Abstract
Drosophila olfactory aversive conditioning has served as a powerful model system with which to elucidate the molecular and neuronal mechanisms underlying memory formation. In the typical protocol, flies are exposed to a constant odor stream while receiving a pulsed electric shock in the conditioning tube of a manual apparatus. We have devised a simple, low-cost semi-automated conditioning apparatus that computationally controls the delivery of odor and shock. A semiconductor-based odor sensor is employed to monitor the change of odor concentration in the training tube. The system thus allows electric shocks to be precisely matched with odor concentration in the training tube. We found that short-term memory performance was improved with a pulsed odor flow protocol, in which odor is presented in short pulses, each paired with electric shock, rather than as a constant flow. The effect of pulsed odor flow might be ascribed to the phenomenon of ‘conditioned approach’, where approach toward an odor is induced when the electric shock is presented before odor pulse ends. Our data shows that the system is applicable to the study of olfactory memory formation and to the examination of conditioning parameters at a level of detail not practical with a manual apparatus.
Keywords: Drosophila melanogaster, olfactory aversive memory, semi-automated conditioning apparatus
INTRODUCTION
Drosophila melanogaster can form an association between an odor (the Conditioned Stimulus, CS) and electric shock (the Unconditioned Stimulus, US), acquiring conditioned avoidance to the odor (Quinn et al., 1974). This conditioned behavior is retained for a certain period of time depending on the details of the training paradigm (Tully et al., 1994). A single co-presentation of an odor with a shock is sufficient to induce short and middle-term memories (STM and MTM), while temporally spaced repetitive training may induce a long-term memory (LTM) that can last for days. Because of the simplicity and reliability of the conditioning procedure and genetic accessibility of Drosophila, this paradigm has been a widely used system for the elucidation of mechanisms underlying memory formation. Genes and neural circuits responsible for Drosophila olfactory memory formation have been identified (Gerber et al., 2004; Davis, 2005; Margulies et al., 2005; Keene and Waddell, 2007). Powerful genetic tools, imaging techniques and electrophysiological techniques enable us to deeply resolve gene functions that are involved in memory (Dubnau et al., 2001; Kitamoto, 2001; McGuire et al., 2001; McGuire et al., 2004).
The conditioning procedure for Drosophila olfactory learning was first developed by Benzer and his colleagues (Quinn et al., 1974; Dudai et al., 1976). The protocol developed by Tully and Quinn (Tully and Quinn, 1985) has been widely used for decades. In the standard protocol, flies are exposed simultaneously to a continuous odor (CS+) stream and pulsed electric shocks (US) for one minute. Following this, flies are exposed to another odor (CS−) without electric shock. This treatment induces a short-term aversive memory to the CS+ odor, which is quantified in a T-maze apparatus presenting the CS+ and CS− odors from opposing directions.
The manual protocol, though relatively simple for the induction of an STM, lacks the automated precision required to systematically explore subtle variations in the training regime. It is also tedious and time-consuming to manually perform the repetitive training protocol required to induce an LTM. Here we report the development of a semi-automated conditioning apparatus with which the timing of odor flow and electric shock presentation can be regulated precisely and reproducibly. Automated conditioning apparatuses were developed in several laboratories and contributed a great deal to studies of Drosophila olfactory memory, especially the identification of genes involved in LTM formation (Tully et al., 1994; Pascual and Preat, 2001; Dubnau et al., 2003; Didelot et al., 2006). However, costly custom-built parts were used to assemble these machines, for which detailed designs are not readily available. We thus sought to design an apparatus with simple and general-purpose parts that are commercially available. The apparatus is easy to expand to permit training multiple specimens simultaneously. The apparatus induces short-term and long-term memory like that of the manual apparatus, and is robust enough for genetic and biochemical studies.
Utilizing our system we reexamined the standard conditioning procedure. Previous studies have shown that a short ten second odor presentation paired with a single electric shock was sufficient to induce conditioned avoidance (Beck et al., 2000) and that the timing of stimuli presentation greatly affects the direction of conditioned behavior (Tanimoto et al., 2004). We found that presentation of odor in pulses (pulsed odor flow) paired with pulsed electric shocks yielded stronger memory than the canonical constant odor procedure.
MATERIALS & METHODS
Fly rearing
Flies were reared on standard medium at 25 °C and 60% relative humidity under a 12 hr L/D cycle. All behavioral experiments were performed in a conditioned room kept at 25 °C or 32°C (for the experiment using shibirets) and 60% relative humidity under dim red light. The Cantonized w1118 strain, w(CS10), was used in all experiments unless otherwise noted. Canton-S and rutabaga2080 were used to examine the rutabaga memory defect. For the inactivation of the mushroom body synaptic activity, flies possessing UAS-shibirets and the Kenyon Cell-specific driver 247-Gal4 were used (kindly provided by H. Tanimoto and M. Heisenberg). Ablation of MB neuroblasts with Hydroxyurea was performed as described previously (Prokop and Technau, 1994; Wolf et al., 1998). One to three day-old flies were used in all experiments. All experiments are n ≥6. Two strains, isoCJ1 and CS-Quinn were also compared for STM score using the semi-automated apparatus.
Semi-automated olfactory conditioning apparatus
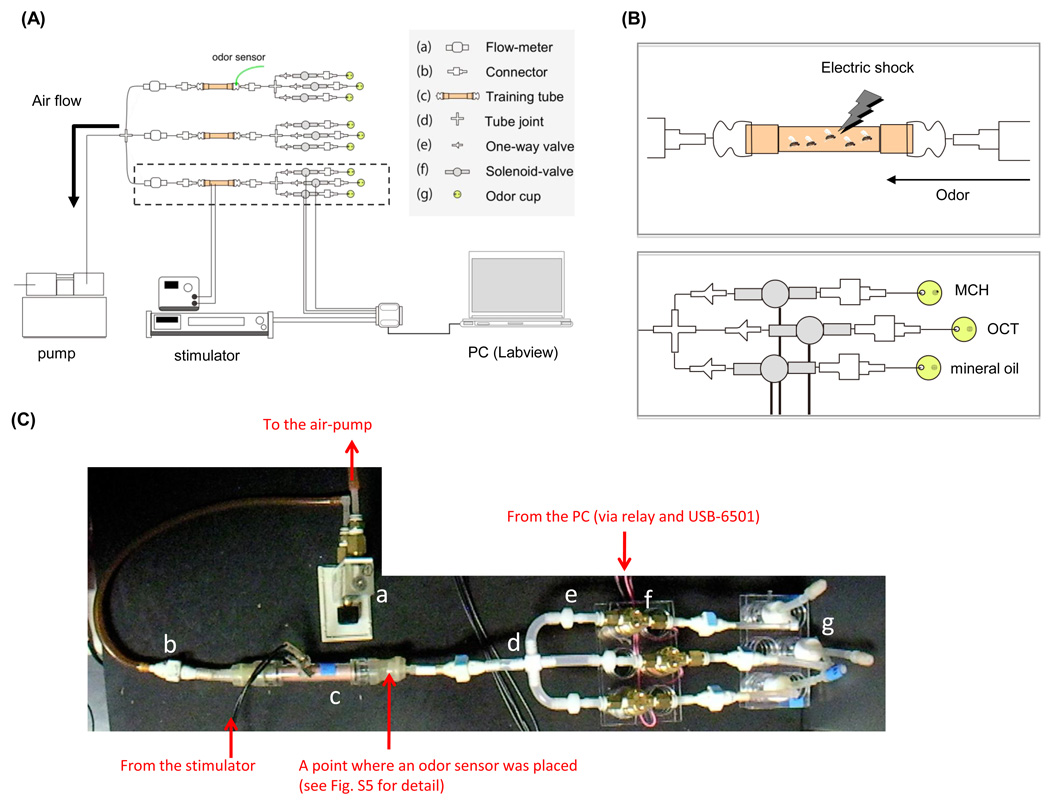
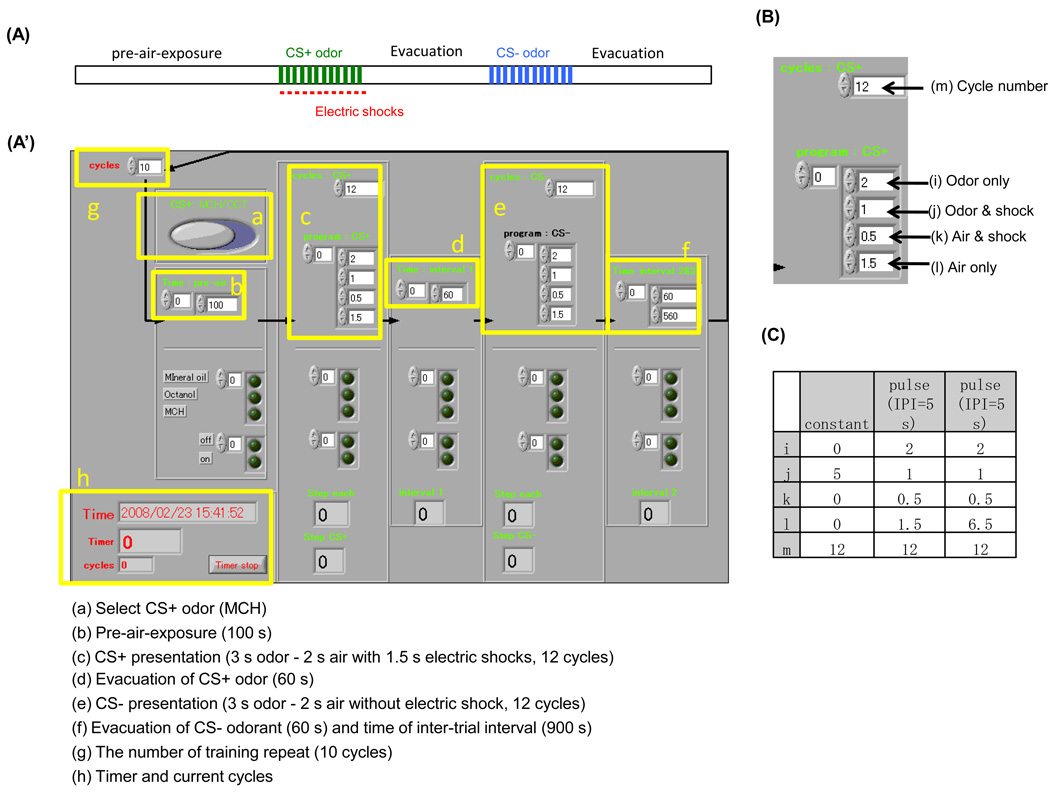
The apparatus consists of 6 units for conducting simultaneous stimulations of 100 flies per unit (Fig.1A). A single unit consists of one training tube with copper grids etched on flexible epoxy backing (Dudai et al., 1976, purchased from IKEDA std., JAPAN) and three solenoid valves (VDW350–6G–2–01, SMC Co.), each of which is connected to a polystyrene-made odor cup (Fig. S3) containing mineral oil (Fluka) and diluted odorants (Aldrich). We constructed two versions of the apparatus, one based on a pump (DA-20D, ULVAC Inc.) that continuously pulls air, generating continuous flow in the training tube from a downstream port. An alternative version of the apparatus uses forward air pressure from a compressed air tank, which is humidified and filtered (Motor Guard, Inc. M-30 filter) upstream of input to the solenoid valves. A large part of the research in this manuscript was done with the former air-supply system, that is, the vacuum-based system. But we found either methods worked equally well. Custom programs employing the Labview software package (Labview 8.2, National Instruments Co.) were utilized to control the gating of solenoid valves that allows air to go through only one of three liquids and into the training tube (Fig. 2,S2,S4). Labview also controlled the delivery of electric shocks through the regulation of a stimulator (Fig. 2,S2,S4, SEN-3301, NIHON KOHDEN co.). An interface (USB-6501, National Instruments Co.) connects the stimulator and solenoid valves to a PC (Fig. S2, National Instruments Co.). The 5V–trigger signals transmitted from USB-6501 were amplified to 12 V by a relay (PS7113–1A–A, NEC Electronics) to control the solenoid valves (Fig. S2A,B). The Labview control panel (Fig. 2) and a corresponding block diagram (Fig. S4) are shown. Additional details for the construction of the apparatus are available in Figure S1–Figure S5. Labview source-file is available online (http://www.iam.u-tokyo.ac.jp/fly/htmls/download.html).
Figure 1. Semi-automated conditioning apparatus.
(A) A schematic diagram of a semi-automated system for Drosophila olfactory conditioning. The system is composed of an air pump, a stimulator, a computer, and conditioning units (outlined by a dotted box). One conditioning unit is composed of three solenoid valves and a training tube with electric grids. Flies are loaded onto the training tube and sequentially receive electric shocks and air flow containing an odorant. Labview controls the gating of solenoid valves and an electric stimulator. To monitor the change in airborne odor concentration, an odor sensor is equipped adjacent to a training tube (green arrow). (B) Magnified illustrations showing an training tube (upper) and odor cups that are connected to solenoid valves (bottom). (C) A photo of a unit of the apparatus (encircled area in A). Letters correspond to parts shown in A. For details on building the apparatus, see supplemental Figures (Fig. S1–5).
Figure 2. Front panel of Labview program for olfactory conditioning.
(A) A schematic diagram showing pulsed odor delivery protocol used in this study. (A’) Labview front panel used for olfactory conditioning. Upper area is used to set experimental parameters (a-h). Lower area is used to monitor ongoing procedures. A current cycle and time are indicated in box h. (B) A magnified image of box c. (C) Parameters used for constant or pulsed odor delivery protocols. Letters (i-m) correspond to those in B. The same parameters were always used for CS+ and CS−.
Semiconductor-based odor sensor
A semiconductor-based odor sensor was employed to monitor odor concentration in the training tube (Fig.1A, 1C and Fig S6; TSG2620, Figaro Co.). Changes in the conductivity of the odor sensor were measured via voltage changes in a resistor connected in parallel to the odor sensor (details are in Fig. S6). Voltages were recorded with a data acquisition device (USB-6212, National Instruments Co.) at 27 Hz 1 hr after the odor sensor is electrified in order to stabilize sensor activity (Fig. S6). The sensor responds faithfully to changes in concentration of airborne odor in less than 0.3s (Fig. S8 and Fig. S10). Voltage change data tables for each trial were generated in Labview (Fig. S7). Data processing was done with Excel (Microsoft) and KaleidaGraph (Synergy Software).
Olfactory conditioning
The odorants 4-methylcyclohexanol (MCH) and 3-octanol (OCT) were diluted in mineral oil. Four ml of diluted odorant or mineral oil was loaded into the odor cup. A 105.0 V electric shock was used in all experiments. Speed of air-flow through each odor cup was adjusted to 1.6 L/min with a flow meter (KOJIMA INSTRUMENTS Inc.) before each experiment. For the training, approximately 100 flies were loaded into each training tube and exposed to sequential stimuli under the regulation of Labview (for detail, see Fig. 2). To fully evacuate any remaining odorant in the training tube during the conditioning, an airstream was delivered for 60 s following each odor presentation, which is longer than the typical protocol. Thirty second evacuation was employed when odor exposure was short (Fig. 3 and Fig. 8C). For spaced training, flies were subjected to ten training sessions with a 900 s interval between each session, unless otherwise noted.
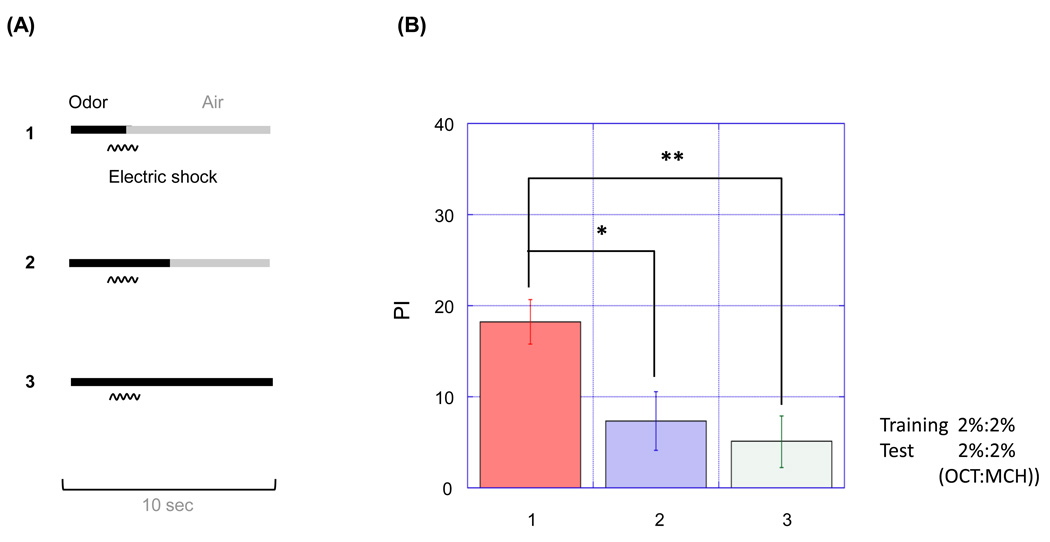
Figure 3. A prolonged odor presentation has a negative effect on memory formation.
(A) Schematic diagrams showing training protocols (protocol 1–3) for the examination of the timing of odor presentation. Animals were exposed to an odor pulse of 3s-, 5s- or 10s-duration (a black bar), which is accompanied with single 1.5 s electric shock (a wavy line). An electric shock was presented at the offset of the odor pulse (protocol 1) or before it (protocol 2 or 3). After 30 s of air-exposure to evacuate CS+ odor, flies were exposed to CS- odor for the same length as CS+. (B) Performance index (PI) of flies trained by protocol 1–3. PI was measured 3 min after the training. Protocol 1 induced conditioning aversion to CS+ odor, which was stronger than those of induced by protocol 2 or 3 (n=6, *p < 0.05, **p<0.01, t-test for each pair). Data are presented as mean ±SEM. The same concentrations of odorants were used in the training and test (2% OCT and 2% MCH).
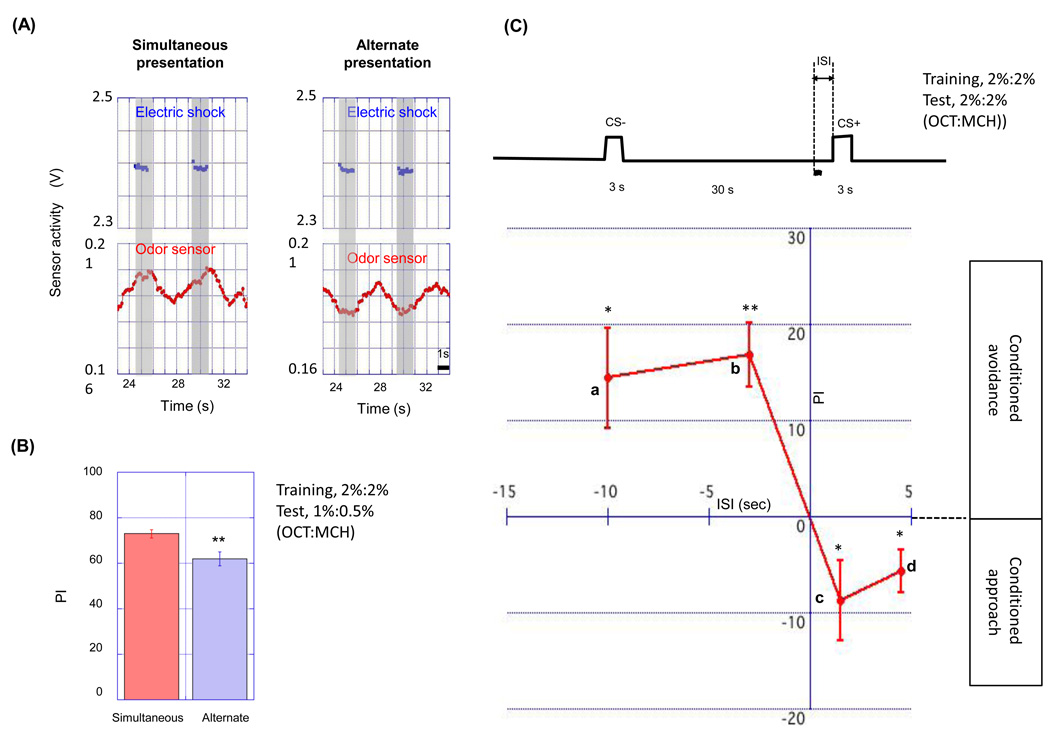
Figure 8. The importance of the timing between electric shocks and odor pulses in pulsed flow.
(A) Plots represent odor sensor activity (red dots) in the pulsed flow protocols. Blue dots represent electric shocks. In the simultaneous presentation program (left) electric shocks were presented when odor concentration was rising and were terminated when odor concentration was dropping. In alternate presentation program (right), odor pulses were presented between electric shocks. (B) PI of flies trained by simultaneous or alternate presentation protocol. PI was measured 3 min after the training. The alternate presentation program resulted in a lower performance than the simultaneous presentation program (n=10, **p<0.01, t-test). (C) The scheme of short training protocol with pulsed stimuli. Flies were pre-exposed to CS- odor 30 s before the CS+ presentation that is paired with an electric shock (a wavy line). The timings of an electric shock (1.5 s duration) and a CS+ odor pulse (3 s duration) were variously changed. Timings are represented by interstimulus interval (ISI). Plus ISI means that an electric shock precedes a CS+ odor pulse. On the other hand, minus ISI means that an electric shock was presented after the onset of CS+ odor pulse. An electric shock preceding odor pulse induced conditioned approach to CS+ (c, d in the graph), while an electric shock presented after the onset of odor pulse resulted in conditioned avoidance to CS+ (a, b in the graph). Data are presented as mean ±SEM (n=6 for each timing, *p<0.05, **p<0.01, t-test).
We first optimized basic parameters for olfactory conditioning such as flow-speed, voltage of electric shock and odor concentrations and found that 1.6L/min air-flow, 105 V electric shock and 2% odor concentration gave PI = 60 for 3-min olfactory aversive memory with the standard constant odor flow protocol. We then optimized stimuli presentation timing and found that pulsed presentation with 10s inter-pulse interval (IPI) gave the highest olfactory aversive memory in our apparatus (for detail, see Results). In order to precisely determine the optimal parameters, the gating valve was controlled according to the time when the odor reached the training tube, which was detected with a semiconductor-based alcohol sensor.
Odor discrimination tests were performed with the manual T-maze apparatus (Tully and Quinn, 1985) (Fig. S2C). Odor flow was continuously presented during the test with 1.4 L/min-airflow for each odor cup. The flow speed was chosen as it gave the highest PI for short-term memory. Odor concentration ratios were established so that naïve flies were evenly distributed in the T-maze. We used 8% OCT and 4% MCH, 2% OCT and 2% MCH, 1% OCT and 0.5% MCH, 0.1% OCT and 0.1% MCH in the test. The performance index (PI) was calculated as the number of flies avoiding the CS+ minus the number of flies avoiding the CS- divided by the total number of flies in the experiment. A single PI value is the average score from the population of flies tested with each odor as CS+ (MCH or OCT). Statistical analysis of PIs was conducted with t-test or ANOVA. ANOVAs were followed by planned pair wise comparisons between the relevant groups with a Tukey’s honestly significant difference post hoc test.
RESULTS
A pulsed odor airflow protocol for the aversive odor training
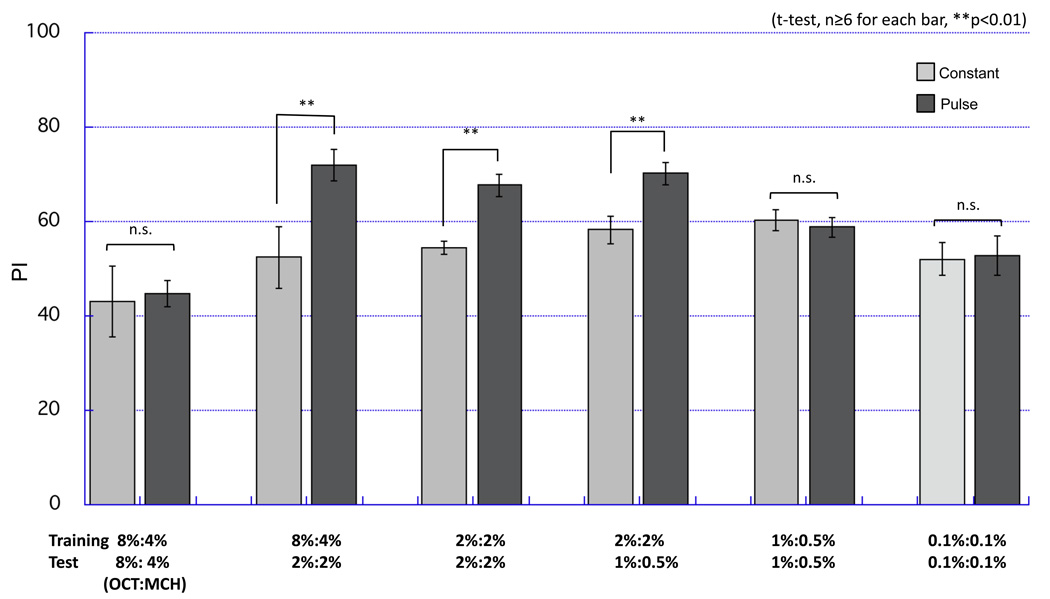
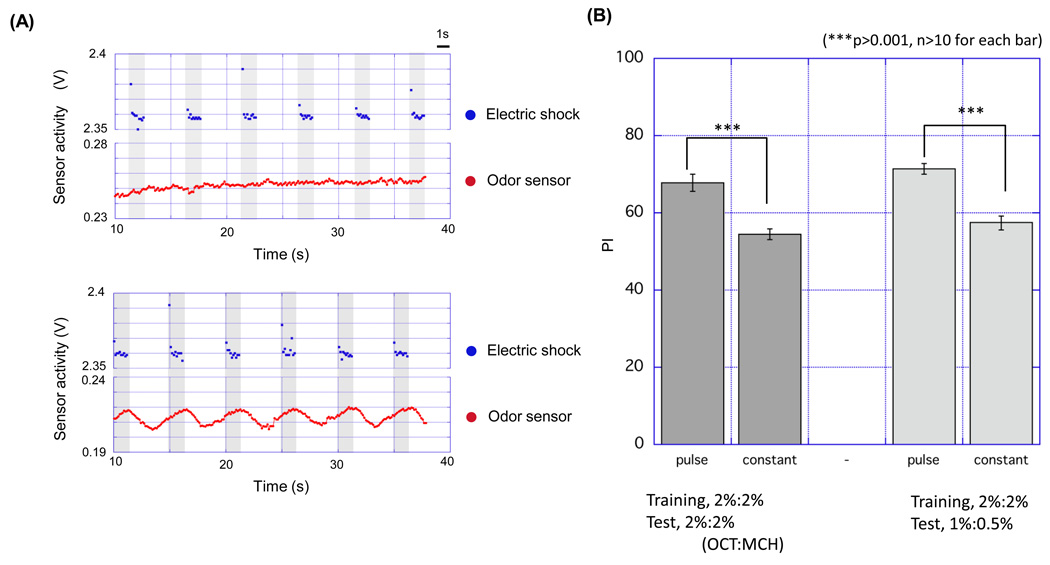
By using a computer-controlled semi-automated conditioning apparatus (Fig. 1,Fig. 2,Fig. S1–Fig. 5) we could adjust the timing of odor presentation during the conditioning protocol to optimize resulting memory performance (Fig. 3,Fig. 4,Fig. 5,Fig. 6). To determine the optimal paradigm for (CS+) odor presentation, flies were subjected to various lengths of odor duration and timing relative to a 1.5 s electric shock (US). In all cases of this assay, the CS− was presented to flies for the same length as CS+, but without electric shock, after 30 s of odor-evacuation. STM was then assayed in the odor-avoidance T-maze. We found that a short 3 s odor presentation induced conditioned avoidance to the CS+ better than longer odor presentations (5 s and 10 s; Fig. 3B). Odor presentation was terminated before the offset of electric shock for the 3 s odor presentation. Odor flow persisted after the termination of the shock in longer odor presentations. The data suggests that prolonged odorant presentation has a negative effect on learning and/or the termination of odor presentation should be synchronized with the termination of the electric shock for effective learning. This is consistent with a previously described method, in which a single electric shock delivered near the offset of 10 s odor presentation resulted in robust STM (Beck et al., 2000). We utilized the result to improve the conditioning protocol. In a typical protocol, odor is presented continuously for 60 s along with 12 pulses of shock in 5 s intervals. These initial results, however, suggested that STM would be greater if odor presentation ceased as each electric shock was terminated. We thus delivered the odor with a pulsed flow, in which the odor flow is presented in pulses synchronized with shocks (Fig. 4A). This pulsed flow protocol, using 2% MCH and 2% OCT concentrations, did indeed result in higher STM scores than with constant odor presentation (Fig. 4B, p<0.01). STM score was higher than constant flow when the length of each odor pulse was 4 s or 3 s (Fig. S11). The pulsed flow paradigm gave similar PI scores to ordinary constant odor flow at either very high (8% MCH and 4% OCT, Fig. 5) or very low (0.1% OCT and 0.1% MCH) odor concentrations (Fig. 5). These data differ from a previous study that found higher concentrations of odorants resulted in greater PI scores (Tully and Quinn, 1985). This discrepancy might be ascribed to differences in odor presentation; our apparatus employs bubbling air through the odorant solution while the previous study utilized odorant vapor pressure in an undisturbed odor cup, an approach that likely results in lower gas phase odor concentrations.
Figure 5. The effect of pulsed flow is odor concentration-dependent.
PI of flies trained by pulsed or constant flow in various odor concentrations. Pulsed flow gave higher scores than constant flow in a limited range of odor concentration. Constant flow did not give average-PI above 60 with odor concentrations we tested. PI was measured 3 min after training. Odor concentrations in the training and the test are indicated. Data are presented as mean ±SEM. n=6, **p< 0.01. t-test. Note that odor concentrations of MCH and OCT were adjusted so that naïve flies are evenly distributed in the T-maze.
Figure 4. Pulsed odor-flow improved learning score.
(A) Plots of odor sensor activity (red dots) and electric shock (blue dots) measured during the training. Airflow containing an odorant was delivered constantly (upper panel) or in pulses (bottom panel) to the training tube, which is accompanied 12 times with 1.5 s periodic electric shocks. In the pulsed flow protocol, electric shock presentations start after the rising of odor concentration and stop immediately before the falling of odor concentration. To generate the pulsed flow, a valve connected to an odor cup was opened for 3 s, followed by opening of a valve for 2 s that is connected to a cup containing mineral oil. (B) PI of flies trained by pulsed or constant flow. PI was examined 3 min after the training. Pulsed flow gave a higher PI than constant flow (n=10, p** < 0.01, t-test). Data are presented as mean ±SEM.
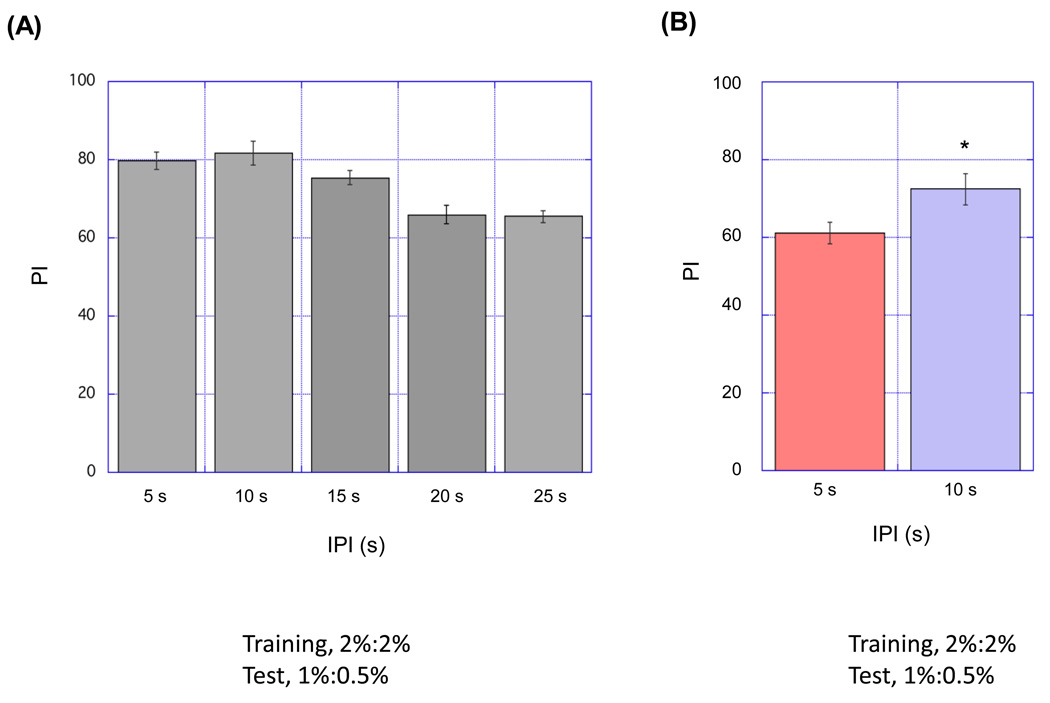
Figure 6. Effect of interpulse interval (IPI) on olfactory aversive learning.
(A) Effect of interpulse interval. PI was examined 3 min after the training. Presentation of a stimuli pair (3 s odor pulse with an electric shock) was repeated 12 times with indicated IPI, followed by 60 s of evacuation step and the same length of CS- pulses without shocks. The timing of electric shocks was the same as protocol 1 in Figure 2. IPI of 5 or 10 s gave a high learning performance. On the other hand, longer IPI (15, 25 or 35 s) resulted in a lower performance. (B) IPI optimization with weaker training protocol. Six stimuli pairs were presented with 5 s or 10 s IPI. PI was examined 3 min after the training. IPI of 10 s resulted in higher performance than 5 s IPI (n=6, *p < 0.05, t-test). Data are presented as mean ±SEM. Odor concentrations of odorants were different in the training and the test (2% MCH and 2%OCT in the training and 0.5% MCH and 1% OCT in the test).
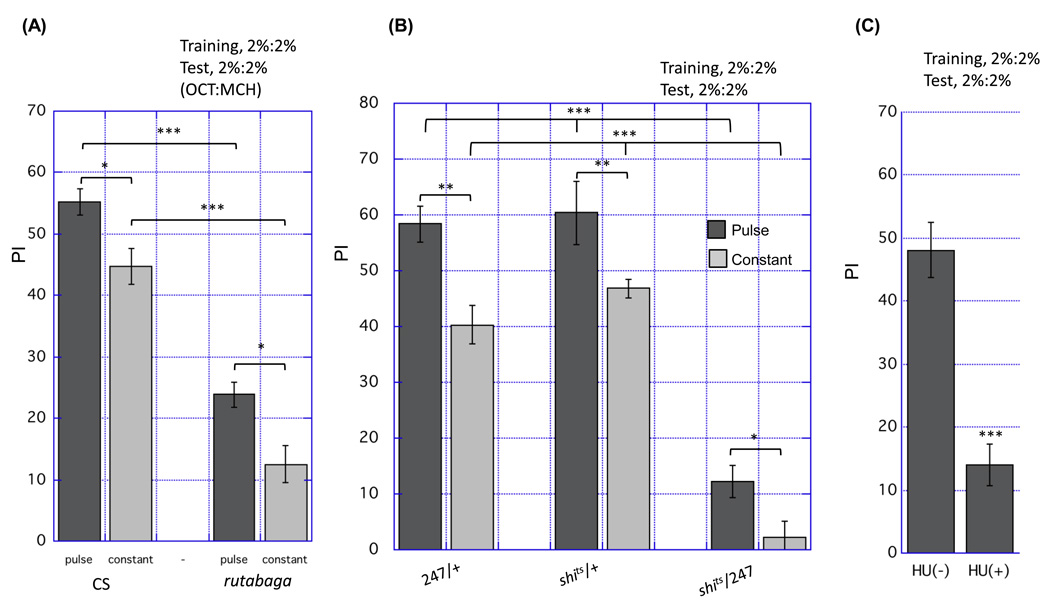
We also considered a second odor presentation parameter in order to optimize the acquisition of STM. The pulsed flow protocol can be considered a sequence of paired presentations of an odor pulse and a shock. We examined the optimal interval between these paired presentations (the interpulse interval, IPI). In a given training session, paired odor and shock pulses were presented 12 times, with various IPI. We first found that an IPI longer than 20 s resulted in a reduction in the performance index (Fig. 6A), whereas the best performance was achieved with either a 5 s or 10 s IPI. To more sensitively detect a possible difference in training with a 5 s or 10 s IPI, we employed a weaker training protocol in which only six paired-pulses were presented. In this case, 10 s IPI was more effective than a 5 s IPI (Fig. 6B). Altogether, increasing synchronization of stimuli with pulsed flow protocol and adjusting the time interval between paired pulses was effective in improving learning efficiency in the automated apparatus. Furthermore, memory formed with this apparatus and optimized protocol had properties described in previous studies; a learning mutant, rutabaga (Livingstone et al., 1984; Tully and Quinn, 1985; Levin et al., 1992; Zars et al., 2000) showed a severe learning defect (Fig. 7A) and memory was dependent on the mushroom body (Fig. 7B,C), which is thought to be the primary neural component in olfactory memory (de Belle and Heisenberg, 1994; Zars et al., 2000; Dubnau et al., 2001; Kitamoto, 2001; McGuire et al., 2001; Pascual and Preat, 2001; Schwaerzel et al., 2002; Heisenberg, 2003). Pulsed odor presentation resulted in significantly higher memory scores than constant odor presentation in rutabata flies and flies with impaired MB function (Fig. 7). These data suggest that the cAMP pathway and the MB are not involved in the molecular or the cellular mechanism underlying an effect of pulsed odor presentation on STM.
Figure 7. Requirement of rutabaga and the MB in the formation of memory induced by the apparatus.
(A) One hour-memory was measured in Canton-S (CS) or rutabaga mutant flies that were trained with pulsed or constant flow protocol. Apparent reduction in memory was observed in rutabaga mutant (n=8 for each, ***p < 0.001, t-test). Pulsed flow gave higher memory score than constant flow in CS or rutabaga mutants (*p<0.05). (B) Flies impaired in MB-output were trained with pulsed or constant flow protocol and 1-hr memory was measured. Neurotransmissions from the MBs were inhibited by a temperature-sensitive form of shibire transgene (shits), which was driven by MB-specific driver Gal4–247. Flies possessing both shits and Gal4–247 showed lower 1 hr-memory at 32 °C than flies having only shits or Gal4–247 (n=6, ***p<0.001, ANOVA). Pulsed flow gave higher memory score than constant flow in control flies or flies impaired in MB-output (n=6, *p<0.05, **p<0.01, t-test). (C) Flies subjected to Hydroxyurea (HU) were trained with pulsed flow protocol. MB-ablated flies with HU resulted in lower 1 hr memory than control flies (n=6, ***p<0.001, t-test).
Odor concentration and conditioned approach
Why does a pulsed presentation increase PI? We noticed that odor concentrations during the training were different between pulsed and constant flow; peak odor concentration of pulsed flow was lower than that of constant flow (Fig. 4). This raised the possibility that a difference in odor concentration between the two methods underlies the increased STM observed with pulsed odor presentation. Previously it was shown that the maximal conditioned avoidance was obtained when the odor concentrations in the training and the test are matched (Masek et al., 2008). This raised the possibility that odor concentration in the test might be more closely matched to training when pulsed odor presentation was employed. We had observed that pulsed odor presentation generated a higher PI than constant odor when the same odorant concentrations were used in both the training and the test (Fig. 4B, Fig. 2% OCT and 2% MCH). When we measured odor concentration in this situation, the concentration in the training tube was lower than in the test tube when training odor was delivered in a pulsed fashion while they were more closely matched when the training odor was delivered constantly (Fig. S9). Thus pulsed odor presentation resulted in a higher PI score than constant presentation, even when the concentrations during training and test were mismatched. Another possibility was that odor concentrations were too high for efficient olfactory learning during constant odor presentation . If this was true, the PI score should be improved by decreasing the odor concentration. However, this was not the case (Fig. 5). Based on these data we believe it is unlikely that pulsed odor presentation improves STM performance as a result of an effectively reduced odor presentation.
We next examined whether stimulus presentation timing contributes to the effect of pulsed odor delivery on STM. As was shown in Figure 3, prolonged odor presentation had a negative effect on olfactory learning. One possible explanation for this negative effect is a phenomenon that has been observed when the US is presented before the offset of the CS+ odor. This has been found to induce a ‘conditioned approach’ to the CS+ odorant in the odor-choice assay (Tanimoto et al., 2004). To examine the contribution of stimulus presentation timing to the effect of pulsed odor delivery, we shifted the timing of odor delivery so that electric shocks were given between odor pulses. If presentation timing is important, this treatment should affect learning performance. Indeed, this “alternate presentation” protocol resulted in a lower level of learning as compared to “simultaneous presentation” protocol (Fig. 8A,B). The total lengths of odorant and shock exposure were equal in both protocols. Therefore the timing between each odor pulse and electric shock must contribute to the effect of pulsed odor delivery on olfactory learning. Since the electric shocks were presented before the increase of odor concentration in the alternate presentation protocol (Fig. 8A, right diagram), its reduced learning score might reflect a higher level of conditioned approach than would occur in the simultaneous presentation protocol. One important basis for this notion is that a single presentation of offset odor, coupled to electric shock, would be sufficient to induce the conditioned approach phenomenon. We examined this directly by shifting the timing of odor pulse and electric shock and found that an electric shock presented before the 3 s odor pulse induced conditioned approach to the odor while an electric shock presented after 3 s odor pulse induced conditioned avoidance of the odor (Fig. 8C). Therefore, a single episode of odor and electric shock presentation is sufficient to induce conditioned approach. These data are consistent with the hypothesis that pulsed odor delivery is efficient because it minimizes the induction of conditioned approach. In the standard procedure, where continuous odor delivery is used, an odor is presented even when electric shocks are absent. Our data raises the possibility that an uncoupled presentation might lead to the induction of a both conditioned approach and conditioned avoidance, resulting in a reduced net avoidance in the T-maze assay and hence reduced apparent learning.
Automated induction of longer-term memory
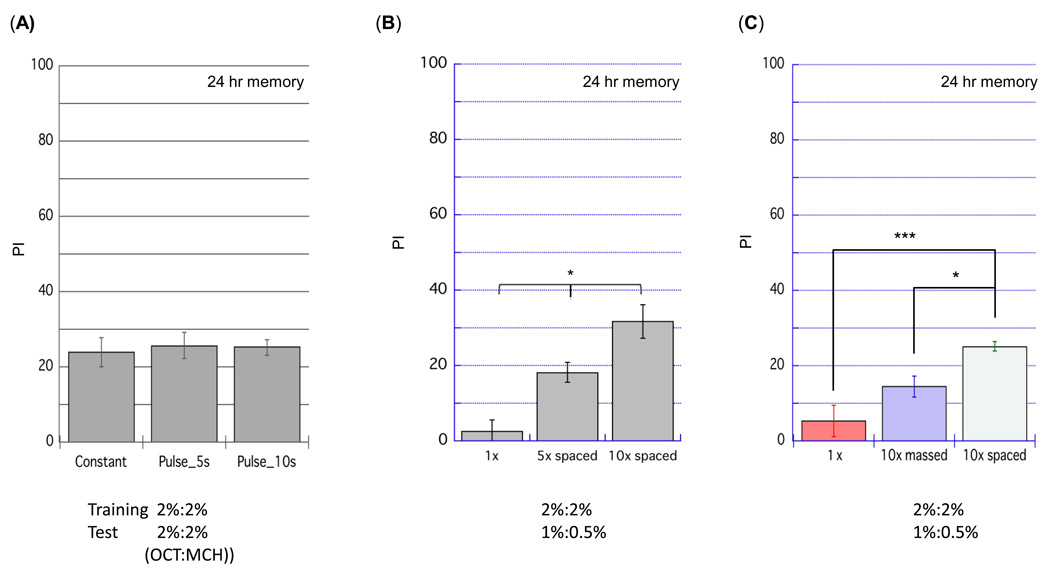
Longer-term memory has been divided into two phases; anesthesia resistant-memory (ARM) and long-term memory (LTM). LTM is induced by a spaced-training protocol in which the training session of the STM protocol is repeated ten times, with each episode separated by a fifteen minute rest period (Tully et al., 1994). Repeated training episodes without the intervening rest period induces ARM, a more labile form of memory. As we observed with STM induction, the semi-automated apparatus induced robust 24-hr memory when it performed a spaced training protocol employing constant CS+ odor presentation (Fig. 9). We then asked whether pulsed delivery of the CS+ odor enhanced 24-hr memory. In contrast to the observation with STM, there was no difference in 24-hr memory scores with the pulsed and constant odor delivery methods (Fig. 9A). We also confirmed that, with either protocol, memory retention was greater in proportion to the number of training sessions (Fig. 9B) and that spaced training induced a higher level of memory than ‘massed’ training, where no rest interval is introduced between the ten training sessions (Fig. 9C). These results demonstrate that the apparatus and the modified training protocol developed above are applicable to the study of longer-term memories.
Figure 9. 24-hr memory induced by semi-automated apparatus.
(A) Flies were conditioned by the spaced training protocol with pulsed flow (IPI= 5 s or 10 s) or constant flow. The pulsed flow protocol induced an identical level of LTM to that with constant flow. (B) Repeated training induced strong 24 hr memory. Memory retention became greater in proportion to the number of training cycles (n=6, *p < 0.05, ANOVA). (C) Spaced training with 15 min interval between training sessions induced stronger 24 hr memory than massed training in which training sessions were repeated without interval. (n=6, *p <0.05, ***p <0.001, ANOVA). Data are presented as mean ±SEM.
DISCUSSION
Associative memory of the coincident presentation of an odor and electric shock has been extensively studied at the cellular, molecular and physiological levels in Drosophila. It is currently one of the best systems in which to seek a comprehensive description of the interplay between the molecular mechanisms and cellular mechanics of information encoding and storage. To facilitate progress in this area, we devised a simple, low-cost semi-automated training apparatus that employs few custom parts. The modular design of the apparatus accommodates scale-up to multiple training chambers, a feature that is especially useful with the long-term training protocol, which proceeds over the course of nearly two hours. The apparatus yields conditioned animals that perform as well in the standard T-maze odor choice task as those obtained with the manual device originally described by Quinn and colleagues (Tully and Quinn, 1985) in either short-term or long-term memory protocols. The apparatus also returned relatively precise results. The standard error of PI from the results of three successive training episodes using the same training chamber was 1.42 (n=3). The standard error was greater (1.81 in PI for STM, n=3) with data from three different chambers. Although air-flow rate and odor concentration were adjusted before the experiments with different training chambers, slight differences in difficult to control variables might result in the larger deviations in this case. We found that results with the apparatus were constant over a period of several months of operation (Fig. S12). Some improvements that might further increase the system’s precision include monitoring electric shock delivery and adjusting air flow rate in the training chamber with a mass flow controller. Introducing feedback elements to monitor these variables would also enable us to successively adjust conditioning parameters in an effort to further improve training results, as well as to explore the effects of specific alterations in the training paradigm on memory formation and retention.
The apparatus is equipped with a semiconductor-based odor sensor, which was used to explore the effect of odor delivery kinetics on the formation of STM. Using the sensor to monitor odorant arrival within the chamber, and an automated delivery system to manipulate it, we found that pulsed delivery of the odorant was more effective than continuous odor delivery in inducing olfactory STM. Prolonged odor presentation that persisted after the termination of electric shock had a negative effect on learning. The significance of presentation timing has been documented in several learning paradigms. In the Aplysia gill withdrawal reflex paradigm, electric shocks to the tail (US) must be given immediately after the stimulation of gills (CS) to induce sensitization (Pinsker et al., 1973). In the fear conditioning paradigm of rodents, the onset of electric shocks (US) are timed to coincide with the offset of a tone (CS) (Quirk et al., 1995). An example of the significance of stimulus timing in Drosophila is the observation of Tanimoto et al (2004) that odor presentation immediately following the termination of electric shock results in a conditioned approach to the odor. That is, the odor (CS+) is associated with the cessation of an aversive stimulus, and thus considered a reward. It was previously suggested that a two-component memory might be induced by olfactory aversive training, which is a mixed memory of conditioned aversion and conditioned approach to the odor (Diegelmann et al., 2006). We speculated that pulsed odor delivery might induce only a “single component” aversive memory while the continuous delivery induces “mixed memory components”. We showed that even a single electric shock pulse preceding an odor pulse was enough to induce conditioned approach. Considering that the procedure with the constant odor delivery has uncoupled odor presentations between electric shocks, this uncoupled presentation might contribute to both conditioned approach and conditioned avoidance. Pulsed odor delivery might minimize the induction of conditioned approach.
The effect of pulsed odor presentation might also be explained by desensitization of olfactory receptor neurons (ORNs), which has been shown to occur under conditions similar to odorant delivery during the standard continuous protocol (Stortkuhl et al., 1999). That is, pulsed odor delivery might increase learning efficiency by attenuating desensitization to the CS+. However, a recent study did not reproduce a decrease of an experience-dependent odor response (Acevedo et al., 2007). Moreover, our data is difficult to interpret as an effect of adaptation, because the enhanced learning with pulsed odor delivery depended on the relative timing of the odor pulse and electric shock (Figure 3 and Figure 8A). Therefore sensory adaptation is unlikely to the enhanced memory performance found with the pulsed odor delivery protocol.
The semi-automated apparatus enabled us to precisely examine stimulus presentation timing at a level that would have been difficult with the manual apparatus. We detected the formation of conditioned approach with a single pulse protocol. Memory formation responded even to a slight difference in presentation timing; there was a sharp transition of conditioned avoidance to conditioned approach. Flies also strictly responded to the timing of odor flow termination. These data reveal the importance of stimulus presentation timing in Drosophila olfactory learning and a temporal acuity of the fly’s capacity for coincidence detection.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Dr. S. Newfeld for his critical reading of the manuscript. We would like to thank Dr. M. Heisenberg, Dr. H. Tanimoto, Dr. M. Saitoe, Dr. R. Kanzaki and Dr. N. Ando for valuable advice and S. Knapek and Y. Aso for coaching us with behavioral experiments. We would also like to thank Dr. Ed Soucy of the Harvard Center for Brain Science for help with computer automation and electrical design. This work was supported by grants-in-aid from the Ministry of Education, Science and Culture of Japan (S.M. and T.T.) and NIH/NIMH grant R01MH081294 to S.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acevedo SF, Froudarakis EI, Tsiorva AA, Skoulakis EM. Distinct neuronal circuits mediate experience-dependent, non-associative osmotactic responses in Drosophila. Mol Cell Neurosci. 2007;34:378–389. doi: 10.1016/j.mcn.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Beck CD, Schroeder B, Davis RL. Learning performance of normal and mutant Drosophila after repeated conditioning trials with discrete stimuli. J Neurosci. 2000;20:2944–2953. doi: 10.1523/JNEUROSCI.20-08-02944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RL. Olfactory memory formation in Drosophila: from molecular to systems neuroscience. Annu Rev Neurosci. 2005;28:275–302. doi: 10.1146/annurev.neuro.28.061604.135651. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Didelot G, Molinari F, Tchenio P, Comas D, Milhiet E, Munnich A, Colleaux L, Preat T. Tequila, a neurotrypsin ortholog, regulates long-term memory formation in Drosophila. Science. 2006;313:851–853. doi: 10.1126/science.1127215. [DOI] [PubMed] [Google Scholar]
- Diegelmann S, Zars M, Zars T. Genetic dissociation of acquisition and memory strength in the heat-box spatial learning paradigm in Drosophila. Learn Mem. 2006;13:72–83. doi: 10.1101/lm.45506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Chiang AS, Grady L, Barditch J, Gossweiler S, McNeil J, Smith P, Buldoc F, Scott R, Certa U, Broger C, Tully T. The staufen/pumilio pathway is involved in Drosophila long-term memory. Curr Biol. 2003;13:286–296. doi: 10.1016/s0960-9822(03)00064-2. [DOI] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Dudai Y, Jan YN, Byers D, Quinn WG, Benzer S. dunce, a mutant of Drosophila deficient in learning. Proc Natl Acad Sci U S A. 1976;73:1684–1688. doi: 10.1073/pnas.73.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber B, Tanimoto H, Heisenberg M. An engram found? Evaluating the evidence from fruit flies. Curr Opin Neurobiol. 2004;14:737–744. doi: 10.1016/j.conb.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- Keene AC, Waddell S. Drosophila olfactory memory: single genes to complex neural circuits. Nat Rev Neurosci. 2007;8:341–354. doi: 10.1038/nrn2098. [DOI] [PubMed] [Google Scholar]
- Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- Levin LR, Han PL, Hwang PM, Feinstein PG, Davis RL, Reed RR. The Drosophila learning and memory gene rutabaga encodes a Ca2+/Calmodulin-responsive adenylyl cyclase. Cell. 1992;68:479–489. doi: 10.1016/0092-8674(92)90185-f. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Sziber PP, Quinn WG. Loss of calcium/calmodulin responsiveness in adenylate cyclase of rutabaga, a Drosophila learning mutant. Cell. 1984;37:205–215. doi: 10.1016/0092-8674(84)90316-7. [DOI] [PubMed] [Google Scholar]
- Margulies C, Tully T, Dubnau J. Deconstructing memory in Drosophila. Curr Biol. 2005;15:R700–R713. doi: 10.1016/j.cub.2005.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masek P, Heisenberg M. Distinct memories of odor intensity and quality in Drosophila. Proc Natl Acad Sci U S A. 2008;105:15985–15990. doi: 10.1073/pnas.0804086105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signaling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McGuire SE, Roman G, Davis RL. Gene expression systems in Drosophila: a synthesis of time and space. Trends Genet. 2004;20:384–391. doi: 10.1016/j.tig.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Pascual A, Preat T. Localization of long-term memory within the Drosophila mushroom body. Science. 2001;294:1115–1117. doi: 10.1126/science.1064200. [DOI] [PubMed] [Google Scholar]
- Pinsker HM, Hening WA, Carew TJ, Kandel ER. Long-term sensitization of a defensive withdrawal reflex in Aplysia. Science. 1973;182:1039–1042. doi: 10.1126/science.182.4116.1039. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. Normal function of the mushroom body defect gene of Drosophila is required for the regulation of the number and proliferation of neuroblasts. Dev biol. 161:321–337. doi: 10.1006/dbio.1994.1034. [DOI] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behavior in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–1039. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- Schwaerzel M, Heisenberg M, Zars T. Extinction antagonizes olfactory memory at the subcellular level. Neuron. 2002;35:951–960. doi: 10.1016/s0896-6273(02)00832-2. [DOI] [PubMed] [Google Scholar]
- Stortkuhl KF, Hovemann BT, Carlson JR. Olfactory adaptation depends on the Trp Ca2+ channel in Drosophila. J Neurosci. 1999;19:4839–4846. doi: 10.1523/JNEUROSCI.19-12-04839.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol [A] 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Wolf R, Wittig T, Liu L, Wustmann G, Eyding D, Heisenberg M. Drosophila mushroom bodies are dispensable for visual, tactile and motor learning. Learn Mem. 1998;5:166–178. [PMC free article] [PubMed] [Google Scholar]
- Zarsn T, Fischer M, Schulz R, Heisenberg M. Localization of a short-term memory in Drosophila. Science. 2000;288:672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.