Abstract
Newly synthesized insect cuticle is soft and pale but becomes stronger (sclerotized) and often darker (pigmented) over several hours or days. The first step in the sclerotization and pigmentation pathways is the hydroxylation of tyrosine to produce 3,4-dihydroxyphenylalanine (DOPA). Tyrosine hydroxylase (TH) is known to catalyze this reaction during pigmentation, but a role for TH in sclerotization has not been documented. The goal of this study was to determine whether TH is required for cuticle sclerotization in the red flour beetle, Tribolium castaneum. We used quantitative RT-PCR to verify that TH expression occurs at the time of cuticle tanning and immunohistochemistry to confirm that TH is expressed in the epithelial cells underlying sclerotized cuticle. In addition, we found that a reduction in TH function (mediated by RNA interference) resulted in a decrease in cuticle pigmentation and a decrease in the hardness of both pigmented and colorless cuticle. These results demonstrate a requirement for TH in sclerotization as well as brown pigmentation of insect cuticle.
Keywords: insect, cuticle, tanning, sclerotization, pigmentation, eye, tyrosine hydroxylase
1. Introduction
Insect cuticle is soft and pale when it is first synthesized. As cuticle matures over a period of several hours to several days, it becomes progressively stronger (sclerotized) due to cross-linking of cuticle proteins and dehydration (Andersen, 2005). Highly sclerotized cuticle is hard and rigid, whereas less sclerotized cuticle retains some degree of flexibility. The sclerotization process is complex, but a simplified model includes the following steps: 1) hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (DOPA), 2) decarboxylation of DOPA to dopamine, 3) N-acylation of dopamine to N-acetyldopamine (NADA) or N-β-alanyldopamine (NBAD), 4) oxidation of NADA and NBAD to their corresponding quinones, and 5) reactions between the quinones or quinone derivatives with cuticle protein side chains resulting in protein cross-linking (Andersen, 2005). Newly synthesized cuticle may remain colorless or become pigmented by one or more poorly understood processes (Andersen, 2005). One type of pigmentation (quinone tanning) is associated with the covalent linkage of cuticular proteins to the ring component of NBAD; in contrast, linkage of proteins to the side chain of NADA (β-sclerotization) is correlated with colorless cuticle (Hopkins and Kramer, 1992; Andersen, 1974). Pigmentation may also arise from the incorporation of melanins (derived from DOPA or dopamine) or other pigments into the cuticle; these pigments may be uniformly dispersed throughout the cuticle or may be present in granules (Kayser, 1985). Like the sclerotization process, the synthesis of most cuticle-associated pigments begins with the production of dopa from tyrosine.
Two kinds of enzymes are known to catalyze the hydroxylation of tyrosine in insects: phenoloxidase (PO) and tyrosine hydroxylase (TH). Insect PO is classified as a tyrosinase (E.C. 1.14.18.1) because it has two catalytic functions: it can hydroxylate monophenols such as tyrosine and oxidize o-diphenols such as DOPA and dopamine (Kanost and Gorman, 2008). PO is expressed as a zymogen (proPO) in hemocytes, which release proPO into hemolymph where it is activated by proteolytic cleavage (Kanost and Gorman, 2008). The role of PO in immune-related melanization is well established, but it is unclear whether PO activity contributes to cuticle sclerotization or pigmentation. PO and tyrosinase activity have been detected in cuticle samples of many insect species; however, expression and activity of PO is not correlated with the cuticle maturation process (Barrett, 1991; Asano and Ashida, 2001; Arakane et al., 2005). Injection of a PO inhibitor into the larval stage of Protophormia terraenovae prevented the formation of black pigment in the pupal cuticle but did not affect the formation of brown pigment or sclerotization, and a similar treatment of Calliphora vomitoria had no observable effect on puparium sclerotization or brown pigmentation (Dennell, 1958). Likewise, an RNAi-mediated reduction of the concentration of PO mRNA in Tribolium castaneum had no effect on cuticle tanning (Arakane et al., 2005). Taken together, these results suggest that PO may play a part in some types of pigmentation but probably not sclerotization.
TH is a pterin-dependent monooxygenase (E.C. 1.14.16.2) (Ulrich and Hofrichter, 2007). Unlike PO, TH is a cytoplasmic enzyme. Several studies have demonstrated that TH is required for cuticle pigmentation. In Papilio xuthus and Pseudolatia separatata, TH is expressed in the epithelial cells underlying darkly pigmented (but flexible) larval cuticle; furthermore, chemical inhibition of P. xuthus TH can completely inhibit pigment formation (Futahashi and Fujiwara, 2005; Ninomiya and Hayakawa, 2007). In Manduca sexta, the amount of TH in various segments of prepupal integument correlates with degree of cuticle pigmentation (Gorman et al., 2007). In Drosophila melanogaster, alternative splicing generates two isoforms of TH; the longer, epidermal form contains a highly acidic region that is lacking in the neural form (Birman et al., 1994). DmTH mutant studies have demonstrated a requirement for TH in the pigmentation of pharate larval structures, most obviously the cephalopharyngeal skeleton and the denticle belts (Budnik and White, 1987; Neckameyer and White, 1993). In addition, patches of epithelial cells lacking DmTH function produced colorless adult cuticle, and ectopic expression of DmTH resulted in ectopic cuticle melanization (True et al., 1999). These studies prove that TH is active in one or more insect pigmentation pathways; however, they do not establish whether TH is required for sclerotization because the studies focused on flexible larval cuticle or loss of function phenotypes in tissues that were too small to evaluate for cuticle hardness.
The goal of this study was to determine whether TH is required for cuticle sclerotization as well as pigmentation. We decided to use the red flour beetle, T. castaneum, for our investigation because this insect is highly sensitive to dsRNA-mediated gene silencing and because considerable information about its cuticle biosynthesis is already known. Our study focused on two types of cuticle: the hard, brown cuticle that covers many parts of the adult insect, and the hard, transparent cuticle that covers the adult eye. Previous studies of T. castaneum cuticle indicate that its brown pigmentation results from a combination of quinone tanning and melanin synthesis (Kramer et al., 1984; Roseland et al., 1987; Arakane et al., 2005; Arakane et al., 2009b). Sclerotization of pigmented cuticle in this beetle requires quinone tanning, dehydration and probably other processes such as β-sclerotization (Roseland et al., 1987; Arakane et al., 2005; Arakane et al., 2009b; Lomakin et al., 2009). Little is known about sclerotization of insect eye cuticle, which is actually an array of colorless corneal lenses. The most abundant protein in the corneal lens of D. melanogaster is drosocrystallin; this 52 kDa polypeptide contains a cuticle protein consensus motif (RR-2 motif) but is much larger than most cuticle proteins (Komori et al., 1992; Janssens and Gehring, 1999). Whether drosocrystallin participates in β-sclerotization or other types of cross-linking is unknown. The proteins that make up the eye cuticle in T. castaneum have not yet been identified. To learn whether TH is required for sclerotization of pigmented and colorless cuticle, we cloned a TcTH cDNA, determined whether TH expression was correlated with sclerotization, and observed RNAi-mediated loss of function phenotypes to ascertain whether sclerotization was affected. Our results demonstrate that TH participates in sclerotization and as well as pigmentation in T. castaneum.
2. Materials and Methods
2.1. Insect culture
The GA-1 strain of T. castaneum was reared at 30°C under standard conditions (Beeman and Stuart, 1990).
2.2. RNA isolation, cDNA synthesis and PCR
Total RNA was isolated from whole insects by using the RNeasy mini kit (Qiagen). Synthesis of cDNA and real time PCR were done as described previously (Arakane et al., 2009b). The ribosomal protein subunit 6 (rpS6) gene was used as a reference gene.
2.3. Cloning a TcTH cDNA
The T. castaneum ortholog of M. sexta TH was identified by performing a BLAST search of the T. castaneum genome. Primers encoding the predicted start and stop codon regions (5′ ATG GCT GCT GTG GCT GCT G 3′ and 5′ TCA CTG ATA CGA CGG CGC 3′) were used to amplify a 1,653 bp cDNA fragment from first strand cDNA synthesized from RNA from adult beetles. The cDNA fragment was cloned into pCR4-TOPO (Invitrogen) and sequenced. The DNA sequence was submitted to GenBank (accession number EF592178).
2.4. Synthesis of double stranded RNA (dsRNA)
The template for TH dsRNA synthesis was a 539 bp fragment of TH cDNA produced by PCR using the primers 5′ TAA TAC GAC TCA TAG GAA GGG GCA GTA TGT CAC CTG 3′ and 5′ TAA TAC GAC TCA CTA TAG GGG TGT GGC GTT TCA AAA AGT 3′ (which encoded T7 polymerase binding sites at the 5′ ends). TH dsRNA was synthesized by using the MEGAscript RNAi kit (Ambion). Double stranded RNA for the T. castaneum vermillion gene (dsV) and enhanced green flurescent protein (dsEGFP) was synthesized as described previously (Arakane et al., 2009b).
2.5. RNAi to determine loss of function phenotypes
Last instar larvae were injected with 200 ng dsRNA. Pupae (2-3 days old) were injected with 2 ng dsRNA. Negative control insects were injected with similar amounts of EGFP dsRNA. After injection, insects were cultured at 28°C, and cuticle sclerotization and pigmentation phenotypes were monitored. Flexibility of adult cuticle was assessed by pressing on the cuticle with forceps until the cuticle became deformed. We considered cuticle to be flexible if the stress applied by the forceps caused an indentation that was reversible (i.e., the cuticle regained its original shape after the stress was removed). We considered the cuticle to be obviously sclerotized if the applied stress caused irreversible damage, for example, a crack or dent.
2.6. Characterization of TH polyclonal antiserum
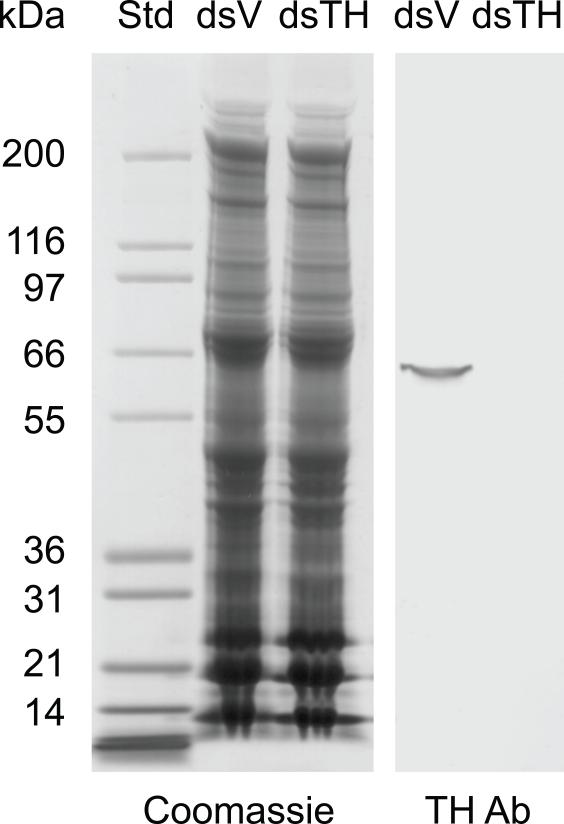
Polyclonal antiserum generated against M. sexta TH was described previously (Gorman et al., 2007). To verify that this antiserum specifically detects TcTH, we performed the following immunoblot analysis. One day old pupae were injected with 200 ng vermillion (V) dsRNA (which should have no effect on the concentration of TH) or TH dsRNA (which should knock down the concentration of TH). After injection, pupae were maintained at 28°C for 5 days at which time they began to molt to the adult stage. Elytra were removed from molting or newly eclosed (<9 hours old) adults. Six pairs of elytra per treatment were homogenized in 90 μl SDS-PAGE sample buffer, heated at 95°C for 10 minutes, and centrifuged for 1 minute to pellet insoluble material. Thirty μl samples were analyzed by SDS-PAGE followed by Coomassie staining or western blotting using the MsTH polyclonal antiserum.
2.7. Immunohistochemistry
Immunostaining was performed as described previously (Arakane et al., 2009a). Transverse sections (5-10 μm) of 0 d old adults were stained with anti-M. sexta TH polyclonal antibody (Gorman et al., 2007), which detects a single protein band of approximately 60 kDa on a western blot of T. castaneum protein extracts (see Figure 3). Cryosections were rinsed with PBST (PBS with 0.1% Tween 20) three times for 5 min to remove the freezing compound and blocked with blocking buffer (PBST containing 2% bovine serum albumin) for 1 h at room temperature. Then the sections were incubated with anti-MsTH or pre-immune serum (1:1000 in blocking buffer) for 2 h at room temperature. After rinsing the sections with PBST, Alexa Fluor 488 goat anti-rabbit IgG secondary antibody (1:500 in blocking buffer, Invitrogen) was applied for 1 h. After rinsing the sections three times with PBST, Rhodamine-conjugated chitin-binding probe (1:100, New England BioLabs) was applied at 4°C for 12 h. The sections were rinsed with PBST three times at room temperature and then stained with 4′,6-diamidine-2-phenylindol dihydrochloride (DAPI, 2 μg/ml). Fluorescence was observed using a Lica CTR6500 inverse microscope with appropriate filters.
Figure 3.
Characterization of the TH polyclonal antiserum. Protein extracts of elytra from dsV and dsTH insects were analyzed by SDS-PAGE and Coomassie staining (left panel) and western blotting (right panel). Commassie staining showed that similar amounts of protein were present in the dsV and dsTH samples. Western blotting demonstrated that the TH antiserum detected a single band of the expected mass (~60 kDa) in dsV but not dsTH protein extract.
3. Results and Discussion
3.1. TH cDNA sequence
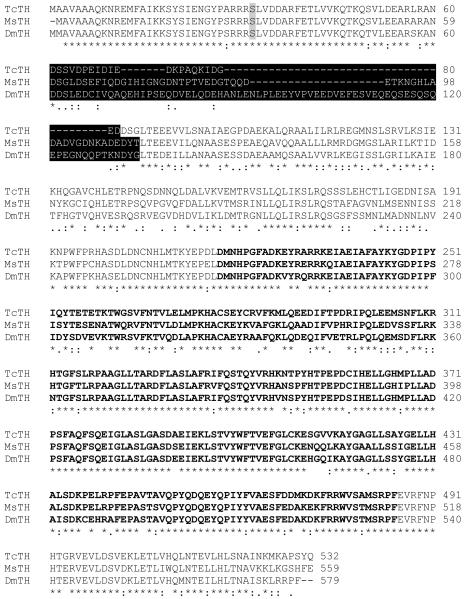
The T. castaneum ortholog of M. sexta TH was identified by performing a BLAST search of the T. castaneum genome. Only one highly similar gene was discovered. Primers encoding the predicted start and stop codon regions were used to amplify a 1,653 bp cDNA that included the entire open reading frame. The predicted amino acid sequence is 532 residues with a predicted mass of 60.4 kDa and a predicted pI of 5.35. The TcTH amino acid sequence is ~70% identical to MsTH and the epidermal form of DmTH (Figure 1). Consistent with our expectation that TcTH is a cytoplasmic protein, we did not identify a signal peptide. Like MsTH and the epidermal form of DmTH, the TcTH sequence encodes an acidic region (pI = 3.51) in the amino-terminal regulatory domain. The acidic region is encoded by one exon in TcTH but two exons in MsTH and DmTH (Gorman et al., 2007; Birman et al., 1994). The precise function of the acidic domain is unknown, but it is associated with higher constitutive activity and lower sensitivity to feed back inhibition of the epidermal form of DmTH (Vie et al., 1999). DmTH is regulated by phosphorylation of Ser32 by a cAMP-dependent protein kinase (Vie et al., 1999), and this serine is conserved in TcTH. TcTH also contains a conserved carboxyl-terminal region that probably acts in tetramer formation (Goodwill et al., 1997).
Figure 1.
Amino acid alignment of TH sequences from T. castaneum, M. sexta and D. melanogaster. DmTH Ser32 (shaded) is phosphorylated by cAMP-dependent protein kinase and is conserved in TcTH. An acidic region with low sequence conservation is highlighted in black. The putative catalytic domain is indicated by bold type. The carboxyl-terminal region (in standard type) is predicted to be a tetramerization domain. (The putative domain boundaries were selected based on the experimentally determined domain boundaries of rat TH [Goodwill et al., 1997].)
3.2. TH expression
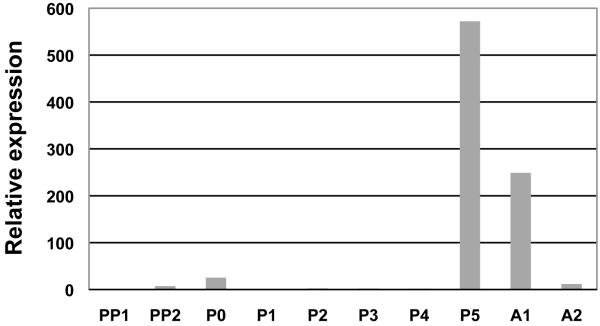
Semiquantitative RT-PCR detected TH transcripts in all developmental stages (eggs, larvae, pupae and adults [data not shown]). To learn whether expression of TH is correlated with cuticle sclerotization and pigmentation, we used quantitative RT-PCR to determine the relative level of TH transcript abundance at one day intervals in the prepupal through adult stages (Figure 2). We found that transcript abundance was highest right before and after molting, when newly formed cuticle begins to sclerotize and darken, and that transcript abundance during the larva to pupa molt was less than during the pupa to adult molt. Because adult cuticle is highly tanned (hard and dark) whereas pupal cuticle is lightly tanned (softer and lighter) (see Figure 5), these data demonstrate a correlation between TH transcript abundance and degree of tanning.
Figure 2.
Developmental expression profile of TH. The level of transcript abundance relative to that of rpS6 in whole insects was determined by quantitative RT-PCR. PP0, 0-1 d-old pharate pupae; PP1, 1-2 d-old pharate pupae; P0, 0 d-old pupae; P1, 1 d-old pupae; P2, 2 d-old pupae; P3, 3 d-old pupae; P4, 4 d-old pupae; P5, 5 d-old pupae; A0, 0 d-old adults and A1, 7 d-old adults. Expression levels for TcTH are presented relative to the levels of expression in the earliest developmental stage analyzed (PP0).
Figure 5.
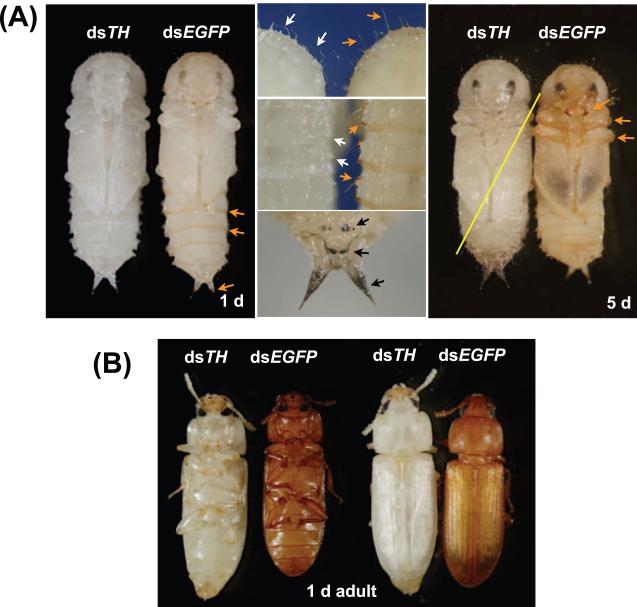
Phenotypes associated with loss of TH function. For each pair of insects, the insect on the left was injected with dsTH and the insect on the right was injected with dsEGFP (as a negative control). Pupae in the A panels were injected with 200 ng dsRNA during the last larval instar. The pupae in the first four panels were one day old, while the pupae in the last panel were five days old. Control pupae had a normal tanning pattern, with brown pigment in the posterior edge of the abdominal segments, urogomphi, bristles, gin traps, mandibles, and legs (orange arrows). The dsTH pupae did not develop this pigmentation pattern (white arrrows indicate bristles [middle, top panel] and gin traps [center]). In addition, the bristles of dsTH were limp and curved rather than stiff and straight (compare bristles of dsTH and dsEGFP insects in middle, top panel). The dsTH pupae developed black spots (middle, bottom panel) that were not seen in control pupae. The yellow line indicates that the insect was very weak and died before adult eclosion. Adults in panel B were injected with 2 ng of dsRNA in the pupal stage. The dsTH adults were very pale compared with dsEGFP controls.
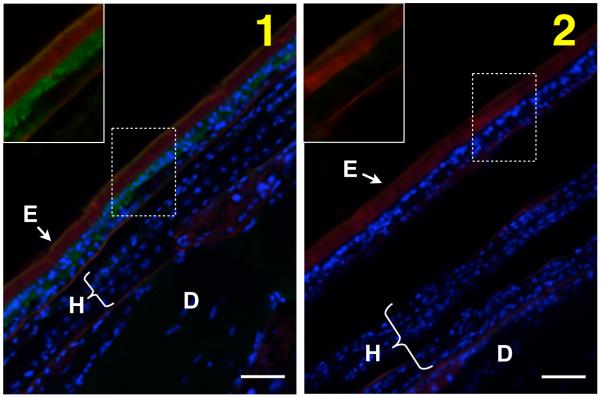
We predicted, based on studies of other insect species, that TcTH would be expressed in epithelial cells underlying pigmented cuticle. To determine the location of TH in newly eclosed adults, we performed immunohistochemistry with anti-TH antibodies. The specificity of the antiserum was first verified by western blot analysis of protein extracts from control (dsV) and dsTH insects (Figure 3). We observed TH staining in the epithelial cells underlying the highly sclerotized and darkly pigmented cuticle of the elytra (forewing) (Figure 4) and in other epithelial cells that secrete hard, dark cuticle (data not shown). We did not detect TH in the cells underlying hind wing cuticle (which is less sclerotized and and lightly pigmented) nor in the cells that secrete dorsal abdominal cuticle (which is flexible and transparent) (Figure 4). In addition, within the elytra, TH was detected in the dorsal layer of epithelial cells, which produce hard, brown cuticle, but not in the ventral epithelial cells, which secrete a pale, membranous cuticle (Figure 4). These results suggest that in newly eclosed adults TH expression is higher in cells that secrete darkly pigmented, highly sclerotized cuticle than it is in cells that secrete paler, more flexible cuticle. We do not know whether the concentration of TH in the latter type of cells is low (below the detection threshold of the technique used) or whether TH is absent in these cells. We did not detect TH in the epithelial cells that secrete the hard, transparent eye cuticle (data not shown), but the eye cuticle and the cells that secrete it become separated during the pupal stage; therefore, if TH participates in sclerotization of eye cuticle, it may be present in pupal rather than adult eye epithelial cells.
Figure 4.
Localization of TH protein. The location of TH protein in newly eclosed adults was determined by immunohistochemistry. The cryosection shown in panel 1 was incubated with polyclonal antiserum made against MsTH. The cryosection shown in panel 2 was incubated with preimmune serum. Anti-TH antibodies were detected with Alexa Fluor 488 conjugated anti-rabbit IgG antibodies (green). Nuclei were stained with DAPI (blue). Cuticle was stained with a Rhodamine-conjugated chitin-binding probe (red). Portions of the images in panels 1 and 2 (dashed lines) are shown without the DAPI signal to increase the visibility of any TH staining (boxed inset image). TH was detected in epithelial cells underlying the cuticle on the dorsal side of the elytra, which becomes highly sclerotized and pigmented after eclosion. TH was not detected in cells of the hind wing, which are less sclerotized and almost colorless, nor was it detected in the epithelial cells underlying the dorsal abdominal cuticle, which is flexible and colorless. E = elytron, H = hindwing, D = dorsal abdomen. Scale bar = 20 μm.
3.3. Loss of function phenotypes
RNAi was used to determine whether TH is required for cuticle pigmentation and sclerotization. Last instar larvae injected with 200 ng dsTH molted to the pupal stage but died before adult eclosion. Control pupae developed a normal pigmentation pattern, which included several brown structures: the posterior edge of the abdominal segments, urogomphi, bristles, gin traps, mandibles, and legs. In addition, in control pupae, dark pigment in the developing adult hind wing could be seen. In contrast, dsTH pupae had none of the usual brown pigment nor the dark hind wing pigment (Figure 5A). Surprisingly, the cuticle of dsTH pupae developed some small black or very dark brown spots, and the urogomphi of dsTH pupae were black rather than brown (Figure 5A). This dark pigment may be melanin. Perhaps an absence of TH activity causes an accumulation of tyrosine in the cuticle where PO could convert it to dopa-quinone and thus initiate melanin synthesis. Normal pupal cuticle is not highly sclerotized, thus it was difficult to determine whether dsTH pupal cuticle was less sclerotized than control pupal cuticle; however, there was an obvious difference in the degree of sclerotization of the bristles. The bristles of dsEGFP pupae were straight and stiff, but many of the bristles of dsTH pupae were curved and limp (Figure 5A). This floppy bristle phenotype suggests that a decrease in TH function leads to a decrease in sclerotization.
We wanted to observe the effect of dsTH treatment on adult cuticle, but larvae injected with 200 ng dsTH died during the pupal stage. To circumvent this problem, we injected pupae with 2 ng dsTH to induce a hypomorphic phenotype in adults. Control adults developed a typical dark brown color within a few days; in contrast, the cuticle of dsTH adults were still pale at the end of the 1 week observation period (Figure 5B). Very slight tanning of dsTH adults was probably the result of incomplete suppression of TH activity. To determine whether the TH knock down had an effect on sclerotization, we examined the hardness of two types of cuticle: the dorsal thoracic cuticle, which is usually hard and dark brown, and the eye cuticle, which is hard and colorless. By pressing on the thoracic or eye cuticle with forceps, we obtained a qualitative assessment of its flexibility (see section 2.5). The cuticle of control adults was still flexible 24 hours post-eclosion; therefore, the sclerotization process was not complete. At 2 days post eclosion, sclerotization was more obvious: all control adults (n = 14) had dorsal thoracic cuticle that cracked when a stress was applied to it, and 64% had eye cuticle that was irreversibly damaged by this treatment. At 3 days post-eclosion, the dorsal thoracic and eye cuticle of all control adults (n = 16) was damaged by the application of stress. In contrast, all dsTH adults had flexible thoracic and eye cuticle at 3 days (n = 14) and 7 days (n = 11) post-eclosion. These data demonstrate that TH is required for hardening of the pigmented thoracic cuticle and unpigmented eye cuticle.
3.4. Conclusions
The goal of this study was to determine whether TH is required for cuticle sclerotization. Using quantitative RT-PCR, we demonstrated that TH expression occurs at the time of cuticle maturation and that TH transcript abundance correlates with degree of cuticle tanning. Immunostaining of TH protein in newly eclosed adults demonstrated that TH is expressed in epithelial cells that secrete brown, sclerotized cuticle. Finally, we found that reducing TH function resulted in decreased cuticle hardness in addition to decreased pigmentation. These results demonstrate a requirement for TH in sclerotization as well as brown pigmentation of insect cuticle.
Our current working model of the role of TH in sclerotization is the following: within cuticle-secreting epithelial cells, TH hydroxylates tyrosine to produce dopa, dopa is decarboxylated to form dopamine, dopamine is converted to NADA and NBAD, and these two substrates are secreted into the cuticle where they participate in protein cross-linking. Our results support this model by demonstrating that TH is present in the cuticle-secreting cells at the expected time and by demonstrating a decrease in sclerotization when TH function is reduced. Previous studies suggest that another tyrosine hydroxylating enzyme, PO, is unlikely to have this role (Dennell, 1958; Barrett, 1991; Asano and Ashida, 2001; Arakane et al., 2005). Our RNAi results demonstrate that TH is required for sclerotization of pigmented and colorless cuticle; however, additional experiments are needed to determine whether TH is expressed in the cells that secrete transparent eye cuticle and whether our model applies to sclerotization of colorless cuticle.
Acknowledgements
We thank Drs. Michael Kanost, Karl Kramer and Richard Beeman for their support of this study and Dr. Michael Kanost for critical reading of the manuscript. We thank Dr. John True for information about DmTH mutant phenotypes. This is contribution 10-043-J from the Kansas Agricultural Experiment Station. The project described was supported by Grant Number R01AI070864 from the National Institute of Allergy and Infectious Diseases and I0S0726425 from the National Science Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen SO. Evidence for two mechanisms of sclerotisation in insect cuticle. Nature. 1974;251:507–508. doi: 10.1038/251507a0. [DOI] [PubMed] [Google Scholar]
- Andersen SO. Cuticular sclerotization and tanning. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 4. Elsevier; Oxford: 2005. pp. 145–170. [Google Scholar]
- Arakane Y, Muthukrishnan S, Beeman RW, Kanost MR, Kramer KJ. Laccase 2 is the phenoloxidase gene required for beetle cuticle tanning. Proc. Natl. Acad. Sci. USA. 2005;102:11337–11342. doi: 10.1073/pnas.0504982102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakane Y, Dixit R, Begum K, Park Y, Specht CA, Merzendorfer H, Kramer KJ, Muthukrishnan S, Beeman RW. Analysis of functions of the chitin deacetylase gene family in Tribolium castaneum. Insect Biochem. Mol. Biol. 2009a;39:355–365. doi: 10.1016/j.ibmb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- Arakane Y, Lomakin J, Beeman RW, Muthukrishnan S, Gehrke SH, Kanost MR, Kramer KJ. Molecular and functional analyses of amino acid decarboxylases involved in cuticle tanning in Tribolium castaneum. J. Biol. Chem. 2009b;284:16584–16594. doi: 10.1074/jbc.M901629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Ashida M. Cuticular pro-phenoloxidase of the silkworm, Bombyx mori: purification and demonsration of its transport from hemolymph. J. Biol. Chem. 2001;14:11100–11112. doi: 10.1074/jbc.M008426200. [DOI] [PubMed] [Google Scholar]
- Barrett FM. Phenoloxidases and the integument. In: Binnington K, Retnakaron A, editors. Physiology of the Insect Epidermis. CSIRO Publications; East Melbourne, Victoria: 1991. [Google Scholar]
- Beeman RW, Stuart JJ. A gene for lindane + cyclodiene resistance in the red flour beetle (Coleoptera, Tenebrionidae) J, Econ, Entomol. 1990;83:1745–1751. [Google Scholar]
- Birman S, Morgan B, Anzivino M, Hirsh J. A novel and major isoform of tyrosine hydroxylase in Drosophila is generated by alternative RNA processing. J. Biol. Chem. 1994;269:26559–26567. [PubMed] [Google Scholar]
- Budnick V, White K. Genetic dissection of dopamine and serotonin synthesis in the nervous system of Drosophila melanogaster. J. Neurogenet. 1987;4:309–314. [PubMed] [Google Scholar]
- Dennell R. The amino acid metabolism of a developing insect cuticle: the larval cuticle and puparium of Calliphora vomitoria III. The formation of the puparium. Proc. R. Soc. Lond. B. Biol. Sci. 1958;149:176–183. doi: 10.1098/rspb.1958.0060. [DOI] [PubMed] [Google Scholar]
- Futahashi R, Fujiwara H. Melanin-synthesis enzymes coregulates stage-specific larval cuticular markings in the swallowtail butterfly, Papilio xuthus. Dev. Genes. Evol. 2005;215:519–529. doi: 10.1007/s00427-005-0014-y. [DOI] [PubMed] [Google Scholar]
- Goodwill KE, Sabatier C, Marks C, Raag R, Fitzpatrick PF, Stevens RC. Crystal structure of tyrosine hydroxylase at 2.3 angstroms and its implications for inherited neurodegenerative diseases. Nat. Struct. Biol. 1997;4:578–585. doi: 10.1038/nsb0797-578. [DOI] [PubMed] [Google Scholar]
- Gorman MJ, An C, Kanost MR. Characterization of tyrosine hydroxylase from Manduca sexta. Insect Biochem. Mol. Biol. 2007;37:1327–1337. doi: 10.1016/j.ibmb.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins TL, Kramer KJ. Insect cuticle sclerotization. Annu. Rev. Entomol. 1992;37:273–302. [Google Scholar]
- Janssens H, Gehring WJ. Isolation and characterization of drosocrystallin, a lens crystallin gene of Drosophila melanogaster. Dev. Biol. 1999;207:204–214. doi: 10.1006/dbio.1998.9170. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Gorman MJ. Phenoloxidases in insect immunity. In: Beckage N, editor. Insect Immunology. Academic Press/Elsevier; San Diego: 2008. pp. 69–96. [Google Scholar]
- Kayser H. Pigments. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Pergamon Press; New York: 1985. pp. 367–415. [Google Scholar]
- Komori N, Usukura J, Matsumoto H. Drosocrystallin, a major 52 kDa glycoprotein of the Drosophila melanogaster corneal lens. J. Cell Sci. 1992;102:191–201. doi: 10.1242/jcs.102.2.191. [DOI] [PubMed] [Google Scholar]
- Kramer KJ, Morgan TD, Hopkins TL, Roseland CR, Aso Y, Beeman RW, Lookhart GL. Catecholamines and β-alanine in the red flour beetle, Tribolium castaneum: roles in cuticle sclerotization and melanization. Insect Biochem. 1984;14:293–298. [Google Scholar]
- Lomakin J, Eichler C, Arakane Y, Kramer KJ, Beeman RW, Kanost MR, Gehrke SH. Mechanical properties of beetle elytral cuticle, a hierarchically ordered, multicomponent biomaterial. Polymeric Materials: Science and Engineering. 2009;100:470–471. [Google Scholar]
- Neckameyer WS, White K. Drosophila tyrosine hydroxylase is encoded by the pale locus. J. Neurogenet. 1993;8:189–199. doi: 10.3109/01677069309083448. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Hayakawa Y. Insect cytokine, growth-blocking peptide, is a primary regulator of melanin-synthesis enzymes in armyworm larval cuticle. FEBS J. 2007;274:1768–1777. doi: 10.1111/j.1742-4658.2007.05724.x. [DOI] [PubMed] [Google Scholar]
- Roseland CR, Kramer KJ, Hopkins TL. Cuticular strength and pigmentation of rust-red and black strains of Tribolium castaneum. Insect Biochem. 1987;17:21–28. [Google Scholar]
- True JR, Edwards KA, Yamamoto D, Carroll SB. Drosophila wing melanin patterns form by vein-dependent elaboration of enzymatic prepatterns. Curr. Biol. 1999;9:1382–1391. doi: 10.1016/s0960-9822(00)80083-4. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Hofrichter M. Enzymatic hydroxylation of aromatic compounds. Cell. Mol. Life Sci. 2007;64:271–293. doi: 10.1007/s00018-007-6362-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vie A, Cigna M, Toci R, Birman S. Differential regulation of Drosophila tyrosine hydroxylase isoforms by dopamine binding and cAMP-dependent phosphorylation. J. Biol. Chem. 1999;274:16788–16795. doi: 10.1074/jbc.274.24.16788. [DOI] [PubMed] [Google Scholar]