Abstract
Mouse oocytes acquire the ability to replicate DNA during meiotic maturation, presumably to ensure that DNA replication does not occur precociously between MI and MII and only after fertilization. Acquisition of DNA replication competence requires protein synthesis, but the identity of the proteins required for DNA replication is poorly described. In Xenopus, the only component missing for DNA replication competence is CDC6, which is synthesized from a dormant maternal mRNA recruited during oocyte maturation, and a similar situation also occurs during mouse oocyte maturation. We report that ORC6L is another component required for acquisition of DNA replication competence that is absent in mouse oocytes. The dormant maternal Orc6l mRNA is recruited during maturation via a CPE present in its 3'UTR. RNAi-mediated ablation of maternal Orc6l mRNA prevents the maturation-associated increase in ORC6L protein and inhibits DNA replication in 1-cell embryos. These results suggest that mammalian oocytes have more complex mechanisms to establish DNA replication competence when compared to their Xenopus counterparts.
Keywords: DNA replication, dormant maternal mRNA, cytoplasmic polyadenylation element, mouse oocyte, mouse 1-cell embryo
Introduction
Somatic cells have developed sophisticated mechanisms to regulate assembly of pre-replication complexes (preRC), license the ability of an origin of replication (ORI) to initiate DNA synthesis, and prevent re-replication of DNA (DePamphilis, 2003; DePamphilis, 2005; DePamphilis et al., 2006). During the M to G1 transition, preRCs are assembled, which entails the assembly of an ORI that is composed of ORC1–6 at many sites in the genome. CDC6 and CDT1 are next recruited to these ORC:chromatin sites and in turn recruit an MCM complex composed of MCM2–7, a DNA helicase. Recruitment of MCM is termed replication licensing because the origin recognition complex (ORC) is now capable of supporting DNA replication. Licensing occurs in an environment of low cyclin-dependent protein kinase (CDK) activity. DNA synthesis is initiated by the further addition of MCM10 and the action of protein kinases, including CDKs, namely, CDK2 associated with either cyclin A or E. Once DNA replication has initiated following recruitment of CDC45 that in turn recruits DNA polymerase-α and DNA primase, re-replication of DNA is prevented by CDKs inhibiting the function of ORCs, CDC6, CDT1 and MCM, thus linking cell cycle progression with DNA replication.
In contrast to somatic cells, oocytes are arrested in the first meiotic prophase and have lost the ability to replicate DNA, ensuring that they maintain a 4C chromosome content. The ability to initiate DNA replication is acquired during oocyte maturation around MI and requires protein synthesis, but DNA replication does not occur until following fertilization or egg activation (Furuno et al., 1994). High levels of CDK1/CDC2A activity are required to maintain metaphase II arrest that in turn inhibits DNA replication (Furuno et al., 1994). The maturation-associated recruitment of Mos mRNA results in synthesis of MOS that is essential to maintain elevated levels of CDK1/CDC2A activity that in turn is required to maintain metaphase arrest (Colledge et al., 1994; Hashimoto et al., 1994). Inhibiting synthesis of MOS during maturation of Xenopus oocytes results in maturing oocytes entering interphase and replicating DNA soon after MI (Furuno et al., 1994). Entry into interphase is presumably because CDK1/CDC2A activity decreases, thereby relieving ORIs from their inhibited state as well as permitting formation of a nuclear membrane that is essential for DNA replication.
Xenopus oocytes contain all of the proteins required to assemble and license an ORI except for CDC6. Recruitment of Cdc6 mRNA during maturation leads to the synthesis of CDC6 protein and restoration of the ability of the cytoplasm to support DNA replication (Lemaitre et al., 2002; Whitmire et al., 2002), i.e., synthesis of CDC6 can solely account for the protein synthesis requirement for acquisition of DNA replication competence during oocyte maturation. For example, blocking the maturation-associated increase in CDC6 protein by injecting anti-sense RNA directed at Cdc6 mRNA in oocytes prior to maturation blocks DNA replication. DNA replication competence is restored, however, when CDC6 protein is also injected (Lemaitre et al., 2002).
Mouse oocytes also lack CDC6 protein and a maturation-associated recruitment of Cdc6 mRNA results in CDC6 protein accumulation by MII (Anger et al., 2005; Lemaitre et al., 2004). A role for newly synthesized CDC6 in DNA replication following fertilization or egg activation could not be established because RNAi-mediated ablation of Cdc6 mRNA inhibited oocyte maturation; oocytes underwent germinal vesicle breakdown but a spindle did not form and although chromosomes condensed, they did not form visible bivalents (Anger et al., 2005). Thus, whether recruitment of Cdc6 mRNA is solely responsible for the maturation-associated acquisition of replication competence, as it is in Xenopus oocytes, remains an open question.
We had previously conducted microarray studies on mouse oocytes and 1-cell embryos and found that the relative abundance of several transcripts is increased in 1-cell embryos relative to oocytes, presumably due to polyadenylation because there is no transcription during this time and poly dT was used to prime the reverse transcription reaction (Zeng et al., 2004; Zeng and Schultz, 2005). We noted that the relative abundance of Orc6l mRNA was increased, making it another candidate whose recruitment would contribute to the maturation-associated acquisition of replication competence. We report here that ORC6L protein is undetectable in oocytes but present in metaphase II-arrested eggs due to a cytoplasmic polyadenylation element (CPE)-mediated recruitment of Orc6l mRNA. RNAi-mediated ablation of Orc6l mRNA prevents accumulation of ORC6L protein in metaphase II eggs and inhibits DNA replication following egg activation.
Materials and methods
Isolation and culture of oocytes and embryos
Full-grown, germinal vesicle intact oocytes (GV) were obtained from pregnant mare serum gonadotropin (PMSG)-primed, 6 week-old, CF-1 female mice (Harlan, Indianapolis, IN) and freed of attached cumulus cells as previously described (Schultz et al., 1983). Germinal vesicle breakdown (GVBD) was inhibited by adding 2.5 μM milrinone to the isolation and culture media (Tsafriri et al., 1996). The collection medium was bicarbonate-free minimal essential medium (Earle's salts) supplemented with 3 mg/ml of polyvinylpyrrolidone (PVP) and 25 mM Hepes (pH 7.3) (MEM-PVP). After collection, oocytes were cultured in CZB medium (Chatot et al., 1989) containing milrinone.
For isolation of metaphase II eggs (MII eggs) and 1-cell embryos, CF-1 female mice were superovulated with the injection of 5 IU of PMSG, followed 48 hours later by 5 IU of human chorionic gonadotropin (hCG). MII eggs were collected 13–16 h post-hCG administration.
For generation of 1-cell embryos, after hCG injection the females were mated with B6D2F1/J male mice (Jackson Lab, Bar Harbor, ME) and embryos were collected 20–24 hs after hCG. The cumulus cells were removed by a brief hyaluronidase treatment (3 mg/ml). One-cell embryos were cultured in 10-μl drops of KSOM supplemented with amino acids (KSOM+AA) under mineral oil (Ho et al., 1995). To generate parthenogenetic embryos, MII eggs were activated with 10 mM SrCl2 in Ca2+- and Mg2+- free CZB for 2.5 h and further cultured in KSOM+AA, except in the experiment described in Fig. 2B in which the eggs were cultured in the presence of SrCl2 for 6 h. When necessary, cycloheximide (20 μg/ml) or MG132 (10 μM) was added to the culture medium to inhibit protein synthesis or proteasome-mediated protein degradation, respectively. All oocytes and embryos were cultured at 37 °C in a humidified atmosphere of 5% CO2 in air.
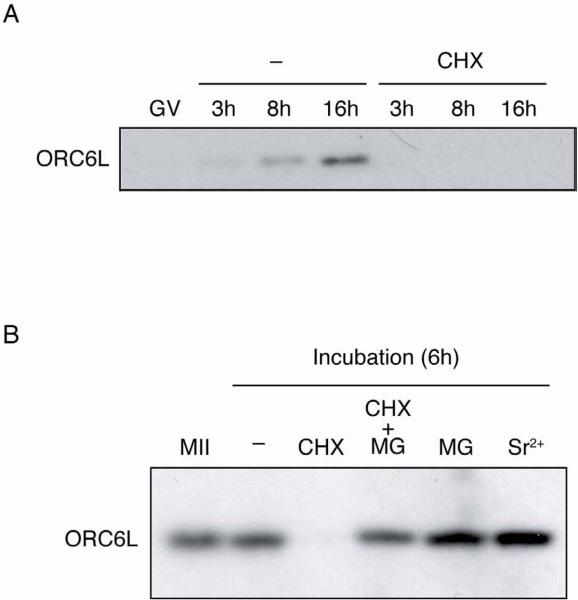
Figure 2.
Protein synthesis is required for accumulation of ORC6L. (A) ORC6L protein levels were determined during oocyte maturation. Full-grown oocytes (GV) were collected and in vitro matured in the absence (−) or presence of 10 μg/ml cycloheximide (CHX). At the indicated times following removal of milrinone samples were collected for immunoblotting. The immunoblots were performed as described in Fig. 3, using 10 oocytes per lane. The experiment was conducted three times and shown is a representative example. (B) Stability of ORC6L protein in unfertilized eggs. Metaphase II eggs (MII) were collected and incubated in CZB with (CHX) or without (−) cycloheximide, cycoheximide and MG132 (CHX+MG) or MG132 alone (MG). Another group of eggs was parthenogenetically activated with 10 mM SrCl2 and incubated for 6 h (Sr2+). ORC6L protein levels were determined by immunoblotting as described above. The experiment was performed two times and similar results were obtained in each case. A similar experiment without adding MG132 was conducted three times and similar results to those shown here were obtained with respect to the extensive degradation of ORC6L in the presence of cycloheximide. In these experiments, however, the apparent increase in the amount of ORC6L in activated eggs when compared to unactivated eggs was not always observed.
Immunoblotting
Oocytes, eggs or embryos were directly lysed in SDS-PAGE sample buffer (100 mM Tris-HCl, pH 6.8, 200 mM dithiothreitol, 4% SDS, 0.2% bromophenol blue, 20% glycerol), and stored at −20°C until use. The samples were boiled for 5 min prior to being subjected to SDS- PAGE in a 12.5% gel. The proteins were then transferred to Immobilon-P (Millipore, Bedford, MA), and the membranes were blocked with 2–5% non-fatty milk in PBST (PBS with 0.2% Tween-20) for 1 h at room temperature, or overnight at 4°C. After blocking, the membranes were washed twice in PBST for 10 min each and then incubated with an anti-ORC6L antibody (1:100 dilution in PBST; Cell Signaling Technology, Danvers, MA) overnight at 4°C. Following incubation with the primary antibody, the membranes were washed 3 times in PBST for 15 min each and then incubated with horseradish peroxidase-conjugated secondary antibody (1:200,000 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 h at room temperature. After washing in PBST for 3 times (15 min each), the membranes were developed using ECL Advance Western Blotting System (GE Healthcare, Piscataway, NY). As a loading control, membranes were stripped and reprobed with a mouse monoclonal anti β-tubulin antibody (cat # T4026, Sigma, St. Louis, MO) at a 1:10,000 dilution.
Immunofluorescence
Oocytes or eggs were fixed in 2% paraformaldehyde for 20 min at room temperature. The cells were permeabilized for 15 min in PBS containing 0.1% Triton X-100, and blocked in PBS containing 0.1% BSA and 0.01% Tween-20 (blocking solution); they were then incubated with the primary antibody for 1 h at room temperature (1:100 dilution in blocking solution). After three washes in blocking solution, the cells were incubated in the appropriate secondary antibody for 1 h (Alexa Fluor 488-conjugated anti-rat IgG was used [Invitrogen, Carlsbad, CA]). DNA was stained with 1.5 mg/ml propidium iodide (Invitrogen). The cells were then washed and mounted under a coverslip with gentle compression in VectaShield antibleaching solution (Vector Laboratories, Burlingame, CA). Fluorescence was detected on a Leica TCS SP laser-scanning confocal microscope.
Preparation of double-stranded RNA (dsRNA)
Total RNA was isolated from 30 GV oocytes using the Picopure RNA isolation kit (Arcturus, Sunnyvale, CA) according to the manufacturer's protocol. A reverse transcription (RT) reaction, primed with oligo dT, was performed using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. PCR was performed using this oocyte cDNA to generate the templates for in vitro transcription. Two dsRNAs were used to target Orc6l mRNA. dsOrc6l corresponds to nucleotides 297 to 1085 (the entire coding region) and dsUTR, to nucleotides 1086 to 1653 (the entire 3'UTR) of mouse Orc6l mRNA (accession number NM_019716.2). For amplification of the coding region of Orc6l, a pair of primers was designed based on the cDNA sequence. The sequence of the upstream primer was 5'-GCGATGGAGTCGGAGCTGG-3' and the downstream primer was 5'-TGCTGTGGCTGTCTGAG-3'. These primers generated a PCR product that was 789 bp in length and corresponded to the entire coding region of Orc6l. For amplification of the 3'UTR of Orc6l, the following primers were used: upstream primer: 5'-TTTCCATCTCACTGCAGGCATG-3', downstream primer: 5'-AATGTTTAAAAAATATTTATTAACTTAGGC-3'. These primers generated a PCR product that was 568 bp in length and corresponded to the entire 3'UTR of Orc6l. Both PCR products were cloned into pGEM-T Easy vector (Promega, Madison, WI), the plasmids were digested and linearized with FspI and served as template for in vitro transcription. Sense and antisense RNAs were transcribed in vitro using SP6 and T7 MEGAscript kits (Ambion, Austin, TX) and mixed. After heating at 75 °C for 5 min, the RNA was cooled and annealed slowly at room temperature. The sample was treated with DNase I and RNase A, and then purified twice by phenol/chloroform extraction and ethanol precipitation. Gfp dsRNA was prepared in the same way and used as a control dsRNA for injections. The purified dsRNAs (1mg/ml) were stored at −20 °C.
DNA constructs, in vitro transcription, and site-directed mutagenesis
Mouse Orc6l 3'UTR was prepared and cloned into pGEM-T Easy vector as described above. Mouse ccb1 3'UTR was amplified by RT-PCR from mouse GV oocyte cDNA using an upstream primer with a MfeI site (5'-CAATTGCTCCAATAGACTGCTACATCTGCA-3') and a downstream primer with a MfeI site (5'-CAATTGAAAGCTTTCCACCAATAAATTTTAT-3'), which were designed based on the cDNA sequence (accession number NM_172301). The PCR product was cloned into pGEM- T Easy vector. pGEM-Orc6l 3'UTR was digested with EcoRI and pGEM-ccnb1 3'UTR was digested with MfeI, and each DNA fragment was cloned into a unique site in pIVT replacing β-globin 3'-UTR. Firefly luciferase was amplified from pGL3 (Promega) and cloned into the expression vector containing the 3'UTR of Orc6l or Ccnb1. Renilla luciferase was amplified from pGL4.70 [hRluc] (Promega) and cloned into pIVT vector (Igarashi et al., 2007). The site directed mutagenesis of CPEs or hexanucleotides in the 3'UTR of Orc6l was performed by a procedure based on long PCR (Imai et al., 1991). Complementary RNAs (cRNAs) of all constructs were synthesized from linearized plasmid DNAs using T7 RNA polymerase and the mMESSAGE mMACHINE kit (Ambion) according to the manufacturer's instructions; the resulting cRNA contains a 33 A residues followed by 12 C residues at the 3' end. The cRNA was cleared and purified by phenol/chloroform extraction and isopropanol precipitation. The final cRNA concentration was determined by spectrophotometry, and cRNA integrity was confirmed by analyzing a sample on a formaldehyde gel.
Microinjection
Injections were done in 10-μl drops of modified Whitten's medium containing 15 mM HEPES, pH 7.2, 7 mM Na2HCO3, 10 μg/ml gentamicin and 0.01% PVA containing 2.5 μM milrinone. Approximately 10 pl of dsRNA or cRNA was injected into the cytoplasm of GV oocytes using a PLI-100 Pico-Injector (Harvard Apparatus, Holliston, MA) on the stage of a Nikon TE2000 microscope equipped with Hoffman optics and Narishige micromanipulators.
BrdU incorporation assay
To detect DNA replication, embryos with a pronucleus were labeled at 7 h after Sr2+ activation with 1 mM BrdU in KSOM+AA for 30 min. The embryos were fixed in 2.5% paraformaldehyde containing 0.5N NaOH for 15 min at room temperature, blocked and permeabilized in PBS containing 10% FBS and 0.1% Triton X-100 for 30 min at 37°C, and then washed with PBS containing 2% FBS and 0.1% Triton X-100. BrdU incorporation to chromatin was detected with mouse monoclonal antibody to bromodeoxyuridine (1:20 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) followed by Alexa 546-conjugated goat anti-mouse IgG (1:100 dilution; cat. # A-11003, Invitrogen). DNA was detected with 0.02% SITOX Green (Invitrogen) for 15 min at room temperature. Embryos were mounted in Vectashield (Vector Laboratories) and observed with a laser-scanning confocal microscope.
Luciferase assay
The cRNAs of luciferase with Orc6l or Ccnb1 3'UTR (10 pg/oocyte) and Renilla luciferase (1.5 pg/oocyte) were co-injected into GV-intact oocytes as described above and incubated in vitro for 16 h with or without maturation. Ten injected oocytes were collected and incubated with Passive Lysis Buffer (1 μl/oocyte) for 15 min at room temperature with shaking. The samples were stored at −80 °C until luciferase activity was assayed using the the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instructions except that 2 μl of sample and 20 μl of Luciferase Assay Reagent II and of Stop & Glo Reagent were used. Signal intensities were measured using a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego, CA). Luciferase activities of constructs with Orc6l 3'UTR were normalized to that of Renilla luciferase.
Results
Maturation-associated increase in ORC6L protein
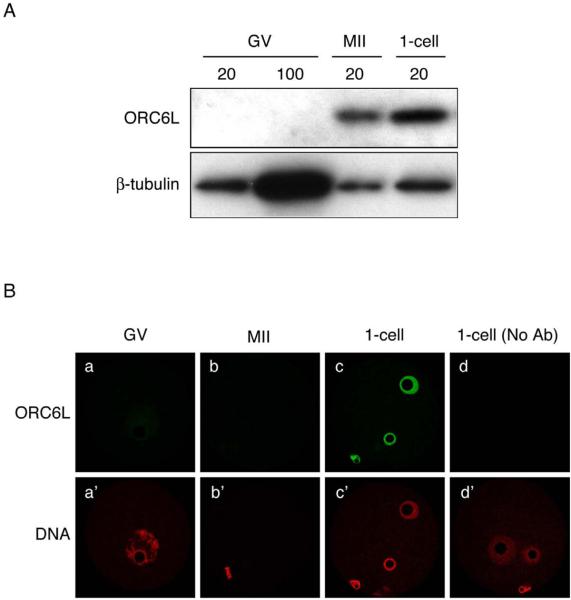
Although oocytes contain Orc6l mRNA (data not shown), they lack ORC6L protein as determined by both immunocytochemical and immunoblotting approaches (Fig. 1); no signal was observed when either 20 or 100 oocytes were used for immunoblotting. Oocyte maturation was associated with a dramatic increase in the amount of ORC6L protein, as determined by immunobloting (Fig. 1A). When normalized to the amount of β-tubulin, the amount of ORC6L protein in 1-cell embryos is similar to that in MII eggs. ORC6L protein is not detected in MII eggs by immunocytochemistry, even on the chromosomes (Fig. 1B). The failure to detect by immunocytochemistry ORC6L protein in MII eggs could reflect the dispersion of the protein in the cytoplasm below threshold detection. Alternatively, if ORC6L as associated with the chromosomes, access of the antibody may be precluded. As anticipated from the immunoblotting results, ORC6L was readily detected in the pronuclei of 1-cell embryos (Fig. 1B).
Figure 1.
Maturation-associated increase in ORC6L protein. (A) Immunoblot analysis of ORC6L in full-grown oocytes (GV), metaphase II eggs (MII), and one-cell embryos (1-cell). The indicated numbers of cells were loaded per lane. The blot was stripped and reprobed with an anti-β-tubulin antibody as a loading control. The experiment was conducted three times and shown is a representative immunoblot. (B) Immunofluorescence detection of ORC6L in full-grown oocytes (GV), metaphase II eggs (MII), and one-cell embryos (1-cell). As a control, the primary antibody was omitted in panel d. The experiment was performed three times and at least eight oocytes/embryos were analyzed in each experiment. Shown are representative examples.
ORC6L protein starts to accumulate shortly after GVBD, progressively accumulates during the course of maturation and requires protein synthesis because addition of cycloheximide to the culture medium prevents ORC6L accumulation (Fig. 2A). Protein synthesis is also required to maintain the newly synthesized ORC6L protein in MII-arrested eggs (Fig. 2B). Virtually no ORC6L was detected in MII eggs cultured for 6 h in medium containing cycloheximide, whereas the amount of ORC6L in control eggs was very similar to that present in MII eggs before culture. Consistent with a relatively short half-life of newly synthesized ORC6L recruited during meiotic maturation was that the proteasome inhibitor MG132 (Tsubuki et al., 1993) prevented the decrease in ORC6L when protein synthesis was inhibited and that the amount of ORC6L protein increased in eggs incubated in the presence of MG132 alone (Fig. 2B). Although the amount of ORC6L protein appeared to increase following egg activation, in other experiments this increase was not observed (see legend to Figure 2B).
Orc6l mRNA is a dormant maternal mRNA recruited during oocyte maturation
The absence of ORC6L protein in oocytes, coupled with the maturation-associated increase is a hallmark of dormant maternal mRNAs, e.g., Mos mRNA. Such maternally recruited mRNAs contain one or more CPEs in their 3'UTR that are typically situated within a few hundred nucleotides of the hexanucleotide polyadenylation element (HEX) (Oh et al., 2000; Pique et al., 2008; Richter, 1991). Examination of the Orc6l mRNA sequence reveals that two potential CPEs (UUUUAUU) reside 26 nucleotides and 53 nucleotides, respectively, upstream from the AAUAA polyadenylation element.
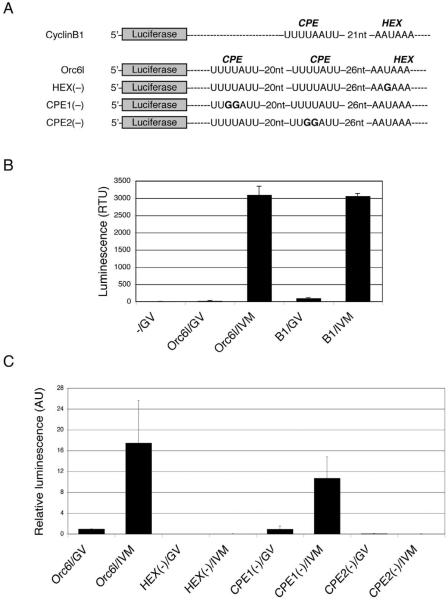
To determine whether either or both of these potential CPEs were responsible for the maturation-associated increase in ORC6L protein, luciferase (Luc) mRNA reporter constructs that contained these CPEs in the 3'UTR were injected in oocytes (Fig. 3A). A cyclin B1 (Ccnb1) reporter that contains one CPE was injected as a positive control. The oocytes were then matured in vitro. Control oocytes were cultured for the same amount of time in the presence of milrinone, which prevents maturation (Tsafriri et al., 1996). Maturation was clearly associated with an increase in luciferase activity following injection of the Luc reporter construct containing both Orc6l CPEs. As anticipated, there was also a dramatic increase in luciferase activity following maturation of oocytes injected with the reporter containing Ccnb1 CPEs (Fig. 3B).
Figure 3.
Functional analysis of the 3'UTR of Orc6l mRNA. (A) Schematic depiction of the firefly luciferase reporters used for microinjection. These cRNAs contain firefly luciferase open reading frame, β-globin 5'UTR, and the 3'UTR of mouse cyclin B1 (Ccnb1) or Orc6l, including the hexanucleotide polyadenylation element (HEX) and the cytoplasmic polyadenylation elements (CPEs). HEX(−): Orc6l cRNA with a point mutation in the HEX; CPE1(−): Orc6l cRNA in which the CPE distant from the HEX has been mutated; CPE2(−):Orc6l cRNA in which the CPE proximal to the HEX has been mutated. Bold letters indicate the mutated nucleotides in these constructs. (B) Full-grown mouse oocytes were microinjected with cRNAs containing firefly luciferase open reading frame and the 3'UTR of either cyclin B1 (B1) or Orc6l. Microinjected oocytes were cultured for 16 h in conditions that prevent meiotic maturation (GV) or they were allowed to undergo in vitro maturation (IVM) prior to assaying for luciferase activity. −/GV: uninjected oocytes. The data are expressed as the mean ± SEM of three experiments. (C) Oocytes were injected with each of the cRNAs depicted in (A), either in vitro matured (IVM) or kept arrested at the GV stage (GV) and luciferase activities were assayed as described in Materials and methods. Firefly luciferase reporter activities were normalized to the co-injected Renilla luciferase control and are shown relative to the activity in oocytes injected with Orc6l mRNA, which was set to 1. The experiment was conducted three times and the data are presented as the mean ± SEM.
To establish that the putative CPEs were indeed functional and if so, what was the relative contribution of each CPE, Luc reporter constructs containing mutations (UUUUAUU —> UUGGAUU) in each of the putative CPEs were injected into oocytes (Fig. 3C). Results of these experiments strongly implicate CPE2, the CPE closer to the polyadenylation element as the CPE that is largely responsible for recruitment of Orc6l mRNA. In all cases, a functional HEX was required for the maturation-associated increase in luciferase activity (Fig. 3C).
Recruitment of maternal Orc6l mRNA during maturation is essential for DNA replication in activated eggs
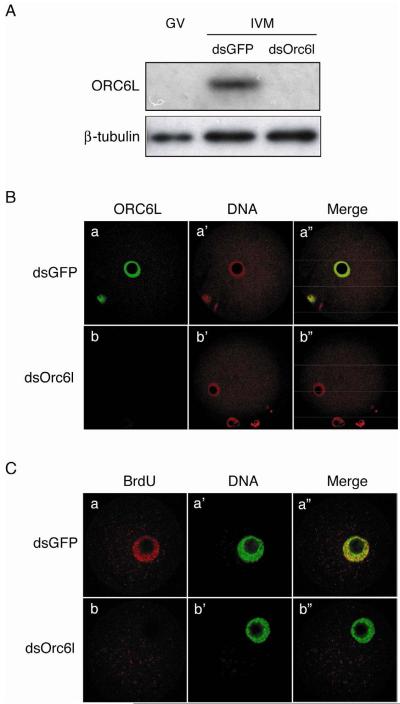
RNAi-mediated ablation of the dormant maternal Orc6l mRNA was used to establish whether the maturation-associated synthesis of ORC6L was required for DNA replication following egg activation. Accordingly, oocytes were injected with dsRNA targeting endogenous Orc6l mRNA and then matured in vitro; control oocytes were injected with Gfp dsRNA. As expected, maturation of oocytes injected with Orc6l dsRNA did not display an increase in ORC6L protein, whereas such an increase was observed in Gfp dsRNA-injected cells (Fig. 4A). Furthermore, following Sr2+ activation of the matured eggs, ORC6L was readily detected in the pronucleus of the Gfp dsRNA-, but not Orc6l dsRNA-, injected eggs (Fig. 4B). Thus, RNAi-mediated targeting of Orc6l mRNA effectively inhibited the maturation-associated increase in ORC6L protein. Furthermore, Sr2+-activated eggs in which accumulation of ORC6L protein was inhibited by RNAi displayed a virtual absence of BrdU incorporation in contrast to their Gfp dsRNA-injected counterparts (Fig. 4C), a result consistent with inhibiting DNA replication in ORC6L-depleted eggs
Figure 4.
Orc6l knockdown abolishes DNA replication in parthenogenetically activated eggs. (A) Full-grown mouse oocytes were microinjected with Orc6l dsRNA (dsOrc6l) or GFP dsRNA (dsGFP) and in vitro matured (IVM) for 16 h. Twenty oocytes per treatment were processed and analysed by immunoblotting. The blot was stripped and reprobed with an anti-β-tubulin antibody for loading control. GV: uninjected full-grown oocytes. The experiment was conducted three times and a representative immunoblot is shown. (B) Oocytes microinjected and in vitro-matured as described in (A) were parthenogenetically activated with SrCl2. After activation, they were further cultured and then processed for immunocytochemical detection of ORC6L (a, b); DNA was stained with propidium iodide (a', b'). The merge images are also shown (a”, b”). The experiment was conducted three times using at least 5 oocytes per group and representative images are shown. (C) dsRNA-injected oocytes were in vitro-matured and parthenogenetically activated as described above. Seven h after Sr2+ activation BrdU incorporation into chromatin was assayed (a, b). DNA was stained with SYTOX Green (a', b'). The merge images are also shown (a”, b”). The experiment was conducted three times using at least 10 oocytes per group and representative images are shown.
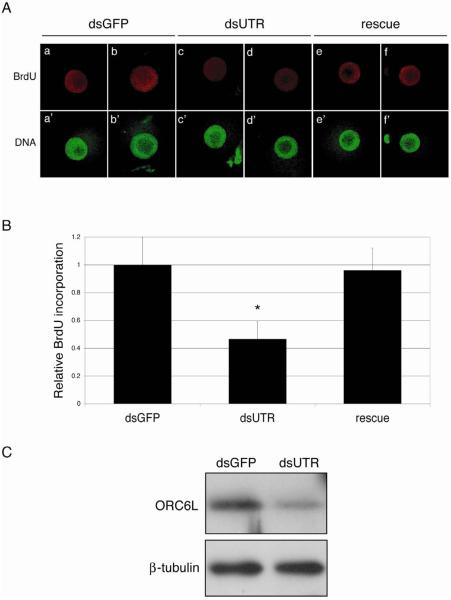
It was formally possible that the observed inhibition of DNA synthesis in ORC6L-depleted activated eggs was due to off-targeting effects in response to the Orc6l dsRNA. Results of a rescue experiment strongly suggest that this was an unlikely scenario. Oocytes were co-injected with a dsRNA directed to the 3'UTR of Orc6l (to inhibit the maturation-associated increase in ORC6L protein) and an Orc6l-encoding cRNA. This cRNA cannot be targeted by the siRNAs generated from the dsRNA directed to the 3'UTR of Orc6l and thereby enhances the likelihood of observing a rescue. Controls were injected with Gfp dsRNA. Following maturation and Sr2+-activation BrdU incorporation was determined. As anticipated, activated eggs in which the maturation-associated increase in ORC6L was inhibited displayed reduced BrdU incorporation when compared to controls and incorporation was fully restored following injection of Orc6l cRNA (Fig. 5A and B).
Figure 5.
Orc6l mRNA can rescue the DNA replication failure of ORC6L knockdown oocytes. (A) Full-grown mouse oocytes were microinjected with a dsRNA against the 3'UTR of Orc6l (dsUTR) or GFP dsRNA (dsGFP). A third group of oocytes was co-injected with dsUTR and a cRNA that contains the coding sequence of Orc6l and the 5' and 3'UTR of β-globin (rescue). All microinjected oocytes were in vitro matured and parthenogenetically activated with 10 mM SrCl2. Seven h after Sr2+ activation BrdU incorporation into chromatin was assayed; DNA was stained with SYTOX Green. The experiment was conducted three times in which at least 10 cells with a PN per group were analyzed; representative images are shown. (B) BrdU incorporation in the three groups of microinjected oocytes described in (A) was quantified using ImageJ software (http://rsb.info.nih.gov/ij). Fluorescence intensity in each nucleus was measured and the background value (an area of the cytoplasm of equal size) was subtracted. The data were analyzed using Prism (GraphPad Software, La Jolla, CA). Comparisons among groups were done using one-way ANOVA, followed by Bonferroni post-test. *, P<0.05. The results are expressed as the mean ± SEM and normalized to the mean fluorescence intensity of GFP dsRNA-injected oocytes. (C) Full-grown mouse oocytes were microinjected with dsUTR or dsGFP and in vitro matured for 16 h. Twenty oocytes per treatment were processed and analysed by immunoblotting. The blot was stripped and reprobed with an anti-β-tubulin antibody for loading control. The experiment was conducted three times and a representative immunoblot is shown.
We noted that in contrast to the virtual total abolition of BrdU incorporation when a dsRNA directed towards the coding region of Orc6l was used (see Fig. 4), detectable BrdU incorporation was observed when a dsRNA directed towards the 3'UTR was used. The likely basis for this observation was that the dsRNA directed towards the 3'UTR of Orc6l was less effective in inhibiting the maturation-associated increase in ORC6L protein (Fig. 5C). This difference presumably reflects differences in accessibility of the siRNAs for their cognate binding sequences.
Discussion
We report here that mouse oocytes lack detectable amounts of ORC6L, which is synthesized during oocyte maturation due to recruitment of Orc6l mRNA and that the maturation-associated synthesis of ORC6L protein is required to establish DNA replication competence in the 1-cell embryo. Thus, mouse oocytes lack at least two components required for preRC formation and ORI licensing, i.e., ORC6L and CDC6. Preliminary immunoblotting and immunocytochemical studies indicate that oocytes contain all of the other proteins of the ORI complex, i.e., ORC1, 2, 3L, 4L, and 5L, as well as all six members of the MCM complex, i.e., MCM2–7 (unpublished observations) and previous studies documented the presence of MCM2, 6, and 7 (Lemaitre et al., 2004; Swiech et al., 2007). In addition, proteins involved in recruiting MCM and initiating DNA replication (CDT1, CDC7, CDC45, MCM10) and regulating licensing, e.g., GMN (Lei and Tye, 2001), are also present (unpublished observations).
The observation that at least two components required for DNA replication are not present in mouse oocytes but synthesized during oocyte maturation contrasts to Xenopus in which CDC6 appears to be the only preRC and licensing component missing in the fully grown oocyte (Lemaitre et al., 2002; Whitmire et al., 2002). In Xenopus, recruitment of Cdc6 mRNA during maturation provides the missing ORI protein and high levels of CDK1/CDC2A activity in the metaphase II egg inhibit licensing of the ORI. That at least two components—CDC6 and ORC6L—required to license DNA replication are missing in mouse oocytes may reflect an evolutionary selective pressure to minimize precocious DNA replication either during maturation or following arrest at metaphase II in species that ovulate only a few eggs; premature DNA replication would clearly compromise reproductive fitness. This constraint may not be as severe in species such as Xenopus that ovulate thousands of eggs.
The presence of the ORC on chromosomes as a function of the cell cycle depends on the species (DePamphilis, 2003). In yeast, ORC remain associated with chromatin throughout the course of the cell cycle. In contrast, in metazoans, ORCs display cell cycle changes in chromosome association, namely, disengaging during S phase and dissociating during entry into M phase. Our not detecting ORC6L on metaphase II chromosomes is consistent with ORCs dissociating from chromosomes during entry into metaphase. The best evidence that ORCs do not associate with metaphase chromosomes in higher eukaryotes comes from experiments that examined Orc2 localization during early Drosophila embryogenesis (Baldinger and Gossen, 2009). In that study a transgenic line was developed that expressed an Orc2-GFP fusion protein to the same level as endogenous Orc2 and furthermore integrated into an ORC as efficiently as endogenous Orc2. Thus, GFP localization accurately reflects ORC localization. ORC was highly enriched in the nucleus during interphase, absent from metaphase chromosomes, and only appeared on chromosomes following entry into anaphase in response to a decrease in CDK activity. The likely absence of ORCs on chromosomes of eggs arrested at metaphase II ensures that precocious DNA replication cannot occur.
Orc6l mRNA is a newly identified dormant maternal mRNA in mouse oocytes. Recruitment of maternal mRNAs is governed by the presence of a CPE in the 3'UTR and Orc6l mRNA contains two CPEs identified by their sequence. Results from our functional studies indicate that the CPE closest to the polyadenylation element confers the ability of Orc6l mRNA to be recruited. In contrast to Mos mRNA whose recruitment is also coupled with its degradation (Medvedev et al., 2008), our microarray data suggest that recruitment of Orc6l mRNA is not coupled with its degradation. ORC6L protein appears fairly unstable, based on its rapid decrease in abundance when protein synthesis is inhibited in MII eggs, and this instability may be the driving force to uncouple recruitment of Orc6l mRNA with its degradation. Fertilization does not appear to stimulate ORC6L synthesis, which is consistent with Orc6l mRNA lacking any polyuridine tract that is associated with mRNA recruitment following fertilization, e.g., dodecauridine (Simon and Richter, 1994; Simon et al., 1992).
Other components of the ORC and licensing machinery have putative CPEs, e,g, Mcm2, 4, and 7, Cdc45, and Cdt1, suggesting that they too could be recruited during maturation. Interestingly, in contrast to ORC6L (and CDC6), these proteins are already readily detectable in the oocyte based on immunocytochemical and immunoblotting analyses. A maturation-associated increase in these components could be important for DNA replication in a fashion similar to the IP3 receptor protein, which is present in oocytes and displays a maturation-associated increase that confers increased sensitivity to IP3-mediated calcium release (Xu et al., 2003). Whether these mRNAs that encode other components of the ORC and licensing apparatus are recruited during maturation and whether such recruitment is necessary for DNA replication following fertilization is not known. Nevertheless, what emerges is a general theme that several components required to initiate DNA replication in 1-cell embryos are up-regulated during oocyte maturation in preparation for fertilization.
In S. cerevisiae ORC6, although not required for DNA binding, is essential for DNA replication, presumably because of failure to recruit CDT1 and hence facilitate loading of the MCM2–7 helicase (Chen et al., 2007). Results in other species and cell types suggest that ORC6 is also essential for DNA replication. Although early evidence pointed to a defect in cytokinesis rather than DNA replication in Orc6 mutants (Chesnokov et al., 2003; Prasanth et al., 2002), more recent findings pinpoint a role of the N-terminal domain in DNA replication, whereas the C-terminal domain appears critical to traverse M phase (Balasov et al., 2009). In contrast, results of experiments using Xenopus egg extracts led to the conclusion that ORC6 is not required for initiation of DNA replication (Gillespie et al., 2001). In Xenopus, ORC6 is loosely associated with ORC1–5 because ORC6 is absent from purified ORC, which is composed of ORC1–5. The purified complex can stimulate DNA synthesis and hence replication licensing in a reconstituted system using activated Xenopus egg extracts and sperm nuclei. The presence of ORC6 in the egg extract, however, could account for the ability of ORC1–5 to stimulate DNA replication; the concentration of ORC components in Xenopus eggs is extraordinarily high (DePamphilis, 2005). Our results strongly suggest that ORC6L is required to initiate DNA replication in 1-cell mouse embryos because preventing the maturation-associated synthesis of ORC6L results in 1-cell embryos that cannot support DNA replication. Whether this is a unique feature of mammalian zygotes or truly reflects a more universal requirement for ORC6L in initiation of DNA replication remains an open question.
Acknowledgements
This research was supported by NIH grants HD22732 and HD22681 to R.M.S. M.G.B. was supported by a grant from the Fogarty International Center Fellowship Program (5D43TW000671). S.M. was supported in part by a Research Project of Toho University School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anger M, Stein P, Schultz RM. CDC6 requirement for spindle formation during maturation of mouse oocytes. Biol Reprod. 2005;72:188–194. doi: 10.1095/biolreprod.104.035451. [DOI] [PubMed] [Google Scholar]
- Balasov M, Huijbregts RP, Chesnokov I. Functional analysis of an Orc6 mutant in Drosophila. Proc Natl Acad Sci U S A. 2009;106:10672–10677. doi: 10.1073/pnas.0902670106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldinger T, Gossen M. Binding of Drosophila ORC proteins to anaphase chromosomes requires cessation of mitotic cyclin-dependent kinase activity. Mol Cell Biol. 2009;29:140–149. doi: 10.1128/MCB.00981-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. Journal of Reproduction and Fertility. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Chen S, de Vries MA, Bell SP. Orc6 is required for dynamic recruitment of Cdt1 during repeated Mcm2–7 loading. Genes Dev. 2007;21:2897–2907. doi: 10.1101/gad.1596807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnokov IN, Chesnokova ON, Botchan M. A cytokinetic function of Drosophila ORC6 protein resides in a domain distinct from its replication activity. Proc Natl Acad Sci U S A. 2003;100:9150–9155. doi: 10.1073/pnas.1633580100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge WH, Carlton MBL, Udy GB, Evans MJ. Disruption of c-mos causes parthenogenetic development of unfertilized mouse eggs. Nature. 1994;370:65–68. doi: 10.1038/370065a0. [DOI] [PubMed] [Google Scholar]
- DePamphilis ML. The `ORC cycle': a novel pathway for regulating eukaryotic DNA replication. Gene. 2003;310:1–15. doi: 10.1016/s0378-1119(03)00546-8. [DOI] [PubMed] [Google Scholar]
- DePamphilis ML. Cell cycle dependent regulation of the origin recognition complex. Cell Cycle. 2005;4:70–79. doi: 10.4161/cc.4.1.1333. [DOI] [PubMed] [Google Scholar]
- DePamphilis ML, Blow JJ, Ghosh S, Saha T, Noguchi K, Vassilev A. Regulating the licensing of DNA replication origins in metazoa. Curr Opin Cell Biol. 2006;18:231–239. doi: 10.1016/j.ceb.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Furuno N, Nishizawa M, Okazaki K, Tanaka H, Iwashita J, Nakajo N, Ogawa Y, Sagata N. Suppression of DNA replication via Mos fuction during meiotic divisions in Xenopus oocytes. EMBO J. 1994;13:2399–2410. doi: 10.1002/j.1460-2075.1994.tb06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PJ, Li A, Blow JJ. Reconstitution of licensed replication origins on Xenopus sperm nuclei using purified proteins. BMC Biochem. 2001;2:15. doi: 10.1186/1471-2091-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N, Watanabe N, Furuta Y, Tamemoto H, Sagata N, Yokoyama M, Okazaki K, Nagayoshi M, Takeda N, Ikawa Y, et al. Parthenogenetic activation of oocytes in c-mos-deficient mice. Nature. 1994;370:68–71. doi: 10.1038/370068a0. [DOI] [PubMed] [Google Scholar]
- Ho Y, Wigglesworth K, Eppig JE, Schultz RM. Preimplantation development of mouse embryos in KSOM: Augmentation by amino acids and analysis of gene expression. Mol. Reprod. Dev. 1995;41:232–238. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- Igarashi H, Knott JG, Schultz RM, Williams CJ. Alterations of PLCbeta1 in mouse eggs change calcium oscillatory behavior following fertilization. Dev Biol. 2007;312:321–330. doi: 10.1016/j.ydbio.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai Y, Matsushima Y, Sugimura T, Terada M. A simple and rapid method for generating a deletion by PCR. Nucleic Acids Res. 1991;19:2785. doi: 10.1093/nar/19.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Tye BK. Initiating DNA synthesis: from recruiting to activating the MCM complex. J Cell Sci. 2001;114:1447–1454. doi: 10.1242/jcs.114.8.1447. [DOI] [PubMed] [Google Scholar]
- Lemaitre JM, Bocquet S, Mechali M. Competence to replicate in the unfertilized egg is conferred by Cdc6 during meiotic maturation. Nature. 2002;419:718–722. doi: 10.1038/nature01046. [DOI] [PubMed] [Google Scholar]
- Lemaitre JM, Bocquet S, Terret ME, Namdar M, Ait-Ahmed O, Kearsey S, Verlhac MH, Mechali M. The regulation of competence to replicate in meiosis by Cdc6 is conserved during evolution. Mol Reprod Dev. 2004;69:94–100. doi: 10.1002/mrd.20153. [DOI] [PubMed] [Google Scholar]
- Medvedev S, Yang J, Hecht NB, Schultz RM. CDC2A (CDK1)-mediated phosphorylation of MSY2 triggers maternal mRNA degradation during mouse oocyte maturation. Dev Biol. 2008;321:205–215. doi: 10.1016/j.ydbio.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. doi: 10.1242/dev.127.17.3795. [DOI] [PubMed] [Google Scholar]
- Pique M, Lopez JM, Foissac S, Guigo R, Mendez R. A combinatorial code for CPE-mediated translational control. Cell. 2008;132:434–448. doi: 10.1016/j.cell.2007.12.038. [DOI] [PubMed] [Google Scholar]
- Prasanth SG, Prasanth KV, Stillman B. Orc6 involved in DNA replication, chromosome segregation, and cytokinesis. Science. 2002;297:1026–1031. doi: 10.1126/science.1072802. [DOI] [PubMed] [Google Scholar]
- Richter JD. Translational control during early development. BioEssays. 1991;13:179–183. doi: 10.1002/bies.950130406. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte maturation: Implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev. Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Simon R, Richter JD. Further analysis of cytoplasmic polyadenylation in Xenopus embryos and identification of embryonic cytoplasmic polyadenylation element-binding proteins. Mol Cell Biol. 1994;14:7867–7875. doi: 10.1128/mcb.14.12.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Tassan JP, Richter JD. Translational control by poly(A) elongation during Xenopus development: differential repression and enhancement by a novel cytoplasmic polyadenylation element. Genes Dev. 1992;6:2580–2591. doi: 10.1101/gad.6.12b.2580. [DOI] [PubMed] [Google Scholar]
- Swiech L, Kisiel K, Czolowska R, Zientarski M, Borsuk E. Accumulation and dynamics of proteins of the MCM family during mouse oogenesis and the first embryonic cell cycle. Int J Dev Biol. 2007;51:283–295. doi: 10.1387/ijdb.062239ls. [DOI] [PubMed] [Google Scholar]
- Tsafriri A, Chun S-Y, Zhang R, Hsueh AJW, Conti M. Oocyte maturation involves compartmentalization and opposing changes in cAMP levels in follicular somatic and germ cells: Studies using selective phosphodiesterase inhibitors. Dev.Biol. 1996;178:393–402. doi: 10.1006/dbio.1996.0226. [DOI] [PubMed] [Google Scholar]
- Tsubuki S, Kawasaki H, Saito Y, Miyashita N, Inomata M, Kawashima S. Purification and characterization of a Z-Leu-Leu-Leu-MCA degrading protease expected to regulate neurite formation: a novel catalytic activity in proteasome. Biochem Biophys Res Commun. 1993;196:1195–1201. doi: 10.1006/bbrc.1993.2378. [DOI] [PubMed] [Google Scholar]
- Whitmire E, Khan B, Coue M. Cdc6 synthesis regulates replication competence in Xenopus oocytes. Nature. 2002;419:722–725. doi: 10.1038/nature01032. [DOI] [PubMed] [Google Scholar]
- Xu Z, Williams CJ, Kopf GS, Schultz RM. Maturation-associated increase in IP3 receptor type 1: role in conferring increased IP3 sensitivity and Ca2+ oscillatory behavior in mouse eggs. Dev Biol. 2003;254:163–171. doi: 10.1016/s0012-1606(02)00049-0. [DOI] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev. Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zeng F, Schultz RM. RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol. 2005;283:40–57. doi: 10.1016/j.ydbio.2005.03.038. [DOI] [PubMed] [Google Scholar]