Abstract
Chronic inflammatory diseases such as autoimmune and bacterial infections are associated with an elevated risk of ocular inflammation. Ciliary epithelial cells that play an important role in maintaining aqueous humor dynamics and homeostasis of anterior segment of eye are continuously exposed to inflammatory markers during infections and injury. Lipopolysachharide (LPS), a gram negative bacterial endotoxin, dysregulates aqueous humor (AqH) homeostasis by inducing inflammatory changes. We have investigated how inhibition of a polyol pathway enzyme, aldose reductase (AR), alters LPS-induced inflammatory changes in human non-pigmented ciliary epithelial cells (hNPECs). The stimulation of hNPECs with LPS (1 μg/ml) caused increased secretion of inflammatory markers such as PGE2 and NO in the culture medium as well as increased expression of COX-2 and iNOS proteins in cell extracts. LPS also increased phosphorylation of MAPKs (ERK1/2) and SAPK/JNK and activation of redox-sensitive transcription factors NF-κB and AP-1 in hNPECs and inhibition of AR by zopolrestat and sorbinil ameliorated these changes. Further, LPS-induced decrease in the expression of Na/K-ATPase in hNPECs was restored by AR inhibitors. Similar results were observed in ciliary bodies of LPS-injected rats. Taken together, our results suggest that AR plays an important role in the LPS-induced inflammatory changes in hNPECs and that inhibition of AR could be a novel therapeutic approach for ocular inflammation.
Keywords: hNPECs, oxidative stress, LPS, Aldose reductase, inflammation
1. Introduction
Ocular inflammation due to injury or inflammatory diseases such as autoimmune and bacterial infections is a major cause of severe visual impairment that accounts for 10–15% of all cases of total blindness in US (Nussenblatt, 1990; Curi et al., 2005). Current therapies such as corticosteroids or other drugs that suppress the immune system to control the inflammation have many serious side effects, and severely diminish the patient’s quality of life (Dukes, 1996; Samudre et al., 2004). Inflammation is invariably associated with increased oxidative stress by elevated reactive oxygen species (ROS), which could alter cellular and molecular targets and pathways crucial to normal tissue homeostasis (Dröge, 2002; Valko et al., 2007). Despite multiple studies showing that ROS and oxidative stress are significant components of such pathological conditions, the mechanisms by which ROS regulate cell functions or induce tissue injury remain unclear, and to date no suitable antioxidant is available with both hydrophilic and hydrophobic properties that could readily be delivered to cells. Further, most antioxidants act as pro-oxidants at higher doses (Meffert, 2008). Therefore, understanding the ROS-mediated signaling mechanisms involved in inflammation is necessary to develop better therapeutic strategies. Lipopolysaccharide (LPS), a major component of the Gram-negative bacterial outer membrane, is a principal mediator of host innate immune responses (Bozinovski et al., 2004; Ulevitch et al., 2004). LPS causes many of the pathologic effects by stimulating host cells to synthesize large quantities of bioactive inflammatory mediators such as nitric oxide, prostaglandins, and cytokines, which by autocrine and paracrine effects propagate ocular inflammatory signals leading to tissue damage and death. This can be a serious threat to the vision and could result in the temporary visual impairment or permanent vision loss.
The ciliary epithelium, a bi-layer structure, consists of two types of cells in an apex to apex orientation. The inner layer of pigmented ciliary epithelium (PE) faces the stromal blood and the outer non-pigmented ciliary epithelial layer faces the aqueous humor. The solute transport across the bilayer gives rise to osmotic water movement, resulting in aqueous humor (AqH) formation. Aqueous humor supplies nutrients to non-vascular tissues of the eye such as the lens and the cornea (Reddy, 2000; Schutte, 1980), and also maintains intraocular pressure (McLaren, 2009). The hNPECs possess tight junctions that act as a blood-aqueous barrier (Hirsch et al, 1977; Raviola, 1974, 1977). AqH synthesis and secretion requires coordinated action of several different ion transport mechanisms and ion channels in the ciliary epithelium (Civan, 1998; To et al., 2002). The major transport proteins that have been identified to be associated with aqueous humor formation include Na/K-ATPase, Na-K-2Cl co-transporter, Na+/H+ and Cl−/HCO3− exchangers and chloride channels (Jacob and Civan, 1996; Civan, 1998; Counillon et al., 2000). In ciliary epithelium both NPE and PE cells express Na/K-ATPase at their basolateral membrane, but Na/K-ATPase activity is two- to three-fold greater in hNPECs cells (Krupin et al., 1984; Riley and Kishida, 1986; Ghosh et al., 1990). The expression of channel proteins is regulated during oxidative stress and disease conditions (Weng et al., 2002; Wills et al., 2000). For example, an impaired Na/K-ATPase activity in RBC has been proposed to be a potential marker of predisposition to diabetic neuropathy (Raccah et al., 1992). A restriction fragment length polymorphism (RFLP) study of the ATP1-A1 gene encoding for the Na/K ATPase’s alpha-1 isoform has shown that a low Na/K-ATPase activity in the red blood cells of type-1 diabetic patients is associated with diabetic neuropathy (Jannot et al., 2002). Although, AR inhibition has been shown to restore Na/K-ATPase activity in peripheral nerve of diabetic rats (Simpson and Hawthorne, 1988), the role of AR in the modulation of ciliary body’s Na/K-ATPase, which is affected by various stimuli and causes modulation in its activation and expression, has not been investigated so far. Therefore, it is pertinent to investigate the role of AR in the bacterial endotoxin-induced oxidative stress that may affect the cellular integrity of ciliary bodies which play an important role in the AqH synthesis and dynamics. We have shown earlier that cytokine, and bacterial endotoxin signaling in human lens epithelial cells (HLEC) is regulated by AR, and inhibition of AR blocks the cytotoxicity induced by these stimuli (Ramana et al., 2003; Pladzyk et al., 2006). Inhibition of AR has been shown to prevent the activation of NF-κB by endotoxin and therefore prevents the transcription of inflammatory markers thereby protects the cells from oxidative stress and cytotoxicity (Pladzyk et al., 2006; Ramana et al., 2006; Yadav et al., 2007). In the present study we have shown that LPS caused the activation of MAPK/PKC/NF-κB pathway that modulates various inflammatory proteins and Na/K-ATPase leading to cytotoxicity in hNPECs. Inhibition of AR prevented these effects suggesting that AR inhibition could be important in maintaining LPS-induced AqH disbalance and it associated inflammatory complications.
2. Material and Methods
2.1. Materials
Dulbecco’s modified Eagle’s medium with 4 mM L-glutamine and 4.5 g/l glucose, phosphate-buffered saline (PBS), gentamicin solution, and trypsin, were purchased from Invitrogen-Gibco (Grand Island, NY). Antibodies against COX-2, iNOS, ICAM-1, Na/K-ATPase-alpha isoform, glyceraldehyde phosphate dehydrogenase (GAPDH) and pan-cadherin were from Santa Cruz Biotechnology (Santa Cruz, CA); and antibodies against phospho-PLCβ3, phospho-PKC-βII phospho-p38, phospho-ERK1/2 and phospho-JNK and total p38, ERK1/2 and JNK were purchased from Cell Signaling Inc. (Beverly, MA). Sorbinil and zopolrestat were the gifts of Pfizer (New York, NY). The transfection reagent (LipofectAMINE Plus) and reduced-serum cell-culturing medium (OptiMEM) were obtained from Invitrogen-Life Technologies (Gaithersburg, MD); consensus oligonucleotides for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) and AP1 (5′-CGCTTGATGAGTCAGCCGGAA-3′) transcription factors from Promega Corp. (Madison, WI); nitrite/nitrate and PGE2 assay kits from Cayman Chemical Inc. (Ann Arbor, MI). 4-hydroxynonenal (HNE) was purchased from EMD Biosciences, Inc. (San Diego, CA). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) and other reagents used in the electrophoretic mobility gel shift assay (EMSA) and Western blot analysis were obtained from Sigma-Aldrich (St. Louis, MO). All other reagents used were of analytical grade.
2.2. Cell culture and Treatment
SV40-transformed hNPECs were kindly provided by Dr M. Coca-Prados (New Haven, CT). Cells were maintained in Dulbecco’s modified Eagle’s medium with high glucose (4.5 g/l) supplemented with 10% heat-inactivated fetal calf serum, and 50 μg/ml gentimycin sulphate. The cells were grown in a humidified incubator at 37°C and 5% CO2. All incubations were performed in serum-free medium. The cells were pretreated with 20 μM AR inhibitors, sorbinil or zopolrestat for overnight in serum-free medium and subsequently stimulated with 1 μg/mL LPS from E. coli for 24 h, unless otherwise stated.
2.3. Western Blot Analysis
The hNPECs were washed twice with ice-cold PBS and lysed in ice-cold lysis buffer containing 50 mM HEPES [pH 7.6], 10 mM KCl, 0.5% NP-40, 1 mM DTT, 1 mM phenylmethylsulfonylfluoride (PMSF), and 1:100 dilution of phosphatase and protease inhibitor cocktail (Sigma, Saint Louise, MO) for 15 min at 4°C. The crude lysates were cleared by centrifugation at 12,000 g for 10 min at 4°C. Aliquots of the lysates containing equal amount of protein (40 μg) were mixed with 5× SDS sample buffer and incubated in boiling water bath for 5 min. Lysates were separated on 10% SDS-polyacrylamide gels and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were then incubated in blocking solution containing 5% wt/vol dried fat-free milk and 0.1% vol/vol Tween-20 in Tris-buffered Saline. Subsequently, the membranes were incubated with anti-COX-2, -iNOS, -ICAM, -Na/K-ATPase, -AR, -phospho-p38, -phospho-ERK1/2 and -phospho-SAPK/JNK and -p38, -ERK1/2 and -SAPK/JNK and -GAPDH antibodies. The membranes were washed and probed with horseradish peroxidase- conjugated secondary antibodies (GE Healthcare, Piscataway, NJ) and visualized by chemiluminescence (Pierce biotechnology, Rockford, IL). To determine the phosphorylation of PLC-β3 and PKC-βII, the membrane fraction was prepared and equal amount of protein (15 μg) was separated on SDS-PAGE followed by immunoblotting using antibodies against phospho-PLC-β3 and -PKC-βII.
2.4. Electrophoretic Mobility Shift Assay (EMSA)
The hNPECs were pretreated with or without AR inhibitors for 24 h in starving medium, followed by treatment with LPS (1 μg/ml) for 2 h at 37°C. The nuclear extracts were prepared as described earlier (Pladzyk et al., 2006). Briefly, hNPECs were harvested and washed with ice-cold PBS and suspended in 0.1 ml of hypotonic lysis buffer containing protease inhibitors for 10 min. The cells were then lysed with 5 μl of 10% Nonidet P-40. The homogenate was centrifuged, and supernatant containing the cytoplasmic extracts was aspirated and nuclear pellet was resuspended in 50 μl ice-cold nuclear extraction buffer containing 50mM HEPES (ph 7.9), 40 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM Na3VO4, 2 mM DTT, 1 mM PMSF, and 1% glycerol and protease inhibitor (1:100 dilution). After 30 min of intermittent mixing, the extract was centrifuged, and supernatants containing nuclear extracts were secured. The protein content was measured by the Bradford method. The Consensus oligonucleotides for NF-κB and AP-1 transcription factors were 5′-end labeled using T4 polynucleotide kinase. EMSA was performed as described (Pladzyk et al., 2006). The specificity of the assay was examined by competition with an excess of unlabeled oligonucleotide and supershift assays with antibodies to p65.
2.5. Transient transfection and NF-κB-Dependent Secretory Alkaline Phosphatase (SEAP) Expression Assay
To examine NF-κB promoter activity in hNPECs in response to LPS treatment, cells (2.5×106 cells/well in 6-well plate) in DMEM (with 10% FBS) were transfected with pNF-κB-SEAP2-construct and pTAL-SEAP control plasmid (Clontech, USA) using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA) following suppliers instructions. After 6 h of transfection, cells were incubated with LPS (1 μg/ml) or HNE, GS-HNE-ester or GS-DHN-ester (1 μM each) in serum-free medium for 48 h. The GS-HNE-ester and GS-DHN-ester were prepared as described before (Ramana et al., 2006). The cell culture media were harvested and centrifuged at 5000 rpm for 5 min and supernatants were stored at −80°C or used immediately. The media were used for chemiluminescent SEAP assay using GreatEscAPe™ SEAP reporter assay system according to protocol essentially as described by the manufacturer, (BD Biosciences, Palo Alto, CA) using a 96-well chemiluminescence plate reader.
2.6. Antisense ablation of AR
The hNPECs were grown to 50% to 60% confluence in DMEM supplemented with 10% FBS in 6-well plate. The cells were incubated with human AR-siRNA (AAC GCA TTG CTG AGA ACT TTA) or scrambled siRNA (AAC ACG GCT TGA ATG ACT ATA; control) to a final concentration of 100 nM and the RNAiFect™ transfection reagent (Qiagen) as suggested by the supplier. Briefly, for each well 2 μg AR or control siRNA were diluted in serum-free medium to give a final volume of 100 μl and incubated with 6 μl RNAiFect® for 15 min at room temperature. The transfection mixture was added drop-wise to the respective wells, each containing fresh 1900 μl complete medium and incubated for 48 h. Changes in the expression of AR were determined by Western blot analysis using anti-AR antibodies.
2.7. Determination of PGE2 and Nitrate/nitrite levels
The hNPECs were grown to confluence in 6-well plates, and were either transfected with AR SiRNA or incubated with ARIs or its vehicles for 24 h followed by incubation with LPS (1 μg/ml) for additional 24 h in serum-free medium. The medium was harvested, cleared by centrifugation, aliquoted and stored at −80°C until used for assays. Nitrate or nitrite, the stable end products of NO metabolism, were determined in cell culture medium by using Lactate dehydrogenase (LDH)-based colorimetric kit (Cayman Chemicals, Ann arbor, MI). PGE2 concentrations in cell culture supernatants were determined using a commercially available enzyme immunoassay kit (Cayman Chemical, Ann Arbor, MI).
2.8. Determination of Na/K-ATPase by Immunofluorescent assay
Approximately 1×105 hNPECs per chamber were plated on 2-chambered slides, growth-arrested by incubating in 0.1% serum medium without or with AR inhibitor for 24 h and were treated with LPS (1 μg/ml) for 24 h. The cells were washed with cold PBS and fixed in methanol (pre-cooled at −20°C) for 10 min, air dried and washed again with PBS. The cells were incubated in blocking solution, containing 2% BSA and 5% normal goat IgG and 0.1% TritonX-100 in PBS, for 30 min followed by incubation with primary antibodies against Na/K-ATPase for overnight at 4°C. The cells were washed and probed with fluorescent secondary antibodies for 1 h at room temperature. After washing, slides were mounted with VECTASHIELD hard set mounting medium (Vector Laboratories Inc., Burlingame, CA) with DAPI and photomicrographs were acquired using Nikon camera fitted to EPI-800 microscope and pixel density for the fluorescent corresponding to Na/K-ATPase immunoreactivity were measured using Adobe CS4 software.
2.9. Animals and treatments
Six to eight-weeks-old male Lewis rats weighing approximately 150–160 g were used in this study (n=4). All animals were kept in the UTMB’s Animal Care Center. All the animal studies were conducted in compliance with the ARVO statement for the use of Animals in Ophthalmic and Vision Research. Escherichia coli LPS (200 μg) dissolved in phosphate-buffered saline (PBS, pH 7.4) was injected subcutaneously. Rats in ARI and LPS + ARI groups were injected intraperitoneally with AR inhibitor zopolrestat (25 mg/kg body weight) dissolved in dimethyl-sulfoxide (DMSO) 24 h before and immediately after LPS injection. Rats in control group received carrier (PBS + 20% DMSO) injection. The rats were euthanized at 24 h after LPS injection and the eyes were enucleated immediately and stored in 4% para-formaldehyde solution for 48 h at 4° C. The eyes were washed in ice-cold PBS twice and kept in 70% alcohol at 4°C until they were embedded in paraffin. Sagittal sections (5 μm) were cut and used for immunohistochemical studies.
2.10. Immunohistochemical studies
The paraffin sections were warmed at 60° C for 1 h and deparafinized in xylene, followed by rehydration by passing through 100%, 95%, 80% and 70% ethanol and finally washed in deionozed water. After peroxidase blocking with 3% H2O2 the sections were rinsed in PBS twice and incubated with blocking buffer (2% BSA, 0.1% Triton-X100, 2% normal rabbit IgG and 2% normal goat serum) for overnight at 4° C followed by incubation with antibodies against Na-K-ATPase-α isoforms (1:100 dilution) for 1 h at room temperature. The sections were stained using universal LSAB+System-HRP (DakoCytomation, CF, USA). The sections were examined under bright field light microscopy (EPI-800 microscope) and photographed with Nikon camera fitted to EPI-800 microscope.
2.11. Statistics
Data presented as mean ± SE and statistical significance was determined by unpaired Student’s t test using Microsoft Office Excel software. The value of P<0.05 was considered statistically significant.
3. Results
3.1. AR inhibition prevents the LPS-induced increase in the pro-inflammatory markers in hNPECs
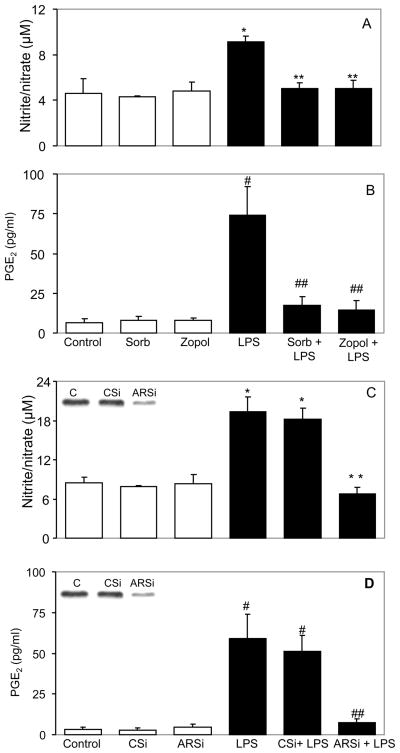
LPS, a major component of the Gram-negative bacterial outer membrane causes many pathologic effects by stimulating host cells to synthesize large quantities of bioactive inflammatory mediators such as nitric oxide, prostaglandins, and cytokines (Ulevitch et al., 2004; Bozinovski et al., 2004). We have shown earlier that AR-mediates cytokine and endotoxin-induced cytotoxic signals in macrophages (Ramana et al., 2006). At first we examined the AR activity in the hNPECs treated with LPS in the presence and absence of AR inhibitor, sorbinil. Our results indicate that LPS increased AR activity by 2 fold in 24 h (9.8 ± 0.95 mU/mg protein) as compared to control hNPECs (4.3 ± 0.52 mU/mg protein). Sorbinil prevented the LPS-induced AR activity significantly by >90% (5.1 ± 0.65). We next examined the effect of AR inhibition on the LPS-induced inflammatory markers in ciliary epithelial cells, which play a major role in anterior uveitis. As shown in Figures 1A and B, treatment of hNPECs with 1 μg/mL LPS for 24 hours caused >2- and 6-fold increase in the synthesis of nitrite/nitrate and PGE2, respectively, and inhibition of AR significantly (>80%) prevented these changes.
Fig 1. Inhibition of AR prevents the LPS-induced nitrate/nitrite and PGE2 level in hNPECs.
Growth-arrested hNPECs were preincubated with sorbinil or zopolrestat (A, B) for 24 h or ablated with Control SiRNA (CSi) or AR siRNA (ARSi) (C, D) for 48 h followed by treatment with LPS (1 μg/ml) for 24 h. At the end of incubation, culture medium was collected and levels of nitrite/nitrate were determined using lactate dehydrogenase (LDH)-based colorimetric assay kit and the levels of PGE2 were determined using ELISA-base PGE2 assay kit essentially as described by the manufacturer. Inset in C and D shows expression of AR protein after siRNA ablation. Bars represent Mean±SD (n=4). *p<0.01 Vs Control; **p<0.01 Vs LPS alone; #p<0.001 Vs Control; ##p<0.001 Vs LPS alone. sorb, sorbinil; zopol, zopolrestat.
Though sorbinil and zopolrestat are fairly specific inhibitors of AR, their nonspecific effect can not be ruled out. Therefore, besides using pharmacological inhibitors, we also ablated the expression of AR protein in hNPECs by AR SiRNA and studied the effect of LPS on NO and PGE2 levels. AR siRNA significantly prevented AR protein (>85%; Fig 1C&D insert) as well as activity (0.9 ± 0.32 mU/mg protein). A two-fold increase in the LPS-induced nitrite or nitrate levels in hNPECs was significantly (>90%) prevented by AR ablation (Fig 1C). Similarly, LPS caused a nearly 6-fold increase in PGE2 in hNPECs culture medium and AR ablation inhibited the increase by >85% (Fig 1D). These results suggest that AR regulates the synthesis of LPS-induced pro-inflammatory markers and inhibition of AR either by pharmacological inhibitors or by gene silencing using AR siRNA prevented the release of pro-inflammatory markers in ciliary epithelial cells.
3.2. AR-inhibition prevents LPS-induced synthesis of inflammatory marker proteins in hNPECs
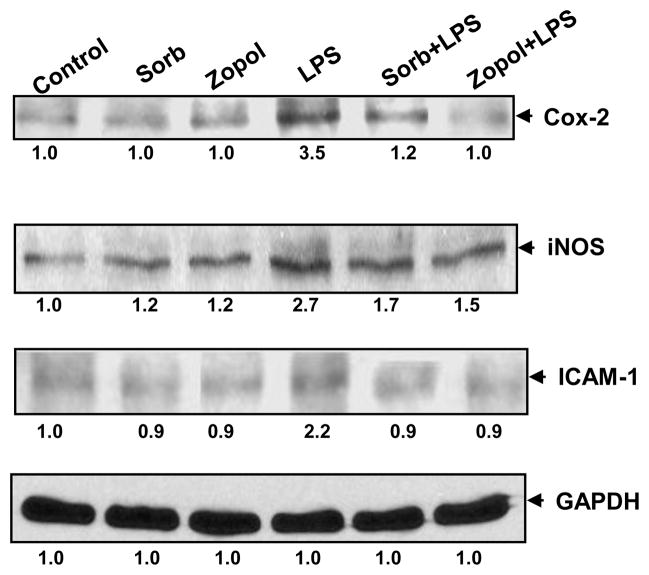
We next examined how AR inhibition prevents LPS-induced release of NO and PGE2 in the hNPECs culture media. Since iNOS and COX-2 catalyze the formation of NO and PGE2, we examined the effect of AR inhibition on the expression of iNOS and COX-2 in hNPECs. As shown in the Fig 2, LPS induced >2-fold increase in the expression of COX-2 and iNOS proteins. Inhibition of AR by sorbinil or zopolrestat significantly (>80%) prevented it (Fig 2). However, AR inhibitors, sorbinil and zopolrestat by themselves did not alter the basal levels in these cells. Collectively, these results suggest that inhibition of AR in hNPECs could prevent the expression of pro-inflammatory enzymes which in turn could prevent the synthesis of pro-inflammatory markers PGE2 and NO. Further, inhibition of AR also prevented LPS-induced expression of ICAM-1, an important adhesion molecule required for adhesion and migration of inflammatory cells in hNPECs.
Fig 2. Inhibition of AR prevents LPS-induced expression of inflammatory marker proteins.
Growth-arrested hNPECs were preincubated with sorbinil or zopolrestat for 24 h followed by treatment with LPS (1 μg/ml) and incubation for additional 24 h. At the end of incubation, cells were lyzed and equal amount of protein (40 μg) was separated on SDS-PAGE followed by immunoblotting using antibodies against COX-2, iNOS, and ICAM-1. Immunoblotting of the stripped membranes with GAPDH antibodies was used to depict equal protein loading. Numbers below the blots show fold changes in the expression of proteins (n=3). sorb, sorbinil; zopol, zopolrestat.
3.3. AR inhibition prevents LPS-induced activation of NF-κB and AP1 in hNPECs
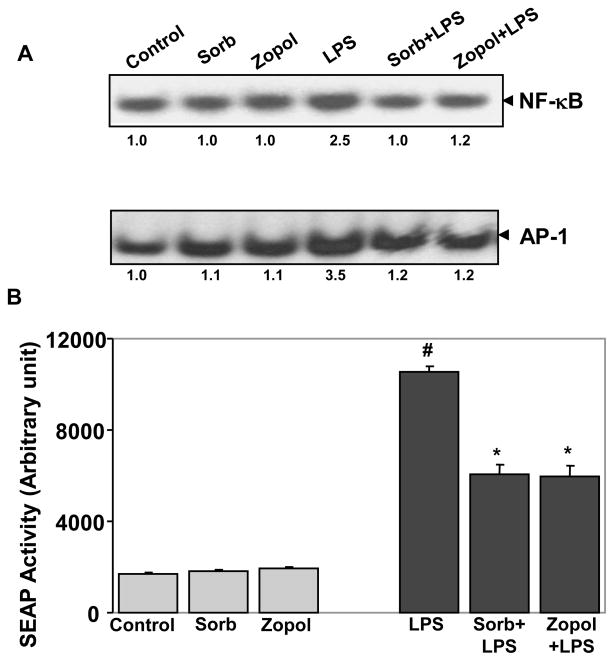
It is well known that redox-sensitive transcription factors such as NF-κB and AP-1 are involved in the transcription of various inflammatory markers including COX-2 and iNOS (Ramana and Srivastava, 2006; Rafi et al., 2007; Chiu and Lin, 2008). We therefore examined the effect of AR inhibition on LPS-induced activation of NF-κB and AP-1. As shown in Fig 3A, LPS caused >2-fold activation of NF-κB, and 2.5-fold activation of AP-1 in hNPECs. Inhibition of AR by sorbinil and zopolrestat significantly (>90%) prevented the LPS-induced NF-κB and AP-1 activation. However, sorbinil or zopolrestat alone did not affect the basal activity of NF-κB and AP-1. In order to further confirm our results, we used a more sensitive secretary alkaline phosphatase (SEAP) reporter assay to examine the effect of AR inhibition on NF-κB-induced SEAP activity. Incubation with LPS caused >5-fold activation in the NF-κB dependent SEAP activity which was significantly inhibited by sorbinil and zopolreastat (Fig 3B). These results suggest that inhibition of AR modulates the LPS-induced activation of redox-sensitive transcription factors, which could be responsible for preventing LPS-induced oxidative stress and production of inflammatory markers.
Fig 3. Inhibition of AR prevents LPS-induced activation of NF-κB in hNPECs.
(A) Growth-arrested hNPECs were preincubated with AR inhibitors sorbinil or zopolrestat or carrier for 24 h followed by treatment with LPS and incubation for 1h. Nuclear extract was prepared and EMSA was performed with equal amount of protein (5 μg). (B) For SEAP assay growth-arrested hNPECs were preincubated with sorbinil, tolrestat or zopolrestat or carrier for 24 h followed by transfection with NF-κB-pSEAP vector or control vector. After 6 h, cells were stimulated with LPS (1 μg/ml) and incubated for 48 h. Cell culture media were harvested, cleared by centrifugation and NF-κB-dependent reporter SEAP activity was measured by chemiluminescence’s method essentially as described by the manufacturer. #p<0.001 Vs Control; *p<0.01 Vs LPS alone (n=3). sorb, sorbinil; zopol, zopolrestat.
3.4. AR inhibition prevents LPS-induced activation of MAPK in hNPECs
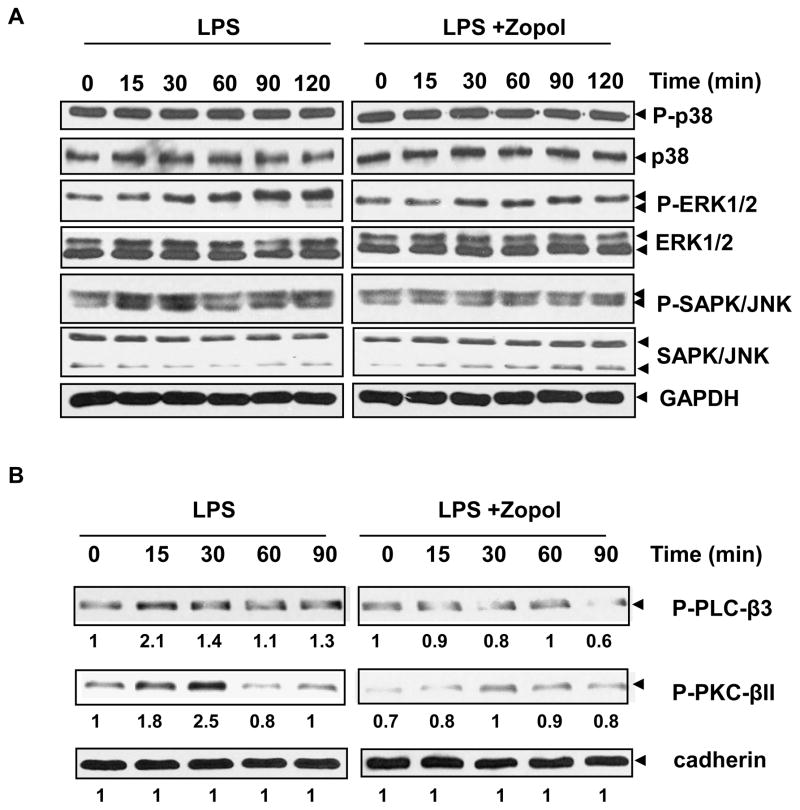
In oxidative stress-induced signaling, mitogen-activated protein kinases (MAPK) are upstream of transcription factors NF-κB and AP-1. We therefore, examined the effect of AR inhibition on LPS-induced activation of MAPK. As shown in Fig 4A, treatment with LPS (1 μg/ml) enhanced phosphorylated forms of ERK1/2- MAPK, and SAPK/JNK in hNPECs without any change in the expression of total ERK1/2 and SAPK/JNK proteins. On the other hand, there was no significant change in the phosphorylated form of p38-MAPK or total p38. Inhibition of AR by zopolrestate prevented LPS-induced phosphorylation of ERK1/2-MAPK and SAPK/JNK (Fig 4A). Collectively, these results suggest that inhibition of AR in hNPECs prevents the phosphorylation of MAPKs and SAPK/JNK and therefore interrupts upstream signaling of the NF-κB induced by LPS-induced oxidative stress.
Fig 4. Inhibition of AR attenuates LPS-induced phosphorylation of (A) MAP-Kinases and (B) PLC-β3 and PKC-βII in hNPECs.
(A) Growth-arrested hNPECs were preincubated with sorbinil, zopolrestat or carrier for 24 h followed by treatment with LPS (1 μg/ml) and incubation for different time periods as indicated. At the end of incubation, cells were lysed and equal amount of protein (40 μg) was separated on SDS-PAGE followed by immunoblotting using antibodies against phospho- and total p38, ERK1/2, and SAPK/JNK. Immunoblotting of the stripped membranes with GAPDH antibodies was used to depict equal protein loading. (B) Growth-arrested hNPECs were preincubated with zopolrestat for 24 h followed by treatment with LPS (1 μg/ml) for different durations as indicated. At the end of incubation, cells were harvested, cell membrane fraction was prepared and equal amount of protein (15 μg) was separated on SDS-PAGE followed by immunoblotting using antibodies against phospho- PLCβ3 and -PKC-βII. Immunoblotting of the stripped membranes with antibodies against pan-cadherin was performed to show equal loading of protein (n=3). zopol, zopolrestat.
3.5. AR inhibition prevents LPS-induced activation of PKC and PLC in hNPECs
We next examined the effect of AR inhibition on PKC activation as PKC is a significant molecule in the LPS-induced signaling and is upstream of NF-κB activation. As shown in Fig 4B, LPS caused time-dependent increase in the phosphorylation and activation of PKC reaching maximum (4-fold) at 30 min after which it returns to basal level. Inhibition of AR prevented the activation of PKC in hNPECs. Similarly PLCβ3, which is upstream of PKC showed time-dependent increase in phosphorylation (maximum at 15 min; 3-fold) and inhibition of AR by zopolrestat significantly, prevented the increase in activation of both PKC and PLC.
3.6. AR inhibition prevents LPS-induced decrease in the expression of Na/K-ATPase in hNPECs and rat ciliary bodies
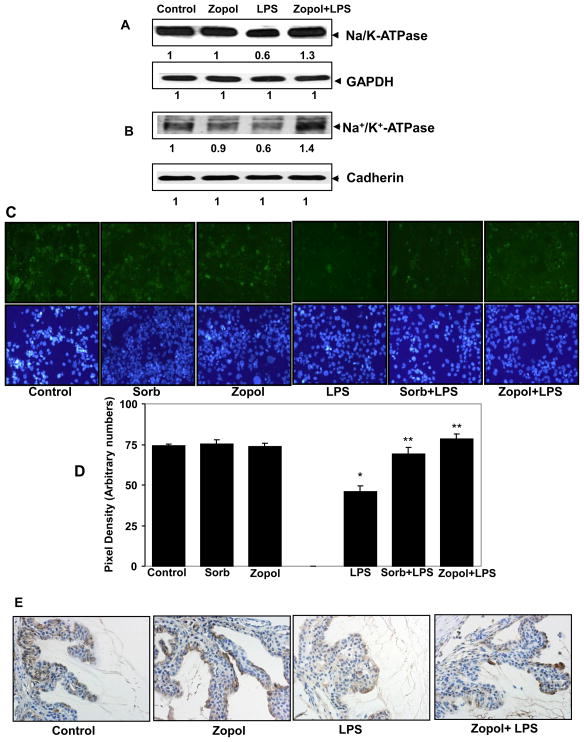
Since the physiological function of hNPECs in-vivo is to maintain the dynamics of AqH in anterior chamber and the secretion of AqH depends on the channel proteins such as Na/K-ATPase (Civan and Macknight, 2004), we next examined the level of Na/K-ATPase in hNPECs whole cell lysate as well as in the cell-membrane fraction by immunoblotting. As shown in Fig 5A and B, unstimulated hNPECs expressed a high level of this transporter protein which was decreased markedly by LPS (1 μg/ml) after 24 h of treatment. Inhibition of AR by zopolrestat prevented the LPS-induced decrease in the expression of Na/K-ATPase. We further studied the expression of Na/K-ATPase in cultured hNPECs by immuno-fluoroscence method. In LPS-treated cells the fluorescence associated with Na/K-ATPase staining was reduced markedly as compared to control cells and treatment with zopolrestat restored the expression level of Na/K-ATPase in hNPECs (Fig 5C).
Fig 5. Inhibition of AR prevents LPS-induced decrease in the expression of Na/K-ATPase transporter protein in hNPECs.
Growth-arrested hNPECs were preincubated with zopolrestat for 24 h followed by incubation with LPS (1 μg/ml) for additional 24 h. At the end of incubation, cells were harvested and either lysed directly (A) or membrane fraction was prepared (B) and equal amount of protein was separated on SDS-PAGE followed by immunoblotting using antibodies against alpha subunit of Na/K-ATPase. Same blots were stripped and immunoblotting using antibodies against GAPDH (A) and cadherin (B) was performed to show equal loading of protein. Numbers below the blots show fold change in the expression of proteins (n=3). (C) For immuno-cytochemistry hNPECs, after incubation with LPS for 24 h, were fixed in prechilled methanol (at −20°C) and air-dried. The cells were washed with cold PBS and immuno-cytochemistry using antibodies against Na/K-ATPase protein was performed using standard protocol. A representative (n=3) fluorescent photomicrograph and corresponding DAPI images have been shown from each group. (D) Pixel density was measured from fluorescent photomicrograph in (C) and corresponding values are shown as bar diagram. *p<0.01 Vs Control; **p<0.01 Vs LPS alone (n=3). (E) Serial sections of paraformaldehyde-fixed rat eyes were immuno-stained with antibodies against alpha subunits of Na/K-ATPase protein. The expression of Na/K-ATPase was observed in the pars plicata region of ciliary bodies of LPS-treated rat. A representative (n=4) photomicrograph is shown from each group. sorb, sorbinil; zopol, zopolrestat.
We further examined the effect of AR inhibition on LPS-induced expression of Na/K-ATPase in rat ciliary bodies. As shown in Fig 5D immunohistochemical examination of ciliary bodies of LPS-injected rats using antibodies against transporter protein Na/K-ATPase showed that the expression of Na/K-ATPase protein was decreased in the outer layer comprising non-pigmented ciliary epithelial cells as compared to the control rats. Administration of rats with AR inhibitor, zopolrestat, restored the expression of Na/K-ATPase in the ciliary bodies. These data suggest that LPS decreases the expression of Na/K-ATPase in non-pigmented ciliary epithelium which could dysregulate the dynamics of AqH and AR inhibition prevents these alterations by restoring the expression of Na/K-ATPase in rat ciliary epithelium.
3.7. AR inhibition prevents HNE- and GS-HNE-induced activation of NF-κB but not by GS-DHN in hNPECs
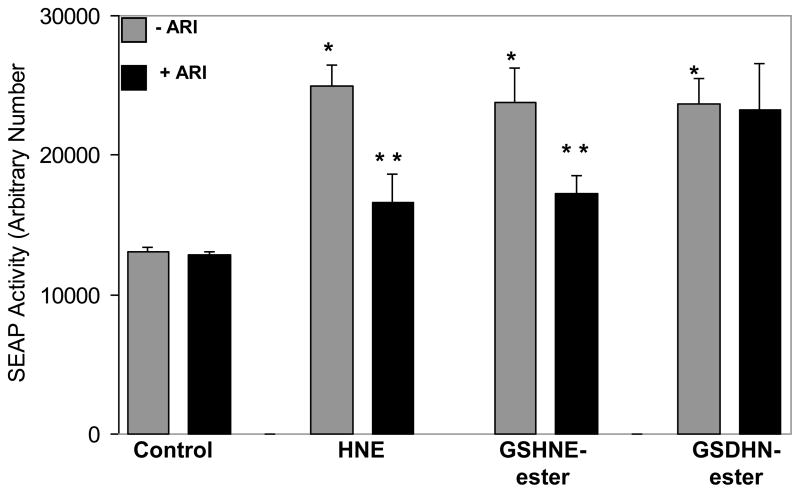
HNE and its conjugates with GSH have shown to be excellent substrates of AR with Km in 10–30 uM (Srivastava et al., 1999). We next examined how AR catalytic activity plays a role of AR HNE and its GSH-conjugates -induced inflammation in hNPECs. We stimulated the hNPECs with substrates of AR, HNE and GS-HNE and AR reduced reaction product, GS-DHN and examined their effects on NF-κB activation by secretary alkaline phosphatase (SEAP) reporter assay. Incubation with HNE and GS-HNE caused >2-fold activation in the NF-κB dependent SEAP activity which was significantly inhibited by AR inhibitor (Fig 6). However, GS-DHN-induced activation of SEAP activity in hNPECs was not changed by AR inhibition, suggesting that the metabolic product of AR could be involved in the activation of redox-sensitive transcription factors, which could be responsible for LPS-induced oxidative stress and production of inflammatory markers.
Fig 6. Inhibition of AR prevents HNE- and GS-HNE-induced activation of NF-κB but not by GS-DHN in hNPECs.
Growth-arrested hNPECs were incubated with AR inhibitor for over-night and transfected with NF-κB-pSEAP vector or control (pTAL SEAP) vector using Lipofectamine 2000. After 6 h, medium was replaced with fresh medium and cells were stimulated with HNE, GS-HNE-ester or GS-DHN-ester (1 μM each) and incubated for 48 h with or without AR inhibitor. Cell culture media were harvested, cleared by centrifugation and NF-κB-dependent reporter SEAP activity was measured by chemiluminescence’s method essentially as described by the manufacturer. *p<0.001 Vs Control; **p<0.01 Vs HNE or GS-HNE-ester alone (n=3).
4. Discussion
The NPE cells play an important role in the production and dynamics of aqueous humor (AqH) (Civan and Macknight, 2004). This function is dysregulated in endotoxin -induced uveitis in rats (Lee and Higginbotham, 1999). The decreased secretion of AqH during bacterial infection hampers the AqH dynamics that could lead to the AqH stagnancy in the anterior chamber and consequent accumulation of toxic byproducts may cause increased oxidative stress and may aggravate the already stressed non-vascular cells in the eye, such as lens epithelial cells, and corneal epithelial cells, which depend heavily on AqH for nutrition. It has been reported that in both acute and chronic anterior uveitis intra-ocular pressure (IOP) decreases significantly (Collins and Moore, 1999). In contrast to this, the IOP is usually abnormally high in glaucoma, which is a major cause of irreversible blindness throughout the world has been attributed, at least in part, to the release of endogenous PGE2 (Quigley, 2005). We observed that the level of PGE2 as well as the expression of COX-2 enzyme significantly increased in the hNPECs by LPS treatment. It appears that LPS cause the increase in the expression of COX-2 which then catalyzes the synthesis of PGE2. Though physiological significance of the increased PGE2 synthesis upon bacterial infection is not very clear, a reduction in the secretion of AqH resulting in reduced IOP is implicated. Identical condition has been observed during the bacterial infection-induced inflammation such as EIU in rats (Samudre et al., 2004). Similarly, the increase of more than two-fold in the NO level in hNPECs after LPS treatment may also contribute to reduction in the secretion of AqH. The cytotoxic actions of excessive NO production leading to apoptosis are well known and have been shown to involve several potential mechanisms (Meij et al., 2004; Langer et al., 2008). Nitric oxide readily reacts with the superoxide radicals to form peroxynitrite, which has been proposed to play a significant role in cellular damage (Beckman et al., 1990). It has been shown that NO treatment of hNPECs decreases the Na/K-ATPase activity (Shahidullah and Delamere, 2006) and also the intraocular pressure, in part because it induces a decrease in aqueous humor production (Ellis et al., 2001). We observed that AR inhibition attenuated both PGE2 and NO increase in hNPECs induced by LPS. These findings suggest that AR is a crucial mediator in LPS-induced production of inflammatory markers. It is well known that NF-κB regulates the transcription of inflammatory genes including COX-2 and iNOS, and we have also shown in our earlier studies that inhibition of AR prevents NF-κB activation in many cell types including HLEC (Pladzyk et al., 2006). It is likely that AR inhibition prevents the LPS-induced increased synthesis of PGE2 and NO by inhibiting the expression of COX-2 and iNOS. The activation of redox sensitive transcription factors, NF-κB and AP-1, is regulated in part by ERK1/2 and stress activated kinases (SAPK/JNK) (Chakraborti and Chakraborti 1998). We observed that LPS activated the p42/44 MAPK and JNK/SAPK in a time-dependent manner, but there was no increase in the LPS-induced phosphorylation of p38-MAPK in hNPECs. On the other hand AR inhibitors have been shown to prevent LPS-induced activation of p38 in murine macrophages. Since JNK and p38 phosphorylations are regulated by different subtypes of stress -activated MAPK kinases (MKK) e.g. p38 is phosphorylated by MKK3/6, while phosphorylation of JNK is catalyzed by MKK4/7 (Johnson and Lapadat, 2002), which explains the differential activation of p38-MAPK and JNK in hNPECs. Prevention of the phosphorylation of ERK1/2 and JNK/SAPKs by AR inhibition indicates that AR-mediated activation of NF-κB is regulated upstream of MAPK. Studies have shown that PGE2 and NO regulate the AqH production by activating Na/K-ATPase (Civan et al., 1994; Shahidullah and Delamere, 2006; Ellis et al., 2001), and in concurrence with these studies we observed that hNPECs when treated with LPS showed a decreased expression of Na/K-ATPase α most likely via up-regulation of the COX-2, iNOS and activation of redox-sensitive molecular signaling in hNPECs. In addition to regulating the production of inflammatory markers, AR inhibition also attenuated the decreased expression of Na/K-ATPase in hNPECs. Although a clear understanding of AR-mediated signaling remains intangible at present, it is likely that AR regulates this cascade at the level of PLC/PKC activation by its metabolic product, glutathionyl-1,4-dihydroxynonanol (GS-DHN) (Ramana et al., 2006). Even though a detailed investigation is warranted, in the present study we observed that AR inhibition prevented the LPS-induced activation of PLCβ3 and PKC βII. Our previous studies have shown that inhibition of AR prevented production of inflammatory markers such as PGE2, COX-2, TNF-α, and NO in HLEC stimulated with LPS (Pladzyk et al., 2006) and in human colon cancer cells stimulated with growth factors (Tammali et al., 2006).
Although precise mechanism of AR in the inflammation is not known, we tested our hypothesis that AR metabolic product could be involved in the activation of key signaling intermediates. Our results indicate that AR reduced reaction product GS-DHN could mediate oxidative stress signals. We have previously demonstrated similar phenomenon in mouse macrophage cells where AR inhibitors prevented HNE and GS-HNE–induced cytotoxic effects but not that induced by GS-DHN (Ramana et al., 2006). These observations bequeath an important role to AR-catalyzed reduced product of glutathione-HNE conjugate, GS-DHN, in the inflammatory signaling and indicate that AR inhibition could be anti-inflammatory (Fig 7). Thus, our results provide evidence that AR inhibition prevents LPS –induced cytotoxic signals by inhibiting the activation of NF-κB and subsequent activation of inflammatory signaling in non-pigmented ciliary epithelial cells. It provides a novel concept that inhibitors of AR could be useful in preventing systemic bacterial infection –induced ocular inflammation.
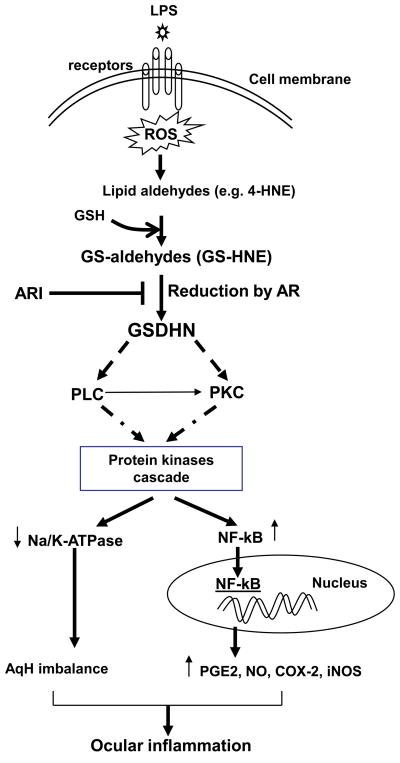
Fig 7. The Role of AR in inflammatory signaling in hNPECs.
The bacterial endotoxin such as LPS is known to induce ROS formation and oxidative stress in cells leading to oxidization of membrane lipids into lipid aldehydes such as 4-hydroxynonenal (HNE), which readily reacts with glutathione and to form GS-HNE. AR catalyzed reduction product, GS-DHN, could activate transcription factor NF-κB via activation of protein kinase cascade which transcribe various inflammatory marker genes such as COX-2 and iNOS leading to ocular inflammation. Further, protein kinases such as MAPK have also shown to negatively regulate Na/K-ATPase leading to AqH imbalance.
Acknowledgments
This work was supported by NIH Grants EY018591 and GM071036 to KVR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozinovski S, Jones J, Beavitt SJ, Cook AD, Hamilton JA, Anderson GP. Innate immune responses to LPS in mouse lung are suppressed and reversed by neutralization of GM-CSF via repression of TLR-4. Am J Physiol Lung Cell Mol Physiol. 2004;286:L877–885. doi: 10.1152/ajplung.00275.2003. [DOI] [PubMed] [Google Scholar]
- Chakraborti S, Chakraborti T. Oxidant-mediated activation of mitogen-activated protein kinases and nuclear transcription factors in the cardiovascular system: a brief overview. Cell Signal. 1998;10:675–683. doi: 10.1016/s0898-6568(98)00014-x. [DOI] [PubMed] [Google Scholar]
- Chiu FL, Lin JK. Tomatidine inhibits iNOS and COX-2 through suppression of NF-kappaB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008;582:2407–2412. doi: 10.1016/j.febslet.2008.05.049. [DOI] [PubMed] [Google Scholar]
- Civan MM, Macknight AD. The ins and outs of aqueous humour secretion. Exp Eye Res. 2004;78:625–631. doi: 10.1016/j.exer.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Civan MM. Transport components of net secretion of the aqueous humour and their integrated regulation. In: Civan MM, editor. The Eye’s Aqueous Humor: from Secretion to Glaucoma. Academic Press; New York: 1998. pp. 1–24. [Google Scholar]
- Civan MM, Coca-Prados M, Peterson-Yantorno K. Pathways signaling the regulatory volume decrease of cultured nonpigmented ciliary epithelial cells. Invest Ophthalmol Vis Sci. 1994;35:2876–2886. [PubMed] [Google Scholar]
- Collins BK, Moore CP. Diseases and surgery of the canine anterior uvea. In: Gelatt KN, editor. Veterinary phthalmology. 3. Lippincott/Williams and Wilkins; Philadelphia: 1999. pp. 755–795. [Google Scholar]
- Counillon L, Touret N, Bidet M, Peterson-Yantorno K, Coca-Prados M, Stuart-Tilley A, Wilhelm S, Alper SL, Civan MM. Na+/H+ and CI−/HCO3−antiporters of bovine pigmented ciliary epithelial cells. Pflugers Arch Eur J Physiol. 2000;440:667–678. doi: 10.1007/s004240000302. [DOI] [PubMed] [Google Scholar]
- Curi A, Matos K, Pavesio C. Acute anterior uveitis. Clin Evid. 2005;14:739–743. [PubMed] [Google Scholar]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dukes MNG. Corticotrophins and corticosteroids. In: Dukes MNG, editor. Meyler’s Side Effects of Drugs. Elsevier; Amsterdam: 1996. pp. 1189–1209. [Google Scholar]
- Ellis DZ, Nathanson JA, Rabe J, Sweadner KJ. Carbachol and nitric oxide inhibition of Na/K-ATPase activity in bovine ciliary processes. Invest Ophthalmol Vis Sci. 2001;42:2625–2631. [PubMed] [Google Scholar]
- Ghosh S, Freitag AC, Martin-Vasallo P, Coca-Prados M. Cellular distribution and differential gene expression of the three alpha subunit isoforms of the Na, K-ATPase in the ocular ciliary epithelium. J Biol Chem. 1990;265:2935–29340. [PubMed] [Google Scholar]
- Hirsch M, Montcourrier P, Renard G. Ultrastructure of the blood-aqueous barrier in normal condition and after paracentesis A freeze-fracture study in the rabbit. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1977;203:169–179. doi: 10.1007/BF00409823. [DOI] [PubMed] [Google Scholar]
- Jacob TJ, Civan MM. Role of ion channels in aqueous humor formation. Am J Physiol. 1996;271:C703–C720. doi: 10.1152/ajpcell.1996.271.3.C703. [DOI] [PubMed] [Google Scholar]
- Jannot MF, Raccah D, De La Tour DD, Coste T, Vague P. Genetic and environmental regulation of Na/K adenosine triphosphatase activity in diabetic patients. Metabolism. 2002;51:284–291. doi: 10.1053/meta.2002.29009. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Krupin T, Reinach PS, Candia OA, Podos SM. Transepithelial electrical measurements on the isolated rabbit iris-ciliary body. Exp Eye Res. 1984;38:115–123. doi: 10.1016/0014-4835(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Langer DA, Das A, Semela D, Kang-Decker N, Hendrickson H, Bronk SF, Katusic ZS, Gores GJ, Shah VH. Nitric oxide promotes caspase-independent hepatic stellate cell apoptosis through the generation of reactive oxygen species. Hepatology. 2008;47:1983–1993. doi: 10.1002/hep.22285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Higginbotham EJ. Glaucoma. In: Lee DA, Higginbotham EJ, editors. Clinical guide to comprehensive ophthalmology. Thieme Medical Publishers; New York: 1999. pp. 333–357. [Google Scholar]
- McLaren JW. Measurement of aqueous humor flow. Exp Eye Res. 2009;88:641–647. doi: 10.1016/j.exer.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Meffert H. Antioxidants - friend or foe? Ger Med Sci. 2008;6 Doc 09. [PMC free article] [PubMed] [Google Scholar]
- Meij JT, Haselton CL, Hillman KL, Muralikrishnan D, Ebadi M, Yu L. Differential mechanisms of nitric oxide- and peroxynitrite-induced cell death. Mol Pharmacol. 2004;66:1043–1053. doi: 10.1124/mol.104.001354. [DOI] [PubMed] [Google Scholar]
- Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14:303–308. doi: 10.1007/BF00163549. [DOI] [PubMed] [Google Scholar]
- Pladzyk A, Reddy AB, Yadav UC, Tammali R, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents lipopolysaccharide-induced inflammatory response in human lens epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5395–5403. doi: 10.1167/iovs.06-0469. [DOI] [PubMed] [Google Scholar]
- Quigley HA. New paradigms in the mechanisms and management of glaucoma. Eye. 2005;19:1241–1248. doi: 10.1038/sj.eye.6701746. [DOI] [PubMed] [Google Scholar]
- Raccah D, Gallice P, Pouget J, Vague P. Hypothesis: low Na/K-ATPase activity in the red cell membrane, a potential marker of the predisposition to diabetic neuropathy. Diabete Metab. 1992;18:236–241. [PubMed] [Google Scholar]
- Tammali R, Ramana KV, Singhal SS, Awasthi S, Srivastava SK. Aldose reductase regulates growth factor-induced cyclooxygenase-2 expression and prostaglandin E2 production in human colon cancer cells. Cancer Res. 2006;66:9705–9713. doi: 10.1158/0008-5472.CAN-06-2105. [DOI] [PubMed] [Google Scholar]
- Rafi MM, Yadav PN, Rossi AO. Glucosamine inhibits LPS-induced COX-2 and iNOS expression in mouse macrophage cells (RAW 264.7) by inhibition of p38-MAP kinase and transcription factor NF-kappaB. Mol Nutr Food Res. 2007;51:587–593. doi: 10.1002/mnfr.200600226. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Fadl AA, Tammali R, Reddy AB, Chopra AK, Srivastava SK. Aldose reductase mediates the lipopolysaccharide-induced release of inflammatory mediators in RAW264.7 murine macrophages. J Biol Chem. 2006;281:33019–33029. doi: 10.1074/jbc.M603819200. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Friedrich B, Bhatnagar A, Srivastava SK. Aldose reductase mediates cytotoxic signals of hyperglycemia and TNF-alpha in human lens epithelial cells. FASEB J. 2003;17:315–317. doi: 10.1096/fj.02-0568fje. [DOI] [PubMed] [Google Scholar]
- Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–122. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviola G. Effects of paracentesis on the blood-aqueous barrier: An electron microscope study on Macaca mulatta using horseradish peroxidase as a tracer. Invest Ophthalmol. 1974;13:828–858. [PubMed] [Google Scholar]
- Reddy VN. A forty-two year voyage through vision research. J Ocul Pharmacol Ther. 2000;16:97–107. doi: 10.1089/jop.2000.16.97. [DOI] [PubMed] [Google Scholar]
- Riley MV, Kishida K. ATPases of ciliary epithelium: cellular and subcellular distribution and probable role in secretion of aqueous humor. Exp Eye Res. 1986;42:559–568. doi: 10.1016/0014-4835(86)90046-1. [DOI] [PubMed] [Google Scholar]
- Samudre SS, Lattanzio FA, Jr, Williams PB, Sheppard JD., Jr Comparison of topical steroids for acute anterior uveitis. J Ocul Pharmacol Ther. 2004;20:533–547. doi: 10.1089/jop.2004.20.533. [DOI] [PubMed] [Google Scholar]
- Schütte E. Evidence for metabolites of the cornea. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;215:113–121. doi: 10.1007/BF00414469. [DOI] [PubMed] [Google Scholar]
- Shahidullah M, Delamere NA. NO donors inhibit Na/K-ATPase activity by a protein kinase G-dependent mechanism in the nonpigmented ciliary epithelium of the porcine eye. Br J Pharmacol. 2006;148:871–880. doi: 10.1038/sj.bjp.0706795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson CM, Hawthorne JN. Reduced Na+/K+-ATPase activity in peripheral nerve of streptozotocin-diabetic rats: a role for protein kinase C? Diabetologia. 1988;31:297–303. doi: 10.1007/BF00277411. [DOI] [PubMed] [Google Scholar]
- Srivastava S, Watowich SJ, Petrashm JM, Srivastava SK, Bhatnagar A. Structural and kinetic determinants of aldehyde reduction by aldose reductase. Biochemistry. 1999;38:42–54. doi: 10.1021/bi981794l. [DOI] [PubMed] [Google Scholar]
- To CH, Kong CW, Chan CY, Shahidullah M, Do CW. The mechanism of aqueous humour formation. Clin Exp Optomet. 2002;85:335–349. [PubMed] [Google Scholar]
- Ulevitch RJ, Mathison JC, da Silva CJ. Innate immune responses during infection. Vaccine. 2004;22:S25–30. doi: 10.1016/j.vaccine.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Weng TX, Godley BF, Jin GF, Mangini NJ, Kennedy BG, Yu AS, Wills NK. Oxidant and antioxidant modulation of chloride channels expressed in human retinal pigment epithelium. Am J Physiol Cell Physiol. 2002;283:C839–C849. doi: 10.1152/ajpcell.00445.2001. [DOI] [PubMed] [Google Scholar]
- Wills NK, Weng T, Mo L, Hellmich HL, Yu A, Wang T, Buchheit S, Godley BF. Chloride channel expression in cultured human fetal RPE cells: response to oxidative stress. Invest Ophthalmol Vis Sci. 2000;41:4247–4255. [PubMed] [Google Scholar]
- Yadav UC, Srivastava SK, Ramana KV. Aldose reductase inhibition prevents endotoxin-induced uveitis in rats. Invest Ophthalmol Vis Sci. 2007;48:4634–42. doi: 10.1167/iovs.07-0485. [DOI] [PMC free article] [PubMed] [Google Scholar]