1172–9. doi: 10.1007/s00018-003-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zandecki M, Berghe P Vanden, Depoortere I, Geboes K, Peeters T, Janssens J, et al. Characterization of myenteric neuropathy in the jejunum of spontaneously diabetic BB-rats. Neurogastroenterol Motil. 2008;20:818–28. doi: 10.1111/j.1365-2982.2008.01091.x. [DOI] [PubMed] [Google Scholar]
- 149.Wrzos HF, Cruz A, Polavarapu R, Shearer D, Ouyang A. Nitric oxide synthase (NOS) expression in the myenteric plexus of streptozotocin-diabetic rats. Dig Dis Sci. 1997;42:2106–10. doi: 10.1023/a:1018830820537. [DOI] [PubMed] [Google Scholar]
- 150.Jeyabal PV, Kumar R, Gangula PR, Micci MA, Pasricha PJ. Inhibitors of advanced glycation end-products prevent loss of enteric neuronal nitric oxide synthase in diabetic rats. Neurogastroenterol Motil. 2008;20:253–61. doi: 10.1111/j.1365-2982.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 151.Dishy V, Pour M Cohen, Feldman L, Naftali T, Baumer M, Efrati S, et al. The effect of sildenafil on gastric emptying in patients with end-stage renal failure and symptoms of gastroparesis. Clin Pharmacol Ther. 2004;76:281–6. doi: 10.1016/j.clpt.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 152.Takahashi T, Kojima Y, Tsunoda Y, Beyer LA, Kamijo M, Sima AA, et al. Impaired intracellular signal transduction in gastric smooth muscle of diabetic BB/W rats. Am J Physiol. 1996;270:G411–7. doi: 10.1152/ajpgi.1996.270.3.G411. [DOI] [PubMed] [Google Scholar]
- 153.Soulie ML, Cros G, Serrano JJ, Bali JP. Impairment of contractile response to carbachol and muscarinic receptor coupling in gastric antral smooth muscle cells isolated from diabetic streptozotocin-treated rats and db/db mice. Mol Cell Biochem. 1992;109:185–8. doi: 10.1007/BF00229775. [DOI] [PubMed] [Google Scholar]
- 154.Nowak TV, Harrington B, Weisbruch JP, Kalbfleisch JH. Structural and functional characteristics of muscle from diabetic rodent small intestine. Am J Physiol. 1990;258:G690–8. doi: 10.1152/ajpgi.1990.258.5.G690. [DOI] [PubMed] [Google Scholar]
- 155.Wang XY, Huizinga JD, Diamond J, Liu JWC. Loss of intramuscular interstitial cells of Cajal and enteric nerves in streptozotocin-induced diabetic rat stomach. Neurogastroenterol Motil. 2006;18:758. [Google Scholar]
- 156.Forster J, Damjanov I, Lin Z, Sarosiek I, Wetzel P, McCallum RW. Absence of the interstitial cells of Cajal in patients with gastroparesis and correlation with clinical findings. J Gastrointest Surg. 2005;9:102–8. doi: 10.1016/j.gassur.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 157.McCallum RW, Lin Z, Damjanov I, Sarosiek I, Forster J. Interstitial cells of Cajal in the stomach of patients with gastroparesis. Neurogastroenterol Motil. 2006;18:758. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 158.Lin Z, Forster JZ, Sarosiek I, Damjanov I, McCallum RW. Association of the status of interstitial cells of Cajal and electrogastrogram parameters, gastric emptying and symptoms in patients with gastroparesis. Neurogastroenterol Motil. 2008;20(Suppl 2):2. doi: 10.1111/j.1365-2982.2009.01365.x. [DOI] [PubMed] [Google Scholar]
- 159.Vinik AI, Anandacoomaraswamy D, Ullal J. Antibodies to neuronal structures: Innocent bystanders or neurotoxins? Diabetes Care. 2005;28:2067–72. doi: 10.2337/diacare.28.8.2067. [DOI] [PubMed] [Google Scholar]
- 160.Di Nardo G, Blandizzi C, Volta U, Colucci R, Stanghellini V, Barbara G, et al. Review article: Molecular, pathological and therapeutic features of human enteric neuropathies. Aliment Pharmacol Ther. 2008;28:25–42. doi: 10.1111/j.1365-2036.2008.03707.x. [DOI] [PubMed] [Google Scholar]
- 161.Hubball A, Martin JE, Lang B, De Giorgio R, Knowles CH. The role of humoral autoimmunity in gastrointestinal neuromuscular diseases. Prog Neurobiol. 2009;87:10–20. doi: 10.1016/j.pneurobio.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 162.Kashyap P, Farrugia G. Enteric autoantibodies and gut motility disorders. Gastroenterol Clin North Am. 2008;37:397–410. vi–vii. doi: 10.1016/j.gtc.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kindt SE, Boesmans W, Berghe P Vanden, Tack J. Neuropeptide imbalance in the myenteric plexus of diabetes prone biobreeding rats is correlated with inflammation, not overt diabetes. Neurogastroenterol Motil. 2008;20(Suppl 1):67–8. [Google Scholar]
- 164.Pardi DS, Miller SM, Miller DL, Burgart LJ, Szurszewski JH, Lennon VA, et al. Paraneoplastic dysmotility: Loss of interstitial cells of Cajal. Am J Gastroenterol. 2002;97:1828–33. doi: 10.1111/j.1572-0241.2002.05851.x. [DOI] [PubMed] [Google Scholar]
- 165.Jackson MW, Gordon TP, Waterman SA. Disruption of intestinal motility by a calcium channel-stimulating autoantibody in type 1 diabetes. Gastroenterology. 2004;126:819–28. doi: 10.1053/j.gastro.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 166.Bhor VM, Raghuram N, Sivakami S. Oxidative damage and altered antioxidant enzyme activities in the small intestine of streptozotocin-induced diabetic rats. Int J Biochem Cell Biol. 2004;36:89–97. doi: 10.1016/s1357-2725(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 167.Russell JW, Feldman EL. Insulin-like growth factor-I prevents apoptosis in sympathetic neurons exposed to high glucose. Horm Metab Res. 1999;31:90–6. doi: 10.1055/s-2007-978704. [DOI] [PubMed] [Google Scholar]
- 168.Russell JW, Sullivan KA, Windebank AJ, Herrmann DN, Feldman EL. Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol Dis. 1999;6:347–63. doi: 10.1006/nbdi.1999.0254. [DOI] [PubMed] [Google Scholar]
- 169.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 170.Cellek S, Qu W, Schmidt AM, Moncada S. Synergistic action of advanced glycation end products and endogenous nitric oxide leads to neuronal apoptosis in vitro: A new insight into selective nitrergic neuropathy in diabetes. Diabetologia. 2004;47:331–9. doi: 10.1007/s00125-003-1298-y. [DOI] [PubMed] [Google Scholar]
- 171.Korenaga K, Micci MA, Taglialatela G, Pasricha PJ. Suppression of nnos expression in rat enteric neurones by the receptor for advanced glycation end-products. Neurogastroenterol Motil. 2006;18:392–400. doi: 10.1111/j.1365-2982.2006.00774.x. [DOI] [PubMed] [Google Scholar]
- 172.Shotton HR, Broadbent S, Lincoln J. Prevention and partial reversal of diabetes-induced changes in enteric nerves of the rat ileum by combined treatment with alpha-lipoic acid and evening primrose oil. Auton Neurosci. 2004;111:57–65. doi: 10.1016/j.autneu.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 173.Horvath VJ, Vittal H, Ordog T. Reduced insulin and igf-i signaling, not hyperglycemia, underlies the diabetes-associated depletion of interstitial cells of Cajal in the murine stomach. Diabetes. 2005;54:1528–33. doi: 10.2337/diabetes.54.5.1528. [DOI] [PubMed] [Google Scholar]
- 174.Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward S, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–8. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- 175.Gibbons SJ, De Giorgio R, Pellegrini MS, Garrity-Park MM, Miller SM, Schmalz PF, et al. Apoptotic cell death of human interstitial cells of Cajal. Neurogastroenterol Motil. 2009;21:85–93. doi: 10.1111/j.1365-2982.2008.01185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mei F, Guo S, He YT, Zhu J, Zhou DS, Niu JQ, et al. Apoptosis of interstitial cells of Cajal, smooth muscle cells, and enteric neurons induced by intestinal ischemia and reperfusion injury in adult guinea pigs. Virchows Arch. 2009 doi: 10.1007/s00428-009-0739-5. [DOI] [PubMed] [Google Scholar]
- 177.Sima AA, Li ZG, Zhang W. The insulin-like growth factor system and neurological complications in diabetes. Exp Diabesity Res. 2003;4:235–56. doi: 10.1155/EDR.2003.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bertrand F, Desbois-Mouthon C, Cadoret A, Prunier C, Robin H, Capeau J, et al. Insulin antiapoptotic signaling involves insulin activation of the nuclear factor kappaB-dependent survival genes encoding tumor necrosis factor receptor-associated factor 2 and manganese-superoxide dismutase. J Biol Chem. 1999;274:30596–602. doi: 10.1074/jbc.274.43.30596. [DOI] [PubMed] [Google Scholar]
- 179.Kuemmerle JF. Endogenous IGF-I protects human intestinal smooth muscle cells from apoptosis by regulation of GSK-3 beta activity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G101–10. doi: 10.1152/ajpgi.00032.2004. [DOI] [PubMed] [Google Scholar]
- 180.Lorincz A, Redelman D, Horvath VJ, Bardsley MR, Chen H, Ordog T. Progenitors of interstitial cells of Cajal in the postnatal murine stomach. Gastroenterology. 2008;134:1083–93. doi: 10.1053/j.gastro.2008.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Beckett EA, Ro S, Bayguinov Y, Sanders KM, Ward SM. Kit signaling is essential for development and maintenance of interstitial cells of Cajal and electrical rhythmicity in the embryonic gastrointestinal tract. Dev Dyn. 2007;236:60–72. doi: 10.1002/dvdy.20929. [DOI] [PubMed] [Google Scholar]
- 182.Ward SM, Ordog T, Bayguinov JR, Horowitz B, Epperson A, Shen L, et al. Development of interstitial cells of Cajal and pacemaking in mice lacking enteric nerves. Gastroenterology. 1999;117:584–94. doi: 10.1016/s0016-5085(99)70451-8. [DOI] [PubMed] [Google Scholar]
- 183.Williams KL, Fuller CR, Fagin J, Lund PK. Mesenchymal IGF-I overexpression: Paracrine effects in the intestine, distinct from endocrine actions. Am J Physiol Gastrointest Liver Physiol. 2002;283:G875–85. doi: 10.1152/ajpgi.00089.2002. [DOI] [PubMed] [Google Scholar]
- 184.Lorincz A, Bardsley M, Izbeki F, Popko L, Gibbons S, Shen R, et al. Gastric dysmotility in eating disorders is associated with depletion of ICC and enteric neurons. Neurogastroenterol Motil. 2008;20(Suppl 1):80. [Google Scholar]
- 185.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, et al. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2b receptors. Gastroenterology. 2007;133:897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 186.Ye J, Zhu Y, Khan WI, Van Snick J, Huizinga JD. IL-9 enhances growth of ICC, maintains network structure and strengthens rhythmicity of contraction in culture. J Cell Mol Med. 2006;10:687–94. doi: 10.1111/j.1582-4934.2006.tb00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Choi KM, Gibbons SJ, Roeder JL, Lurken MS, Zhu J, Wouters MM, et al. Regulation of interstitial cells of Cajal in the mouse gastric body by neuronal nitric oxide. Neurogastroenterol Motil. 2007;19:585–95. doi: 10.1111/j.1365-2982.2007.00936.x. [DOI] [PubMed] [Google Scholar]