Abstract
We discuss a current controversy regarding the relative role of phosphorylation sites on cardiac troponin I (cTnI) (Figure 1) in physiological and patho-physiological cardiac function. Studies with mouse models and in vitro studies indicate that multi-site phosphorylations are involved in both control of maximum tension and sarcomeric responsiveness to Ca2+. Thus one hypothesis is that cardiac function reflects a balance of cTnI phosphorylations and a tilt in this balance may be maladaptive in acquired and genetic disorders of the heart. Studies on human heart samples taken mainly at end stage heart failure, and in depth proteomic analysis of human and rat heart samples demonstrate that Ser23/Ser24 are the major and perhaps the only sites likely to be relevant to control cardiac function. Thus functional significance of Ser23/Ser24 phosphorylation is taken as fact, whereas the function of some other sites is treated as fancy. Maybe the extremes will meet: in any case we both agree that further work needs to be carried out with relatively large mammals and with determination of the time course of changes in phosphorylation to identify transient modifications that may be relevant at a beat-to-beat basis. Moreover, we agree that the changes and effects of cTnI phosphorylation need to be fully integrated into the effects of other phosphorylations in the cardiac myocyte.
1. Solaro Point: Despite controversy regarding the functional significance and presence of some sites of phosphorylation on cardiac troponin I in the heart, we should not abandon thorough molecular, cellular and physiological investigation of these sites
1.1 Despite the long history of multi-site cTnI phosphorylation, the functional significance remains unclear
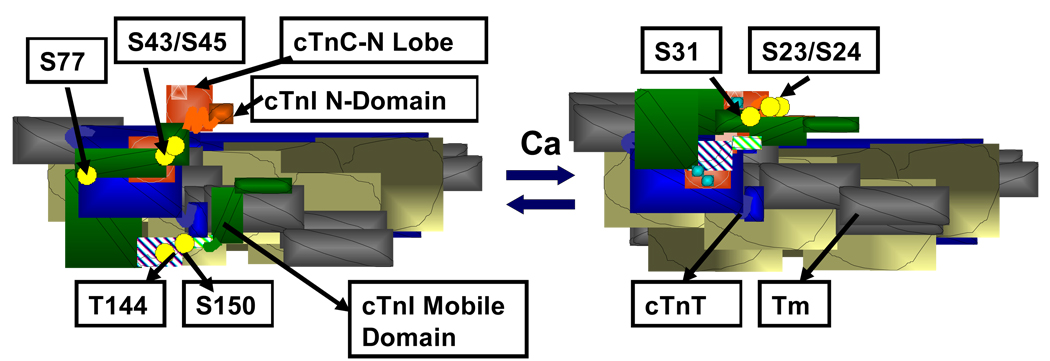
Reports by Stull et al. [1] and Pratje and Heilmeyer [2] in 1972 were the first to demonstrate a sarcomeric protein (cardiac troponin I; cTnI) as a substrate for a protein kinase (PKA). In the 37 years since these findings, although it took much effort to convince some skeptics, we have come a long way in the appreciation that the phosphorylation of troponin is a highly significant element in the control of cardiac contractility and a potential point of vulnerability in cardiac disorders. This link to contractility is most accepted among investigators by functional effects of cTnI phosphorylation at Ser 23 and Ser 24 [3, 4]. Important lines of evidence supporting the functional significance of this post-translational modification include the location of S23, S24 in a unique N-terminal extension of the cTnI variant (Fig. 1) [5, 6], the effect of the phosphorylation to increase the kinetics of Ca2+ exchange with cardiac troponin C [7], and the demonstration of altered contractility in transgenic models with gain or loss of function modifications in the effects of cTnI phosphorylation at these sites [8]. These data support the generally well-accepted hypothesis that phosphorylation of cTnI plays a significant role in the enhanced relaxation rate and abbreviation of the contraction-relaxation cycle time, which is critical to maintain homeostasis in hearts beating at the relatively fast rates associated with adrenergic stimulation. As illustrated in Figure 1, there are a number of other phosphorylation sites on cTnI that have been identified, but the functional significance of these other phosphorylation sites on cTnI remains unclear and controversial.
Figure 1.
Portion of a cardiac thin filament regulatory unit indicating the location troponin units and tropomyosin (Tm) in the transition from diastole to systole, and demonstrating the multi-site phosphorylations (yellow balls) of cardiac troponin I (cTnI). Left Panel. Thin filament in diastole showing troponin C (cTnC) as a dumbbell shaped protein with the N-lobe containing a single regulatory Ca-binding site. cTnI (green) is shown tethered to an actin strand by an inhibitory peptide and a second actin binding region. The distal C-terminal end of cTnI drapes across azimuthal actins and may interact with Tm. cTnT is shown in blue. An N-peptide of cTnI interacts with the N-lobe of cTnC, but is released upon phosphorylation.
Sites other than S23/S24 had been first determined by phospho-peptide analysis of rabbit cTnI in the laboratory of S. V. Perry in which cTnI-S150 [9] was identified as a endogenous site and cTnI-S77 was also identified as a substrate for phosphorylase kinase [9]. cTnI-S150 has since been identified as a substrate for p21-activated kinase (Pak). In vitro phosphorylation induces structural changes modifying the interaction of cTnI with cTnC, and an increase in response to Ca2+ [10]. Yet further studies have reported that activation of Pak in cardiac myocytes leads to dephosphorylation via activation of protein phosphatase 2A [11]. Recent evidence has also identified cTnI-Ser 31 as a substrate for Mst1 (mammalian sterile 20-like kinase 1)[12]. This kinase, when activated, promotes apoptotic signaling and a dilated cardiomyopathy. Extensive studies in the laboratory of J. F. Kuo also demonstrated that, in addition to PKA, cTnI could also be phosphorylated in vitro by pan-protein kinase C (PKC) isolated from brain [13–15]. Phospho-peptide mapping and studies with mutant recombinant cTnI identified the major sites at S43, S45 and at T144 of cTnI [14, 16]. As illustrated in Figure 1, these sites are strategically located in the region of interaction among the troponin units as well as in the inhibitory peptide. Further studies employing specific PKC isoforms supported and extended these initial findings. PKC-epsilon phosphorylated S43, S45, and T144, whereas, PKC-delta phosphorylated these sites as well as S23, S24. Other isoforms, such as PKC-betaII, demonstrated specificity for T144. Moreover, PKC-delta, when Tyr phosphorylated at a specific site by Src-dependent tyrosine, switched its preference from S23, S24 to T144 [17]. It is generally agreed that in vitro phosphorylation or pseudo-phosphorylation of S43 and S45, with S45 having the predominant effect [18], induce a depression in responsiveness of myofilaments to activation by Ca2+ both in terms of sub-maximal and maximal force generation and ATPase rate [14, 19]. The effect of in vitro phosphorylation or pseudo-phosphorylation of T144 is less clear. Our study [19] comparing controls with reconstituted thin filament preparations regulated by cTnI-T144E, revealed a depression in filament sliding in the motility assay, but with little effect of the pseudo-phosphorylation on Ca2+ sensitivity or maximum tension of skinned fibers regulated by T144E. On the other hand, studies in the laboratory of Jeffrey Walker of cardiac myocytes made permeable, treated with PKC-betaII, and rapidly skinned revealed an increase in Ca2+ sensitivity associated with phosphorylation of T144 [20]. This finding fits with earlier studies of Walker and colleagues demonstrating a positive inotropic effect in cardiac myocytes infected with PKC-epsilon and PKC-beta and treated with phorbol esters [21, 22]. Investigations of skinned fibers in mouse models expressing a cardiac directed and relatively low expression level of PKC-epsilon also revealed an increase in Ca2+-sensitivity, which occurred at 3 months of age [23]. By 12 months of age there was no change in Ca2+-sensitivity, but a severe dilated cardiomyopathy. Interestingly, this phenotype was rescued in a double transgenic model generated by cross-breeding the PKC-epsilon over-expressing mouse with the mice expressing cTnI-S43A/S45A [24]. Thus, it is apparent that the mechanism of rescue may be the enhanced phosphorylation of cTnI-S23/S24, which occurred in the cTnI-S43A/S45A transgenic model.
1.2 Animal models reveal data indicating a role for phosphorylation sites on cTnI other than Ser23/Ser24
Naturally these exciting findings from in vitro studies spawned investigations into the role of the cTnI “PKC sites” in control of the integrated function of hearts in health and disease. As with the studies on the S23, S24 sites, the approaches include determination of cardiac phenotype in gain and loss of function mouse models with cardiac specific expression of cTnI variants (either pseudo-phosphorylated switching serine or threonine residues with either glutamic or aspartic acid or with alanine). Other approaches include determination of the relative phosphorylation of the cTnI PKC sites, and correlations with function in models of hypertrophy and failure. Although there are some disagreements in the interpretation of results of the studies employing the transgenic mouse models expressing cTnI variants, these studies generally support the hypothesis inferred from the in vitro studies that PKC site phosphorylation is functionally significant. For example studies by Pi et al. [21, 22] employed a mouse model expressing a mutant cTnI with S43/S45/T144/S23/S24 all replaced with Ala. Stimulation of the beta-adrenergic pathway, which enhanced relaxation in controls, was about 50% reduced in the transgenic hearts. On the other hand, endothelin induced slowing of relaxation and tau in controls, and this effect was greatly reduced in the mouse hearts lacking cTnI phosphorylation sites. This led the authors to conclude that depending on the sites phosphorylated post-translational modifications of cTnI could either promote or diminish relaxation kinetics. A complimentary “gain of function” study supported this conclusion. Sakthivel et al. [25] employed a transgenic model expressing either cTnI-PP (S23D/S24D) or cTnI-AllP (S23D/S24D/S43D/S45D/T144D). Whereas the cTnI-PP hearts demonstrated enhanced contraction/relaxation kinetics and lusitropy compared to controls, the cTnI-AllP hearts did not. These results indicated that the PKC sites have a dominant negative effect able to offset the lusitropic effects of cTnI-PP. Our own studies [26] employing a mouse model expressing cTnI-S43A/S45A also revealed inter-dependence among the phosphorylation sites of cTnI. Hearts of these mice beating in situ demonstrated an increase in contractility reaching near maximal levels and similar to that of controls given maximally effective doses of isoproterenol. Analysis of the sites of phosphorylation in cTnI-S43A/S45A hearts revealed a significant increase in phosphorylation of S23/S24, which appeared to account for the increase in contractility.
Despite the disparity in results from in vitro experiments studies with the transgenic models described above support the general concept that the homeostatic control of cardiac function requires an appropriate balance of cTnI phosphorylations. For example it is reasonable to assume, for example, homoeostasis requires appropriate phosphorylation at sites which increase Ca2+-sensitivity (T144) and sites that diminish Ca2+-sensitivity (S43/S45 or S23/S24). In a scheme described in Solaro and de Tombe [27], we emphasize this balance of phosphorylations as a nodal point in homeostatic control of cardiac contractility. We also point out the possibility and state the hypothesis that a disturbance in the balance is a substantial mechanism in the responses to pathological stresses leading to heart failure. Based on considerable evidence that mutations in sarcomeric proteins including cTnI lead to hypertrophic and dilated cardiomyopathies [28], we argued that this disordered balance of phosphorylation may be an early trigger amplifying and promoting hypertrophic signaling.
Although data obtained from rodent models of heart failure support this hypothesis, data from human studies do not. Results of our studies of rat models of heart failure induced either by pressure overload or by myocardial infarction, produced evidence of a correlation of enhanced cTnI phosphorylation with a depression in force generation [29, 30]. We did not determine the exact sites where the increased phosphorylation occurred. One major challenge to this hypothesis has come from studies comparing levels of phosphorylation of samples of control and end stage failed human hearts. These studies have not demonstrated an increase in PKC site phosphorylation at end-stage heart failure. Moreover, in depth proteomic analysis of post-translational modifications in cTnI has not detected phosphorylation of T144, S43 or S45. A discussion [31] of the relative merits of samples obtained from explanted hearts at end stage failure has been previously published. The major issue with regard to the present discussion is that “top down” proteomic analysis of commercially obtained cTnI from a human heart sample, showed phosphorylation only at S22, S23 and at S76/T77 [32]. In the case of top down proteomic analysis of rat hearts flash frozen in the basal states, the data show un-, mono- and bis-phosphorylated forms of 41%, 46%, and 13% with no evidence of phosphorylation at sites other than S23, S24 [33, 34]. Moreover, a general finding has been that cTnI levels of phosphorylation are low and Ca2+ sensitivity is high in skinned myocytes isolated from explanted human hearts compared to controls [35, 36]. These findings have led to the question as to whether sites other than S23, S24 play a role in control mechanisms in the normal human heart and whether phosphorylation of the S43, S45, and T144 sites are of any significance with regard to pathologies in the human heart [36].
1.3 It is premature to suggest that the findings from in vitro studies and from mouse and rat models of cardiac pathologies are irrelevant
In the case of studies with human heart samples, there is the distinct advantage that the measurements are closest to the human cardiac disorders, but, apart from the difficulty of obtaining matched controls, there is the disadvantage that the course of events leading to the end stage is over. Thus, key information is missing on the time course of altered phosphorylation during the progression of human cardiac pathology. Newer approaches are certain to shed light on the time course of changes in cTnI phosphorylation, but this will be difficult in the human for obvious reasons. Importantly there is evidence that treatment of detergent extracted myocytes from end stage failed hearts with PKC-epsilon or PKC-alpha is able to reduce tension and Ca2+-sensitivity and to increase phosphorylation of cTnI, but also myosin binding protein C (cMyBP-C) [37]. There is no evidence I know of that PKC dependent phosphorylation of cMyBP-C is able to reduce tension. Extensive and detailed analysis during the progression of cardiac disease even in animal models has not been generally done. One study published in abstract form was carried out by Sumandea and colleagues [38], who measured cardiac troponin T (cTnT) phosphorylation during the progression to heart failure in well phenotyped SHR rats. Cardiac TnT phosphorylation transiently increased during the progression, but was lower than the controls at end stage heart failure. We need to know whether these sorts of changes are occurring in animal models as well as in humans and whether the phosphorylation changes are adaptive or maladaptive. It could be that S43/S45 and S150 of cTnI are never phosphorylated in situ, but these and other sites, such as S77, which has been known for many years and forgotten, as pointed out by Marston and Walker [36], may change transiently.
Apart from the lack of information on the time course of changes, there is a need to develop fail safe preparative methods to retain labile or in some cases even relatively stable phosphorylation sites so that in situ phosphorylation is preserved. One of the most difficult steps in proteomic analysis of post-translational modification is retention of the in situ phosphorylation sites. It would be great to have an intracellular and vital probe reporting site specific cTnI phosphorylation, but we don’t. It is difficult to test for retention of in situ phosphorylation sites inasmuch as we don’t know exactly what’s going on in situ, and can only guess intelligently based on data obtained in careful analytical studies. One approach would be to add cTnI phosphorylated at specific sites in vitro to heart samples during processing for proteomic analysis. This has not generally been done or reported.
2. Van der Velden Counterpoint: Molecular, cellular and physiological investigation of phosphorylation sites on cardiac troponin I in the heart should be performed in large animal models and human hearts, as studies in transgenic mice models distract our attention from the relevant regulatory protein phosphorylation sites involved in myofilament function
Troponin I is a powerful regulator of myofilament function in the heart. Next to titin and cMyBP-C, it is a target of protein kinase A, the down-stream kinase of the β-adrenergic receptor. Increased stimulation of the β-adrenergic receptors increases cTnI phosphorylation on Ser 23/24, which contributes to the lusitropic effect on the heart muscle required for improved ventricular filling during increased cardiac stress. Apart from PKA, many other kinases are able to phosphorylate cTnI as discussed above by John. Until now most studies investigating the role of cTnI phosphorylation on Ser 23/24 and other sites were performed using transgenic mice models. Undoubtedly these transgenic models provided incredible insight in the role of troponin phosphorylation in regulation of myofilament function, that is, in the rodent heart. The backside of these fashionable models is that the almost overwhelming amount of information has withdrawn attention from the crucial questions, which sites are phosphorylated in human cTnI and how is cTnI phosphorylation regulated in vivo (i.e. which kinases and phosphatases target cTnI)?
2.1 Troponin exchange experiments have proven that modifications of cTnI do not alter the maximal force generating capacity of cardiomyocytes. Thus, there is little evidence for a patho-physiological role of cTnI, being truncated or phosphorylated, in the depression of maximal force
Depressed maximal force generating capacity and altered Ca2+-sensitivity of myofilaments in cardiac disease have been ascribed to changes in troponin phosphorylation. While the direction of the change in myofilament Ca2+-sensitivity in diseased myocardium is a matter of an ongoing debate [31], it is generally agreed that phosphorylation of cTnI on different sites is centrally involved. However, it is less evident that depressed maximal force of the myofilaments in cardiac disease can be ascribed to altered troponin phosphorylation.
A clear example of rodent studies, which could not be translated to large animals or human myocardium involves the functional role of truncated cTnI, in particular in reducing myofilament force development. The specific and selective proteolysis of cTnI has been proposed to play a key role in human myocardial ischemia/reperfusion injury, including stunning [39, 40] and in acute pressure overload [41]. The decrease in maximal force generating capacity of cardiac muscle is among the main features of reversible ischemia/reperfusion injury (stunning) in rodents and has been attributed to degradation of cTnI, primarily at its C-terminus [42–44]. The functional significance of C-terminally truncated cTnI was studied in exchange experiments using whole troponin complex, in which endogenous full-length cTnI was replaced by truncated cTnI. Truncated cTnI decreased maximal force upon exchange in rat trabeculae [45]. However, the impact of truncated cTnI on maximal force seems to depend on the species used within the exchange protocol, i.e. a 50% reduction in maximal force was observed upon exchange of truncated mouse cTnI in rat trabeculae, while this reduction was only 20% with truncated human cTnI in rat trabeculae and absent upon exchange of truncated human cTnI in human cardiomyocytes [46]. It should be noted that one cannot exclude that the discrepant observations may be related to differences in experimental conditions (type of cardiac preparation, temperature). In both rat and human cardiac preparations, incorporation of truncated cTnI in the myofilaments resulted in an increase in Ca2+-sensitivity [45, 46], which will impair diastolic rather than systolic function of the heart. In support for the increased Ca2+-sensitivity, recent interaction studies using electron microscopy revealed that the C-terminus constrains tropomyosin in a position that interferes with myosin binding on actin in the relaxed state [47]. Truncated cTnI appeared to promote the transition of tropomyosin from the Ca2+-induced closed position on actin toward the myosin-induced open state [48]. The release of tropomyosin from its inhibitory position on actin by truncated cTnI in the presence of Ca2+ may well explain the observed increase in myofilament Ca2+-sensitivity upon exchange of truncated cTnI. Whereas, the latter mechanism may be universal, the reduction in maximal force generating capacity may be species-dependent and rudimentary or even lost during human development.
Several rodent and large animal studies of cardiac disease revealed reduced maximal force generating capacity of the myofilaments, which may impair systolic performance. A reduction in maximal myofilament force has been reported in cardiomyocytes isolated from the post-infarct remodeled myocardium in mice, rat and pig models of myocardial infarction [29, 30, 49, 50]. Belin et al. [30] observed increased phosphorylation of cTnI in two distinct rat models. More, recently Belin and colleagues [29] proposed that increased PKC-alpha-mediated phosphorylation of myofilament proteins underlies reduced maximal force in end-stage congestive heart failure. A number of transgenic models and troponin reconstituted heart preparations indicated that phosphorylation of cTnI and cTnT by PKC reduces the maximal force generating capacity of myofilaments [19, 51, 52]. An in vitro motility assay to study the function of thin filaments from human myocardium showed a significant decrease in maximal calcium-activated force in failing compared to non-failing preparations, which could be corrected with phosphatase treatment. The authors suggested that the decrease in maximal force in failing human myocardium was mediated by PKC [53]. However, elegant troponin exchange experiments in rat myocardium showed that the reduction in maximal force in failing myocardium was not rescued upon exchange of the diseased cTn complex by cTn complex isolated from healthy hearts, while the disease-induced change in Ca2+-sensitivity was corrected to normal values upon incorporation of healthy cTn in the sarcomeres [30]. Studies in large animals did not find evidence favoring a role of cTnI in reducing the maximal force generating capacity of myofilaments [50,54]. No marked differences were found in cTnI phosphorylation and PKC alpha in remodeled hearts three weeks after myocardial infarction in pigs despite a significant reduction in maximal force [50]. Moreover, force measurements in human cardiomyocytes incubated with the catalytic domain of PKC or the active catalytic subunit of PKC alpha could not explain the reduction in maximal force observed in cardiac disease [37, 55]. As the latter studies in pig and human are purely associative, future studies should aim to reveal causality between functional sarcomeric properties and troponin composition using troponin exchange experiments as done previously by Belin et al. in rodent myocardium [30].
2.2 Although altered cTnI phosphorylation may underlie changes in myofilament Ca2+-sensitivity in pathological conditions it may as well be an innocent bystander
One of the major topics of discussion during past years has been the direction of the change in myofilament Ca2+-sensitivity in cardiac disease and the role of cTnI phosphorylation therein [31, 35]. An increase in Ca2+-sensitivity of force has been related with reduced PKA-mediated phosphorylation of cTnI in end-stage human heart failure [35, 56, 57], while a decrease has been assigned to increased PKC-mediated phosphorylation of cTnI [29, 30]. The decrease in Ca2+-sensitivity in rat end-stage congestive heart failure was corrected upon exchange of failing troponin complex with troponin isolated from non-failing hearts [30]. Moreover, careful analysis of cTnI phosphorylation in rodent heart failure models showed alterations on PKC sites [30, 58]. These studies convincingly showed that increased PKC-mediated cTnI phosphorylation may reduce Ca2+-sensitivity in cardiac disease. But what about reduced PKA-mediated phosphorylation leading to an increased Ca2+-sensitivity? In a recent study we took extra precautions to preserve phosphorylation status of the myofilament apparatus to address the question if Ca2+-sensitivity is decreased or increased three weeks after myocardial infarction in a large animal model, and if altered PKA-phosphorylation of cTnI is involved [54]. Trans-mural cardiac biopsies were taken from pigs with a myocardial infarction under well-controlled conditions at the same time as hemodynamic assessment of cardiac function. Cardiac biopsies were directly frozen in liquid nitrogen to arrest the actions of kinases and phosphatases and fix the phosphorylation status of myofilament proteins. Similar to our previous finding in human end-stage heart failure, Ca2+-sensitivity was significantly higher in infarct compared to sham animals. However, much to our surprise, our study did not reveal a change in phosphorylation of cTnI on either PKA or PKC sites, but showed reduced myosin light chain 2 phosphorylation through enhanced protein phosphatase 1 expression. In contrast to our observations in end-stage human heart failure, we have consistently observed increased myofilament Ca2+-sensitivity and unaltered total cTnI phosphorylation in heart failure models in mice, rat and pig [35].
The discussion as to whether Ca2+-sensitivity is increased or decreased in cardiac disease and involves altered cTnI phosphorylation may be largely explained by differences in stage of the disease and/or underlying cause. Only recently, Walker et al.[58] showed transient changes in cTnI phosphorylation after myocardial infarction in mice (initial decline on PKA sites, and a subsequent increase on PKC sites Ser 43/45). Most studies in human were performed in end-stage heart failure (NYHA class IV). Indeed, it has to be admitted that phosphorylation on Ser 23/24 in non-failing donor myocardium is extremely high, in comparison to controls from other studies [35]. On the other hand, it was surprising to find a larger functional effect of PKC alpha and PKC epsilon (i.e. reduction in Ca2+-sensitivity) in failing than in non-failing donor cardiomyocytes, as higher baseline phosphorylation of cTnI on PKC sites was expected in failing hearts on the basis of increased PKC expression/activity [37]. Apart from disease stage, changes in Ca2+-sensitivity and cTnI phosphorylation may be very dynamic. An increase in heart rate has been shown to change Ca2+-sensitivity and phosphorylation of cTnI [59, 60]. Small changes in PKC-mediated cTnI phosphorylation may occur on a beat-to-beat basis, and as pointed out by Marston and Walker [36] need to be “trapped” in vivo.
A major step forward in understanding myofilament dysfunction in human heart disease has been the studies in human biopsies from patients with moderate forms of cardiac disease (NYHA class II-III) [61]. Force measurements in single demembranated human cardiomyocytes provided insight in the mediators underlying diastolic myocardial dysfunction. Increased cardiomyocyte stiffness was found in failing human heart preparations, which could be corrected to values found in non-failing hearts with PKA. Evidence [62] suggests that impaired titin phosphorylation most likely underlies the increased stiffness, which contributes to the diastolic dysfunction observed in heart failure patients. A similar approach may be used to understand changes in Ca2+-sensitivity during progression of human cardiac disease. However, serious limitations intrinsic to human studies should be taken into account. Studies in human preparations are complicated by differences in medication, genetic background, life style and co-morbidities, and emphasize the need for longitudinal studies in large animal models.
A change in myofilament Ca2+-sensitivity has been proposed as a disease mechanism underlying development of primary cardiomyopathies. Familial dilated (DCM) and hypertrophic (HCM) cardiomyopathies are frequently caused by mutations in genes encoding sarcomeric proteins. Again, most data originate from experiments with either engineered mouse models or recombinant proteins. Based on in vitro and transgenic animal studies it has been put forward that HCM mutations in sarcomeric proteins cause an increase in Ca2+-sensitivity, while DCM associated mutations have been proposed to reduce Ca2+-sensitivity. However, there have been few reports to date that actually document the intrinsic abnormalities present in human HCM [63] and DCM hearts. Hence, this paradigm (increased vs. decreased Ca2+-sensitivity) needs to be tested properly in the human setting and in my opinion will turn out to be too simplistic, as myofilament function will not only depend on expression of the mutant, but may be modified by the many phosphorylation sites of cTnI.
2.3 Changes in cTnI phosphorylation and Ca2+-sensitivity may be dynamic and disease stage-specific and may depend on other proteins
Although I think it is a fact that cTnI phosphorylation is not involved in altered maximal force, I do fancy the idea of cTnI as determinant of altered Ca2+-sensitivity in cardiac disease. As cTnI is the dominant protein involved in the PKA-mediated reduction in myofilament Ca2+-sensitivity, one might forget about other proteins, which may exert a modulatory role or play even a central role. Exchange experiments in human cardiomyocytes with PKA-phosphorylated cTn complex revealed that changes in Ca2+-sensitivity depend on background myofilament protein phosphorylation and showed a coordinate role for the other PKA-target proteins in reducing myofilament Ca2+-sensitivity [64]. As the focus has been on troponin, in particular cTnI, other players, such as cMyBP-C and myosin light chain 2 have received less attention. Although, the functional relevance of some cTnI phosphorylation sites may be overestimated, efforts should be made to translate results obtained in vitro and in transgenic rodent models to human myocardium, as in the end we aim to understand and design treatment modalities for man.
3. Solaro Point: Studies of the role of troponin and troponin phosphorylation should move equally on all fronts without dominance of human studies
I am in full agreement with Jolanda regarding the need to do studies in larger mammals including the human. What we disagree on is the value of the studies with other species and of the relative importance of sites of phosphorylation on cTnI other than Ser23/Ser24. In our most recent studies [65] of this question done in collaboration with Jonathan Kirk and the laboratory of Sanjeev Shroff, we found significant depressive effects of expression of a pseudo-phosphorylated (by Glu substitutions) cTnI at Ser 43, S45, and T144 in a transgenic mouse model. This was not surprising to us in view of our previous data [19, 24, 26], but what is surprising is that only ~7% of the native cTnI had been replaced with the mutant pseudo-phosphorylated form. These data together with findings pointed out above indicate that there are transient changes in protein phosphorylation and function of the sarcomeres responding to stress and provide strong evidence for the need to use animal models. Moreover, an advantage of rodent genetic models is that they offer the possibility to determine whether phosphorylation of cTnI, for example, correlates with the maladaptive effects and expression of a mutant protein linked to a cardiomyopathy or whether the phosphorylation is an adaptive response. Another rationale for pursuing studies in animal models is the complexity of signaling through kinases and phosphatases. Multiple targets and dose and time dependence of agonist effects all contribute to this complexity. Sorting out the relative significance of these complex reactions is impractical in large animals and essentially impossible in the human. It is also worth noting at this point that studies with rabbit models also provide an approach, which involves an animal model closer to the human in terms of electrical properties and myosin isoform population. The disadvantage is the slow and relative expensive throughput of experiments. The problems with the studies of human samples remain and have been emphasized previously [31]. Apart from difficulty with well controlled studies, sampling problems remain significant. As pointed in a recent “white paper” written as a result of an NIH workshop, sampling in human studies is among the most prominent pitfalls [66].
4.0 Van der Velden counterpoint: To extrapolate the knowledge from transgenic animals present studies of the role of troponin and troponin phosphorylation should focus on human
I think John and I fully agree that we need to focus our studies on transient changes of the contractile machinery during onset and progression of cardiac disease. Indeed, studies in end-stage human myocardium have provided a rather narrow view and do not reflect the changes which occur during cardiac disease. I believe our recent studies in cardiac biopsies from patients with an earlier stage of heart failure advanced our understanding of sarcomeric dysfunction as they established the link between increased passive stiffness of sarcomeres and diastolic heart failure [67]. Investigation of genetically manipulated rodent models is indispensable for advancing our general understanding of sarcomeric protein function, but observations from these models need to be validated in human. I fear the devotion to transgenic models as these studies may further increase the gap between mice and men. We should take action and join efforts to overcome the pitfalls of human studies. One exciting novel approach to investigate (patho)physiologic mechanisms in human are human induced pluripotent stem (iPS) cells, which can be generated from somatic cells from patients who suffer from cardiovascular disease. The iPS cells can be differentiated into beating cardiomyocytes and may serve as a human cardiovascular disease model [68]. As the iPS cells have a similar genotype to that of the patient, they may be of particular interest to study monogenetic cardiac diseases such as HCM and DCM which are caused by mutations in genes encoding sarcomeric proteins. Although there will be several technical hurdles to take, cardiomyocytes derived from human iPS cells may be used to gain insight in site-specific phosphorylation of cTnI and the functional consequences and thereby extend the tools to investigate sarcomeric function in men. We have reached a point in sarcomeric studies where we should convince medical ethical committees and cardiac surgeons of the importance of human cardiac studies, which will allow us to check if inferences from transgenic animal studies are fact or fancy.
Acknowledgements
Work described in this paper was supported by grants from the National Institutes of Health, National Heart. Lung, and Blood Institute.
The authors wish to dedicate this article to the memory of Professor S. V. Perry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stull JT, Brostrom CO, Krebs EG. Phosphorylation of the inhibitor component of troponin by phosphorylase kinase. J Biol Chem. 1972 Aug 25;247(16):5272–5274. [PubMed] [Google Scholar]
- 2.Pratje E, Heilmeyer LM. Phosphorylation of rabbit muscle troponin and actin by a 3', 5'-c-AMP-dependent protein kinase. FEBS Lett. 1972 Oct 15;27(1):89–93. doi: 10.1016/0014-5793(72)80416-2. [DOI] [PubMed] [Google Scholar]
- 3.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004 Feb 6;94(2):146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi T, Solaro RJ. Calcium, thin filaments, and the integrative biology of cardiac contractility. Annu Rev Physiol. 2005;67:39–67. doi: 10.1146/annurev.physiol.67.040403.114025. [DOI] [PubMed] [Google Scholar]
- 5.Solaro RJ, Moir AJ, Perry SV. Phosphorylation of troponin I and the inotropic effect of adrenaline in the perfused rabbit heart. Nature. 1976 Aug 12;262(5569):615–617. doi: 10.1038/262615a0. [DOI] [PubMed] [Google Scholar]
- 6.Moir AJ, Solaro RJ, Perry SV. The site of phosphorylation of troponin I in the perfused rabbit heart. The effect of adrenaline. Biochem J. 1980 Feb 1;185(2):505–513. doi: 10.1042/bj1850505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robertson SP, Johnson JD, Holroyde MJ, Kranias EG, Potter JD, Solaro RJ. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982 Jan 10;257(1):260–263. [PubMed] [Google Scholar]
- 8.Takimoto E, Soergel DG, Janssen PM, Stull LB, Kass DA, Murphy AM. Frequency- and afterload-dependent cardiac modulation in vivo by troponin I with constitutively active protein kinase A phosphorylation sites. Circ Res. 2004 Mar 5;94(4):496–504. doi: 10.1161/01.RES.0000117307.57798.F5. [DOI] [PubMed] [Google Scholar]
- 9.Moir AJ, Perry SV. Phosphorylation of rabbit cardiac-muscle troponin I by phosphorylase kinase. The effect of adrenaline. Biochem J. 1980 Nov 1;191(2):547–554. doi: 10.1042/bj1910547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MX, Wang X, Lindhout DA, Buscemi N, Van Eyk JE, Sykes BD. Phosphorylation and mutation of human cardiac troponin I deferentially destabilize the interaction of the functional regions of troponin I with troponin C. Biochemistry. 2003 Dec 16;42(49):14460–14468. doi: 10.1021/bi035408y. [DOI] [PubMed] [Google Scholar]
- 11.Ke Y, Wang L, Pyle WG, de Tombe PP, Solaro RJ. Intracellular localization and functional effects of P21-activated kinase-1 (Pak1) in cardiac myocytes. Circ Res. 2004 Feb 6;94(2):194–200. doi: 10.1161/01.RES.0000111522.02730.56. [DOI] [PubMed] [Google Scholar]
- 12.You B, Yan G, Zhang Z, Yan L, Li J, Ge Q, et al. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem J. 2009 Feb 15;418(1):93–101. doi: 10.1042/BJ20081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noland TA, Jr, Raynor RL, Jideama NM, Guo X, Kazanietz MG, Blumberg PM, et al. Differential regulation of cardiac actomyosin S-1 MgATPase by protein kinase C isozyme-specific phosphorylation of specific sites in cardiac troponin I and its phosphorylation site mutants. Biochemistry. 1996 Nov 26;35(47):14923–14931. doi: 10.1021/bi9616357. [DOI] [PubMed] [Google Scholar]
- 14.Noland TA, Jr, Guo X, Raynor RL, Jideama NM, Averyhart-Fullard V, Solaro RJ, et al. Cardiac troponin I mutants. Phosphorylation by protein kinases C and A and regulation of Ca(2+)-stimulated MgATPase of reconstituted actomyosin S-1. J Biol Chem. 1995 Oct 27;270(43):25445–25454. doi: 10.1074/jbc.270.43.25445. [DOI] [PubMed] [Google Scholar]
- 15.Mazzei GJ, Kuo JF. Phosphorylation of skeletal-muscle troponin I and troponin T by phospholipid-sensitive Ca2+-dependent protein kinase and its inhibition by troponin C and tropomyosin. Biochem J. 1984 Mar 1;218(2):361–369. doi: 10.1042/bj2180361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989 Dec 5;264(34):20778–20785. [PubMed] [Google Scholar]
- 17.Sumandea MP, Rybin VO, Hinken AC, Wang C, Kobayashi T, Harleton E, et al. Tyrosine phosphorylation modifies protein kinase C delta-dependent phosphorylation of cardiac troponin I. J Biol Chem. 2008 Aug 15;283(33):22680–22689. doi: 10.1074/jbc.M802396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathur MC, Kobayashi T, Chalovich JM. Negative charges at protein kinase C sites of troponin I stabilize the inactive state of actin. Biophys J. 2008 Jan 15;94(2):542–549. doi: 10.1529/biophysj.107.113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burkart EM, Sumandea MP, Kobayashi T, Nili M, Martin AF, Homsher E, et al. Phosphorylation or glutamic acid substitution at protein kinase C sites on cardiac troponin I differentially depress myofilament tension and shortening velocity. J Biol Chem. 2003 Mar 28;278(13):11265–11272. doi: 10.1074/jbc.M210712200. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Grant JE, Doede CM, Sadayappan S, Robbins J, Walker JW. PKC-betaII sensitizes cardiac myofilaments to Ca2+ by phosphorylating troponin I on threonine-144. J Mol Cell Cardiol. 2006 Nov;41(5):823–833. doi: 10.1016/j.yjmcc.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003 Nov 1;552(Pt 3):845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pi Y, Kemnitz KR, Zhang D, Kranias EG, Walker JW. Phosphorylation of troponin I controls cardiac twitch dynamics: evidence from phosphorylation site mutants expressed on a troponin I-null background in mice. Circ Res. 2002 Apr 5;90(6):649–656. doi: 10.1161/01.res.0000014080.82861.5f. [DOI] [PubMed] [Google Scholar]
- 23.Goldspink PH, Montgomery DE, Walker LA, Urboniene D, McKinney RD, Geenen DL, et al. Protein kinase Cepsilon overexpression alters myofilament properties and composition during the progression of heart failure. Circ Res. 2004 Aug 20;95(4):424–432. doi: 10.1161/01.RES.0000138299.85648.92. [DOI] [PubMed] [Google Scholar]
- 24.Scruggs SB, Walker LA, Lyu T, Geenen DL, Solaro RJ, Buttrick PM, et al. Partial replacement of cardiac troponin I with a non-phosphorylatable mutant at serines 43/45 attenuates the contractile dysfunction associated with PKC epsilon phosphorylation. Journal of Molecular and Cellular Cardiology. 2006;40(4):465–473. doi: 10.1016/j.yjmcc.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 25.Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, et al. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005 Jan 7;280(1):703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 26.Roman BB, Goldspink PH, Spaite E, Urboniene D, McKinney R, Geenen DL, et al. Inhibition of PKC phosphorylation of cTnI improves cardiac performance in vivo. Am J Physiol Heart Circ Physiol. 2004 Jun;286(6):H2089–H2095. doi: 10.1152/ajpheart.00582.2003. [DOI] [PubMed] [Google Scholar]
- 27.Solaro RJ, de Tombe PP. Review focus series: sarcomeric proteins as key elements in integrated control of cardiac function. Cardiovascular Research. 2008;77(4):616–618. doi: 10.1093/cvr/cvn004. [DOI] [PubMed] [Google Scholar]
- 28.Morimoto S. Sarcomeric proteins and inherited cardiomyopathies. Cardiovasc Res. 2008 Mar 1;77(4):659–666. doi: 10.1093/cvr/cvm084. [DOI] [PubMed] [Google Scholar]
- 29.Belin RJ, Sumandea MP, Allen EJ, Schoenfelt K, Wang H, Solaro RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007 Jul 20;101(2):195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 30.Belin RJ, Sumandea MP, Kobayashi T, Walker LA, Rundell VL, Urboniene D, et al. Left ventricular myofilament dysfunction in rat experimental hypertrophy and congestive heart failure. Am J Physiol Heart Circ Physiol. 2006 Nov;291(5):H2344–H2353. doi: 10.1152/ajpheart.00541.2006. [DOI] [PubMed] [Google Scholar]
- 31.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008 Nov;45(5):603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zabrouskov V, Ge Y, Schwartz J, Walker JW. Unraveling molecular complexity of phosphorylated human cardiac troponin I by top down electron capture dissociation/electron transfer dissociation mass spectrometry. Mol Cell Proteomics. 2008 Oct;7(10):1838–1849. doi: 10.1074/mcp.M700524-MCP200. [DOI] [PubMed] [Google Scholar]
- 33.Ayaz-Guner S, Zhang J, Li L, Walker JW, Ge Y. In Vivo Phosphorylation Site Mapping in Mouse Cardiac Troponin I by High Resolution Top-Down Electron Capture Dissociation Mass Spectrometry: Ser22/23 Are the Only Sites Basally Phosphorylated. Biochemistry. 2009 Jul 28; doi: 10.1021/bi900739f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sancho Solis R, Ge Y, Walker JW. Single amino acid sequence polymorphisms in rat cardiac troponin revealed by top-down tandem mass spectrometry. J Muscle Res Cell Motil. 2008;29(6–8):203–212. doi: 10.1007/s10974-009-9168-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, et al. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29(6–8):189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 36.Marston SB, Walker JW. Back to the future: new tecdhniques show that forgotten phosphorylation sites are present in contractile proteins of the heart whilst intensively studied sites appear to be absent. J Muscle Res Cell Motil. 2009 doi: 10.1007/s10974-009-9184-y. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kooij V, Boontje N, Zaremba R, Jaquet K, Dos Remedios C, Stienen GJ, et al. Protein kinase C alpha and epsilon phosphorylation of troponin and myosin binding protein C reduce Ca(2+) sensitivity in human myocardium. Basic Res Cardiol. 2009 Aug 5; doi: 10.1007/s00395-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminsky M, Afari-Armah N, Dike U, Solaro RJ, Chen-Izu Y, Sumandea MP. Differential phosphorylation of cTnT, cTnI and MLC2 with heart disease progression from pre-hypertension to heart failure. Biophysical Journa. 2005 153/PosB528. [Google Scholar]
- 39.McDonough JL, Labugger R, Pickett W, Tse MY, MacKenzie S, Pang SC, et al. Cardiac troponin I is modified in the myocardium of bypass patients. Circulation. 2001 Jan 2;103(1):58–64. doi: 10.1161/01.cir.103.1.58. [DOI] [PubMed] [Google Scholar]
- 40.Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, et al. Transgenic mouse model of stunned myocardium. Science. 2000 Jan 21;287(5452):488–491. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- 41.Feng J, Schaus BJ, Fallavollita JA, Lee TC, Canty JM., Jr Preload induces troponin I degradation independently of myocardial ischemia. Circulation. 2001 Apr 24;103(16):2035–2037. doi: 10.1161/01.cir.103.16.2035. [DOI] [PubMed] [Google Scholar]
- 42.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997 Mar;80(3):393–399. [PubMed] [Google Scholar]
- 43.McDonough JL, Arrell DK, Van Eyk JE. Troponin I degradation and covalent complex formation accompanies myocardial ischemia/reperfusion injury. Circ Res. 1999 Jan 8–22;84(1):9–20. doi: 10.1161/01.res.84.1.9. [DOI] [PubMed] [Google Scholar]
- 44.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res. 1998 Feb 9;82(2):261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 45.Tachampa K, Kobayashi T, Wang H, Martin AF, Biesiadecki BJ, Solaro RJ, et al. Increased cross-bridge cycling kinetics after exchange of C-terminal truncated troponin I in skinned rat cardiac muscle. J Biol Chem. 2008 May 30;283(22):15114–15121. doi: 10.1074/jbc.M801636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narolska NA, Piroddi N, Belus A, Boontje NM, Scellini B, Deppermann S, et al. Impaired diastolic function after exchange of endogenous troponin I with C-terminal truncated troponin I in human cardiac muscle. Circ Res. 2006 Oct 27;99(9):1012–1020. doi: 10.1161/01.RES.0000248753.30340.af. [DOI] [PubMed] [Google Scholar]
- 47.Galinska-Rakoczy A, Engel P, Xu C, Jung H, Craig R, Tobacman LS, et al. Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J Mol Biol. 2008 Jun 20;379(5):929–935. doi: 10.1016/j.jmb.2008.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Galinska-Rakoczy A, Hatch V, Craig R, Murphy AM, Van Eyk JE, Wang CLA, et al. EM and 3D-reconstruction of thin filaments reconstituted with truncated troponin I. Biophys J. 2009;96 502a. [Google Scholar]
- 49.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res. 2007 Apr 13;100(7):1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 50.Duncker DJ, Boontje NM, Merkus D, Versteilen A, Krysiak J, Mearini G, et al. Prevention of myofilament dysfunction by beta-blocker therapy in postinfarct remodeling. Circ Heart Fail. 2009 May;2(3):233–242. doi: 10.1161/CIRCHEARTFAILURE.108.806125. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery DE, Chandra M, Huang Q, Jin J, Solaro RJ. Transgenic incorporation of skeletal TnT into cardiac myofilaments blunts PKC-mediated depression of force. Am J Physiol Heart Circ Physiol. 2001 Mar;280(3):H1011–H1018. doi: 10.1152/ajpheart.2001.280.3.H1011. [DOI] [PubMed] [Google Scholar]
- 52.Sumandea MP, Pyle WG, Kobayashi T, de Tombe PP, Solaro RJ. Identification of a functionally critical protein kinase C phosphorylation residue of cardiac troponin T. J Biol Chem. 2003 Sep 12;278(37):35135–35144. doi: 10.1074/jbc.M306325200. [DOI] [PubMed] [Google Scholar]
- 53.Noguchi T, Hunlich M, Camp PC, Begin KJ, El-Zaru M, Patten R, et al. Thin-filament-based modulation of contractile performance in human heart failure. Circulation. 2004 Aug 24;110(8):982–987. doi: 10.1161/01.CIR.0000139334.43109.F9. [DOI] [PubMed] [Google Scholar]
- 54.Colantonio DA, Van Eyk JE, Przyklenk K. Stunned peri-infarct canine myocardium is characterized by degradation of troponin T, not troponin I. Cardiovasc Res. 2004 Aug 1;63(2):217–225. doi: 10.1016/j.cardiores.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 55.van der Velden J, Narolska NA, Lamberts RR, Boontje NM, Borbely A, Zaremba R, et al. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006 Mar 1;69(4):876–887. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 56.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007 Jan;42(1):247–259. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 57.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996 Jul 1;98(1):167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker LA, Walker JS, Ambler SK, Buttrick PM. Stage-specific changes in myofilament protein phosphorylation following myocardial infarction in mice. J Mol Cell Cardiol. 2009 Sep 30; doi: 10.1016/j.yjmcc.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, et al. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol. 2007 Jul 15;582(Pt 2):695–709. doi: 10.1113/jphysiol.2007.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Varian KD, Kijtawornrat A, Gupta SC, Torres CA, Monasky MM, Hiranandani N, et al. Impairment of diastolic function by lack of frequency-dependent myofilament desensitization rabbit right ventricular hypertrophy. Circ Heart Fail. 2009 Sep;2(5):472–481. doi: 10.1161/CIRCHEARTFAILURE.109.853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Heerebeek L, Borbely A, Niessen HW, Bronzwaer JG, van der Velden J, Stienen GJ, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006 Apr 25;113(16):1966–1973. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 62.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009 Mar 27;104(6):780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 63.van Dijk SJ, Dooijes D, dos Remedios C, Michels M, Lamers JM, Winegrad S, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009 Mar 24;119(11):1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 64.Kooij V, van der Velden J, Stienen G. Effect fo troponin I Ser23/24 bis-phosphorylation on Ca-sensitivity is dependent on PKA phosphorylation of other contractile proteins. Biophys J. 2009;96 501a–502a. [Google Scholar]
- 65.Kirk JA, Macgowan GA, Evans C, Smith SH, Warren CM, Mamidi R, et al. Left Ventricular and Myocardial Function in Mice Expressing Constitutively Pseudophosphorylated Cardiac Troponin I. Circ Res. 2009 Oct 22; doi: 10.1161/CIRCRESAHA.109.205427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rudy Y, Ackerman MJ, Bers DM, Clancy CE, Houser SR, London B, et al. Systems approach to understanding electromechanical activity in the human heart: a national heart, lung, and blood institute workshop summary. Circulation. 2008 Sep 9;118(11):1202–1211. doi: 10.1161/CIRCULATIONAHA.108.772715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borbely A, van der Velden J, Papp Z, Bronzwaer JG, Edes I, Stienen GJ, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005 Feb 15;111(6):774–781. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 68.Freund C, Davis RP, Gkatzis K, Ward-van Oosterwaard D, Mummery CL. The first reported generation of human induced pluripotent stem cells (iPS cells) and iPS cell-derived cardiomyocytes in the Netherlands. Neth Heart J. 2010 Jan;18(1):51–54. [PMC free article] [PubMed] [Google Scholar]