Abstract
Ca2+ desensitization of myofilaments is indicated as a primary mechanism for the pathogenesis of familial dilated cardiomyopathy (DCM) associated with the deletion of lysine 210 (ΔK210) in cardiac troponin T (cTnT). ΔK210 knock-in mice closely recapitulate the clinical phenotypes documented in patients with this mutation. Considerable evidence supports the proposition that phosphorylation of cardiac sarcomeric proteins is a key modulator of function and may exacerbate the effect of the deletion. In this study we investigate the impact of K210 deletion on phosphorylation propensity of sarcomeric proteins. Analysis of cardiac myofibrils isolated from ΔK210 hearts identified a decrease in phosphorylation of cTnI (46%), cTnT (30%) and MyBP-C (32%) compared with wild type controls. Interestingly, immunoblot analyses with phospho-specific antibodies show augmented phosphorylation of cTnT-Thr203 (28%) and decreased phosphorylation of cTnI-Ser23/24 (41%) in mutant myocardium. In vitro kinase assays indicate that ΔK210 increases phosphorylation propensity of cTnT-Thr203 three fold, without changing cTnI-Ser23/24 phosphorylation. Molecular modeling of cTnT-ΔK210 structure reveals changes in the electrostatic environment of cTnT helix (residues 203–224) that lead to a more basic environment around Thr203, which may explain the enhanced PKC-dependent phosphorylation. In addition, yeast two-hybrid assays indicate that cTnT-ΔK210 binds stronger to cTnI compared with cTnT-wt. Collectively, our observations suggest that cardiomyopathy-causing ΔK210 has far-reaching effects influencing cTnI-cTnT binding and posttranslational modifications of key sarcomeric proteins.
Keywords: cardiomyopathy, sarcomeric, cardiac troponin T, K210, phosphorylation
INTRODUCTION
Cardiac myofilaments’ response to fluctuating intracellular Ca2+ conditions forms a mechanistic basis for contractile dysfunction associated with a variety of pathological conditions. Cardiac troponin (cTn) is the Ca2+ sensor of the cardiac contractile apparatus. As a heterotrimeric complex, it consists of a calcium-binding protein, troponin C (cTnC), an actomyosin MgATPase-inhibiting protein, troponin I (cTnI) and a tropomyosin-binding protein, troponin T (cTnT) [1]. At low [Ca2+]i, cTnI is anchored to the actin filament and through the action of cTnT holds tropomyosin (Tm) in a position that inhibits myosin binding to actin thus preventing force-generating cross-bridge formation. At high [Ca2+], cTnC undergoes a Ca2+-induced conformational change that enhances its affinity for cTnI, relieving it’s binding to actin. This movement is transmitted through cTnT to Tm, which then slides deeper into the groove formed by the actin filament, facilitating myosin-actin cross-bridge formation [2].
Advances in molecular genetic studies during the past decade have identified 37 mutations in cTnT associated with different forms of cardiomyopathies, either dilated (DCM) or hypertrophic (HCM) (www.cardiogenomics.med.harvard.edu). The underlying molecular mechanism by which missense or deletion mutations in cTnT lead to the pathogenesis of distinct and often lethal forms of cardiomyopathy remains undetermined [3]. Functional analyses indicate that HCM- and DCM-causing mutations in cTnT have distinct and divergent effects on the Ca2+ sensitivity of myofilaments [4]. It was found that at least 80% of HCM mutations increased Ca2+ sensitivity of force development. In contrast, a reduced Ca2+ sensitivity seems to be an essential property of the DCM-causing mutations [5].
Deletion of lysine 210 (ΔK210) was the first mutation in the human cTnT gene that had been linked to familial primary DCM [6]. Du et al [7], generated a knock-in mouse model in which the three base pairs coding for the residue K210 were deleted from endogenous TNNT2 genes by gene-targeting technology. The knock-in mice closely recapitulated the clinical phenotypes documented in patients with this mutation, i.e. enlarged hearts, heart failure, and a high incidence of premature death. Yet, the precise molecular mechanism by which ΔK210 leads to DCM remains unresolved. Mechanical experiments with permeabilized (skinned) fibers from mutant hearts demonstrated that ΔK210 has a Ca2+-desensitizing effect on cardiac myofilaments without affecting maximum force [7]. Unlike permeabilized fibers, intact cardiomyocytes isolated from the same hearts showed a depressed contractile response to Ca2+ that could not be adequately explained by the deletion of K210 residue [7]. We propose that phosphorylation of cardiac sarcomeric proteins, a key modulator of function, may be responsible for the discrepant results obtained with skinned fibers and intact cardiomyocytes. In general, mechanical experiments with cardiac fibers containing cardiomyopathy causing cTnT mutants (e.g. R92W, R92L) show a surprisingly mild effect that by itself may not account for the full clinical phenotype associated with those mutations [3]. Phosphorylation moieties being labile, it is no surprising that experiments with permeabilized fibers, where no special precaution is taken to preserve the endogenous phosphorylation state, miss this important functional effect observed with intact cardiomyocytes.
Phosphorylation of cardiac troponin has been shown to influence the myofilaments response to Ca2+ [8, 9]. cTnI has three main phosphorylation clusters (Ser23/Ser24, Ser43/Ser45 and Thr144) that exert distinct effects on function[8]. Phosphorylation of Ser23/Ser24, desensitizes the myofilaments to Ca2+, without changing maximal force. On the other hand, Thr144 phosphorylation seems to attenuate the desensitizing effect of Ser23/Ser24 phosphorylation [10]. Phosphorylation of Ser43/Ser45 cluster has a depressing effect on maximal actomyosin Mg-ATPase rate and Ca2+-activated force [11]. cTnT also contains three phosphorylation clusters: Ser1; Thr194/Ser198/Thr203; and Ser275/Thr284 – human sequence notation [2]. T203 was identified as the functionally significant phosphorylation site of cTnT; the other sites do not seem to have a considerable functional effect by themselves [12]. Phosphorylation of T203 leads to a significant myofilament Ca2+ desensitization and a decrease in maximal force, actomyosin Mg-ATPase rate and tension cost.
The aim of the present study is to understand better the mechanism by which ΔK210 exerts its detrimental effect on cardiac myofilament function. Using isolated myofibrils from the hearts of WT and ΔK210 mutant mice, we ask whether the presence of cTnT-ΔK210 in the myofilament milieu alters the phosphorylation propensity of key sarcomeric proteins. We find a decrease in phosphorylation of cTnI-Ser23/Ser24 and MyBP-C, and an increase in cTnT-Thr203 phosphorylation. In vitro kinase assays also show augmentation of Thr203 phosphorylation in cTn-ΔK210 compared with WT. In addition, using a yeast two-hybrid assay we show that cTnT-ΔK210 has a higher affinity for cTnI compared with cTnT-WT. Molecular modeling indicates that ΔK210 may lead to changes in the electrostatic environment of cTnT helix 203-224 that: i) alter intra-troponin I–T binding dynamics, and ii) directly or indirectly affect phosphorylation propensity of cTnT, cTnI and MyBP-C.
MATERIALS AND METHODS
Generation of cTnT-ΔK210 construct
To produce the cTnT-ΔK210, QuickChange site-directed mutagenesis (Stratagene) was first carried out according to the manufacturer’s recommendations, using mouse cardiac cTnT-wt cDNA as template and following primers: 5'-GAGAGAGAAGAAGAAGATCCTGGCAGAGAGG-3' and 5'-CCTCTCTGCCAGGATCTTC TTCTTCTCTCTC-3'. The identity of construct was verified by DNA sequencing.
Troponin Expression and Purification
Recombinant mouse cardiac cTnT, cTnI and cTnC were expressed and purified as previously described [11, 12]. Briefly, cTnT-ΔK210 was expressed in BL21(DE3) cells grown overnight in Luria Broth supplemented with 30µg/ml kanamycin. The cell pellet was collected by centrifugation at 6000×g for 10 min at 4 °C and then resuspended in TnT-Buffer A containing 25 mM Tris (pH 8.0), 6 M urea, 1 mM EDTA, 0.1 mM AEBSF, 1 mM benzamidine, 1 mM DTT and 0.5% Triton X-100. The cells were lysed by sonication on ice, followed by 60 min of centrifugation at 48,000×g at 4°C. The supernatant fraction was subjected to ammonium sulfate fractionation. The obtained pellet was solubilized in TnT-Buffer A, and then dialyzed at 4°C against the same buffer. The dialyzed sample was applied on a DEAE Fast Flow Sepharose column (GE Healthcare) connected to a FPLC System (Amersham Biosciences). cTnT-ΔK210 was eluted with a 0.0–0.5 M KCl gradient in TnT-Buffer A. The fractions containing cTnT were analyzed by SDS-PAGE and those containing more than 90% pure cTnT were pooled, extensively dialyzed against 0.4% formic acid in H2O, lyophilized and stored in powder form at -80°C.
Recombinant Troponin Complex Reconstitution
Recombinant heterotrimeric troponin complexes were prepared according to stringent conditions designed to avoid the presence of any contaminants, as previously described [12]. Recombinant heterotrimeric cTn complexes were reconstituted by mixing equimolar amounts of cTnT (either wt or ΔK210), cTnI and cTnC in a solubilization buffer containing 6 M Urea, 50 mM Tris pH 8.0, 1 M NaCl, 5 mM MgCl2, 1 mM CaCl2, 1 mM DTT. The solution was subjected to sequential dialysis using 0.7 M NaCl, 0.4 M NaCl, and finally 0.05 M NaCl in the above buffer minus urea (CaCl2 was omitted from the final dialysis step). A high performance Resource Q column (GE Healthcare) was then used to further purify the cTn complex from any contaminants. Troponin complex elutes as a sharp peak well separated from any unincorporated monomeric TnI or TnC subunits.
PKC expression, purification and specific activity determination
Human PKC isoforms α, βII,ε ,δ cDNAs were purchased from American Type Culture Collection and subcloned into pVL1392 or pVL1393 baculovirus vectors (BD Biosciences). The recombinant proteins were produced using the BaculoGold expression system (BD Biosciences) in Sf9 cells, as previously reported [12]. Specific activity of each purified PKC was determined using myelin basic protein (MBP), an equally preferred substrate to all 4 isozymes. The specific activities determined were 6,015 units/mg PKC-α, 873 units/mg PKC-βII, 2,454 units/mg PKC-ε, 3,844 units/mg PKC-δ. Units of PKC activity were defined as 1nmol of 32P incorporated into MBP per minute. In vitro kinase assays with cTnT-WT (various concentrations) as substrate were carried for 30 min at 30°C using 0.15 units PKC/reaction.
Phosphorylation of cTnT and cTn by PKC-α
In vitro kinase assays were performed using 1ng/reaction recombinant PKC-α [12] in the presence of cTnT-wt, cTnT-ΔK210, cTn-wt or cTn-ΔK210. Various amounts of proteins (1–6 µM) were incubated with PKC-α in the presence of 20 mM Hepes pH 7.5, 150 mM NaCl (or 300mM NaCl for cTnT samples), 5mM MgCl2, 0.1 mM CaCl2, 20 mM NaF, 0.5 mM AEBSF, 2µg/ml Pepstatin, 10µg/ml Leupeptin, 2mM DTT, 0.3 mM phosphatidylserine, 0.02 mM diacylglycerol, and 0.4 mM ATP (Sigma) in a VorTemp 56™ Shaking Incubator (Alpco Diagnostics). All the reactions were carried out at 30°C for 15min (20 µl final volume). Phosphorylation was shown to occur at a constant rate over the first 30 min of the reaction for cTnT sites Thr203 and Thr284, but not cTnI sites Ser23/Ser24.
Myofibrillar isolation
Myofibrils were isolated from left ventricular tissue of 4 and 8 week-old wild type (WT), 8 and 24 week-old TNNT2+/ΔK210 heterozygous (HT), and 4 week-old TNNT2ΔK210/ΔK210 homozygous (HM) mice hearts as previously reported [13]. Briefly, left ventricular tissue was homogenized with a dounce homogenizer in an ice cold buffer containing 75 mM KCl, 10 mM Imidazole (pH 7.2), 2mM MgCl2, 2 mM EGTA, 1 mM NaN3, 1% Triton X-100, 1mM DTT and a cocktail of protease (0.25mM AEBSF, 1.25 µg/ml Leupeptin, 1.25 µg/ml Pepstatin A, 1.25 µg/ml Aprotinin) and phosphatase inhibitors (50mM NaF, 0.25mM Na3VO4). After a series of centrifugations and washes in the same buffer, the pure myofibrillar pellet was resuspended in 50% glycerol, 30 mM KCl, 10 mM MOPS (pH 7.0), 1 mM MgCl2, 1 mM DTT and a cocktail of protease and phosphatase inhibitors (see above), and were either used for analysis or stored at −20°C. There were no differences in phosphorylation pattern between fresh or −20°C stored samples. It is worth mentioning that due to the high glycerol content the myofibrils do not freeze during storage at −20°C.
Sarcomeric proteins’ phosphorylation
For analysis, myofibrils were resuspended in loading buffer (8M Urea, 2M thiourea, 50 mM Tris-HCl (pH 6.8), 75 mM DTT and 0.05% bromophenol blue). Note that SDS was omitted from the sample-loading buffer because it was found to interfere with ProQ Diamond staining, giving false positives (personal observation). The RC DC Kit (BioRad) was used according to the manufacturer’s instructions to determine protein concentrations of isolated mouse cardiac myofibrils. 8µg of denatured myofibrils were then separated on a homemade 12.5% Tri-Gly gel. For phosphoproteins detection, the ProQ Diamond staining protocol (Molecular Probes) was used with minor modifications. Phosphoproteins were visualized in gel using a Typhoon-9410 (GE Healthcare). Same gels were then incubated overnight with SYPRO Ruby stain (Molecular Probes) and rescanned for the determination of total proteins optical densities. Phosphorylation levels of sarcomeric proteins were determined from the optical density ratio of phosphorylated to total protein using ImageQuant TL software (GE Healthcare). Actin levels were used to verify equal loading of each sample and if necessary to correct for small variations. All samples were run simultaneously on the same gel.
Immunoblot analysis
Site-specific phosphorylation of cTnT at Thr203 and Thr284 (control) was followed using custom made phospho-specific antibodies purified by peptide-affinity chromatography, as previously described [14]. Phospho-specific antibodies were thoroughly characterized for specificity by peptide competition assays as described [14]. The phosphor-antibodies are specific to phosphorylated residues and have no reactivity with dephosphorylated (following phosphatase treatment) or non-phosphorylated cTnT. Moreover, the antibodies are inhibited by the phospho-peptide used as immunogen but not the non-phospho-peptide [14]. Total cTnT was determined by immunoblotting with either CT3 antibody (provided by the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the Department of Biological Sciences, University of Iowa) or JLT12 antibody (Sigma). cTnI-S23/24 phospho-antibody was from Cell Signaling Technology and pan-cTnI-C5 antibody was from Fitzgerald Industries. Anti-actin (Sigma) antibody was used to verify and correct for small variations in loading. Unless otherwise noted in the text, proteins were separated by 12.5% SDS-PAGE, transferred to a Hybond-LF membrane (GE Healthcare), blocked for 1–2 hr at room temperature in 5% ECF blocking agent (GE Healthcare) and incubated overnight in primary antibodies. After respective secondary antibody incubation, reactivity was detected using the ECF kit (GE Healthcare) according to the manufacturer’s recommendations. Immunoblots were the analyzed with the ImageQuant TL software. The efficiency of protein transfer from the gel to the membrane was determined by staining the gel with Coomassie brilliant blue R-250.
Yeast two-hybrid assays
cTnI and cTnT (WT or –ΔK210) cDNA were subcloned into the Matchmaker Gal4 two-hybrid system 3 (Clontech) vectors pGADT7 and pGBKT7 [15]. pGAD-cTnI and pGBK-cTnT were co-transformed into the lacZ phenotype yeast strain AH109, using a lithium acetate method. Only yeast cells carrying both plasmids grew on the selective plates lacking nutritional markers Leu/Trp. For the β-gal (or colony-lift) assay, freshly transformed yeast cells that grew on selective media were replica-plated to filter papers, immersed into liquid nitrogen to lyse the cells, placed on filters pre-soaked in 49 mM X-β-gal solution and incubated at 30°C for up to 4 hr. In the α-gal assays yeast transformants were spread on plates containing X-α-gal (40mg/mL, Sigma), incubated at 30°C and then checked for color development.
Protein Modeling
Three dimensional modeling of cTnT helix 1 (residues 202–224) containing phosphorylation residue Thr203 and lacking Lys210, was performed with the 3D-JIGSAW program [16–18] based on the homologous structure of the partial cardiac troponin solved by x-ray diffraction [19]. cTnT-H1-WT and modeled cTnT-H1-ΔK210 graphical models were constructed with the WebLab ViewerPro molecular simulations software.
Statistical Analysis
All data are presented as mean ± SEM, and were analyzed for statistical significance using a Student’s t-test (KaleidaGraph Software). Values of p < 0.05 were considered significant.
RESULTS
Differential phosphorylation of sarcomeric proteins
To determine whether K210 deletion induces alterations in phosphorylation of troponin and other sarcomeric proteins, we investigated total and site-specific phosphorylation of myofilament proteins in myofibrils isolated from wild-type (WT), heterozygous TNNT2+/ΔK210 (HT) and homozygous TNNT2ΔK210/ΔK210 (HM) mice hearts [7]. Cardiac myofibrils were prepared under conditions shown to preserve phosphorylation status of the sarcomeric proteins (in the presence of protease and phosphatase inhibitors). Total phosphorylation levels were determined for myosin light chain 2 (MLC2), troponin I (cTnI), troponin T (cTnT), desmin and myosin binding protein-C (MyBP-C). Tropomyosin (Tm) and α-actinin were below the detection limit of ProQ Diamond phosphostain in these samples. However, increasing the amount of myofibrils to 15µg/lane allowed clear detection of the two phospho-proteins. There were no differences in Tm or α-actinin phosphorylation levels in wt and mutant hearts. Data presented in Figure 1 (and Table 1) show significant decrease of cTnT (by 29%), cTnI (by 46%) and MyBP-C (by 31%) basal phosphorylation levels in HM hearts, but not HT either 8-wk or 24-wk, compared with WT hearts. MLC2 phosphorylation levels were unchanged. Interestingly, interfilament protein desmin shows a 33% phosphorylation decrease in the 24-wk HT hearts but no changes in the HM hearts. Table 1 indicates that 4-wk and 8-wk wild type hearts have similar levels of myofilament protein phosphorylation.
FIGURE 1.
Differential phosphorylation of sarcomeric proteins in isolated myofibrils from ΔK210 homozygous hearts. A, cardiac myofibrillar proteins (8 µg/lane) were resolved by 12.5% SDS-PAGE and stained with ProQ Diamond for phospho-proteins detection and Sypro Ruby for total protein detection. Main sarcomeric phospho-proteins are indicated, together with actin, to show equal protein content (Sypro) and disparity in phosphorylation levels (ProQ) in the WT and ΔK210 myofilaments. Data shown are from 4 week-old animals. There are no significant differences in phosphorylation pattern of sarcomeric proteins in 4 and 8 week-old WT hearts. B, Optical densities of phospho/total protein ratio (n = 5 hearts/group). The results are normalized by setting the mean value for WT to 100. Data are means ± SEM. Values marked with asterisk were deemed statistically significant (p<0.05). A.U., arbitrary units.
TABLE I.
Differential phosphorylation of sarcomeric proteins in wild-type (WT), heterozygous ΔK210 (HT), and homozygous ΔK210 (HM) hearts.
| Sample | Total phosphorylation (relative units) | ||||
|---|---|---|---|---|---|
| MLC2 | cTnI | cTnT | Desmin | MyBP-C | |
| WT 4-wk | 0.40 ± 0.06 | 2.07 ± 0.18 | 1.04 ± 0.04 | 0.48 ± 0.05 | 1.50 ± 0.03 |
| WT 8-wk | 0.36 ± 0.05 | 2.09 ± 0.21 | 0.94 ± 0.05 | 0.53 ± 0.04 | 1.46 ± 0.02 |
| HT 8-wk | 0.45 ± 0.06 | 2.46 ± 0.19 | 1.01 ± 0.05 | 0.48 ± 0.04 | 1.30 ± 0.06 |
| HT 24-wk | 0.39 ± 0.03 | 1.8 ± 0.08 | 0.94 ± 0.05 | 0.36 ± 0.03* | 1.35 ± 0.09 |
| HM 4-wk | 0.35 ± 0.04 | 1.12 ± 0.15* | 0.67 ± 0.09* | 0.48 ± 0.06 | 1.02 ± 0.06* |
Data are means of at least three separate experiments from five different hearts. Phosphorylation values were determined from the analysis of optical density corresponding to the respective sarcomeric protein, as described in Experimental procedures. All data are presented as means ± SEM, and were analyzed for statistical significance using a Student’s t-test (= p < 0.05).
Site-specific phosphorylation of cTnT and cTnI in isolated myofibrils
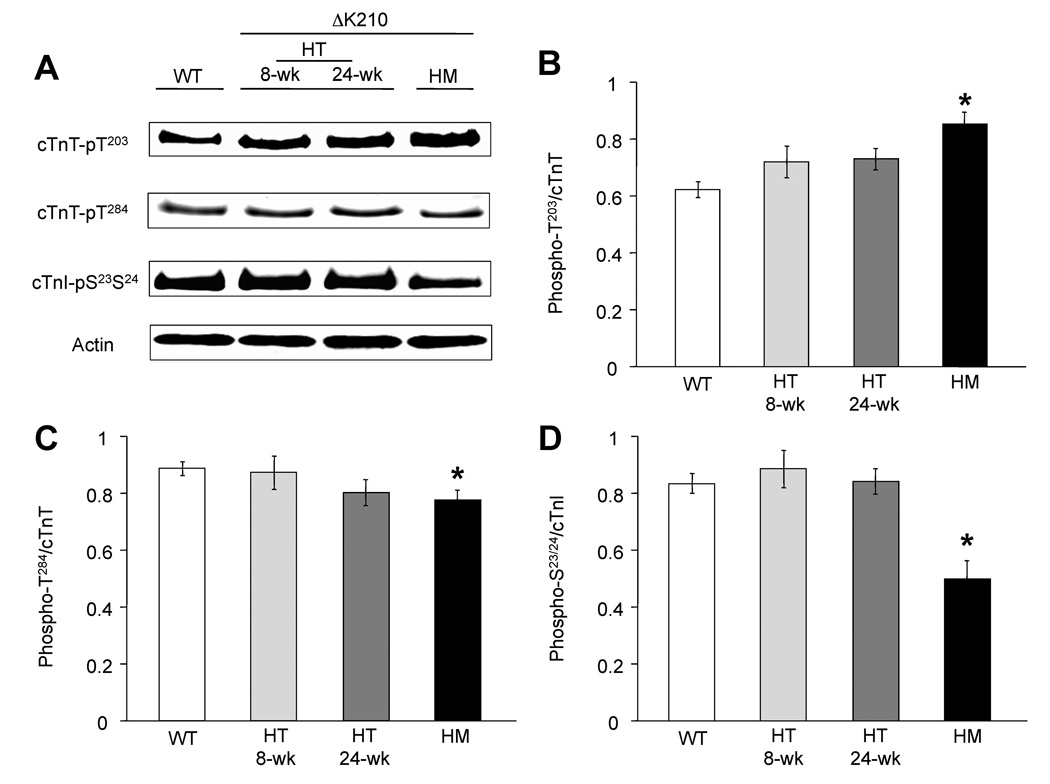
Next we investigated changes at specific phosphorylation sites (cTnT and cTnI) in isolated myofibrils from mutant and wild type hearts. The functionally critical phosphorylation residue Thr203 of cTnT showed enhanced phosphorylation in all the mutant hearts when compared with WT (figure 2 A, B). Since there were no differences between 4 and 8 week-old WT hearts, only the 8-wk data is shown. Heterozygous hearts displayed only modest increases compared with a 28% augmentation of Thr203 phosphorylation in HM myocardium. This is surprising since total cTnT phosphorylation was significantly lower in HM hearts compared with controls (Figure 1, Table 1). Considering that cTnT has 5 other potential phosphorylation sites apart from Thr203 (see figure 3A) it is possible for one site to increase (i.e Thr203) while the overall phosphorylation to decrease. This is supported by the fact that phosphorylation of two other cTnT sites, Thr284 (figure 2C) and Ser275 (data not shown), decreased noticeably in HM. In agreement with the trend noted for total cTnI phosphorylation (Table 1), cTnI-Ser23/24 phosphorylation dropped significantly in the HM (~40%) but not the heterozygous hearts (figure 2D). Other cTnI phosphorylation sites showed no changes (data not shown).
FIGURE 2.
Analysis of site-specific phosphorylation of troponin T and I in cardiac myofibrils isolated from WT, heterozygous (HT), and homozygous (HM) hearts. A, representative immunoblots show reactivity of anti-phosphorylation specific antibodies cTnT-pT203, cTnT–pT284 (control) and cTnI-pS23/24 with freshly isolated cardiac myofibrils. Actin was used to verify and correct for small variations in loading. B, C, D bar graphs show the phosphorylation profiles of cTnT-T203, cTnT-T284 and cTnI-S23/24, respectively. cTnT-T203 phosphorylation increased while T284 and cTnI-S23/24 decreased in mutant hearts. Data are means of the values calculated from the optical density of the bands obtained by Western blotting normalized against pan-cTnT or pan-cTnI densities (n = 5 hearts/group). Values marked with asterisk were deemed statistically significant (p< 0.05).
FIGURE 3.
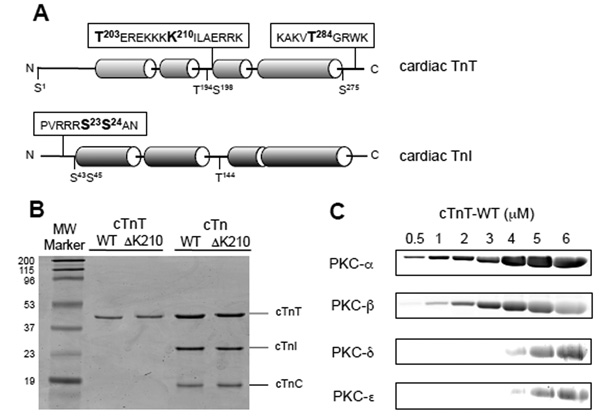
Cardiac troponin preparations and concentration dependent phosphorylation by PKC isozymes. A, schematic representation of cTnT and cTnI, showing main phosphorylation sites and the location of K210 relative to Thr203. Amino acid residues near studied phosphorylation sites are shown using the 1 letter abbreviation. cTnT is numbered according to the human sequence. B, Recombinant cardiac troponins were expressed in E. Coli, purified to near homogeneity (as shown by SDS-PAGE) and were used to reconstitute troponin complexes. TnT-wt and cTnT-ΔK210 are shown as purified individual proteins or as part of the troponin complexes in the presence of wild type murine cTnI and cTnC. Molecular weight markers (Bio-Rad) are shown on the left. Gel was stained with Coomassie brilliant blue. C, in vitro kinase assays with cTnT-WT as substrate were carried out for 30 min at 30°C using 0.15 units PKC/reaction in the presence of phosphatidylserine and diacylglycerol, and Ca2+ for PKC-α andβ. Phosphorylated proteins were resolved by SDS-PAGE and stained with ProQ Diamond. Data indicate that PKC-α is a better cTnT kinase than the other PKCs.
Phosphorylation of cTnT-WT by PKC isozymes
To determine whether ΔK210 has a direct or indirect effect on troponin phosphorylation we performed in vitro kinase assays. Comparative specific activities of four recombinant PKC isozymes (α, βII, ε,δ) were tested toward an equally preferred substrate, myelin basic protein (MBP). Kinase assays performed under conditions of equal PKC activity (0.15 units/reaction) in the presence of increasing concentration of cTnT-wt (figure 3C) indicate that conventional PKCs (α > βII) are better cTnT kinases than the novel PKCs (α andε). Subsequent phosphorylation studies used solely PKC-α.
cTnT and cTnI phosphorylation by PKC-α
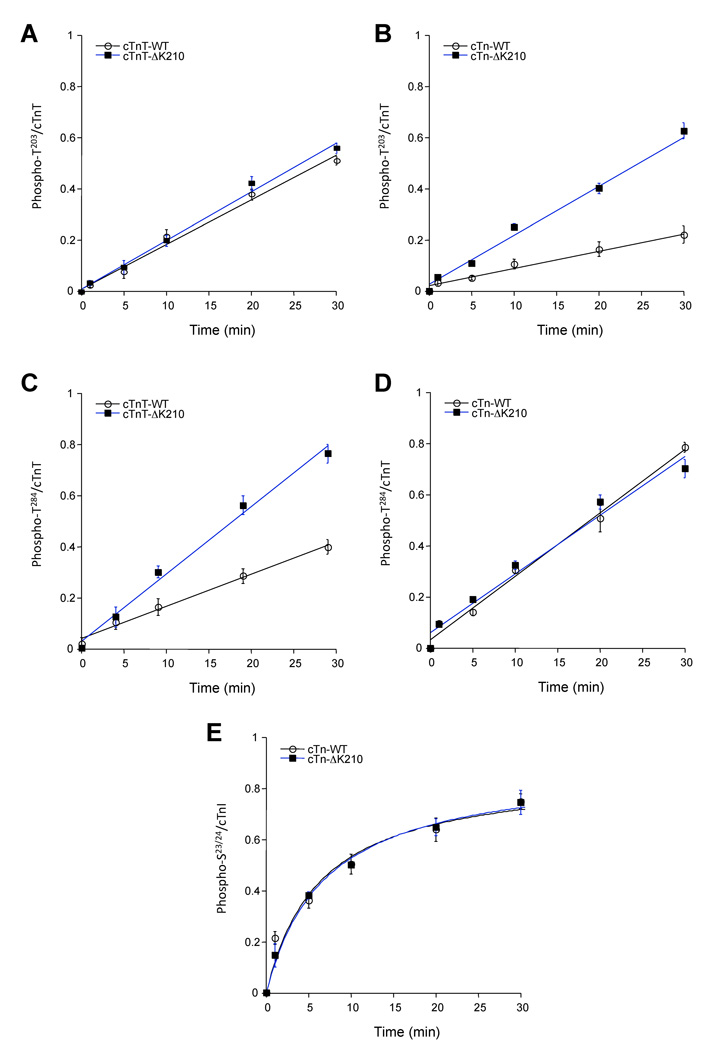
In vitro kinase assays, in the presence of low concentrations of PKC (1ng/reaction) and excess substrate, were used to examine whether ΔK210 influences PKC-α phosphorylation kinetics of cTnT-T203 or cTnT-T284 (as control). Experiments where cTnT by itself (WT or ΔK210) served as substrate for PKC-α show that deletion of K210 has no effect on T203 while enhancing T284 phosphorylation (figure 4A,C). However, when more physiological substrates were used, troponin complexes containing equimolar concentrations of cTnI-wt, cTnC-wt and either cTnT-wt or cTnT-ΔK210 (see figure 3B), ΔK210 augmented T203 but not T284 phosphorylation (figure 4B,D). On the other hand, cTnI-S23/24 phosphorylation was not affected by deletion of K210 in cTnT (figure 4E).
FIGURE 4.
Time-course analysis of site-specific phosphorylation of WT and ΔK210 cTnT or cTn complexes. In vitro kinase assays show time dependent PKC-α phosphorylation of cTnT sites T203 and T284, and cTnI-S23/24. ΔK210 has no effect on T203 phosphorylation in cTnT alone (A) but it speeds up the rate of phosphorylation reaction when incorporated in the troponin complex (B). Interestingly, T284 phosphorylation rates are opposite from those observed with T203 (C, D). Phosphorylations of cTnI sites S23/24 are not affected by ΔK210 (E). Data are means of optical density values (± SEM) determined by analyzing the band intensities from immunoblots using ImageQuant TL software and normalized to total cTnT (A–D) or cTnI (E) detected by an anti-TnT or anti-cTnI pan antibodies. All reactions contained 1 ng PKC-α and 1 µM substrate, and were carried out at 30°C.
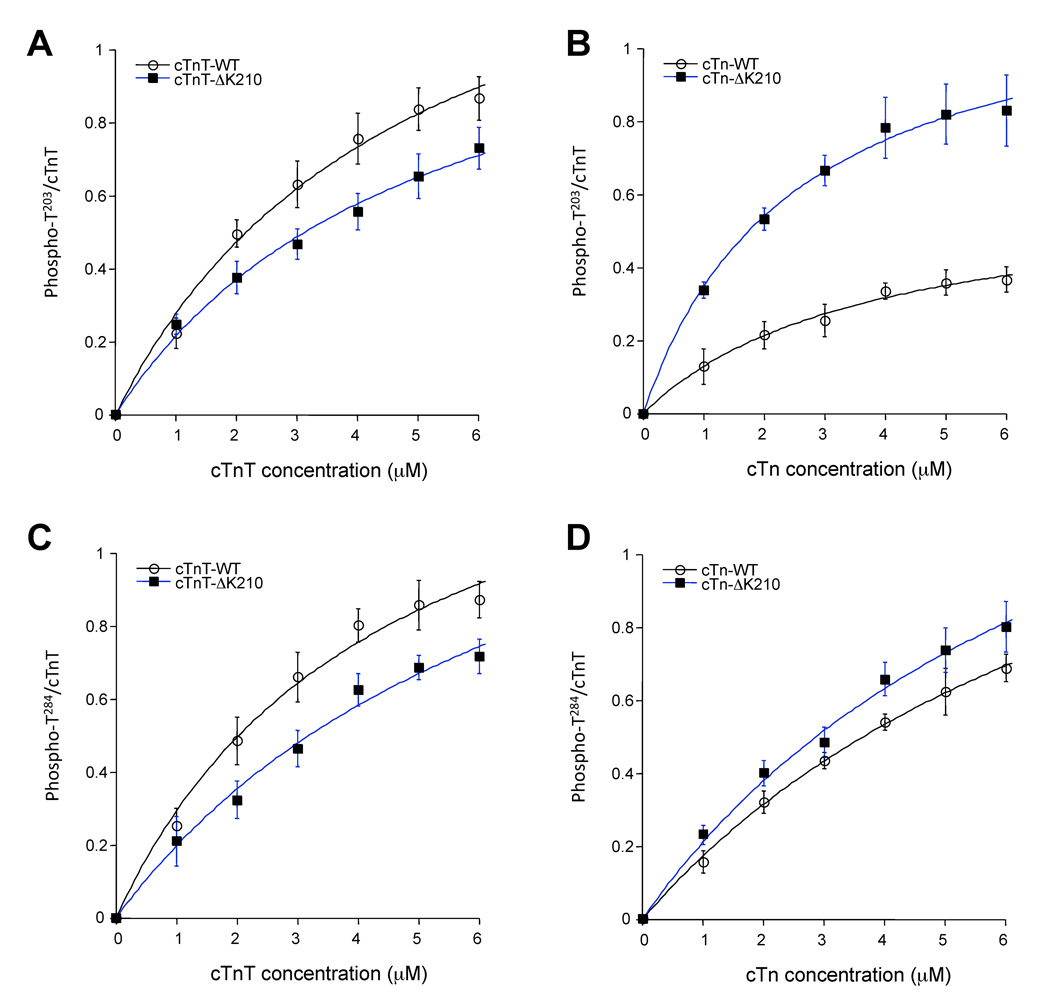
To further examine the differences in T203 and T284 phosphorylations induced by ΔK210, kinetic parameters were determined under conditions where initial velocity could be determined (Figure 5 and Table 2). The apparent Michaelis-Menten constant (Km) values for T203 phosphorylation increased in the order Tn-ΔK210 < Tn-wt < TnT-wt < TnT-ΔK210. Tn-ΔK210 complexes display Vmax values 2-fold higher that those determined for Tn-wt, indicating faster reaction rates for Tn-ΔK210 compared with Tn-wt. As judged from the ratio of Vmax/Km the relative efficacy of T203 sites of wt or ΔK210 proteins to serve as PKC-α substrates, in descending order, were: Tn-ΔK210 > TnT-wt > TnT-ΔK210 > Tn-wt. Deletion of K210 residue had little effect on the kinetics of phosphorylation of T284 site.
FIGURE 5.
In vitro kinase assays for determination of kinetic constants. Increase in substrate concentrations differentially affect phosphorylation of T203 in cTnT alone compared with cTn complexes. ΔK210 enhance PKC phosphorylation of T203 site when present in troponin complex (B) but not when in cTnT alone (A). Phosphorylation of T284 follows the same trend as T203 for cTnT alone (C) but not cTn (D). Data are means of optical density values (± SEM) determined by analyzing the band intensities from immunoblots using ImageQuant TL software and normalized to total cTnT detected by an anti-TnT pan antibody. All reactions contained 1 ng PKC-α in the presence of varying substrate concentrations, and were carried out at 30°C.
Table II.
Summary of kinetic constants for phosphorylation of cTnT-Thr203 and cTnT-Thr284 by PKC-α.
| Sample | Phosphorylated | Km(app) | Vmax | Vmax/Km(app) |
|---|---|---|---|---|
| Residue | (µM) | (units•µL−1) | (units•µL−1•µM−1) | |
| cTnT-wt | T203 | 4.85 ± 0.83 | 1.62 ± 0.18 | 0.33 ± 0.08 |
| cTnT-ΔK210 | T203 | 5.09 ± 0.97 | 1.31 ± 0.12 | 0.26 ± 0.05 |
| cTn-wt | T203 | 3.74 ± 0.67 | 0.615 ± 0.05 | 0.16 ± 0.03 |
| cTn-ΔK210 | T203 | 2.49 ± 0.29 | 1.22 ± 0.06 | 0.49 ± 0.06 |
| cTnT-wt | T284 | 4.43 ± 0.96 | 1.59 ± 0.18 | 0.36 ± 0.09 |
| cTnT-ΔK210 | T284 | 7.40 ± 0.93 | 1.66 ± 0.29 | 0.22 ± 0.07 |
| cTn-wt | T284 | 9.24 ± 0.75 | 1.76 ± 0.13 | 0.19 ± 0.02 |
| cTn-ΔK210 | T284 | 8.04 ± 0.88 | 1.90 ± 0.26 | 0.24 ± 0.06 |
In vitro kinase assays (Fig 6) were used to estimate the apparent Michaelis-Menten constant Km(app), Vmax (the maximum rate of reaction at infinite substrate concentration) and the ratio Vmax/Km(app) which denotes catalytic efficiency. Values are means ± SEM.
Yeast two-hybrid binding assay
A yeast two-hybrid assay was used to evaluate whether ΔK210 propagates its noxious effect through altered cTnT-cTnI binding. Figure 6A, shows schematically the main steps of the assay. The strength of the interaction between cTnT and cTnI is directly proportional to the color development determined by two different colorimetric assays, α-gal (identical to β-gal, data not shown) and β-gal (i.e. dark blue indicates enhanced interaction between the two proteins, figure 6B, 6C). Our data suggests that cTnT-ΔK210 binds stronger to cTnI compared with the cTnT-WT counterpart.
FIGURE 6.
Yeast two-hybrid assay. A, schematic representation of the main steps of the yeast two-hybrid assay. cTnI (subcloned into the pGADT7 – activation domain vector) and cTnT (subcloned into the pGBKT7 – DNA binding vector) were co-transformed into the AH109 yeast strain. B, β-gal assay shows the interaction between cTnT and cTnI as monitored by color blue development. The intense blue color developed by yeast cells containing cTnT-ΔK210 and cTnI indicate a stronger interaction between these two proteins compared with WT control (lighter blue). C, Color intensity of blue colonies was determined using Image J software and normalized to that of control colonies (white). Data shown is from a single experiment and is representative of 4 separate experiments.
DISCUSSION
Our study provides novel evidence that deletion of K210 in cTnT, linked to familial dilated cardiomyopathy, may propagate its deleterious effect through alterations in cTnI-cTnT interaction and basal phosphorylation levels of key contractile proteins. Isolated cardiac myofibrils from homozygous ΔK210 hearts indicate differential phosphorylation of cTnT (augmented T203 phosphorylation), cTnI (decrease S23/24 phosphorylation) and reduced overall MyBP-C phosphorylation. An important finding of our study is that deletion of K210 enhances PKC phosphorylation kinetics for T203. Our previous analysis of the functional role of each cTnT phosphorylation site identified a distinct region centered on T203 that when modified by phosphorylation controls force, actomyosin Mg-ATPase rate, Ca2+ sensitivity and cross bridge cycling of mouse cardiac fiber bundles [12]. Apparently phosphorylations of the other cTnT sites have no detectable direct functional role (although they probably act indirectly to enhance the effect of Thr203 phosphorylation) [12]. Our results suggest that augmentation of cTnT-T203 phosphorylation on a background of decreased overall sarcomeric proteins phosphorylation may exacerbate the myofilaments Ca2+ desensitization and may explain the contractile deficit documented in the ΔK210 hearts.
To understand better the mechanism by which deletion of K210 confers a higher phosphorylation propensity to T203, we modeled the region of cTnT containing these two residues. Since the high-resolution x-ray structure of the core cardiac troponin complex contains the fragment of cTnT spanning residues 202–285 (human sequence), we used this structure for our model building [19]. Figure 7 shows a surface representation of helix 203–224 of cTnT-wt and ΔK210. The native structure shows the protruding T203 residue on the same face of the helix as K210, flanked by two glutamate residues E204 and E206. Interestingly, this structure points to a possible unique feature of K210: a helix stabilizing interaction with E214. Another helix stabilizing electrostatic bond is shown between E204 and K207. It is well documented that two of the five amino acids (M, A, L, E, K) with the highest helical forming propensity K(−)-E(+) when located at positions i, i+4 ( e.g. K210 and E214) and i, i+3 (e.g. E204 and K207) have the tendency to form helix stabilizing salt-bridges (electrostatic bonds) [20]. The molecular model of ΔK210 (generated by the use of 3D-Jigsaw program) shows some interesting local changes, without affecting the helical content. This model is in good agreement with circular dichroism data showing the α-helical content of cTnT-ΔK210 was nearly identical to that of cTnT-wt [21]. In the absence of K210 there is a rearrangement of E/K electrostatic interactions. E214 engages in a new bond with K217, the E204-K207 bond is broken and the two residues are pulled apart from each other. K210 deletion reorganizes the electrostatic microenvironment near T203 leading to a clustering of basic charges on the same side of the helix (fig. 7). This may explain the enhanced phosphorylation kinetics by PKC-α, which preferentially phosphorylates sites bordered by highly basic residues [22].
FIGURE 7.
Molecular graphics views of cTnT helix 202–224 WT or lacking K210. Partial cardiac troponin core structure (1J1E.pdb) solved by x-ray diffraction [19] was used as the starting point for modeling the partial structure of cTn-ΔK210. Modeling was performed with the 3D-JIGSAW program. Solvent-accessible surface representations of cTnT helix 202–224, shows the position and charge of acidic (red color) and basic (blue color) residues in WT (left) and ΔK210 model (right). While the disappearance of K210 does not induce gross structural changes (right) it does alter the orientation of basic residues (R205, K207–K209), breaks the electrostatic interactions E204-K207 and K210-E214, and facilitates the formation of the novel E214-K217 bond. These microenvironment alterations enhance the basic electrostatic potential near T203 making it a much better substrate for PKC-α.
The idea that a cardiomyopathy mutation could influence phosphorylation propensity of a nearby site has been proposed for some time. However, until now, there was no systematic analysis to demonstrate this effect. Equally interesting is the fact that deletion of a residue in cTnT has far reaching effects, influencing PKA-dependent phosphorylation of neighboring contractile proteins cTnI and MyBP-C. Recent advances in MyBP-C structure suggest that N-terminal domains of MyBP-C (including the m-domain containing the cardiac specific PKA phosphorylation sites) bind F-actin [23–25]. In this case, the m-domain of MyBP-C would be anchored on the thin filament in the vicinity of cTnT and cTnI. The question is how could the deletion of K210 lead to alterations in PKA-dependent phosphorylation of cTnI and MyBP-C? Although experiments proposed here do not directly address this question, recent evidence from our laboratory point to a potential scenario. Using a yeast-two hybrid system we have recently identified a novel function for cTnT as an anchoring protein for cAMP-dependent protein kinase A (PKA)[15]. Sequence homology followed by mutagenesis studies confirmed the existence of a cTnT PKA binding site on the same helix harboring T203 and K210. Yeast-two hybrid data indicate that PKA affinity for cTnT-ΔK210 is lower than for cTnT-WT [15]. Low PKA-cTnT affinity would diminish thin filament sequestration of PKA, resulting in decrease phosphorylation of its two main substrates cTnI and MyBP-C. Taken together these observations suggest that deletion of K210 on one hand impairs cTnI and MyBP-C phosphorylation by weakening PKA docking to cTnT, and on the other hand enhance PKC-dependent phosphorylation of cTnT-T203. Increased T203 phosphorylation [12] and decreased MyBP-C phosphorylation [26] may work in synergy to exacerbate the effect of K210 deletion and may explain the defect in contractile dynamics seen in the HM knock-in myocardium.
ΔK210 also enhances cTnT-cTnI binding affinity, as demonstrated by yeast two-hybrid assays (fig. 6). The implication is that a stronger cTnT-cTnI interaction may lead to a more rigid troponin with impaired Ca2+ sensing/transmitting properties. As a result the ΔK210 myofilaments would be less reactive to Ca2+. The yeast two-hybrid system (Y2H) is a sensitive eukaryotic assay widely used to detect novel interactions between proteins and to map the specific interacting domains of two proteins known to form a complex [27]. The strength of the interaction between two proteins is associated with the level of expression of an appropriate reporter gene. The work of Estojak, et al [28] indicates that there is a good correlation between the strength of interaction of two proteins predicted by Y2H assay with that determined in vitro by biochemical assays.
Collectively, our observations suggest that cardiomyopathy-causing ΔK210 has far-reaching effects influencing cTnI-cTnT binding and posttranslational modifications of key sarcomeric proteins, which may underlie the dysregulation of myofilament function and DCM progression. This novel effect of ΔK210 is likely to represent a more general trend of cardiomyopathy-inducing mutations positioned near critical phosphorylation sites.
ACKNOWLEDGMENTS
This work was supported by AHA-SDG 0335199N, and by NIH grants HL071865, HL68733 and AG032009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000 Apr;80(2):853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 3.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005 Sep;10(3):237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 4.Chang AN, Parvatiyar MS, Potter JD. Troponin and cardiomyopathy. Biochem Biophys Res Commun. 2008 Apr 25;369(1):74–81. doi: 10.1016/j.bbrc.2007.12.081. [DOI] [PubMed] [Google Scholar]
- 5.Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, et al. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem. 2005 Aug 5;280(31):28498–28506. doi: 10.1074/jbc.M412281200. [DOI] [PubMed] [Google Scholar]
- 6.Kamisago M, Sharma SD, DePalma SR, Solomon S, Sharma P, McDonough B, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000 Dec 7;343(23):1688–1696. doi: 10.1056/NEJM200012073432304. [DOI] [PubMed] [Google Scholar]
- 7.Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, et al. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res. 2007 Jul 20;101(2):185–194. doi: 10.1161/CIRCRESAHA.106.146670. [DOI] [PubMed] [Google Scholar]
- 8.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005 Apr 1;66(1):12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 9.Sumandea MP, Burkart EM, Kobayashi T, de Tombe P, Solaro RJ. Molecular and Integrated Biology of Thin Filament Protein Phosphorylation in Heart Muscle. In: Sideman S, Beyar R, editors. Cardiac Engineering: From Genes and Cells to Structure and Function: Ann. NY Acad. Sci. 2004. [DOI] [PubMed] [Google Scholar]
- 10.Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, et al. Tyrosine Phosphorylation Modifies Protein Kinase C {delta}-dependent Phosphorylation of Cardiac Troponin I. J Biol Chem. 2008 Aug 15;283(33):22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, et al. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003 Mar 28;278(13):11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 12.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003 Sep 12;278(37):35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 13.Arteaga GM, Warren CM, Milutinovic S, Martin AF, Solaro RJ. Specific enhancement of sarcomeric response to Ca2+ protects murine myocardium against ischemia-reperfusion dysfunction. Am J Physiol Heart Circ Physiol. 2005 Nov;289(5):H2183–H2192. doi: 10.1152/ajpheart.00520.2005. [DOI] [PubMed] [Google Scholar]
- 14.Sumandea MP, Vahebi S, Sumandea CA, Garcia-Cazarin ML, Staidle J, Homsher E. Impact of cardiac troponin T N-terminal deletion and phosphorylation on myofilament function. Biochemistry. 2009 Aug 18;48(32):7722–7731. doi: 10.1021/bi900516n. [DOI] [PubMed] [Google Scholar]
- 15.Sumandea CA, Garcia-Cazarin ML, Bozio CH, Balke CW, Sumandea MP. Cardiac troponin T: a novel scaffold protein, anchors PKA to the myofilaments (in review) 2009 [Google Scholar]
- 16.Bates PA, Kelley LA, MacCallum RM, Sternberg MJ. Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins. 2001 Suppl 5:39–46. doi: 10.1002/prot.1168. [DOI] [PubMed] [Google Scholar]
- 17.Bates PA, Sternberg MJ. Model building by comparison at CASP3: using expert knowledge and computer automation. Proteins. 1999 Suppl 3:47–54. doi: 10.1002/(sici)1097-0134(1999)37:3+<47::aid-prot7>3.3.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Contreras-Moreira B, Bates PA. Domain fishing: a first step in protein comparative modelling. Bioinformatics. 2002 Aug;18(8):1141–1142. doi: 10.1093/bioinformatics/18.8.1141. [DOI] [PubMed] [Google Scholar]
- 19.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca(2+)-saturated form. Nature. 2003 Jul 3;424(6944):35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 20.Marqusee S, Baldwin RL. Helix stabilization by Glu-…Lys+ salt bridges in short peptides of de novo design. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatraman G, Harada K, Gomes AV, Kerrick WG, Potter JD. Different functional properties of troponin T mutants that cause dilated cardiomyopathy. J Biol Chem. 2003 Oct 24;278(43):41670–41676. doi: 10.1074/jbc.M302148200. [DOI] [PubMed] [Google Scholar]
- 22.Nishikawa K, Toker A, Johannes FJ, Songyang Z, Cantley LC. Determination of the specific substrate sequence motifs of protein kinase C isozymes. J Biol Chem. 1997 Jan 10;272(2):952–960. doi: 10.1074/jbc.272.2.952. [DOI] [PubMed] [Google Scholar]
- 23.Whitten AE, Jeffries CM, Harris SP, Trewhella J. Cardiac myosin-binding protein C decorates F-actin: implications for cardiac function. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18360–18365. doi: 10.1073/pnas.0808903105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeffries CM, Whitten AE, Harris SP, Trewhella J. Small-angle X-ray scattering reveals the N-terminal domain organization of cardiac myosin binding protein C. J Mol Biol. 2008 Apr 4;377(4):1186–1199. doi: 10.1016/j.jmb.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009 May 1;284(18):12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong CW, Stelzer JE, Greaser ML, Powers PA, Moss RL. Acceleration of crossbridge kinetics by protein kinase A phosphorylation of cardiac myosin binding protein C modulates cardiac function. Circ Res. 2008 Oct 24;103(9):974–982. doi: 10.1161/CIRCRESAHA.108.177683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields S, Sternglanz R. The two-hybrid system: an assay for protein-protein interactions. Trends Genet. 1994 Aug;10(8):286–292. doi: 10.1016/0168-9525(90)90012-u. [DOI] [PubMed] [Google Scholar]
- 28.Estojak J, Brent R, Golemis EA. Correlation of two-hybrid affinity data with in vitro measurements. Mol Cell Biol. 1995 Oct;15(10):5820–5829. doi: 10.1128/mcb.15.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]