Abstract
The perifornical-lateral hypothalamic area (PF-LHA) has been implicated in the regulation of behavioral arousal. The PF-LHA predominantly contains neurons that are active during behavioral and cortical activation and quiescent during non-rapid eye movement (nonNREM) sleep, i.e., are nonREM-off neurons. Some in vitro and in vivo studies indicate that PF-LHA neurons, including hypocretin-expressing neurons, are under GABAergic control. However, a role of GABA in suppressing the discharge of PF-LHA neurons during spontaneous nonREM sleep has not been confirmed. We recorded the sleep-wake discharge profiles of PF-LHA neurons and simultaneously assessed the contributions of local GABAA receptor activation and blockade on their wake- and nonREM sleep-related discharge activities by delivering GABAA receptor agonist, muscimol (500nm, 5μM, and 10μM) and its antagonist, bicuculline (5μM, 10μM, and 20μM), adjacent to the recorded neurons via reverse microdialysis. Muscimol dose-dependently decreased the discharge of PF-LHA neurons including nonREM-off neurons. Muscimol-induced suppression of discharge during nonREM sleep was significantly weaker than the suppression produced during waking. In the presence of bicuculline, PF-LHA neurons, including nonREM-off neurons, exhibited elevated discharge, which was dose-dependent and was significantly higher during nonREM sleep, compared to waking. These results suggest that GABAA receptor mediated increased GABAergic tone contributes to the suppression of PF-LHA neurons, including nonREM-off neurons, during spontaneous nonREM sleep.
Keywords: Perifornical-lateral hypothalamus, Hypocretin, GABA, Posterior-lateral hypothalamus, sleep
INTRODUCTION
The perifornical-lateral hypothalamic area (PF-LHA) has been implicated in the regulation of behavioral arousal (Gerashchenko and Shiromani, 2004, Datta and Maclean, 2007, McCarley, 2007, Szymusiak and McGinty, 2008). The PF-LHA predominantly contains neurons that are active during behavioral and cortical activation and quiescent during non rapid eye movement (nonREM) sleep, i.e., are nonREM-off neurons (Alam et al., 2002, Koyama et al., 2003, Suntsova et al., 2007). Stimulation of the PF-LHA evokes locomotor activity, EEG activation, and arousal, whereas its lesions increase sleep (Stock et al., 1981, Sinnamon et al., 1999, Gerashchenko et al., 2003, Alam and Mallick, 2008). Neurochemically, the PF-LHA is a heterogeneous structure and includes populations of neurons expressing hypocretin (HCRT), melanin-concentrating hormone (MCH), gamma-aminobutyric acid (GABA), and glutamate (Bittencourt et al., 1992, Peyron et al., 1998, Abrahamson and Moore, 2001, Gerashchenko and Shiromani, 2004, Ohno and Sakurai, 2008). Amongst these neuronal groups, HCRT neurons have been implicated in the facilitation and/or maintenance of arousal (Ohno and Sakurai, 2008). These neurons exhibit wake-associated discharge and c-Fos expression (Fos-IR) and are quiescent during both nonREM and REM sleep (Estabrooke et al., 2001, Espana et al., 2003, Lee et al., 2005, Mileykovskiy et al., 2005, Takahashi et al., 2008). A loss of HCRT signaling is linked with symptoms of narcolepsy in human and animals (Lin et al., 1999, Peyron et al., 2000, Thannickal et al., 2000). While glutamateric neurons have been implicated in the regulation of HCRT neuronal excitability and are wake-active, GABAergic and MCH neurons in the PF-LHA have been implicated in the regulation of sleep, especially REM sleep (Li et al., 2002, Kumar et al., 2005, Hassani et al., 2009). Although, in vitro studies have identified several neurotransmitters and neuromodulators that influence the activity of PF-LHA neurons including HCRT neurons (Kukkonen et al., 2002, Ohno and Sakurai, 2008), the neurotransmitter(s) that regulate the suppression of PF-LHA neurons during nonREM sleep remains poorly understood.
GABAergic neurons are major contributors to inhibitory tone throughout the mammalian brain and acts via two different types of receptors, viz., GABAA and GABAB (Cooper et al., 1996). A role of GABAergic system in sleep regulation is evident from the fact that most commonly used hypnotics are GABAA receptor analogues, although GABAB receptor has also been implicated (Gottesmann, 2002, Mohler, 2006, Matsuki et al., 2009, Winsky-Sommerer, 2009). Administration of selective GABAA receptor agonists, e.g., muscimol and THIP, increase the duration of nonREM and REM sleep and EEG delta frequency in rats and humans (Lancel and Faulhaber, 1996, Faulhaber et al., 1997, Lancel et al., 1997). Majority of sleep-active neurons in the preoptic (POA) region are GABAergic (Gong et al., 2004, Gvilia et al., 2006). It has been hypothesized that GABAergic system is a predominant contributor of the inhibitory tone to multiple arousal systems during sleep (Saper et al., 2005, Szymusiak and McGinty, 2008).
Evidence suggests that PF-LHA neurons, including HCRT neurons are subject to GABAergic inhibitory tone. For example, a) PF-LHA contains local GABAergic neurons and receives projections from sleep-active GABAergic neurons in the POA region (Abrahamson and Moore, 2001, Kumar et al., 2005, Uschakov et al., 2007); b) both GABAA and GABAB receptors are localized on HCRT neurons and modulate HCRT neuronal activity in vitro (Li et al., 2002, Eggermann et al., 2003, Backberg et al., 2004, Xie et al., 2006); c) GABA levels in the posterior hypothalamus are higher during nonREM and REM sleep (Nitz and Siegel, 1996); d) THIP administration into the PF-LHA increases sleep (Thakkar et al., 2008); and e) in presence of bicuculline into the PF-LHA, Fos-IR is increased in HCRT and non-HCRT neurons and animals spent more time in waking and less time in nonREM/REM sleep (Alam et al., 2005, Goutagny et al., 2005). However, the contributions of GABAergic inhibitory tone in suppressing the discharge of PF-LHA neurons during spontaneous nonREM sleep has not been directly confirmed. In this study, we recorded the sleep-wake discharge profiles of the PF-LHA neurons and simultaneously assessed the contributions of local GABAA receptor activation and blockade on their wake- and nonREM sleep-related discharges by delivering GABAA receptor agonist, muscimol, and its antagonist, bicuculline, adjacent to the recorded neurons via reverse microdialysis.
MATERIALS AND METHODS
A. Experimental procedure
Experiments were conducted on 6 Sprague-Dawley, unanesthetized, unrestrained male rats weighing between 300-350g and in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals. These rats were maintained on 12:12h light-dark cycle (lights on from 8.00 AM) and with food and water available ad libitum. The experiments were conducted during light-on phase so that the extracellular discharge activity of PF-LHA neurons could be recorded through multiple sleep-wake cycles during baseline and drug treatment.
The experimental procedure has been described in detail earlier (Alam et al., 1999, Kumar et al., 2007). In brief, under surgical anesthesia (Ketamine + Xylazine: 80:10 mg/kg; i.p.) and aseptic conditions, rats were stereotaxically implanted with electroencephalogram (EEG) and electromyogram (EMG) electrodes for polygraphic determination of sleep-waking states. A microdrive-microdialysis guide cannula assembly, consisting of a single barrel mechanical microdrive and an adjacent guide cannula for microdialysis probe insertion, was implanted such that their tips rested 3mm above the dorsal aspect of the PF-LHA (A, −3.0 to −3.20; L, 1.40 to 1.60; H, 4.5 to 5.0 from bregma)(Paxinos and Watson, 1998). Five pairs of microwires, each consisting of two 20μm insulated stainless steel wires glued together except for 2.0mm at the tip, were passed through the microdrive barrel such that their tips projected into the PF-LHA.
B. Data Acquisition
Experiments were started at least 10 days after surgery and acclimatization of rats with the recording chamber. At least 12h before the experiment, the stylet of the microdialysis guide cannula was replaced by a microdialysis probe (semi-permeable membrane tip length 1mm; outer diameter, 0.22mm; molecular cut off, 50kDa; Eicom, Japan), which was fixed in place with dental cement. After implantation, the probe was flushed with artificial cerebrospinal fluid (aCSF; composition in mM, 145 Na+, 2.7 K+, 1.0 Mg++, 1.2 Ca++, 1.5 Cl− and 2 Na2HPO4, pH, 7.2) at a flow rate of ~2 μl/min for 3-4h. In this study, the microdialysis probe was fixed and microwires were advanced adjacent to the microdialysis probe to minimize the tissue trauma and ensure maximum stability of the unit recording. The length of the probe was set such that the microwires were within 200-500μm from the semi-permeable membrane.
The experimental paradigm included recording of EEG, EMG, and unit activity during baseline, during drug delivery and during washout. The microdrive was advanced in 20-30μm steps until an isolated single unit was found. First, the discharge rate of an isolated PF-LHA neuron was recorded through 2-3 stable sleep-wake cycle with aCSF perfusion as a baseline. After baseline recording, known concentrations of muscimol (500nM, 5μM, and 10μM), a GABAA receptor agonist, or bicuculline (5μM, 10μM, and 20μM), a GABAA receptor antagonist, was dialyzed adjacent to the recorded neurons for 10min so that the transient effects of each drug on the discharge activity of neuron(s) could be studied without triggering a strong behavioral response. After delivery of drugs, the perfusion medium was switched back to aCSF and the recording continued for another 45-90 min as wash out or recovery. During the entire recording session, the animal was undisturbed except, if necessary, a stable episode of waking was achieved by mild auditory stimuli or gentle touch. EEG, EMG, and raw microwire signals were digitized (Cambridge Electronic Design 1401, London; supporting software, Spike 2) and stored on a disc for subsequent analyses. Multiple spikes, if present, were sorted on the basis of spike shape parameters from the amplified unprocessed microwire signals.
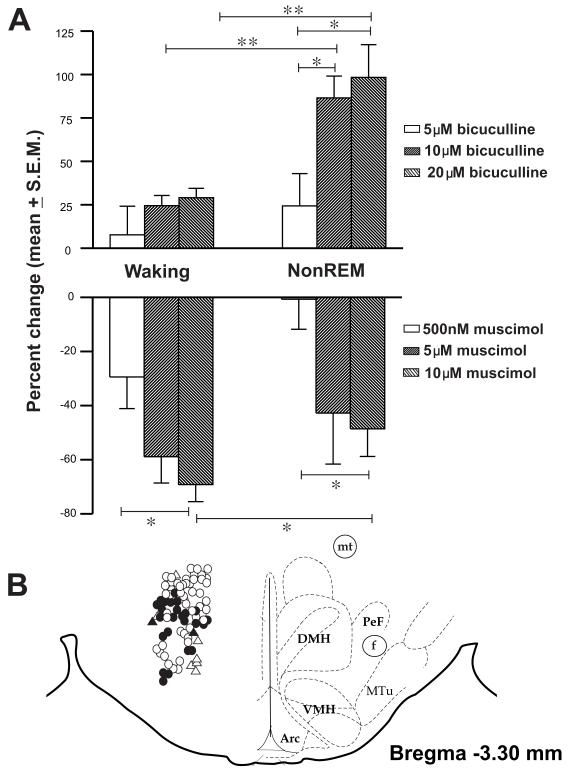
At the end of the recording session, under deep anesthesia (100mg/kg, i.p., pentobarbital), rats were perfused and the brain tissue was processed for histology. The location of the microdialysis probe and the microwire tracts were histologically confirmed and the locations of the various neuronal types encountered were plotted (Figure-1).
Figure 1.
(A). Effects of microdialysis perfusion of various doses of muscimol and bicuculline on percentage changes in discharge during waking and nonREM sleep. **, ≤ 0.01; *, ≤ 0.05 level of significance.
B. Line drawing of a representative brain section through the PF-LHA showing the anatomical distribution and sleep-wake profiles of the recorded neurons. Although theses neurons were recorded from areas ± 200μm from bregma −3.3, they are shown on one plane for convenience. Filled circle, wake-active, open circle, wake/REM-active, triangle, state-indifferent and filled triangle, REM-active neurons. The neurons were scanned during waking and therefore, fewer state-indifferent and REM-active neurons were recorded. Arc, arcuate hypothalamic nucleus; DMH, dorsomedial hypothalamus; f, fornix; mt, mammillothalamic tract; Mtu, medial tuberal nucleus; PeF, perifornical nucleus; VMH, ventromedial hypothalamus; 3V, third ventricle.
C. Data analysis
The sleep-wake discharge profiles of neurons were determined by the criteria adopted earlier (Alam et al., 2002). Neurons were classified as “wake-active” if their nonREM/active-wake as well as REM/active-wake discharge ratios were <0.5. Neurons were classified as “wake/REM-active” if REM/active-wake ratio was >0.5 and <1.5. Neurons were classified as “REM-active” if the REM/active-wake and REM/nonREM ratios were >1.5. Neurons exhibiting a change of less than 50% in all states were classified as “state-indifferent” neurons.
This study was aimed at determining GABAergic contribution on the suppression of PF-LHA neurons during nonREM sleep. Since wake- and wake/REM-active neurons are quiescent during nonREM sleep, we specifically focused on these two neuronal types, which were grouped together as “nonREM-off” neurons. Effects of muscimol or bicuculline on the discharge activity of PF-LHA neurons were determined by comparing the average discharge rates from 2-4 episodes of at least >30s of waking or nonREM sleep during aCSF perfusion and within the first 20min after 5min of drug delivery near the recorded neurons. If an episode of nonREM sleep did not occur during the first 20min, the discharge during the second 20min was considered. The discharge rates of neurons during baseline vs. drug delivery in waking or nonREM sleep were compared using either t test or one-way ANOVA followed by Holm-Sidak test for multiple comparisons. In those cases, where a normality test failed, comparable non-parametric tests were used. REM sleep was not consistently observed during the first 20-30min of muscimol/bicuculline perfusion and therefore their effects on REM sleep discharge was not considered for analysis.
RESULTS
The anatomical distributions of the 83 neurons that were characterized in terms of their sleep-wake discharge profiles and responsiveness to different doses of muscimol or bicuculline are shown in figure-1. The recorded neurons were localized in dorso-medial, perifornical, and lateral hypothalamic areas. During aCSF, bicuculline or muscimol perfusion, neuronal recordings exhibited stable signal/noise ratios. The microdialysis perfusion of muscimol and bicuculline affected neuronal discharge in a reciprocal and dose dependent manner. The details of the results are as follows:
Effects of muscimol on PF-LHA neurons
The effects of muscimol on the discharge of an individual PF-LHA neuron across sleep-wake cycle and on the mean discharge of PF-LHA neurons during waking and nonREM sleep are shown in table-1 and figures 1-2. Of 32 neurons studied, the effects of 500nM, 5μM and 10μM muscimol were examined on 9, 11 and 12 neurons, respectively. Overall, there was a significant difference in the percent decrease in discharge of the PF-LHA neurons amongst different muscimol treatment groups during waking (p<0.05) and nonREM sleep (p<0.05). While 500nM and 5μM muscimol suppressed the discharge of PF-LHA neurons during waking and produced only marginal effects during nonREM sleep, 10μM suppressed their discharge during both waking and nonREM sleep. Of the three doses used, 10μM produced significantly stronger suppression as compared to 500nM during both waking and nonREM sleep.
Table:1.
Effects of various doses of muscimol and bicuculline on the dischrge activity of PF-LHA neurons
| DRUGS USED / # of cells | B A S E L I N E | T R E A T M E N T | |||||
|---|---|---|---|---|---|---|---|
| Waking | nonREM | REM | NonREM/W | NonREM/REM | Waking | NREM | |
| Muscimol (500nM)/ n=9 | 1.94 ± 0.58 | 0.10 ± 0.03 | 0.82 ± 0.15 | 0.07± 0.03 | 0.12 ± 0.04 | 1.17 ± 0.30, p<0.05 | 0.10 ± 0.03 |
| Muscimol (5 μM)/n=11 | 2.53 ± 0.70 | 0.33 ± 0.12 | 1.55 ± 0.63 | 0.12 ± 0.03 | 0.28 ± 0.09 | 0.72 ± 0.19, p<0.001 | 0.24 ± 0.12 |
| Muscimol (10 μM)/n=12 | 2.96 ± 0.73 | 0.51 ± 0.21 | 2.41 ± 0.71 | 0.12 ± 0.04 | 0.26 ± 0.11 | 0.97 ± 0.26, p<0.001 | 0.20 ± 0.08, p<0.001 |
| Total = 32 | 2.52 ± 0.39 | 0.34 ± 0.09 | 1.66 ± 0.35 | 0.11 ± 0.02 | 0.23 ± 0.05 | 0.94 ± 0.14 p<0.001 | 0.19 ± 0.05, p <0.01 |
| Bicuculline (5 μM)/n=6 | 14.36 ± 3.40 | 7.83 ± 2.47 | 8.66 ± 2.15 | 0.63± 0.18 | 0.85 ± 0.07 | 15.70 ± 4.22 | 9.36 ± 2.82 |
| Bicuculline (10 μM)/n=16 | 4.29 ± 0.85 | 0.66 ± 0.13 | 3.85 ± 0.86 | 0.21 ± 0.04 | 0.23 ± 0.03 | 5.28 ± 1.04, p<0.01 | 1.26 ± 0.25 p<0.001 |
| Bicuculline (20 μM)/n=29 | 3.71 ± 0.61 | 0.92 ± 0.34 | 2.42 ± 0.41 | 0.26 ± 0.04 | 0.40 ± 0.09 | 4.61 ± 0.82, p<0.001 | 1.46 ± 0.49, p<0.001 |
| Total = 51 | 5.14 ± 0.74 | 1.65 ± 0.46 | 3.60 ± 0.51 | 0.28 ± 0.04 | 0.40 ± 0.06 | 6.12 ± 0.88, p<0.001 | 2.31 ± 0.55, p<0.001 |
Figure-2.
Continuous recording traces showing the discharge of a neuron during baseline (A) and during muscimol perfusion (B). This was a typical Wake/REM-active neuron found in this area. The discharge rate of this neuron increased during each arousal state indicated by elevation of EMG activity and desynchronized EEG as well as during REM sleep. Muscimol strongly suppressed the discharge of this neuron during both waking and nonREM sleep. EEG, electroencephalogram; EMG, electromyogram; Unit, extracellular neuronal activity.
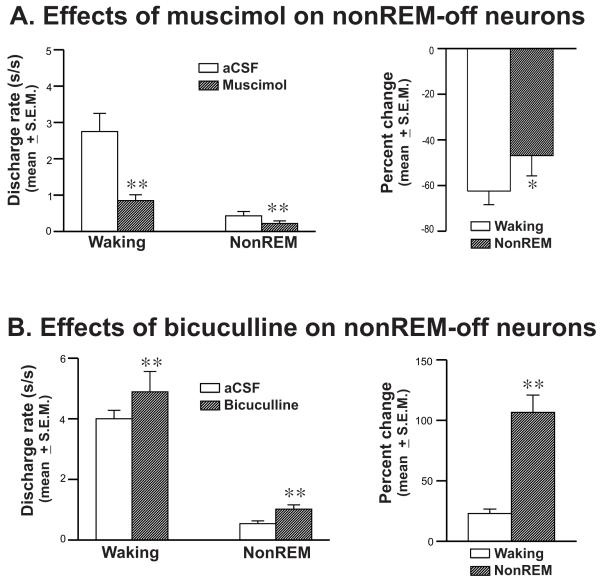
There were no significant differences in the magnitude of changes produced by 5μM and 10μM muscimol on the discharge of neurons during both waking and nonREM sleep. Therefore, data of neuronal discharge from these studies were pooled together for further quantifying effects of muscimol on the discharge of nonREM-off neurons. NonREM-off neurons as a group (wake-active=7; wake/REM-active=13; total=20) exhibited nonREM/active-wake and nonREM/REM discharge ratios of 0.13 ± 0.02 and 0.23 ± 0.05, respectively. Muscimol significantly suppressed the discharge of these neurons during waking as well as during nonREM sleep. However, the suppression produced during nonREM sleep was significantly lower as compared to that induced during waking (Figure-4).
Figure-4.
The effects of muscimol (5+10μM) and bicuculline (10+20μM) on the mean (±SEM) discharge activity (s/s) and on the percent changes compared to aCSF control, during waking and nonREM sleep in nonREM-off neurons. **, ≤ 0.01; *, ≤ 0.05 level of significance.
Effects of bicuculline on PF-LHA neurons
The effects of bicuculline on the discharge of an individual PF-LHA neuron across sleep-wake cycle and on the mean discharge of PF-LHA neurons during waking and nonREM sleep are shown in table-1 and figures-1&3. Of 51 neurons, the effects of 5μM, 10μM and 20μM bicuculline were examined on 6, 16 and 29 neurons, respectively. While there was no significant difference in the magnitude of changes produced by bicuculline amongst 5μM, 10μM, and 20μM treatment groups during waking, there was significant difference amongst these groups in percent increase in discharge produced by bicuculline during nonREM sleep (p<0.05, figure-1). While 5μM bicuculline did not affect the discharge of PF-LHA neurons, both 10μM and 20μM bicuculline significantly increased their discharge during both waking and nonREM sleep. The percent increases in discharge in both 10μM and 20μM bicuculline treated groups during nonREM sleep were significantly higher than that produced during waking.
Figure-3.
Continuous recording traces showing the discharge of one wake-active neuron (unit-1) and one wake/REM-active neuron (unit-2) recorded simultaneously during baseline (A) and during bicuculline perfusion (B). In the presence of bicuculline, both neurons exhibited elevated discharge, albeit stronger during nonREM sleep. EEG, electroencephalogram; EMG, electromyogram; Unit, extracellular neuronal activity
As compared to 10μM, the effects of 20μM bicuculline were not significantly different during both waking and nonREM sleep. Therefore, neurons examined for the effects of 10μM and 20μM of bicuculline were pooled together for further quantifying its effects on nonREM-off neurons (figure-4). The nonREM-off neurons as a group (wake-active=9; wake/REM-active=29; total=38) exhibited a nonREM/active-wake and nonREM/REM discharge ratios of 0.17 ± 0.02 and 0.23 ± 0.04. In the presence of bicuculline, nonREM-off neurons as a group exhibited significantly elevated discharge during waking as well as during nonREM sleep. However, the percent increase exhibited during nonREM sleep was significantly higher than that observed during waking (figure-4).
DISCUSSION
This study demonstrates that GABAA receptor stimulation suppressed the discharge of PF-LHA neurons including nonREM-off neurons. This suppression was stronger during waking as compared to nonREM sleep. On the other hand, in the presence of GABAA receptor blockade, these neurons exhibited increased discharge. This increase was larger during nonREM sleep as compared to waking. The findings of this study are consistent with a hypothesis that PF-LHA neurons are subject to increased GABAergic inhibitory tone during nonREM sleep. This further compliments an established role of GABA as a sleep-promoting neurotransmitter and PF-LHA neurons as one of the targets where GABA acts via GABAA receptor to promote sleep.
In this study, GABAA receptor agonist and antagonist produced consistent, reversible, dose-dependent, and opposite effects on the discharge activity of PF-LHA neurons during waking and nonREM sleep. Computer generated templates, based on spike shape parameters used for spike sorting, excluded any artifactual spikes and ensured that the activity of the same neuron was recorded across baseline, drug delivery and washout periods. The whole experimental paradigm for each cell was completed in 2-4h recording session. The microdialysis drug administration method we used, compared to microinjection or microiontophoresis, is less likely to elicit inflammatory responses (Quan and Blatteis, 1989). Therefore, we conclude that the observed effects of muscimol and bicuculline were likely to be physiological. However, since the microdialysis probe provides a bathing medium for the entire extracellular environment of recorded neurons, the site of drug action cannot be pinpointed.
The PF-LHA is critically involved in the regulation of behavioral arousal (see introduction) and most of its neurons are quiescent during nonREM sleep. This study was aimed at evaluating the proportion of changes in the discharge of PF-LHA neurons during spontaneous nonREM sleep vs. waking that could be attributed to local GABAA receptor mediated mechanisms. In earlier studies we and others have shown that behavioral changes could be elicited by long-term delivery of GABAergic agents into the PF-LHA (Alam et al., 2005, Thakkar et al., 2008). To avoid the effect of behavioral changes on the discharge activity, in the present study, lower concentrations of GABAA agonist and antagonist were perfused for short duration and adjacent to the recorded neurons.
In this study, while GABAA receptor blockade significantly attenuated the discharge suppression of PF-LHA neurons during nonREM sleep, it failed to completely block it. This could be due to one or combination of factors: a) that the concentration of bicuculline perfused was not sufficient enough to completely attenuate the discharge suppression; and b) that the state-dependent discharge of PF-LHA neurons is modulated by multiple neurochemical inputs (Kukkonen et al., 2002, Li et al., 2002, Ohno and Sakurai, 2008) and GABAA receptor mediated inhibitory tone is one of the factors contributing to the quiescence of PF-LHA neurons during nonREM sleep. For example, it is likely that GABAB receptor mediated transmission also contributes significantly. GABAB receptor agonist inhibits the activity of HCRT neurons in vitro (Xie et al., 2006). Mice with selective deletion of GABAB1 gene specifically from HCRT neurons exhibited severe fragmentation of sleep-wake states, suggesting that GABAB receptors on HCRT neurons are essential for stabilizing and consolidating sleep-wake states (Matsuki et al., 2009). Microdialysis perfusion of THIP, a selective agonist for extrasynaptic GABAA receptors, into the PF-LHA has been shown to reduce waking with a concomitant increase in nonREM sleep (Thakkar et al., 2008). Recently we found that A1 receptor mediated adenosinergic inhibition may also contribute to the suppression of PF-LHA neurons (Alam et al., 2009).
In this study, a lower concentration of muscimol suppressed discharge during waking and produced minimal effect during nonREM sleep and a higher concentration produced a relatively weak suppression of discharge during nonREM sleep. The weak effect of GABAA receptor agonist during nonREM sleep could occur if these neurons were already under GABAergic inhibitory tone and could be due to a ceiling effect. Consistent with this idea, we found that in the presence of bicuculline, PF-LHA neurons exhibited much stronger activation during nonREM sleep than during waking. These findings suggest that PF-LHA neurons are subject to significantly stronger GABAergic tone during nonREM sleep.
In this study, nonREM-off neurons, which included both wake- and wake/REM-active neurons were affected by GABAA receptor manipulations, suggesting that GABAergic effects are not specific to wake-active including HCRT neurons. This is consistent with earlier studies where both HCRT and non-HCRT neurons have been shown to exhibit Fos-IR during GABAA receptor blockade in the PF-LHA (Alam et al., 2005, Goutagny et al., 2005). In our earlier study, we also found that GABAA receptor blockade produced only marginal effects on Fos-IR in MCH neurons, compared to HCRT and other wake-active PF-LHA neurons. Since our unit recording method does not permit identification of neurotransmitter phenotypes of recorded cells, we could not assess a hypothesis that GABA exerts relatively weaker inhibitory influences on the discharge activity of REM-active MCH neurons (Hassani et al., 2009).
The PF-LHA contains local GABAergic interneurons and receives afferents from sleep-active GABAergic neurons from POA region (Gong et al., 2004, Uschakov et al., 2007). Recently we found that MnPN stimulation suppressed the discharge of most of the PF-LHA neurons including practically all of the wake/REM and wake-active neurons encountered, whereas MnPN inactivation led to the activation of HCRT and other PF-LHA neurons in anesthetized rats (Suntsova et al., 2007, Kumar et al., 2008). These finding suggests that at least a subset of PF-LHA neurons including HCRT neurons are under direct MnPN inhibitory control during sleep. We also found that a subset of GABAergic neurons in the PF-LHA exhibited Fos-IR during recovery sleep after 6h of sleep deprivation, i.e., are sleep-active (Kumar et al., 2005). The present study cannot differentiate if bicuculline-induced attenuation in suppression in discharge of PF-LHA neurons during nonREM sleep was due to the blockade of GABAergic transmission from MnPN sleep-active neurons or that of the local GABAergic interneurons. However, it is reasonable to assume that both GABAergic sources are involved.
In conclusion, present study demonstrates that local GABAA receptor activation suppressed the discharge of PF-LHA neurons, including nonREM-off neurons which was significantly weaker during nonREM sleep as compared to waking. In contrast, in the presence of GABAA receptor blockade, the suppression in the discharge of PF-LHA neurons, including nonREM-off neurons, was strongly attenuated during nonREM sleep. These results support that PF-LHA neurons, including nonREM-off neurons, are subject to increased GABAA receptor-mediated GABAergic inhibitory tone during nonREM sleep.
ACKNOWLEDGEMENTS
This work was supported by the US Department of Veteran Affairs Medical Research Service and US National Institutes of Health grants, NS-050939, MH63323, and MH075076.
ABBREVIATIONS
- aCSF
Artificial cerebrospinal fluid
- Fos-IR
c-fos protein immunoreactivity
- GABA
Gamma-aminobutyric acid
- HCRT
Hypocretin
- MCH
Melanin-concentrating hormone
- NonREM
Non-rapid eye movement sleep
- PF-LHA
Perifornical-lateral hypothalamic area
- TBS
Tris buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abrahamson EE, Moore RY. The posterior hypothalamic area: chemoarchitecture and afferent connections. Brain Res. 2001;889:1–22. doi: 10.1016/s0006-8993(00)03015-8. [DOI] [PubMed] [Google Scholar]
- Alam MA, Mallick BN. Glutamic acid stimulation of the perifornical-lateral hypothalamic area promotes arousal and inhibits non-REM/REM sleep. Neurosci Lett. 2008;439:281–286. doi: 10.1016/j.neulet.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Alam MN, Gong H, Alam T, Jaganath R, McGinty D, Szymusiak R. Sleep-waking discharge patterns of neurons recorded in the rat perifornical lateral hypothalamic area. J Physiol. 2002;538:619–631. doi: 10.1113/jphysiol.2001.012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Kumar S, Bashir T, Suntsova N, Methippara MM, Szymusiak R, McGinty D. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–582. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Rai S, Kumar S, Szymusiak R, McGinty D. Role of adenosine A1 receptor in the regulation of hypocretin neurons in freely behaving rats. Abstract, Society for Neuroscience. 2009 doi: 10.1016/j.neuroscience.2010.01.044. 277.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam MN, Szymusiak R, Gong H, King J, McGinty D. Adenosinergic modulation of rat basal forebrain neurons during sleep and waking: neuronal recording with microdialysis. J Physiol. 1999;521(Pt 3):679–690. doi: 10.1111/j.1469-7793.1999.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backberg M, Ultenius C, Fritschy JM, Meister B. Cellular localization of GABA receptor alpha subunit immunoreactivity in the rat hypothalamus: relationship with neurones containing orexigenic or anorexigenic peptides. J Neuroendocrinol. 2004;16:589–604. doi: 10.1111/j.1365-2826.2004.01207.x. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. doi: 10.1002/cne.903190204. [DOI] [PubMed] [Google Scholar]
- Cooper JR, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. Oxford University Press; New York: 1996. [Google Scholar]
- Datta S, Maclean RR. Neurobiological mechanisms for the regulation of mammalian sleep-wake behavior: reinterpretation of historical evidence and inclusion of contemporary cellular and molecular evidence. Neurosci Biobehav Rev. 2007;31:775–824. doi: 10.1016/j.neubiorev.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espana RA, Valentino RJ, Berridge CW. Fos immunoreactivity in hypocretin-synthesizing and hypocretin-1 receptor-expressing neurons: effects of diurnal and nocturnal spontaneous waking, stress and hypocretin-1 administration. Neuroscience. 2003;121:201–217. doi: 10.1016/s0306-4522(03)00334-8. [DOI] [PubMed] [Google Scholar]
- Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci. 2001;21:1656–1662. doi: 10.1523/JNEUROSCI.21-05-01656.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulhaber J, Steiger A, Lancel M. The GABAA agonist THIP produces slow wave sleep and reduces spindling activity in NREM sleep in humans. Psychopharmacology (Berl) 1997;130:285–291. doi: 10.1007/s002130050241. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Blanco-Centurion C, Greco MA, Shiromani PJ. Effects of lateral hypothalamic lesion with the neurotoxin hypocretin-2-saporin on sleep in Long-Evans rats. Neuroscience. 2003;116:223–235. doi: 10.1016/s0306-4522(02)00575-4. [DOI] [PubMed] [Google Scholar]
- Gerashchenko D, Shiromani PJ. Different neuronal phenotypes in the lateral hypothalamus and their role in sleep and wakefulness. Mol Neurobiol. 2004;29:41–59. doi: 10.1385/MN:29:1:41. [DOI] [PubMed] [Google Scholar]
- Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–946. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesmann C. GABA mechanisms and sleep. Neuroscience. 2002;111:231–239. doi: 10.1016/s0306-4522(02)00034-9. [DOI] [PubMed] [Google Scholar]
- Goutagny R, Luppi PH, Salvert D, Gervasoni D, Fort P. GABAergic control of hypothalamic melanin-concentrating hormone-containing neurons across the sleep-waking cycle. Neuroreport. 2005;16:1069–1073. doi: 10.1097/00001756-200507130-00008. [DOI] [PubMed] [Google Scholar]
- Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci. 2006;26:9426–9433. doi: 10.1523/JNEUROSCI.2012-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassani OK, Lee MG, Jones BE. Melanin-concentrating hormone neurons discharge in a reciprocal manner to orexin neurons across the sleep-wake cycle. Proc Natl Acad Sci U S A. 2009;106:2418–2422. doi: 10.1073/pnas.0811400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Takahashi K, Kodama T, Kayama Y. State-dependent activity of neurons in the perifornical hypothalamic area during sleep and waking. Neuroscience. 2003;119:1209–1219. doi: 10.1016/s0306-4522(03)00173-8. [DOI] [PubMed] [Google Scholar]
- Kukkonen JP, Holmqvist T, Ammoun S, Akerman KE. Functions of the orexinergic/hypocretinergic system. Am J Physiol Cell Physiol. 2002;283:C1567–1591. doi: 10.1152/ajpcell.00055.2002. [DOI] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Rai S, McGinty D, Alam MN. Effects of serotonin on perifornical-lateral hypothalamic area neurons in rat. Eur J Neurosci. 2007;25:201–212. doi: 10.1111/j.1460-9568.2006.05268.x. [DOI] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Bashir T, Suntsova N, Rai S, McGinty D, Alam MN. Inactivation of median preoptic nucleus causes c-Fos expression in hypocretin- and serotonin-containing neurons in anesthetized rat. Brain Res. 2008;1234:66–77. doi: 10.1016/j.brainres.2008.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Szymusiak R, Methippara MM, Seema R, Suntsova N, McGinty D, Alam MN. GABAergic and glutamatergic neurons in the perifornical lateral hypothalamic area exhibit differential Fos expression after sleep deprivation vs. recovery sleep. Sleep. 2005;29:A146. [Google Scholar]
- Lancel M, Faulhaber J. The GABAA agonist THIP (gaboxadol) increases non-REM sleep and enhances delta activity in the rat. Neuroreport. 1996;7:2241–2245. doi: 10.1097/00001756-199609020-00036. [DOI] [PubMed] [Google Scholar]
- Lancel M, Faulhaber J, Schiffelholz T, Mathias S, Deisz RA. Muscimol and midazolam do not potentiate each other’s effects on sleep EEG in the rat. J Neurophysiol. 1997;77:1624–1629. doi: 10.1152/jn.1997.77.3.1624. [DOI] [PubMed] [Google Scholar]
- Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–6720. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Gao XB, Sakurai T, van den Pol AN. Hypocretin/Orexin excites hypocretin neurons via a local glutamate neuron-A potential mechanism for orchestrating the hypothalamic arousal system. Neuron. 2002;36:1169–1181. doi: 10.1016/s0896-6273(02)01132-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M, Sakurai T. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci U S A. 2009;106:4459–4464. doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–798. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recept Signal Transduct Res. 2006;26:731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Nitz D, Siegel JM. GABA release in posterior hypothalamus across sleep-wake cycle. Am J Physiol. 1996;271:R1707–1712. doi: 10.1152/ajpregu.1996.271.6.R1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K, Sakurai T. Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol. 2008;29:70–87. doi: 10.1016/j.yfrne.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain: in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, Li R, Hungs M, Pedrazzoli M, Padigaru M, Kucherlapati M, Fan J, Maki R, Lammers GJ, Bouras C, Kucherlapati R, Nishino S, Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan N, Blatteis CM. Microdialysis: a system for localized drug delivery into the brain. Brain Res Bull. 1989;22:621–625. doi: 10.1016/0361-9230(89)90080-4. [DOI] [PubMed] [Google Scholar]
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Sinnamon HM, Karvosky ME, Ilch CP. Locomotion and head scanning initiated by hypothalamic stimulation are inversely related. Behav Brain Res. 1999;99:219–229. doi: 10.1016/s0166-4328(98)00106-5. [DOI] [PubMed] [Google Scholar]
- Stock G, Rupprecht U, Stumpf H, Schlor KH. Cardiovascular changes during arousal elicited by stimulation of amygdala, hypothalamus and locus coeruleus. J Auton Nerv Syst. 1981;3:503–510. doi: 10.1016/0165-1838(81)90083-7. [DOI] [PubMed] [Google Scholar]
- Suntsova N, Guzman-Marin R, Kumar S, Alam MN, Szymusiak R, McGinty D. The median preoptic nucleus reciprocally modulates activity of arousal-related and sleep-related neurons in the perifornical lateral hypothalamus. J Neurosci. 2007;27:1616–1630. doi: 10.1523/JNEUROSCI.3498-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–286. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Lin JS, Sakai K. Neuronal activity of orexin and non-orexin waking-active neurons during wake-sleep states in the mouse. Neuroscience. 2008;153:860–870. doi: 10.1016/j.neuroscience.2008.02.058. [DOI] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. Effect of microdialysis perfusion of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol in the perifornical hypothalamus on sleep-wakefulness: role of delta-subunit containing extrasynaptic GABAA receptors. Neuroscience. 2008;153:551–555. doi: 10.1016/j.neuroscience.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R. Role of GABAA receptors in the physiology and pharmacology of sleep. Eur J Neurosci. 2009;29:1779–1794. doi: 10.1111/j.1460-9568.2009.06716.x. [DOI] [PubMed] [Google Scholar]
- Xie X, Crowder TL, Yamanaka A, Morairty SR, Lewinter RD, Sakurai T, Kilduff TS. GABA(B) receptor-mediated modulation of hypocretin/orexin neurones in mouse hypothalamus. J Physiol. 2006;574:399–414. doi: 10.1113/jphysiol.2006.108266. [DOI] [PMC free article] [PubMed] [Google Scholar]