Abstract
The increased use of silver nanoparticles in consumer and medical products has led to elevated human and environmental exposures. Silver nanoparticles act as antibacterial/antifungal agents by releasing Ag+ and recent studies show that Ag+ impairs neural cell replication and differentiation in culture, suggesting that in vivo exposures could compromise neurodevelopment. To determine whether Ag+ impairs development in vivo, we examined the effects of exposure on survival, morphological, and behavioral parameters in zebrafish embryos and larvae.
Methods
We exposed zebrafish from 0–5 days post-fertilization to concentrations of Ag+ ranging from 10 nM to 100 µM in order to assess effects on survival and early embryonic development. We then tested whether concentrations below the threshold for dysmorphology altered larval behavior and subsequent survival. Ag+ concentrations ≥3 µM significantly reduced embryonic survival, whereas 1 µM delayed hatching with no effect on survival. Reducing the concentration to as low as 0.1 µM delayed the inflation of the swim bladder without causing gross dysmorphology or affecting hatching. At this concentration, swimming activity was impaired, an effect that persisted past the point where swim bladder inflation became normal; in contrast, general motor function was unaffected. The early behavioral impairment was then predictive of subsequent decreases in survival. Ag+ is a developmental toxicant within concentrations only slightly above allowable levels. At low concentrations, Ag+ acts as a neurobehavioral toxicant even in the absence of dysmorphology.
Keywords: Developmental toxicity/neurotoxicity, Silver, Zebrafish
INTRODUCTION
Silver, a heavy metal historically found at low concentrations in environmental and human populations, is rapidly being incorporated into new consumer and medical products as nanoparticles (AgNPs) [8]. This has already led to higher human and environmental exposures, and levels are predicted to increase even further [5,8]. AgNPs are designed to release monovalent silver (Ag+) to provide an antimicrobial/antifungal effect [9,11], and while exposure to heavy metals is detrimental at any life stage, developmental periods are especially vulnerable to such toxic insults [37]. There are reasons to suspect that developmental Ag+ exposure may be especially problematic since it evokes antimicrobial effects by inhibiting DNA synthesis and cell replication [13], by elevating oxidative stress [21], and by interfering with ion homeostasis and electron transport required for energy utilization [7,33]. All of these processes are known targets for toxicants that affect embryonic development and especially those that damage the developing nervous system, which has heightened vulnerability relative to the rest of the organism [6,12,14,25]. Developmental exposure of rodents to high Ag+ concentrations (250 mg/kg AgCl per day, given to the pregnant dam p.o., e.g. mM range) disrupts embryo morphology and results in neonatal death [34]; yet, little is known about persistent effects of lower, nonlethal exposures. Importantly, even apparently nontoxic exposures lead to Ag+ accumulation in the brain [29], suggesting a specific targeting of the central nervous system. In the current study, we have performed some of the first studies to explore whether developmental Ag+ exposure below the threshold for dysmorphology produces neurobehavioral deficits.
We made our evaluations in the zebrafish, a model of vertebrate development that is increasingly important for studies of toxicant effects that impact environmental and human populations [22]. Zebrafish show effects of toxicants and pharmaceuticals similar to those found in mammals, supporting the growing use of zebrafish as a model to identify agents which pose a risk to human health and the environment [18,26]. Just as in rodents, high concentrations of Ag+ produce mortality in zebrafish, with a 48h LD50 of 92 µM [16]. Similarly, high concentrations of AgNPs decrease survival, delay hatching, and evoke dysmorphology in embryonic zebrafish [2,3,16]; however, it is not yet clear whether these reflect the actions of Ag+ released by the AgNPs, nor did these reports deal with longer-term effects from low-level exposures, ones that are highly relevant to environmental impact. Behavioral assessments provide particularly sensitive endpoints to reveal the heightened vulnerability of the developing nervous system [6] and here, we used the zebrafish to contrast the dose-effect relationships for survival, dysmorphology, and behavioral measures of Ag+ exposure.
MATERIALS AND METHODS
Reagents
Both silver nitrate (AgNO3) and sodium nitrate (NaNO3) were purchased from Sigma Chemical Co. (St. Louis, MO). AgNO3 was stored in a light-tight container to avoid photooxidation to Ag2+. Test concentrations were prepared in 30% Danieau’s Solution: 58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5 mM Hepes, pH 7.2.
Animals
All experiments were carried out humanely and with regard for alleviation of suffering, with protocols approved by the Duke University Institutional Animal Care and Use Committee. Adult wild-type zebrafish of the AB* strain were bred in our facility and maintained in deionized water containing final concentrations of 0.013% SeaChem (SeaChem Laboratories Inc., Madison, GA) and 0.05% InstantOcean (InstantOcean, Cincinnati, OH). Fish were kept on a 14/10 h light/dark cycle at 28.5 °C, with continuous fluid recirculation. In each experiment, embryos were obtained by covering glass dishes with a plastic mesh and placing them into tanks the evening prior to embryo collection. Twelve to 18 tanks containing 10 to 25 fish per tank were used in each experiment. Dishes were removed from tanks 1.5 h after the start of embryo fertilization to standardize the timing. After removal from the tank, embryos were rinsed thoroughly with, and maintained in 30% Danieau’s Solution; embryos and larval fish were kept under the same temperature and lighting conditions as adults. For larval testing, fish were removed from the exposure solutions at 5 days post-fertilization (dpf). Water was changed daily from 5–14 dpf and larvae were fed GP Reef and Larval Diet (Brine Shrimp Direct, Ogden, UT) and baby brine shrimp (Great Salt Lake Brine Shrimp, Salt Lake City, UT). After 14 dpf, animals were housed as described for adults.
Embryonic toxicity
All embryos were examined under a light microscope for proper cell division and general health at 3.5 h post-fertilization (hpf) as described previously [20]. Healthy embryos were placed into 60-mm dishes containing 12-ml of test solution for exposure, with each experiment constituting a total of 10 to 20 embryos per dish and two dishes per treatment group; within a given experiment, the same number of embryos was included for each treatment group. Treatment was initiated at 4 hpf by introducing test solutions containing H2O, AgNO3 or NaNO3. The solutions were replaced at 24 h intervals through 5 dpf. Each experiment contained duplicate dishes for each treatment and experiments were repeated several times. Embryos were imaged using either an Axiovert 100 TV microscope or Nikon AZ100 microscope. Dead embryos were removed on a daily basis.
Larval testing
Embryos were treated as already described and were raised in 12-well dishes with one fish per well. For testing, the larvae were placed in fresh solution without the test substances; all testing took place between 13:30 and 16:00 in lighted conditions. Motor function was assessed on 5 and 10 dpf by transferring fish into a 12-well test plate (2 ml fluid volume per well), with each well containing a single fish. Fish were allowed to acclimate to the well for 2 min and then movements were recorded for the ensuing 3 min using a Noldus tracking device (Noldus Information Technology, Wageningen, Netherlands) and Media Cruiser recording software (Canopus Corporation, Kobe, Japan). Fish were then returned to a home 12-well plate until the subsequent test period.
Videos of fish movement were assessed using EthoVision 3.1.14 software (Noldus Information Technology, Wageningen, Netherlands). After we acquired data from each video, tracks of individual wells were assessed for completeness and accuracy of the fish’s path. Where necessary, missing segments of the fish path were filled in using the interpolation tool in EthoVision so that the track matched the fish path in the video recording. The “minimum distance moved” filter was used in order to discriminate between swimming and small vibrations in stationary fish. Computerized results were spot-checked by visual evaluations of swimming distances from the original videotape.
Data analysis
Survival, hatching and swim bladder inflation were assessed using χ2 analysis for observed vs. expected frequencies for all comparisons where the expected frequency was at 5 or greater; where the expected frequency was less than 5, we used Fisher’s Exact Test. The expected frequency was determined from the H2O controls but significant differences due to AgNO3 treatment were also compared to the corresponding NaNO3 group. Significance for these parameters was assessed one-tailed, since exposures were expected only to impair or delay the developmental indices. Swimming distances were compiled as means and standard errors, with treatment comparisons carried out by two-factor ANOVA (treatment, age) followed by Fisher’s Protected Least Significant Difference Test for post-hoc comparisons of individual treatments; for these tests, p-values were assessed two-tailed, since activity measures could be either decreased or increased by the treatments. Significance for all tests was assumed at p < 0.05.
RESULTS
Embryonic toxicity
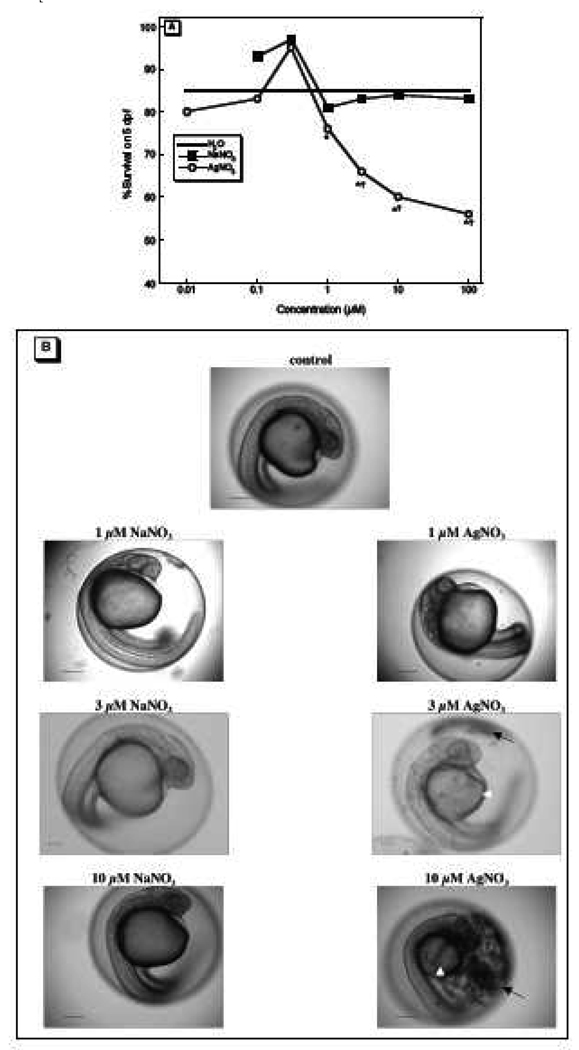
We examined a large Ag+ concentration range (10 nM to 100 µM) to determine the threshold for effects on embryonic survival and dysmorphology, exposing zebrafish from 4 hpf through 5 dpf. Survival was impaired at concentrations of 3 µM and higher (Figure 1A); although we also saw a significant difference between 1 µM Ag+ and the H2O controls, the Ag+ effect at this concentration was not significant from the corresponding NaNO3 group. We also observed dysmorphology with the same threshold (Figure 1B). The affected embryos were smaller, had dark aggregates in the chorion (likely to be condensed yolk sac protein or precipitates of Ag compounds such as Ag2S), and exhibited a white pigmentation compared to the translucent controls. Importantly, these effects were not due to NO3, since the NaNO3 group showed no signs of dysmorphology. The dose-effect relationship for delayed hatching showed a lower threshold (Figure 2A): by 3 dpf, only 81% of embryos exposed to 1 µM Ag+ hatched compared to 98% of H2O controls, with fewer embryos hatched as the concentration was raised further; raising the concentration to 100 µM exceeded the threshold for adverse effects of NO3, since the NaNO3 group also showed delayed hatching at that concentration. Nevertheless, embryos exposed to 1 or 3 µM Ag+ did hatch by 4 dpf, indicating that the exposure only delayed hatching, rather than compromising viability. The delay in hatching was accompanied by persistent dysmorphology with continued formation of aggregrates in the chorion and decreased embryo size, effects that were pronounced at 10 µM Ag+ and higher (Figure 2B).
Figure 1.
Effects of Ag+ on embryonic survival and on morphology at 1 dpf: (A) survival after exposure on 0–5 dpf (B) representative micrographs showing embryo morphology at 1 dpf. The number of fish used in each group were as follows: 204 for H2O; NaNO3, 40 at 0.1 µM, 39 at 0.3 µM, 64 at 1 µM, 64 at 3 µM, 100 at 10 µM, 60 at 100 µM; AgNO3, 40 at 0.01 µM, 80 at 0.1 µM, 40 at 0.3 µM, 124 at 1 µM, 64 at 3 µM, 122 at 10 µM, 100 at 100 µM. In (A), the solid line shows the survival for control embryos (H2O); asterisks denote treatments significantly different from control (χ2 analysis) and daggers denote treatments significantly different from NaNO3. In (B), white arrow heads point to decreased yolk size and black arrows point to aggregates forming in the chorion. Scale bar = 100 µm.
Figure 2.
Effects of Ag+ on hatching and on morphology at 3 dpf: (A) percent of total embryos hatched on 3 dpf (left) and 4 dpf (right); (B) representative micrographs showing embryo morphology at 3 dpf. The number of fish in each group on 3 dpf and 4 dpf were as follows: 289 and 287 for H2O; NaNO3, 56 and 55 at 0.03 µM, 60 and 60 at 0.1 µM, 129 and 127 at 0.3 µM, 52 and 52 at 1 µM, 56 and 53 at 3 µM, 87 and 84 at 10 µM, 50 and 50 at 100 µM; AgNO3, 32 and 32 at 0.01 µM, 56 and 56 at 0.03 µM, 143 and 143 at 0.1 µM, 149 and 149 at 0.3 µM, 81 and 83 at 1 µM, 43 and 46 at 3 µM, 74 and 79 at 10 µM, 57 and 64 at 100 µM. In (A), the solid line shows the values for control embryos (H2O); asterisks denote treatments significantly different from control (χ2 test or Fisher’s Exact Test; see Materials and Methods) and daggers denote where the AgNO3 group is significantly different from NaNO3. In (B), note the lack of hatching in the AgNO3 groups at 1 µM or above and the much larger scale for the 10 µM group. Scale bar = 100 µm.
At concentrations below the threshold for these impairments, we noted a more subtle effect on the timing of swim bladder inflation (Figure 3). In controls, inflation was nearly complete by 4 dpf, whereas exposure to 0.1 or 0.3 µM Ag+ delayed inflation in a substantial proportion of the population. Inflation in larvae exposed to 0.1 µM Ag+ was indistinguishable from controls by 6 dpf while those exposed to 0.3 µM did not achieve complete inflation until 9 dpf. Sodium nitrate had no significant effect on swim bladder inflation and the effects of AgNO3 were equally distinguishable from this additional control group.
Figure 3.
Effects of Ag+ on swim bladder inflation. The number of fish used in each group were as follows: 101 for H2O; NaNO3, 24 at 0.1 µM, 77 at 0.3 µM; AgNO3, 24 at 0.1 µM, 98 at 0.3 µM. Asterisks denote treatments significantly different from H2O (χ2 test or Fisher’s Exact Test; see Materials and Methods) and daggers denote where the AgNO3 group is significantly different from NaNO3.
Embryonic and larval behavior
In embryonic stages from 1dpf to hatching, we observed spontaneous movement each day for each embryo, over a 20 sec period, as well as the response to touching the embryos with a metal probe; compared to the H2O control group, embryos exposed to 0.1 µM NaNO3, 0.1 µM AgNO3 or 0.3 µM AgNO3 were indistinguishable (not significant by χ2 analysis, categorizing responses as “moved” or “didn’t move;” 23–24 fish per group, data not shown). These observations were repeated in larvae from hatching to 10 dpf with the same results. Despite the lack of an underlying deficit in motor function, exposure to either 0.1 or 0.3 µM Ag+ altered larval swimming performance. We measured the distance larvae swam on 5 and 10 dpf to span the time points where swim bladder inflation was significantly impaired, and where it became comparable to controls. There was a significant decrease in the distance swum in the Ag+ group at both 5 and 10 dpf, effects which were again distinct from the lack of effect seen with comparable concentrations of NaNO3 (Figure 4A). Thus, while swim bladder inflation recovered by 6 dpf or 9 dpf, behavioral deficits persisted.
Figure 4.
Effects of AgNO3 on larval swimming behavior and survival: (A) distance swum on 5 dpf and 10 dpf after AgNO3 exposure 0–5 dpf, (B) percentage of fish surviving through 40 dpf. In (A) data represent means and standard errors. The number of fish used in each group on 5 and 10 dpf were as follows: 48 and 40 for H2O; NaNO3, 24 and 21 at 0.1 µM, 23 and 19 at 0.3 µM; AgNO3, 24 and 20 at 0.1 µM, 49 and 38 at 0.3 µM. ANOVA across all treatments is shown at the top of the panel; asterisks below the x-axis denote treatments significantly different from H2O and daggers denote where the AgNO3 group is significantly different from NaNO3. Abbreviation: NS, not significant. In (B) the number of fish used in each group were as follows: 24 for H2O; 23 for NaNO3; 23 for 0.1 µM AgNO3, 24 for 0.3 µM AgNO3. Asterisks denote treatments significantly different from H2O (χ2 test or Fisher’s Exact Test; see Materials and Methods) and daggers denote where the AgNO3 group is significantly different from NaNO3.
Finally, we followed the survival of larvae exposed to 0.1 or 0.3 µM Ag+ after the conclusion of behavioral testing. Neither concentration affected survival up to 10 dpf but there was a subsequent decrease in the Ag+-exposed groups relative to either the H2O or NaNO3 controls over the ensuing month (Figure 4B). To some extent, though, the effect represented an acceleration of the natural attrition rate. When we examined survival at about three months of age, the H2O control group exhibited 32% survival and the figure for the 0.3 µM NaNO3 group was 40%; the value for the the group exposed to 0.1 µM AgNO3 was similar (30%) but the fish exposed to the higher, 0.3 µM AgNO3 concentration was still significantly lower (11%).
DISCUSSION
To our knowledge, this paper presents the first assessment of the neurobehavioral teratology of Ag+ in the zebrafish model. We found that low-concentrations of Ag+, which are below the threshold for dysmorphology or effects on embryonic viability, nevertheless alter swimming performance. While the behavioral changes were preceded by delayed swim bladder inflation, they persisted beyond the point where this parameter became normal, indicating that these are separate phenomena. Further, the behavioral changes were predictive of decreased survival later in life, suggesting that developmental Ag+ exposure has persistent effects not immediately observable during embryonic development. Such subtle effects are in clear contrast with those seen at progressively higher concentrations, which delayed hatching, decreased embryonic survival and evoked frank dysmorphology. Thus, developmental Ag+ exposure has effects in vivo that are highly concentration-dependent, with apparently lower, otherwise nontoxic exposures resulting in behavioral impairments and ultimately compromising long-term survival.
Embryonic exposure to Ag+ at 3 µM or higher concentrations clearly decreased embryo viability and altered morphology. These findings recapitulate the effects of high Ag+ exposure in rats [34], further supporting the zebrafish as a complementary model for evaluations of developmental toxicity. The gross impairment of development is not unexpected, given the ability of Ag+ to compromise DNA and protein synthesis and to evoke oxidative stress, as demonstrated in mammalian neural cells [27] and in prokaryotes [13,21]. Reducing the concentration to 1 µM did not affect embryonic survival but still delayed zebrafish hatching, indicating that Ag+ can elicit more subtle effects on development. Although the specific mechanisms for this effect have yet to be identified, it is notable that similar delays in hatching occur when neurotransmitter levels are affected, specifically involving excess dopamine [32]. Our work in PC12 cells [27] similarly shows that Ag+ exposure can promote expression of the dopaminergic phenotype at the expense of the cholinergic phenotype; if this happens in vivo, the neurotransmitter effect could then lead directly to altered hatching. Clearly, studies quantifying dopamine levels are needed to prove this relationship. Alternatively, or perhaps in combination with a neural effect, Ag+ may interfere with the enzymatic action of chorinase, the protease critical for hatching to occur [17], since Ag+ readily binds thiol groups present in most enzymes [24] and thereby interferes with protein function. Interestingly, others found that Cu+ directly inhibits chorinase activity in rainbow trout and impairs hatching in zebrafish [17,19]; given that Cu+ transporters take up Ag+ [4], the two metals may bind to other proteins in a similar fashion, allowing Ag+ to impair chorinase. Finally, although Ag+ did not interfere with spontaneous embryonic movement at the lower concentrations, it is possible that higher concentrations might do so, which would also interfere with hatching. In any case, the delay in hatching is consistent with the view that Ag+ targets critical developmental events even at a concentration that does not alter embryonic survival or produce other signs of gross toxicity.
Below the concentrations that affected hatching, we found a delay in swim bladder inflation. Recent work points to cholinergic signaling as a key step in this developmental event [28], again suggesting involvement of neurodevelopmental disruption. Indeed, our findings in PC12 cells indicate that Ag+ suppresses expression of the cholinergic phenotype, which would be consistent with this explanation. Regardless of the mechanism, this could have serious consequences for fish populations, since swim bladder inflation shortly after hatching is thought to decrease energy demand by minimizing the mass-to-volume ratio of larvae as they begin swimming up to the water surface to find food [28].
However, our most notable finding was that low concentrations of Ag+ decreased behavioral performance in the swim test on both 5 dpf and 10 dpf, with the latter point well past the end of Ag+ exposure and at a stage when swim bladder inflation was completed in the exposed fish. Given that there was no underlying motor incapacity and that the Ag+-exposed group responded normally to touch, it is highly likely that Ag+ alters the development of the central circuits governing swimming. Again, this is consistent with recent work showing that developmental exposures to chemicals or pharmaceuticals that alter neurotransmitter levels in the brain result in decreased motor activity [10,23,30,31,35]. We are currently acquiring data to assess neurotransmitter levels after developmental Ag+ exposure so as to confirm this relationship. Importantly, the behavioral changes we observed on 5 and 10 dpf were an early indicator of subsequent, impaired survival, either accelerating natural attrition (0.1 µM Ag+) or compromising the net survival rate (0.3 µM Ag+); whether these differences lie in further defects in swim bladder maturation [28], defects in the development or functioning of other organs, or continued behavioral or functional impairments remains to be seen.
Based on the acute toxicity of Ag+ in adult fish populations, the U.S. EPA has set exposure limits of 2–3 µg/L [15,36]. Although the lowest concentration we studied was higher (0.1 µM = 11 µg/L), we did not identify the no observed adverse effect level, which of course will be lower. Further, our assessment was conducted at a substantially lower concentration than that required for acute toxicity in adult zebrafish, 22 µg/L [16], pointing to the heightened vulnerability of the developing organism; developmental differences were apparently not considered in the setting the exposure limits. Our findings point to the likelihood of adverse developmental effects at otherwise “acceptable” environmental concentrations. Indeed, the presence of salts which can complex Ag+ in the 30% Danieau’s Solution used for our studies, makes it probable that the actual exposure concentrations were substantially lower than the nominal concentrations added to the wells; such complexation probably occurs in the environment [15] but in any case, our results point to the need to consider damage resulting from low levels of free Ag+ released by AgNPs. Perhaps most importantly, though, our results corroborate our earlier in vitro findings that low-level Ag+ impairs neurodevelopment in a standard mammalian neural cell model [27]. Given that the current limit for Ag in drinking water is 100 µg/L (≈1 µM) [1], the continued expansion of the use of AgNPs may increase human exposures beyond the threshold for adverse neurodevelopmental effects.
In conclusion, our results with zebrafish indicate that the adverse neurodevelopmental effects of Ag+ exposure first identified with in vitro models [27], also extend to developing organisms in vivo. We found clear evidence of behavioral impairment at exposures below the threshold for overt embryotoxicity or delayed hatching, and independent of delays in swim bladder inflation. In turn, the compromise of behavioral function was predictive of mortality later in life. We are currently conducting studies comparing the effects of Ag+, the bioeffective ion released by AgNPs, with those elicited by the AgNPs themselves.
Acknowledgments/disclaimers
Research was supported by NIH ES10356 and GM007105. The authors thank Susan Donerly for helpful advice. The authors state that they have no conflicts of interest. TAS has provided expert witness testimony in the past three years at the behest of the following law firms: The Calwell Practice (Charleston WV), Frost Brown Todd (Charleston WV), Weltchek Mallahan & Weltchek (Lutherville MD), Finnegan Henderson Farabow Garrett & Dunner (Washington DC), Frommer Lawrence Haug (Washington DC), Carter Law (Peoria IL), Corneille Law (Madison WI), Angelos Law (Baltimore MD), Kopff, Nardelli & Dopf (New York NY), Gutglass Erickson Bonville & Larson (Madison WI) and Pardieck Law (Seymour IN).
Abbreviations
- ANOVA
analysis of variance
- dpf
days post-fertilization
- hpf
hours post-fertilization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry. Silver CAS #7440-22-4. 1999. [Google Scholar]
- 2.Asharani P, Wu Y, Gong Z, Valiyaveettil S. Toxicity of silver nanoparticles in zebrafish models. Nanotechnology. 2008;19:255102–255110. doi: 10.1088/0957-4484/19/25/255102. [DOI] [PubMed] [Google Scholar]
- 3.Bar-Ilan O, Albrecht RM, Fako VE, Furgeson DY. Toxicity assessments of multisized gold and silver nanoparticles in zebrafish embryos. Small. 2009;5:1897–1920. doi: 10.1002/smll.200801716. [DOI] [PubMed] [Google Scholar]
- 4.Bertinato J, Swist E, Plouffe LJ, Brooks SP, L'Abbé MR. Ctr2 is partially localized to the plasma membrane and stimulates copper uptake in COS-7 cells. Biochem J. 2008;409:731–740. doi: 10.1042/BJ20071025. [DOI] [PubMed] [Google Scholar]
- 5.Blaser SA, Scheringer M, Macleod M, Hungerbuhler K. Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ. 2008;390:396–409. doi: 10.1016/j.scitotenv.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Bondy SC, Campbell A. Developmental neurotoxicology. J Neurosci Res. 2005;81:605–612. doi: 10.1002/jnr.20589. [DOI] [PubMed] [Google Scholar]
- 7.Bragg PD, Rainnie DJ. The effect of silver ions on the respiratory chain of Escherichia coli. Can J Microbiol. 1974;20:883–889. doi: 10.1139/m74-135. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Damm C, Munstedt H, Rosch A. The antimicrobial efficacy of polyamide 6/silver-nano- and microcomposites. Mater Chem Physics. 2008;108:61–66. [Google Scholar]
- 10.Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evanoff DDJ, Chumanov G. Synthesis and optical properties of silver nanoparticles and arrays. Chemphyschem. 2005;6:1221–1231. doi: 10.1002/cphc.200500113. [DOI] [PubMed] [Google Scholar]
- 12.Fantel AG. Reactive oxygen species in developmental toxicity: review and hypothesis. Teratology. 1996;53:196–217. doi: 10.1002/(SICI)1096-9926(199603)53:3<196::AID-TERA7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J Biomed Mater Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Finnell RH, Waes JG, Eudy JD, Rosenquist TH. Molecular basis of environmentally induced birth defects. Annu Rev Pharmacol Toxicol. 2002;42:181–208. doi: 10.1146/annurev.pharmtox.42.083001.110955. [DOI] [PubMed] [Google Scholar]
- 15.Ford L. Development of chronic aquatic water quality criteria and standards for silver. Water Environ Res. 2001;73:248–253. doi: 10.2175/106143001x139245. [DOI] [PubMed] [Google Scholar]
- 16.Griffitt RJ, Luo J, Gao J, Bonzongo JC, Barber DS. Effects of particle composition and species on toxicity of metallic nanomaterials in aquatic organisms. Environ Toxicol Chem. 2008;27:1972–1978. doi: 10.1897/08-002.1. [DOI] [PubMed] [Google Scholar]
- 17.Hagenmaier HE. The hatching process in fish embryos. IV. The enzymological properties of a highly purified enzyme (chorionase) from the hatching fluid of the rainbow trout, Salmo gairdneri Rich. Comp Biochem Physiol B. 1974;49:313–324. doi: 10.1016/0305-0491(74)90166-7. [DOI] [PubMed] [Google Scholar]
- 18.Irons TD, Macphail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicol Teratol. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- 19.Johnson A, Carew E, Sloman KA. The effects of copper on the morphological and functional development of zebrafish embryos. Aquat Toxicol. 2007;84:431–438. doi: 10.1016/j.aquatox.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 21.Le Pape H, Solano-Serena F, Contini P, Devillers C, Maftah A, Leprat P. Involvement of reactive oxygen species in the bactericidal activity of activated carbon fibre supporting silver; Bactericidal activity of ACF(Ag) mediated by ROS. J Inorg Biochem. 2004;98:1054–1060. doi: 10.1016/j.jinorgbio.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 22.Lele Z, Krone PH. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol Adv. 1996;14:57–72. doi: 10.1016/0734-9750(96)00004-3. [DOI] [PubMed] [Google Scholar]
- 23.Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Liau SY, Read DC, Pugh WJ, Furr JR, Russell AD. Interaction of silver nitrate with readily identifiable groups: relationship to the antibacterial action of silver ions. Lett Appl Microbiol. 1997;25:279–283. doi: 10.1046/j.1472-765x.1997.00219.x. [DOI] [PubMed] [Google Scholar]
- 25.Mattson MP, Gleichmann M, Cheng A. Mitochondria in neuroplasticity and neurological disorders. Neuron. 2008;60:748–766. doi: 10.1016/j.neuron.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parng C, Roy NM, Ton C, Lin Y, McGrath P. Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods. 2007;55:103–112. doi: 10.1016/j.vascn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Powers CM, Wrench N, Ryde IT, Smith AM, Seidler FJ, Slotkin TA. Silver impairs neurodevelopment: studies in PC12 cells. Environ Health Perspect. 2010;118:73–79. doi: 10.1289/ehp.0901149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robertson GN, McGee CA, Dumbarton TC, Croll RP, Smith FM. Development of the swimbladder and its innervation in the zebrafish, Danio rerio. J Morphol. 2007;268:967–985. doi: 10.1002/jmor.10558. [DOI] [PubMed] [Google Scholar]
- 29.Rungby J, Danscher G. Neuronal accumulation of silver in brains of progeny from argyric rats. Acta Neuropathol. 1983;61:258–262. doi: 10.1007/BF00691995. [DOI] [PubMed] [Google Scholar]
- 30.Sallinen V, Sundvik M, Reenila I, Peitsaro N, Khrustalyov D, Anichtchik O, Toleikyte G, Kaslin J, Panula P. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J Neurochem. 2009;109:403–415. doi: 10.1111/j.1471-4159.2009.05986.x. [DOI] [PubMed] [Google Scholar]
- 31.Sallinen V, Torkko V, Sundvik M, Reenila I, Khrustalyov D, Kaslin J, Panula P. MPTP and MPP+ target specific aminergic cell populations in larval zebrafish. J Neurochem. 2009;108:719–731. doi: 10.1111/j.1471-4159.2008.05793.x. [DOI] [PubMed] [Google Scholar]
- 32.Schoots AF, Meijer RC, Denuce JM. Dopaminergic regulation of hatching in fish embryos. Dev Biol. 1983;100:59–63. doi: 10.1016/0012-1606(83)90200-2. [DOI] [PubMed] [Google Scholar]
- 33.Schreurs WJ, Rosenberg H. Effect of silver ions on transport and retention of phosphate by Escherichia coli. J Bacteriol. 1982;152:7–13. doi: 10.1128/jb.152.1.7-13.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shavlovski MM, Chebotar NA, Konopistseva LA, Zakharova ET, Kachourin AM, Vassiliev VB, Gaitskhoki VS. Embryotoxicity of silver ions is diminished by ceruloplasmin — further evidence for its role in the transport of copper. Biometals. 1995;8:122–128. doi: 10.1007/BF00142011. [DOI] [PubMed] [Google Scholar]
- 35.Thirumalai V, Cline HT. Endogenous dopamine suppresses initiation of swimming in prefeeding zebrafish larvae. J Neurophysiol. 2008;100:1635–1648. doi: 10.1152/jn.90568.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.U.S. Environmental Protection Agency. Water Quality Criteria. 2009 [Google Scholar]
- 37.Zelikoff JT, Bertin JE, Burbacher TM, Hunter ES, Miller RK, Silbergeld EK, Tabacova S, Rogers JM. Health risks associated with prenatal metal exposure. Fundam Appl Toxicol. 1995;25:161–170. doi: 10.1006/faat.1995.1052. [DOI] [PubMed] [Google Scholar]