Abstract
The Frank-Starling law of the heart describes the interrelationship between end-diastolic volume and cardiac ejection volume, a regulatory system that operates on a beat-to-beat basis. The main cellular mechanism that underlies this phenomenon is an increase in the responsiveness of cardiac myofilaments to activating Ca2+ ions at a longer sarcomere length, commonly referred to as myofilament length dependent activation. This review focuses on what molecular mechanisms may underlie myofilament length dependency. Specifically, the roles of inter-filament spacing, thick and thin filament based regulation, as well as sarcomeric regulatory proteins are discussed. Although the “Frank-Starling law of the heart” constitutes a fundamental cardiac property that has been appreciated for well over a century, it is still not known in muscle how the contractile apparatus transduces the information concerning sarcomere length to modulate ventricular pressure development.
Keywords: Frank-Starling Law of The Heart, Length-Tension Relationship, Sarcomere length, Regulation
1. Frank-Starling’s Law of the Heart
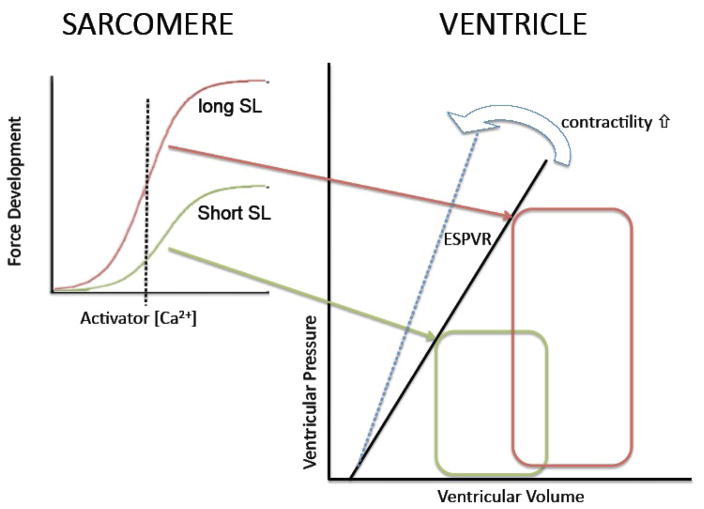
Over a century ago, Otto Frank in Germany and Ernest Starling in England reported on the relationship between the extent of ventricular filling and pump function of the heart, a phenomenon collectively referred to as Frank-Starling’s Law of the Heart. A modern view of this phenomenon[1] (illustrated in Figure 1) holds that there is a unique relationship between end-systolic volume and end-systolic pressure in the heart that is solely determined by contractile state. As a consequence, for a given contractile state, ventricular stroke volume is i) proportional to diastolic filling (i.e. preload), and ii) stroke volume can be maintained in the face of increased aortic pressures (i.e. afterload) simply by increasing preload as illustrated by the two pressure-volume loops in Figure 1. Contractile state, within this framework, can be viewed as any factor that alters end-systolic pressure independently of end-systolic volume and can conveniently be estimated semi-quantitatively by the slope of the end-systolic pressure-volume relationship (ESPVR; cf. solid line -- control state -- and dashed blue line – enhanced contractile state-- in the right panel of Figure 1). The ESPVR-slope is a very useful index of cardiac contractility that can be measured in situ by various methods; a convenient and popular approach is the use of the pressure-volume conductance catheter[2]. The cellular mechanisms that underlie the ESPVR are discussed in the following section.
Figure 1. The Frank-Starling mechanism and myofilament length dependent activation.
The Frank-Starling Law of the Heart describes a fundamental property of the heart (figure on the right). That is, for a given contractile state there is a unique relationship between end-systolic pressure reached in the heart and end-systolic pressure (ESPVR); increased contractility results in an increased slope of the ESPVR (cf. blue arrow). Increased ventricular filling (pre-load; red PV loop) leads to an increase in ventricular pressure development at end-systole which allows for i) increased stroke volume for a given systolic pressure (after-load) and ii) sustained stroke volume at elevated systolic pressure. The Frank-Starling mechanism has, as its basis, a modulation of myofilament Ca2+ sensitivity upon a change in sarcomere length as illustrated in the left graphs. Myofilament force development is the result of activation by Ca2+ ions. The relationship between force development and activator [Ca2+] is shifted up and to the left at longer sarcomere length (short SL, green; long SL, red). For a given contractile state (and, thus, cytosolic [Ca2+]; dashed vertical line), more myofilament force is developed at the longer SL (red) leading to a higher ventricular pressure at higher end-systolic volume (red PV loop). Thus, the Frank-Starling Law of the heart is a direct consequence of the myofilament length dependent activation properties of the cardiac sarcomere.
2. Relationship between whole heart property and myofilament length dependent activation
Pump function of the heart is intimately related to force generation, active shortening, and regulation of cardiac sarcomere activation and relaxation[1, 3]. The relationship between biomechanical properties of the cardiac sarcomere and mechanical behavior of the heart’s chamber is complex[3]. It is determined not only by the orientation and density of the constituent cardiac muscle fibers (i.e. spatial parameters), but also by the timing of cardiac muscle fiber activation and relaxation (i.e. temporal parameters). Nevertheless, there is ample evidence to support the notion that the biochemical properties of the cardiac cell, and indeed the cardiac sarcomere itself, are directly responsible for many, if not most, of the mechanical properties of the heart[1, 3–8]. Indeed, twitch force in isolated cardiac muscle is directly proportional to systolic sarcomere length; furthermore, the shape of the force-sarcomere length relationship is modulated by contractile state such that more force is generated at a given sarcomere length when contractile activation is elevated (e.g. by raising extracellular [Ca2+])[9]. At first sight, it may appear logical to suggest that variation of contractile filament overlap underlies the Frank-Starling Law of the Heart. However, the relationship between contractile twitch force and sarcomere length is too steep and too variable between contractile states to be solely explained by such a simple mechanism[8–12]. Activation of the contractile apparatus is initiated upon a transient increase in the cytosolic calcium concentration[13]. Under normal physiological conditions, calcium entry during the plateau phase of the cardiac action potential is not sufficient to directly activate the myofilaments, but instead serves as a trigger to release calcium from the sarcoplasmic reticulum[13]. The mechanisms that underlie this excitation-contraction coupling process are beyond the scope of this overview (for excellent reviews on this topic see[6, 13]). Nevertheless, it is important to note here that, in general, there is a direct relationship between the magnitude of the calcium transient and the contractile state of the cardiac cell and, therefore, the ventricle[13–15]. However, the level of myofilament activation is by no means a simple proportional function of cytosolic calcium concentration. Rather, it constitutes a complex and dynamic signal transduction process that itself is also subject to regulation and modulation by both intrinsic (mechanical loading, sarcomere length) and extrinsic (neuro-hormonal) factors. Early experiments by Fabiato suggested that the released amount of this activator calcium varies with sarcomere length[16], but these results have not since been confirmed. Instead, more recent experiments[11, 17, 18] clearly demonstrated that it is the level of activation of the cardiac contractile apparatus itself that is sensitive to changes in sarcomere length[3, 10]. These early experiments were performed on chemically permeabilized (skinned) isolated cardiac muscle, a preparation that allows direct access to the contractile apparatus such that steady state force can be measured as function of activator calcium concentration and sarcomere length; more recent experiments on intact twitching isolated myocardium employing fluorescent Ca2+ probes have confirmed these results[19]. Therefore, there is a direct proportionality between sarcomere length and the sensitivity of the cardiac sarcomere to Ca2+ ions, such that more force is generated at a given concentration of activator Ca2+ as sarcomere length is increased (the curves are both shifted to the left on the activator [Ca2+] axis and up to higher forces at the higher sarcomere length. Hence, it can be said that the myofilaments possess a length dependency property that is termed “myofilament length dependent activation”. This phenomenon is illustrated in the left panel of Figure 1: because of the sarcomere length modulation of sarcomeric properties, myofilament force development for a given level of activator [Ca2+] during the cardiac cycle (left panel; dashed vertical line) is also modulated and this, in turn, results in modulation of ventricular pressure development at end-systole (right panel; the two colored arrows indicate the connections between peak myofilament twitch force and end-systolic ventricular pressure). Thus, the whole heart Frank-Starling property has, as its basis, the myofilament length dependent activation property of the cardiac sarcomere. Incidentally, intact twitching cardiac muscle responds to a change in length in two distinct phases: an immediate change in twitch force, and a slower phase that develops over the course of several minutes. Experimental evidence suggests that the latter phase is due to a change in sarcoplasmic reticulum calcium release secondary to altered calcium loading of the cell, while the immediate response is due to the change in calcium sensitivity of the cardiac sarcomere described above[12]; this review is focused on the immediate response to a change in sarcomere length. Despite the importance for the Frank-Starling mechanism, the molecular mechanisms that may underlie the immediate response of myofilament force generation upon a change in sarcomere length have not been entirely elucidated[3, 8].
3. Myofilament length dependent activation and the sarcomere
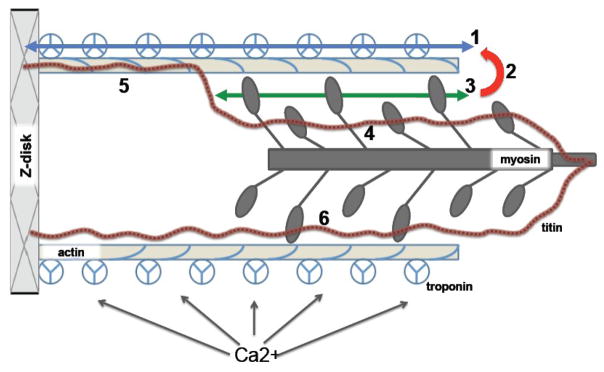
The change in myofilament force upon variation of sarcomere length is due to a change in the number of active cycling, force producing cross-bridges in the cardiac sarcomere[12, 20, 21]. The major proteins that make up the striated muscle sarcomere are: in the thin filament actin, troponin, and tropomyosin, and in the thick filament, myosin, myosin light chains, and myosin binding protein C (for a detailed review see[3, 7, 8, 22–24]. Figure 2 shows a schematic diagram illustrating the structure of the sarcomere as well as some of the possible molecular mechanisms that underlie myofilament length dependent activation. Dimers of the asymmetric molecule myosin are located in the thick filament. Their tail portions form the backbone of this filament, while the globular heads protrude from the thick filament reaching out towards actin. These globular heads, or cross-bridges, cyclically attach to the actin filament during contraction[25]. When strongly bound, the cross-bridges produce a pulling force on actin and, through the thin filament, on the proteins that make up the Z-band. The Z-bands are mechanically coupled via a multitude of proteins that include the integrin receptors at the cell surface to the extracellular collagen network thereby transmitting actin-myosin produced force to the tendons in skeletal muscle or towards the heart’s cavity so as to produce pressure. It should be noted that an additional sarcomeric protein has been identified, titin, a high molecular weight giant protein that spans the entire half sarcomere from the Z-band to the M-line and that binds tightly with the myosin thick filament[26–29]. As will be discussed below, recent evidence has implicated titin in myofilament length dependent activation. Striated muscle contraction is regulated by troponin and the tropomyosin (Tm) filament, both located on the thin filament and these proteins are essential for the regulation of myofilament contractile activation[3, 7, 8, 22–24, 30]. The heteromeric protein complex troponin (Tn) is composed of troponin-C (TnC), the calcium receptor; troponin-I (TnI), an inhibitor of contraction that switches between being bound to actin and cTnC; and troponin-T (TnT), a Tm binding protein that relays the Ca2+ binding signal from TnC to the thin filament by interacting with TnI and Tm. All striated muscle (skeletal and cardiac) are fundamentally identical in terms of the type of proteins that make up the sarcomere, but differ in terms of the specific isoforms expression of these proteins (which usually derive from different gene products in different muscle types)[7, 8].
Figure 2. Schematic of the sarcomere and putative mechanisms underlying myofilament length dependent activation.
Schematic diagram depicting the striated muscle sarcomere and some of the possible molecular mechanisms underlying myofilament length dependent activation (indicated by bold numbers). Changes in sarcomere length (SL) occur by means relative sliding between the thin filament (slender tope colored bars) and thick filament (dark grey bars). Thin filaments contains actin, tropomyosin (blue coil) and the troponin complex (composed of TnT, TnC, and TnI; three segmented circles). The globular portion of myosin interacts with actin to generate a mechanical force that is transmitted via the thin filament to the Z-disk and from there to the extra-cellular matrix. Ca2+ binding to troponin activates the thin filament to initiate contraction. Titin is a large molecular weight filament protein that spans between the Z-disk and the M-band of the thick filament. Myofilament length dependent activation could be the result of: i) modulation of pathways involving thin filament activation by troponin, either by directly modulating troponin-troponin cooperative interaction (1) or via a titin-actin interaction modulating the level of thin filament activation, either directly or by altered troponin Ca2+ binding affinity (5). ii) alterations in the cooperative interaction between force generating cross-bridges because the number of attached cross-bridges varies with SL (3); in addition, attached cross-bridges may affect thin filament activation and Ca2+ binding affinity (red arrow; 2), causing an indirect strong binding cross-bridges-thin filament activation feedback modulation that depends on SL. iii) titin interaction with myosin in the A-band may alter the structure of the globular domain of myosin heads that are interacting with actin in a length dependent manner (6). iv) finally, titin interaction with myosin in the pre-force state of the cross-bridge cycle may affect the structure and/or distribution of these weakly bound cross-bridges so as to affect the number of cross-bridges entering the force-generating strongly bound state in a length dependent manner (4).
In diastole, cross-bridges are either blocked from interacting with actin or in a weak, rapid binding-unbinding state with actin without generating force[3, 7, 8, 31–33]. In systole, activation of the thin filament allows for a strong-binding cross-bridge state, associated with force generation and high rate of ATP hydrolysis. Ca2+ is the physiological activator of the contractile machinery in both skeletal and cardiac muscle. Skeletal muscle TnC contains two high affinity and two low affinity binding sites[34]. The cardiac isoform (cTnC) also has two high affinity sites, but only one low affinity binding site[35]. The nature of Ca2+ regulation is such that activation of the sarcomere occurs by binding to the low affinity site(s). Ca2+ always occupies the high affinity sites of TnC under physiological conditions[36]; hence, these sites may serve only a structural function allowing for the anchoring of TnC to the troponin complex. In either cardiac muscle or skeletal muscle, the relationship between free Ca2+ and isometric tension is very steep, much greater than would be expected on the basis of equilibrium binding of Ca2+ to one or two binding sites on TnC[18, 37–41]. Hence, the transduction processes induced by Ca2+ binding that lead to activation of muscle contraction clearly involve cooperative interactions. In the absence of Ca2+, TnI exerts an inhibitory effect, preventing actin-myosin interaction. Ca2+ binding induces a conformational change in TnC such that a hydrophobic region becomes exposed thereby allowing interaction with TnI[42, 43]. However, the affinity of skeletal TnC for TnI in the presence of Ca2+ is much greater than the affinity of cardiac TnC for TnI in the presence of Ca2+[8, 44] which may be related to the diminished ability of Ca2++ to fully activate cardiac thin filaments at low (or initial) levels of cross-bridge binding[22, 45–49]. This phenomenon may also explain the greater myofilament length dependent activation observed in cardiac muscle compared to skeletal muscle[50]. Prior to Ca2+ activation, Tn constrains Tm in a position that sterically hinders myosin-S1 binding[7, 8, 51]. The movement of the troponin complex induces a conformational change in Tm, increasing its binding to actin, thereby revealing myosin-S1 binding sites on actin. Furthermore, upon forming strongly-bound cross-bridges, the binding of additional cross-bridges is greatly enhanced in a cooperative manner, presumably by initiating the movement of tropomyosin to a third, more favorable position[7, 8, 32, 52–54].
4. Myofilament length dependent activation and inter-filament spacing
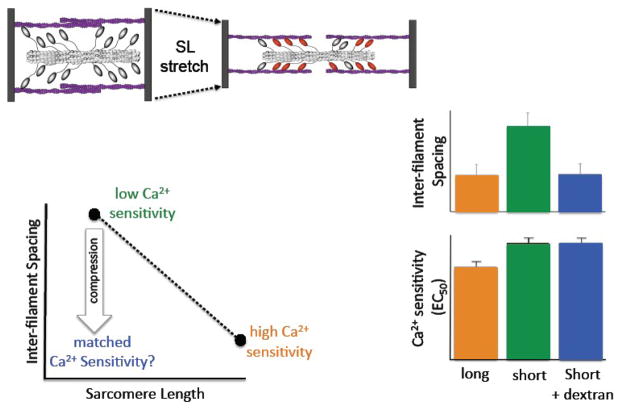
A unifying theory that has gained acceptance proposes that the impact of sarcomere length on myofilament Ca2+ sensitivity is due to changes in the spacing between the thick and thin filaments[45, 55–57]. This theory is schematically illustrated in Figure 3. Because myofibrils maintain close to constant volume[58–65], elongation of the sarcomere is expected to lead to a reduction of the distance between the thick and thin filaments. A closer approximation of the myosin heads to actin may be expected to increase the probability of strong cross-bridge formation at a given concentration of activator calcium (as illustrated by the red myosin heads in figure 3). Thus, one would predict an increase in myofilament Ca2+ sensitivity at the higher sarcomere length. Several experiments have provided support for this theory. Osmotic compression of the myofilaments by high molecular weight molecules that cannot enter the myofilament lattice structure, such as dextran, induces an increase in myofilament Ca2+ sensitivity concomitant with a reduction of muscle diameter[55–57]. A reduction in muscle diameter occurs in parallel to a reduction in myofilament lattice spacing in muscle[58–65], hence, the increase in myofilament Ca2+ sensitivity following dextran treatment without a change in sarcomere length is consistent with the myofilament lattice spacing theory. More direct support was provided by Fuchs et al, who showed that myofilament Ca2+ sensitivity could be rendered length independent when muscle diameter was kept constant by applying an appropriate amount dextran at each sarcomere length[57]. In addition, selective removal of titin by mild treatment with trypsin has been shown to affect both myofilament Ca2+ sensitivity and inter-filament spacing[66]; however, whether these observations are causally linked could not be determined in that study. To shed further light on these issues, we have reexamined the inter-filament spacing hypothesis, this time employing x-ray diffraction to directly measure myofilament lattice spacing in a comprehensive series of experiments[10, 59, 67–72]. First, we found an inverse relation between inter-filament spacing and sarcomere length in isolated cardiac[59] as well as fast and slow skeletal muscle preparations[72], a finding that is overall consistent with, although not proof of, the inter-filament spacing hypothesis. Second, we found that muscle diameter was not a consistent indicator of inter-filament spacing[71]. Third, in a study comparing skinned myocardium obtained from a transgenic murine model in which cTnI was replaced by ssTnI to wild-type myocardium, we found profound and opposing changes in inter-filament spacing upon PKA treatment that were not correlated to myofilament Ca2+ sensitivity[70]. Fourth, a detailed comparison between fast and slow skeletal muscle of the rat revealed no differences in inter-filament spacing as function of sarcomere length, despite large differences in myofilament length dependency[72]. Finally, in experiments illustrated in Figure 2, we used osmotic compression to reduce myofilament inter-filament spacing at a short sarcomere length to match inter-filament recorded at a high sarcomere length (i.e. despite the fact that sarcomere length had not changed) and asked the question whether myofilament Ca2+ sensitivity at the short sarcomere length would match that recorded at the high sarcomere length[71, 73]. A similar study, based on cell diameter not inter-filament spacing, had previously been reported by McDonald et al[56], and their results were in support of the inter-filament spacing hypothesis. As illustrated by the bar graphs in Figure 3, despite the matching of inter-filament spacing, myofilament Ca2+ sensitivity was only affected by sarcomere length and not by inter-filament spacing per se. The apparent discrepancy between those experiments and the previous experiments may be explained by i) the difference impact of osmotic compression on inter-filament spacing compared to muscle diameter; to mimic sarcomere length induced changes in inter-filament spacing much less osmotic compression is required than to mimic the changes in muscle diameter[71], ii) dextran induced osmotic compression causes increased myofilament Ca2+ sensitivity, but this effect appears to be due to a a direct effect on weakly bound cross-bridges rather than inter-filament spacing per se[69] (see also below). Nevertheless, whether inter-filament spacing underlies myofilament length dependent activation still remains controversial[73, 74].
Figure 3. The inter-filament spacing hypothesis.
Modulation of myofilament inter-filament spacing has been proposed as a unifying mechanism for myofilament length dependent activation. Stretch of the sarcomere (top left) is expected to result in a closer approximation of the thick and filaments within the sarcomere (dashed arrows) resulting in a more favorable disposition of myosin heads to interact with actin (illustrated by red myosin heads on the right). Direct measurement[59, 68] of inter-filament spacing by x-ray diffraction has confirmed an inverse relationship between inter-filament spacing and sarcomere length (bottom left). A short sarcomere length is associated with a large inter-filament spacing and low myofilament Ca2+ sensitivity (green), while the opposite is true for a long sarcomere length (orange). Compression of the myofilament lattice (e.g. by dextran) at a short sarcomere length is expected, within the framework of the inter-filament spacing hypothesis, to lead to matched myofilament Ca2+ sensitivity[56, 74] even though sarcomere length has not changed (cf. blue arrow). The bar graphs (lower right) show the results of such an experiment: inter-filament spacing, as measured by x-ray diffraction, was varied by either a change in sarcomere length (long SL, orange; short SL, green) or by compression with 1% dextran (blue). Even though compression resulted in an inter-filament spacing that was matched to that attained at the long sarcomere length (top bars), myofilament Ca2+ sensitivity (as indexed by EC50) was not affected by the osmotic compression (bottom bars). These data, and other data discussed in the text, suggest that inter-filament spacing may not be primary mechanism that underlies myofilament length dependent activation (modified from [71]).
5. Myofilament length dependent activation: filament based mechanisms
Myofilament activation is highly cooperative, that is, force development increases steeply over a very narrow concentration range of activator calcium[7, 8, 11, 12, 17, 18, 22, 30, 75–78]. Cooperative myofilament activation may be due to i) enhanced probability of calcium binding to TnC, either when neighboring TnC sites are occupied by calcium or when proximate cross-bridges are bound to actin (mechanisms #1& #2 in figure 2), ii) cooperative feedback between troponin sites in activating the thin filament (mechanism #1), iii) promotion of further cross-bridge formation by near-neighbor actively cycling cross-bridges[7, 8, 48, 75, 76, 79–83] (mechanism #3 in Fig. 2), or iv, increased Ca2+ binding affinity in proportion to the number of force generating cross-bridges (mechanism #2). Manipulation of cooperative activation by various agents or substrates may affect length dependent activation[45, 77, 84–87]. In comparing various striated muscle types, we found a correlation between the level of cooperativity and length dependent activation among fast, slow, and cardiac muscle of the rat[72]. However, the level of cooperative activation itself does not appear to be length dependent, as we have shown in chemically permeabilized rat myocardium[18]. Thus, the precise role of cooperative activation in length dependent activation is, at best, incompletely understood. Reports by the Moss group[77, 88], have shown that activation of thin-filament activation by non-cycling myosin cross-bridges (NEM-S1) virtually eliminates length dependency. These results suggest a mechanism for length dependency via a feedback pathway through strongly bound cross-bridges. Consistent with this notion, modulation of force development has been shown to alter myofilament length dependent activation properties[45, 46, 84, 85]. Together, these studies support the hypothesis that i) strongly bound cross-bridges activate the thin filament, and, ii) that myofilament length dependent activation involves, as a final common signal transduction pathway, also thin filament activation. Hence, alteration of thin filament activation, strongly bound cross-bridge, or the feedback between strongly bound cross-bridges either directly or via modulation of Ca2+ binding affinity would all be expected to affect length dependency[46, 48, 56, 57, 77, 83–85, 89, 90].
An alternative mechanism underling length dependent activation may not be related to cross-bridges entering the strongly bound state, but rather the transition to the weakly bound state[45, 46, 91]. Indeed, data in isolated skinned myocardium suggests that the number of cross-bridges in the weakly bound state increases when sarcomere length is increased, or, when relaxed skinned muscle is treated with dextran. Enhanced weakly bound cross-bridges were detected both mechanically by high-speed stiffness and by x-ray diffraction[69, 91, 92]. Since cardiac muscle thin filament activation appears to be sub-maximal under normal conditions[22, 45, 47–49, 91], expansion of the pool of weakly bound cross-bridges at the longer sarcomere length that can contribute to thin-filament activation via feedback could give rise to a robust length dependent activation. Consistent with this notion, dextran application induces increases in the intensity ratios of the 1,1 and 1,0 equatorial reflections in x-ray diffraction measurements, indicative of alterations in myosin structure upon dextran compression[69, 91]; incidentally, in our study relaxed cross-bridge disposition correlates with myofilament Ca2+ sensitivity, while inter-filament spacing does not[69]. Finally, it has been suggested that titin may be involved in myofilament length dependent activation[28, 29, 93–96]. It is perceivable that titin strain influences the number of weakly bound cross-bridges since titin is known to possess both actin and myosin binding domains[28, 29, 94, 97] (mechanisms #4,#5,#6 in figure 2). Indeed, release of the passive tension borne by the titin molecule, either by mild treatment with trypsin or by brief over-stretching of the sarcomere, markedly reduces myofilament length dependency. Moreover, regional differences between the epi- and endo-cardial layer of the heart have been identified in passive stiffness[98, 99]. The larger endo-myocardial stiffness was found to correlate with enhanced myofilament length dependent activation in several species[98, 99]. Of interest, these regional differences are diminished in heart failure, but can be restored by exercise[100]. The mechanisms that underlie this regional phenomenon are not fully understood. Differences in titin isoform distribution were but almost certainly excluded; rather, marked regional differences in contractile protein phosphorylation were suggested as the possible mechanism that underlies regional variation in length dependent activation[99]; which contractile protein(s), and whether titin itself is among the affected contractile protein remains to be investigated. Finally, the group of Greaser[101] has recently identified a strain of rats in which the developmentally regulated splice variation of the titin gene is disrupted in the heart. As a result, the normal reduction in the titin persistence length upon aging does not occur in the hearts of these animals, leading to low passive stiffness and unusually long resting sarcomere length. Whether myofilament length dependence is affected in these animals and whether the Frank-Starling property of the ventricle is altered remains to be investigated. The availability of this animal model, however, will provide for a unique opportunity to investigate the role of titin in length dependency of the sarcomere[102].
6. Myofilament length dependent activation and troponin-I
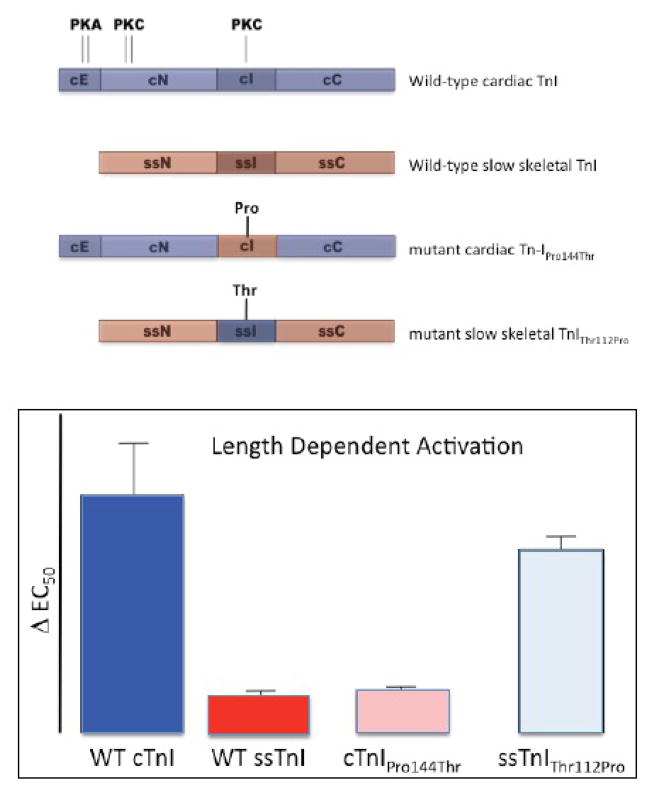
Myofilament length dependency is significantly blunted in slow skeletal muscle[72]. The protein composition of the cardiac sarcomere shares some similarities with slow skeletal muscle, such as the cardiac isoform of TnC and b-myosin. In contrast, in the heart the slow skeletal isoform of TnI is replaced by a unique, cardiac, isoform of Tn-I shortly after birth in most species[103]. We have previously demonstrated that the blunted length dependency seen in slow skeletal muscle is recapitulated when cardiac TnI is replaced by ssTnI in the hearts of a transgenic mouse model[70], indicating that cardiac TnI plays a pivotal role in the length signal transduction process within the sarcomere. The cardiac isoform of TnI differs from slow skeletal TnI in several significant ways as illustrated in Figure 4[23, 24, 104] [103]: a unique 33 amino-acid N-terminus extension (cE) that contains two serines that are the target for protein kinase A (PKA). In addition, two protein-kinase C (PKC) target sites that are located in the cardiac N-terminus domain (cN) are lacking/non-functional in the equivalent domain in slow skeletal TnI. Finally, a threonine at position 144 within the cardiac inhibitory domain (cI) that is also a PKC target, is replaced by a proline residue in the equivalent domain of slow skeletal TnI (ssI). Of interest, this is the only difference between the cardiac and slow skeletal inhibitory domain of TnI. Using a protein exchange technique in chemically permeabilized isolated myocardium it is possible to replace the endogenous troponin complex with a recombinant derived troponin complex[105, 106]. As illustrated in by the bar graphs in Figure 4, exchange with recombinant troponin complex containing wild-type cardiac resulted in robust length dependency (dark blue bar), while exchange with troponin containing wild-type slow skeletal TnI virtually eliminated length dependency (red bar); In these experiments, length dependent activation was indexed by ΔEC50, the difference between myofilament Ca2+ sensitivity at the long and short sarcomere length. These results are consistent with the properties of cardiac versus slow skeletal muscle[72] as well as our transgenic mouse model results discussed above[70]. Earlier data, published in abstract form[107], had already suggested that the critical region for length dependency may be located within the inhibitory domain of cardiac TnI. Indeed, substitution of threonine with proline residue at the 144 position in cardiac TnI virtually eliminated length dependency (pink bar), while introduction of a threonine at the equivalent (122) position in slow skeletal TnI markedly increased length dependency, almost to the level that is seen in cardiac muscle (light blue bar). Thus, the inhibitory region of cTnI plays a prominent role in the length transducing process of the sarcomere and, furthermore, this property is governed by the presence of the threonine residue at position 144 in cardiac TnI. The molecular mechanisms underlying this phenomenon, however, are presently unknown. Although TnI-Thr144 is clearly important for myofilament length dependent activation, the regional differences in this parameter observed within the heart[99, 108] indicate that other factors must also play a significant role in modulating length sensitivity. Of interest, a gradient of myosin light chain phosphorylation has long been recognized to exist in the heart[109], and phosphorylation of this contractile protein is know to be involved in stretch dependent regulation of cardiac contraction[109–111].
Figure 4. The inhibitory region of cardiac troponin-I plays a pivotal role in myofilament length dependent activation.
The adult heart expresses a unique isoform of troponin-I (wild-type cardiac TnI, top diagram; shaded in dark blue) that contains a unique N-terminus 33 amino acid extension (cE). In addition, cardiac TnI contains both PKA and PKC phosphorylation target sites that are lacking in slow skeletal TnI (wild-type slow skeletal TnI; shaded in red). The inhibitory region differs between cardiac TnI and slow skeletal TnI by a single amino acid residue: a threonine at position 144 in cardiac TnI, and a proline at position 112 in slow skeletal TnI. Exchange for recombinant wild-type cardiac troponin containing cardiac TnI into isolated chemically permeabilized myocardium retained the robust myofilament length dependency that is characteristic of this muscle type (cf. bar graphs; DEC50, index of length dependency; WT cTnI; dark blue bar), while exchange for cardiac troponin containing slow skeletal TnI virtually abolished length dependency (WT ssTnI; dark red bar). Exchange for troponin containing a mutant cardiac TnI in which threonine 144 was replaced by a proline (as is found in ssTnI) also virtually eliminated length dependency (cTnIPro144Thr; pink bar), while introduction of a threonine at the equivalent position in slow skeletal TnI markedly enhanced length dependency (ssTnIThr112Pro). These data suggest that presence of a threonine at position 144 is both necessary and sufficient to impart length dependent activation properties upon the cardiac sarcomere (modified from [67]).
8. Concluding remarks
The “Frank-Starling law of the heart” constitutes a fundamental cardiac property that has been appreciated for well over a century. At its basis, this ventricular property is the result of a modulation of myofilament Ca2+ sensitivity upon a change in sarcomere length. Despite intense investigations, the molecular mechanisms that underlie this phenomenon are still not known. As discussed in this review, controversy remains whether inter-filament spacing constitutes the molecular strain sensing mechanism of the sarcomere. Rather, evidence points to a length modulated regulation of thin filament activation state via regulation of thin filament activation state. How the thin filament is regulated by sarcomere length is not known; some of the potential mechanisms are illustrated in figure 3 that include cooperative feedback between force generating cross-bridges, Ca2+ binding affinity, as well as the giant cytoskeleteal protein titin as the mechanical linker protein that transmits information regarding sarcomere length to the contractile apparatus. Clearly, the question as to as to how myofilament Ca2+ sensitivity is regulated by sarcomere length is far from being solved. Future biophysical, biochemical, and detailed ultra-structural investigations are clearly required to solve this intriguing cellular molecular physiology puzzle.
Acknowledgments
Our work was supported by NIH grants PO1-HL62426, RO1-HL75494, T32-007692, and the American Heart Association. We would like to acknowledge the help, assistance and extensive discussion with our colleagues at the University of Illinois Chicago, Loyola University Chicago Medical School, the Illinois Institute of Technology, and the Argonne Advanced Photon Source.
Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Energy Research, under Contract No. W-31-109-ENG-38. BioCAT is a U.S. National Institutes of Health-supported Research Center RR08630.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sagawa K, Maughan L, Suga H, Sunagawa K. Cardiac contraction and the pressure-volume relationship. New York, Oxford: Oxford University Press; 1988. [Google Scholar]
- 2.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–34. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Tombe PP. Cardiac myofilaments: mechanics and regulation. J Biomech. 2003 May;36(5):721–30. doi: 10.1016/s0021-9290(02)00450-5. [DOI] [PubMed] [Google Scholar]
- 4.Solaro RJ. Integration of myofilament response to Ca2+ with cardiac pump regulation and pump dynamics. Am J Physiol. 1999 Dec;277(6 Pt 2):S155–63. doi: 10.1152/advances.1999.277.6.S155. [DOI] [PubMed] [Google Scholar]
- 5.ter Keurs HEDJ, Tyberg JV. Mechanics of the Circulation. Dordrecht: Martinus Nijhoff; 1986. [Google Scholar]
- 6.Fozzard HA, Haber E, Jennings RB, Katz AM. Scientific foundations. 2. New York: Raven Press; 1992. The heart and cardiovascular system. [Google Scholar]
- 7.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovasc Res. 2008 Mar 1;77(4):616–8. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi T, Jin L, de Tombe PP. Cardiac thin filament regulation. Pflugers Arch. 2008 Oct;457(1):37–46. doi: 10.1007/s00424-008-0511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ter Keurs HEDJ, Rijnsburger WH, van Heuningen R, Nagelsmit MJ. Tension development and sarcomere length in rat cardiac trabeculae: Evidence of length-dependent activation. Circulation Research. 1980;46:703–14. doi: 10.1161/01.res.46.5.703. [DOI] [PubMed] [Google Scholar]
- 10.Konhilas JP, Irving TC, De Tombe PP. Frank-Starling law of the heart and the cellular mechanisms of length-dependent activation. Pflugers Arch. 2002 Dec;445(3):305–10. doi: 10.1007/s00424-002-0902-1. [DOI] [PubMed] [Google Scholar]
- 11.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–68. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 12.Allen DG, Kentish JC. The cellular basis of the length-tension relation in cardiac muscle. J Mol Cell Cardiol. 1985 Sep;17(9):821–40. doi: 10.1016/s0022-2828(85)80097-3. [DOI] [PubMed] [Google Scholar]
- 13.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2. Dordrecht, The Netherlands: Kluwer Academic Press; 2001. [Google Scholar]
- 14.Gwathmey JK, Hajjar RJ. Intracellular calcium related to force development in twitch contraction of mammalian myocardium. Cell Calcium. 1990;11:531–8. doi: 10.1016/0143-4160(90)90029-t. [DOI] [PubMed] [Google Scholar]
- 15.Backx PH, Gao WD, Azan-Backx MD, Marban E. The relationship between contractile force and intracellular [Ca2+] in intact rat cardiac trabeculae. J Gen Physiol. 1995 Jan;105(1):1–19. doi: 10.1085/jgp.105.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fabiato A, Fabiato F. Dependence of the contractile activation of skinned cardiac cells on the sarcomere length. Nature. 1975 Jul 3;256(5512):54–6. doi: 10.1038/256054a0. [DOI] [PubMed] [Google Scholar]
- 17.Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. JPhysiol. 1982;329:527–40. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobesh DP, Konhilas JP, de Tombe PP. Cooperative activation in cardiac muscle: impact of sarcomere length. Am J Physiol Heart Circ Physiol. 2002 Mar;282(3):H1055–62. doi: 10.1152/ajpheart.00667.2001. [DOI] [PubMed] [Google Scholar]
- 19.Claflin DR, Morgan DL, Julian FJ. The effect of length on the relationship between tension and intracellular [Ca2+] in intact frog skeletal muscle fibres. J Physiol. 1998 Apr 1;508( Pt 1):179–86. doi: 10.1111/j.1469-7793.1998.179br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wannenburg T, Janssen PM, Fan D, de Tombe PP. The Frank-Starling mechanism is not mediated by changes in rate of cross-bridge detachment. Am J Physiol. 1997;273(5 Pt 2):H2428–35. doi: 10.1152/ajpheart.1997.273.5.H2428. [DOI] [PubMed] [Google Scholar]
- 21.Wannenburg T, Heijne GH, Geerdink JH, Van Den Dool HW, Janssen PM, De Tombe PP. Cross-bridge kinetics in rat myocardium: effect of sarcomere length and calcium activation. Am J Physiol Heart Circ Physiol. 2000;279(2):H779–90. doi: 10.1152/ajpheart.2000.279.2.H779. [DOI] [PubMed] [Google Scholar]
- 22.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000 Apr;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 23.Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998 Sep 7;83(5):471–80. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 24.Solaro RJ, Montgomery DM, Wang L, Burkart EM, Ke Y, Vahebi S, et al. Integration of pathways that signal cardiac growth with modulation of myofilament activity. J Nucl Cardiol. 2002 Sep-Oct;9(5):523–33. doi: 10.1067/mnc.2002.127626. [DOI] [PubMed] [Google Scholar]
- 25.Huxley HE. The mechanism of muscular contraction. Science. 1969;164(886):1356–65. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, McClure J, Tu A. Titin: major myofibrillar components of striated muscle. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3698–702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maruyama K, Kimura S, Ohashi K, Kuwano Y. Connectin, an elastic protein of muscle. Identification of “titin” with connectin. J Biochem (Tokyo) 1981 Mar;89(3):701–9. doi: 10.1093/oxfordjournals.jbchem.a133249. [DOI] [PubMed] [Google Scholar]
- 28.Granzier H, Labeit S. Cardiac titin: an adjustable multi-functional spring. J Physiol. 2002 Jun 1;541(Pt 2):335–42. doi: 10.1113/jphysiol.2001.014381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linke WA. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc Res. 2008 Mar 1;77(4):637–48. doi: 10.1016/j.cardiores.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 30.de Tombe PP, Solaro RJ. Integration of cardiac myofilament activity and regulation with pathways signaling hypertrophy and failure. Ann Biomed Eng. 2000 Aug;28(8):991–1001. doi: 10.1114/1.1312189. [DOI] [PubMed] [Google Scholar]
- 31.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993 Aug;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehrer SS, Geeves MA. The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol. 1998;277(5):1081–9. doi: 10.1006/jmbi.1998.1654. [DOI] [PubMed] [Google Scholar]
- 33.Maytum R, Lehrer SS, Geeves MA. Cooperativity and switching within the three-state model of muscle regulation. Biochemistry. 1999 Jan 19;38(3):1102–10. doi: 10.1021/bi981603e. [DOI] [PubMed] [Google Scholar]
- 34.Potter JD, Gergely J. The calcium and magnesium binding sites on troponin and their role in the regulation of myofribrillar adenosine triphosphatase. Journal of Biological Chemistry. 1975;250:4628–33. [PubMed] [Google Scholar]
- 35.Holroyde MJ, Robertson SP, Johnson JD, Solaro RJ, Potter JD. The calcium and magnesium binding sites on cardiac troponin and their role in the regulation of myofribillar adenosine triphosphatase. Journal of Biological Chemistry. 1980;255:11688–93. [PubMed] [Google Scholar]
- 36.Pan BS, Solaro RJ. Calcium-binding properties of troponin C in detergent-skinned heart muscle fibers. J Biol Chem. 1987 Jun 5;262(16):7839–49. [PubMed] [Google Scholar]
- 37.Rice JJ, Stolovitzky G, Tu Y, De Tombe PP. Ising model of cardiac thin filament activation with nearest-neighbor cooperative interactions. Biophys J. 2003 Feb;84(2):897–909. doi: 10.1016/S0006-3495(03)74907-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice JJ, Tu Y, Poggesi C, De Tombe PP. Spatially-compressed cardiac myofilament models generate hysteresis that is not found in real muscle. Pac Symp Biocomput. 2008:366–77. [PubMed] [Google Scholar]
- 39.Rice JJ, Wang F, Bers DM, de Tombe PP. Approximate model of cooperative activation and crossbridge cycling in cardiac muscle using ordinary differential equations. Biophys J. 2008 Jan 30; doi: 10.1529/biophysj.107.119487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill TL. Cooperativity theory in Biochemistry: steady-state and equilibrium systems. New York: Springer-Verlag; 1985. [Google Scholar]
- 41.Shiner JS, Solaro RJ. The hill coefficient for the Ca2+-activation of striated muscle contraction. Biophys J. 1984 Oct;46(4):541–3. doi: 10.1016/S0006-3495(84)84051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slupsky CM, Sykes BD. NMR solution structure of calcium-saturated skeletal muscle troponin C. Biochemistry. 1995 Dec 12;34(49):15953–64. doi: 10.1021/bi00049a010. [DOI] [PubMed] [Google Scholar]
- 43.Gagne SM, Tsuda S, Li MX, Smillie LB, Sykes BD. Structures of the troponin C regulatory domains in the apo and calcium-saturated states. Nat Struct Biol. 1995 Sep;2(9):784–9. doi: 10.1038/nsb0995-784. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 45.Smith L, Tainter C, Regnier M, Martyn DA. Cooperative cross-bridge activation of thin filaments contributes to the Frank-Starling mechanism in cardiac muscle. Biophys J. 2009 May 6;96(9):3692–702. doi: 10.1016/j.bpj.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adhikari BB, Regnier M, Rivera AJ, Kreutziger KL, Martyn DA. Cardiac length dependence of force and force redevelopment kinetics with altered cross-bridge cycling. Biophys J. 2004 Sep;87(3):1784–94. doi: 10.1529/biophysj.103.039131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clemmens EW, Entezari M, Martyn DA, Regnier M. Different effects of cardiac versus skeletal muscle regulatory proteins on in vitro measures of actin filament speed and force. J Physiol. 2005 Aug 1;566(Pt 3):737–46. doi: 10.1113/jphysiol.2005.084194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Regnier M, Martin H, Barsotti RJ, Rivera AJ, Martyn DA, Clemmens E. Cross-bridge versus thin filament contributions to the level and rate of force development in cardiac muscle. Biophys J. 2004 Sep;87(3):1815–24. doi: 10.1529/biophysj.103.039123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Regnier M, Rivera AJ, Chen Y, Chase PB. 2-deoxy-ATP enhances contractility of rat cardiac muscle. Circ Res. 2000 Jun 23;86(12):1211–7. doi: 10.1161/01.res.86.12.1211. [DOI] [PubMed] [Google Scholar]
- 50.Konhilas JP, Irving TC, Smith SH, de Tombe PP. Length-dependent activation compared in three striated muscle types of the rat. Biophysical Journal. 2001 Jan;80(1):1053. [Google Scholar]
- 51.Lehman W, Craig R, Vibert P. Ca(2+)-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994;368(6466):65–7. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- 52.Swartz DR, Moss RL. Influence of a strong-binding myosin analogue on calcium-sensitive mechanical properties of skinned skeletal muscle fibers. J Biol Chem. 1992;267(28):20497–506. [PubMed] [Google Scholar]
- 53.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000 Sep 22;302(3):593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 54.Tobacman LS, Butters CA. A new model of cooperative myosin-thin filament binding. J Biol Chem. 2000 Sep 8;275(36):27587–93. doi: 10.1074/jbc.M003648200. [DOI] [PubMed] [Google Scholar]
- 55.Godt RE, Maughan DW. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pflugers Arch. 1981;391(4):334–7. doi: 10.1007/BF00581519. [DOI] [PubMed] [Google Scholar]
- 56.McDonald KS, Moss RL. Osmotic compression of single cardiac myocytes eliminates the reduction in Ca2+ sensitivity of tension at short sarcomere length. Circulation Research. 1995;77:199–205. doi: 10.1161/01.res.77.1.199. [DOI] [PubMed] [Google Scholar]
- 57.Fuchs F, Smith SH. Calcium, cross-bridges, and the frank-starling relationship. News Physiol Sci. 2001;16:5–10. doi: 10.1152/physiologyonline.2001.16.1.5. [DOI] [PubMed] [Google Scholar]
- 58.Rome E. X-ray diffraction studies of the filament lattice of striated muscle in various bathing media. J Mol Biol. 1968;37(2):331–44. doi: 10.1016/0022-2836(68)90272-6. [DOI] [PubMed] [Google Scholar]
- 59.Irving TC, Konhilas J, Perry D, Fischetti R, de Tombe PP. Myofilament lattice spacing as a function of sarcomere length in isolated rat myocardium. Am J Physiol Heart Circ Physiol. 2000;279(5):H2568–H73. doi: 10.1152/ajpheart.2000.279.5.H2568. [DOI] [PubMed] [Google Scholar]
- 60.Matsubara I, Elliott GF. X-ray diffraction studies on skinned single fibres of frog skeletal muscle. J Mol Biol. 1972 Dec 30;72(3):657–69. doi: 10.1016/0022-2836(72)90183-0. [DOI] [PubMed] [Google Scholar]
- 61.Matsubara I. Light and x-ray diffraction studies on chick skeletal muscle under controlled physiological conditions. J Physiol. 1974 May;238(3):473–86. doi: 10.1113/jphysiol.1974.sp010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsubara I, Millman BM. X-ray diffraction patterns from mammalian heart muscle. J Mol Biol. 1974 Feb 5;82(4):527–36. doi: 10.1016/0022-2836(74)90246-0. [DOI] [PubMed] [Google Scholar]
- 63.Matsubara I, Goldman YE, Simmons RM. Changes in lateral filament spacing of skinned msucle fibres when cross-bridges attach. JMolBiol. 1984;173:15–33. doi: 10.1016/0022-2836(84)90401-7. [DOI] [PubMed] [Google Scholar]
- 64.Matsubara I, Umazume Y, Yagi N. Lateral filamentary spacing in chemically skinned murine muscles during contraction. J Physiol. 1985 Mar;360:135–48. doi: 10.1113/jphysiol.1985.sp015608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farman GP, Tachampa K, Mateja R, Cazorla O, Lacampagne A, de Tombe PP. Blebbistatin: use as inhibitor of muscle contraction. Pflugers Arch. 2008 Mar;455(6):995–1005. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- 66.Wikman-Coffelt J, Refsum H, Hollosi G, Rouleau L, Chuck L, Parmley WW. Comparitive force-velocity relation and analyses of myosin of dog atria and ventricles. AMJPHYSIOL. 1991;243:H391. doi: 10.1152/ajpheart.1982.243.3.H391. [DOI] [PubMed] [Google Scholar]
- 67.Tachampa K, Wang H, Farman GP, de Tombe PP. Cardiac troponin I threonine 144: role in myofilament length dependent activation. Circ Res. 2007 Nov 26;101(11):1081–3. doi: 10.1161/CIRCRESAHA.107.165258. [DOI] [PubMed] [Google Scholar]
- 68.Farman GP, Allen EJ, Gore D, Irving TC, de Tombe PP. Interfilament spacing is preserved during sarcomere length isometric contractions in rat cardiac trabeculae. Biophys J. 2007 May 1;92(9):L73–5. doi: 10.1529/biophysj.107.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farman GP, Walker JS, de Tombe PP, Irving TC. Impact of osmotic compression on sarcomere structure and myofilament calcium sensitivity of isolated rat myocardium. Am J Physiol Heart Circ Physiol. 2006 Oct;291(4):H1847–55. doi: 10.1152/ajpheart.01237.2005. [DOI] [PubMed] [Google Scholar]
- 70.Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, et al. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol. 2003 Mar 15;547(Pt 3):951–61. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konhilas JP, Irving TC, de Tombe PP. Myofilament calcium sensitivity in skinned rat cardiac trabeculae: role of inter-filament spacing. Circulation Research. 2002;90:59–65. doi: 10.1161/hh0102.102269. [DOI] [PubMed] [Google Scholar]
- 72.Konhilas JP, Irving TC, de Tombe PP. Length-dependent activation in three striated muscle types of the rat. J Physiol. 2002 Oct 1;544(Pt 1):225–36. doi: 10.1113/jphysiol.2002.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moss RL, Fitzsimons DP. Frank-Starling relationship: long on importance, short on mechanism. Circ Res. 2002 Jan 11;90(1):11–3. [PubMed] [Google Scholar]
- 74.Fuchs F, Martyn DA. Length-dependent Ca(2+) activation in cardiac muscle: some remaining questions. J Muscle Res Cell Motil. 2005;26(4–5):199–212. doi: 10.1007/s10974-005-9011-z. [DOI] [PubMed] [Google Scholar]
- 75.Campbell KB, Razumova MV, Kirkpatrick RD, Slinker BK. Nonlinear myofilament regulatory processes affect frequency-dependent muscle fiber stiffness. Biophys J. 2001 Oct;81(4):2278–96. doi: 10.1016/S0006-3495(01)75875-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Razumova MV, Bukatina AE, Campbell KB. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys J. 2000 Jun;78(6):3120–37. doi: 10.1016/S0006-3495(00)76849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fitzsimons DP, Moss RL. Strong binding of myosin modulates length-dependent Ca2+ activation of rat ventricular myocytes. Circ Res. 1998;83(6):602–7. doi: 10.1161/01.res.83.6.602. [DOI] [PubMed] [Google Scholar]
- 78.Regnier M, Rivera AJ, Wang CK, Bates MA, Chase PB, Gordon AM. Thin filament near-neighbour regulatory unit interactions affect rabbit skeletal muscle steady-state force-Ca(2+) relations. J Physiol. 2002 Apr 15;540(Pt 2):485–97. doi: 10.1113/jphysiol.2001.013179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kreutziger KL, Piroddi N, Scellini B, Tesi C, Poggesi C, Regnier M. Thin filament Ca2+ binding properties and regulatory unit interactions alter kinetics of tension development and relaxation in rabbit skeletal muscle. J Physiol. 2008 Aug 1;586(Pt 15):3683–700. doi: 10.1113/jphysiol.2008.152181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno-Gonzalez A, Gillis TE, Rivera AJ, Chase PB, Martyn DA, Regnier M. Thin-filament regulation of force redevelopment kinetics in rabbit skeletal muscle fibres. J Physiol. 2007 Mar 1;579(Pt 2):313–26. doi: 10.1113/jphysiol.2006.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kreutziger KL, Gillis TE, Davis JP, Tikunova SB, Regnier M. Influence of enhanced troponin C Ca2+-binding affinity on cooperative thin filament activation in rabbit skeletal muscle. J Physiol. 2007 Aug 15;583(Pt 1):337–50. doi: 10.1113/jphysiol.2007.135426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gillis TE, Martyn DA, Rivera AJ, Regnier M. Investigation of thin filament near-neighbour regulatory unit interactions during force development in skinned cardiac and skeletal muscle. J Physiol. 2007 Apr 15;580(Pt. 2):561–76. doi: 10.1113/jphysiol.2007.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moreno-Gonzalez A, Fredlund J, Regnier M. Cardiac troponin C (TnC) and a site I skeletal TnC mutant alter Ca2+ versus crossbridge contribution to force in rabbit skeletal fibres. J Physiol. 2005 Feb 1;562(Pt 3):873–84. doi: 10.1113/jphysiol.2004.077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fukuda N, Kajiwara H, Ishiwata S, Kurihara S. Effects of MgADP on length dependence of tension generation in skinned rat cardiac muscle. Circ Res. 2000 Jan 7;86(1):E1–6. doi: 10.1161/01.res.86.1.e1. [DOI] [PubMed] [Google Scholar]
- 85.Fukuda N, JOU, Sasaki D, Kajiwara H, Ishiwata S, Kurihara S. Acidosis or inorganic phosphate enhances the length dependence of tension in rat skinned cardiac muscle. J Physiol. 2001 Oct 1;536(Pt 1):153–60. doi: 10.1111/j.1469-7793.2001.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith SH, Fuchs F. Effect of ionic strength on length-dependent Ca(2+) activation in skinned cardiac muscle. J Mol Cell Cardiol. 1999;31(12):2115–25. doi: 10.1006/jmcc.1999.1043. [DOI] [PubMed] [Google Scholar]
- 87.Arteaga GM, Kobayashi T, Solaro RJ. Molecular actions of drugs that sensitize cardiac myofilaments to Ca2+ Ann Med. 2002;34(4):248–58. doi: 10.1080/078538902320322510. [DOI] [PubMed] [Google Scholar]
- 88.Stelzer JE, Larsson L, Fitzsimons DP, Moss RL. Activation dependence of stretch activation in mouse skinned myocardium: implications for ventricular function. J Gen Physiol. 2006 Feb;127(2):95–107. doi: 10.1085/jgp.200509432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martyn DA, Gordon AM. Influence of length on force and activation-dependent changes in troponin c structure in skinned cardiac and fast skeletal muscle. Biophys J. 2001 Jun;80(6):2798–808. doi: 10.1016/S0006-3495(01)76247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liang B, Chen Y, Wang CK, Luo Z, Regnier M, Gordon AM, et al. Ca2+ regulation of rabbit skeletal muscle thin filament sliding: role of cross-bridge number. Biophys J. 2003 Sep;85(3):1775–86. doi: 10.1016/S0006-3495(03)74607-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martyn DA, Adhikari BB, Regnier M, Gu J, Xu S, Yu LC. Response of equatorial x-ray reflections and stiffness to altered sarcomere length and myofilament lattice spacing in relaxed skinned cardiac muscle. Biophys J. 2004 Feb;86(2):1002–11. doi: 10.1016/S0006-3495(04)74175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu S, Martyn D, Zaman J, Yu LC. X-ray diffraction studies of the thick filament in permeabilized myocardium from rabbit. Biophys J. 2006 Nov 15;91(10):3768–75. doi: 10.1529/biophysj.106.088971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001 May 25;88(10):1028–35. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- 94.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004 Feb 20;94(3):284–95. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 95.Fukuda N, Wu Y, Farman G, Irving TC, Granzier H. Titin isoform variance and length dependence of activation in skinned bovine cardiac muscle. J Physiol. 2003 Nov 15;553(Pt 1):147–54. doi: 10.1113/jphysiol.2003.049759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Helmes M, Lim CC, Liao R, Bharti A, Cui L, Sawyer DB. Titin determines the Frank-Starling relation in early diastole. J Gen Physiol. 2003 Feb;121(2):97–110. doi: 10.1085/jgp.20028652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Soteriou A, Gamage M, Trinick J. A survey of interactions made by the giant protein titin. J Cell Sci. 1993 Jan;104( Pt 1):119–23. doi: 10.1242/jcs.104.1.119. [DOI] [PubMed] [Google Scholar]
- 98.Cazorla O, Szilagyi S, Le Guennec JY, Vassort G, Lacampagne A. Transmural stretch-dependent regulation of contractile properties in rat heart and its alteration after myocardial infarction. FASEB J. 2005 Jan;19(1):88–90. doi: 10.1096/fj.04-2066fje. [DOI] [PubMed] [Google Scholar]
- 99.Ait Mou Y, le Guennec JY, Mosca E, de Tombe PP, Cazorla O. Differential contribution of cardiac sarcomeric proteins in the myofibrillar force response to stretch. Pflugers Arch. 2008 Oct;457(1):25–36. doi: 10.1007/s00424-008-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mou YA, Reboul C, Andre L, Lacampagne A, Cazorla O. Late exercise training improves non-uniformity of transmural myocardial function in rats with ischaemic heart failure. Cardiovasc Res. 2009 Feb 15;81(3):555–64. doi: 10.1093/cvr/cvn229. [DOI] [PubMed] [Google Scholar]
- 101.Greaser ML, Warren CM, Esbona K, Guo W, Duan Y, Parrish AM, et al. Mutation that dramatically alters rat titin isoform expression and cardiomyocyte passive tension. J Mol Cell Cardiol. 2008 Jun;44(6):983–91. doi: 10.1016/j.yjmcc.2008.02.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cazorla O, de Tombe PP. Some rat: a very special rat with a rather special titin. J Mol Cell Cardiol. 2008 Jun;44(6):976–8. doi: 10.1016/j.yjmcc.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kruger M, Kohl T, Linke WA. Developmental changes in passive stiffness and myofilament Ca2+ sensitivity due to titin and troponin-I isoform switching are not critically triggered by birth. Am J Physiol Heart Circ Physiol. 2006 Aug;291(2):H496–506. doi: 10.1152/ajpheart.00114.2006. [DOI] [PubMed] [Google Scholar]
- 104.Solaro RJ. Protein phosphorylation in heart muscle. Boca Raton: CRC Press; 1986. [Google Scholar]
- 105.Brenner B, Chalovich JM. Kinetics of thin filament activation probed by fluorescence of N-((2-(Iodoacetoxy)ethyl)-N-methyl)amino-7-nitrobenz-2-oxa-1, 3-diazole-labeled troponin I incorporated into skinned fibers of rabbit psoas muscle: implications for regulation of muscle contraction. Biophys J. 1999 Nov;77(5):2692–708. doi: 10.1016/S0006-3495(99)77103-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chandra M, Kim JJ, Solaro RJ. An improved method for exchanging troponin subunits in detergent skinned rat cardiac fiber bundles. Biochem Biophys Res Commun. 1999 Sep 16;263(1):219–23. doi: 10.1006/bbrc.1999.1341. [DOI] [PubMed] [Google Scholar]
- 107.Smith SH, Solaro RJ, Martin AF, de Tombe PP. Chimeric Troponin I and the Sarcomere Length Dependence of Calcium Sensitivity in Skinned Rat Trabeculae. Biophys J. 2003;84:251a. [Google Scholar]
- 108.Cazorla O, Le Guennec JY, White E. Length-tension relationships of sub-epicardial and sub-endocardial single ventricular myocytes from rat and ferret hearts. J Mol Cell Cardiol. 2000 May;32(5):735–44. doi: 10.1006/jmcc.2000.1115. [DOI] [PubMed] [Google Scholar]
- 109.Davis JS, Hassanzadeh S, Winitsky S, Lin H, Satorius C, Vemuri R, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001 Nov 30;107(5):631–41. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]
- 110.Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, et al. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with PKCepsilon phosphorylation. J Mol Cell Cardiol. 2006 Apr;40(4):465–73. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 111.Stelzer JE, Patel JR, Moss RL. Acceleration of stretch activation in murine myocardium due to phosphorylation of myosin regulatory light chain. J Gen Physiol. 2006 Sep;128(3):261–72. doi: 10.1085/jgp.200609547. [DOI] [PMC free article] [PubMed] [Google Scholar]