Abstract
RNA-dependent protein kinase is an interferon-induced, double-stranded (ds), RNA-activated serine/threonine protein kinase involved in the eukaryotic response to viral infection. While PKR also functions in cellular differentiation, growth control and apoptosis, its role in human cancer remains poorly understood. To explore a role for PKR in human cancer, we evaluated PKR expression and function in a series of cancer cell lines from different tumor types. We observed that PKR protein expression is high in various cancer cells and low in normal cells. Knockdown of PKR protein expression by PKR siRNA induced cell death, indicating a PKR-dependent survival pathway under normal growth conditions. Inhibition of PKR signaling using a dominant negative adenoviral PKR mutant (Ad-Δ6PKR) also induced cancer cell apoptosis via a mechanism that blocks activation of AKT-mediated survival while simultaneously inducing ER stress. ER stress-mediated apoptosis was evidenced by unregulated expression of phosphorylated JNK (p-JNK), phosphorylated cJun (p-cJun), and caspase-4 and was significantly reduced in cancer cells treated with JNK and caspase-4 inhibitors. We further demonstrated that inhibition of PKR signaling via either siRNA or Ad-Δ6PKR sensitizes cancer cells to etoposide or cisplatin-mediated cell death. Our results suggest a rationale to develop therapeutic strategies that target PKR signaling in human cancer cells.
Keywords: PKR, gene therapy, adenovirus
Introduction
RNA-dependent protein kinase (PKR) is an interferon-induced, double-stranded (ds), RNA-activated serine/threonine protein kinase that has a well-established role in anti-viral defense mechanisms.1–3 In response to dsRNAs and interferons, activated PKR inhibits cellular protein translation by phosphorylation of eIF-2α, an event that induces cellular apoptosis. In the absence of viral infections, however, PKR also plays a role in other important cellular functions including growth control, apoptosis regulation, cell proliferation, signal transduction and differentiation.1–6 While PKR controls the expression of multiple genes and signaling pathways, its function in suppressing or promoting mammalian cell growth is somewhat controversial.
In support of its role in growth suppression, for example, treatment of murine cell lines with dsRNA, TNFα or lipopolycacharide leads to a PKR-dependent apoptosis, which may be due to phosphorylation of eIF-2α, but also to the expression of other pro-apoptotic factors such as Fas.7,8 In addition, PKR induces apoptosis in epithelial cancer cell lines in response to adenoviral overexpression of TNFα, E2F-1 and the melanoma differentiation-associated gene 7 (mda7), a novel tumor suppressor gene.9–12 Lastly, in studies of primary human cancers from the head and neck, thyroid and colon, increasing PKR expression has been correlated with more well-differentiated tumors and diminished proliferative activity, suggesting that PKR regulates tumor suppression.13–15
Other studies, however, do not support a tumor suppressor role for PKR and raise the alternative hypothesis that PKR functions in growth promotion. For example, PKR knockout mice do not show increased spontaneous rates of tumor development.16,17 In addition, increased expression and activity of PKR has been reported in some human tumor types and correlates with neoplastic progression.18,19 Biochemical studies have shown that PKR can phosphorylate IKK, leading to activation of the NFκB anti-apoptotic signaling pathway.20 PKR can also induce the expression of several key survival genes known to be dependent on NFκB, such as c-IAP1, c-IAP2 and A20.21 PKR also “crosstalks” with multiple signaling pathways that activate and engage a number of transcription factors implicated in oncogenesis.22
Several recent studies from our group further support the hypothesis that PKR promotes cellular survival. First, using PKR wild-type (+/+) and knockout (−/−) MEFs to deduce PKR function, treatment with tumor necrosis factor (TNF) activated AKT and NFκB in a PKR-dependent manner.23 In conjunction with this, PKR was required for TNF-induced NFκB-dependent anti-apoptotic protein expression.24 Secondly, PKR was shown to positively regulate an AKT-dependent survival pathway in human lung cancer cells.24 Thirdly, PKR mediates resistance to radiation therapy in mouse embryo fibroblasts through the activation of AKT and NFκB-dependent signaling pathways.25 Collectively, these results suggest that PKR can function to enhance cell survival, although this phenotype may depend on the stress signal, cell-type and physiologic context.
In the present study, we explored the role for PKR in promoting resistance to chemotherapy. We show that inhibition of PKR signaling using a dominant negative PKR mutant (Ad-Δ6PKR) acts synergistically with chemotherapy to induce cell death in human cancer cell lines. The induction of cell death was due in part to the activation of an endoplasmic reticulum (ER) stress pathway. Our results suggest a therapeutic strategy to target PKR signaling in the treatment of human cancer.
Results
Knock down of PKR by siRNA in cancer cells induces cell death
We first investigated the expression and cellular localization of PKR in human cancer cells as well as normal cells. Figure 1A shows that PKR protein expression is high in multiple cell lines derived from human lung (A549, H460), prostate (PC3, LNCaP), breast (MCF7, T47D) and colon (SW620, HT-29, KM12L4) cancers. In contrast, PKR expression was low in normal human bronchial epithelial cells (NHBEs) and normal human mammary epithelial cells (HMECs).
Figure 1.
Knockdown of PKR by siRNA induces cell death in cancer cell lines. (A) Western blot analysis of PKR protein expression in human lung (A549 and H460), prostate (PC3 and LNcaP), breast (MCF-7 and T47D), and colon (SW620, HT-29 and KM12L4) cancer cell lines and normal cells (NHBEs and HMECs). The expression of actin was used as a loading control. (B) Immunofluorescence microscopy with antibodies against PKR (green) demonstrated cytosolic expression of PKR in A549 and PC3 cancer cells and diffuse expression of PKR in normal cells (NHBEs and HMECs). (C) Western blot and Flow cytometric analyses of PKR protein expression and cell death in human lung (A549), prostate (PC3), breast (T47D) cancer cell lines and normal cells (NHBEs) cells after 72 hrs of treatment with PKR siRNA and luc SiRNA. Indicated at the bottom is the percent cell death induced by PKR siRNA and Luc siRNA treatment in each of these cell lines. Actin expression was analyzed as a loading control.
We next investigated the pattern of PKR expression in two cancer cell lines (A549 and PC3) and the two normal cell lines (NHBEs and HMECs) by confocal immunofluorescence analysis. We observed both cytosolic and nuclear localization of PKR in A549 and PC3 cancer cells with the predominant signal in the cytosol (Fig. 1B). In contrast, we observed a diffuse expression pattern of PKR in NHBEs and HMECs normal cells (Fig. 1B).
We next investigated the effect of silencing PKR protein expression on cell viability. As revealed by immunoblotting and FACS analysis, treatment of A549, PC3 and T47 cancer cells with PKR siRNA effectively knocked down expression of PKR protein and induced apoptosis (Fig. 1C). Conversely, treatment with luc siRNA did not knock down expression of PKR protein nor induce apoptosis (Fig. 1C). Normal NHBEs cells treated with PKR siRNA also underwent apoptosis, but to a much lesser extent than the cancer cells (12% versus ~45–50% in normal and cancer cells, respectively) (Fig. 1C). Taken together, these results suggest differential function of PKR between normal and cancer cells.
Inhibition of PKR by adenoviral mutant PKR (Ad-Δ6PKR) induces cell death in cancer cells
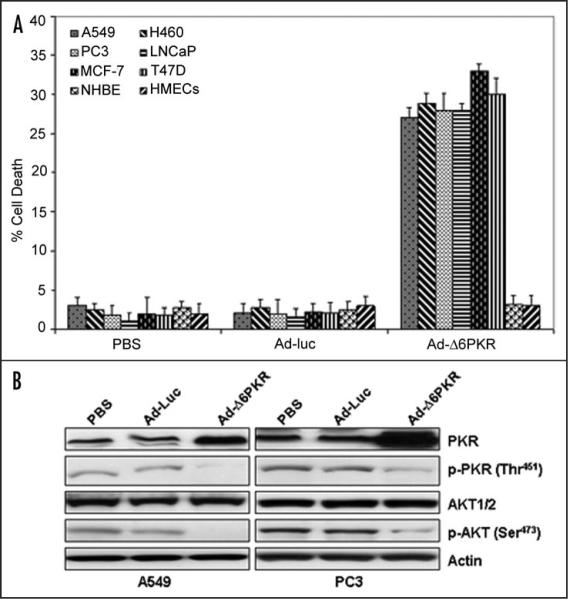
We have previously shown that a deletion mutant of PKR (Δ6PKR) can act as a dominant negative inhibitor of PKR function.8 As shown by flow cytometric analysis of human lung (A549 and H460), prostate (PC3 and LNCaP) and breast (MCF-7 and T47D) cancer cells, adenoviral infection with Δ6PKR (Ad-Δ6PKR) induced cell death within 72 hrs of infection (Fig. 2A). In contrast, Ad-Δ6PKR was not toxic to NHBE or HMEC normal cells (Fig. 2A).
Figure 2.
Inhibition of PKR by Adenoviral mutant PKR (Ad-Δ6PKR) vector induces cell death in cancer cells. (A) Flow cytometric analysis of cell death in A549, H460, PC3, LNCaP, MCF-7, T47D, NHBEs and HMECs cells 72 hours after treatment with PBS, Ad-luc (3000 vp) or Ad-Δ6PKR (3000 vp). Experiments were performed in triplicate; data are presented as the means (error bars, SDs). (B) Western blot analysis of phosphorylated PKR (p-PKR), PKR, phosphorylated AKT (p-AKT), and AKT1/2 protein expression in A549 and PC3 cell lysates 72 hours after treatment with PBS, Ad-luc (3000 vp) or Ad-Δ6PKR (3000 vp). β-Actin expression was analyzed as a loading control.
Our previous studies in PKR+/+MEFs indicated that PKR activates an AKT-dependent anti-apoptotic survival pathway by upregulating levels of phospho-AKT (Ser473).23,25 Conversely, Ad-Δ6PKR inhibited phospho-AKT (Ser473) in PKR+/+MEFs.25 To test whether Ad-Δ6PKR would also inhibit AKT phosphorylation in cancer cells, we infected A549 and PC3 cells with Ad-Δ6PKR. As revealed by immunoblotting, a high level of PKR expression was observed in A549 and PC3 cancer cells after being infected with Ad-Δ6PKR (Fig. 2B), whereas low levels of endogenous PKR protein were observed in cells infected with the control contructs. Ad-Δ6PKR infected cancer cells demonstrated decreased expression of phospho-PKR (Thr451) and phospho-AKT (Ser473). We could not detect decreased expression of phosphorylated PKR and AKT1/2 in Ad-Luc infected cancer cells (Fig. 2B). These results further confirmed that Ad-Δ6PKR can inhibit phospho-AKT (Ser473) in cancer cells.
Activation of the endoplasmic reticulum (ER) stress response contributes to mutant PKR (Ad-Δ6PKR)-induced apoptosis
The endoplasmic reticulum functions in normal cellular homeostasis by ensuring proper protein folding in response to different environmental and metabolic stimuli.26,27 Under conditions of stress (for example with hypoxia and/or nutrient deprivation) misfolded/unfolded proteins accumulate in the ER and stimulate a signaling pathway known as the “unfolded protein response” (UPR). The UPR permits cells to either adapt to the stress until the stress resolves, or alternatively, promotes apoptosis when the stress related damage becomes irreversible. Previous results suggested that PKR plays an important role in ER stress-induced retinal neuron damage in mice.28
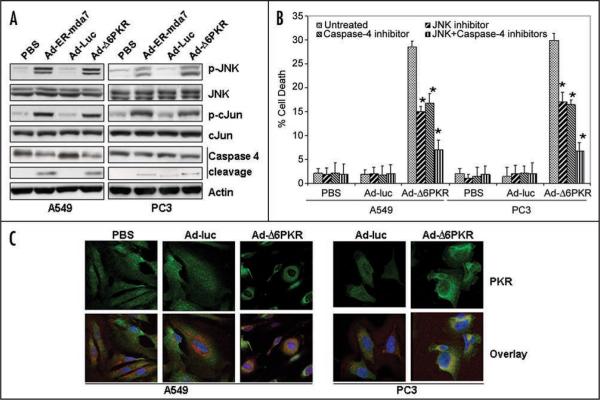
We next tested whether Ad-Δ6PKR-mediated apoptosis of cancer cell lines resulted from activation of ER-stress. To do this, we measured downstream target proteins that are activated via phosphorylation in response to ER-stress, including JNK and c-Jun. A549 and PC3 cancer cell lines were treated for 72 hours with either PBS, Ad-Luc, Ad-Δ6PKR or endoplasmic reticulum targeting melanoma differentiation-associated gene 7 (Ad-ER-mda7). Ad-ER-mda7 served as a positive control. As shown in Figure 3A, expression of phospho-JNK and phospho-cJun was upregulated after Ad-Δ6PKR and Ad-ER-mda7 transduction but not after Ad-Luc transduction (Fig. 3A), suggesting that activation of JNK or cJun was the mechanism by which Ad-Δ6PKR mediated cell death.
Figure 3.
Adenoviral mutant PKR (Ad-Δ6PKR) induces the endoplasmic reticulum (ER) stress-mediated cell death pathway. (A) Western blot analysis of phosphorylated JNK (p-JNK), JNK, phosphorylated cJun (p-cJun), cJun and caspase-4 protein expression in A549 and PC3 cell lysates 72 hrs after treatment with Ad-luc (3000 vp), Ad-ER-mda7 (3000 vp) or Ad-Δ6PKR (3000 vp). β-Actin expression was analyzed as a loading control. (B) Flow cytometric analysis of cell death in A549 and PC3 cancer cells 72 hrs after treatment with PBS, Ad-Luc (3000 vp) or Ad-Δ6PKR (3000 vp) with or without caspase-4 (Ac-LEVD-CHO, 10 μmol/L) or JNK chemical inhibitors (10 μmol/L). Experiments were performed in triplicate; data are presented as the means (error bars, SDs) *p < 0.05 vs. untreated control, Wilcoxon rank-sum test. (C) Immunofluorescent confocal microscopic analysis of PKR protein expression. Analysis of antibody against PKR stained green, ER stained red with ER-Tracker dyes, and nuclei stained blue with DAPI, demonstrated colocalization (orange) of PKR protein and the ER marker in Ad-Δ6PKR-treated A549 and PC3 cancer cells 72 hrs after transfection.
Human caspase-4 is localized to the ER membrane and is specifically activated by and required for ER stress-induced apoptosis.33 We next investigated whether caspase-4 was involved in Ad-Δ6PKR-induced cell death. Caspase-4 was cleaved to its active form in A549 and PC3 cancer cells treated with Ad-Δ6PKR or Ad-ER-mda7 (positive control) but not in those treated with PBS or Ad-Luc (Fig. 3A). To more directly determine the importance of JNK and caspase-4 activation in Ad-Δ6PKR-mediated cell death, A549 and PC3 cancer cells were infected with Ad-Δ6PKR in the presence or absence of a chemical JNK inhibitor (SP600125, 10 μmol/L), a peptide inhibitor of caspase-4 (Ac-LEVD-CHO, 10 μmol/L) or combination of the two inhibitors. In both cancer cell lines, inhibitory treatment reduced the amount of AdΔ6PKR-induced cell death as measured by FACS. In A549 cells, apoptosis was reduced from 28.5% to 15% after JNK inhibition, 16.8% after caspase 4 inhibition, and 7% after treatment with both inhibitors (p < 0.05, significantly different from untreated). Similarly in PC3 cells, apoptosis was reduced from 29.9% to 17% after JNK inhibition, 16.4% after caspase 4 inhibition, and 6.7% after treatment with both inhibitors (Fig. 3B) (p < 0.05, significantly different from untreated). Conversely, neither inhibitor reduced the amount of cell death induced by overexpression of Ad-Bak, a Bcl-2 family member that induces apoptosis in a PKR-independent manner (data not shown). Together, these results suggest that Ad-Δ6PKR cell death is mediated in part through activation of ER stress and downstream effectors such as JNK and caspase-4.
We next analyzed the pattern of PKR expression in A549 lung cancer and PC3 prostate cancer cells after treatment with PBS, Ad-Luc or Ad-Δ6PKR. Confocal immunofluorescence analysis revealed that PKR levels were markedly higher in the cytosol of A549 and PC3 cells after treatment with Ad-Δ6PKR (Fig. 3C). The precise subcellular localization of the PKR (green) was further confirmed by comparing its expression pattern with an ER molecular marker (red) known to reside within the ER compartment (Fig. 3C). Treatment with Ad-Δ6PKR led to an increase in PKR within the ER, as evidenced by enhanced colocalization fluorescence (yellow-orange) in the overlay images. These results further support a role for the endoplasmic reticulum in Ad-Δ6PKR induced cellular apoptosis.
Adenoviral mutant PKR (Ad-Δ6PKR) sensitizes cancer cells to some chemotherapeutic agents
In a single study previously published by Bergeron et al., PKR-deficient (PKR−/−) mouse-embryonic fibroblasts (MEFs) were more sensitive than PKR-proficient (PKR+/+) MEFs to bulky adduct DNA damage caused by UV radiation or alkylating chemotherapeutic agents such as cisplatin and melphalan.30 To confirm and expand on these results, we first compared the viability of PKR+/+ and PKR−/−MEFs following treatment with DNA damaging agents (etoposide, cisplatin, mitomycin), an anti-metabolite (5-fluouracil [5FU]) and an anti-tubulin (Taxol). In agreement with Bergeron et al., we found that PKR−/−MEFs cells are much more susceptible than PKR+/+MEFs to cell death induced by cisplatin or melphalan (Fig. 4A) (p < 0.05). In contrast, there was no difference following treatment with Mitomycin C. The enhanced sensitivity to DNA-damage induced apoptosis was not restricted to akylating agents, as PKR−/−MEFs were also more sensitive to DNA damage by etoposide, a Topoisomerase II poison. Interestingly, PKR−/−MEFs were also more susceptible than PKR+/+MEFs to cell death induced by 5FU but not Taxol.
Figure 4.
PKR-deficient mouse-embryonic fibroblasts (PKR−/−) are susceptible to chemotherapeutic agents. (A) Flow cytometric analysis of apoptosis in PKR+/+ and PKR−/−MEF cells after 72 hrs of treatment with etoposide, cisplatin, 5-FU, melphalan, Taxol or mitomycin. Experiments were performed in triplicate; data are presented as the means (error bars, SDs) *p < 0.05 vs. corresponding control, Wilcoxon rank-sum test. (B) Flow cytometric analysis of apoptosis in PKR+/+ and PKR−/−MEF cells after 72 hrs of treatment with PKR siRNA and luc SiRNA with or without etoposide (ET) or cisplatin (CDDP) treatment. Experiments were performed in triplicate; data are presented as the means (error bars, SDs) *p < 0.05 vs. corresponding control, Wilcoxon rank-sum test.
To control for the possibility that PKR−/−MEFs acquired genetic change(s) outside the PKR locus that could account for these results, we next transfected PKR+/+MEFs with PKR siRNA and then treated them with cisplatin or etoposide. PKR+/+MEFs transfected with PKR siRNA underwent chemotherapy-induced apoptosis to a similar degree as PKR−/−MEFs (Fig. 4B). In contrast, PKR+/+MEFs transfected with a control (Luciferase) siRNA retained a relative resistance to etoposide or cisplatin when compared to PKR−/−MEFs. Collectively, these results demonstrate that a PKR-dependent survival pathway mediates resistance to some chemotherapeutic agents.
We next wanted to test whether inhibition of PKR signaling would confer a similar chemosensitization effect in human cancer cells. To do this, we examined the apoptotic effects of Ad-Δ6PKR alone or in combination with etoposide (10 μmol/L), cisplatin (10 μmol/L), or both drugs in A549 and PC3 cancer cells after 72 hrs treatment. By flow cytometric analysis, Ad-Δ6PKR alone resulted in cell death rates of 25% in A549 cells and 24% in PC3 cells (Fig. 5). Treatment with etoposide or cisplatin as single agents resulted in cell death rates of 10% and 8.6% respectively in A549 cells and 11% and 9% respectively in PC3 cells (Fig. 5). Importantly, the combination of Ad-Δ6PKR with either etoposide or cisplatin resulted in a synergistic enhancement of apoptosis in both A549 (55% with etoposide and 50% with cisplatin) and PC3 (57% with etoposide and 48% with cisplatin) cancer cells (Fig. 5) (p < 0.05, significantly different from untreated or Ad-luc treated). In contrast, Ad-luc did not have any significant effects on cell viability, either alone or in combination with chemotherapy.
Figure 5.
Adenoviral mutant PKR (Ad-Δ6PKR) enhanced etoposide (ET)- or cisplatin (CDDP)-mediated cell death in cancer cells. Flow cytometric analysis of apoptosis in Ad-Δ6PKR-transfected A549 and PC3 cancer cells, with or without etoposide or cisplatin treatment after 72 hrs. Experiments were performed in triplicate; data are presented as the means (error bars, SDs) *p < 0.05 by Wilcoxon rank-sum test compared with corresponding control (ET, CDDP, Ad-Δ6PKR, Ad-Luc + ET or Ad-Luc + CDDP).
Discussion
PKR is a stress-activated kinase induced in response to multiple different stimuli including viral infections, interferons, interleukins, TNFα, lipopolysaccharide and ionizing radiation.1–6,22 In the present study, we tested whether knockdown of PKR by PKR siRNA or inhibition of PKR by adenoviral mutant PKR (Ad-Δ6PKR) alters cell viability or chemotherapy-mediated apoptosis in human cancer cells. We demonstrated that PKR protein expression is high in various cancer cell lines and low in normal cells (Fig. 1A). We have investigated the pattern of PKR expression in two cancer cell lines (A549 and PC-3) and two normal cell lines (NHBE and HMECs) by confocal immunofluorescence analysis. We observed both the cytosol (most) and nuclei (less) localization of PKR in A549 and PC-3 cancer cells. We observed a diffuse expression pattern of PKR in NHBE and HMECs normal cells. Once activated, PKR translocates to the nucleus. Unlike the known cytoplasmic function of PKR, the role of PKR in the nucleus has yet to be defined. Dr. Barber's laboratory has demonstrated that both NFAR-1 and -2 (nuclear factors associated with dsRNA) are substrates for PKR.22 Dr. Barber's results indicate that the NFARs may facilitate double-stranded RNA-regulated gene expression at the level of post-transcription and possibly contribute to host defense-related mechanisms in the cell.22 Knockdown of PKR by PKR siRNA induced cell death in these cancer cells under normal growth conditions, underscoring the dependence of these cells on PKR signaling even in the absence of exogenous stress stimuli (Fig. 1C). Inactivation of PKR by dominant negative adenoviral mutant PKR (Ad-Δ6PKR) selectively and effectively induces cell death may partly due to reduction of phospho-AKT (Ser473) protein (Fig. 2A and B).
We have also determined the long-term effect of PKR siRNA or Ad-Δ6PKR treatment on cell growth using a clonogenic assay. Both PKR siRNA and Ad-Δ6PKR treatments caused significant reduction in clonogenic potential of A549 and PC3 cells (data not shown). Despite similar phenotypes, however, we postulate that inactivation of PKR signaling using siRNA may not be functionally equivalent to inactivation using dominant negative Ad-Δ6PKR. Downregulation of PKR protein by siRNA would be predicted to inhibit both apoptotic and survival pathways equally. In this case, cell fate (survival versus death) would reflect the balance of pro-apoptotic versus anti-apoptotic pathways and depend on which pathway was dominant in a particular cancer cell (cell-type dependent). In contrast, inactivation of PKR by dominant negative PKR may result predominantly in cell death because Ad-Δ6PKR preferentially induces ER-stress mediated apoptosis in cancer cells (cell-type independent). Future studies aim to elucidate the precise mechanism(s) of PKR siRNA and Δ6PKR mediated apoptosis in cancer cells versus normal cells.
PKR associates with ribosomes, mainly to the 40S subunits.1–3 In a recent study by Onuki et al., PKR was shown to be involved in ER stress-mediated apoptosis induced by tunicamycin treatment of SK-N-SH human neuroblastoma cells.31 In that study, tunicamycin treatment increased expression levels of phosphoylated PKR in the nucleus. Another recent study showed a role for PKR in endoplasmic reticulum (ER) stress-induced neuronal cell death in cases of Alzheimer's disease and Huntington's disease.32 In our study, we demonstrated that activation of ER stress occurs as a consequence of deregulating PKR signaling with mutant PKR (Δ6PKR). This was evidenced by increased expression of phospho-JNK and phosphor-Jun, and increased cleavage of caspase-4. Taken together, these results suggest significant “crosstalk” between the PKR and ER-stress signaling pathways.
The ER stress-mediated cell death pathway involves recruitment of the cytosolic adaptor TRAF2 to the ER membrane, where TRAF2 activates the apoptosis-signaling kinase 1 (ASK1). Activation of ASK1 leads in turn to activation of JNK and mitochondria-dependent caspases.33 In this study, we demonstrated that activation of JNK and caspase-4 is essential for Ad-Δ6PKR-mediated cell death. We hypothesize two possible mechanisms for this. First, inhibition of PKR by Ad-Δ6PKR reduces expression of survival factors such as phospho-AKT. Secondly, inactivation of PKR by Ad-Δ6PKR disrupts normal PKR dimerization such that inactive PKR dimers or monomers translocate to the ER and induce stress. PKR exists in the cell in at least three forms: an inactive monomer associated with ribosomes, an inactive dimer in the cytoplasm, and an active dimer. PKR can also switch from an inactive dimer to an active dimer by a mechanism involving dsRBM interactions.1–6
We previously demonstrated that PKR plays a critical role in radio-resistance.25 In that study, we found that PKR-deficient (PKR−/−) mouse-embryonic fibroblasts (MEFs) were more susceptible to radiation-induced cell death than PKR-proficient (PKR+/+) MEFs.25 This resistance was mediated in part through PKR-dependent upregulation of AKT1/2.25 In the present study, we provide evidence that PKR also contributes to chemotherapy resistance. First, in agreement with Bergeron et al., PKR−/−MEFs were more sensitive than PKR+/+ MEFs to apoptosis due to bulky adduct DNA damage caused by cisplatin and melphalan.30 The increased susceptibility of PKR−/−MEFs to cytotoxic chemotherapy was not due increased proliferation (and hence potentially increased exposure to the drug), since PKR−/−MEFs actually grow ~50% more slowly than PKR+/+MEFs (data not shown). Secondly, inhibition of PKR signaling using a dominant-negative mutant sensitized cancer cells to etoposide and cisplatin-mediated cell death. As with MEFs, the increased chemosensitivity of Ad-Δ6PKR infected cells was not due increased proliferation since Ad-Δ6PKR caused growth inhibition (data not shown). Our working hypothesis is that PKR regulates an AKT and NFκB-dependent survival pathway in response to chemotherapy treatment. Since this mechanism mediates PKR-dependent radioresistance, future studies will address if AKT1/2 and NFκB play a similar role in promoting chemoresistance.25
Our data add to a growing body of evidence that support a role for PKR in promoting tumor cell survival. It has been shown that PKR activation contributes to neoplastic progression and decreased sensitivity to conventional chemotherapy agents in melanoma and colon cancer cells, presumably through upregulation of pro-survival pathways such as NFκB.34 Nussbaum et al., have proposed that high PKR activity in breast cancer cells was responsible for activation of protooncogenes such as the platelet-derived growth factor (PDGF) and c-fos that stimulate cell proliferation.35 We have previously demonstrated that PKR can indirectly regulate AKT1/2 and NFκB-dependent survival pathways.23,25 This regulation is likely critical for the tumor promoting properties of PKR, since AKT is known to promote cellular survival and NFκB blocks apoptosis induced by chemotherapeutic agents.36–38 It is possible; therefore, that PKR promotes cancer cell growth through activation of survival factors such as AKT1/2, NFκB or activation of protooncogenes such as c-fos in human cancers. We are now examining whether the Ad-Δ6PKR inhibits activation of NFκB or c-fos.
Lastly, our results suggest a therapeutic strategy to target PKR signaling in the treatment of human cancer. As proof of principal for this concept, injection of melanoma tumor cells transfected with a PKR-specific short hairpin RNA (shRNA) expressing plasmid resulted in inhibition of pulmonary metastatic nodules in mice.39 In primary human thyroid carcinomas, Terada et al., reported significant correlations between high PKR expression, vascular invasion, and the presence of satellite tumor nodules.40 These data suggest that PKR may play a role in cancer cell metastases. Further investigation is required to determine the effect of PKR inhibition on the development of metastases.
Materials and Methods
Cell lines and reagents
Human lung (A549 and H460), prostate (PC3 and LNcaP), breast (MCF-7 and T47D), colon (SW620, HT-29 and KM12L4) cancer cell lines and normal cell lines (normal human bronchial epithelial cells [NHBEs] and normal human mammary epithelial cell [HMECs]) were obtained from the American Type Culture Collection (Manassas, VA). PKR+/+ and PKR−/− MEFs were obtained from Dr. Glen Barber (University of Miami School of Medicine, Miami, FL.7–9 Caspase-4 and JNK inhibitors were obtained from Calbiochem (San Diego, CA). Final working solutions were diluted in medium to contain <0.01% of dimethyl sulfoxide. All experiments using these compounds were performed under subdued lighting conditions.
Adenovirus production and plasmid constructs
We have developed an adenoviral vector expressing a mutant form (Δ6PKR) of PKR. The Δ6PKR protein has a deletion of six amino acids (AAs 361–366, LFIQME) between catalytic domains IV and V, cannot autophosphorylate or activate substrates, and acts as a dominant negative inhibitor of PKR function.8 Construction of the adenoviral luciferase (Ad-luc) and Ad-Δ6PKR vectors has been reported previously.23,25 The transduction efficiencies of adenoviral vectors in cancer cell lines were determined by infecting cells with Ad-LacZ and then quantifying the titers needed to transduce at least 70% of the cells.
Flow cytometric analysis
We measured apoptotic cells by propidium iodide staining and fluorescence-activated cell sorting (FACS) analysis.12 Cells were harvested; pelleted by centrifugation; resuspended in phosphate-buffered saline (PBS) containing 50 μg/mL propidium iodide, 0.1% Triton X-100, and 0.1% sodium citrate; and subjected to vortex mixing prior to FACS analysis (FL-3 channel, FACScan; Becton-Dickinson, Mountain View, CA).
Immunoblot analysis
At 72 hrs after transfection, the cell extracts were prepared and immunoblot assays performed as previously described.12 Antibodies to JNK, phosphorylated JNK (phospho-JNK), cJun, phosphorylated cJun (phospho-cJun), caspase 4, PKR (K-17), phosphorylated PKR (Thr451), AKT1/2, phosphorylated AKT (Ser473), and β-actin (control) were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Small interfering RNA transfections
PKR and luc siRNA were obtained from Santa Cruz Biotechnology. Transfections were performed according to the manufacturer's instructions. Briefly, 1 × 104 cells were seeded onto 12-well plates with antibiotic-free medium overnight. Solution A was prepared by adding 3.6 μL of the desired 10 μM siRNA to 60 μL of the siRNA transfection medium, and solution B, by adding 3.6 μL of the transfection reagent to 14.5 μL of the siRNA transfection medium. Both solutions were mixed gently and kept at room temperature for 5 min, after which they were combined to form an siRNA-siRNA transfection reagent complex. This complex was added to cells for 5 hrs. Luciferase siRNA was used as a control.
Immunofluorescent cellular localization studies
A549 lung cancer cells and PC3 prostate cancer cells were grown on chamber slides to 70% confluence (5 × 104 cells/well) and then infected with Ad-luc or Ad-Δ6PKR or treated with PBS as a negative control. Seventy-two hours later, cells were washed with PBS and fixed with 4% paraformaldehyde/PBS for confocal microscopic analysis as previously described.12 In brief, cells were blocked with 1% normal goat serum for 1 hr and then incubated overnight at a dilution of 1:100 with the primary PKR antibody. Next, the slides were washed to remove primary antibody, rinsed with PBS, and placed in a prewarmed staining solution containing ER-Tracker red dyes (Molecular Probes) for 15–20 min at 37°C. Then the slides were washed and incubated with a fluorescein isothiocyanate-conjugated secondary antibody (Invitrogen, Eugene, OR) for 1 hr. Next, the slides were mounted with ProLong Gold antifade reagent containing 4',6-diamidino-2-phenylindole (DAPI; Invitrogen) and analyzed with an Olympus FluoView FV500 laser confocal microscope (Olympus America, Melville, NY) after adjustment for background staining.
Statistical analysis
Data reported in the figures are the means and standard deviations (SD) of three independent experiments. Differences from untreated controls were considered statistically significant in all experiments at p < 0.05.
Acknowledgements
We thank Bingbing Wang for her technical assistance and Debbie Smith for her assistance in preparing the manuscript.
This work was supported by grants from the National Cancer Institute, National Institutes of Health: P01 CA78778-01A1 (J.A.R., S.G.S.), SPORE 2P50-CA70970-04, SBIR S/C 1R43 CA86587-1 (S.G.S.), and The University of Texas M.D. Anderson Cancer Center Support Core Grant (CA 16672); by gifts to the Division of Surgery from Tenneco and Exxon for the Core Laboratory Facility; by the W.M. Keck Foundation; by a sponsored research agreement with Introgen Therapeutics, Inc., (SR93-004-1); and by support from the Homer Flower Gene Therapy Fund, the Charles Rogers Gene Therapy Fund, and the George P. Sweeney Esophageal Research Fund.
References
- 1.Barber GN. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–26. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 2.Williams BRG. Signal integration via PKR. Sci STKE. 2001;89:2. doi: 10.1126/stke.2001.89.re2. [DOI] [PubMed] [Google Scholar]
- 3.Katze MG. The war against the interferon-induced dsRNA-activated protein kinase: can viruses win? J Interferon Res. 1992;12:241–8. doi: 10.1089/jir.1992.12.241. [DOI] [PubMed] [Google Scholar]
- 4.Jagus R, Joshi B, Barber GN. PKR, apoptosis and cancer. Int J Biochem Cell Biol. 1999;31:123–38. doi: 10.1016/s1357-2725(98)00136-8. [DOI] [PubMed] [Google Scholar]
- 5.Gil J, Esteban M. Induction of apoptosis by the dsRNA-dependent protein kinase (PKR): mechanism of action. Apoptosis. 2000;5:107–14. doi: 10.1023/a:1009664109241. [DOI] [PubMed] [Google Scholar]
- 6.Gil J, Alcamí J, Esteban M. Induction of apoptosis by double-stranded-RNA-dependent protein kinase (PKR) involves the alpha subunit of eukaryotic translation initiation factor 2 and NFkappaB. Mol Cell Biol. 1999;9:4653–63. doi: 10.1128/mcb.19.7.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Der SD, Yang YL, Weissmann C, Williams BR. A double-stranded RNA-activated protein kinase-dependent pathway mediating stress-induced apoptosis. Proc Natl Acad Sci USA. 1997;94:3279–83. doi: 10.1073/pnas.94.7.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balachandran S, Kim CN, Yeh WC, Mak TW, Bhalla K, Barber GN. Activation of the dsRNA-dependent protein kinase, PKR, induces apoptosis through FADD-mediated death signaling. EMBO J. 1998;17:6888–902. doi: 10.1093/emboj/17.23.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pataer A, Vorburger SA, Barber GN, Chada S, Mhashilkar AM, Zou-Yang H, Stewart AL, Balachandran S, Roth JA, Hunt KK, Swisher SG. Adenoviral transfer of the melanoma differentiation-associated gene 7 (mda7) induces apoptosis of lung cancer cells via upregulation of the double-stranded RNA-dependent protein kinase (PKR) Cancer Res. 2002;62:2239–43. [PubMed] [Google Scholar]
- 10.Vorburger SA, Pataer A, Yoshida K, Barber GN, Xia W, Chiao P, Ellis LM, Hung MC, Swisher SG, Hunt KK. Role for the double-stranded RNA activated protein kinase PKR in E2F-1-induced apoptosis. Oncogene. 2002;21:6278–88. doi: 10.1038/sj.onc.1205761. [DOI] [PubMed] [Google Scholar]
- 11.Holzen UV, Bocangel D, Pataer A, Vorburger SA, Liu Y, Lu X, Hunt KK, Swisher SG. Role for the double-stranded RNA-activated protein kinase PKR in Ad-TNFalpha gene therapy in esophageal cancer. Surgery. 2005;138:261–8. doi: 10.1016/j.surg.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Pataer A, Vorburger SA, Chada S, Balachandran S, Barber GN, Roth JA, Hunt KK, Swisher SG. Melanoma differentiation-associated gene-7 protein physically associates with the double-stranded RNA-activated protein kinase PKR. Mol Ther. 2005;11:717–23. doi: 10.1016/j.ymthe.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Haines GK, 3rd, Becker S, Ghadge G, Kies M, Pelzer H, Radosevich JA. Expression of the double-stranded RNA-dependent protein kinase (p68) in squamous cell carcinoma of the head and neck region. Arch Otolaryngol Head Neck Surg. 1993;119:1142–7. doi: 10.1001/archotol.1993.01880220098012. [DOI] [PubMed] [Google Scholar]
- 14.Haines GK, 3rd, Panos RJ, Bak PM, Brown T, Zielinski M, Leyland J, Radosevich JA. Interferon-responsive protein kinase (p68) and proliferating cell nuclear antigen are inversely distributed in head and neck squamous cell carcinoma. Tumour Biol. 1998;19:52–9. doi: 10.1159/000029974. [DOI] [PubMed] [Google Scholar]
- 15.Terada T, Maeta H, Endo K, Ohta T. Protein expression of double-stranded RNA activated protein kinase in thyroid carcinomas: correlations with histologic types, pathologic parameters and Ki-67 labeling. Hum Pathol. 2000;31:817–21. doi: 10.1053/hupa.2000.8443. [DOI] [PubMed] [Google Scholar]
- 16.Yang YL, Reis YF, Pavlovic J, Aguzzi A, Schäfer R, Kumar A, Williams BR, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abraham N, Stojdl DF, Duncan PI, Méthot N, Ishii T, Dubé M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem. 1999;274:5953–62. doi: 10.1074/jbc.274.9.5953. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Gunnery S, Choe JK, Mathews MB. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene. 2002;21:8741–8. doi: 10.1038/sj.onc.1205987. [DOI] [PubMed] [Google Scholar]
- 19.Roh MS, Kwak JY, Kim SJ, Lee HW, Kwon HC, Hwang TH, Choi PJ, Hong YS. Expression of double-stranded RNA-activated protein kinase in small-size peripheral adeno-carcinoma of the lung. Pathol Int. 2005;55:688–93. doi: 10.1111/j.1440-1827.2005.01892.x. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Shillinglaw W, Henzel WJ, Beg AA. The Rela(p65) subunit of NFkappaB is essential for inhibiting double-stranded RNA-induced cytotoxicity. J Biol Chem. 2001;276:1185–94. doi: 10.1074/jbc.M006647200. [DOI] [PubMed] [Google Scholar]
- 21.Donze O, Deng J, Curran J, Sladek R, Picard D, Sonenberg N. The protein kinase PKR: a molecular clock that sequentially activates survival and death programs. EMBO J. 2004;23:564–71. doi: 10.1038/sj.emboj.7600078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barber GN. The dsRNA-dependent protein kinase, PKR and cell death. Cell Death Differ. 2005;12:563–70. doi: 10.1038/sj.cdd.4401643. [DOI] [PubMed] [Google Scholar]
- 23.Takada Y, Ichikawa H, Pataer A, Swisher SG, Aggarwal BB. Genetic deletion of PKR abrogates TNF-induced activation of IκBα kinase, JNK, Akt and cell proliferation but potentiates p44/p42 MAPK and p38 MAPK activation. Oncogene. 2006;21:1–12. doi: 10.1038/sj.onc.1209906. [DOI] [PubMed] [Google Scholar]
- 24.Pataer A, Bocangel D, Chada S, Roth JA, Hunt KK, Swisher SG. Enhancement of adenoviral mda-7 mediated cell killing in human lung cancer cells by geldanamycin and its 17-allylamino-17-demethoxy analogue. Cancer Gene Ther. 2006;10:1–7. doi: 10.1038/sj.cgt.7700989. [DOI] [PubMed] [Google Scholar]
- 25.von Holzen U, Pataer A, Raju U, Bocangel D, Vorburger SA, Liu Y, Lu X, Roth JA, Aggarwal BB, Barber GN, Keyomarsi K, Hunt KK, Swisher SG. The double-stranded RNA-activated protein kinase mediates radiation resistance in mouse embryo fibroblasts through nuclear factor kappaB and Akt activation. Clin Cancer Res. 2007;13:6032–9. doi: 10.1158/1078-0432.CCR-06-2932. [DOI] [PubMed] [Google Scholar]
- 26.Moenner M, Pluquet O, Bouchecareilh M, Chevet E. Integrated endoplasmic reticulum stress responses in cancer. Cancer Res. 2007;67:10631–4. doi: 10.1158/0008-5472.CAN-07-1705. [DOI] [PubMed] [Google Scholar]
- 27.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;12:2277–93. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 28.Shimazawa M, Ito Y, Inokuchi Y, Hara H. Involvement of double-stranded RNA-dependent protein kinase in ER stress-induced retinal neuron damage. Invest Ophthalmol Vis Sci. 2007;48:3729–36. doi: 10.1167/iovs.06-1122. [DOI] [PubMed] [Google Scholar]
- 29.Kim R, Emi M, Tanabe K, Murakami S. Role of the unfolded protein response in cell death. Apoptosis. 2006;11:5–13. doi: 10.1007/s10495-005-3088-0. [DOI] [PubMed] [Google Scholar]
- 30.Bergeron J, Benlimame N, Zeng-Rong N, Xiao D, Scrivens PJ, Koromilas AE, Alaoui-Jamali MA. Identification of the interferon-inducible double-stranded RNA-dependent protein kinase as a regulator of cellular response to bulky adducts. Cancer Res. 2000;60:6800–4. [PubMed] [Google Scholar]
- 31.Onuki R, Bando Y, Suyama E, Katayama T, Kawasaki H, Baba T, Tohyama M, Taira K. An RNA-dependent protein kinase is involved in tunicamycin-induced apoptosis and Alzheimer's disease. EMBO J. 2004;23:959–68. doi: 10.1038/sj.emboj.7600049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bando Y, Onuki R, Katayama T, Manabe T, Kudo T, Taira K, Tohyama M. Double-strand RNA dependent protein kinase (PKR) is involved in the extrastriatal degeneration in Parkinson's disease and Huntington's disease. Neurochem Int. 2005;46:11–8. doi: 10.1016/j.neuint.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ. 2006;13:374–84. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 34.Kim SH, Gunnery S, Choe JK, Mathews MB. Neoplastic progression in melanoma and colon cancer is associated with increased expression and activity of the interferon-inducible protein kinase, PKR. Oncogene. 2002;21:8741–8. doi: 10.1038/sj.onc.1205987. [DOI] [PubMed] [Google Scholar]
- 35.Nussbaum JM, Major M, Gunnery S. Transcriptional upregulation of interferon-induced protein kinase, PKR, in breast cancer. Cancer Lett. 2003;196:207–16. doi: 10.1016/s0304-3835(03)00276-3. [DOI] [PubMed] [Google Scholar]
- 36.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 37.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 38.Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934–49. doi: 10.1038/sj.onc.1207515. [DOI] [PubMed] [Google Scholar]
- 39.Delgado André N, De Lucca FL. Knockdown of PKR expression by RNAi reduces pulmonary metastatic potential of B16-F10 melanoma cells in mice: Possible role of NFkappaB. Cancer Lett. 2007;258:118–25. doi: 10.1016/j.canlet.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 40.Terada T, Maeta H, Endo K, Ohta T. Protein expression of double-stranded RNA-activated protein kinase in thyroid carcinomas: correlations with histologic types, pathologic parameters and Ki-67 labeling. Hum Pathol. 2000;31:817–21. doi: 10.1053/hupa.2000.8443. [DOI] [PubMed] [Google Scholar]