Abstract
Social defeat stress is an ethologically salient stressor which activates dopaminergic areas and, when experienced repeatedly, has long-term effects on dopaminergic function and related behavior. The mechanism for these long-lasting consequences remains unclear. A potential candidate for mediating these effects is brain-derived neurotrophic factor (BDNF), a neurotrophin involved in synaptic plasticity and displaying alterations in dopaminergic regions in response to various types of stress. In this study, we sought to determine whether repeated social defeat stress altered BDNF mRNA and protein expression in dopaminergic brain regions either immediately after the last stress exposure or four weeks later. Male Sprague-Dawley rats were subjected to social defeat stress consisting of brief confrontation with an aggressive male rat every third day for 10 days; control rats were handled according to the same schedule. Animals were euthanized either 2 h or 28 days after the last stress or handling episode. Our results show that 2 h after stress, BDNF protein and mRNA expression increased in the medial prefrontal cortex. At this time-point, BDNF mRNA increased in the amygdala and protein expression increased in the substantia nigra. Twenty-eight days after stress, BDNF protein and mRNA expression were elevated in the medial amygdala and ventral tegmental area. Given the role of BDNF in neural plasticity, BDNF alterations that are long-lasting may be significant for neural adaptations to social stress. The dynamic nature of BDNF expression in dopaminergic brain regions in response to repeated social stress may therefore have implications for lasting neurochemical and behavioral changes related to dopaminergic function.
Keywords: Mesolimbic, ventral tegmental area, prefrontal cortex, amygdala, dopamine, neurotrophin
Social defeat stress is an ethologically salient stressor which provides a relevant model to investigate the etiology of stress-related disorders in humans (Koolhaas et al., 1997; Koolhaas et al., 1999). The effects of social defeat stress are long-lasting, as even a single stress episode is sufficient to alter behaviors such as exploratory activity and sensitivity to other stressors for several weeks (Koolhaas et al., 1999).
Various types of stressors can dramatically alter neurochemistry specifically within the mesocorticolimbic dopamine circuit (Fadda et al., 1978; Dunn and File, 1983; Deutch et al., 1985; Louilot et al., 1986; Abercrombie et al., 1989; Imperato et al., 1992; Tidey and Miczek, 1996; Nikulina et al., 1998, 2001; Bland et al., 2005; Covington et al., 2005), which mediates general reward and reinforcement. Intermittent social defeat in particular produces persistent neurochemical changes lasting for days and sometimes months after the last stress episode. These effects include increased μ-opioid receptor mRNA in the ventral tegmental area (VTA), and enhanced functional activation of neurons in the VTA and amygdala after amphetamine administration (Covington and Miczek, 2001; Nikulina et al., 2004, 2008; Covington et al., 2005) . In addition, intermittent or chronic social defeat stress is known to influence various behaviors that persist long after stress termination, including anxiety- and depressive-like behavior, social avoidance, analgesia, and conditioned defeat (Miczek et al., 1982; Huhman et al., 2003; Avgustinovich et al., 2005). Intermittent social defeat stress also enhances drug-related behaviors such as psychomotor sensitization and self-administration of cocaine, which persist for up to 2 months after stress exposure (Covington and Miczek, 2001; Nikulina et al., 2004; Covington et al., 2005). Taken together, these findings suggest that social defeat stress produces neuroadaptations within the mesocorticolimbic system that may have long-lasting functional consequences.
One potential candidate for sustaining persistent stress-induced behavioral effects is the neurotrophin brain-derived neurotrophic factor (BDNF), which promotes plasticity and neuronal survival (Thoenen, 1995) and is expressed in regions that both produce and receive dopamine (Seroogy et al., 1994; Conner et al., 1997). Several mesocorticolimbic regions exhibit altered BDNF after social stress, including the frontal cortex and amygdala, where BDNF mRNA is decreased after a single social defeat stress episode (Pizarro et al., 2004). Both acute and chronic social threat stress, defined as exposure to a dominant animal without physical contact, also increase BDNF expression in the hippocampus (Pardon et al., 2005). These studies demonstrate that social stress effects on BDNF are not uniform across brain regions.
Changes in mesocorticolimbic BDNF after chronic social stress may also be significant for adaptive behavior, as virus-induced deletion of BDNF in the VTA attenuates social defeat stress-dependent social avoidance behavior in mice for up to 28 days after removal of the stressor (Berton et al., 2006). In addition, mice that show enhanced nucleus accumbens (NAc) BDNF expression after chronic social defeat stress are more susceptible to social stress-induced behavioral changes (Krishnan et al., 2007). These studies indicate that BDNF in dopaminergic regions may be particularly important to general behavioral changes associated with social stress. In addition, delayed and prolonged alterations of mesocorticolimbic BDNF have been demonstrated after other stimuli that, like social stress exposure, increase dopamine transmission (Tidey and Miczek, 1996). For example, cocaine administration produced delayed (30 and 90 days) increases in BDNF expression in the NAc, amygdala, and VTA which occurred at the same time as peak drug-seeking behavior (Grimm et al., 2003).
To date, the long-term impact of repeated, intermittent social stress on mesocorticolimbic BDNF remains unknown. In this study, we examined the short- and long-term effects of intermittent social defeat stress on BDNF expression in mesocorticolimbic circuits. BDNF mRNA and protein levels in various mesocorticolimbic regions were measured 2 h and 28 days after stress exposure.
EXPERIMENTAL PROCEDURES
Animals
Subjects were male Sprague-Dawley rats (Charles River Laboratories; Kingston, RI, USA), weighing 150-200 g upon arrival. Experimental rats were housed individually under a reverse light/dark cycle (12:12 h, lights off at 1000 h) with unlimited access to food (Purina Rodent Diet, Brentwood, MO, USA) and water. They were allowed at least one week to habituate after arrival before the start of any behavioral procedures. Stimulus male Long-Evans rats, termed “residents,” were pair-housed with females on a separate rack where social defeat procedures were carried out as described below. All females underwent surgical tubal ligation prior to pair-housing with males to maintain cycling and prevent pregnancy. Resident pairs were housed in large Plexiglas cages (36.5 × 25.7 × 20.5cm). Each resident was screened for consistency of aggressive reactions towards a novel stimulus intruder rat, which was characterized by repeated attacks towards the intruder. Resident rats were exposed to a novel stimulus intruder rat every 1-2 weeks (Miczek, 1979), and residents not eliciting a social defeat from an intruder for 3 consecutive screening sessions were not utilized in the study. All experimental procedures were approved by the Tufts University Institutional Animal Care and Use Committee, and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996). All efforts were made to minimize suffering and to limit the number of animals used.
Social Defeat Stress Procedure
Rats were randomly assigned to either the experimental “social defeat stress” intruder group or the non-stressed “handled” control group (n = 8-9 per group). Stressed rats were exposed to social defeat once every 72 h over the course of 10 days (i.e., four stress exposures); control rats were handled and weighed on the same days their counterparts were stressed and weighed (Tornatzky and Miczek, 1993).
During the social stress procedure, the intruder was placed into the home cage of the resident following removal of the female. For the first 5 min, the intruder remained under a stainless steel protective cage (25 × 15 × 15 cm) for exposure to threats from the resident. The protective cage was then removed and the resident displayed aggressive behavior, which usually happened after 1-2 min of latent period and lasted no more than 5 min; “defeat” was considered to have occurred when the intruder exhibited a supine posture for at least 4 sec, after which the intruder was placed under the protective cage within the resident's home cage where he remained for an additional 20 min. After each social stress episode, intruders were immediately returned to their home cage. Intruders were never exposed to the same residents on consecutive days, or more than twice during the procedure.
Tissue Preparation and Histological Procedures
Two hours or 28 days after termination of the last social stress episode, rats were deeply anesthetized with an overdose of sodium pentobarbital (100 mg/kg) and perfused transcardially with 15 ml of 10% heparin in 0.1 M phosphate buffered saline (pH 7.4) followed by 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer at 4°C (PB; pH 7.4). Brains were removed, post-fixed for 1.5 h in the same fixative at 4°C, then placed into graded concentrations of sucrose in 0.1 M phosphate buffer at 4°C. Coronal sections (20 μm) were taken from the following brain regions (Paxinos and Watson, 2005) on a sliding microtome: prefrontal cortex (PFC; +3.2 to +2.8 mm from bregma), NAc (+1.2 to + 1.0 mm), amygdala (-2.5 to -2.8 mm), and VTA and substantia nigra (SN; -4.8 to -5.2 mm). Sections were collected in 0.1 M chilled phosphate buffer and mounted onto glass slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA), which were dried and stored at -35°C prior to processing.
Immunohistochemistry (IHC)
Sections were washed in 0.05 M potassium phosphate-buffered saline (KPBS) to remove any fixative, then were blocked for one hour in solution of 5% normal goat serum, 0.4% Triton X-100 in 0.05 M KPBS. Sections were then incubated with rabbit polyclonal antiserum directed against BDNF (1:300 dilution of 1779 SP; Chemicon/Millipore; Temecula, CA) for 48 hours at 4°C. Sections were then washed again in KPBS and incubated for 1 h in biotin-conjugated goat anti-rabbit serum (1:40 dilution in normal goat serum solution, Vectastain ABC kit; Vector Laboratories, Burlingame, CA) at room temperature. Sections were then washed in KPBS and incubated with an avidin-biotin-peroxidase complex (Vectastain ABC kit) for 45 min. After washing again in KPBS, sections were processed using the DAB with nickel-intensified substrate kit (Vector Laboratories). Sections were allowed to dry overnight at room temperature and coverslipped.
Image analysis for IHC
Tissue sections were examined using a Zeiss Axioskop microscope for the presence of chromagen reaction product. For each subject, data were obtained from at least three sections through each brain region, and the mean of these data determined the regional value for that animal. Counts of immunolabeled profiles were determined without knowledge of the experimental group, and were quantified using computerized image analysis (Image Pro Plus, version 4.1.0.0; Media Cybernetics, Silver Spring, MD, USA). Selected areas were captured and digitized using a Cool Snap Pro video camera interfaced to the microscope using a 20× objective. The mean density of non-specific background labeling was measured in a cell-free area of each experimental region, and digitally subtracted so that any background staining was eliminated. Specific threshold values were then used to detect only those labeled objects of appropriate size and staining density. Similar threshold criteria were used to assess every tissue section. This semi-quantitative approach allows detection of labeled neurons containing immunolabeling that is greater than a fixed intensity threshold value in all cases. Sections from stressed and handled groups were processed simultaneously within each time-point, but not across time-points, throughout all stages of the immunohistochemical procedure. The number of labeled profiles in an area of standard size was determined in the prelimbic (PrL), infralimbic (IL), and anterior cingulate (ACg) subregions of the PFC, NAc, medial, central and basolateral amygdaloid nuclei (MeA, CeA, BLA, respectively), VTA, and SN pars compacta. Data were transformed to represent the number of labeled profiles / mm2.
In Situ hybridization histochemistry (ISHH)
Sections were hybridized using a 50 mer oligonucleotide (synthesized by GenScript, Piscataway, NJ) complementary to bases 745-795 of rat BDNF cDNA. The BDNF probe was 3’ end labeled with [35S]dATP (PerkinElmer Life Sciences; Waltham, MA) utilizing terminal deoxynucleotidyl transferase (Roche Applied Sciences; Indianapolis, IN), and purified and diluted with hybridization buffer containing 50% formamide, 500 μg/ml sheared salmon sperm DNA, 250 μg/ml yeast tRNA, 4× saline-sodium citrate (SSC) solution, 1× Denhardt's solution and 10% dextran sulfate combined with DTT (2 μL 5 M DTT per 100 μL solution) in order to yield a 3 × 107 cpm/mL hybridization solution. Slides were incubated in 20 μl/section of the hybridization solution under sterile coverslips for 16 h at 37°C. Coverslips were removed in 1× SSC and slides were rinsed in 2× SSC for 1 h, 1× SSC for 1 h, 0.5× SSC for 30 min, and 0.1× SSC at 37°C for 30 min, then rinsed in 0.1× SSC solution, washed twice in 1× SSC solution for 30 min and dehydrated in a series of graded ethanol solutions before air-drying for 1 h. Hybridization was visualized by X-ray film (Kodak Biomax MR; Carestream Health, Rochester, NY) autoradiography, with an exposure time of 3 days at room temperature.
Image analysis for ISHH
Regional autoradiographs were digitized using a CCD camera connected to a Macintosh computer. BDNF mRNA expression was measured in the PrL, IL, and Cg cortices, NAc (2h only), MeA, CeA, BLA, VTA, and SN. Sub-regions were defined manually, and quantification of optical density was assessed with image-analysis software (ImageJ; created by Wayne Rasband, National Institute of Mental Health, Bethesda, MD, and available on the internet at: http://rs.info.nih.gov/ij/) using calibrated radiostandards (American Radiolabeled Chemicals, St. Louis, MO) co-exposed on film to generate a 14C calibration curve in cpm/mg. Data from the right and left hemispheres of three to five adjacent sections were combined to generate a mean value for each selected brain region in each subject.
Statistics
The mean number of BDNF-like immunoreactive neurons or average film labeling intensity for each experimental group was compared within each time-point for each brain region. Homogeneity of variance was determined using Levene's test, and was considered violated when this test yielded p < 0.05. If variance was homogeneous, data were assessed using a one-way ANOVA (stressed vs. handled). If variance was not homogeneous, data were subject to a Welsh ANOVA (stressed vs. handled), which provides a corrected F statistic and degrees of freedom. In both cases, differences between the means were considered statistically significant if p ≤ 0.05. Some cases were deleted from analysis due to poor tissue quality, resulting in variability in the number of cases analyzed for each brain region. All data are represented as mean ± standard error of the mean (SEM).
RESULTS
Effects of intermittent social defeat stress on regional BDNF expression
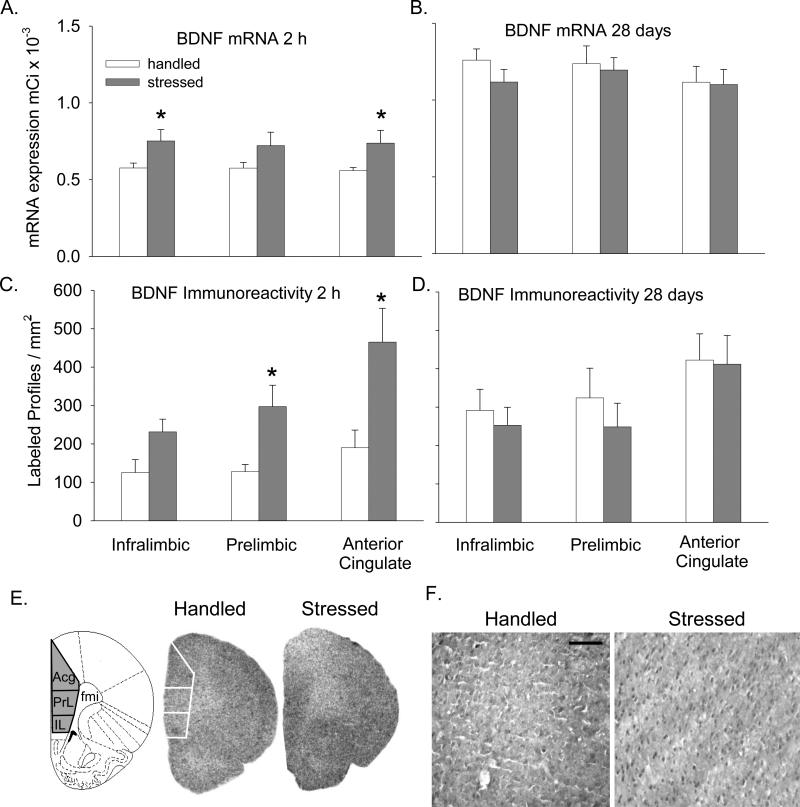
Two hours after stress termination, BDNF mRNA increased significantly in the IL (Fig. 1A and E; F(1,9)=5.31; p < 0.05) and ACg (Fig 1A and E; F(1,9)=5.24; p < 0.05) cortices, but not in the PrL cortex (Fig. 1A and E). Twenty-eight days later, this effect was no longer apparent in any subregion of the PFC (Fig. 1B). Similarly, 2 hr after stress termination, BDNF-like immunoreactivity was significantly elevated after stress in all subregions of the PFC (Fig. 1C and F; PrL, F(1,8) = 5.964; p < 0.05; IL, F(1,8) = 5.579; p < 0.05; ACg, F(1,8) = 6.57; p < 0.05), and this difference was no longer present 28 days after stress (Fig. 1D).
Figure 1. Effect of intermittent stress on BDNF in the PFC.
BDNF mRNA labeling in the IL, PrL, and ACg subregions of PFC in film autoradiographs expressed as μCi/g of calibrated radiostandard: A - 2 h after termination of stress (n=5-6) and B - 28 days later (n=5-6). BDNF immunolabeling expressed as labeled profiles / mm2 in the IL, PrL, and ACg subregions of the PFC: C - 2 h after termination of stress (n=4-6) and D - 28 days later (n=5-6). All values represent mean ± SEM. E - Schematic representation of locations of PFC subregions; fmi – forceps minor. Representative autoradiographs of PFC showing BDNF mRNA signal intensity 2 h after stress; white lines indicate boundaries of regions analyzed. F - Representative photomicrographs taken from ACg region showing BDNF immunolabeling 2 h after stress. Scale bar in F indicates 100 μm. *- p<0.05.
Repeated intermittent defeat stress did not affect BDNF protein expression in the NAc core or shell regions either 2 h or 28 days after stress (Table 1). Likewise, no changes in BDNF mRNA were observed in the NAc core or shell 2 h after the last stress (Table 1).
Table 1.
Short- and long-term effect of intermittent social stress on BDNF mRNA and protein expression in the nucleus accumbens core and shell.
| BDNF-labeled profiles / mm2 | BDNF mRNA expression (mCi−3) | ||||
|---|---|---|---|---|---|
| Time after stress: | 2 h | 28 days | 2 h | 28 days | |
| NAc core | handled | 72.6 ± 7.1 | 183.6 ± 58.4 | 0.84 ±0.04 | nd |
| stressed | 69.0 ± 13.0 | 192.8 ± 62.9 | 0.93 ±0.04 | nd | |
| NAc shell | handled | 81.9 ± 10.9 | 247.2 ± 67.3 | 0.94 ±0.03 | nd |
| stressed | 107.4 ± 28.6 | 210.6 ± 56.7 | 1.02 ±0.04 | nd | |
N=4-7; nd indicates not done.
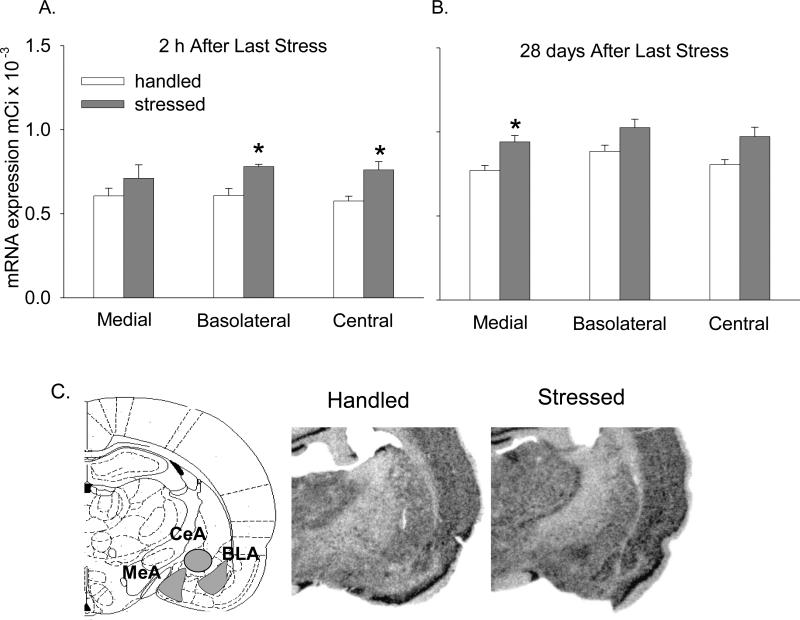
In the amygdala, BDNF mRNA exhibited a dynamic and time-dependent response to intermittent stress. Two hours after termination of stress, BDNF mRNA was elevated in the BLA and CeA (Fig. 2A; BLA, F(1,4.96) = 14.45; p < 0.05; CeA, F(1,7) = 10.20; p < 0.05), but not in the MeA. Twenty-eight days later, there was an increase of BDNF mRNA in the MeA (Figs. 2B and C, F(1,10) = 7.84; p < 0.05). Although there was not a significant difference of BDNF mRNA between the stress and handled groups in the BLA and CeA 28 days after stress, both nuclei exhibited a trend towards increased BDNF in the stressed group (Figs. 2B and C; BLA, F(1,10) = 3.21; p = 0.10; CeA, F(1,7.37) = 4.38; p = 0.07).
Figure 2. Effect of intermittent stress on BDNF mRNA in amygdala.
BDNF mRNA labeling in film autoradiographs expressed as μCi/g of calibrated radiostandard: A - 2 h after termination of stress (n=4-5), and B - 28 days later (n=6). Values represent mean ± SEM. C - Schematic representation of amygdalar nuclei (left panel). Representative autoradiographs of amygdala showing BDNF mRNA signal intensity 28 days after stress (right two panels). * - p<0.05
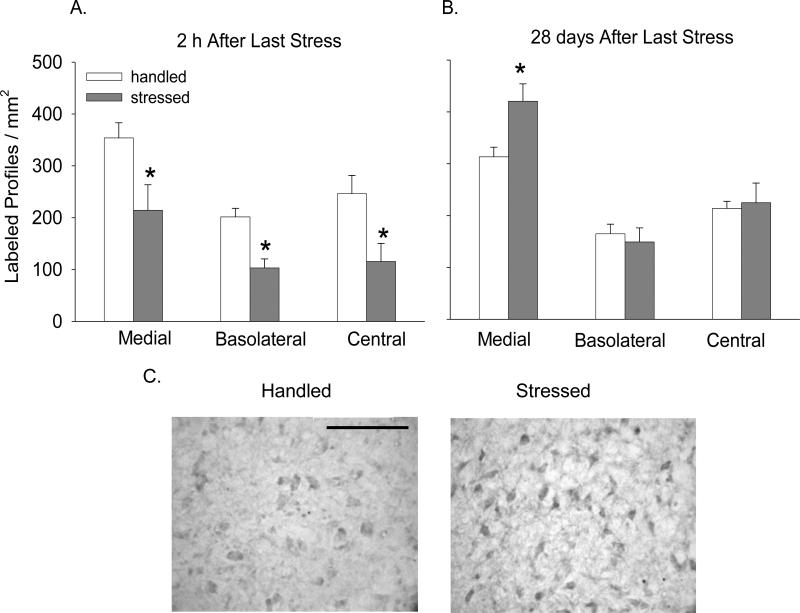
In contrast, BDNF protein expression was significantly reduced 2 h after stress in all amygdalar nuclei (Fig. 3A; CeA: F(1,7) = 6.89; p < 0.05; BLA: F(1,7) = 16.75; p < 0.05; and MeA: F(1,7) = 5.86; p < 0.05). However, not only did this effect disappear 28 days later in the CeA and BLA (Fig. 3B), but it reversed in the MeA, wherein BDNF protein expression significantly increased 28 days after stress (Figs. 3B and C, F(1,7.83) = 7.95; p < 0.05).
Figure 3. Effect of intermittent stress on BDNF immunoreactivity in amygdala.
BDNF immunolabeling expressed as the number of labeled profiles / mm2 in the MeA, BLA, and CeA: A - 2 h after termination of stress (n=4-5), and B - 28 days later (n=6). C - Representative photomicrographs taken from MeA showing BDNF immunolabeling 28 days after stress. Scale bar in C indicates 100 μm. * - p<0.05
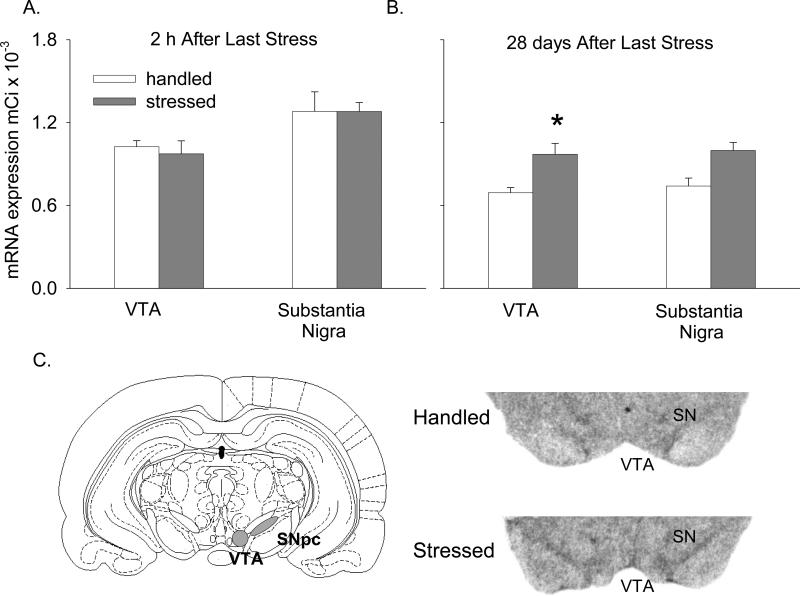
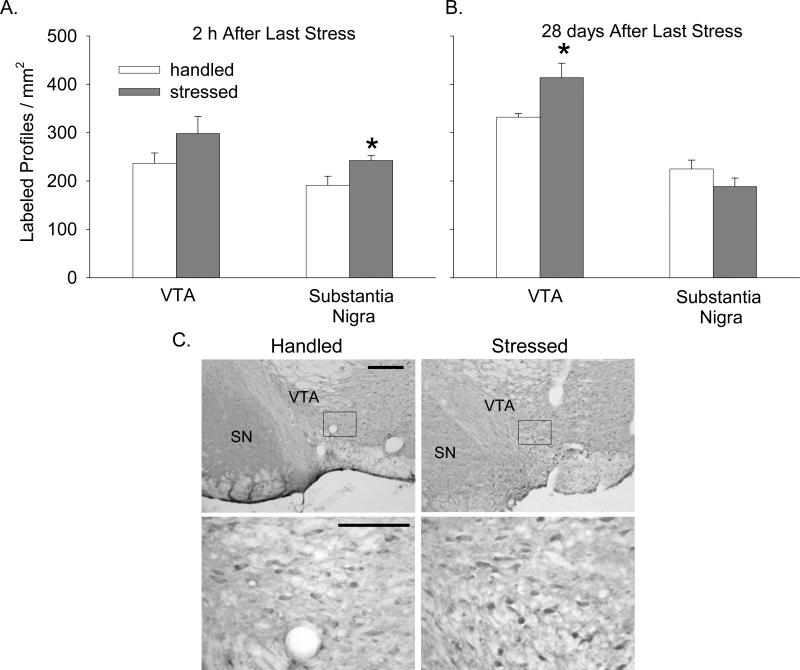
In the VTA, BDNF mRNA was unchanged 2 h after termination of stress exposure (Fig. 4A), but increased significantly 28 days later (Figs. 4B and C, F(1,8.33) = 10.21; p < 0.05). Similarly, there was no change in BDNF protein expression 2 h after termination of stress exposure (Fig. 5A), but a significant increase was observed 28 days later (Figs. 5B and C, F(1,5.58) = 7.02; p < 0.05).
Figure 4. Effect of intermittent stress on BDNF mRNA in the midbrain.
BDNF mRNA labeling in film autoradiographs expressed as μCi/g of calibrated radiostandard: A - 2 h after termination of stress (n=4-6), and B - 28 days later (n=5-7). C - Schematic representation of VTA and SN pars compacta (left), and representative film autoradiographs of midbrain showing BDNF mRNA signal intensity 28 days after stress (right). * - p<0.05
Figure 5. Effect of intermittent stress on BDNF immunoreactivity in the midbrain.
BDNF immunolabeling expressed as as the number of labeled profiles / mm2 in the VTA and SN pars compacta: A - 2 h after termination of stress (n=5-6), and B - 28 days later (n=5-6). C - Representative photomicrographs taken from VTA showing BDNF immunolabeling 28 days after stress. Outlined region (top row; scale bar = 200 μm) indicates magnified region (bottom row; scale bar = 100 μm). * - p<0.05
In the SN, there was no significant change of BDNF mRNA either 2 h or 28 days after termination of stress exposure (Figs. 4A and B). However, BDNF protein expression increased 2 h after stress exposure in the SN (Fig. 5A, F(1,9) = 5.65; p < 0.05), an effect which was no longer present 28 days later (Fig. 5B).
Stress effect on weight gain
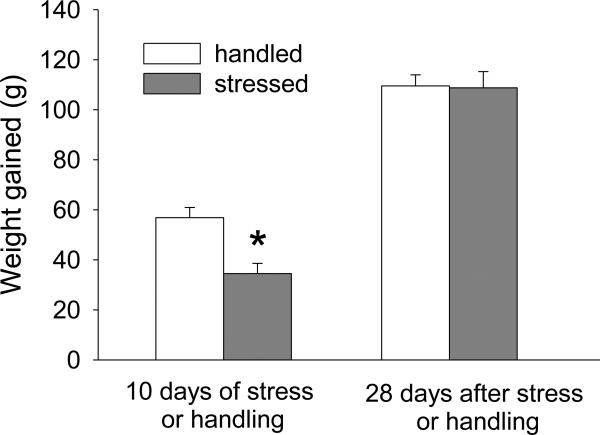
Stressed rats gained significantly less weight than their handled counterparts during the period of stress exposure when assessed 2 hr after termination of stress or handling (Fig. 6; F(1,14)=13.35; p < 0.05). However, 28 days later the effect of stress on weight gain was no longer present (Fig. 6).
Figure 6. Effect of intermittent social defeat stress on weight gain in rats during stress and 28 days after stress.
Weight gain between the first and last day of stress or control handling (left), and during 28 days later (n=8). * - p<0.05
DISCUSSION
Our data reveal that repeated, intermittent social defeat stress produced dynamic, time-dependent alterations of BDNF expression in mesocorticolimbic regions. BDNF is implicated in synaptic plasticity, particularly in long-term potentiation associated with learning and memory (Kang and Schuman, 1995; Linnarsson et al., 1997; Ma et al., 1998; Mizuno et al., 2000), which highlights its role as a potential regulator of neuroadaptation in response to external stimuli. Most noteworthy is our finding that BDNF is altered long after termination of repeated social stress in amygdala and VTA.
Mechanistically, alteration of BDNF following repeated social stress may be related to elevated levels of corticosterone. Social defeat is known to increase circulating corticosterone and to affect glucocorticoid receptor expression in the hippocampus (Covington and Miczek, 2005; Patel et al., 2008). Mesocorticolimbic regions such as the PFC, amygdala, NAc, and striatum exhibit fairly dense expression of the glucocorticoid receptor (Cintra et al., 1994), and glucocorticoid signaling can differentially affect BDNF expression in brain regions responsive to stress, such as the amygdala and hippocampus (Schulte-Herbrüggen et al., 2006). This implies that regulation of BDNF can occur downstream from stimulation of the glucocorticoid receptor, which might be mediated by induction of c-Fos (Dong et al., 2006). The breadth of signaling pathways affected by glucocorticoids may in part account for the diverse and region-dependent effects of stress on BDNF expression.
We also observed that repeated social defeat produced transient restriction of weight gain, consistent with the findings of others (de Jong et al., 2005; Krishnan et al., 2007). This might be related to the weight loss seen in individuals under chronic stress, such as those with stress-related mood disorders.
Our results reveal that BDNF protein and mRNA levels were elevated in the PFC immediately after the last social stress exposure, in line with the induction of cortical BDNF observed after exposure to various types of stressors. We also noticed a potential upward trend in baseline levels of BDNF in the mPFC in the 28 day handled control group. This may be a result of isolation housing, which presents a mild stressor, as rats in the 28 day groups were single housed 28 days longer than those in the 2 h groups. Our findings are similar to those of other studies, which show that acute tail-shock, immobilization, or restraint stress result in transient induction of BDNF mRNA expression in the PFC (Molteni et al., 2001; Bland et al., 2005; Lee et al., 2006; Bland et al., 2007). Chronic intermittent social threat also induces BDNF protein expression in mouse PFC measured 1 h after the last stress episode (Pardon et al., 2005). However, a single social stress episode reduced cortical BDNF mRNA when measured 24 h later in mouse frontal cortex (Pizarro et al., 2004), suggesting that cortical induction may be transient. Finally, our observation that both BDNF protein and mRNA expression in the PFC were elevated after repeated social stress exposure suggests that the increase of BDNF protein level is a result of BDNF produced by PFC neurons.
We observed no changes of BDNF immunolabeling in the NAc core or shell either 2 h or 28 days after repeated social stress exposure, and no changes in BDNF mRNA expression 2 h after repeated social stress. It has been reported that BDNF mRNA is not present in the NAc at basal conditions (Conner et al., 1997), and that chronic social stress does not alter BDNF mRNA in the NAc of mice (Krishnan et al., 2007). By contrast, more severe chronic social stress in mice produces both a short- (24 h) and long-term (28 days) increase of total BDNF protein level in NAc tissue homogenates, which results from increased BDNF release by terminals of mesolimbic neurons in the VTA (Berton et al., 2006; Krishnan et al., 2007). In our case, it is possible that we did not observe altered BDNF protein expression in the NAc after social defeat stress because such BDNF is contained mostly in neuron terminals in the NAc, which would be difficult to quantify using immunohistochemistry.
We found that repeated social stress increased BDNF mRNA in all regions of the amygdala 2 h after termination of social stress, and concurrently reduced BDNF protein expression in the BLA and CeA. Such a disparity between BDNF mRNA and protein expression has been previously observed, as one study reports a mismatch in BDNF mRNA and protein levels in limbic regions (Nawa et al., 1995). The authors of this study suggest that this may be due to poorly understood BDNF post-translational modifications or other sub-cellular BDNF processing. Consistent with our findings, BDNF mRNA increased in the amygdala 1 h after the final exposure to intermittent water immersion stress (Aguilar-Valles et al., 2005). However, repeated restraint or acute social stress exposure reduced amygdalar BDNF mRNA 24 h later (Smith et al., 1995; Pizarro et al., 2004). This suggests that stress-induced changes in BDNF are transient in the amygdala as in the PFC. We also observed a persistent increase of BDNF mRNA and protein expression in the MeA 28 days after repeated social defeat stress.
Functionally, the amygdala is a heterogeneous structure involved in the regulation of various aspects of stress. The CeA and MeA are thought to govern neuroendocrine responses to stress (Dayas et al., 1999), and the CeA may specifically mediate submissive behaviors associated with conditioned social defeat in hamsters (Jasnow et al., 2004). The BLA is more intimately involved in mesocorticolimbic responses, regulating stress-induced dopamine release in the PFC and striatum (Stevenson and Gratton, 2003). Specifically, BDNF in the BLA is implicated in fear conditioning (Rattiner et al., 2004), a behavioral consequence of stressful stimuli. In addition, deletion of BDNF in the MeA induces anxiety-like behavior in rodents (Pandey et al., 2006). Together, these findings suggest that amygdalar BDNF may be involved in certain behavioral adaptations to stress.
In the VTA, both BDNF protein and mRNA expression increased 28 days after repeated social defeat stress, but not 2 h after the last stress exposure, reflecting a long-term molecular response. Also, similar to the mPFC, we observed a slight increase in BDNF protein expression in the 28 day control handled group, which again, may reflect a mild stress effect due to isolated housing. It is known that social defeat stress increases μ-opioid receptor mRNA expression within minutes after an acute episode and lasting for at least 7 days after repeated episodes (Nikulina et al., 1999; 2008). In fact, μ-opioid receptor signaling has been shown to up-regulate BDNF mRNA in the frontal cortex, hippocampus, and amygdala (Zhang et al., 2006), indicating that social stress-induced expression of μ-opioid receptors might precede BDNF induction in the VTA. Moreover, BDNF gene deletion in the VTA attenuates stress-induced behaviors, such as social avoidance in mice (Berton et al., 2006; Krishnan et al., 2007). VTA BDNF might also be involved in stress-induced cross-sensitization, as suggested by the co-occurrence of increased VTA BDNF following repeated social defeat stress with persistent cross-sensitization to amphetamine 28 days after stress termination (our unpublished observations), and the ability of enhanced VTA BDNF to augment behavioral sensitization to cocaine (Horger et al., 1999). Finally, a recent study suggests that social stress-induced changed in VTA BDNF may regulate behavior differently depending on the severity and duration of stress exposures (Miczek et al., 2010). Given the role of the VTA in motivation and reinforcement, altered VTA BDNF might provide a substrate underlying stress-induced response to drugs of abuse, as well as depressive-like behaviors, as previously hypothesized (Nestler and Carlezon, 2006).
Repeated social defeat stress also increased BDNF protein expression 2 h after stress in the SN without affecting mRNA expression, although this difference was no longer apparent 28 days later. Infusion of BDNF into the SN increases dopamine turnover in the striatum, electrical activity of dopamine neurons and associated behavioral activity (Altar et al., 1992; Shen et al., 1994; Martin-Iverson and Altar, 1996). However, it is unlikely that a transient increase of SN BDNF is related to activation of the nigrostriatal system during social defeat stress because dorsal striatal dopamine release is not altered by social defeat stress (Tidey and Miczek, 1996).
Due to the ubiquitous nature of BDNF throughout the brain and its significant role in synaptic plasticity, the rapid and persistent regional alterations of BDNF observed herein may be important for adaptation to stress. Delayed, persistent changes of mesocorticolimbic BDNF are also present after chronic cocaine self-administration (Grimm et al., 2003) and exposure to social threat (Pardon et al., 2005), emphasizing the potential role of mesocorticolimbic BDNF in neural adaptation.
ACKNOWLEDGEMENTS
This research was supported by USPHS awards R03 DA024817 and R01 DA026451 (E.M.N.), R01 MH073930 (R.P.H.), and F31 DA022830 (S.F.).
Abbreviations
- ACg
Anterior cingulate cortex
- BLA
Basolateral Amygdala
- BDNF
Brain-Derived Neurotrophic Factor
- CeA
Central Amygdala
- IHC
Immunohistochemistry
- ISHH
In situ Hybridization Histochemistry
- IL
Infralimbic cortex
- MeA
Medial Amygdala
- NAc
Nucleus Accumbens
- PB
Phosphate Buffer
- KPBS
Potassium Phosphate Buffered Saline
- PFC
Prefrontal Cortex
- PrL
Prelimbic cortex
- SSC
Saline-sodium citrate
- SEM
Standard error of the mean
- SN
Substantia Nigra
- VTA
Ventral Tegmental Area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. J Neurochem. 1989;52:1655–1658. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Aguilar-Valles A, Sánchez E, de Gortari P, Balderas I, Ramírez-Amaya V, Bermúdez-Rattoni F, Joseph-Bravo P. Analysis of the stress response in rats trained in the water-maze: differential expression of corticotropin-releasing hormone, CRH-R1, glucocorticoid receptors and brain-derived neurotrophic factor in limbic regions. Neuroendocrinology. 2005;82:306–319. doi: 10.1159/000093129. [DOI] [PubMed] [Google Scholar]
- Altar CA, Boylan CB, Jackson C, Hershenson S, Miller J, Wiegand SJ, Lindsay RM, Hyman C. Brain-derived neurotrophic factor augments rotational behavior and nigrostriatal dopamine turnover in vivo. Proc Natl Acad Sci U S A. 1992;89:11347–11351. doi: 10.1073/pnas.89.23.11347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A Model of Anxious Depression: Persistence of Behavioral Pathology. Neurosci Beh Phys. 2005;35:917–924. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, DiLeone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005;1051:90–99. doi: 10.1016/j.brainres.2005.05.065. [DOI] [PubMed] [Google Scholar]
- Bland ST, Tamlyn JP, Barrientos RM, Greenwood BN, Watkins LR, Campeau S, Day HE, Maier SF. Expression of fibroblast growth factor-2 and brain-derived neurotrophic factor mRNA in the medial prefrontal cortex and hippocampus after uncontrollable or controllable stress. Neuroscience. 2007;44:1219–1228. doi: 10.1016/j.neuroscience.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintra A, Zoli M, Rosen L, Agnati LF, Okret S, Wikstrom AC, Gustaffsson JA, Fuxe K. Mapping and computer assisted morphometry and microdensitometry of glucocorticoid receptor immunoreactive neurons and glial cells in the rat central nervous system. Neuroscience. 1994;62:843–897. doi: 10.1016/0306-4522(94)90481-2. [DOI] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Repeated social defeat stress, cocaine, or morphine: Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology. 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacol. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Covington HE, Kikusui T, Goodhue J, Nikulina EM, Hammer RP, Miczek KA. Brief social defeat stress: long lasting effects on cocaine taking during a binge and zif268 mRNA expression in the amygdala and prefrontal cortex. Neuropsychopharmacology. 2005;30:310–321. doi: 10.1038/sj.npp.1300587. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Buller KM, Day TA. Neuroendocrine responses to an emotional stressor: evidence for involvement of the medial but not the central amygdala. Eur J Neurosci. 1999;11:2312–2322. doi: 10.1046/j.1460-9568.1999.00645.x. [DOI] [PubMed] [Google Scholar]
- de Jong JG, van der Vegt BJ, Buwalda B, Koolhaas JM. Social environment determines the long-term effects of social defeat. Physiol and Behav. 2005;84:87–95. doi: 10.1016/j.physbeh.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Tam SY, Roth RH. Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985;333:143–146. doi: 10.1016/0006-8993(85)90134-9. [DOI] [PubMed] [Google Scholar]
- Dong M, Wu YW, Fan Y, Xu M, Zhang J. c-fos modulates brain-derived neurotrophic factor mRNA expression in mouse hippocampal CA3 and dentate gyrus neurons. Neurosci Letters. 2006;400(1-2):177–80. doi: 10.1016/j.neulet.2006.02.063. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, File SE. Cold restraint alters dopamine metabolism in frontal cortex, nucleus accumbens and neostriatum. Physiol Behav. 1983;31:511–513. doi: 10.1016/0031-9384(83)90074-4. [DOI] [PubMed] [Google Scholar]
- Fadda F, Argiolas A, Melis MR, Tissari AH, Onali PL, Gessa GL. Stress-induced increase in 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and in n. accumbens: reversal by diazepam. Life Sci. 1978;23:2219–2224. doi: 10.1016/0024-3205(78)90207-2. [DOI] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, S.M. L, Israel JE, Jasnow AM. Conditioned defeat in male and female syrian hamsters. Hormones and Behavior. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Imperato A, Angelucci L, Casolini P, Zocchi A, Puglisi-Allegra S. Repeated stressful experiences differently affect limbic dopamine release during and following stress. Brain Res. 1992;577:194–199. doi: 10.1016/0006-8993(92)90274-d. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behavioral Neurosci. 2004;118:1052–1061. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J Physiol. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Meerlo P, de Boer SF, Strubbe JH, Bohus B. The temporal dynamics of the stress response. Neurosci Biobehav Rev. 1997;21:775–782. doi: 10.1016/s0149-7634(96)00057-7. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Lee Y, Duman R, GJ M. The mGlu2/3 receptor agonist LY354740 suppresses immobilization stress-induced increase in rat prefrontal cortical BDNF mRNA expression. Neurosci Letters. 2006;398:328–332. doi: 10.1016/j.neulet.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Linnarsson S, Bjorklund A, Ernfors P. Learning deficit in BDNF mutant mice. Eur J Neurosci. 1997;9:2581–2587. doi: 10.1111/j.1460-9568.1997.tb01687.x. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Differential reactivity of dopaminergic neurons in the nucleus accumbens in response to different behavioral situations. An in vivo voltammetric study in free moving rats. Brain Res. 1986;397:395–400. doi: 10.1016/0006-8993(86)90646-3. [DOI] [PubMed] [Google Scholar]
- Ma YL, Wang HL, Wu HC, Wei CL, Lee EH. Brain-derived neurotrophic factor antisense oligonucleotide impairs memory retention and inhibits long-term potentiation in rats. Neuroscience. 1998;82:957–967. doi: 10.1016/s0306-4522(97)00325-4. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Altar CA. Spontaneous behaviours of rats are differentially affected by substantia nigra infusions of brain-derived neurotrophic factor and neurotrophin-3. Eur J Neurosci. 1996;8:1696–1706. doi: 10.1111/j.1460-9568.1996.tb01313.x. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology. 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215:1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- Miczek K, Nikulina E, Shimamoto A, Covington H. Escalated or suppressed cocaine reward, tegmental BDNF and accumbal dopamine due to episodic vs. continuous social stress in rats. Proc Natl Acad Sci U S A. 2010 doi: 10.1523/JNEUROSCI.0637-11.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Olariu A, Nawa H, Nabeshima T. Involvement of brain-derived neurotrophic factor in spatial memory formation and maintenance in a radial arm maze test in rats. J Neurosci. 2000;20:7116–7121. doi: 10.1523/JNEUROSCI.20-18-07116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Lipska BK, Weinberger DR, Racagni G, Riva MA. Developmental and stress-related changes of neurotrophic factor gene expression in an animal model of schizophrenia. Mol Psychiatry. 2001;6:285–292. doi: 10.1038/sj.mp.4000865. [DOI] [PubMed] [Google Scholar]
- Nawa H, Carnahan J, Gall C. BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur J Neurosci. 1995;7:1527. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Miczek KA, Hammer RP. Repeated social stress sensitized Fos response to amphetamine in mesocorticolimbic dopamine regions of the rat brain. Biol Psychiatry. 2001;49:120S. [Google Scholar]
- Nikulina EM, Marchand JE, Kream RM, Miczek KA. Behavioral sensitization to cocaine after a brief social stress is accompanied by changes in fos expression in the murine brainstem. Brain Res. 1998;810:200–210. doi: 10.1016/s0006-8993(98)00925-1. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Hammer RP, Miczek KA, Kream RM. Social defeat stress increases expression of mu-opioid receptor mRNA in rat ventral tegmental area. Neuroreport. 1999;10:3015–3019. doi: 10.1097/00001756-199909290-00026. [DOI] [PubMed] [Google Scholar]
- Nikulina EM, Arrillaga-Romany IA, Miczek KA, Hammer RP. Long-lasting alteration in mesocorticolimbic structures after repeated social defeat stress in rats: time course of mu-opioid receptor mRNA and FosB/DeltaFosB immunoreactivity. Eur J Neurosci. 2008;27:2272–2284. doi: 10.1111/j.1460-9568.2008.06176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikulina EM, Covington HE, Ganschow L, Hammer RP, Miczek KA. Long-term behavioral and neuronal cross-sensitization to amphetamine induced by repeated brief social defeat stress: Fos in the ventral tegmental area and amygdala. Neuroscience. 2004;123:857–865. doi: 10.1016/j.neuroscience.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Misra K. Central and medial amygdaloid brain-derived neurotrophic factor signaling plays a critical role in alcohol-drinking and anxiety-like behaviors. J Neurosci. 2006;26:8320–8331. doi: 10.1523/JNEUROSCI.4988-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardon MC, Roberts RE, Marsden CA, Bianchi M, Latif ML, Duxon MS, Kendall DA. Social threat and novel cage stress-induced sustained extracellular-regulated kinase1/2 (ERK1/2) phosphorylation but differential modulation of brain-derived neurotrophic factor (BDNF) expression in the hippocampus of NMRI mice. Neuroscience. 2005;132:561–574. doi: 10.1016/j.neuroscience.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Patel PD, Katz M, Karssen AM, Lyons DM. Stress-induced changes in corticosteroid receptor expression in primate hippocampus and prefrontal cortex. Psychoneuroendocrinology. 2008;33:360–367. doi: 10.1016/j.psyneuen.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos A, Watson C. The Rat Brain in Stereotactic Coordinates. Elsevier Academic Press; New York, NY: 2005. [Google Scholar]
- Pizarro JM, Lumley LA, Medina W, Robison CL, Chang WE, Alagappan A, Bah MJ, Dawood MY, Shah JD, Mark B, Kendall N, Smith MA, Saviolakis GA, Meyerhoff JL. Acute social defeat reduces neurotrophin expression in brain cortical and subcortical areas in mice. Brain Res. 2004;1025:10–20. doi: 10.1016/j.brainres.2004.06.085. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004;24:4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Herbrüggen O, Chourbaji S, Ridder S, Brandwein C, Gass P, Hörtnagl H, Hellweg R. Stress-resistant mice overexpressing glucocorticoid receptors display enhanced BDNF in the amygdala and hippocampus with unchanged NGF and serotonergic function. Psychoneuroendocrinology. 2006;31:1266–1277. doi: 10.1016/j.psyneuen.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Lundgren KH, Tran TM, Guthrie KM, Isackson PJ, Gall CM. Dopaminergic neurons in rat ventral midbrain express brain-derived neurotrophic factor and neurotrophin-3 mRNAs. J Comp Neurol. 1994;342:321–334. doi: 10.1002/cne.903420302. [DOI] [PubMed] [Google Scholar]
- Shen RY, Altar CA, Chiodo LA. Brain-derived neurotrophic factor increases the electrical activity of pars compacta dopamine neurons in vivo. Proc Natl Acad Sci U S A. 1994;91:8920–8924. doi: 10.1073/pnas.91.19.8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kim SY, Kvetnansky R. Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology. 1995;136:3743–3750. doi: 10.1210/endo.136.9.7649080. [DOI] [PubMed] [Google Scholar]
- Stevenson CW, Gratton A. Basolateral amygdala modulation of the nucleus accumbens dopamine response to stress: role of the medial prefrontal cortex. Eur J Neurosci. 2003;17:1287–1295. doi: 10.1046/j.1460-9568.2003.02560.x. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol and Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Zhang H, Torregrossa MM, Jutkiewicz EM, Shi YG, Rice KC, Woods JH, Watson SJ, Ko MC. Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects. Eur J Neurosci. 2006;23:984–994. doi: 10.1111/j.1460-9568.2006.04621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]