Abstract
Background
Postmenopausal women with osteoporosis/osteopenia are at increased risk of fracture. Aromatase inhibitors further increase bone loss in these patients. This study evaluates whether zoledronic acid prevents the bone loss expected when these patients initiate letrozole.
Patients and Methods
Postmenopausal women with estrogen and/or progesterone receptor-positive breast cancer and a bone mineral density (BMD) T-score < −2.0 were given letrozole 2.5 mg/vitamin D 400 international units daily, calcium 500 mg twice daily, and 4 mg zoledronic acid every 6 months. The BMD was assessed at baseline and 1 year. The primary endpoint was the mean change in lumbar spine (LS) BMD at 1 year.
Results
46 patients completed 1 year of treatment. LS BMD increased by 2.66% (p=0.01), femoral neck (FN) by 4.81% (p=0.01), and any measured endpoint by 4.55% (p=0.0052).
Conclusions
Zoledronic acid prevents bone loss in postmenopausal women with osteoporosis/osteopenia starting letrozole and is associated with improvements in BMD.
Introduction
Postmenopausal women are at increased risk of bone loss due to the failure of ovarian function and corresponding decline in estrogen levels. The loss of bone mineral density (BMD) occurs as the estrogen-mediated effect on osteoclast activity is withdrawn, leading to a decline in osteoclast apoptosis and an increase in osteoclast differentiation (1). Estrogen therapy (with or without progesterone) has been shown to prevent bone loss and reduce the risk of hip and vertebral fractures (2–4). Women with breast cancer are commonly advised to avoid this therapy, however, due to the presence of estrogen and progesterone receptors (ER, PR) in the majority of these malignancies.
Adjuvant endocrine therapy for postmenopausal women with breast cancer may utilize tamoxifen, a selective estrogen receptor modulator known to increase BMD in postmenopausal women(5), or aromatase inhibitors, which have improved disease-free survival outcomes (6–10) when compared to tamoxifen. Aromatase inhibitors are also beneficial as extended adjuvant therapy following 5 years of tamoxifen (11) and are commonly used as first-line therapy. Aromatase inhibitors reduce peripheral estrogen production, an effect that is accompanied by a corresponding decline in BMD (12–14), further increasing the risk of fracture in women with a pre-existing history of osteoporosis or osteopenia.
Postmenopausal women with breast cancer and a history of osteoporosis or osteopenia are therefore at increased risk of fractures, due to multiple factors. Therapy for osteoporosis is standard for women with a BMD T-score of ≤ −2.5. Treatment is also warranted for women with a T-score of ≤ −2.0, which exceeds the fracture threshold, particularly in the presence of risk factors for ongoing bone loss (such as aromatase inhibitor therapy). Bisphosphonates have been utilized for the treatment of women with breast cancer because they have no known interaction with estrogen or progesterone receptors and have been shown to increase BMD in normal postmenopausal women (15). Zoledronic acid has also been shown to stabilize BMD in postmenopausal women with a T-score of ≥ −2.0 who are initiating adjuvant therapy with letrozole for breast cancer (14, 16). However, the effect of zoledronic acid in women already at increased fracture risk due to the presence of pre-existing moderate-to-severe osteopenia (T-score − −2.0) or osteoporosis, and initiating therapy with an aromatase inhibitor, has not been well studied. To document the effect of zoledronic acid on BMD in this population, the current study was developed.
Patients and Methods
This study was conducted by the Mayo Clinic Cancer Research Consortium (MCCRC) after approval by the local Institutional Review Boards from participating sites. Written informed consent was obtained from all participants prior to enrollment. This study was funded by Novartis, who reviewed the protocol but then had no further involvement in the conduct of the study.
Study Population
Eligible participants were postmenopausal women with a history of localized Stage I, II, or IIIa breast cancer, ER and/or PR positive cancers without evidence of recurrent or metastatic disease, an Eastern Cooperative Oncology Group (ECOG) Performance Status of 0–2, a life expectancy of at least 5 years, and accessible for follow-up. Oral bisphosphonates, if used previously, must have been discontinued greater than 3 weeks prior to registration, and the baseline total lumbar spine (LS) or femoral neck (FN) BMD T-score must have been less than −2.0 (more than 2 standard deviations below the normal BMD for a young healthy woman). Osteoporosis was defined as a standardized BMD T-score of at least 2.5 standard deviations below the normal BMD for a young healthy woman, while osteopenia was defined as a standardized BMD T-score of less than 2 standard deviations below the normal BMD for a young healthy woman.
Participants were excluded if they had recent treatment with any drugs known to affect the skeleton, prior treatment with intravenous bisphosphonates or aromatase inhibitors, prior exposure (within the prior 6 months) to anabolic steroids or growth hormone, or other malignancy within 5 years (except adequately treated non-melanoma skin cancer or cervical carcinoma in-situ). Patients with other malignancies ≥ 5 years prior must have been disease free at registration. Certain non-malignant diseases were also considered contra-indicated, including uncontrolled infections, diabetes mellitus, thyroid dysfunction, or seizure disorders associated with falls, any diseases affecting bone metabolism, malabsorption syndrome, or mental illnesses that would have precluded the patient from providing informed consent. Participants with a known hypersensitivity to zoledronic acid, other bisphosphonates, letrozole, calcium, or vitamin D were excluded, as were those with hypercalcemia, hypocalcemia, or considered unreliable for follow-up or compliance with the study requirements. Participants were also excluded if the dual x-ray absorptiometry (DXA) at the spine was contraindicated. Patients with current active dental problems, dental or fixture trauma, or current or prior diagnosis of osteonecrosis of the jaw (ONJ), exposed bone in the mouth or slow healing after dental procedures were also excluded, as were those with recent or planned dental or jaw surgery (≤ 6 weeks).
Study Design
This was an open-label, single arm, observational trial designed to assess the changes in BMD in postmenopausal women with significant osteopenia or osteoporosis and hormone receptor positive breast cancer who initiated letrozole and zoledronic acid concurrently. All patients were assigned to take letrozole 2.5 mg daily for 5 years, zoledronic acid 4 mg intravenously over 15 minutes every 6 months (until disease progression or for 5 years), calcium 500 mg twice daily, and vitamin D 400 international units daily. The dose of zoledronic acid was adjusted for a creatinine clearance < 60 mL/minute. Patients who discontinued letrozole or zoledronic acid were withdrawn from the study. Prohibited concomitant therapy included any other bisphosphonates, calcitonin, sodium fluoride, parathyroid hormone, mithramycin, gallium nitrate, or tibolone.
Participants underwent annual BMD as measured by DXA at baseline and for 5 years. The reliability of BMD assessments was ensured by calculating the change in BMD over a specified time interval for each individual participant (intra-patient change) using the same bone site for measurement at each time-point. Quality assurance measures such as the regular calibration of the machines used for scanning or use of the same machine for each scan within an individual patient were left to the discretion of the individual participating sites. Other measures such as a common phantom across sites or central analysis of all BMD results were not performed. The primary endpoint was prospectively defined as the average intra-patient change in BMD in the LS from baseline to 1 year, expressed as a percentage of the baseline value. Secondary prospectively defined endpoints included the average intra-patient change in LS BMD from baseline to years 2 through 5, and the change in FN BMD from baseline to years 1 through 5. Although many studies have reported changes in the total hip rather than the FN, previous investigators (17) have shown that half of all hip fractures occur at the FN, with a significant correlation existing between changes in FN BMD and hip fracture risk. Therefore, changes in FN BMD were used as a secondary endpoint for this study. The frequency and severity of toxicity reported using the NCI’s Common Terminology Criteria for Adverse Events (CTCAE) was calculated. Disease progression was assessed at each patient visit. Any patients with disease progression were removed from the study. The time to disease progression was also calculated.
Statistical Analysis
Analysis of the primary endpoint was accomplished by calculating the average intra-patient change in LS BMD from baseline to 1 year. As a single-arm study using Kruskall-Wallis p-value methodology as the primary method of assessment, the analysis plan was primarily descriptive. A sample size of 60 patients was estimated to provide percentage statistics accuracy to within 13% with 95% confidence. Analysis of the secondary endpoints was performed in a manner analogous to the primary analysis.
Results
Sixty participants were enrolled between June, 2006 and July, 2007. One participant subsequently refused to start therapy, 4 were later deemed ineligible (1- LS BMD contraindicated, 3-baseline BMD T score ≥ −2.0), and 1 was not included for analysis due to a major violation (letrozole was not dispensed for the first 6 months) (figure 1). This left 54 participants for analysis. Of these 54, 6 refused further treatment (see figure 1) before reaching the 1 year time point, 1 patient had an adverse event, and 1 missed the 1 year BMD measurement, leaving 46 patients with 1 year follow-up data. Among these 46 patients, some reported BMD data at baseline and 1 year for the LS, some reported BMD data at baseline and 1 year for the FN, and some reported data at both sites. Eight patients reported baseline and 1 year BMD data, but not at the same sites and thus were not included in the analysis. Baseline patient characteristics are outlined in Table 1. Overall, 44% reported a prior history of tamoxifen usage.
Figure 1.
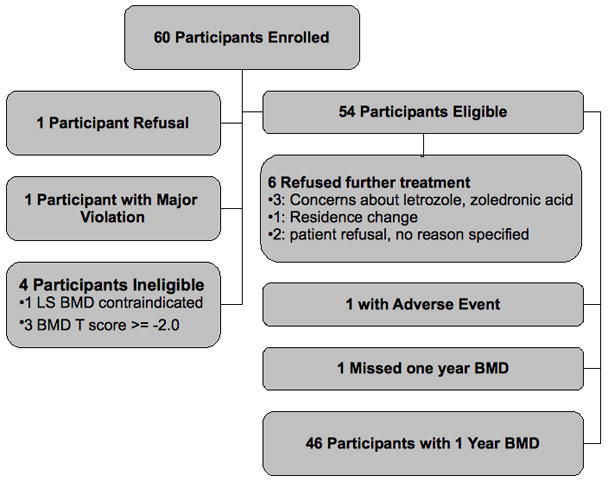
Patient Flow
Table 1.
Baseline Participant Characteristics
| Total (N=54) | |
|---|---|
| Age | |
| N | 54 |
| Mean (SD) | 66.7 (10.13) |
| Range | (45.0–84.0) |
| Prior Tamoxifen | |
| Yes | 24 (44%) |
| Duration of Tamoxifen Use | |
| Missing | 30 |
| <=2 Years | 4 (17%) |
| >2 Years | 20 (83%) |
| Time Since Tamoxifen Ended | |
| Missing | 30 |
| <1 Year | 20 (83%) |
| >=1 Year | 4 (17%) |
| Prior Chemotherapy | |
| Yes | 21 (39%) |
| Previous Fracture by history or x-ray | |
| Yes | 7 (13%) |
| Race | |
| White | 51 (94%) |
| Black or African American | 1 (2%) |
| Asian | 2 (4%) |
Bone Mineral Density Results
The LS BMD improved significantly with an increase of 2.66% (p=0.01) from baseline to 1 year among patients reporting LS BMD data at both time points (Table 2). There was no difference in the change or percent change in LS BMD among patients who had any prior tamoxifen exposure vs. those without prior tamoxifen usage (p = 0.80 and 0.50, respectively). A similar finding was noted among those patients reporting BMD data at the FN at both time points, with an increase of 4.81% (p=0.01). Among the 38 patients who had any BMD site measured at baseline and 1 year, there was a 4.55% improvement in BMD (p=0.005). When those patients are considered individually, 30 experienced a positive change in their BMD versus 8 whose BMD declined. Among those whose BMD declined, there was no significant difference in age, prior tamoxifen exposure, duration of tamoxifen use, time since tamoxifen use, prior chemotherapy, or race compared to those whose BMD increased. Patients whose BMD decreased were more likely to report a prior history of fracture (38 vs 9%, p = 0.025) than those who experienced an increase in their BMD.
Table 2.
BMD Change and Percent Change at Year 1
| BMD Measurement | N | Baseline BMD | 1 Year BMD | Difference | % Change | p-value |
|---|---|---|---|---|---|---|
| LS | 30 | 0.86 | 0.88 | 0.02 | 2.66 | 0.01 |
| FN | 32 | 0.69 | 0.73 | 0.033 | 4.81 | 0.01 |
| Any Endpoint | 38 | 0.78 | 0.82 | 0.036 | 4.55 | 0.005 |
Fractures and Disease Progression
Participants were assessed for fracture at 6 month intervals. There were 4 reports of bone fracture; this constitutes 7% of patients experiencing a fracture in this high risk population. No participants experienced disease progression within the first year of therapy.
Toxicity profile
Several toxicities reported within the first year were felt to be at least possibly related to therapy (Table 3) and were consistent with the previously-reported adverse effects of either letrozole or zoledronic acid. The most commonly reported symptom was arthralgia, a known effect of aromatase inhibitors, with 12 patients reporting grade 1 or 2 severity. Hot flashes, another known effect of aromatase inhibitors, also occurred in 2 patients, with only 1 to 2 patients reporting problems with creatinine, headache, rash, fever, nausea, or vomiting. There were no reports of osteonecrosis of the jaw (ONJ).
Table 3.
Maximum-Grade Toxicity Incidence at Least Possibly Related to Study Medications
| Toxicity | Grade 1 | Grade 2 | Total |
|---|---|---|---|
| Arthralgia | 8 | 4 | 12 |
| Creatinine | 1 | 0 | 1 |
| Rash | 0 | 1 | 1 |
| Headache | 0 | 1 | 1 |
| Hot Flashes | 0 | 2 | 2 |
| Nausea | 2 | 0 | 2 |
| Fever | 2 | 0 | 2 |
| Vomiting | 1 | 0 | 1 |
| Total | 14 | 8 | 22 |
Discussion
This single-arm study supports that women with moderate-to-severe osteopenia or osteoporosis and increased risk of bone fractures do not lose additional BMD when zoledronic acid is started concurrently with letrozole for hormone responsive breast cancer. Bone density stabilized at all sites measured, with an overall increase in BMD at 1 year for the LS and the FN. These data corroborate the hypothesis that concurrent therapy with zoledronic acid prevents bone loss in a high-risk population of women who are initiating therapy known to promote ongoing bone loss and increase fracture risk.
Letrozole counter-acts the effect of estrogen on bone by decreasing estrogen production, thereby increasing the risk of bone loss (and fracture). Aromatase inhibitors have been documented to reduce LS and total hip BMD by approximately 4% at 2 years as well as to increase biomarkers of bone turnover, increase the risk of osteoporosis, and increase the risk of fractures (11–12). Anastrozole significantly increased the odds ratio of fracture, by 49%, versus tamoxifen in the Anastrozole and Tamoxifen Alone or in Combination (ATAC) trial, with 11% of all participants on anastrozole experiencing a fracture. Patients taking letrozole after tamoxifen also experienced a slight increase in risk for fracture, despite 88% of patients not having a pre-existing diagnosis of osteoporosis (11).
Zoledronic acid may counter-act this effect by inhibiting osteoclast activity and has been documented to prevent the bone loss associated with aromatase inhibitors in women with breast cancer and a BMD T-score > −2.0. Two randomized, controlled trials (14, 16) have recently documented a protective effect of immediate zoledronic acid on bone density in women with breast cancer starting letrozole. However, participants in these trials were excluded if they had a BMD T-score of <−2.0. Based on the World Health Organization, a 50 year old Caucasian woman in the United States with a BMD T-score of < −2.0 would have a greater than 8–56% ten-year probability of a major osteoporotic fracture, depending on the number of clinical risk factors for osteoporosis (18). Therefore, the possibility of further increasing the loss of BMD and the fracture risk may serve as a deterrent to the use of aromatase inhibitors in these patients.
The documented loss of bone density associated with aromatase inhibitor therapy appears to be abolished with the use of concurrent zoledronic acid in this population; its effect on fracture risk, nonetheless, is not clear. Although 7% of participants experienced a fracture in this study, 13% had a pre-existing history of fracture, which supports the underlying premise that this population is at high risk. Among the known risk factors for fracture in this population are the pre-existing low BMD (which meets or exceeds the fracture threshold), the postmenopausal status, contra-indication for estrogen therapy, concomitant aromatase inhibitor therapy, and history of breast cancer, which has been reported to increase the risk of fracture. Additional risk factors for fracture were not collected as part of this study. There was no placebo arm in this trial to determine what frequency of fractures would have been seen in women with moderate-to-severe osteopenia or osteoporosis receiving treatment with aromatase inhibitors. Therefore, it is difficult to find a baseline level of fracture expected for this population. However, women with this level of bone loss starting aromatase inhibitor therapy would clinically be considered candidates for empirical therapy with bisphosphonates. This study substantiates the strategy of using zoledronic acid to stabilize BMD in these high risk patients.
The strengths of this study include the fact that this is the first known report of the effect of zoledronic acid on BMD in breast cancer patients with a pre-existing history of moderate-to-severe bone loss who were receiving an aromatase inhibitor. The results from this study are consistent with previously published findings on zoledronic acid. However, these findings provide evidence that can be applied clinically to a population of patients at higher risk for fracture. Other bisphosphonates, such as alendronate and risedronate, have been proven to reduce the risk of fracture in women with a history of low BMD (20–23). In addition, two bisphosphonates have been tested for their effect on bone loss in this setting (postmenopausal women with osteoporosis/osteopenia starting an aromatase inhibitor for breast cancer). The study of anastrozole with the bisphosphonate risedronate (SABRE) evaluated the effect of risedronate 35 mg weekly on BMD in women with hormone receptor positive breast cancer treated with anastrozole. Among the 37 women at high-risk for fracture (T score < −2.0), BMD increased significantly at the LS and TH at 1 year (24). The ARIBON trial examined the effect of ibandronate in a similar population. Although patients with osteopenia (T score between −1.0 and −2.5) randomized to take 150 mg of monthly ibandronate (in addition to anastrozole) also experienced an increase in BMD, the median BMD T score in this group was only −1.4 at the LS and −1.2 at the TH. Among the 13 osteoporotic patients (T score <−2.5) treated with open-label ibandronate for 2 years, BMD increased at the LS by 3.52% and at the TH by 2.49% compared to baseline. These findings are consistent with the documented effect of zoledronic acid in this study and confirm the beneficial effect of bisphosphonates in aromatase inhibitor-treated women with a pre-existing history of low BMD (25).
Weaknesses of this study include its relatively small size. It was designed as an adjunct to the larger trials looking at BMD in women without a history of severe bone loss. However, >10% of patients were found to have missing data at baseline or 1 year and could not be included in the final analysis. This and the higher than expected level of patient drop-out (at enrollment) limit the strength of the study and are difficult to explain. Patients were enrolled at individual sites participating in the consortium and treated/evaluated under the direction of their local physicians; thus certain participants may have been subject to individual changes that would not affect other participating sites in the consortium. The study was designed as a single arm intervention, primarily to provide all of the patients the predicted benefit of bisphosphonate therapy; the lack of placebo arm may also limit the conclusions that can be drawn (i.e. relative effect on fracture risk and disease progression). Data on bone marker changes were not collected in sufficient numbers to determine whether the dose and schedule were sufficient to normalize bone turnover. However, other studies (14, 16) using zoledronic acid have reported improvements in BMD with a similar dosing regimen. Finally, vitamin D levels, which could have an impact on BMD/response to therapy, were not assessed. The presence of vitamin D deficiency among these patients could limit the effect of zoledronic acid, and thus the true benefit may be underestimated.
Conclusions
Postmenopausal women with a BMD T-score of < −2.0 are at increased risk of fracture. Therapy with aromatase inhibitors have been shown to improve disease-free survival in women with hormone responsive breast cancer but are also associated with worsening bone loss and increased fracture rates. Concurrent therapy with intravenous zoledronic acid is associated with a preservation-to-improvement of BMD in this population. Effects on fracture rates and disease progression cannot be calculated based on this study alone. However, recent data from other studies suggest that these may represent additional benefits.
Acknowledgments
This study was conducted as a trial of the Mayo Cancer Center Research Consortium in part by Public Health Service grants CA-35431, CA-35195, and CA-52352. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
This work was supported, in part, by the following United States National Institutes of Health Grant-CA 124477 (PI Charles Loprinzi MD)
Footnotes
This study was conducted as a trial of the Mayo Cancer Center Research Consortium in part by Public Health Service grants CA-35431, CA-35195, and CA-52352. The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Additional participating institutions include: Carle Cancer Center CCOP, Urbana, IL 61801 (Kendrith M. Rowland, Jr., M.D.); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, M.D.)
Conflict of Interest
Dr. Hines, Dr. Perez, and Dr. Loprinzi report some research funding from Novartis. Otherwise, no conflicts of interest are present.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Perez EA, Weilbaecher K. Aromatase Inhibitors and Bone Loss. Oncology. 2006;20(9):1029–1039. [PMC free article] [PubMed] [Google Scholar]; Prince RL, Smith M, Dick IM, Price RI, Webb PG, Henderson NK, Harris MM. Prevention of postmenopausal osteoporosis. A comparative study of exercise, calcium supplementation, and hormone replacement therapy. NEJM. 1991;325(17):1189–95. doi: 10.1056/NEJM199110243251701. [DOI] [PubMed] [Google Scholar]
- 2.Riis B, Thomsen K, Christiansen C. Does calcium supplementation prevent postmenopausal bone loss? A double-blind, controlled clinical study. NEJM. 1987;316(4):173–7. doi: 10.1056/NEJM198701223160401. [DOI] [PubMed] [Google Scholar]
- 3.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 4.Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B, Chlebowski R, Curb D, Gass M, Hays J, Heiss G, Hendrix S, Howard BV, Hsia J, Hubbell A, Jackson R, Johnson KC, Judd H, Kotchen JM, Kuller L, LaCroix AZ, Lane D, Langer RD, Lasser N, Lewis CE, Manson J, Margolis K, Ockene J, O’Sullivan MJ, Phillips L, Prentice RL, Ritenbaugh C, Robbins J, Rossouw JE, Sarto G, Stefanick ML, Van Horn L, Wactawski-Wende J, Wallace R, Wassertheil-Smoller S. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–12. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 5.Powles T, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of Tamoxifen on Bone Mineral Density Measured by Dual-Energy X-Ray Absorptiometry in Healthy Premenopausal and Postmenopausal Women. J Clin Oncol. 1996;14(1):78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 6.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, et al. A randomized Trial of Exemestane after Two to Three Years of Tamoxifen Therapy in Postmenopasual Women with Primary Breast Cancer. NEJM. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 7.The ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early brest cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2131–39. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- 8.The ATAC Trialists’ Group. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol. 2008;9:45–53. doi: 10.1016/S1470-2045(07)70385-6. [DOI] [PubMed] [Google Scholar]
- 9.Coates AS. Five Years of Letrozole Compared With Tamoxifen As Initial Adjuvant Therapy for Postmenopausal Women With Endocrine-Responsive Early Breast Cancer: Update of Study BIG 1–98. JCO. 2007;25(5):486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 10.Coombes RC, et al. Survival and Safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (Intergroup Exemestane Study): a randomized controlled trial. Lancet. 2007;369:559–70. doi: 10.1016/S0140-6736(07)60200-1. [DOI] [PubMed] [Google Scholar]
- 11.Goss PE, et al. Randomized Trial of Letrozole Following Tamoxifen as Extended Adjuvant Therapy in Receptor-Positive Breast Cancer: Updated Findings from the NCIC CTG MA.17. JNCI. 2005;97(17):1262–1271. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 12.Eastell R, Hannon RA, Cuzick J, Dowsett M, Clack G, Adams JE. Effect of an Aromatase Inhibitor on BMD and Bone Turnover Markers: 2-Year Results of the Anastrozole, Tamoxifen, Alone or in Combination Trial. J of Bone and Mineral Research. 2006;21(8):1215–23. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 13.Perez EA, Josse RG, Pritchard KI, Ingle JN, Martino S, Findlay BP, et al. Effect of Letrozole versus Placebo on Bone Mineral Density in Women with Primary Breast Cancer Completing 5 or More Years of Adjuvant Tamoxifen: A Companion Study to NCIC CTG MA.17. J Clin Oncol. 2006;24(22):3629–35. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 14.Brufsky A, Harker WG, Beck JT, Carroll R, Tan-Chiu E, Seidler C, et al. Zoledronic Acid Inhibits Adjuvant Letrozole-Induced Bone Loss in Postmenopausal Women with Early Breast Cancer. J Clin Oncol. 2007;25(7):829–836. doi: 10.1200/JCO.2005.05.3744. [DOI] [PubMed] [Google Scholar]
- 15.Reid IR, Brown JP, Burckhardt P, Horowitz A, Richardson P, Trechsel U, et al. Intravenous Zoledronic Acid in postmenopausal women with low bone mineral density. NEJM. 2002;346(9):653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 16.Hines SL, Mincey BA, Dentchev T, Sloan JA, Perez EA, Johnson DB, et al. Immediate vs. Delayed Zoledronic Acid for Prevention of Bone Loss in Postmenopausal Women with Breast Cancer Starting Letrozole After Tamoxifen. Breast Cancer Research and Treatment. 2009 doi: 10.1007/s10549-009-0332-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, et al. Bone density at various sites for prediction of hip fractures. Lancet. 1993;341:72–75. doi: 10.1016/0140-6736(93)92555-8. [DOI] [PubMed] [Google Scholar]
- 18.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnant M, et al. Adjuvant ovarian suppression combined with tamoxifen or anastrozole, alone or in combination with zoledronic acid, in premenopausal women with hormone-responsive, stage I and II breast cancer: First efficacy results from ABCSG-12. JCO. 2008;26(supplement):LBA4. (abstract) [Google Scholar]
- 20.Harrington JT, Ste-Marie LG, Brandi ML, Civitelli R, Fardellone P, Grauer A, et al. Risedronate Rapidly Reduces the Risk for Nonvertebral Fractures in Women with Postmenopausal Osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 21.Watts NB, Cooper C, Lindsay R, Eastell R, Manhart MD, Barton IP, et al. Relationship Between Changes in Bone Mineral Density and Vertebral Fracture Risk Associated with Risedronate. Journal of Clinical Densitometry. 2004;7(3):255–261. doi: 10.1385/jcd:7:3:255. [DOI] [PubMed] [Google Scholar]
- 22.Crandall C. Risedronate: A Clinical Review. Arch Intern Med. 2001;161:353–60. doi: 10.1001/archinte.161.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of Alendronate on Risk of Fracture in Women With Low Bone Density but Without Vertebral Fractures. JAMA. 1998;280(24):2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 24.Van Poznak C, Hannon RA, Clack G, Campone M, Mackey JR, Apffelstaedt J, et al. The SABRE (Study of Anastrozole with the Bisphosphonate Risedronate) study: 12-month analysis. Breast Cancer Res Treat. 2007;106(Supple 1):S37. (abstract 502) [Google Scholar]
- 25.Lester JE, Dodwell D, Purohit OP, Gutcher SA, Ellis SP, Thorpe R, et al. Prevention of Anastrozole-Induced Bone Loss with Monthly Oral Ibandronate during Adjuvant Aromatase Inhibitor Therapy for Breast Cancer. Clin Cancer Res. 2008;14(19):6336–6342. doi: 10.1158/1078-0432.CCR-07-5101. [DOI] [PubMed] [Google Scholar]


