Abstract
Background
Systemic modulation of Group I and II metabotropic glutamate receptors (mGluRs) regulate ethanol self-administration in a variety of animal models. Although these receptors are expressed in reward-related brain regions, the anatomical specificity of their functional involvement in ethanol self-administration remains to be characterized. This study sought to evaluate the functional role of Group I (mGluR5) and Group II (mGluR2/3) in mesocorticolimbic brain regions in ethanol self-administration.
Methods
Alcohol-preferring (P) rats, a genetic model of high alcohol drinking, were trained to self-administer ethanol (15% v/v) versus water in operant conditioning chambers. Effects of brain site-specific infusion of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) and the mGluR2/3 agonist were then assessed on the maintenance of self-administration.
Results
Microinjection of the mGluR5 antagonist MPEP in the nucleus accumbens reduced ethanol self-administration at a dose that did not alter locomotor activity. By contrast, infusion of the mGluR2/3 agonist LY379268 in the nucleus accumbens reduced self-administration and produced nonspecific reductions in locomotor activity. The mGluR5 involvement showed anatomical specificity as evidenced by lack of effect of MPEP infusion in the dorsomedial caudate or medial prefrontal cortex on ethanol self-administration. To determine reinforcer specificity, P-rats were trained to self-administer sucrose (.4% w/v) versus water, and effects of intra-accumbens MPEP were tested. The MPEP did not alter sucrose self-administration or motor behavior.
Conclusions
These results suggest that mGluR5 activity specifically in the nucleus accumbens is required for the maintenance of ethanol self-administration in individuals with genetic risk for high alcohol consumption.
Keywords: Alcohol drinking, alcoholism, caudate, LY379268, mGluR2/3, mGluR5, MPEP, nucleus accumbens, P-rats, prefrontal cortex, reinforcement, self-administration
The amino acid glutamate is the primary excitatory transmitter in the mammalian brain. Fast excitatory actions of glutamate are mediated by ionotropic N-methyl-D-aspartate (NMDA), α-amino-3-hydroxi-5-methyl-ioxyzole-4-propionic acid (AMPA), and kainite (KA) receptors. Metabotropic glutamate receptors (mGluRs) mediate slower glutamate responses through G-protein coupling to various intracellular signaling cascades that can modulate ionotropic receptor function (1). In addition to its well-known effects on ionotropic glutamate receptor function (2–9), ethanol modulates general metabotropic receptor activity as evidenced by reduced basal and stimulated phosphoinositide hydrolysis (10).
The mGluRs comprise eight subtypes categorized into three groups according to similar properties, such as sequence homology, molecular structure, selective pathways of signal transduction, and pharmacological profiles (11–13). Group I receptors (mGluR1 and mGluR5) are coupled to Gq, stimulate phospholipase C and phosphoinositide hydrolysis (14,15), are predominantly located postsynaptically, and modulate neuronal signaling and cellular excitability (16). The Group II (mGluR2 and 3) and Group III (mGluR4, 6, 7, and 8) family of receptors couple to Gi, a pathway that inhibits cyclic adenosine monophosphate formation upon activation (16,17), and are predominantly located presynaptically but have also been localized postsynaptically (18) and function to regulate glutamate release to appropriate physiological levels (17). The mGluRs regulate a variety of neurobiological processes, such as learning and memory, synaptic plasticity, and modulation of ionotropic glutamate and γ-aminobutyric acid (GABA) receptor activity, as well as facilitating glutamate release (16,19–22). Low concentrations of ethanol selectively alter neuronal firing rates (23) and Ca2+ levels (24) mediated by mGluRs in vitro, which suggests that ethanol has direct actions on mGluR activity.
Although the neurobiological substrates that regulate the positive reinforcing effects of ethanol remain to be fully characterized, the mesolimbic system has received considerable attention because of its general involvement in reinforcement (25–28) and drug self-administration (29–32). Early studies focused on dopamine neurotransmission within this system, because acute injection of ethanol was found to increase extracellular dopamine levels in the nucleus accumbens (33). Infusion of dopamine agonists in the nucleus accumbens increases ethanol-reinforced responding, whereas D1 and D2 receptor antagonists produce decreases (29,34,35). Similarly, microinjection studies have shown that intra-accumbens systems that interact with dopamine—including GABA-A (36,37), opiate (38,39), and endocannabinoid CB1 (40) receptors—regulate ethanol reinforcement. Evidence also shows that ethanol injection increases glutamate levels in the nucleus accumbens (41) and NMDA receptor antagonist infusion in the nucleus accumbens decreases ethanol-reinforced responding (42,43), which underscores the potential importance of mesolimbic glutamate in this process.
Group I and II mGluRs are highly expressed in the mesocorticolimbic system. mGluR5s and mGluR2/3s are abundant in regions such as the nucleus accumbens, lateral septum, striatum, amygdala, and hippocampus (15,44–46). Alternatively, mGluR1s show low expression in most limbic brain regions but are highly expressed in the cerebellum where they regulate motor coordination (47). This differential mesocorticolimbic distribution pattern of mGluR subtypes suggests that mGluR5 and mGluR2/3 might regulate ethanol reinforcement, but mGluR1 might not. Indeed, systemically administered mGluR5 antagonists and mGluR2/3 agonists have been shown to modulate cocaine, nicotine, and ethanol reinforcement and drug-seeking behavior in reinstatement models (48–55). By contrast, mGluR1 receptor modulation of ethanol self-administration is attributable to nonspecific motor impairment (56,57). Furthermore, evidence from site-specific infusion studies shows that ethanol self-administration is modulated by activity of specific brain regions, including the nucleus accumbens and frontal cortex (29,35,36,42,58), that express high levels of mGluR5 and mGluR2/3. Accordingly, the present study was designed to examine the direct functional involvement of mGluR5 or mGluR2/3 expressed in mesocorticolimbic brain regions in ethanol reinforcement.
Because individuals with variation in the mGluR5 gene (GRM5) show significant risk for developing alcohol dependence (59), we sought to examine the functional involvement of mesocorticolimbic mGluR5 and mGluR2/3 in ethanol reinforcement in alcohol-preferring (P) rats, a prominent genetic model of high alcohol intake (60). The P-rat line has been found to fulfill the requirements of an animal model of alcoholism (61), because these rats voluntarily consume alcohol in quantities that produce significant blood alcohol concentrations, develop tolerance and dependence through voluntary drinking (62–64), and maintain preference for ethanol when palatable solutions are presented as an alternative (65). Briefly, P-rats were trained to self-administer ethanol (15% v/v) and then received surgical implantation of bilateral injector cannulae aimed at the nucleus accumbens core; however, no attempt was made to differentiate between subnuclei of the accumbens (e.g., core vs. shell), because the extent of drug diffusion is greater than the anatomical distance between these structures (66). After surgery, effects of intra-accumbens infusion of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) and the mGluR2/3 agonist (1R,4R,5S,6R)-4-amino-2-oxabicyclo(3.1.0)hexane-4,6-dicarboxylic acid (LY379268) on the maintenance of ethanol self-administration were determined. The mGluR5 antagonist was also tested in the dorsomedial striatum and medial prefrontal cortex (mPFC) to further examine anatomical specificity of mGluR5 involvement in ethanol-reinforced responding. To assess reinforcer specificity, the mGluR5 antagonist was administered in the nucleus accumbens of P-rats trained to self-administer sucrose (.4% w/v) versus water. Finally, locomotor assessments were conducted after site-specific infusion of mGluR5 or mGluR2/3 compounds to determine whether reductions in self-administration were associated with nonspecific motor impairments.
Methods and Materials
See Supplement 1 for detailed methods.
Procedure
Ethanol Self-Administration Training
Male ethanol-preferring rats were trained to self-administer ethanol (15% v/v) versus water during daily (Monday–Friday) 30-min sessions in operant conditioning chambers with a sucrose-fading procedure (67) as previously described (36,50,57,68). After sucrose fading, self-administration sessions with ethanol (15% v/v) and water as the reinforcers continued for the remainder of the study. After completion of 28 baseline self-administration sessions with 15% ethanol, rats underwent surgery for cannulae implantation
Testing of mGluR Compounds in the Nucleus Accumbens on Ethanol Self-Administration
Rats were habituated to the microinjection procedure by receiving a sham infusion as the initial test. For testing of the mGlu receptor compounds, rats received a bilateral microinjection of a single MPEP (0 μg, 1 μg, 3 μg, 10 μg; n = 6) or LY379268 (0 μg, .03 μg, .1 μg, .17 μg; n = 9) dose in randomized order. After injectors were removed, rats were placed in the chambers for a self-administration session (30 min). Test sessions were interspersed with training sessions with at least two self-administration sessions between tests. The MPEP and LY379268 dose order was randomized.
Testing of mGluR Compounds in the Nucleus Accumbens on Locomotor Activity
After the mGlu receptor compound evaluation on ethanol self-administration, the lowest effective dose of MPEP and LY379268 that reduced ethanol self-administration was evaluated to determine whether the reductions in ethanol self-administration were accompanied by motor impairments. Rats were administered the same mGluR compound tested in self-administration, either MPEP (0 μg, 10 μg) or LY379268 (0 μg, .17 μg), and immediately placed in the activity chambers for 30-min sessions. Dose order was randomly assigned, and each rat experienced two locomotor sessions. Locomotor sessions were interspersed with self-administration sessions with at least 3 days between tests. Self-administration sessions were withheld on the days of the locomotor assessments.
Testing of mGluR5 Antagonist in the Dorsomedial Caudate Putamen and mPFC on Ethanol Self-Administration and Locomotor Activity
Rats received implantation of bilateral cannulae aimed at the dorsomedial caudate putamen (n = 9) or the mPFC (n = 7). Rats were habituated to the microinjection procedure and then administered MPEP (0 μg, 1 μg, 3 μg, 10 μg; dorsomedial caudate) or MPEP (0 μg, 1 μg, 3 μg, 10 μg, 30 μg; mPFC) in random dose order with at least two baseline sessions between tests. After the self-administration assessment, effects of MPEP (dorsomedial caudate: 0 μg, 10 μg; mPFC: 0 μg, 30 μg) were tested on motor activity with the same procedure as previously described.
Testing of mGluR5 Antagonist in the Nucleus Accumbens on Sucrose Self-Administration and Locomotor Activity
Rats were trained to self-administer sucrose (.4% w/v) versus water with procedures as described for ethanol self-administration with the exception that ethanol was never present in the solution. This concentration of sucrose was chosen because we have previously shown that this dose results in similar baseline responding as 15% v/v ethanol (56,57). After 22 baseline sessions, rats received implantation of bilateral cannulae aimed at the nucleus accumbens. Effects of presession administration of MPEP (0 μg, 1 μg, 3 μg, 10 μg, 30 μg; n = 8) in nucleus accumbens was then evaluated on sucrose self-administration as described for ethanol. After the self-administration assessment, effects of MPEP (0 μg, 30 μg) were tested on motor activity with the same procedure as previously described.
Drugs
Ethanol (95%) was diluted in distilled water. The MPEP (Sigma Aldrich, St. Louis, Missouri), a selective antagonist of mGluR5 (69), and LY379268 (Tocris, Ellisville, Missouri), a selective Group II (mGluR2/3) agonist (70), were dissolved in artificial cerebrospinal fluid (aCSF).
Data Analysis
Total responses on the ethanol (or sucrose) and water levers and ethanol intake (g/kg) were analyzed by a one-way repeated measures analysis of variance (RM ANOVA) with Dunnett post hoc analyses. Cumulative ethanol responses and locomotor activity during the 30-min sessions were analyzed with a two-way RM ANOVA with Tukey post hoc comparisons to extract significant main effects and interactions. Statistical significance was declared at p ≤ .05.
Results
Testing of mGluR Compounds in the Nucleus Accumbens on Ethanol Self-Administration and Motor Activity
Average baseline data (mean ± SEM) for the 2 days preceding the sham test of MPEP and LY379268 on ethanol self-administration are shown in Table 1. Histological verification showed that injection sites were in the nucleus accumbens core (Figures 1A and 1B). Intra-accumbens infusion of the mGluR5 antagonist MPEP significantly reduced total session ethanol (15% v/v) responding [F(3,15) = 4.65, p < .02] (Figure 2A). No MPEP-induced changes in water responses were evident. Ethanol intake (g/kg) was also significantly reduced by MPEP [F(3,15) = 4.65, p = .02], with significantly reduced ethanol intake at the highest MPEP dose (10 μg; Table 2). Cumulative ethanol responses were examined to determine the pattern of ethanol responding across the 30-min session (Figure 2B). The two-way RM ANOVA showed a significant main effect of time [F(5,25) = 45.25, p < .001], a significant main effect of MPEP dose [F(3,15) = 4.97, p < .01], and a significant interaction [F(15,75) = 1.88, p < .04], with a significant MPEP (10 μg)-induced reduction in ethanol-reinforced responding during the last half of the session (p values < .03). In the locomotor assessment, the dose of MPEP (10 μg) that reduced ethanol-reinforced responding did not alter activity across the 30-min session (Figure 2C). The two-way RM ANOVA showed a significant increase in activity over time [F(14,56) = 28.81, p < .001], with no significant main effect of MPEP dose or dose × time interaction.
Table 1.
Baseline Self-Administration Parameters (Mean ± SEM) Before Testing of the mGlu Receptors Compounds
| Group | Responses | Ethanol Intake (g/kg) |
|
|---|---|---|---|
| Ethanol Lever | Water Lever | ||
| Ethanol Reinforcement | |||
| Nucleus Accumbens (MPEP) | 180.67 ± 44.90 | 20.33 ± 7.76 | .96 ± .22 |
| Nucleus Accumbens (LY379268) | 202.42 ± 40.38 | 6.00 ± 1.68 | 1.32 ± .29 |
| Dorsomedial Caudate (MPEP) | 185.36 ± 27.93 | 23.86 ± 8.74 | 1.15 ± .18 |
| Medial Prefrontal Cortex (MPEP) | 187.00 ± 12.46 | 23.40 ± 11.32 | 1.02 ± .08 |
| Sucrose Reinforcement | |||
| Nucleus Accumbens (MPEP) | 171.93 ± 21.21 | 25.43 ± 7.83 | |
mGlu, metabotropic glutamate; MPEP, 2-methyl-6-(phenylethynyl)pyridine hydrochloride.
Figure 1.
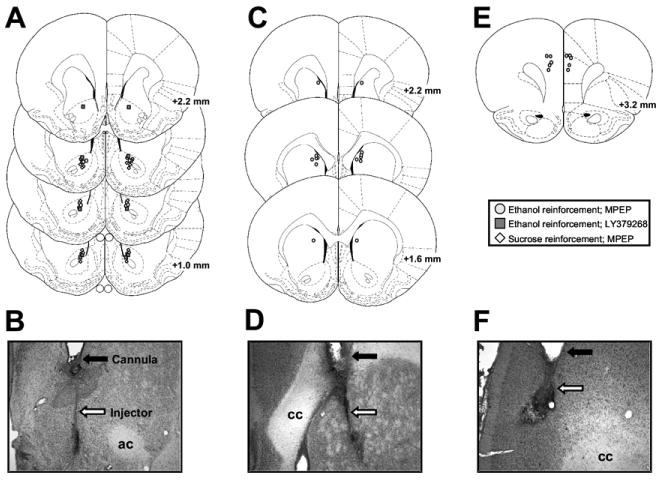
Illustrations of cannulae and injector placements. Injector placements from individual rats with accurate bilateral placements in the (A) nucleus accumbens, (C) dorsomedial caudate, and (E) medial prefrontal cortex. Circles represent animals trained on ethanol self-administration tested with 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP); squares represent animals trained on ethanol self-administration tested with LY379268; and diamonds represent animals trained on sucrose self-administration tested with MPEP. Representative photomicrographs showing cannula (closed arrows) and injector (open arrows) tracks in nucleus accumbens (B), dorsomedial caudate (D), and medial prefrontal cortex (F). ac, anterior commissure; cc, corpus callosum. Figures A, C, and E published in The Rat Brain in Stereotaxic Coordinates, 4th ed. (CD-ROM) by Paxinos and Watson, Copyright Elsevier (1998).
Figure 2.
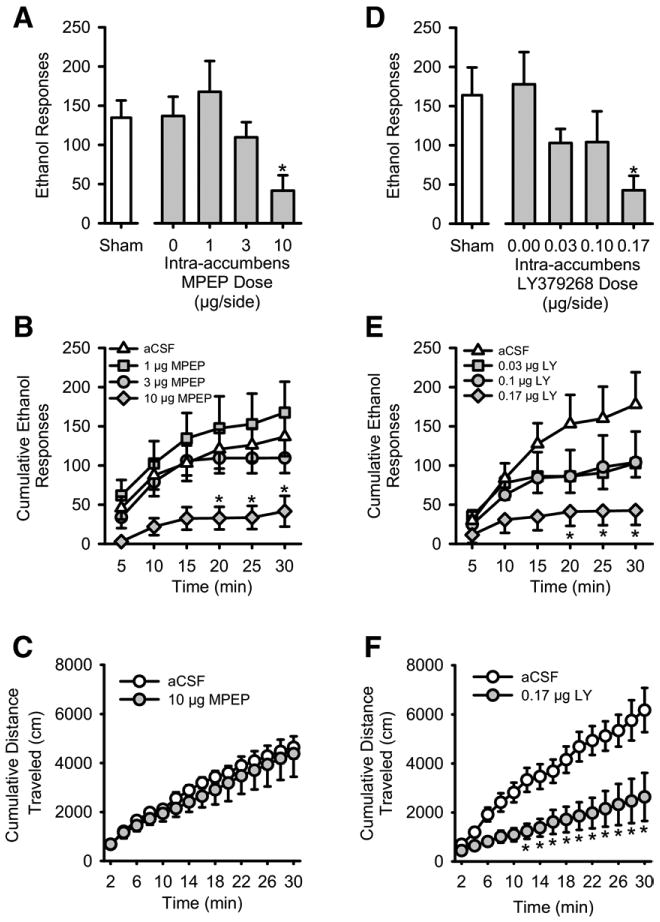
Intra-accumbens metabotropic glutamate 5 (mGlu5) antagonism reduces ethanol self-administration without affecting spontaneous motor activity. (A) Mean (± SEM) responses on the ethanol (15% v/v) lever after intra-accumbens administration of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP) and a sham infusion (to left of axis break). (B) Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after intra-accumbens MPEP administration. (C) Mean (± SEM) cumulative distance traveled (cm) during the 30-min locomotor assessment after intra-accumbens administration of MPEP (10 μg). (D) Mean (± SEM) responses on the ethanol (15% v/v) lever after intra-accumbens administration of the mGluR2/3 agonist LY379268 (LY) and a sham infusion (to left of axis break). (E) Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after intra-accumbens LY administration. (F) Mean (± SEM) cumulative distance traveled (cm) during the 30-min locomotor assessment after intra-accumbens administration of LY (.17 μg). aCSF, artificial cerebrospinal fluid. *Significant difference from vehicle (Tukey, p < .05).
Table 2.
Ethanol Intake (g/kg) for Each of the mGlu Receptor Compound Tests (mean ± SEM)
| MPEP Dose (μg) | |||||
|---|---|---|---|---|---|
| Region | 0 | 1 | 3 | 10 | 30 |
| Nucleus Accumbens | .74 ± .14 | .87 ± .19 | .55 ± .10 | .24 ± .12a | |
| Dorsomedial Caudate | 1.17 ± .17 | 1.22 ± .20 | 1.22 ± .16 | 1.01 ± .25 | |
| Medial Prefrontal Cortex | .83 ± .09 | .80 ± .16 | .53 ± .24 | .71 ± .14 | .67 ± .14 |
| LY379268 (μg) | |||||
| 0 | .03 | .1 | .17 | ||
| Nucleus Accumbens | 1.10 ± .23 | .62 ± .11 | .60 ± .22 | .25 ± .11a | |
mGlu, metabotropic glutamate; MPEP, 2-methyl-6-(phenylethynyl)pyridine hydrochloride.
p < .05 relative to 0 (Dunnett).
Intra-accumbens administration of the mGluR2/3 agonist LY379268 also significantly reduced total session ethanol responses [F(3,17) = 3.76, p = .03] (Figure 2D) and ethanol intake [F(3,17) = 4.19, p = .02] (Table 2). Water responses were unchanged by LY379268 pretreatment. As shown in Figure 2E, analysis of the cumulative ethanol responses showed a significant main effect of time [F(5,25) = 12.90, p < .001] and a significant interaction [F(15,75) = 2.99, p < .001], with reduced ethanol responding relative to vehicle during the last half of the session (p values ≤ .02) after treatment with the highest LY379268 dose (.17 μg). The dose of LY379268 (.17 μg) that decreased ethanol-reinforced responding produced a significant reduction in locomotor activity, because examination of the cumulative distance traveled across the 30-min session showed a significant main effect of LY379268 dose [F(1,5) = 8.42, p = .03], a significant main effect of time [F(14,70) = 46.10, p < .001], and a significant LY379268 dose × time interaction [F(14,70) = 5.21, p < .001] (Figure 2F). The LY379268-induced reduction in cumulative distance emerged at min 12 and continued throughout the session (p values ≤ .04).
Testing of mGluR5 Antagonist in the Dorsomedial Caudate Putamen and the mPFC on Ethanol Self-Administration and Motor Activity
Average baseline data (mean ± SEM) for both the dorsomedial caudate and mPFC group for the 2 days preceding the sham infusion are shown in Table 1. Dorsomedial caudate administration (Figures 1C and 1D) of MPEP did not alter ethanol (15% v/v) self-administration (Figure 3A) or ethanol intake (g/kg; Table 2). Analysis of cumulative ethanol responses across the 30-min session indicated a significant main effect of time [F(5,25) = 29.27, p < .001] but no main effect of MPEP dose or interaction (Figure 3B). Interestingly, intracaudate MPEP induced a significant increase in total session water responding (Table 3) at the highest MPEP dose [10 μg; F(3,17) = 3.28, p = .046]. Intracaudate administration of the highest dose of MPEP (10 μg/μL) did not alter cumulative locomotor activity (Figure 3C). An ANOVA showed a significant effect of time only [F(14,84) = 57.50, p < .001], with no effect of MPEP and no MPEP × time interaction.
Figure 3.
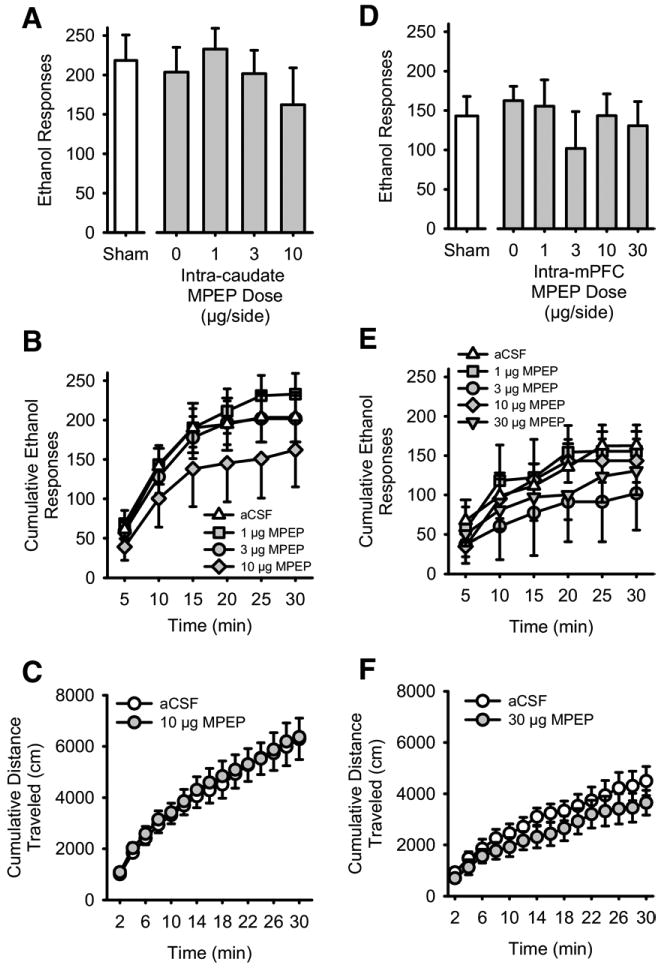
Intracaudate or medial prefrontal cortex (mPFC) antagonism of mGluR5 does not alter ethanol self-administration or spontaneous motor activity. (A) Mean (± SEM) responses on the ethanol (15% v/v) lever after intra-dorsomedial caudate putamen administration of the mGluR5 antagonist MPEP and a sham infusion (to left of axis break). (B) Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after intracaudate MPEP administration. (C) Mean (± SEM) cumulative distance traveled (cm) during the 30-min locomotor assessment after intracaudate administration of MPEP (10 μg). (D) Mean (± SEM) responses on the ethanol (15% v/v) lever after intra-mPFC administration of the mGluR5 antagonist MPEP and a sham infusion (to left of axis break). (E) Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after intra-mPFC MPEP administration. (F) Mean (± SEM) cumulative distance traveled (cm) during the 30 min locomotor assessment after intra-mPFC administration of MPEP (30 μg). Abbreviations as Figures 1 and 2.
Table 3.
Total Session Water Responses for Each of the mGlu Receptor Compound Tests (Mean ± SEM)
| Region | MPEP Dose (μg) | ||||
|---|---|---|---|---|---|
| 0 | 1 | 3 | 10 | 30 | |
| Ethanol Reinforcement | |||||
| Nucleus accumbens | 7.83 ± 1.80 | 17.33 ± 8.04 | 4.50 ± .92 | 3.50 ± 1.31 | |
| Dorsomedial caudate | 8.29 ± 2.91 | 11.00 ± 3.57 | 11.29 ± 3.99 | 25.00 ± 7.85a | |
| Medial prefrontal cortex | 6.40 ± 2.62 | 6.20 ± 1.86 | 3.00 ± 1.27 | 6.40 ± 3.67 | 24.6 ± 22.87 |
| LY379268 (μg) | |||||
| 0 | .03 | .1 | .17 | ||
| Nucleus accumbens | 8.43 ± 3.0 | 10.43 ± 3.70 | 5.14 ± 1.99 | 8.33 ± 3.35 | |
| MPEP Dose (μg) | |||||
| 0 | 1 | 3 | 10 | 30 | |
| Sucrose Reinforcement | |||||
| Nucleus accumbens | 14.86 ± 4.17 | 11.29 ± 3.80 | 11.00 ± 3.92 | 14.83 ± 1.62 | 16.14 ± 7.71 |
Abbreviations as in Table 2.
p < .05 relative to 0 (Dunnett).
Intra-mPFC administration of MPEP in the mPFC (Figures 1E and 1F) did not significantly alter total ethanol (Figure 3D) or water responses (Table 3). Analysis of the pattern of response across time showed a significant main effect of time [F(5,20) = 17.85, p < .001]. The main effect of intra-mPFC MPEP dose and the interaction were not significant (Figure 3E). The highest dose of MPEP (30 μg/μL) tested in the mPFC produced no effect on cumulative locomotor activity (Figure 3F), with ANOVA confirming only a significant effect of time [F(14,56) = 59.57, p < .001]. No significant main effect of MPEP dose or interaction was observed on locomotor activity.
Testing of mGluR5 Antagonist in the Nucleus Accumbens on Sucrose Self-Administration and Motor Activity
Average baseline data (mean ± SEM) for the 2 days preceding the sham test of MPEP on sucrose self-administration are shown in Table 1. Nucleus accumbens administration (Figures 1A and 1D) of MPEP did not alter sucrose self-administration (.4% w/v; Figure 4A). Water-responding was also not altered by MPEP pretreatment (Table 3). Analysis of cumulative sucrose responses across the 30-min session indicated a significant main effect of time [F(5,25) = 31.94, p < .001] but no main effect of MPEP dose (Figure 4B). A significant interaction was found [F(20,100) = 1.98, p = .01]; however, sucrose-reinforced responding after intra-accumbens MPEP administration did not significantly differ from aCSF at any time point throughout the session.
Figure 4.
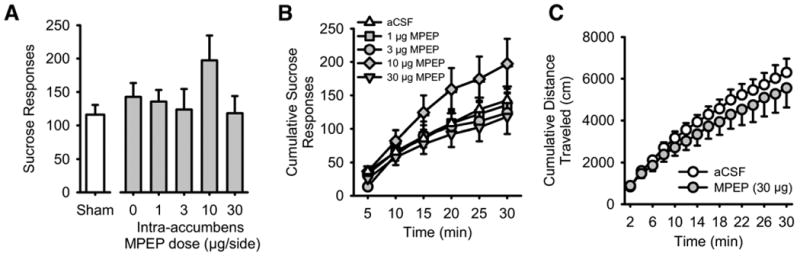
Intra-accumbens mGluR5 antagonism does not alter sucrose self-administration or spontaneous motor activity. (A) Mean (± SEM) responses on the sucrose (.4% w/v) lever after intra-accumbens administration of the mGluR5 antagonist MPEP and a sham infusion (to left of axis break). (B) Mean (± SEM) cumulative ethanol responses across the 30-min self-administration session after intra-accumbens MPEP administration. (C) Mean (± SEM) cumulative distance traveled (cm) during the 30-min locomotor assessment after intra-accumbens administration of MPEP (10 μg). Abbreviations as Figures 1 and 2.
Intra-accumbens administration of the highest MPEP dose tested (30 μg) did not alter spontaneous motor activity in sucrose-exposed rats (Figure 4C). Analysis of the cumulative distance traveled across the 30-min session showed a significant main effect of time [F(14,84) = 44.18, p < .001] but no main effect of MPEP dose or interaction.
Discussion
This study was designed to investigate functional involvement of nucleus accumbens mGluR5 and mGluR2/3 in ethanol reinforcement in the alcohol-preferring P-rat line, which is a prominent genetic model of excessive alcohol-drinking (64). Accordingly, P-rats self-administered relatively high levels of ethanol (range .74 g/kg–1.32 g/kg) during 30-min operant sessions, for which we have shown results in blood ethanol levels of approximately 80 mg/dL (57). Results showed that pharmacological inhibition of mGluR5 in the nucleus accumbens produced a 70% reduction in ethanol self-administration in the absence of nonspecific effects on motor activity. Functional specificity of mGluR5 was confirmed, because activation of mGluR2/3 in the nucleus accumbens did not selectively alter ethanol self-administration. Blockade of mGluR5 in two other brain regions (mPFC and dorsomedial caudate) produced no effect on ethanol self-administration, indicating anatomical specificity of mGluR5 function in this behavior. Furthermore, ethanol specificity was confirmed, because intra-accumbens mGluR5 antagonism did not alter self-administration of another reinforcing solution (i.e., sucrose). These results indicate mGluR5 activity in the nucleus accumbens is required for the full expression of ethanol's reinforcing effects in individuals with a genetic predisposition for heavy alcohol-drinking.
Previous work has determined a role for mGluR5 in ethanol reinforcement as evidenced by reductions in ethanol self-administration after systemic administration of mGluR5 antagonists (48–51,56,71). This study shows that infusion of the mGluR5 antagonist MPEP (0 μg–10 μg) in the nucleus accumbens reduces ethanol-reinforced responding. The mGluR5 antagonism, or gene knockout, has been shown to potentiate ethanol's acute sedative-hypnotic effects and produce motor impairments after moderate alcohol doses (72–74); thus, examination of the temporal pattern of responding was critical for the interpretation of the total session reductions in ethanol self-administration. Intra-accumbens mGluR5 antagonism produced a general suppression of ethanol-reinforced responding throughout the 30-min self-administration session, indicating that MPEP effects were independent of consumed ethanol. Furthermore, the prolonged suppression of ethanol-reinforced responding across the 30-min session is consistent with the time course of mGluR5 occupancy after systemic MPEP injection (i.e., full receptor occupancy sustained for at least 1 hour (75). Finally, the reduction in ethanol self-administration is likely not related to drug substitution, because mGluR5 antagonism itself does not produce ethanol-like properties and does not alter the subjective properties of low ethanol dose (76–78).
The results of this study complement other evidence showing that glutamate transmission in the nucleus accumbens regulates ethanol reinforcement. Microinjection of the competitive NMDA receptor antagonist AP-5 into the nucleus accumbens of Wistar rats reduced ethanol-reinforced responding (42). The present study extends this work by showing that inhibition of mGluR5 but not mGluR2/3 activity specifically in the nucleus accumbens reduces the maintenance of ethanol-reinforced responding in P-rats that were self-administering relatively high doses of ethanol under control conditions (mean 1.04 g/kg/30-min session). Thus, it seems that disrupting glutamate neurotransmission either through blockade of postsynaptic ionotropic NMDA or metabotropic mGluR5 in the nucleus accumbens is sufficient to prevent the full expression of ethanol's reinforcing properties.
mGluR5s are widely expressed throughout the striatum (44), with similar expression in the nucleus accumbens and caudate putamen (76,79,80). However, infusion of MPEP in the dorsomedial caudate, contrary to the nucleus accumbens infusion, had no effect on ethanol self-administration. The dorsal striatum has been shown to regulate cocaine self-administration by dopamine and AMPA/kainate receptors (81) and ethanol self-administration by NMDA (NR2B) receptors (82). Furthermore, the dorsal striatum has been implicated in learning stimulus-response relations associated with habit formation (83). Given that compulsive/habitual drug use—a hallmark of drug addiction—can be regarded as maladaptive stimulus-response relations, the dorsal striatum has become a region of significant interest in the drug abuse field (84). Although the present findings suggest that mGluR5 within this region do not modulate the maintenance of ethanol-reinforced responding, a partial trend was observed at the highest dose of MPEP tested, suggesting that an extended dose range of MPEP or another mGluR5 antagonist such as 3-((2-methyl-1,3-thiazol-4-yl)ethynyl)pyridine might show efficacy. It will also be both interesting and important for future work to determine whether mGluR5 in the dorsal striatum regulate other aspects of ethanol-seeking behavior that rely on conditioned reinforcement, such as cue-induced reinstatement.
Functional specificity of nucleus accumbens mGluR5 was further evaluated by examining the effects of MPEP infusion in the mPFC on the maintenance of ethanol self-administration. The mPFC was tested for several reasons. First, this brain region has been shown to modulate ethanol self-administration (58,85,86), and low doses of ethanol increase extracellular glutamate levels in the mPFC of alcohol-preferring Lewis rats (87). Second, mGluR5 regulate firing rate of mPFC neurons (88), which is a neural correlate of reward prediction (89). Third, the mPFC sends glutamatergic projections to the nucleus accumbens, and inactivation of the mPFC reduces the firing rate of nucleus accumbens neurons in response to reward-predictive cues (90). Thus, inhibition of postsynaptic mGluR5 in the mPFC might alter ethanol self-administration via local mechanisms or by dampening mPFC glutamatergic inputs to the nucleus accumbens. Results showed, however, that infusion of the mGluR5 antagonist MPEP in the mPFC had no effect on ethanol self-administration. This was evident even though a higher MPEP dose range was tested in the mPFC compared with the nucleus accumbens. These findings suggest that mGluR5 in the mPFC are not involved in the regulation of the maintenance of ethanol self-administration. However, because the mPFC regulates response to predictive cues, mGluR5 in this brain region might influence behaviors that are regulated by associative learning, such as cue-induced reinstatement of ethanol seeking.
Specificity of nucleus accumbens mGluR5 was further confirmed by testing the role of mGluR2/3 in ethanol self-administration. Intra-accumbens mGluR2/3 activation reduced ethanol self-administration. This was evident by a reduction in total session ethanol-reinforced responding and a pattern of suppression similar to that observed after mGluR5 antagonism (i.e., general suppression of responding throughout the session). These results are consistent with evidence showing that intra-accumbens infusion of the mGlu2/3 agonist APDC reduced ethanol-drinking in a 4-bottle home-cage procedure by C57Bl/6 J and DBA2/J mice (91). However, results from the present study showed that LY379268 also reduced spontaneous motor activity, suggesting that reductions in ethanol-reinforced responding were likely due to a motor impairment. This finding is consistent with the findings of a study by Backstrom and Hyytia (92) in which a systemically administered mGluR2/3 agonist produced a significant reduction in ethanol self-administration at a dose that was accompanied by a motor impairment (but see [93]). Given that LY379268 decreased ethanol self-administration only at a dose that produced nonspecific reductions in locomotor activity, these findings indicate that mGluR2/3 activation in the nucleus accumbens does not selectively modulate the maintenance of ethanol self-administration. However, because systemically administered mGluR2/3 agonists exhibit efficacy in other behavioral procedures, such as models of relapse, anxiety, and stress reactivity during abstinence (93–95), future work might determine a more selective role of intra-accumbens mGluR2/3 in these pathological behaviors.
To determine whether mGluR5 in the nucleus accumbens produces a reduction in the maintenance of self-administration of a rewarding substance in general, intra-accumbens mGluR5 antagonism was assessed in rats trained to self-administer sucrose versus water. Baseline level of sucrose (.4% w/v) self-administration was similar to the baseline ethanol self-administration, consistent with our previous findings (56,57). Accordingly, differences in self-administration behavior between the sucrose and ethanol group cannot be attributed to differential effects of the mGluR5 antagonist on response rate. Intra-accumbens antagonism was without effect on sucrose self-administration, even as an mGluR5 antagonist dose range higher than for ethanol self-administration was tested. This finding suggests specificity of the effects of mGluR5 antagonism to ethanol reinforcement and also confirms the lack of antagonist-induced nonspecific motor effects. Furthermore, the dissociation of mGluR5 involvement in ethanol versus sucrose reinforcement is similar to the findings of another study in which motivation to self-administer ethanol but not sucrose was reduced after systemic mGluR5 antagonism (57). Overall, some studies have shown reductions in food-reinforced behavior after mGluR5 antagonism (96,97), whereas other studies have not (53,98,99). However, the present results are consistent with another study that showed no change in sucrose self-administration after intra-accumbens mGluR5 antagonism (100). Together, this data pattern supports a role for intra-accumbens mGluR5 in the selective modulation of ethanol self-administration.
One issue that should be discussed when considering mGlu receptor subtype specificity relates to the selectivity of mGlu receptor compounds tested. Studies have emerged showing that the mGluR5 antagonist MPEP modulates the activity of other receptor systems. For instance, MPEP has been shown to blunt NMDA-evoked currents (101), inhibit the norepinephrine transporter (102), and act as a positive allosteric modulator of mGlu4 receptors (103). The mGluR2/3 agonist LY379268 has recently been shown to stimulate dopamine D2 receptors (104). Moreover, MPEP infused in the nucleus accumbens inhibits intra-accumbens mGluR5 agonist-induced GABA release in the ventral pallidum (105). Because these receptor systems have been shown to modulate ethanol self-administration or voluntary ethanol-drinking (29,82,106–108), it is possible that mGluR5 inhibition in the nucleus accumbens reduced ethanol self-administration via nonspecific pharmacological effects or regulation of associated neural circuit(s).
In conclusion, there is growing interest in developing mGlu receptor compounds as therapeutics for drug and alcohol use disorders (109), because these compounds have been shown to reduce self-administration and relapse to drug-taking of several drugs of abuse (54,110–112), including alcohol (48–51,56,57,71,91–93,113). The results of this study extend the previous literature to show that inhibition of mGluR5 activity in the nucleus accumbens, a key component of the brain's reward pathway, specifically reduces operant ethanol self-administration. These data confirm the importance of mGluR5 activity in the nucleus accumbens in regulating drug reinforcement and emphasize the potential therapeutic utility of targeting this receptor system in individuals with genetic risk for excessive drinking.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism to JB (AA016009) and CWH (AA014983 and AA011605).
Footnotes
The authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Benquet P, Gee CE, Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J Neurosci. 2002;22:9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschner M, Mutkus L, Allen JW. Aspartate and glutamate transport in acutely and chronically ethanol exposed neonatal rat primary astrocyte cultures. Neurotoxicology. 2001;22:601–605. doi: 10.1016/s0161-813x(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 3.Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000;24:706–715. [PubMed] [Google Scholar]
- 4.Dodd PR, Beckmann AM, Davidson MS, Wilce PA. Glutamate-mediated transmission, alcohol, and alcoholism. Neurochem Int. 2000;37:509–533. doi: 10.1016/s0197-0186(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 5.Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S. Role of polyamines and NMDA receptors in ethanol dependence and withdrawal. Alcohol Clin Exp Res. 2001;25:132S–136S. doi: 10.1097/00000374-200105051-00023. [DOI] [PubMed] [Google Scholar]
- 6.Mihic SJ. Acute effects of ethanol on GABAA and glycine receptor function. Neurochem Int. 1999;35:115–123. doi: 10.1016/s0197-0186(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 7.Tabakoff B, Hoffman PL. Ethanol, sedative hypnotics, and glutamate receptor function in brain and cultured cells. Behav Genet. 1993;23:231–236. doi: 10.1007/BF01067428. [DOI] [PubMed] [Google Scholar]
- 8.Weight FF, Peoples RW, Wright JM, Lovinger DM, White G. Ethanol action on excitatory amino acid activated ion channels. Alcohol Alcohol Suppl. 1993;2:353–358. [PubMed] [Google Scholar]
- 9.Woodward JJ. Ionotropic glutamate receptors as sites of action for ethanol in the brain. Neurochem Int. 1999;35:107–113. [PubMed] [Google Scholar]
- 10.Gonzales RA, Theiss C, Crews FT. Effects of ethanol on stimulated inositol phospholipid hydrolysis in rat brain. J Pharmacol Exp Ther. 1986;237:92–98. [PubMed] [Google Scholar]
- 11.Benarroch EE. Metabotropic glutamate receptors: Synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008;70:964–968. doi: 10.1212/01.wnl.0000306315.03021.2a. [DOI] [PubMed] [Google Scholar]
- 12.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 14.Houamed KM, Kuijper JL, Gilbert TL, Haldeman BA, O'Hara PJ, Mulvihill ER, et al. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- 16.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 17.Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: Behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- 18.Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- 19.Altinbilek B, Manahan-Vaughan D. A specific role for group II metabotropic glutamate receptors in hippocampal long-term depression and spatial memory. Neuroscience. 2009;158:149–158. doi: 10.1016/j.neuroscience.2008.07.045. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: Physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 21.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 22.Xiao MY, Gustafsson B, Niu YP. Metabotropic glutamate receptors in the trafficking of ionotropic glutamate and GABA(A) receptors at central synapses. Curr Neuropharmacol. 2006;4:77–86. doi: 10.2174/157015906775202986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Netzeband JG, Parsons KL, Sweeney DD, Gruol DL. Metabotropic glutamate receptor agonists alter neuronal excitability and Ca2 + levels via the phospholipase C transduction pathway in cultured Purkinje neurons. J Neurophysiol. 1997;78:63–75. doi: 10.1152/jn.1997.78.1.63. [DOI] [PubMed] [Google Scholar]
- 24.Gruol DL, Parsons KL, DiJulio N. Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res. 1997;773:82–89. doi: 10.1016/s0006-8993(97)00912-8. [DOI] [PubMed] [Google Scholar]
- 25.Burns LH, Everitt BJ, Kelley AE, Robbins TW. Glutamate-dopamine interactions in the ventral striatum: Role in locomotor activity and responding with conditioned reinforcement. Psychopharmacology. 1994;115:516–528. doi: 10.1007/BF02245576. [DOI] [PubMed] [Google Scholar]
- 26.Fiorino DF, Coury A, Fibiger HC, Phillips AG. Electrical stimulation of reward sites in the ventral tegmental area increases dopamine transmission in the nucleus accumbens of the rat. Behav Brain Res. 1993;55:131–141. doi: 10.1016/0166-4328(93)90109-4. [DOI] [PubMed] [Google Scholar]
- 27.Le Moal M, Simon H. Mesocorticolimbic dopaminergic network: Functional and regulatory roles. Physiol Rev. 1991;71:155–234. doi: 10.1152/physrev.1991.71.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Wise RA, Bauco P, Carlezon WA, Jr, Trojniar W. Self-stimulation and drug reward mechanisms. Ann N Y Acad Sci. 1992;654:192–198. doi: 10.1111/j.1749-6632.1992.tb25967.x. [DOI] [PubMed] [Google Scholar]
- 29.Hodge CW, Samson HH, Haraguchi M. Microinjections of dopamine agonists in the nucleus accumbens increase ethanol-reinforced responding. Pharmacol Biochem Behav. 1992;43:249–254. doi: 10.1016/0091-3057(92)90665-3. [DOI] [PubMed] [Google Scholar]
- 30.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 31.Porrino LJ, Williams-Hemby L, Whitlow C, Bowen C, Samson HH. Metabolic mapping of the effects of oral alcohol self-administration in rats. Alcohol Clin Exp Res. 1998;22:176–182. [PubMed] [Google Scholar]
- 32.Weiss F, Hurd YL, Ungerstedt U, Markou A, Plotsky PM, Koob GF. Neurochemical correlates of cocaine and ethanol self-administration. Ann N Y Acad Sci. 1992;654:220–241. doi: 10.1111/j.1749-6632.1992.tb25970.x. [DOI] [PubMed] [Google Scholar]
- 33.Imperato A, Di Chiara G. Preferential stimulation of dopamine release in the nucleus accumbens of freely moving rats by ethanol. J Pharmacol Exp Ther. 1986;239:219–228. [PubMed] [Google Scholar]
- 34.Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: Further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge CW, Samson HH, Tolliver GA, Haraguchi M. Effects of intraaccumbens injections of dopamine agonists and antagonists on sucrose and sucrose-ethanol reinforced responding. Pharmacol Biochem Behav. 1994;48:141–150. doi: 10.1016/0091-3057(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 36.Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 37.June HL, Torres L, Cason CR, Hwang BH, Braun MR, Murphy JM. The novel benzodiazepine inverse agonist RO19-4603 antagonizes ethanol motivated behaviors: Neuropharmacological studies. Brain Res. 1998;784:256–275. doi: 10.1016/s0006-8993(97)01380-2. [DOI] [PubMed] [Google Scholar]
- 38.Heyser CJ, Roberts AJ, Schulteis G, Koob GF. Central administration of an opiate antagonist decreases oral ethanol self-administration in rats. Alcohol Clin Exp Res. 1999;23:1468–1476. [PubMed] [Google Scholar]
- 39.Hyytia P, Kiianmaa K. Suppression of ethanol responding by centrally administered CTOP and naltrindole in AA and Wistar rats. Alcohol Clin Exp Res. 2001;25:25–33. doi: 10.1111/j.1530-0277.2001.tb02123.x. [DOI] [PubMed] [Google Scholar]
- 40.Malinen H, Hyytia P. Ethanol self-administration is regulated by CB1 receptors in the nucleus accumbens and ventral tegmental area in alcohol-preferring AA rats. Alcohol Clin Exp Res. 2008;32:1976–1983. doi: 10.1111/j.1530-0277.2008.00786.x. [DOI] [PubMed] [Google Scholar]
- 41.Yan QS, Reith ME, Yan SG, Jobe PC. Effect of systemic ethanol on basal and stimulated glutamate releases in the nucleus accumbens of freely moving Sprague-Dawley rats: A microdialysis study. Neurosci Lett. 1998;258:29–32. doi: 10.1016/s0304-3940(98)00840-4. [DOI] [PubMed] [Google Scholar]
- 42.Rassnick S, D'Amico E, Riley E, Pulvirenti L, Zieglgansberger W, Koob GF. GABA and nucleus accumbens glutamate neurotransmission modulate ethanol self-administration in rats. Ann N Y Acad Sci. 1992;654:502–505. doi: 10.1111/j.1749-6632.1992.tb26013.x. [DOI] [PubMed] [Google Scholar]
- 43.Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- 44.Shigemoto R, Nomura S, Ohishi H, Sugihara H, Nakanishi S, Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci Lett. 1993;163:53–57. doi: 10.1016/0304-3940(93)90227-c. [DOI] [PubMed] [Google Scholar]
- 45.Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- 46.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: Differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 47.Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 48.Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 49.Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- 50.Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology. 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology. 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 53.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology. 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 54.Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49(suppl 1):167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-Fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Effects of mGlu1-receptor blockade on ethanol self-administration in inbred alcohol-preferring rats. Alcohol. 2008;42:13–20. doi: 10.1016/j.alcohol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hodge CW, Chappelle AM, Samson HH. Dopamine receptors in the medial prefrontal cortex influence ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1996;20:1631–1638. doi: 10.1111/j.1530-0277.1996.tb01709.x. [DOI] [PubMed] [Google Scholar]
- 59.Schumann G, Johann M, Frank J, Preuss U, Dahmen N, Laucht M, et al. Systematic analysis of glutamatergic neurotransmission genes in alcohol dependence and adolescent risky drinking behavior. Arch Gen Psychiatry. 2008;65:826–838. doi: 10.1001/archpsyc.65.7.826. [DOI] [PubMed] [Google Scholar]
- 60.Lumeng L, Hawkins DT, Li TK. New strains of rats with alcohol preference and nonpreference. In: Thurman RG, Williamson JR, Drott H, Chance B, editors. Alcohol and Aldehyde Metabolizing Systems. Vol. 3. New York: Academic Press; 1977. pp. 537–544. [Google Scholar]
- 61.Lester D, Freed EX. Criteria for an animal model of alcoholism. Pharmacol Biochem Behav. 1973;1:103–107. doi: 10.1016/0091-3057(73)90062-2. [DOI] [PubMed] [Google Scholar]
- 62.Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: Changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcohol Clin Exp Res. 2000;24:278–284. [PubMed] [Google Scholar]
- 63.Lumeng L, Li TK. The development of metabolic tolerance in the alcohol-preferring P rats: Comparison of forced and free-choice drinking of ethanol. Pharmacol Biochem Behav. 1986;25:1013–1020. doi: 10.1016/0091-3057(86)90079-1. [DOI] [PubMed] [Google Scholar]
- 64.Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- 65.Lankford MF, Roscoe AK, Pennington SN, Myers RD. Drinking of high concentrations of ethanol versus palatable fluids in alcohol-preferring (P) rats: Valid animal model of alcoholism. Alcohol. 1991;8:293–299. doi: 10.1016/0741-8329(91)90417-u. [DOI] [PubMed] [Google Scholar]
- 66.Perez de la Mora M, Lara-Garcia D, Jacobsen KX, Vazquez-Garcia M, Crespo-Ramirez M, Flores-Gracia C, et al. Anxiolytic-like effects of the selective metabotropic glutamate receptor 5 antagonist MPEP after its intra-amygdaloid microinjection in three different non-conditioned rat models of anxiety. Eur J Neurosci. 2006;23:2749–2759. doi: 10.1111/j.1460-9568.2006.04798.x. [DOI] [PubMed] [Google Scholar]
- 67.Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 68.Schroeder JP, Overstreet DH, Hodge CW. The neuropeptide-Y Y5 receptor antagonist L-152,804 decreases alcohol self-administration in inbred alcohol-preferring (iP) rats. Alcohol. 2005;36:179–186. doi: 10.1016/j.alcohol.2005.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gasparini F, Lingenhohl K, Stoehr N, Flor PJ, Heinrich M, Vranesic I, et al. 2-methyl-6-(phenylethynyl)-pyridine (MPEP), a potent, selective and systemically active mGlu5 receptor antagonist. Neuropharmacology. 1999;38:1493–1503. doi: 10.1016/s0028-3908(99)00082-9. [DOI] [PubMed] [Google Scholar]
- 70.Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo(3.1.0) hexane-2,6-dicarboxylic acid ( LY354740): Identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 71.Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 72.Bird MK, Kirchhoff J, Djouma E, Lawrence AJ. Metabotropic glutamate 5 receptors regulate sensitivity to ethanol in mice. Int J Neuropsychopharmacol. 2008;11:765–774. doi: 10.1017/S1461145708008572. [DOI] [PubMed] [Google Scholar]
- 73.Blednov YA, Adron HR. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: Relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharko AC, Hodge CW. Differential modulation of ethanol-induced sedation and hypnosis by metabotropic glutamate receptor antagonists in C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:67–76. doi: 10.1111/j.1530-0277.2007.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson JJ, Bradbury MJ, Giracello DR, Chapman DF, Holtz G, Roppe J, et al. In vivo receptor occupancy of mGlu5 receptor antagonists using the novel radioligand [3H]3-methoxy5-(pyridine-2-ylethynyl)pyridine) Eur J Pharmacol. 2003;473:35–40. doi: 10.1016/s0014-2999(03)01935-6. [DOI] [PubMed] [Google Scholar]
- 76.Besheer J, Grondin JJM, Salling MC, Spanos M, Stevenson RA, Hodge CW. Interoceptive properties produced by alcohol require mGlu5 receptor activity in the nucleus accumbens. J Neurosci. 2009;29:9582–9591. doi: 10.1523/JNEUROSCI.2366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Besheer J, Hodge CW. Pharmacological and anatomical evidence for an interaction between mGluR5- and GABA(A) alpha1-containing receptors in the discriminative stimulus effects of ethanol. Neuropsychopharmacology. 2005;30:747–757. doi: 10.1038/sj.npp.1300616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Besheer J, Stevenson RA, Hodge CW. mGlu5 receptors are involved in the discriminative stimulus effects of self-administered ethanol in rats. Eur J Pharmacol. 2006;551:71–75. doi: 10.1016/j.ejphar.2006.08.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mao L, Wang JQ. Differentially altered mGluR1 and mGluR5 mRNA expression in rat caudate nucleus and nucleus accumbens in the development and expression of behavioral sensitization to repeated amphetamine administration. Synapse. 2001;41:230–240. doi: 10.1002/syn.1080. [DOI] [PubMed] [Google Scholar]
- 80.Simonyi A, Ngomba RT, Storto M, Catania MV, Miller LA, Youngs B, et al. Expression of groups I and II metabotropic glutamate receptors in the rat brain during aging. Brain Res. 2005;1043:95–106. doi: 10.1016/j.brainres.2005.02.046. [DOI] [PubMed] [Google Scholar]
- 81.Vanderschuren LJ, Di Ciano P, Everitt BJ. Involvement of the dorsal striatum in cue-controlled cocaine seeking. J Neurosci. 2005;25:8665–8670. doi: 10.1523/JNEUROSCI.0925-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Carnicella S, Phamluong K, Jeanblanc J, Ronesi JA, Chaudhri N, et al. Ethanol induces long-term facilitation of NR2B-NMDA receptor activity in the dorsal striatum: Implications for alcohol drinking behavior. J Neurosci. 2007;27:3593–3602. doi: 10.1523/JNEUROSCI.4749-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 84.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 85.Samson HH, Chappell A. Dopaminergic involvement in medial prefrontal cortex and core of the nucleus accumbens in the regulation of ethanol self-administration: A dual-site microinjection study in the rat. Physiol Behav. 2003;79:581–590. doi: 10.1016/s0031-9384(03)00126-4. [DOI] [PubMed] [Google Scholar]
- 86.Samson HH, Chappell A. Muscimol injected into the medial prefrontal cortex of the rat alters ethanol self-administration. Physiol Behav. 2001;74:581–587. doi: 10.1016/s0031-9384(01)00607-2. [DOI] [PubMed] [Google Scholar]
- 87.Selim M, Bradberry CW. Effect of ethanol on extracellular 5-HT and glutamate in the nucleus accumbens and prefrontal cortex: Comparison between the Lewis and Fischer 344 rat strains. Brain Res. 1996;716:157–164. doi: 10.1016/0006-8993(95)01385-7. [DOI] [PubMed] [Google Scholar]
- 88.Homayoun H, Moghaddam B. Bursting of prefrontal cortex neurons in awake rats is regulated by metabotropic glutamate 5 (mGlu5) receptors: Rate-dependent influence and interaction with NMDA receptors. Cereb Cortex. 2006;16:93–105. doi: 10.1093/cercor/bhi087. [DOI] [PubMed] [Google Scholar]
- 89.Pratt WE, Mizumori SJ. Neurons in rat medial prefrontal cortex show anticipatory rate changes to predictable differential rewards in a spatial memory task. Behav Brain Res. 2001;123:165–183. doi: 10.1016/s0166-4328(01)00204-2. [DOI] [PubMed] [Google Scholar]
- 90.Ishikawa A, Ambroggi F, Nicola SM, Fields HL. Dorsomedial prefrontal cortex contribution to behavioral and nucleus accumbens neuronal responses to incentive cues. J Neurosci. 2008;28:5088–5098. doi: 10.1523/JNEUROSCI.0253-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: A role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- 92.Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 93.Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 94.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- 95.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: A comparison of efficacy and side-effect profiles. Psychopharmacology. 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- 97.Paterson NE, Markou A. The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology. 2005;179:255–261. doi: 10.1007/s00213-004-2070-9. [DOI] [PubMed] [Google Scholar]
- 98.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 99.van der Kam EL, de Vry J, Tzschentke TM. Effect of 2-methyl-6-(phenylethynyl) pyridine on intravenous self-administration of ketamine and heroin in the rat. Behav Pharmacol. 2007;18:717–724. doi: 10.1097/FBP.0b013e3282f18d58. [DOI] [PubMed] [Google Scholar]
- 100.Backstrom P, Hyytia P. Involvement of AMPA/kainate, NMDA, and mGlu5 receptors in the nucleus accumbens core in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2007;192:571–580. doi: 10.1007/s00213-007-0753-8. [DOI] [PubMed] [Google Scholar]
- 101.O'Leary DM, Movsesyan V, Vicini S, Faden AI. Selective mGluR5 antagonists MPEP and SIB-1893 decrease NMDA or glutamate-mediated neuronal toxicity through actions that reflect NMDA receptor antagonism. Br J Pharmacol. 2000;131:1429–1437. doi: 10.1038/sj.bjp.0703715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heidbreder CA, Bianchi M, Lacroix LP, Faedo S, Perdona E, Remelli R, et al. Evidence that the metabotropic glutamate receptor 5 antagonist MPEP may act as an inhibitor of the norepinephrine transporter in vitro and in vivo. Synapse. 2003;50:269–276. doi: 10.1002/syn.10261. [DOI] [PubMed] [Google Scholar]
- 103.Mathiesen JM, Svendsen N, Brauner-Osborne H, Thomsen C, Ramirez MT. Positive allosteric modulation of the human metabotropic glutamate receptor 4 (hmGluR4) by SIB-1893 and MPEP. Br J Pharmacol. 2003;138:1026–1030. doi: 10.1038/sj.bjp.0705159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seeman P, Guan HC. Phencyclidine and glutamate agonist LY379268 stimulate dopamine D2High receptors: D2 basis for schizophrenia. Synapse. 2008;62:819–828. doi: 10.1002/syn.20561. [DOI] [PubMed] [Google Scholar]
- 105.Diaz-Cabiale Z, Vivo M, Del Arco A, O'Connor WT, Harte MK, Muller CE, et al. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci Lett. 2002;324:154–158. doi: 10.1016/s0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 106.Holter SM, Danysz W, Spanagel R. Novel uncompetitive N-methyl-D-aspartate (NMDA)-receptor antagonist MRZ 2/579 suppresses ethanol intake in long-term ethanol-experienced rats and generalizes to ethanol cue in drug discrimination procedure. J Pharmacol Exp Ther. 2000;292:545–552. [PubMed] [Google Scholar]
- 107.June HL, Foster KL, McKay PF, Seyoum R, Woods JE, Harvey SC, et al. The reinforcing properties of alcohol are mediated by GABA(A1) receptors in the ventral pallidum. Neuropsychopharmacology. 2003;28:2124–2137. doi: 10.1038/sj.npp.1300239. [DOI] [PubMed] [Google Scholar]
- 108.Ventura R, De Carolis D, Alcaro A, Puglisi-Allegra S. Ethanol consumption and reward depend on norepinephrine in the prefrontal cortex. Neuroreport. 2006;17:1813–1817. doi: 10.1097/01.wnr.0000239964.83566.75. [DOI] [PubMed] [Google Scholar]
- 109.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: Target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 110.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 111.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: Comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 113.Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: Blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


