Abstract
The performance of type-I high-speed counter-current chromatography was evaluated by changing the column inclination against the rotating centrifugal force field. The separations were performed with two different solvent systems composed of 1-butanol-acetic acid-water (4.75:0.25:5, v/v) (BAW) and hexane-ethyl acetate-methanol-0.1 M HCl (1:1:1:1, v/v) (HEMW) using dipeptides and DNP-amino acid as test samples, respectively. A set of short coiled columns connected in series is mounted around the holder hub in two different ways: in the parallel orientation, all column units are arranged in parallel to each other and mounted on the holder at various angles against the horizontal plane. In the zigzag configuration, the neighboring units of the same column are mounted symmetrically forming various angles apart. In the parallel configuration, for both the BAW and HEMW systems, the retention of stationary phase first increased as the column angle decreased from 90° to 60° and then decreased, as the column angle further decreased from 60° to 0°, while Rs (peak resolution) continually declined over the entire column angle range from 90° to 0°. But, for both solvent systems, with the zigzag configuration, retention of stationary phase and resolution both decreased as the column angle decreased from 90° to 0°. In general, Sf and Rs for separation of dipeptides in the BAW system, from 90° to 15°, is better for the parallel orientation than for the zigzag configuration. However, at 0°, Sf and Rs are better for the zigzag orientation. In the DNP-amino acid separation with the HEMW system, retention of the stationary phase and Rs for the parallel orientation is better than that for the zigzag orientation from 90° to 30°, whereas from 30° to 0° the results are opposite. Over all results of our studies revealed that the formally used column orientation [5] at 90 degree inclination yields the highest peak resolution in both solvent systems.
Keywords: Type-I counter-current chromatography, coil planet centrifuge, the angle against the rotating centrifugal force field, retention of the stationary phase, resolution, dipeptide, DNP-amino acid
1. Introduction
Among various types of coil planet centrifuges for counter-current chromatography (CCC) for the separation and purification of natural products [1-4], the type-I coil planet centrifuge was developed in 1971[5]. Although it has produced high separation efficiency, the study was limited on the original bulky centrifuge design [5, 6].
This original type-I coil planet centrifuge was built by modifying a large floor-model centrifuge with the revolution radius of 37 cm and column height of ca 50 cm that limits the maximum rotational speed at 700 rpm [5, 6]. In order to further study and learn about its performance, recently we have developed a compact type-I coil planet centrifuge with the revolution radius of 10 cm and the column holder height at 5 cm [7]. In this new design the rotational speed can be increased to 1200 rpm which enormously widened application ranges of type-I counter-current chromatography.
In the previous studies on the original type-I coil planet centrifuge, the results showed that the coiled column mounted at 60° angle of inclination produced a higher level of stationary phase retention than that of the vertical (90°) coiled column for a polar butanol solvent system while the peak resolution in this column orientation was not reported[6]. Our previous studies on the separation of dipeptides and DNP-amino acids using the compact type-I coil planet centrifuge showed that both separation efficiency and separation time have been remarkably improved over those obtained from the original apparatus. More recently, retention of the stationary phase and resolution with 90° and 45° angle coiled columns were investigated in the compact type-I, indicating that the retention of stationary phase with a 45° angle coiled columns was better than that with a 90° angle coiled column, but the peak resolution declined [7].
In order to clarify the rules of the performance with the various column inclinations of type-I CCC, the effect of column inclination against the horizontal plane was investigated in the present study using a set of short coiled columns connected in series. Using two typical solvent systems with suitable sets of dipeptide and DNP-amino acid samples, the performance, in terms of Rs and Sf, of type-I CCC was investigated using various column inclinations of two different column configurations (parallel and zigzag, Fig. 1) in terms of peak resolution and retention of the stationary phase.
Figure 1.
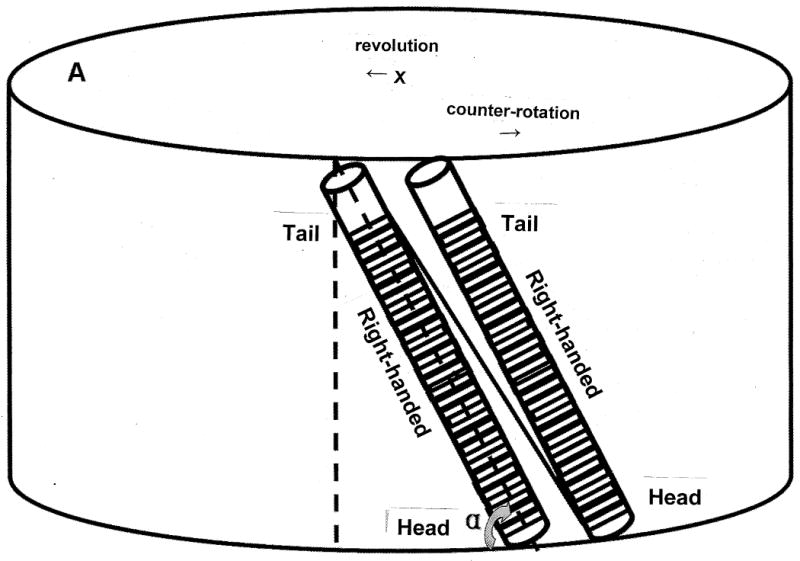
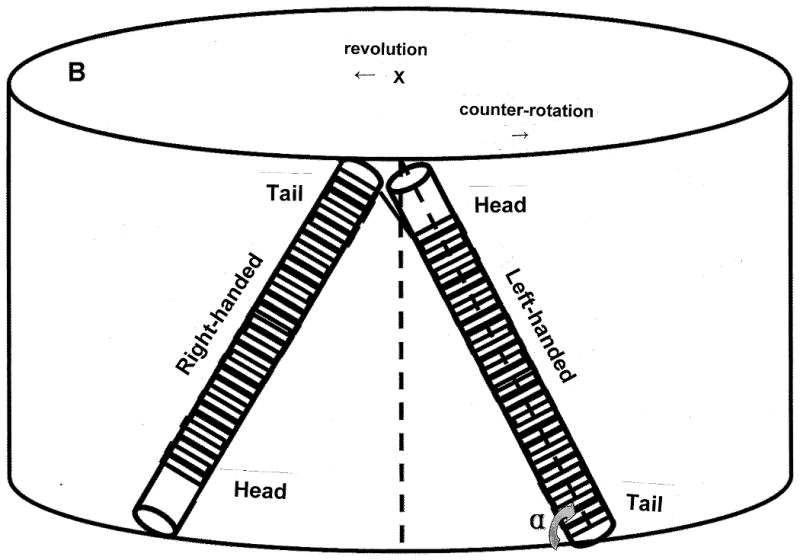
The orientation of the coiled column mounted on the holder. A) parallel configuration; B) angular configuration.
2. Experimental
2.1. Apparatus
The apparatus used in the present study is a type-I coil planet centrifuge fabricated at the NIH Machine Shop, Bethesda, MD, USA. It holds a separation column on one side and a counterweight on the other side of the rotor each 10 cm from the central axis of the apparatus. The column holder undergoes type-I synchronous planetary motion, i.e., the holder counter-rotates about its own axis once during each revolution around the central axis of the centrifuge. This unique planetary motion is produced by coupling a pair of identical toothed pulleys, one fixed on the bottom of the central axis of the centrifuge (stationary pulley) and the other around the lower end of the column holder shaft (planetary pulley) with a toothed belt. This planetary motion forms a centrifugal force field uniformly circulating around every point on the column holder. The separation column was made by winding a single piece of 0.85 mm I.D. tubing (PTFE SW No. 20, Zeus Industrial Products, Orangeburg, SC, USA) onto 47 pieces of 5 mm O.D. and 5 cm long nylon pipe in such a way that all coil units are in the same handedness (Fig. 1A) or every other coil unit has opposite handedness (Fig. 1B) so that the head of one coil unit is connected to the tail of the next unit. The length of the tubing is approximately 20 m with a total capacity of 12 ml [7]. The coiled column was mounted around the holder hub at various angles (against the horizontal plane) with all coil units arranged in parallel or zigzag configuration as illustrated in Fig. 1A and B. respectively. The revolution speed of the apparatus is regulated from 600 to 1200 rpm with a speed controller (Bodine Electric, Chicago, IL, USA).
A metering HPLC pump (Shimadzu LC-10ADVP, Columbia, MD, USA) was used for pumping the solvents, and the effluent was continuously monitored with a UV detector (LKB Instruments, Stockholm, Sweden).
2.2. Reagents
1-Butanol, hexane, ethyl acetate and methanol were all HPLC grade and purchased from Fisher Scientific, Fair Lawn, NJ, USA and other solvents such as acetic acid and hydrochloric acid were analytical grade from Mallinckrodt Chemicals, Phillipsburg, NJ, USA. Test samples including tryptophyl-tyrosine (Trp-Tyr), valyl-tyrosine (Val-Tyr), N-2,4-dinitrophenyl-L-alanine (DNP-L-ala), N-2,4-dinitrophenyl-β-alanine (DNP-β-ala), N-2,4-dinitrophenyl-DL-glutamic acid (DNP-DL-glu) were all obtained from Sigma Chemicals, St. Louis, MO, USA.
2.3 Distribution Coefficient Measurement [8]
The distribution coefficients (KD) of each sample in the two-phase solvent system were determined using the conventional test tube method with a UV spectrophotometer (Genesis 10 UV, Thermo Spectronic, Rochester, NY, USA) at 280 nm. The UV absorbance of the upper phase was recorded as AU and that of the lower phase as AL. Then, the distribution coefficient (KD) was calculated according to the following equation: KU =AU/AL (see Table 1).
Table 1.
Two-phase solvent systems and test samples
| Two-phase solvent systems* (volume ratio) | Settling time (s) | Test samples | KD (UP/LP)** |
|---|---|---|---|
| 1-BuOH/AcOH/H2O (19:1:20) | 50 | Trp-Tyr | 1.91 |
| Val-Tyr | 0.23 | ||
| Hexane/EtOAc/MeOH/0.1M HCl (1:1:1:1) | 19 | DNP-L-Ala | 2.36 |
| DNP-β-ala | 1.18 | ||
| DNP-DL-glu | 0.44 |
BuOH: buthanol; AcOH: acetic acid; EtOAc: ethyl acetate; MeOH: methanol; H2O: water
KD: distribution coefficient; UP: upper phase; LP: lower phase
2.4 Two-phase Solvent Systems and Sample Solutions
Two typical two-phase solvent systems including 1-butanol-acetic acid-water (4.75:0.25:5, v/v) (BAW) and hexane-ethyl acetate-methanol-0.1 M HCl (1:1:1:1, v/v) (HEMW) were used to separate the dipeptide and DNP-amino acid test samples, respectively. Each solvent mixture was thoroughly equilibrated in a separatory funnel by repeated vigorous shaking and degassing, and the phases separated shortly before use. Sample solution 1 was prepared by dissolving 25 mg of Trp-Tyr and 100 mg of Val-Tyr in 20 ml of the upper phase of 1-butanol-acetic acid-water. Sample solution 2 was prepared by dissolving 5.7mg of DNP-L-ala, 5.1 mg of DNP-β-ala and 5.3 mg of DNP-DL-glu in 10 ml of the upper phase of hexane-ethyl acetate-methanol-0.1 M HCl (1:1:1:1, v/v).
2.5 Separation Procedure
In each separation, the separation column was entirely filled with the stationary phase, followed by sample injection, and the column was rotated at a given revolution speed while the mobile phase was pumped into the coiled column at a given flow rate. The effluent from the outlet of the coiled column was continuously monitored with a Uvicord IIS (LKB, Stockholm, Sweden) at 280 nm and the elution curve was traced using a strip-chart recorder (Pharmacia, Stockholm, Sweden). After the desired peaks were eluted, the run was stopped and the column contents were collected into a graduated cylinder by pressured air to determine the volume of the stationary phase retained in the column. The retention of the stationary phase was computed by dividing the volume of the retained stationary phase by the total column volume.
2.6 Evaluation of Partition Efficiency
The partition efficiency of the separation column was evaluated by computing the theoretical plate number (N) for each peak and the peak resolution (Rs) between the peaks using the following conventional equations:
| (1) |
| (2) |
where tR and W indicate respectively the retention time and the baseline peak width in Eq 1 and those for the specified peaks in Eq 2 subscript, respectively.
3. Results and discussion
There are two column configurations in this study. One is parallel configuration (Fig. 1A) and the other is zigzag configuration (Fig. 1B), each placed at various angles against the horizontal plane as described earlier. At first, the effect of column angle was evaluated with the parallel column orientation from 90° and 0°. Table 2 shows the effect of different column angles on the separation of dipeptides and DNP-amino acids in type-I countercurrent chromatography using a parallel column configuration and the BAW and HEMW solvent systems with a mobile upper phase. In both solvent systems, the retention, Sf, of lower stationary phase first increased gradually for inclination angles from 90° to 60° and then decreased from 60° to 0°, while resolution (Rs) decreased all along from 90° to 0° These results agree with our previous reports [6,7]. This suggests that when the column angle is decreased, the decrease in Rs, particularly in the initial decline from 90° to 60° results largely from decreased phase mixing.
Table 2.
Effect of column angle on sample separation for parallel column configuration.
| Angle | Lower phase flow rate (ml/min) | BAW system (dipeptides) | HEMW system (DNP-amino acids) | ||||
|---|---|---|---|---|---|---|---|
| Sf (%) | Rs | N | Sf (%) | Rs | N | ||
| 90° | 0.2 | 32.2 | 2.00 | 1264/347 | 40.0 | 2.34/1.89 | 912/827/435 |
| 0.4 | 22.7 | 2.29 | 1255/729 | 30.4 | 2.75/2.16 | 1771/1354/716 | |
| 75° | 0.2 | 38.6 | 1.89 | 1225/257 | 40.5 | 2.26/1.74 | 1006/698/371 |
| 0.4 | 24.6 | 1.67 | 1015/179 | 31.2 | 2.23/1.93 | 1600/1174/796 | |
| 60° | 0.2 | 41.3 | 1.61 | 701/343 | 41.7 | 2.19/1.62 | 828/565/313 |
| 0.4 | 25.0 | 1.59 | 1276/351 | 31.9 | 2.03/1.85 | 1587/1037/754 | |
| 45° | 0.2 | 40.9 | 1.60 | 384/109 | 41.3 | 2.13/1.58 | 952/658/359 |
| 0.4 | 23.8 | 1.45 | 1541/353 | 30.4 | 2.00/1.82 | 1024/1156/616 | |
| 30° | 0.2 | 36.4 | 1.57 | 396/165 | 33.4 | 1.83/1.64 | 1032/770/372 |
| 0.4 | 22.7 | 1.33 | 364/295 | 30.2 | 1.96/1.76 | 1575/1231/591 | |
| 15° | 0.2 | 20.9 | 1.48 | 1219/542 | 19.1 | 1.13/1.02 | 1315/924/602 |
| 0.4 | 18.2 | 1.24 | 1248/471 | 12.4 | 1.15/1.04 | 2371/1099/968 | |
| 0° | 0.2 | 5.5 | 0.53 | 1131/207 | 4.6 | 1.12/0.96 | 4593/1862/1492 |
| 0.4 | 4.4 | 0.95 | 3287/643 | 4.3 | 0.61/0.70 | 4444/3322/2031 | |
Note: Sample in BAW system: Val-Tyr, Trp-Tyr; Sample in HEMW system: DNP-DL-glu, DNP-β-ala, DNP-L-ala; Sample size: 200 μl; Rotational speed: 800 rpm.
Table 3 summarizes similar tests of the zigzag column configuration, where the retention of lower phase (Sf) and peak resolution (Rs) decreased over the entire column inclination range from 90° to 0°. On the whole, the BAW solvent system performs better than the zigzag configuration for angles from 90° to 15°. But, the zigzag configuration is better at 0° due to significantly greater loss of stationary phase in the parallel configuration. The HEMW system likewise performs better in the parallel configuration except at both 15° and 0° where the zigzag configuration provides slightly better Rs.
Table 3.
Effect of column angle on sample separation for zigzag column configuration.
| Angle | Lower phase Flow rate (ml/min) | BAW system (dipeptides) | HEMW system (DNP-amino acids) | ||||
|---|---|---|---|---|---|---|---|
| Sf (%) | Rs | N | Sf (%) | Rs | N | ||
| 90° | 0.2 | 32.2 | 2 | 1264/347 | 40.0 | 2.34/1.89 | 1112/827/435 |
| 0.4 | 22.7 | 2.29 | 1255/729 | 30.4 | 2.75/2.16 | 1771/1354/716 | |
| 75° | 0.2 | 30.9 | 1.91 | 1280/357 | 35.2 | 2.10/1.50 | 1073/865/369 |
| 0.4 | 21.8 | 1.81 | 1134/439 | 28.6 | 2.23/1.76 | 1613/1321/593 | |
| 60° | 0.2 | 27.3 | 1.83 | 794/344 | 33.4 | 1.86/1.36 | 1116/676/352 |
| 0.4 | 20.8 | 1.79 | 1142/469 | 26.7 | 2.03/1.73 | 1237/1272/599 | |
| 45° | 0.2 | 21.1 | 1.76 | 1296/373 | 32.2 | 1.83/1.33 | 976/804/353 |
| 0.4 | 16.2 | 1.74 | 1056/514 | 26.1 | 1.91/1.70 | 1156/1134/627 | |
| 30° | 0.2 | 18.2 | 1.74 | 1008/423 | 31.3 | 1.77/1.29 | 951/687/316 |
| 0.4 | 14.6 | 1.37 | 1286/631 | 24.8 | 1.86/1.49 | 1248/1089/614 | |
| 15° | 0.2 | 17.3 | 1.52 | 1362/450 | 27.4 | 1.65/1.24 | 914/900/415 |
| 0.4 | 11.8 | 1.33 | 1220/400 | 20.9 | 1.67/1.47 | 1306/1832/666 | |
| 0° | 0.2 | 16.4 | 1.08 | 2191/492 | 10.8 | 1.21/1.18 | 1486/1225/1472 |
| 0.4 | 10.9 | 1.25 | 1951/322 | 8.2 | 0.85/0.79 | 1178/1349/924 | |
Note: Sample in BAW system: Val-Tyr, Trp-Tyr; Sample in HEMW system: DNP-DL-glu, DNP-β-ala, DNP-L-ala; Sample size: 200 μl; Rotational speed: 800 rpm.
On the whole, the performance including Sf and Rs of parallel orientation is better than zigzag configuration from 90° to 15° column inclination in the BAW system. But performance of zigzag configuration is better at 0° due to sharply reduced retention of stationary phase in the parallel configuration. In the HEMW system, the performance of parallel configuration is also better than zigzag configuration except for 15° and 0° column inclination.
4. Conclusions
The overall results of our experiments indicated that the retention of stationary phase was increased for the parallel configuration by slightly decreasing the column angle. But for the zigzag configuration, both retention of stationary phase and resolution decreased.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ito Y. J Chromatogr A. 2005;1065:145–168. doi: 10.1016/j.chroma.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 2.Yang Y, Gu D, Wu H, Aisa HA, Zhang T, Ito Y. J Liq Chromatogr Rel Technol. 2008;31:3012. doi: 10.1080/10826070802424956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutherland I, Fisher D. J Chromatogr A. 2009;1216:740. doi: 10.1016/j.chroma.2008.11.095. [DOI] [PubMed] [Google Scholar]
- 4.Berthod A, Ruiz-Angel MJ, Carda-Broch S. J Chromatogr A. 2009;1216:4206. doi: 10.1016/j.chroma.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 5.Ito Y, Bowman RL. Science. 1971;173:420. doi: 10.1126/science.173.3995.420. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Bowman RL. J Chromatogr Sci. 1973;11:284. doi: 10.1093/chromsci/11.6.284. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Gu D, Liu Y, Aisa HA, Ito Y. J Chromatogr A. 2009;1217:1313–1319. 2010. doi: 10.1016/j.chroma.2009.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Y, Aisa HA, Ito Y. J Liq Chromatogr Rel Technol. 2009;32:2030. doi: 10.1080/10826070903126856. [DOI] [PMC free article] [PubMed] [Google Scholar]


