SUMMARY
T cells can reject established tumors when adoptively transferred into patients, thereby demonstrating that the immune system can be harnessed for cancer therapy. However, such passive immunotherapy is unlikely to maintain memory T cells that might control tumor outgrowth on the long term. Active immunotherapy with vaccines has the potential to induce tumor-specific effector and memory T cells. Vaccines act through dendritic cells (DCs) which induce, regulate and maintain T cell immunity. Clinical trials testing first generation DC vaccines pulsed with tumor antigens provided a proof-of-principle that therapeutic immunity can be elicited. The increased knowledge of the DC system, including the existence of distinct DC subsets is leading to new trials which aim at improved immune and clinical outcomes.
Keywords: dendritic cells, cancer, vaccines, priming
INTRODUCTION
Vaccination relies on dendritic cells (DCs), the professional antigen presenting cells that induce and regulate immune responses. To allow resistance to infection and tolerance to self, DCs have developed functional plasticity and subsets [1]. The two major subsets are the myeloid DCs (mDCs) and the plasmacytoid DCs (pDCs). This diversity permits the adaptive immune system to mount functionally distinct types of responses. In this review, we will briefly summarize our progresses on the topic of DCs and cancer, and discuss how this new knowledge will permit us to design improved cancer vaccines.
DENDRITIC CELLS IN VACCINATION AGAINST CANCER
Ex vivo-generated DCs have been used as therapeutic vaccines in patients with cancer for over a decade and early studies have been discussed in detail elsewhere [2]. While a fraction of patients can experience durable tumor regressions [3], the most common outcome of the current DC vaccination protocols is a demonstration of expanded antigen-specific immunity, most often using IFN-γ ELISPOT, but no durable objective tumor regression.
Altogether, three outcomes emerge from our studies:
1) Patients who do not mount immune response to melanoma antigens presented on DC vaccines. These patients are usually early clinical progressors. Their responses to control antigens such as KLH or viral peptides (Flu-M1 or CMV) are preserved in most cases, indicating that they cannot mount tumor-specific responses. In vitro experiments indicated that T cells of several patients can be primed to differentiate into CTLs with specificity for multiple melanoma antigens [4]. Thus, tumor antigen-specific CD8+ T cells are kept anergic rather than deleted. 2) Patients who show melanoma-antigen specific immunity, but do not experience durable objective tumor regression. The most common outcome of current DC vaccination protocols is the induction of immune responses in the absence of clinical responses. Improved immunomonitoring is expected to provide insights into the mechanisms of immune efficacy as discussed hereunder. 3) Patients who mount immune responses and experience clinical benefit. Vaccination with DCs can elicit therapeutic immunity and our challenge is to identify approaches that will increase the fraction of patients who experience durable tumor regression and/or prolonged survival.
The quality of elicited antigen-specific immune responses
Establishing causative links in clinical studies is a difficult task which often requires large patient cohorts. The current data suggest an association between the tumor-specific CD8+ T cell responses and clinical outcomes. In our view, four critical components will determine whether the induced immune response will be therapeutic: 1) the quality of elicited CTLs; 2) the quality of induced CD4+ helper T cells; 3) the elimination and/or non-activation of Tregs; and 4) the breakdown of immunosuppressive tumor microenvironment.
Indeed, the immune responses elicited by earlier DC vaccines might not be of the quality needed to allow the rejection of the tumors, for example low avidity T cells which are susceptible to suppressive factors and cells within the tumor environment [5]. Furthermore, the induced T cells might not migrate into the tumor lesions [5,6], for example the expression of CXCR3 in blood CD8+ T cells might be necessary, as it appears to correlate with survival in patients with melanoma [7]. Finally, the tumor micro-environment might inhibit effector T cell functions, for example by action of myeloid derived suppressor cells [8] and Tregs [9].
The recent progresses in immunomonitoring of specific immune responses in the blood and at the tumor site should help us address these questions [3,10]. Modern approaches including polychromatic flow cytometry rather than the analysis of a single cytokine (e.g., IFN-γ ELISPOT) and/or frequency of tetramer positive cells will contribute to a better assessment of the quality of the immune responses elicited in the patients. Indeed, several studies, mostly performed in the context of HIV vaccines, have led to the conclusion that a mere measurement of the frequency of IFN-γ secreting CD8+ T cells is insufficient to evaluate the quality of vaccine-elicited immunity [5].
HUMAN MYELOID DENDRITIC CELL SUBSETS
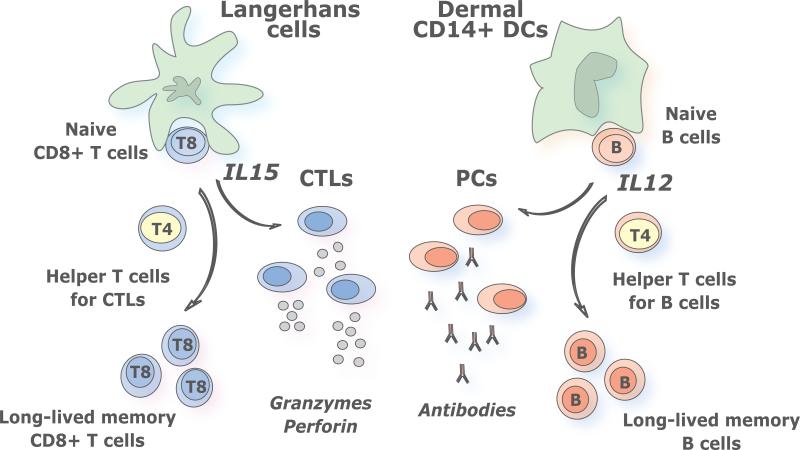
The best studied human mDC subsets are those from skin, where three subsets can be identified. The epidermis hosts only LCs while the dermis displays two mDC subsets, CD1a+ DCs and CD14+ DCs, as well as macrophages [11,12]. LCs and CD14+ DCs differ in their molecular profiles (Figure 1) including cytokines they secrete upon activation. While CD14+ DCs produce a large set of soluble factors including IL-12, LCs produce only a few cytokines, including IL-15 [13]. As we discuss hereunder, this distinct cytokine secretion pattern might contribute to the unique biological functions of these DC subsets.
Figure 1. Distinct subsets of myeloid dendritic cells induce distinct types of immune responses.
Human LCs induce potent CTL responses, possibly via IL-15: At least four lines of evidence indicate that LCs are remarkable at inducing CTL responses: 1) LCs loaded with an HLA class I peptide potently induce the proliferation of peptide-specific naïve CD8+ T cells; 2) LCs expand naïve CD8+ T cells with high avidity against peptide/HLA-I complex; 3) Naive CD8+ T cells primed by LCs express high levels of cytotoxic molecules, such as granzymes A, B, and perforin, and display a high cytotoxicity; and 4) LCs are efficient at cross-presentation of antigens. Human CD14+ dermal DCs induce potent humoral responses via IL-12: When DCs form the complex with T cells and B cells at extrafollicular sites, IL-12 derived from activated DCs promotes B cells to differentiate into ASCs by two different paths: a direct path via DC-B interaction, and an indirect path through induction of IL-21-producing Tfh-like cells. We envision that targeting antigens and activation of distinct mDC subsets, with different specializations, will result in the generation of a broad and long lived immune protection. Thus, the most efficient vaccines might be those that will target both LCs and dermal CD14+ DCs thereby allowing the maximal stimulation of cellular and humoral immune responses and the generation of long-term memory protection.
Dermal DCs, antibody responses and IL-12
In the mid 90's, we observed that CD14+ DCs derived from CD34+ hematopoietic progenitor cells (HPCs) induce CD40-activated naïve B cells to differentiate into IgM-producing plasma cells through the secretion of IL-6 and IL-12 [14]. A decade later, we found that CD14+ DCs induce naïve CD4+ T cells to differentiate into cells with properties of T follicular helper cells (Tfh), a CD4+ T cell subset specialized in B cell help [15]. There, CD4+ T cells primed by CD14+ DCs help naïve B cells to produce large amounts of IgM, and switch isotypes towards IgG and IgA. This ability to regulate B cell differentiation appears unique to CD14+ DCs, as LCs are unable to do so. Acquisition of Tfh phenotype and function depends on IL-12p70, which endows activated CD4+ T cells with the capacity to help the differentiation of antibody secreting cells (ASCs) via IL-21 [16].
Thus, IL-12 appears to contribute to humoral immunity in humans through two different paths: a direct path in DC-B interaction, and an indirect path through DC-T cell interaction and induction of Tfh cells (Figure 1). These two paths might act simultaneously in vivo, through the “ménage à trois” formation of antigen-presenting DCs with antigen-specific T cells and B cells at extrafollicular sites, as recently illustrated through in vivo imaging in mice [17].
These findings might explain only very modest clinical efficacy of systemic IL-12 administration in cancer patients [18,19]. Furthermore, the injection of IL-12 into tumor sites of head and neck cancer patients resulted in the activation of B cells in the draining lymph nodes, which was associated with their infiltration into tumor sites and tumor regression [20]. Thus, adjuvants that promote the secretion of IL-12 might improve vaccines aimed at induction of neutralizing antibodies in humans, not necessarily those aimed at induction of CD8+ T cell responses.
LCs and CD8+ T cell responses
LCs induce a robust proliferation of naïve allogeneic CD8+ T cells when compared to CD14+ DCs [13]. When pulsed with MHC class I peptides derived from tumor or viral antigens, LCs are far more efficient than CD14+ DCs in the priming of antigen-specific CD8+ T cells. LCs are also efficient in cross-presenting peptides from protein antigens to CD8+ T cells. CD8+ T cells primed by LCs show high avidity in tetramer binding assays and express higher levels of cytotoxic molecules, such as granzymes and perforin. Accordingly, they are remarkably more efficient in killing target cells; in particular tumor cells that express low levels of peptide/HLA complexes [13]. Our preliminary studies suggest that IL-15 might explain the remarkable effects of LCs on the development of CTL responses.
BUILDING ON DENDRITIC CELL SUBSETS TO IMPROVE VACCINES
The results summarized above prompted us to hypothesize that the two different arms of adaptive immunity, i.e., the humoral and cellular arms, are differentially regulated by the two skin mDC subsets. In this view, humoral immunity is preferentially initiated by CD14+ dermal DCs, while cellular immunity is preferentially regulated by LCs (Figure 1).
It has become apparent that the cytokine combinations used to differentiate monocytes into DCs are critical for the quality of the elicited T cell responses (Figure 2). For example, DCs generated with GM-CSF and IL-15 include cells with the phenotype and characteristics of LCs and are far more efficient in vitro in priming melanoma-antigen specific CD8+ T cells than DCs derived with GM-CSF and IL-4 [21,22]. Thus, vaccination with IL15-DCs might yield improved clinical responses and we are initiating such clinical trials. Another critical parameter is the DC activation pathway. For example, IL-4 DCs activated with a cocktail of IFN-α, polyI:C, IL-1β, TNF, and IFN-γ induce up to 40 times more melanoma-specific CTLs in vitro than DCs matured with the “standard” cocktail of IL-1β/TNF/IL-6/prostaglandin E2 (PGE2)[23]. Additional studies will be necessary to establish the therapeutic value of these newer ex vivo generated DC vaccines in patients. These studies are critical to the understanding of the human immune system because they permit us to assess in vivo the type of immune responses elicited by human DCs generated in different cytokine environments.
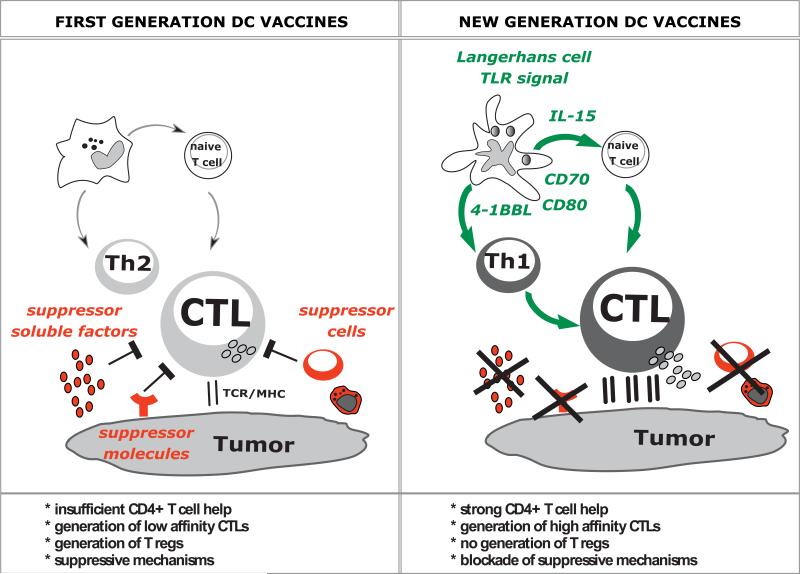
Figure 2. Building on dendritic Cell Subsets to Improve Cancer Vaccines.
First generation vaccines: Early clinical trials with first generation DC vaccines (left panel) showed the induction of immune responses to vaccine antigens. However, the clinical responses are still infrequent. Possible contributing factors can be grouped into: i) vaccine features, and ii) suppressive pathways established by tumors. Vaccine features include: i) insufficient CD4+ T cell help; ii) generation of low affinity CTLs; and iii) generation of T regs. Suppressive pathways involve: i) suppressor cells, both T regs as well as myeloid suppressor cells; ii) suppressor molecules expressed by tumors such as PD-L1; and iii) suppressive factors secreted by tumors, for example TGF-β or VEGF. All these act in concert to inhibit the CTL function including inhibiting the release of cytotoxic effector molecules Granzyme B and perforin.
New generation DC vaccines: Improved next generation DC vaccines will harness Langerhans cells and microbial activation signals leading to: i) secretion of high amounts of cytokines such as IL-12, which will generate strong Th1 response and helper function for generation of memory T cells; and IL-15 which will help generation of high avidity CTLs that might be resistant to tumor microenvironment; and ii) strong costimulation mediated via at least three molecular pathways such as CD80, CD70 and 4-1BB. This in combination with therapies that will permit to eliminate T regs and block tumor microenvironment will results in the full activity of elicited CTLs and tumor rejection.
This in turn is essential to building a novel approach to vaccination based on delivering antigens directly to DCs in vivo using chimeric proteins that are composed of an anti-DC receptor antibody and an antigen (DC targeting). Studies in mice demonstrate that the specific targeting of antigen to DCs in vivo results in considerable potentiation of antigen-specific CD4+ and CD8+ T cell immunity [24,25]. These pioneering studies have been already extended to demonstrate the targeting of tumor antigens to DCs [26] and Langerhans cells (LCs) in animal models [27,28] and the generation of anti-tumor immunity [29]. The therapeutic success of these vaccines will build on the recent knowledge and progresses in our understanding of the biology of human DC subsets, cutaneous myeloid DCs (mDCs) in particular.
Our studies suggest that targeting LCs for antigen delivery will be optimal for the induction of potent antigen-specific CTL response. There, LC-specific molecule, such as Langerin, can be used as a target DC receptor [30]. Dermal CD14+ DCs might represent the appropriate target for the induction of potent humoral responses. Selection of an appropriate adjuvant is also a critical parameter for the induction of the immunity of the desired type. For example, although TLR-ligands are widely considered to promote protective immunity against infectious agents, selecting the appropriate ligand will be critical. For instance, TLR2 ligation, which promotes the induction of Tregs rather than Th1 or Th17 cells [31], does not appear to be a preferred option for cancer vaccines. Furthermore, certain lectins [32-37], including Dectin-1, LOX-1 and DC-SIGN, deliver intracellular signaling to activate DCs. These features of DC-lectins may place them as gatekeepers for controlling the early stage of immune responses. Thus, the challenge is to match the molecular target on DCs with the desired immune outcome, mimicking in many ways the natural role of these DC receptors to fine tune responses appropriate to the infection.
CONCLUDING REMARKS
The considerable progresses made in knowledge of DC biology as well as effector/regulatory T cell biology clearly open the avenues for development of vastly improved clinical protocols (Figure 3). Importantly, rather than the quantity of IFN-γ secreting CD8+ T cells, we should aim at generating high quality and high avidity poly-functional effector CD8+ T cells able to reject tumors and long-lived memory CD8+ T cells able to prevent relapse. The capacity of LCs and CD14+ DCs to preferentially prime, respectively, cellular immunity and humoral immunity has significant implications, most particularly in the context of novel human vaccines. Targeting LCs will be important for the design of vaccines that aim at eliciting strong cellular immunity. Therapeutic vaccination in patients with non-resectable metastatic cancer will require combination therapies. These will be tailored to the patient and to the specific suppressive pathways that the patient displays. These pathways will be determined at the antigen-non-specific level via genomic studies on blood and tumor samples as well as proteomics, and at the antigen-specific level (T regs) using EPIMAX and related methods.
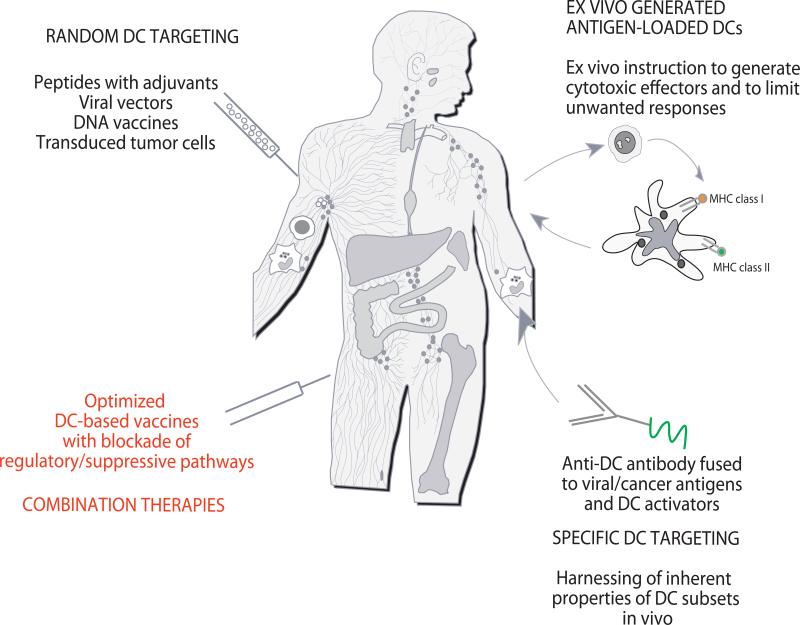
Figure 3. Approaches to DC-based immune intervention in cancer.
1) Vaccines based on antigen with or without adjuvant that target DCs randomly. That might result in vaccine antigens being taken up by a “wrong” type of DCs in the periphery which might lead to “unwanted” type of immune response. Vaccine antigens could also flow to draining lymph nodes where they can be captured by resident DCs; 2) Vaccines based on ex-vivo generated tumor antigen-loaded DCs that are injected back into patients; and 3) specific in vivo DC targeting with anti-DC antibodies fused with antigens and with DC activators. 4) Next generation clinical trials will test combination therapies to offset tumor-induced suppression.
ACKNOWLEDGMENTS
Dedicated to patients and volunteers who participated in our studies. We thank former and current members of the Institute for their contributions. Supported by the NIH (P01 CA084514, U19 AIO57234, R01 CA089440 and CA078846), the Dana Foundation, the Susan Komen Foundation, the Baylor Health Care System; the Baylor Health Care System Foundation, the ANRS and the INSERM. KP holds the Michael A. Ramsay Chair for Cancer Immunology Research. JB holds the Caruth Chair for Transplant Immunology Research.
REFERENCES
- 1.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. [DOI] [PubMed] [Google Scholar]
- 2.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 3.Palucka AK, Ueno H, Connolly J, Kerneis-Norvell F, Blanck JP, Johnston DA, Fay J, Banchereau J. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 4.Berard F, Blanco P, Davoust J, Neidhart-Berard EM, Nouri-Shirazi M, Taquet N, Rimoldi D, Cerottini JC, Banchereau J, Palucka AK. Cross-Priming of Naive CD8 T Cells against Melanoma Antigens Using Dendritic Cells Loaded with Killed Allogeneic Melanoma Cells. J Exp Med. 2000;192:1535–1544. doi: 10.1084/jem.192.11.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5***.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [Outstanding review of the field and the analysis of the desired characteristics of CD8+ T cell immunity in Cancer and HIV] [DOI] [PubMed] [Google Scholar]
- 6**.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [The first demonstration of a link between the signature in the tumor environment and the recruitment of effector T cells] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- 8**.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [Excellent review of the field] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9**.Menetrier-Caux C, Gobert M, Caux C. Differences in tumor regulatory T-cell localization and activation status impact patient outcome. Cancer Res. 2009;69:7895–7898. doi: 10.1158/0008-5472.CAN-09-1642. [The first demonstration that T cell localization within the tumor environment is related to T cell function] [DOI] [PubMed] [Google Scholar]
- 10.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–20889. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 12.Nestle FO, Di Meglio P, Qin JZ, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. 2009;9:679–691. doi: 10.1038/nri2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klechevsky E, Morita R, Liu M, Cao Y, Coquery S, Thompson-Snipes L, Briere F, Chaussabel D, Zurawski G, Palucka AK, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510. doi: 10.1016/j.immuni.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caux C, Massacrier C, Vanbervliet B, Dubois B, Durand I, Cella M, Lanzavecchia A, Banchereau J. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor alpha: II. Functional analysis. Blood. 1997;90:1458–1470. [PubMed] [Google Scholar]
- 15.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmitt N, Morita R, Bourdery L, Bentebibel SE, Zurawski SM, Banchereau J, Ueno H. Human dendritic cells induce the differentiation of interleukin-21-producing T follicular helper-like cells through interleukin-12. Immunity. 2009;31:158–169. doi: 10.1016/j.immuni.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17***.Germain RN, Bajenoff M, Castellino F, Chieppa M, Egen JG, Huang AY, Ishii M, Koo LY, Qi H. Making friends in out-of-the-way places: how cells of the immune system get together and how they conduct their business as revealed by intravital imaging. Immunol Rev. 2008;221:163–181. doi: 10.1111/j.1600-065X.2008.00591.x. [Outstanding review of the field and contributions to the understanding of how the immune responses are initiated] [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Rakhit A, Thompson JA, Nemunaitis J, Murphy BA, Ellerhorst J, Schwartz LH, Berg WJ, Bukowski RM. Randomized multicenter phase II trial of subcutaneous recombinant human interleukin-12 versus interferon-alpha 2a for patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2001;21:257–263. doi: 10.1089/107999001750169934. [DOI] [PubMed] [Google Scholar]
- 19**.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [Excellent review of existing clinical grade drugs that could be exploited for immunotherapy] [DOI] [PubMed] [Google Scholar]
- 20.van Herpen CM, van der Voort R, van der Laak JA, Klasen IS, de Graaf AO, van Kempen LC, de Vries IJ, Boer TD, Dolstra H, Torensma R, et al. Intratumoral rhIL-12 administration in head and neck squamous cell carcinoma patients induces B cell activation. Int J Cancer. 2008;123:2354–2361. doi: 10.1002/ijc.23756. [DOI] [PubMed] [Google Scholar]
- 21.Mohamadzadeh M, Berard F, Essert G, Chalouni C, Pulendran B, Davoust J, Bridges G, Palucka AK, Banchereau J. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–1020. doi: 10.1084/jem.194.7.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naive CD8(+) T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–1690. doi: 10.1002/eji.200636329. [DOI] [PubMed] [Google Scholar]
- 23.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 24***.Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–1638. doi: 10.1084/jem.20021598. [Seminal study demonstrating targeting of DC in vivo for generation and regulation of T cell immunity] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonifaz LC, Bonnyay DP, Charalambous A, Darguste DI, Fujii S, Soares H, Brimnes MK, Moltedo B, Moran TM, Steinman RM. In Vivo Targeting of Antigens to Maturing Dendritic Cells via the DEC-205 Receptor Improves T Cell Vaccination. J Exp Med. 2004;199:815–824. doi: 10.1084/jem.20032220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caminschi I, Lahoud MH, Shortman K. Enhancing immune responses by targeting antigen to DC. Eur J Immunol. 2009;39:931–938. doi: 10.1002/eji.200839035. [DOI] [PubMed] [Google Scholar]
- 27.Flacher V, Douillard P, Ait-Yahia S, Stoitzner P, Clair-Moninot V, Romani N, Saeland S. Expression of langerin/CD207 reveals dendritic cell heterogeneity between inbred mouse strains. Immunology. 2008;123:339–347. doi: 10.1111/j.1365-2567.2007.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flacher V, Sparber F, Tripp CH, Romani N, Stoitzner P. Targeting of epidermal Langerhans cells with antigenic proteins: attempts to harness their properties for immunotherapy. Cancer Immunol Immunother. 2009;58:1137–1147. doi: 10.1007/s00262-008-0563-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, Wang S, Zhang D, Hou S, Qian W, Li B, Guo H, Kou G, He J, Wang H, et al. Targeted delivery of tumor antigens to activated dendritic cells via CD11c molecules induces potent antitumor immunity in mice. Clin Cancer Res. 2009;15:4612–4621. doi: 10.1158/1078-0432.CCR-08-3321. [DOI] [PubMed] [Google Scholar]
- 30.Idoyaga J, Cheong C, Suda K, Suda N, Kim JY, Lee H, Park CG, Steinman RM. Cutting edge: langerin/CD207 receptor on dendritic cells mediates efficient antigen presentation on MHC I and II products in vivo. J Immunol. 2008;180:3647–3650. doi: 10.4049/jimmunol.180.6.3647. [DOI] [PubMed] [Google Scholar]
- 31.Manicassamy S, Ravindran R, Deng J, Oluoch H, Denning TL, Kasturi SP, Rosenthal KM, Evavold BD, Pulendran B. Toll-like receptor 2-dependent induction of vitamin A-metabolizing enzymes in dendritic cells promotes T regulatory responses and inhibits autoimmunity. Nat Med. 2009;15:401–409. doi: 10.1038/nm.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6:33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 33.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 34.Jeannin P, Bottazzi B, Sironi M, Doni A, Rusnati M, Presta M, Maina V, Magistrelli G, Haeuw JF, Hoeffel G, et al. Complexity and complementarity of outer membrane protein A recognition by cellular and humoral innate immunity receptors. Immunity. 2005;22:551–560. doi: 10.1016/j.immuni.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Delneste Y, Magistrelli G, Gauchat J, Haeuw J, Aubry J, Nakamura K, Kawakami-Honda N, Goetsch L, Sawamura T, Bonnefoy J, et al. Involvement of LOX-1 in dendritic cell-mediated antigen cross-presentation. Immunity. 2002;17:353–362. doi: 10.1016/s1074-7613(02)00388-6. [DOI] [PubMed] [Google Scholar]
- 36.Geijtenbeek TB, van Vliet SJ, Engering A, t Hart BA, van Kooyk Y. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu Rev Immunol. 2004;22:33–54. doi: 10.1146/annurev.immunol.22.012703.104558. [DOI] [PubMed] [Google Scholar]
- 37.Caparros E, Munoz P, Sierra-Filardi E, Serrano-Gomez D, Puig-Kroger A, Rodriguez-Fernandez JL, Mellado M, Sancho J, Zubiaur M, Corbi AL. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–3958. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]