Abstract
Increased myofilament Ca2+ sensitivity, a common attribute of inherited and acquired cardiomyopathies, is often associated with cardiac arrhythmias. Accumulating evidence supports that increased myofilament Ca2+ sensitivity is an independent risk factor for arrhythmias, but the underlying molecular mechanism remains unclear. This review focuses on potential mechanisms how myofilament Ca2+ sensitivity may affect cardiac excitation and leads to the generation of arrhythmias. We discuss in detail the downstream effects of increased myofilament Ca2+ sensitivity, i.e. altered Ca2+ buffering/handling, impaired energy metabolism and increased mechanical stretch, and how they may contribute to the proarrhythmic effect.
Keywords: sudden cardiac death, reentry arrhythmias, conduction velocity, Ca2+ handling, energetic deficit, mechanical stretch
1. Regulation of myofilament Ca2+ sensitivity
In heart muscle, cyclic interactions between thin (actin) filaments and thick (myosin) filaments result in muscle shortening and force production. Initiation of contraction occurs when Ca2+ binds to troponin C in the troponin complex on the thin filament [1]. The troponin complex is comprised of troponin C (TnC or the Ca2+ binding subunit), troponin I (TnI, involved in inhibition of actin–myosin interaction in the absence of Ca2+), and troponin T (TnT which binds the troponin complex to tropomyosin, another thin filament protein) in a 1:1:1 stoichiometric ratio [2]. During diastole when cytosolic Ca2+ levels are low, tropomyosin is positioned such that actin and myosin cannot interact. Once Ca2+ binds to TnC, tropomyosin shifts and allows actin and myosin to form strong cross-bridges. The thin and thick filaments slide along each other driven by ATP hydrolysis and the heart contracts. Myofilament activation is directly dependent on the amount of activating Ca2+, but is also determined by the Ca2+ dependence of force production (=myofilament Ca2+ sensitivity), which can be regulated within certain limits. The Ca2+ sensitivity of the myofilament is usually determined by the steady-state force-calcium relationship, which is well described by a Hill equation. The myofilament Ca2+ sensitivity is increased when the relationship is shifted to the left, towards lower concentrations on the [Ca2+] axis.
Myofilament Ca2+ sensitivity is dynamically regulated by a number of processes that relate calcium cycling to myofilament force production: Ca2+ binding to TnC, de-inhibition of actin-myosin interaction by the thin filament and actin-myosin cross-bridge properties [3]. For example, Ca sensitivity changes during each cardiac cycle with sarcomere length [4, 5], an effect in part responsible for the immediate adaptation in cardiac output during beat-to-beat changes in ventricular filling (Frank-Starling response [6]). Longer lasting regulation of myofilament Ca2+ sensitivity is achieved by phosphorylation (for overview see [7]), most strikingly by phosphorylation of TnI [8]. The phosphorylation of two N-terminal serines by cAMP dependent protein kinase A (PKA) decreases myofilament Ca2+ sensitivity and contributes to the positive lusitropic effect of beta agonists [9, 10]. The same serines are also phosphorylated by protein kinase D (PKD) [11] thereby allowing multiple signaling pathways to regulate Ca2+ dependence of force production via this route.
During acute myocardial ischemia, myofilament Ca2+ sensitivity decreases largely due to a combined effect of acidic pH and increased [PO4] (as a consequence of the decline in high energy phosphates) [12–14]. Myofilament Ca2+ sensitivity remains decreased in post-ischemic or "stunned" myocardium even once the intracellular milieu is restored, likely because of modification of or proteolytic injury to contractile proteins [15, 16]. Myofilament Ca2+ sensitization has been proposed as an attractive drug target to increase pump function in failing hearts without deleterious effects on energetic efficiency [17, 18]. In response several “Ca2+ sensitizers” were developed and studied in the 1990s [19–21], but often impaired cardiac relaxation [22, 23] and only Levosimendan is currently in clinical use in Europe [24].
2. Inherited and acquired conditions with increased myofilament Ca2+ sensitivity are often associated with arrhythmias
Mutations in genes encoding sarcomeric proteins have been identified in several inherited cardiomyopathies, one of which is Familial Hypertrophic Cardiomyopathy (FHC) [25]. FHC mutations are associated with an increased risk for cardiac arrhythmias leading to sudden cardiac death [26, 27]. Especially several mutations in cardiac TnT are associated with a high incidence of sudden death, with the majority of deaths (75%) occurring in patients under the age of 45 [28–30], despite little or no cardiac hypertrophy [31]. Generally, the genotype/phenotype relationships of sarcomeric mutations are weak [32], suggesting that mechanisms in addition to hypertrophy, myofibrillar disarray and fibrosis play a significant role for the risk of ventricular arrhythmias and sudden cardiac death, especially in young patients with only minor structural remodeling of the heart.
FHC-mutations of the thin filament proteins almost universally increase myofilament Ca2+ sensitivity of force development, as we and others have reviewed [33–36]. This was studied in vitro [37–46] and in transgenic models expressing the mutant thin filament proteins [41, 42, 47–54]. In contrast the effects of these sarcomeric mutations on other myofilament properties are rather diverse, i.e. differential effects on myofilament Ca2+-ATPase activity, maximal force, troponin complex binding affinity to the thin filament and pH-regulation. While less well studied than the thin filament proteins, reports suggest that many FHC-linked mutations in myosin heavy chain (MHC) [55–57], in myosin binding protein C (MyBP-C) [58, 59] and in regulatory light chain [60, 61] can also increase Ca2+ sensitivity of force development. These data indicate that FHC-linked sarcomeric mutations disrupt the tight inhibitory regulation exerted by the thin filament, with a common downstream effect of increased Ca2+ sensitivity of force development.
In addition to inherited conditions, acquired diseases can also be associated with changes in myofilament Ca2+ sensitivity. Substantial evidence exists for increased Ca2+ sensitivity of force production in animals after myocardial infarction (MI) [62]. This disease is also characterized by a high incidence of ventricular tachycardia and sudden cardiac death. Interestingly, exercise training after MI, which normalizes the increased myofilament Ca2+ sensitivity [63], also has been shown to reduce mortality and the rate of ventricular arrhythmias in dog [64, 65] and human studies [66](reviewed in [67]). Treatment with the Ca2+ sensitizer levosimendan significantly increased the incidence of ventricular tachycardia in one clinical study [68], further linking increased myofilament Ca2+ sensitivity with arrhythmias.
Myofilament Ca2+ sensitivity appears to be also increased in end-stage human heart failure [69–71], another condition associated with a high incidence of ventricular arrhythmia. The myofilament sensitization is at least partially due to decreased phosphorylation of troponin I [69, 70, 72–74]. While results from human tissues can be somewhat problematic as the tissue is not collected under controlled conditions and there are inherent differences between donor and recipient treatment [75], increased myofilament Ca2+ sensitivity has also been observed in experimental models where samples were obtained under controlled conditions [62, 76, 77].
3. Increased myofilament Ca2+ sensitivity – a novel mechanism of arrhythmogenesis?
The above observations support the hypothesis that Ca2+ sensitized myofilaments are a risk factor for developing ventricular tachyarrhythmias. We recently tested this hypothesis using different lines of transgenic mice where a range of myofilament Ca2+ sensitivity was produce by expressing different TnT mutants or TnI isoforms [78]. Compared to control mice with no change in myofilament Ca sensitivity (non-transgenic littermates (NTG), human TnT-WT, TnT-R278C), mice with Ca2+ sensitized myofilaments (TnT-F110I, TnT-I79N or slow skeletal TnI expression) demonstrate an increase in the rate of premature ventricular complexes when injected with the β-agonist isoproterenol. The occurrence of ventricular tachycardia (VT) in the myofilament Ca2+ sensitized groups was directly proportional to the degree of shift in Ca2+ sensitivity, whereas VT did not occur in controls. Similarly, the average pacing frequency required to induce sustained VT in isolated perfused hearts was lower in Ca2+ sensitized TnT mutants. All transgenic mice used have been extensively phenotyped and do not exhibit cardiac hypertrophy, fibrosis, or myofibrillar disarray [41, 49, 79]. However, we cannot completely rule out that small defects in anatomy contribute to the arrhythmia susceptibility. Therefore we also investigated the effect of the Ca2+ sensitizing compound EMD 57033 at a concentration with minimal phosphodiesterase inhibition [19, 80]. Presence of EMD rendered NTG hearts susceptible to VT induction, an effect that was completely reversible upon washout. These experiments suggest that myofilament Ca2+ sensitization per se is proarrhythmic and raise the question if a reduction in myofilament Ca2+ sensitivity to control level is anti-arrhythmic [78]. However, Ca2+ de-sensitizers are not readily available to test this hypothesis, as until now there was no known purpose for such a compound. Blebbistatin (BLEB), an actin-myosin uncoupler, has been shown to shift the Ca2+ dependence of force development to the right with only negligible effects on cardiac ion channels [81, 82]. We reproduced this effect and showed that BLEB reduces myofilament Ca2+ sensitivity in TnT mutant mice and antagonizes the Ca2+ sensitizing effect of EMD [78]. In accordance with that result BLEB completely prevented the increased occurrence of VT in all Ca2+ sensitized groups (TnT-mutants and EMD-treated). This showed, for the first time, that a reduction of Ca2+ sensitivity in myofilaments is anti-arrhythmic, which may be beneficial for individuals with hypertrophic cardiomyopathy.
4. How does myofilament Ca2+ sensitivity affect cardiac electrical activity?
Our experimental results clearly demonstrate that increasing Ca2+ sensitivity of myofilaments alters the electrical activity of the heart [78]. However, the underlying mechanisms are much less clear. In optical recordings using a voltage sensitive dye, we typically found long lasting rotors forming repetitive activation patterns [78]. These results suggest that Ca2+ sensitization causes a substrate for reentrant activation. The functional reentry appears to be caused by the regional slowing of impulse propagation (increased spatial dispersion of conduction velocity (CV)) during rapid pacing. This was observed in myofilament Ca2+ sensitized transgenic hearts, but also during treatment of control hearts with the Ca2+ sensitizer EMD, clearly demonstrating that this effect does not require an anatomical substrate. How can increased myofilament Ca2+ sensitivity cause the observed electrical heterogeneities? Myofilament Ca2+ sensitization may mediate the effect on cardiac excitation via three principal pathways [83]: (1) through an effect on the intracellular Ca2+ homeostasis (e.g. decreased Ca2+ transient amplitude and slowed Ca2+ transient decline [84]), (2) energy metabolism (energy depletion due to increased energy demand combined with decreased energy utilization, as described in FHC [47, 85, 86] ) or (3) increased mechanical stress (hearts are hypercontractile [49], but fail under conditions of increased demand).
(1) Ca2+ -handling
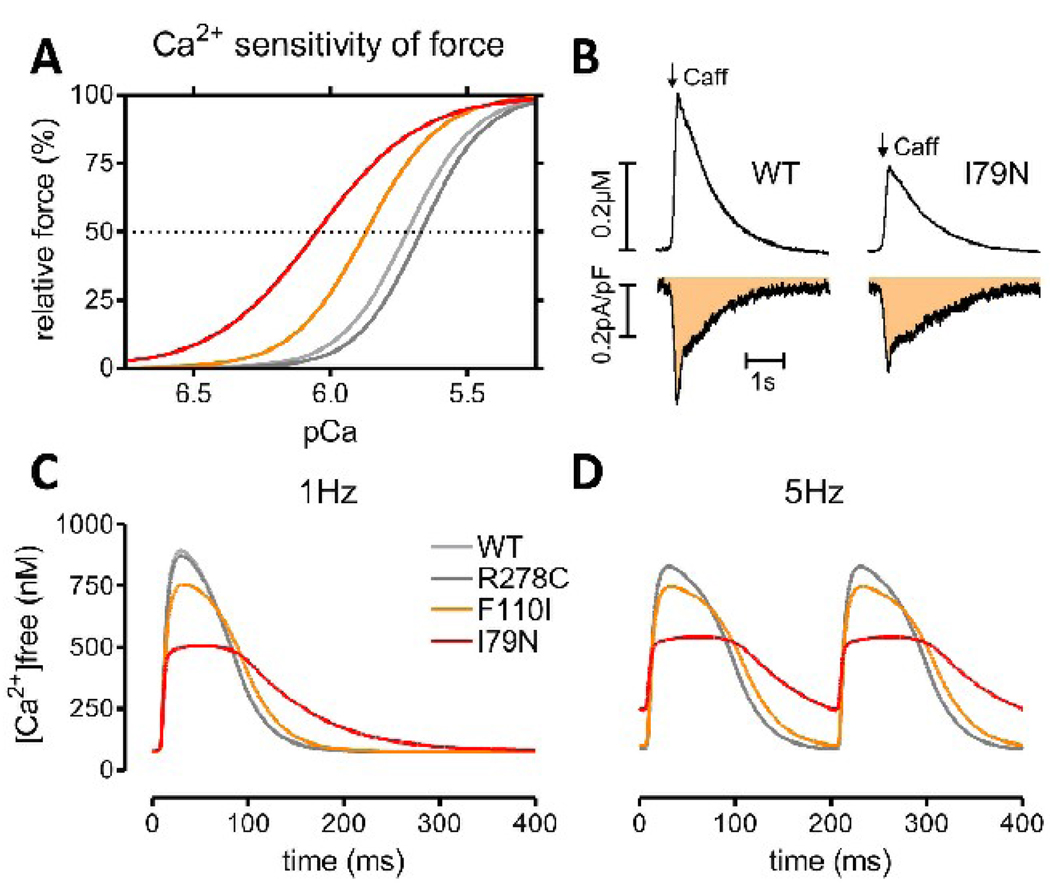
In intact myocytes, free [Ca2+] is determined by the rate of sarcolemmal and trans-sarcoplasmic reticulum Ca2+ fluxes and the Ca2+ buffering properties of the cytosol (reviewed in detail in [87]). TnC represents a substantial portion of cytoplasmic Ca2+ buffering, binding in the order of 50% of Ca2+ released from the sarcoplasmic reticulum during a typical heart beat [88]. Hence, an increase in myofilament Ca2+ sensitivity, if mediated by increasing the Ca2+ affinity of TnC (see above), can be predicted to decrease peak free [Ca2+] [50, 83]. This prediction has been confirmed experimentally by introducing exogenous Ca2+ buffers into ventricular myocytes: Two Ca2+ buffers both decreased peak systolic [Ca2+] and also slowed Ca2+ transient decline (while the exact kinetic effects were dependent on each buffers properties) [89]. Recently, we directly measured cytosolic Ca2+ buffering in myocytes from TnT mutant mice using two established techniques [90, 91]. The increase in free [Ca2+] in response to a matched total Ca2+ influx was significantly lower in TnT-I79N myocytes compared to control (Fig. 1, published in abstract form [92]). Consistent with this finding, in field-stimulated myocytes from TnT-I79N mice Ca2+ transient amplitude is reduced and decay is prolonged, while the diastolic [Ca2+] was increased in the presence of isoproterenol or when the cells were rapidly paced [84]. This result supports the idea that at peak [Ca2+] levels more Ca2+ is bound to the myofilaments, but as [Ca2+] decreases the Ca2+ dissociation from the myofilaments becomes the rate limiting step for Ca2+ transient decline. Fig. 1 illustrates this concept of how increased myofilament Ca2+ sensitivity may change cytosolic [Ca2+] during a typical heart beat. Slow Ca2+ transient decay kinetics were also found in papillary muscles from transgenic mice expressing a mutant regulatory light chain (R58Q) with increased myofilament Ca2+ sensitivity [61].
Fig. 1.
The effect of myofilament Ca2+ sensitization on myocyte Ca2+ handling. Panel (A) shows the effect of three TnT mutations on myofilament Ca2+ sensitivity measured in skinned fibers of transgenic mice [78]. Panel (B) shows the effect myofilament Ca2+ sensitization on apparent Ca2+ buffering. The change in total Ca2+ was measured by the Na/Ca exchanger integral (bottom, shaded area) simultaneously with the change in [Ca2+]free (top panel) in voltage clamped fluo-4 loaded myocytes [90, 92]. Note that the peak [Ca2+] is lower in TnT-I79N despite a similar change in total Ca2+, consistent with increased Ca2+ buffering. (C)+(D) Ca2+ transients simulated with LabHEART 5.0 [176] containing a newly integrated force response [177]. Ionic current conductances were adjusted to reproduce a typical mouse action potential. The myofilament Ca2+ sensitivity curves in (A) were reproduced by changing the TnC Ca2+ on/off rates and Ca2+ binding cooperativity. Ca2+ transients are simulated at 1 Hz (C) and 5 Hz (D) pacing frequency. Note that peak [Ca2+] is lower and Ca2+ transients are prolonged in Ca2+ sensitized mutants, while the diastolic [Ca2+] is additionally increased in TnT-I79N at 5 Hz. Panel (C)+(D) were kindly provided by Dr. J. Puglisi, UC Davis.
Myofilament Ca2+ sensitizing drugs can be expected to affect Ca2+ homeostasis similarly to some sarcomeric mutations, that is if they indeed increase Ca2+ binding affinity of TnC. Ca2+ sensitizers may also stabilize the Ca2+-induced conformation of TnC or have other downstream effects [93], e.g. direct effects on cross-bridge interaction as for example caffeine [94]. The mechanism of Ca2+ sensitization for each drug cannot be simply determined by measuring peak [Ca2+], but often requires direct assessment of Ca2+ binding to TnC. This is necessary because most Ca2+ sensitizers inhibit to some degree cyclic nucleotide phosphodiesterases (PDE), which in parallel enhances Ca2+ cycling through activation of PKA, like e.g. pimobendan [95]. However, EMD57033, together with CGP-48506 [96], seems to be a Ca2+ sensitizer with only negligible PDE inhibition at effective concentrations [80]. In ferret cardiac muscle, EMD57033 produced an enhanced and prolonged contraction, while it decreased Ca2+ transient amplitude, consistent with increased Ca2+ binding to TnC [80]. In the same study it appeared to shorten the Ca2+ transient at higher concentrations (20 µM), while 50 µM prolonged time to 80% Ca2+ decay and increased diastolic [Ca2+] without an effect on peak [Ca2+] in human ventricular muscle [22]. Thus, while it is unclear if EMD57033 increases TnC Ca2+ buffering, it likely has some effect on Ca2+ homeostasis in cardiomyocytes, while the exact effects still need to be determined.
Ca2+ handling and action potential (AP)
There is a clear interdependence between intracellular Ca2+ signaling and membrane potential. Ca2+ ion movements can affect the AP during the plateau phase (phase 2) in ventricular myocytes. L-type Ca2+ channel current is the major inward current during that phase and the Ca2+ that enters triggers Ca2+ induced Ca2+ release (CICR) from the SR. The inactivation of the L-type Ca2+ current greatly depends on Ca2+ induced inactivation, i.e. the higher the amplitude of the Ca2+ transient the more accelerated is the inactivation, creating a negative feedback loop (when the Ca2+ release is large Ca2+ entry is reduced) [97]. Therefore, if peak [Ca2+] is blunted due to increased myofilament Ca2+ sensitivity, the negative feedback is reduced and more Ca2+ enters during each AP. In TnT-I79N myocytes we failed to observe any changes in L-type Ca2+ current magnitude or inactivation, despite lower peak [Ca2+] [84]. However, a decrease in whole cell peak [Ca2+] due to increased myofilament Ca2+ buffering may not affect Ca2+ levels in the junctional cleft, where RyRs and L-type Ca2+ channels communicate.
Another opportunity for Ca2+ handling to affect the AP is through the activity of the Na/Ca exchanger (NCX). NCX is also active during the plateau phase producing a net-inward current by transporting Ca2+ out of the cell. Large NCX inward currents will therefore prolong the plateau phase. Since the Ca2+ transients are prolonged in myofilament Ca2+ sensitized myocytes, Ca2+ comes off more slowly, therefore potentially resulting in decreased NCX peak activity and slower activity decay. The result would be that the plateau phase is shorter, but overall AP duration may not necessarily be affected. This is exactly what we found before in TnT-I79N: the AP duration at 70% repolarization levels (APD70) is reduced at 1 and 5 Hz, while AP duration at 90% repolarization levels (APD90) is reduced at 1 Hz, but not at 5 Hz [84]. The AP differences were abolished by perfusion with Li+, which can enter though Na+ channels, but cannot be transported by the NCX, confirming the role of NCX activity. Similarly, we found a decreased APD70 after perfusion with EMD57033, consistent with the TnT mutant results, which was reversible upon washout of EMD57033. Thus it appears that APs are shorter and assume a more triangular shape (AP “triangulation”) when myofilaments are Ca2+ sensitized, due to a different time-course of NCX activity. It should be noted that APDs of higher mammals are less dependent on NCX activity and the application of the above mechanism may be limited [98, 99]. However, we also confirmed APD shortening of APD30, APD50 and APD70 in cat myocytes, which have AP shapes closely resembling human APs.
Ca2+ handling and ventricular arrhythmias
In order to discuss how Ca2+ handling may contribute to ventricular arrhythmia susceptibility, we briefly review several characteristics of arrhythmias found in association with increased myofilament Ca2+ sensitivity. First, arrhythmias can be initiated by fast pacing rates (hearts with highest degree of Ca2+ sensitization required the lowest pacing frequencies for induction) [78], which is consistent with the observation that fast heart rates often precede arrhythmic events in patients with hypertrophic cardiomyopathy [100, 101]. Second, optical mapping studies revealed that the spatial dispersion of ventricular activation (determined by standard deviation of conduction velocity (CV) at 10 different angles) is increased in myofilament Ca2+ sensitized TnT mutants at higher pacing frequencies. This regional slowing of CV was similarly observed after perfusion of control hearts with EMD57033, confirming that this is not due to intrinsic structural abnormalities [78]. Third, the repetitive activation patterns observed during VT, e.g. stationary rotors, are indicative of reentry-type arrhythmia [78].
These findings support the notion that the observed regional abnormalities of conduction provide the substrate for the arrhythmia [102], possibly forming zones of cells that are refractory and temporarily unexcitable (conduction block). Once initiated, the arrhythmia is then maintained by a regenerative circuit of electrical activity around relatively inexcitable tissue (re-entry). Local alterations in channel function or passive tissue properties can also disrupt the normal activation pattern and contribute to altered conduction patterns and reentrant arrhythmias.
One of the best supported possibilities how altered intracellular Ca2+ handling may support reentrant arrhythmias is the occurrence of Ca2+ transient alternans [103–105]. Briefly, myocytes with prolonged Ca2+ transients (e.g. induced by myofilament Ca2+ sensitization) are more likely to develop Ca2+ transient alternans at fast heart rates [106]. As discussed earlier, Ca2+ handling will affect APD via Ca2+-sensitive membrane currents and APD will also begin to alternate (also visa versa). This bidirectional coupling between Ca2+ homeostasis and APD can drive the Ca2+ transient of two neighboring cells to be out-of-phase, which will result in spatially discordant alternans on the tissue level (independent of CV restitution) [107]. The spatial gradients in APD or Ca2+ transient amplitude are very steep at the nodal line, which separates the out-of-phase regions, predisposing to localized conduction block [108–111]. Under these conditions, ectopic beats have a high probability of inducing re-entry [111].
Another possibility is based on the shortening of APD as a consequence of lower peak [Ca2+ ] observed in TnT mutants myocytes. Shorter APD, or AP “triangulation”, if associated with beat-to-beat instability (as demonstrated in cat heart after EMD57033 perfusion [78]), has been shown to be a strong predictor of drug-induced proarrhythmia in vivo [112]. Mechanistically, the AP “triangulation” may be associated with a shallow terminal AP repolarization slope, which could lead to incomplete and varying recovery of Na+ channels and subsequently to APD alternans during fast pacing.
Together, these two mechanisms may be responsible to provide a substrate that is able to sustain VT, but in most cases an additional triggering mechanism is still required. A possible hypothesis is that the increased diastolic [Ca2+ ] at fast pacing rates shifts into the SR during pauses and causes greater post-rest Ca2+ transient potentiation. This in turn will lead to considerable post-pause APD prolongation and, if long enough for the L-type Ca2+ current to recover from inactivation, the generation of early afterdepolarizations (EADs) [113]. In addition to a mechanism based on reactivation of L-type current, early or delayed afterdepolarizations can be induced by spontaneous Ca2+ release from the above steady-state filled SR after a pause and activate the NCX inward current (reviewed in [114]). However, post-rest increase of the SR load is species dependent and both mechanisms may only apply to rodents [115]. Another consideration is that the slow Ca2+ transient decay and/or increased diastolic [Ca2+ ] activates calmodulin-dependent kinase 2 (CaMKII), which has been shown to cause EADs in mice [116] and in a dog model [117].
(2) energy metabolism
There are several reports documenting an energetic deficits in FHC and, just like myofilament Ca2+ sensitization, it appears to be a common feature (reviewed in [85, 118]). The positive inotropic effect associated with myofilament Ca2+ sensitization, expected when Ca2+ cycling remains unaltered, can be predicted to proportionally increase ATP utilization as a direct consequence of increased myosin ATPase activity [83]. In addition, inefficient energy utilization, i.e. increased Ca2+ activated ATPase rate divided by tension (increased “tension cost“ [119]), or simply put, more ATP is hydrolyzed to generate the same amount of force, may further raise the energy demand. This particular feature does not appear to require an increase in myofilament Ca2+ sensitivity, considering that an increased tension cost was demonstrated in some FHC models with increased [51] as well as decreased [120, 121] myofilament Ca2+ sensitivity. However, in animals [86, 122, 123] and humans alike [124–126], the increased energy use leads to a decrease in high-energy phosphates in the FHC heart (predominantly phosphocreatine). This demonstrates that the increased energy utilization creates an energetic deficit and possible metabolic remodeling in vivo, without a primary defect in energy supply.
Ca2+ sensitizers are expected to improve the energetic economy of contraction when compared to other positive inotropic agents such as catecholamines [127–132]. Nevertheless, any intervention that increases contractile force by increasing myosin ATPase activity will consume more energy and increase energy demand (unless an agent actually decreases tension cost [133]). Thus, increased myofilament Ca2+ sensitivity is expected to increase myosin ATPase activity and the increased ATP utilization may cause deficits in energy supply. The effect of energy depletion on cardiac electrical activity has been intensively studied in the context of hypoxia and ischemia, but it is less clear to which extent these results are applicable to a potentially chronic energetic deficit induced by increased myofilament Ca2+ sensitivity.
Energetic deficit and AP
It is easily conceivable that increased ATP consumption by myosin ATPase subsequently affects other high energy consuming processes, e.g. the SR Ca2+ ATPase (SERCA) responsible for Ca2+ uptake into the SR. SERCA operates close to its theoretical thermodynamic limit and therefore is most vulnerable to decreases in free energy released from ATP hydrolysis (ΔGATP↓; that is expected if e.g. [ADP] increases) [134]. Decreased SERCA function would enhance the direct effect of increased myofilament Ca2+ sensitivity on Ca2+ homeostasis, by further decreasing Ca2+ transient amplitude and exacerbating transient prolongation as shown in Fig. 1 [135]. This may affect the AP via NCX as outlined above (prolonged Ca2+ transients).
The NCX activity is directly and indirectly dependent on [Ca2+], but also depends on [Na+ ] [136]. Thus, the effect of energy depletion on NCX becomes more complex if also Na+/K+ ATPase (NKA) activity, responsible for maintaining the Na+ and K+ gradient across the sarcolemma, becomes limited by insufficient energy supply. The primary consequence of NKA inhibition will be a rise in intracellular [Na+], which favors Ca2+ influx through the NCX during an AP, in contrast to the almost exclusive Ca2+ extrusion under physiological conditions [87]. This therefore would then generate first a transient outward current and the additional Ca2+ influx later enhances the transient inward current during the plateau phase, both resulting in altered AP shape.
A decrease in ΔGATP can also activate ATP-dependent K+ channels (KATP), which are present in high density in cardiac myocytes [137]. The single channel conductance is large and therefore the opening of very few channels (<1%) appears to be sufficient to shorten APD by as much as 50% [138, 139]. The opening of these channels may also contribute to the loss of intracellular [K+] and subsequent increase in extracellular [K+] during ischemia, which is an important factor for the formation of lethal ventricular arrhythmias (see below). Secondary to an elevation in extracellular [K+], the resting membrane potential increases (due to an increase in EK) and proportionally inactivates the inward Na+ current, causing slowing of AP upstroke and a decrease in AP amplitude [140]. The role of KATP channels in the net-loss of intracellular K+ however is controversial, and may depend more on an increase in intracellular [Na+] [141].
Recent findings also put forward a role of unpaired connexin43 (Cx43) hemichannels in disturbing ionic balances in the heart during conditions when energy supply is limited, e.g. ischemia and metabolic inhibition [142–145]. Hemichannels (or connexons) are inserted into the membrane to partner with other hemichannels from neighboring cells to form functional gap junction channels [146]. Based on theoretical estimates several thousand hemichannels exist in cardiac myocytes at any given time [143] and it has been demonstrated that metabolically sensitive hemichannels exist in cultured neonatal and isolated adult myocytes [144, 147]. Similar to the KATP channels, their unitary conductance for cations is high and the opening of just a few channels can be predicted to cause significant loss of intracellular K+ and intracellular loading of Na+ and Ca2+, with profound consequences for excitability and action potential shape. Hemichannel opening has been demonstrated during ischemia in neurons and likely contributes to neuronal death during stroke [148].
Energetic deficit and arrhythmias
In the case of compromised SERCA function the generation of arrhythmias is triggered by disturbances in Ca2+ homeostasis (see under Ca2+ handling and arrhythmias), but in the other cases discussed above it is due to primary disturbances in Na+/K+ homeostasis (inhibition of NKA, opening of KATP or Cx43 hemichannels). These later possibilities would all require a decrease in subsarcolemmal [ATP], but it is currently unclear which target would be most sensitive under conditions of a rising energetic deficit elicited by increased energy demand.
The consequences of intracellular Na+ overload, as a consequence of NKA inhibition and/or Cx43 hemichannel opening, has been recently reviewed [149]. Increased intracellular [Na+] is a solid predictor of ventricular fibrillation during hypoxia and therefore seems to contribute to electrical instability [150]. The increase in [Na+] secondarily promotes passive net K+ loss, which may be necessary to maintain electroneutrality and osmotic balance (sum of cations [K+] + [Na+] inside and outside remain unchanged) [141]. Extracellular K+ accumulation depolarizes the membrane, slows conduction and alters refractoriness and altogether these factors increase the probability of reentrant arrhythmias [151]. KATP channels contribute to the K+ loss, but their activation may also render a cell non-excitable (at membrane potentials above −70 mV sodium channels do not recover from inactivation [152]). This may be generally a protective mechanism that allows an energetically compromised cell to conserve energy. For example, KATP (Kir 6.2) is required for cellular adaptation during periods of stress [153] and may have a protective role during ischemia/reperfusion [154]. However, once non-excitable cells accumulate in one region this can cause conduction block and promote reentrant arrhythmias. Furthermore, epicardial cells may be more susceptible to KATP opening and APD shortening during metabolic inhibition than endocardial cells, i.e. epicardial APDs shortens more drastically than endocardial APDs [155]. This may further increase heterogeneity of repolarization, which has long been recognized to play a crucial role in the induction or maintenance of ventricular tachyarrhythmias [156, 157].
In addition to the role of Cx43 hemichannels in the formation of arrhythmias, alteration of Cx43 gap junction function may also contribute by influencing the precisely orchestrated patterns of impulse propagation. Gap junctions are intercellular channels that permit the transfer of electrical current and small molecules between directly adjacent cells [158]. Essentially all forms of cardiomyopathy are associated with changes in the expression and distribution of Cx43, e.g. in human hypertrophic cardiomyopathy a redistribution from the intercalated disks to the sides of the myocytes (lateralization) has been described [159]. Under control conditions myocardial Cx43 is highly phosphorylated at multiple carboxyterminal residues [160], but during global ischemia rapid Cx43 dephosphorylation paralleled the loss of gap junctional coupling within 30 min [161]. This dephosphorylation of Cx43 is reversible and tightly linked to cellular levels of ATP in neonatal rat cardiomyocytes [162]. This result is consistent with the observation that gap junctional current rapidly declined in adult rat cardiomyocytes due to dephosphorylation when no ATP was added to the patch pipette solution [163]. Thus, cellular uncoupling is a potential mechanism that contributes to abnormal impulse conduction under conditions of increased energy demand. It may not be in itself sufficient to initiate arrhythmias, as coupling has to be reduced to very low levels to slow conduction [164], but it may be a contributing factor by enhancing APD heterogeneity [165].
(3) mechanical stress/strain
Ca2+ sensitized myofilaments will by definition produce more force for a given [Ca2+]. Therefore, as expected, systolic function was enhanced in myofilament Ca2+ sensitized TnT-I79N mice under baseline conditions in vivo [166] and ex vivo (physiological [Ca2+]) and in control hearts after treatment with EMD57033 [167]. However, at fast heart rates and/or in the presence of β-adrenergic stimulation myofilament Ca2+ sensitized hearts show impaired systolic and diastolic performance [167]. The diastolic failure can be directly explained by the increased myofilament Ca2+ sensitivity induced by the TnT-I79N mutation, causing delayed diastolic separation of Ca2+ from the troponin complex and therefore slowing of relaxation. In vivo the pressure-volume relationship is shifted to the right (towards larger volume) and upwards (towards higher diastolic pressure) during isoproterenol stress, indicating increased mechanical stretch of the ventricular wall under those conditions [166].
Mechanical stress/strain and AP/arrhythmias
The crosstalk between mechanical stretch and AP is well established and has been demonstrated in various mammalian hearts and human (reviewed in [168, 169]). This relationship is often referred to as mechano-electric feedback (MEF) [170]. Generally an inverse relationship is found, such that increased intraventricular pressure causes shorter APDs. In addition to altering AP shape, increasing stretch raises the membrane potential until the depolarization reaches threshold and an action potential is triggered [170]. In intact canine hearts, both isolated and in situ, increases in ventricular volume and pressure resulted in a decrease in AP plateau duration (APD20) and appearance of early afterdepolarizations [171]. In rabbit heart the occurrence of triggered premature ventricular excitations was dependent on the amplitude and velocity of stretch application [172]. Regions that experience greater relative stretch may be susceptible to focal excitation and trigger stretch-activated arrhythmias, a theory that was supported by the observed spatial variability of AP properties [172]. In particular, moderate stretch may induce heterogeneous strain and focal excitation rather than larger stretch that causes synchronous excitation [173]. In intact rabbit hearts these focal excitations developed into reentrant arrhythmias.
5. Conclusions
Accumulative evidence suggest that increased myofilament Ca2+ sensitivity contributes to the risk for ventricular arrhythmias, but the underlying molecular mechanism remains unclear. Direct consequences of increased myofilament Ca2+ sensitivity, i.e. altered Ca2+ buffering/handling, impaired energy metabolism and increased mechanical stretch, each by itself or together in varying degree may be responsible for the proarrhythmic effect. Blebbistatin prevents arrhythmias in Ca2+ sensitized hearts by reducing myofilament Ca2+ sensitivity to control level [78] and can be expected to simultaneously prevent all molecular consequences (it interferes with actin-myosin interaction downstream of Ca2+ binding [174], by inhibition of myosin ATPase it preserves cellular ATP [175] and it minimizes stretch by inhibiting contraction [81]). Therefore it does not provide additional insights into the signaling pathway responsible for ventricular arrhythmias and additional detailed experiments are required.
This is an exciting area of research that may lead to development of improved Ca2+ sensitizers for inotropic support of failing hearts, or may clarify that Ca2+ desensitization is a life prolonging treatment. However, since Ca2+ desensitization might decrease heart pump function, other downstream targets are of therapeutic interest. The successful dissection of the signaling chain from myofilament Ca2+ sensitization to ventricular arrhythmias will allow the identification of new targets for the treatment of cardiac arrhythmias.
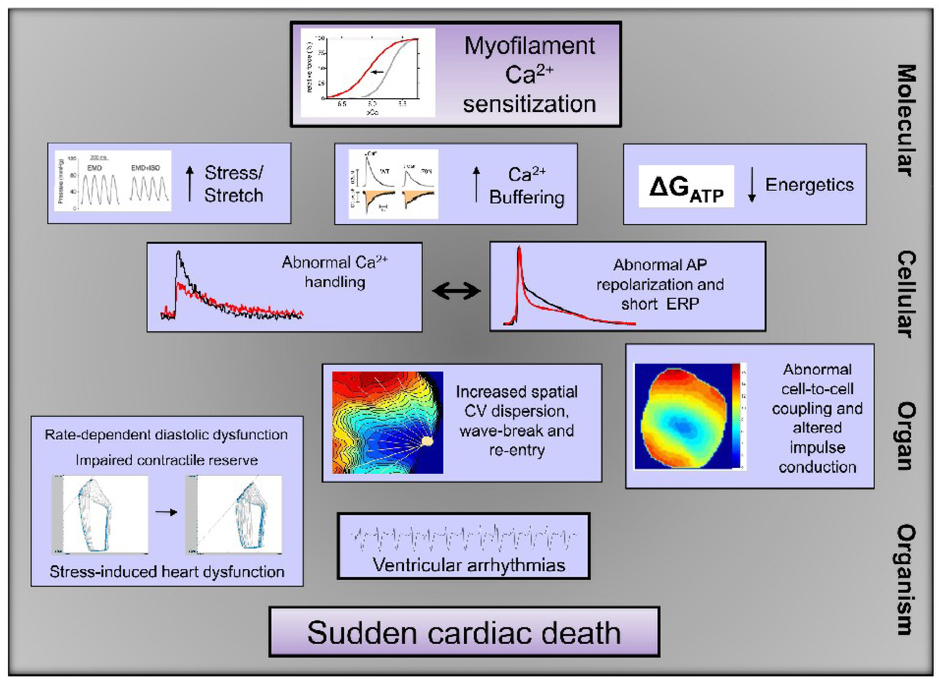
Fig. 2.
Mechanism of arrhythmogenesis caused by myofilament Ca2+ sensitization. The cartoon illustrates components likely involved in the signaling pathway from myofilament Ca2+ sensitization to sudden cardiac death. On the molecular level increased mechanical stress [166], increased Ca2+ buffering (published in abstract form [92]) and/or an energetic deficit (reduced free energy from ATP hydrolysis (ΔGATP)) [83] may contribute to the observed cellular changes in Ca2+ handling [84] and AP repolarization and effective refractory period (ERP)[78, 84]. On the organ level this results in stress-induced contractile dysfunction [49, 166]and abnormalities in propagation of excitation (increased conduction velocity (CV) dispersion [78]), possibly supported by abnormal cell-to-cell coupling (published in abstract form [178]). Together, these factors contribute to ventricular tachyarrhythmias and sudden death.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
NONE
References
- 1.Ebashi S, Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- 2.Gomes AV, Potter JD, Szczesna-Cordary D. The role of troponins in muscle contraction. IUBMB Life. 2002 Dec;54(6):323–333. doi: 10.1080/15216540216037. [DOI] [PubMed] [Google Scholar]
- 3.Huxley AF. Muscle structure and theories of contraction. Prog Biophys Biophys Chem. 1957;7:255–318. [PubMed] [Google Scholar]
- 4.Hibberd MG, Jewell BR. Calcium- and length-dependent force production in rat ventricular muscle. J Physiol. 1982 Aug;329:527–540. doi: 10.1113/jphysiol.1982.sp014317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circ Res. 1986 Jun;58(6):755–768. doi: 10.1161/01.res.58.6.755. [DOI] [PubMed] [Google Scholar]
- 6.Patterson SW, Piper H, Starling EH. The regulation of the heart beat. J Physiol. 1914 Oct 23;48(6):465–513. doi: 10.1113/jphysiol.1914.sp001676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solaro RJ. Modulation of cardiac myofilament activity by protein phosphorylation. New York: Oxford University Press; 2002. [Google Scholar]
- 8.Mope L, McClellan GB, Winegrad S. Calcium sensitivity of the contractile system and phosphorylation of troponin in hyperpermeable cardiac cells. J Gen Physiol. 1980 Mar;75(3):271–282. doi: 10.1085/jgp.75.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang R, Zhao J, Mandveno A, Potter JD. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995 Jun;76(6):1028–1035. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Desantiago J, Chu G, Kranias EG, Bers DM. Phosphorylation of phospholamban and troponin I in beta-adrenergic-induced acceleration of cardiac relaxation. Am J Physiol Heart Circ Physiol. 2000 Mar;278(3):H769–H779. doi: 10.1152/ajpheart.2000.278.3.H769. [DOI] [PubMed] [Google Scholar]
- 11.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, et al. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004 Nov 26;95(11):1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard EM, Solaro RJ. Inhibition of the activation and troponin calcium binding of dog cardiac myofibrils by acidic pH. Circ Res. 1984 Sep;55(3):382–391. doi: 10.1161/01.res.55.3.382. [DOI] [PubMed] [Google Scholar]
- 13.Fabiato A, Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kentish JC. The effects of inorganic phosphate and creatine phosphate on force production in skinned muscles from rat ventricle. J Physiol. 1986 Jan;370:585–604. doi: 10.1113/jphysiol.1986.sp015952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao WD, Atar D, Liu Y, Perez NG, Murphy AM, Marban E. Role of troponin I proteolysis in the pathogenesis of stunned myocardium. Circ Res. 1997 Mar;80(3):393–399. [PubMed] [Google Scholar]
- 16.Van Eyk JE, Powers F, Law W, Larue C, Hodges RS, Solaro RJ. Breakdown and release of myofilament proteins during ischemia and ischemia/reperfusion in rat hearts: identification of degradation products and effects on the pCa-force relation. Circ Res. 1998 Feb 9;82(2):261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 17.Namba T, Takaki M, Araki J, Ishioka K, Akashi T, Zhao LY, et al. Ca2+ sensitivity of contractile machinery and Ca2+ handling energy. Simulation. Jpn Heart J. 1993 Sep;34(5):601–616. doi: 10.1536/ihj.34.601. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen-Kudsk JE, Aldershvile J. Will calcium sensitizers play a role in the treatment of heart failure? J Cardiovasc Pharmacol. 1995;26 Suppl 1:S77–S84. [PubMed] [Google Scholar]
- 19.Solaro RJ, Gambassi G, Warshaw DM, Keller MR, Spurgeon HA, Beier N, et al. Stereoselective actions of thiadiazinones on canine cardiac myocytes and myofilaments. Circ Res. 1993 Dec;73(6):981–990. doi: 10.1161/01.res.73.6.981. [DOI] [PubMed] [Google Scholar]
- 20.Wolska BM, Kitada Y, Palmiter KA, Westfall MV, Johnson MD, Solaro RJ. CGP-48506 increases contractility of ventricular myocytes and myofilaments by effects on actin-myosin reaction. Am J Physiol. 1996 Jan;270(1 Pt 2):H24–H32. doi: 10.1152/ajpheart.1996.270.1.H24. [DOI] [PubMed] [Google Scholar]
- 21.Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res. 1995 Jul;77(1):107–113. doi: 10.1161/01.res.77.1.107. [DOI] [PubMed] [Google Scholar]
- 22.Hajjar RJ, Schmidt U, Helm P, Gwathmey JK. Ca++ sensitizers impair cardiac relaxation in failing human myocardium. J Pharmacol Exp Ther. 1997 Jan;280(1):247–254. [PubMed] [Google Scholar]
- 23.Webster KA, Bodi I, McNamara JP, Tracy M, Discher DJ, Bishopric NH. Negative lusitropy and abnormal calcium handling in hypoxic cardiac myocytes exposed to the calcium-sensitizer EMD 53998. J Mol Cell Cardiol. 1993 Jul;25(7):747–751. doi: 10.1006/jmcc.1993.1087. [DOI] [PubMed] [Google Scholar]
- 24.Kivikko M, Antila S, Eha J, Lehtonen L, Pentikainen PJ. Pharmacodynamics and safety of a new calcium sensitizer, levosimendan, and its metabolites during an extended infusion in patients with severe heart failure. J Clin Pharmacol. 2002 Jan;42(1):43–51. doi: 10.1177/0091270002042001005. [DOI] [PubMed] [Google Scholar]
- 25.Keren A, Syrris P, McKenna WJ. Hypertrophic cardiomyopathy: the genetic determinants of clinical disease expression. Nat Clin Pract Cardiovasc Med. 2008 Mar;5(3):158–168. doi: 10.1038/ncpcardio1110. [DOI] [PubMed] [Google Scholar]
- 26.Seggewiss H, Blank C, Pfeiffer B, Rigopoulos A. Hypertrophic cardiomyopathy as a cause of sudden death. Herz. 2009 Jun;34(4):305–314. doi: 10.1007/s00059-009-3248-z. [DOI] [PubMed] [Google Scholar]
- 27.Watkins H. Sudden death in hypertrophic cardiomyopathy. N Engl J Med. 2000 Feb 10;342(6):422–424. doi: 10.1056/NEJM200002103420609. [DOI] [PubMed] [Google Scholar]
- 28.Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, et al. Sudden death due to troponin T mutations. J Am Coll Cardiol. 1997 Mar 1;29(3):549–555. doi: 10.1016/s0735-1097(96)00530-x. [DOI] [PubMed] [Google Scholar]
- 29.Varnava A, Baboonian C, Davison F, de Cruz L, Elliott PM, Davies MJ, et al. A new mutation of the cardiac troponin T gene causing familial hypertrophic cardiomyopathy without left ventricular hypertrophy. Heart. 1999 Nov;82(5):621–624. doi: 10.1136/hrt.82.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O'Donoghue A, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995 Apr 20;332(16):1058–1064. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 31.Varnava AM, Elliott PM, Baboonian C, Davison F, Davies MJ, McKenna WJ. Hypertrophic cardiomyopathy: histopathological features of sudden death in cardiac troponin T disease. Circulation. 2001 Sep 18;104(12):1380–1384. doi: 10.1161/hc3701.095952. [DOI] [PubMed] [Google Scholar]
- 32.Van Driest SL, Ellsworth EG, Ommen SR, Tajik AJ, Gersh BJ, Ackerman MJ. Prevalence and spectrum of thin filament mutations in an outpatient referral population with hypertrophic cardiomyopathy. Circulation. 2003 Jul 29;108(4):445–451. doi: 10.1161/01.CIR.0000080896.52003.DF. [DOI] [PubMed] [Google Scholar]
- 33.Gomes AV, Potter JD. Molecular and cellular aspects of troponin cardiomyopathies. Ann N Y Acad Sci. 2004 May;1015:214–224. doi: 10.1196/annals.1302.018. [DOI] [PubMed] [Google Scholar]
- 34.Knollmann BC, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. Trends Cardiovasc Med. 2001 Jul;11(5):206–212. doi: 10.1016/s1050-1738(01)00115-3. [DOI] [PubMed] [Google Scholar]
- 35.Tardiff JC. Sarcomeric proteins and familial hypertrophic cardiomyopathy: linking mutations in structural proteins to complex cardiovascular phenotypes. Heart Fail Rev. 2005 Sep;10(3):237–248. doi: 10.1007/s10741-005-5253-5. [DOI] [PubMed] [Google Scholar]
- 36.Gomes AV, Potter JD. Cellular and molecular aspects of familial hypertrophic cardiomyopathy caused by mutations in the cardiac troponin I gene. Mol Cell Biochem. 2004 Aug;263(1–2):99–114. doi: 10.1023/B:MCBI.0000041852.42291.aa. [DOI] [PubMed] [Google Scholar]
- 37.Bottinelli R, Coviello DA, Redwood CS, Pellegrino MA, Maron BJ, Spirito P, et al. A mutant tropomyosin that causes hypertrophic cardiomyopathy is expressed in vivo and associated with an increased calcium sensitivity. Circ Res. 1998 Jan 9–23;82(1):106–115. doi: 10.1161/01.res.82.1.106. [DOI] [PubMed] [Google Scholar]
- 38.Elliott K, Watkins H, Redwood CS. Altered regulatory properties of human cardiac troponin I mutants that cause hypertrophic cardiomyopathy. J Biol Chem. 2000 Jul 21;275(29):22069–22074. doi: 10.1074/jbc.M002502200. [DOI] [PubMed] [Google Scholar]
- 39.Harada K, Potter JD. Familial hypertrophic cardiomyopathy mutations from different functional regions of troponin T result in different effects on the pH and Ca2+ sensitivity of cardiac muscle contraction. J Biol Chem. 2004 Apr 9;279(15):14488–14495. doi: 10.1074/jbc.M309355200. [DOI] [PubMed] [Google Scholar]
- 40.Harada K, Takahashi-Yanaga F, Minakami R, Morimoto S, Ohtsuki I. Functional consequences of the deletion mutation deltaGlu160 in human cardiac troponin T. J Biochem. 2000 Feb;127(2):263–268. doi: 10.1093/oxfordjournals.jbchem.a022603. [DOI] [PubMed] [Google Scholar]
- 41.Hernandez OM, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, et al. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem. 2005 Nov 4;280(44):37183–37194. doi: 10.1074/jbc.M508114200. [DOI] [PubMed] [Google Scholar]
- 42.Kruger M, Zittrich S, Redwood C, Blaudeck N, James J, Robbins J, et al. Effects of the mutation R145G in human cardiac troponin I on the kinetics of the contraction-relaxation cycle in isolated cardiac myofibrils. J Physiol. 2005 Apr 15;564(Pt 2):347–357. doi: 10.1113/jphysiol.2004.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morimoto S, Yanaga F, Minakami R, Ohtsuki I. Ca2+-sensitizing effects of the mutations at Ile-79 and Arg-92 of troponin T in hypertrophic cardiomyopathy. Am J Physiol. 1998 Jul;275(1 Pt 1):C200–C207. doi: 10.1152/ajpcell.1998.275.1.C200. [DOI] [PubMed] [Google Scholar]
- 44.Nakaura H, Morimoto S, Yanaga F, Nakata M, Nishi H, Imaizumi T, et al. Functional changes in troponin T by a splice donor site mutation that causes hypertrophic cardiomyopathy. Am J Physiol. 1999 Aug;277(2 Pt 1):C225–C232. doi: 10.1152/ajpcell.1999.277.2.C225. [DOI] [PubMed] [Google Scholar]
- 45.Nakaura H, Yanaga F, Ohtsuki I, Morimoto S. Effects of missense mutations Phe110Ile and Glu244Asp in human cardiac troponin T on force generation in skinned cardiac muscle fibers. J Biochem. 1999 Sep;126(3):457–460. doi: 10.1093/oxfordjournals.jbchem.a022473. [DOI] [PubMed] [Google Scholar]
- 46.Szczesna D, Zhang R, Zhao J, Jones M, Guzman G, Potter JD. Altered regulation of cardiac muscle contraction by troponin T mutations that cause familial hypertrophic cardiomyopathy. J Biol Chem. 2000 Jan 7;275(1):624–630. doi: 10.1074/jbc.275.1.624. [DOI] [PubMed] [Google Scholar]
- 47.Chandra M, Rundell VL, Tardiff JC, Leinwand LA, De Tombe PP, Solaro RJ. Ca(2+) activation of myofilaments from transgenic mouse hearts expressing R92Q mutant cardiac troponin T. Am J Physiol Heart Circ Physiol. 2001 Feb;280(2):H705–H713. doi: 10.1152/ajpheart.2001.280.2.H705. [DOI] [PubMed] [Google Scholar]
- 48.James J, Zhang Y, Osinska H, Sanbe A, Klevitsky R, Hewett TE, et al. Transgenic modeling of a cardiac troponin I mutation linked to familial hypertrophic cardiomyopathy. Circ Res. 2000 Oct 27;87(9):805–811. doi: 10.1161/01.res.87.9.805. [DOI] [PubMed] [Google Scholar]
- 49.Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, et al. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001 Mar 30;276(13):10039–10048. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- 50.Miller T, Szczesna D, Housmans PR, Zhao J, de Freitas F, Gomes AV, et al. Abnormal contractile function in transgenic mice expressing a familial hypertrophic cardiomyopathy-linked troponin T (I79N) mutation. J Biol Chem. 2001 Feb 9;276(6):3743–3755. doi: 10.1074/jbc.M006746200. [DOI] [PubMed] [Google Scholar]
- 51.Montgomery DE, Tardiff JC, Chandra M. Cardiac troponin T mutations: correlation between the type of mutation and the nature of myofilament dysfunction in transgenic mice. J Physiol. 2001 Oct 15;536(Pt 2):583–592. doi: 10.1111/j.1469-7793.2001.0583c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muthuchamy M, Pieples K, Rethinasamy P, Hoit B, Grupp IL, Boivin GP, et al. Mouse model of a familial hypertrophic cardiomyopathy mutation in alpha-tropomyosin manifests cardiac dysfunction. Circ Res. 1999 Jul 9;85(1):47–56. doi: 10.1161/01.res.85.1.47. [DOI] [PubMed] [Google Scholar]
- 53.Sanbe A, James J, Tuzcu V, Nas S, Martin L, Gulick J, et al. Transgenic rabbit model for human troponin I-based hypertrophic cardiomyopathy. Circulation. 2005 May 10;111(18):2330–2338. doi: 10.1161/01.CIR.0000164234.24957.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, et al. A truncated cardiac troponin T molecule in transgenic mice suggests multiple cellular mechanisms for familial hypertrophic cardiomyopathy. J Clin Invest. 1998 Jun 15;101(12):2800–2811. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kirschner SE, Becker E, Antognozzi M, Kubis HP, Francino A, Navarro-Lopez F, et al. Hypertrophic cardiomyopathy-related beta-myosin mutations cause highly variable calcium sensitivity with functional imbalances among individual muscle cells. Am J Physiol Heart Circ Physiol. 2005 Mar;288(3):H1242–H1251. doi: 10.1152/ajpheart.00686.2004. [DOI] [PubMed] [Google Scholar]
- 56.Palmer BM, Fishbaugher DE, Schmitt JP, Wang Y, Alpert NR, Seidman CE, et al. Differential cross-bridge kinetics of FHC myosin mutations R403Q and R453C in heterozygous mouse myocardium. Am J Physiol Heart Circ Physiol. 2004 Jul;287(1):H91–H99. doi: 10.1152/ajpheart.01015.2003. [DOI] [PubMed] [Google Scholar]
- 57.Palmiter KA, Tyska MJ, Haeberle JR, Alpert NR, Fananapazir L, Warshaw DM. R403Q and L908V mutant beta-cardiac myosin from patients with familial hypertrophic cardiomyopathy exhibit enhanced mechanical performance at the single molecule level. J Muscle Res Cell Motil. 2000;21(7):609–620. doi: 10.1023/a:1005678905119. [DOI] [PubMed] [Google Scholar]
- 58.Witt CC, Gerull B, Davies MJ, Centner T, Linke WA, Thierfelder L. Hypercontractile properties of cardiac muscle fibers in a knock-in mouse model of cardiac myosin-binding protein-C. J Biol Chem. 2001 Feb 16;276(7):5353–5359. doi: 10.1074/jbc.M008691200. [DOI] [PubMed] [Google Scholar]
- 59.Yang Q, Sanbe A, Osinska H, Hewett TE, Klevitsky R, Robbins J. A mouse model of myosin binding protein C human familial hypertrophic cardiomyopathy. J Clin Invest. 1998 Oct 1;102(7):1292–1300. doi: 10.1172/JCI3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Szczesna-Cordary D, Guzman G, Zhao J, Hernandez O, Wei J, Diaz-Perez Z. The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J Cell Sci. 2005 Aug 15;118(Pt 16):3675–3683. doi: 10.1242/jcs.02492. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Xu Y, Kerrick WG, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol. 2006 Aug 11;361(2):286–299. doi: 10.1016/j.jmb.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 62.van der Velden J, Merkus D, Klarenbeek BR, James AT, Boontje NM, Dekkers DH, et al. Alterations in myofilament function contribute to left ventricular dysfunction in pigs early after myocardial infarction. Circ Res. 2004 Nov 26;95(11):e85–e95. doi: 10.1161/01.RES.0000149531.02904.09. [DOI] [PubMed] [Google Scholar]
- 63.de Waard MC, van der Velden J, Bito V, Ozdemir S, Biesmans L, Boontje NM, et al. Early exercise training normalizes myofilament function and attenuates left ventricular pump dysfunction in mice with a large myocardial infarction. Circ Res. 2007 Apr 13;100(7):1079–1088. doi: 10.1161/01.RES.0000262655.16373.37. [DOI] [PubMed] [Google Scholar]
- 64.Billman GE, Kukielka M. Effects of endurance exercise training on heart rate variability and susceptibility to sudden cardiac death: protection is not due to enhanced cardiac vagal regulation. J Appl Physiol. 2006 Mar;100(3):896–906. doi: 10.1152/japplphysiol.01328.2005. [DOI] [PubMed] [Google Scholar]
- 65.Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation. 1984 Jun;69(6):1182–1189. doi: 10.1161/01.cir.69.6.1182. [DOI] [PubMed] [Google Scholar]
- 66.Jolliffe JA, Rees K, Taylor RS, Thompson D, Oldridge N, Ebrahim S. Exercise-based rehabilitation for coronary heart disease. Cochrane Database Syst Rev. 2001;(1):CD001800. doi: 10.1002/14651858.CD001800. [DOI] [PubMed] [Google Scholar]
- 67.Billman GE. Cardiac autonomic neural remodeling and susceptibility to sudden cardiac death: effect of endurance exercise training. Am J Physiol Heart Circ Physiol. 2009 Oct;297(4):H1171–H1193. doi: 10.1152/ajpheart.00534.2009. [DOI] [PubMed] [Google Scholar]
- 68.Flevari P, Parissis JT, Leftheriotis D, Panou F, Kourea K, Kremastinos DT. Effect of levosimendan on ventricular arrhythmias and prognostic autonomic indexes in patients with decompensated advanced heart failure secondary to ischemic or dilated cardiomyopathy. Am J Cardiol. 2006 Dec 15;98(12):1641–1645. doi: 10.1016/j.amjcard.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 69.van der Velden J, Papp Z, Zaremba R, Boontje NM, de Jong JW, Owen VJ, et al. Increased Ca2+-sensitivity of the contractile apparatus in end-stage human heart failure results from altered phosphorylation of contractile proteins. Cardiovasc Res. 2003 Jan;57(1):37–47. doi: 10.1016/s0008-6363(02)00606-5. [DOI] [PubMed] [Google Scholar]
- 70.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J Clin Invest. 1996 Jul 1;98(1):167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brixius K, Savvidou-Zaroti P, Mehlhorn U, Bloch W, Kranias EG, Schwinger RH. Increased Ca2+-sensitivity of myofibrillar tension in heart failure and its functional implication. Basic Res Cardiol. 2002;97 Suppl 1:I111–I117. doi: 10.1007/s003950200039. [DOI] [PubMed] [Google Scholar]
- 72.Ardelt P, Dorka P, Jaquet K, Heilmeyer LM, Jr, Kortke H, Korfer B, et al. Microanalysis and distribution of cardiac troponin I phospho species in heart areas. Biol Chem. 1998 Mar;379(3):341–347. doi: 10.1515/bchm.1998.379.3.341. [DOI] [PubMed] [Google Scholar]
- 73.Bodor GS, Oakeley AE, Allen PD, Crimmins DL, Ladenson JH, Anderson PA. Troponin I phosphorylation in the normal and failing adult human heart. Circulation. 1997 Sep 2;96(5):1495–1500. doi: 10.1161/01.cir.96.5.1495. [DOI] [PubMed] [Google Scholar]
- 74.Messer AE, Jacques AM, Marston SB. Troponin phosphorylation and regulatory function in human heart muscle: dephosphorylation of Ser23/24 on troponin I could account for the contractile defect in end-stage heart failure. J Mol Cell Cardiol. 2007 Jan;42(1):247–259. doi: 10.1016/j.yjmcc.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 75.Marston SB, de Tombe PP. Troponin phosphorylation and myofilament Ca2+-sensitivity in heart failure: increased or decreased? J Mol Cell Cardiol. 2008 Nov;45(5):603–607. doi: 10.1016/j.yjmcc.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wolff MR, Whitesell LF, Moss RL. Calcium sensitivity of isometric tension is increased in canine experimental heart failure. Circ Res. 1995 May;76(5):781–789. doi: 10.1161/01.res.76.5.781. [DOI] [PubMed] [Google Scholar]
- 77.Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, et al. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol. 2007 Jul 15;582(Pt 2):695–709. doi: 10.1113/jphysiol.2007.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest. 2008 Dec;118(12):3893–3903. doi: 10.1172/JCI36642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, et al. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol. 1999 May 15;517(Pt 1):143–157. doi: 10.1111/j.1469-7793.1999.0143z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.White J, Lee JA, Shah N, Orchard CH. Differential effects of the optical isomers of EMD 53998 on contraction and cytoplasmic Ca2+ in isolated ferret cardiac muscle. Circ Res. 1993 Jul;73(1):61–70. doi: 10.1161/01.res.73.1.61. [DOI] [PubMed] [Google Scholar]
- 81.Dou Y, Arlock P, Arner A. Blebbistatin specifically inhibits actin-myosin interaction in mouse cardiac muscle. Am J Physiol Cell Physiol. 2007 Sep;293(3):C1148–C1153. doi: 10.1152/ajpcell.00551.2006. [DOI] [PubMed] [Google Scholar]
- 82.Fedorov VV, Lozinsky IT, Sosunov EA, Anyukhovsky EP, Rosen MR, Balke CW, et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007 May;4(5):619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 83.Kataoka A, Hemmer C, Chase PB. Computational simulation of hypertrophic cardiomyopathy mutations in troponin I: influence of increased myofilament calcium sensitivity on isometric force, ATPase and [Ca2+]i. J Biomech. 2007;40(9):2044–2052. doi: 10.1016/j.jbiomech.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 84.Knollmann BC, Kirchhof P, Sirenko SG, Degen H, Greene AE, Schober T, et al. Familial hypertrophic cardiomyopathy-linked mutant troponin T causes stress-induced ventricular tachycardia and Ca2+-dependent action potential remodeling. Circ Res. 2003 Mar 7;92(4):428–436. doi: 10.1161/01.RES.0000059562.91384.1A. [DOI] [PubMed] [Google Scholar]
- 85.Ashrafian H, Redwood C, Blair E, Watkins H. Hypertrophic cardiomyopathy:a paradigm for myocardial energy depletion. Trends Genet. 2003 May;19(5):263–268. doi: 10.1016/S0168-9525(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 86.Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003 Sep;112(5):768–775. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Second Edition ed. Dordrecht: KLUWER Academic Publishers; 2001. [Google Scholar]
- 88.Shannon TR, Ginsburg KS, Bers DM. Reverse mode of the sarcoplasmic reticulum calcium pump and load-dependent cytosolic calcium decline in voltage-clamped cardiac ventricular myocytes. Biophys J. 2000 Jan;78(1):322–333. doi: 10.1016/S0006-3495(00)76595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diaz ME, Trafford AW, Eisner DA. The effects of exogenous calcium buffers on the systolic calcium transient in rat ventricular myocytes. Biophys J. 2001 Apr;80(4):1915–1925. doi: 10.1016/S0006-3495(01)76161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Trafford AW, Diaz ME, Eisner DA. A novel, rapid and reversible method to measure Ca buffering and time-course of total sarcoplasmic reticulum Ca content in cardiac ventricular myocytes. Pflugers Arch. 1999 Feb;437(3):501–503. doi: 10.1007/s004240050808. [DOI] [PubMed] [Google Scholar]
- 91.Bassani RA, Shannon TR, Bers DM. Passive Ca2+ binding in ventricular myocardium of neonatal and adult rats. Cell Calcium. 1998 Jun;23(6):433–442. doi: 10.1016/s0143-4160(98)90100-2. [DOI] [PubMed] [Google Scholar]
- 92.Gryshchenko O, Huke S, Baudenbacher F, Potter JD, Knollmann BC. Ca2+ sensitizing troponin T mutations linked to hypertrophic cardiomyopathy increase apparent cytosolic Ca2+ binding. Biophysical Journal. 2009 Suppl.96:513a. [Google Scholar]
- 93.Haikala H, Pollesello P. Calcium sensitivity enhancers. IDrugs. 2000 Oct;3(10):1199–1205. [PubMed] [Google Scholar]
- 94.Powers FM, Solaro RJ. Caffeine alters cardiac myofilament activity and regulation independently of Ca2+ binding to troponin C. Am J Physiol. 1995 Jun;268(6 Pt 1):C1348–C1353. doi: 10.1152/ajpcell.1995.268.6.C1348. [DOI] [PubMed] [Google Scholar]
- 95.Takahashi R, Endoh M. Increase in myofibrillar Ca2+ sensitivity induced by UD-CG 212 Cl, an active metabolite of pimobendan, in canine ventricular myocardium. J Cardiovasc Pharmacol. 2001 Feb;37(2):209–218. doi: 10.1097/00005344-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Neumann J, Eschenhagen T, Grupp IL, Haverich A, Herzig JW, Hirt S, et al. Positive inotropic effects of the calcium sensitizer CGP 48506 in failing human myocardium. J Pharmacol Exp Ther. 1996 Jun;277(3):1579–1585. [PubMed] [Google Scholar]
- 97.Puglisi JL, Yuan W, Bassani JW, Bers DM. Ca(2+) influx through Ca(2+) channels in rabbit ventricular myocytes during action potential clamp: influence of temperature. Circ Res. 1999 Sep 17;85(6):e7–e16. doi: 10.1161/01.res.85.6.e7. [DOI] [PubMed] [Google Scholar]
- 98.duBell WH, Boyett MR, Spurgeon HA, Talo A, Stern MD, Lakatta EG. The cytosolic calcium transient modulates the action potential of rat ventricular myocytes. J Physiol. 1991 May;436:347–369. doi: 10.1113/jphysiol.1991.sp018554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.London B. Cardiac arrhythmias: from (transgenic) mice to men. J Cardiovasc Electrophysiol. 2001 Sep;12(9):1089–1091. doi: 10.1046/j.1540-8167.2001.01089.x. [DOI] [PubMed] [Google Scholar]
- 100.Cha YM, Gersh BJ, Maron BJ, Boriani G, Spirito P, Hodge DO, et al. Electrophysiologic manifestations of ventricular tachyarrhythmias provoking appropriate defibrillator interventions in high-risk patients with hypertrophic cardiomyopathy. J Cardiovasc Electrophysiol. 2007 May;18(5):483–487. doi: 10.1111/j.1540-8167.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 101.Pablo Kaski J, Tome Esteban MT, Lowe M, Sporton S, Rees P, Deanfield JE, et al. Outcomes after implantable cardioverter-defibrillator treatment in children with hypertrophic cardiomyopathy. Heart. 2007 Mar;93(3):372–374. doi: 10.1136/hrt.2006.094730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boersma L, Zetelaki Z, Brugada J, Allessie M. Polymorphic reentrant ventricular tachycardia in the isolated rabbit heart studied by high-density mapping. Circulation. 2002 Jun 25;105(25):3053–3061. doi: 10.1161/01.cir.0000019407.35848.af. [DOI] [PubMed] [Google Scholar]
- 103.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008 Jan;44(1):31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laurita KR, Rosenbaum DS. Cellular mechanisms of arrhythmogenic cardiac alternans. Prog Biophys Mol Biol. 2008 Jun–Jul;97(2–3):332–347. doi: 10.1016/j.pbiomolbio.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006 May 26;98(10):1244–1253. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 106.Goldhaber JI, Xie LH, Duong T, Motter C, Khuu K, Weiss JN. Action potential duration restitution and alternans in rabbit ventricular myocytes: the key role of intracellular calcium cycling. Circ Res. 2005 Mar 4;96(4):459–466. doi: 10.1161/01.RES.0000156891.66893.83. [DOI] [PubMed] [Google Scholar]
- 107.Sato D, Shiferaw Y, Garfinkel A, Weiss JN, Qu Z, Karma A. Spatially discordant alternans in cardiac tissue: role of calcium cycling. Circ Res. 2006 Sep 1;99(5):520–527. doi: 10.1161/01.RES.0000240542.03986.e7. [DOI] [PubMed] [Google Scholar]
- 108.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001 Feb;12(2):196–206. doi: 10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 109.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999 Mar 16;99(10):1385–1394. doi: 10.1161/01.cir.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 110.Henry H, Rappel WJ. Dynamics of conduction blocks in a model of paced cardiac tissue. Phys Rev E Stat Nonlin Soft Matter Phys. 2005 May;71(5 Pt 1):051911. doi: 10.1103/PhysRevE.71.051911. [DOI] [PubMed] [Google Scholar]
- 111.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000 Oct 3;102(14):1664–1670. doi: 10.1161/01.cir.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 112.Hondeghem LM, Carlsson L, Duker G. Instability and triangulation of the action potential predict serious proarrhythmia, but action potential duration prolongation is antiarrhythmic. Circulation. 2001 Apr 17;103(15):2004–2013. doi: 10.1161/01.cir.103.15.2004. [DOI] [PubMed] [Google Scholar]
- 113.January CT, Moscucci A. Cellular mechanisms of early afterdepolarizations. Ann N Y Acad Sci. 1992 Jan 27;644:23–32. doi: 10.1111/j.1749-6632.1992.tb30999.x. [DOI] [PubMed] [Google Scholar]
- 114.Sipido KR, Bito V, Antoons G, Volders PG, Vos MA. Na/Ca exchange and cardiac ventricular arrhythmias. Ann N Y Acad Sci. 2007 Mar;1099:339–348. doi: 10.1196/annals.1387.066. [DOI] [PubMed] [Google Scholar]
- 115.Bers DM, Bassani RA, Bassani JW, Baudet S, Hryshko LV. Paradoxical twitch potentiation after rest in cardiac muscle: increased fractional release of SR calcium. J Mol Cell Cardiol. 1993 Sep;25(9):1047–1057. doi: 10.1006/jmcc.1993.1117. [DOI] [PubMed] [Google Scholar]
- 116.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, et al. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002 Sep 3;106(10):1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 117.Tanskanen AJ, Greenstein JL, O'Rourke B, Winslow RL. The role of stochastic and modal gating of cardiac L-type Ca2+ channels on early after-depolarizations. Biophys J. 2005 Jan;88(1):85–95. doi: 10.1529/biophysj.104.051508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004 Jul 23;95(2):135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 119.de Tombe PP, Stienen GJ. Impact of temperature on cross-bridge cycling kinetics in rat myocardium. J Physiol. 2007 Oct 15;584(Pt 2):591–600. doi: 10.1113/jphysiol.2007.138693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Frey N, Brixius K, Schwinger RH, Benis T, Karpowski A, Lorenzen HP, et al. Alterations of tension-dependent ATP utilization in a transgenic rat model of hypertrophic cardiomyopathy. J Biol Chem. 2006 Oct 6;281(40):29575–29582. doi: 10.1074/jbc.M507740200. [DOI] [PubMed] [Google Scholar]
- 121.Sweeney HL, Feng HS, Yang Z, Watkins H. Functional analyses of troponin T mutations that cause hypertrophic cardiomyopathy: insights into disease pathogenesis and troponin function. Proc Natl Acad Sci U S A. 1998 Nov 24;95(24):14406–14410. doi: 10.1073/pnas.95.24.14406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Spindler M, Saupe KW, Christe ME, Sweeney HL, Seidman CE, Seidman JG, et al. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest. 1998 Apr 15;101(8):1775–1783. doi: 10.1172/JCI1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Luedde M, Flogel U, Knorr M, Grundt C, Hippe HJ, Brors B, et al. Decreased contractility due to energy deprivation in a transgenic rat model of hypertrophic cardiomyopathy. J Mol Med. 2009 Apr;87(4):411–422. doi: 10.1007/s00109-008-0436-x. [DOI] [PubMed] [Google Scholar]
- 124.Jung WI, Sieverding L, Breuer J, Hoess T, Widmaier S, Schmidt O, et al. 31P NMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation. 1998 Jun 30;97(25):2536–2542. doi: 10.1161/01.cir.97.25.2536. [DOI] [PubMed] [Google Scholar]
- 125.Jung WI, Hoess T, Bunse M, Widmaier S, Sieverding L, Breuer J, et al. Differences in cardiac energetics between patients with familial and nonfamilial hypertrophic cardiomyopathy. Circulation. 2000 Mar 28;101(12):E121. doi: 10.1161/01.cir.101.12.e121. [DOI] [PubMed] [Google Scholar]
- 126.Crilley JG, Boehm EA, Blair E, Rajagopalan B, Blamire AM, Styles P, et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J Am Coll Cardiol. 2003 May 21;41(10):1776–1782. doi: 10.1016/s0735-1097(02)03009-7. [DOI] [PubMed] [Google Scholar]
- 127.Grandis DJ, DelNido PJ, Koretsky AP. Functional and energetic effects of the inotropic agents EMD-57033 and BAPTA on the isolated rat heart. Am J Physiol. 1995 Aug;269(2 Pt 1):C472–C479. doi: 10.1152/ajpcell.1995.269.2.C472. [DOI] [PubMed] [Google Scholar]
- 128.Holubarsch C, Hasenfuss G, Just H, Blanchard E, Mulieri LA, Alpert NR. Influence of the positive inotropic substance pimobendan (UD-CG 115 BS) on contractile economy of guinea pig papillary muscles. J Cardiovasc Pharmacol. 1989;14 Suppl 2:S13–S17. [PubMed] [Google Scholar]
- 129.Onishi K, Sekioka K, Ishisu R, Abe Y, Tanaka H, Nakamura M, et al. MCI-154, a Ca2+ sensitizer, decreases the oxygen cost of contractility in isolated canine hearts. Am J Physiol. 1997 Oct;273(4 Pt 2):H1688–H1695. doi: 10.1152/ajpheart.1997.273.4.H1688. [DOI] [PubMed] [Google Scholar]
- 130.Mori M, Takeuchi M, Takaoka H, Hata K, Hayashi Y, Yamakawa H, et al. Oxygen-saving effect of a new cardiotonic agent, MCI-154, in diseased human hearts. J Am Coll Cardiol. 1997 Mar 1;29(3):613–622. doi: 10.1016/s0735-1097(96)00534-7. [DOI] [PubMed] [Google Scholar]
- 131.Grandis DJ, MacGowan GA, Koretsky AP. Comparison of the effects of ORG 30029, dobutamine and high perfusate calcium on function and metabolism in rat heart. J Mol Cell Cardiol. 1998 Dec;30(12):2605–2612. doi: 10.1006/jmcc.1998.0817. [DOI] [PubMed] [Google Scholar]
- 132.MacGowan GA. The myofilament force-calcium relationship as a target for positive inotropic therapy in congestive heart failure. Cardiovasc Drugs Ther. 2005 May;19(3):203–210. doi: 10.1007/s10557-005-2465-9. [DOI] [PubMed] [Google Scholar]
- 133.Teerlink JR. A novel approach to improve cardiac performance: cardiac myosin activators. Heart Fail Rev. 2009 Feb 21; doi: 10.1007/s10741-009-9135-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tian R. Thermodynamic limitation for the sarcoplasmic reticulum Ca(2+)-ATPase contributes to impaired contractile reserve in hearts. Ann N Y Acad Sci. 1998 Sep 16;853:322–324. doi: 10.1111/j.1749-6632.1998.tb08290.x. [DOI] [PubMed] [Google Scholar]
- 135.Periasamy M, Huke S. SERCA pump level is a critical determinant of Ca(2+)homeostasis and cardiac contractility. J Mol Cell Cardiol. 2001 Jun;33(6):1053–1063. doi: 10.1006/jmcc.2001.1366. [DOI] [PubMed] [Google Scholar]
- 136.Bers DM, Despa S. Na(+) transport in cardiac myocytes; Implications for excitation-contraction coupling. IUBMB Life. 2009 Mar 61;(3):215–221. doi: 10.1002/iub.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983 Sep 8–14;305(5930):147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- 138.Nichols CG, Ripoll C, Lederer WJ. ATP-sensitive potassium channel modulation of the guinea pig ventricular action potential and contraction. Circ Res. 1991 Jan;68(1):280–287. doi: 10.1161/01.res.68.1.280. [DOI] [PubMed] [Google Scholar]
- 139.Wu TJ, Yashima M, Doshi R, Kim YH, Athill CA, Ong JJ, et al. Relation between cellular repolarization characteristics and critical mass for human ventricular fibrillation. J Cardiovasc Electrophysiol. 1999 Aug;10(8):1077–1086. doi: 10.1111/j.1540-8167.1999.tb00280.x. [DOI] [PubMed] [Google Scholar]
- 140.Whalley DW, Wendt DJ, Starmer CF, Rudy Y, Grant AO. Voltage-independent effects of extracellular K+ on the Na+ current and phase 0 of the action potential in isolated cardiac myocytes. Circ Res. 1994 Sep;75(3):491–502. doi: 10.1161/01.res.75.3.491. [DOI] [PubMed] [Google Scholar]
- 141.Shivkumar K, Deutsch NA, Lamp ST, Khuu K, Goldhaber JI, Weiss JN. Mechanism of hypoxic K loss in rabbit ventricle. J Clin Invest. 1997 Oct 1;100(7):1782–1788. doi: 10.1172/JCI119705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN. Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem. 1999 Jan 1;274(1):236–240. doi: 10.1074/jbc.274.1.236. [DOI] [PubMed] [Google Scholar]
- 143.John S, Cesario D, Weiss JN. Gap junctional hemichannels in the heart. Acta Physiol Scand. 2003 Sep;179(1):23–31. doi: 10.1046/j.1365-201X.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 144.Kondo RP, Wang SY, John SA, Weiss JN, Goldhaber JI. Metabolic inhibition activates a non-selective current through connexin hemichannels in isolated ventricular myocytes. J Mol Cell Cardiol. 2000 Oct;32(10):1859–1872. doi: 10.1006/jmcc.2000.1220. [DOI] [PubMed] [Google Scholar]
- 145.Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003 Apr;4(4):285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- 146.Solan JL, Lampe PD. Connexin phosphorylation as a regulatory event linked to gap junction channel assembly. Biochim Biophys Acta. 2005 Jun 10;1711(2):154–163. doi: 10.1016/j.bbamem.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 147.Clarke TC, Williams OJ, Martin PE, Evans WH. ATP release by cardiac myocytes in a simulated ischaemia model: inhibition by a connexin mimetic and enhancement by an antiarrhythmic peptide. Eur J Pharmacol. 2009 Mar 1;605(1–3):9–14. doi: 10.1016/j.ejphar.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 148.Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006 May 12;312(5775):924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- 149.Barth AS, Tomaselli GF. Cardiac metabolism and arrhythmias. Circ Arrhythm Electrophysiol. 2009 Jun;2(3):327–335. doi: 10.1161/CIRCEP.108.817320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neubauer S, Newell JB, Ingwall JS. Metabolic consequences and predictability of ventricular fibrillation in hypoxia. A 31P- and 23Na-nuclear magnetic resonance study of the isolated rat heart. Circulation. 1992 Jul;86(1):302–310. doi: 10.1161/01.cir.86.1.302. [DOI] [PubMed] [Google Scholar]
- 151.Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- 152.Beeler GW, Reuter H. Reconstruction of the action potential of ventricular myocardial fibres. J Physiol. 1977 Jun;268(1):177–210. doi: 10.1113/jphysiol.1977.sp011853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zingman LV, Hodgson DM, Bast PH, Kane GC, Perez-Terzic C, Gumina RJ, et al. Kir6.2 is required for adaptation to stress. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13278–13283. doi: 10.1073/pnas.212315199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suzuki M, Sasaki N, Miki T, Sakamoto N, Ohmoto-Sekine Y, Tamagawa M, et al. Role of sarcolemmal K(ATP) channels in cardioprotection against ischemia/reperfusion injury in mice. J Clin Invest. 2002 Feb;109(4):509–516. doi: 10.1172/JCI14270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Furukawa T, Kimura S, Furukawa N, Bassett AL, Myerburg RJ. Role of cardiac ATP-regulated potassium channels in differential responses of endocardial and epicardial cells to ischemia. Circ Res. 1991 Jun;68(6):1693–1702. doi: 10.1161/01.res.68.6.1693. [DOI] [PubMed] [Google Scholar]
- 156.Misier AR, Opthof T, van Hemel NM, Vermeulen JT, de Bakker JM, Defauw JJ, et al. Dispersion of 'refractoriness' in noninfarcted myocardium of patients with ventricular tachycardia or ventricular fibrillation after myocardial infarction. Circulation. 1995 May 15;91(10):2566–2572. doi: 10.1161/01.cir.91.10.2566. [DOI] [PubMed] [Google Scholar]
- 157.Han J, Moe GK. Nonuniform Recovery of Excitability in Ventricular Muscle. Circ Res. 1964 Jan;14:44–60. doi: 10.1161/01.res.14.1.44. [DOI] [PubMed] [Google Scholar]
- 158.Jansen JA, van Veen TA, de Bakker JM, van Rijen HV. Cardiac connexins and impulse propagation. J Mol Cell Cardiol. 2009 Aug 31; doi: 10.1016/j.yjmcc.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 159.Sepp R, Severs NJ, Gourdie RG. Altered patterns of cardiac intercellular junction distribution in hypertrophic cardiomyopathy. Heart. 1996 Nov;76(5):412–417. doi: 10.1136/hrt.76.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009 Apr 15;419(2):261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Beardslee MA, Lerner DL, Tadros PN, Laing JG, Beyer EC, Yamada KA, et al. Dephosphorylation and intracellular redistribution of ventricular connexin43 during electrical uncoupling induced by ischemia. Circ Res. 2000 Oct 13;87(8):656–662. doi: 10.1161/01.res.87.8.656. [DOI] [PubMed] [Google Scholar]
- 162.Turner MS, Haywood GA, Andreka P, You L, Martin PE, Evans WH, et al. Reversible connexin 43 dephosphorylation during hypoxia and reoxygenation is linked to cellular ATP levels. Circ Res. 2004 Oct 1;95(7):726–733. doi: 10.1161/01.RES.0000144805.11519.1e. [DOI] [PubMed] [Google Scholar]
- 163.Verrecchia F, Duthe F, Duval S, Duchatelle I, Sarrouilhe D, Herve JC. ATP counteracts the rundown of gap junctional channels of rat ventricular myocytes by promoting protein phosphorylation. J Physiol. 1999 Apr 15;516(Pt 2):447–459. doi: 10.1111/j.1469-7793.1999.0447v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Jongsma HJ, Wilders R. Gap junctions in cardiovascular disease. Circ Res. 2000 Jun 23;86(12):1193–1197. doi: 10.1161/01.res.86.12.1193. [DOI] [PubMed] [Google Scholar]
- 165.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004 Oct;287(4):H1762–H1770. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 166.Westermann D, Knollmann BC, Steendijk P, Rutschow S, Riad A, Pauschinger M, et al. Diltiazem treatment prevents diastolic heart failure in mice with familial hypertrophic cardiomyopathy. Eur J Heart Fail. 2006 Mar;8(2):115–121. doi: 10.1016/j.ejheart.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 167.Sirenko SG, Potter JD, Knollmann BC. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts--role of myofilament Ca2+ sensitivity increase. J Physiol. 2006 Aug 15;575(Pt 1):201–213. doi: 10.1113/jphysiol.2006.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ravens U. Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol. 2003 May–Jul;82(1–3):255–266. doi: 10.1016/s0079-6107(03)00026-9. [DOI] [PubMed] [Google Scholar]
- 169.Taggart P, Lab M. Cardiac mechano-electric feedback and electrical restitution in humans. Prog Biophys Mol Biol. 2008 Jun–Jul;97(2–3):452–460. doi: 10.1016/j.pbiomolbio.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 170.Kaufmann R, Theophile U. Autonomously promoted extension effect in Purkinje fibers, papillary muscles and trabeculae carneae of rhesus monkeys. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;297(3):174–189. [PubMed] [Google Scholar]
- 171.Franz MR, Burkhoff D, Yue DT, Sagawa K. Mechanically induced action potential changes and arrhythmia in isolated and in situ canine hearts. Cardiovasc Res. 1989 Mar;23(3):213–223. doi: 10.1093/cvr/23.3.213. [DOI] [PubMed] [Google Scholar]
- 172.Franz MR, Cima R, Wang D, Profitt D, Kurz R. Electrophysiological effects of myocardial stretch and mechanical determinants of stretch-activated arrhythmias. Circulation. 1992 Sep;86(3):968–978. doi: 10.1161/01.cir.86.3.968. [DOI] [PubMed] [Google Scholar]
- 173.Seo K, Inagaki M, Nishimura S, Hidaka I, Sugimachi M, Hisada T, et al. Structural Heterogeneity in the Ventricular Wall Plays a Significant Role in the Initiation of Stretch-Induced Arrhythmias in Perfused Rabbit Right Ventricular Tissues and Whole Heart Preparations. Circ Res. 2009 Nov 5; doi: 10.1161/CIRCRESAHA.109.203828. [DOI] [PubMed] [Google Scholar]
- 174.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004 Aug 20;279(34):35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 175.Allingham JS, Smith R, Rayment I. The structural basis of blebbistatin inhibition and specificity for myosin II. Nat Struct Mol Biol. 2005 Apr;12(4):378–379. doi: 10.1038/nsmb908. [DOI] [PubMed] [Google Scholar]
- 176.Puglisi JL, Bers DM. LabHEART: an interactive computer model of rabbit ventricular myocyte ion channels and Ca transport. Am J Physiol Cell Physiol. 2001 Dec;281(6):C2049–C2060. doi: 10.1152/ajpcell.2001.281.6.C2049. [DOI] [PubMed] [Google Scholar]
- 177.Negroni JA, Lascano EC. Simulation of steady state and transient cardiac muscle response experiments with a Huxley-based contraction model. J Mol Cell Cardiol. 2008 Aug;45(2):300–312. doi: 10.1016/j.yjmcc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 178.Huke S, Venkataraman R, Sidorov VY, Baudenbacher F, Knollmann BC. Decreased Connexin43 phosphorylation and reduced lateral conduction velocity contribute to arrhythmia risk in hypertrophic cardiomyopathy due to troponin T mutations. Circulation. 2009;120 Suppl. II:S682. [Google Scholar]