Abstract
Noise-induced hearing loss is a highly prevalent occupational injury, yet little is known concerning the signals controlling normal cochlear sensitivity and susceptibility to noise-induced trauma. While the corticotropin-releasing factor (CRF) system is involved in activation of the classic hypothalamic-pituitary-adrenal axis, it is also involved in local physiological responses to stress in many tissues, and is expressed in the inner ear. We demonstrate that mice lacking the CRF receptor CRFR2 exhibit a significantly lower auditory threshold than wild type mice, but this gain of function comes at the price of increased susceptibility to acoustic trauma. We further demonstrate that glutamatergic transmission, purinergic signaling, and activation of Akt (PKB) pathways within the cochlea are misregulated, which may underlie the enhanced sensitivity and trauma susceptibility observed in CRFR2-/- mice. Our data suggest that CRFR2 constitutively modulates hearing sensitivity under normal conditions, and thereby provides protection against noise-induced hearing loss.
Introduction
Corticotropin-releasing factor (CRF) is a 41 amino acid peptide critically important to hypothalamic-pituitary-adrenal (HPA) axis function (Vale et al., 1981). While three receptors have been cloned (Grammatopoulos and Chrousos, 2002; Bale and Vale, 2004), only CRFR1 and CRFR2 are expressed in mammals. In addition to its role in HPA axis physiology, CRF and its receptors are expressed in the central nervous system (Sawchenko et al., 1993; Van Pett et al., 2000), suggesting functions for CRF beyond its classic hormonal role. CRF receptors are involved in sensitivity to stress and anxiety (Smith et al., 1998; Bale et al., 2000; Kishimoto et al., 2000; Bale et al., 2002; Vetter et al., 2002), cellular stress responses of the skin (Slominski et al., 1998; Slominski et al., 1999; Slominski et al., 2000; Slominski et al., 2001), mood disorders (Nemeroff, 1988; Nemeroff et al., 1988; Nemeroff, 1992; Bale and Vale, 2003), energy balance and metabolism (Pelleymounter et al., 2000), hemodynamics (Brown et al., 1986), vascularization (Bale et al., 2003) and differentiation of neuronal dendrites within the hippocampus (Chen et al., 2004).
Within the cochlea, hair cells are responsible for encoding auditory stimuli, while various support cells are important for homeostatic regulation of the endolymph, a specialized fluid of the scala media bathing the hair cell apices. Endolymph is an unusual extracellular fluid by virtue of its high potassium content, and relatively low calcium level. The exact ionic composition of the endolymph can be altered by acoustic overexposure (Marcus et al., 1998; Jentsch et al., 2000; Housely et al., 2006) and by other endogenous signals, that alter cochlear sensitivity, thereby serving a protective role against release of potentially excitotoxic levels of glutamate from the hair cells.
Because cochlear hair cells are spontaneously active, and are constantly stimulated by the environment, the cochlea is under constant physical and metabolic stress. Damage and subsequent loss of cochlear hair cells results in permanent hearing loss in mammals. Although noise-induced hearing loss is the most prevalent occupational injury reported in the US, knowledge concerning mechanisms underlying susceptibility to noise-induced hearing loss, and general protein expression changes that take place within the cochlea in response to noise exposure, is incomplete. We have previously demonstrated the existence of urocortin, a CRF-related peptide, and CRFR1 and CRFR2 in the murine cochlea (Vetter et al., 2002) in regions involved with homeostatic regulatory functions of the inner ear, as well as neural processing of hair cell responses. Given that other systems such as the skin use local CRF signaling to maintain homeostasis and protect against physical damage, we hypothesized that the CRF system may play a similar role in the inner ear, and may serve to protect against pathologies such as noise-induced hearing loss. We therefore investigated whether CRF is expressed in the cochlea, determined the role of CRFR2 activity in cochlear function, and attempted to define some of the possible mechanisms by which CRFR2 may exert its protective effects using a CRFR2 null mouse line.
Materials and Methods
Animals, housing, and noise assessment
CRFR2-/- mice have been described previously (Bale et al., 2000). Mice were raised under standard vivarium conditions (12hr light/dark cycles) in ventilated Thoren cage racks. Alternatively, some mice were raised in an IAC acoustic chamber on static shelving. One octave filter measurements of sound intensities over a spectrum of frequencies from 63 to 16000Hz were measured in the standard vivarium to assess ambient sound levels. Two measures were taken; one on the actual shelf the cages are suspended from, and one from the room as an open field measure approximately four feet from the floor. A Bruel and Kjaer SLM model 2231 audiometer was calibrated on site just prior to measurements using a Bruel and Kjaer pistonphone. The audiometer was equipped with a Filter set type 1625 with a ½ inch microphone, model 4125. Linear unfiltered measures were also obtained (see Supplemental data). As expected, the most intense sound was at the lowest frequency (63Hz, 74dB SPL). Intensities fell linearly with increasing frequency, and stabilized between 58dB (at 500Hz) and 50dB (at 16000Hz). Examination of the mouse ABRs reveal that mice generally have very poor hearing at frequencies below 8-10kHz, and therefore the most intense sounds do not reach threshold and are not of concern for data interpretation. However, at 16kHz, mice exhibit very sensitive hearing, and thus one may assume that the mice held in the standard vivarium are sensitive to the 50dB constant noise from the ventilated housing racks.
Immunolabeling
Cochleae were fixed in 4% paraformaldehyde for 1 hour at room temperature and decalcified overnight at 4°C in solution containing 8% EDTA buffered in 1× (final) PBS. Cochleae were then handled either as whole mount dissections, or embedded for cryosectioning. For whole mount processing, decalcified cochleae were stripped of the surrounding bone, and the lateral wall was trimmed down to the basilar membrane level using microdissection scissors. The turns were then separated into an apical and a basal turn (hook region was generally not recovered) and put into PBS in an eppendorf tube, in which the remaining immunolabeling and wash steps were carried out. For cryosectioning, decalcified cochleae were cryoprotected with 15% sucrose in 1× PBS for 2-4 hours at 4°C, and then transferred to a 30% sucrose solution made in 1× PBS overnight on a rotator. Cochleae were then moved to OCT embedding medium and slowly injected with OCT via round and oval windows using a 10cc syringe and an 18 gauge needle. Cochleae were embedded in fresh OCT in peel away paraffin embedding molds and frozen in isopentane previously cooled with, and maintained on, dry ice. Cryostat sections were cut 10μm thick and only mid-modiolar sections were used for examination. All tissue was incubated in blocking solution (5% normal goat serum and 0.5% Triton X-100 in 1× PBS) for 1 hour at room temperature and incubated in primary antibody solution (1% normal goat serum, 0.1% Triton X-100) overnight at room temperature. Primary antibodies included: polyclonal rabbit anti-CRFR2 (1:200, Chemicon/Millipore, Billerica, MA), polyclonal rabbit and monoclonal mouse anti-calbindin (1:1000, Swant, Bellinzona, Switzerland), mouse anti-CtBP2 (1:1000, Millipore), polyclonal rabbit anti-CRF (1:200, Chemicon/Millipore, Billerica, MA), monoclonal mouse anti-TuJ1 (1:1000, Neuromics, Edina, MN), rabbit monoclonal anti-total Akt1 (1:100), anti-Akt1 pThr 308 (1:50), and anti-Akt1 pSer473 (1:50) (all Akt antibodies from Cell Signaling Technology (Danvers, MA). Following primary incubation, tissue was washed three times in 1× PBS and then incubated in secondary antibody for 1 hour at room temperature. Secondary antibodies used for fluorescent immunolabeling were either goat anti-mouse Alexa488, goat anti-rabbit Alexa594, and goat anti-rabbit Oregon Green (1:200, Invitrogen, Eugene, OR). For DAB immunostaining (calbindin), biotinylated goat anti-rabbit secondary (1:100 Jackson Immunoresearch Labs, West Grove, PA) was used, followed by incubation in standard ABC (Vector Labs, Burlingame, CA). For CRF immunolabeling, 15% (v/v) saturated picric acid was added to the 4% paraformaldehyde fixative. Images were gathered using a Leica TCS SP2 AOBS confocal microscope. Controls for each primary antibody consisted of a no primary step in which primary antibody was replaced with PBS. All other steps were as described above.
Auditory Physiology
Briefly, mice were anesthetized with xylazine (20 mg kg-1 intraperitoneally, i.p.) and ketamine (100 mg kg-1 i.p.). For auditory brainstem responses, needle electrodes were inserted at vertex and pinna, with a ground near the tail. Stimuli were 5-ms tone pips delivered at 35s-1. At each test frequency, the sound-pressure level was varied in 5 dB steps. DPOAEs were measured with an ER-10C system. Two primary tones (f2:f1 = 1.2) were presented with f2 level 10 dB < f1. We computed a fast Fourier transform and extracted sound pressures at f1, f2 and 2f1 - f2 after spectral averaging from five serial waveform traces. We interpolated the iso-response contours for DPOAEs from the amplitude-versus-level functions: the criterion response was a 2f1 - f2 DPOAE of 0 dB SPL.
Assessment of susceptibility to noise-induced auditory threshold shifts
Awake and unrestrained mice were exposed to sounds free-field in a small reverberant chamber. Acoustic trauma consisted of a 2-hr exposure to an 8-16 kHz octave band noise presented at 100 dB SPL. The exposure stimulus was generated by a custom white-noise source, filtered (Brickwall Filter with a 60 dB/octave slope), amplified (Crown power amplifier), and delivered (JBL compression driver) through an exponential horn fitted securely to a hole in the top of a reverberant box. Sound pressure levels previously measured at 4 positions within the holding cage (using a 0.25 inch Bruel and Kjaer condenser microphone) was found to vary by less than 0.5 dB across all positions.
Western Blot
Whole cochlear lysates were prepared from at least 4 mice (8 cochleae). Mice were raised either in continuous noise (standard vivarium conditions, referred to as moderate noise environment), or in an acoustic chamber (referred to as quiet condition). Homogenates were prepared in buffer containing either T-Per plus protease inhibitor cocktail (Pierce, Rockford, IL) on ice or 2% SDS at 95-100°C. Protein concentration was quantified using a Micro BCA kit (Pierce, Rockford, IL) and either 75μg (or 150 μg for GluR4 analysis) of total protein (100μg for phosphorylation assays) was loaded onto an 8% polyacrylamide gel (15% for connexin detection). Proteins were resolved using SDS-Page and transferred to a PVDF membrane. The membrane was blocked with 10% dry nonfat milk in TBST (50mM Tris, 150mM NaCl, pH 7.6, .05% Tween-20) for 1 hour at room temperature. Primary antibodies were diluted into solution containing 1% non-fat milk in TBST and incubated with the membrane overnight at 4°C. Primary antibodies included: polyclonal rabbit anti-GluR4 (1:500, Millipore, Billerica, MA), polyclonal rabbit anti-P2X2 (1:2000, Abcam, Cambridge, MA), polyclonal rabbit anti-P2Y4 (1:1000, Sigma, St. Louis, MO), monoclonal mouse anti-Connexin 26 or 30 (1:500, Zymed, Carlsbad, CA). Following primary antibody incubation, membranes were washed 3× 5 minutes with TBST and incubated with HRP-labeled goat anti-rabbit or goat anti-mouse secondary (1:2000, Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) for 1 hour at room temperature. Secondary antibodies were diluted into solution containing 1% non-fat milk powder in 0.5% TBST. Following secondary incubation, blots were washed 2 × 10 minutes in distilled water and then 1× 5 minutes in TBST. Following the TBST wash, blots were rinsed with distilled water and incubated for 2 minutes with SuperSignal West Dura Extended Duration Substrate (Pierce, Rockford, IL). Bands were visualized using a Kodak Image Station 2000. Minimally, 3 blots were generated from the same lysate for each protein for statistical analysis. Band densities were assessed using Image J or Kodak ImageStation software.
GluR4 is expressed at low levels in the cochlea. However, a typical enrichment of the GluR4 target using immunoprecipitation prior to western blotting was not possible with the antibody used. Given these issues, large amounts of total protein (150ug) were initially loaded onto the gel to obtain a detectable signal. As a result, standard loading controls, which are typically abundant proteins such as actin, were massively overloaded, causing loading control protein to diffuse between lanes across the entire blot. Quantification of these signals for any single lane was therefore impossible, and we therefore expressed GluR4 signal relative to input protein, based on the initial protein estimation.
To probe the Akt signaling pathway, western blotting was performed on cochlear lysates generated by homogenization of whole cochlea in 2% boiling SDS. Expression data were obtained from blots performed on three separate lysates (each generated from at least 4 mice). Antibodies used were rabbit monoclonals obtained from Cell Signal Technologies (Danvers, MA): anti-phospho-Akt (Ser473), anti-phospho-Akt (Thr308), anti-phospho-PTEN (Ser308), anti-phospho-PDK1 (Ser241), Akt1, PI3kinase, and p70S6Kinase, all used at 1:1000 dilution as described above.
Immunoprecipitation
Due to the relatively low abundance of GluR2/3 in the cochlea, immunoprecipitation procedures were used to enrich the signal. For each sample, 500μg total protein was diluted in immunoprecipitation buffer (25mM Tris, 150mM NaCl, pH 7.2) to a final volume of 700μl. Monoclonal rabbit anti-GluR2/3 antibody (1:70, Abcam) was added to the diluted solution and incubated overnight at 4°C with gentle shaking. Following primary antibody incubation, 100μl Protein A/G Plus Agarose bead slurry (Pierce, Rockford, IL) was added to each sample, yielding 50μl settled beads per sample. The beads incubated with the mixture overnight at 4°C with gentle shaking. After incubation, the samples were spun at 2000×g for 3 minutes and the supernatant removed. The beads were washed 5 times with immunoprecipitation buffer then boiled for 5 min at 100°C in Laemmli's SDS sample buffer (2× upon 1:1 dilution with distilled water, 250mM Tris-HCL, pH6.8, 8%SDS, 40% glycerol, 8% βME, 0.02% bromophenol blue). The samples were spun at 2000×g for 3 minutes and the supernatant loaded onto a 10% polyacrylamide gel. Enriched proteins were resolved, blotted, and detected using SDS-PAGE and western blotting procedures described above. The primary antibody used for detection was the same used to immunoprecipitate but the concentration was reduced to 1:200. Because immunoprecipitation procedures were used to reveal GluR2/3 expression levels, a loading control consisting of a housekeeping protein, or structural protein such as actin, would not be reliable, since only the enriched GluR2/3 protein captured by the immunoprecipitation was loaded onto the gel. Flow-through proteins are not always reliable, as nothing is known of non-specific retention during the immunoprecipitation step. We therefore used the initial protein estimation to ensure that equal amounts of protein were initially loaded into the immunoprecipitation reaction.
Results
CRF is expressed in the murine cochlea
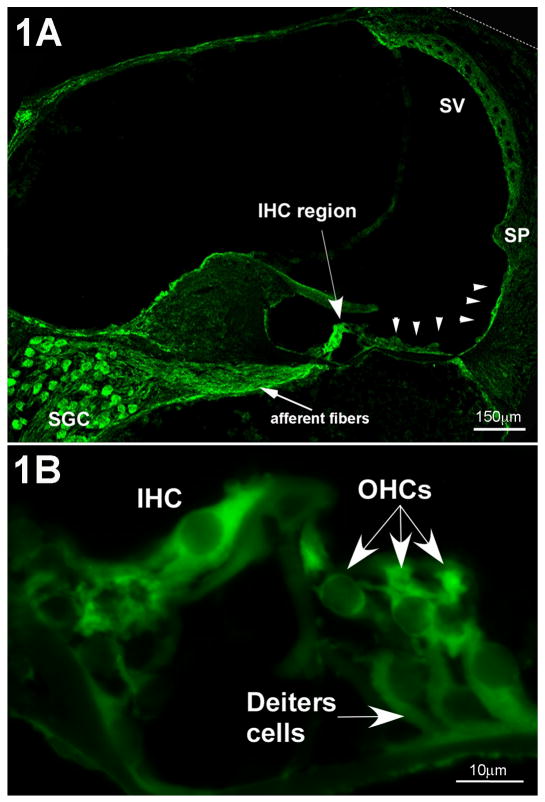
The only CRF-like ligand previously reported in the cochlea is urocortin (Vetter et al., 2002). However, given the mismatch between the relatively restricted expression pattern of urocortin in the lateral olivocochlear terminals and the widespread distribution of its receptors, we hypothesized that other CRF-like ligands may also exist in the cochlea. Immunofluorescent labeling of adult mouse cochleae localized CRF to cells lining the lumen of the scala media, including lateral support cells along the basilar membrane, inner sulcus cells of the spiral limbus, outer sulcus cells to the spiral prominence, and the stria vascularis, where it was especially prominent at the apical (luminal) surface of the marginal cells (Fig. 1A). Robust expression was also observed in spiral ganglion cells and interdental cells of the spiral limbus (Fig. 1A), and both hair cell populations (Fig. 1B). Based on no primary control experiments (which yielded no discernable immunofluorescent labeling), lower intensity immunofluorescence observed in Deiter's cells, root processes of Type IV fibrocytes, and along Reissner's membrane were considered to be indicative of positive labeling, albeit of lesser expression levels. Moderate labeling was found in the intermediate and basal cells of the stria vascularis.
Figure 1.
Immunolocalization of CRF in the mouse cochlea. 1A- CRF is localized in numerous regions of the cochlea. The highest immunofluorescence intensities are located in the inner hair cell region (IHC), the spiral ganglion cells (SGC) and the associated afferent fibers, and in the lateral support cells along the basilar membrane (arrowheads) extending up the lateral wall to the spiral prominence (SP). Also, the stria vascularis (SV) is moderately labeled for CRF. 1B- In the organ of Corti, inner hair cells (IHC) and outer hair cells (OHCs) are immunolabeled for CRF. The Deiter's cells are also immunopositive, but are less intensely labeled.
CRFR2 is expressed widely throughout the murine cochlea
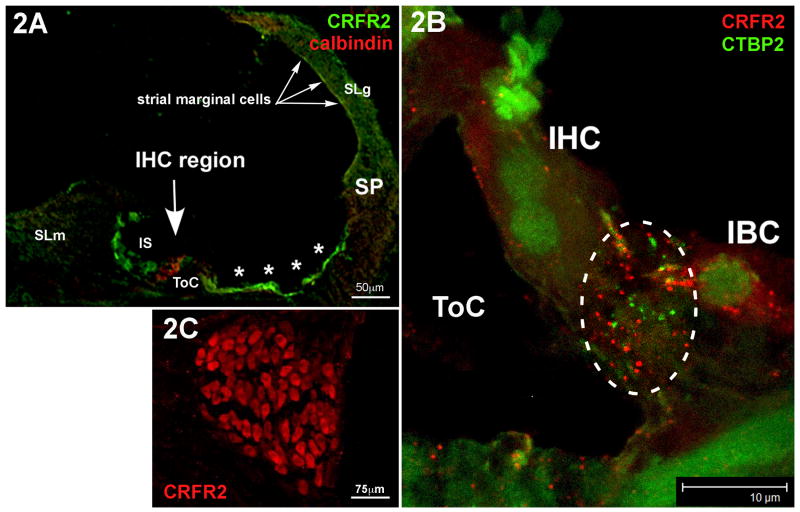
The localization of CRFR2 protein has not been previously reported in the cochlea. Therefore, we examined its expression pattern using immunolabeling methods. The most prominent regions of CRFR2 immunofluorescent labeling were the various “support” cells along the cochlear duct, including the inner sulcus cells of the spiral limbus, and the lateral support cells (Fig. 2A). Cells within the spiral ligament, including regions containing Type I, II, and IV fibrocytes, were labeled with anti-CRFR2 antibodies (Fig. 2A). Cells in intimate contact with hair cells, including the inner border cell at the medial aspect of the inner hair cell (IHC) (Fig. 2B), and the Deiter's cells, which cup the basal pole of the outer hair cells (OHCs), were immunofluorescently labeled. Spiral ganglion cell bodies also expressed CRFR2 (Fig. 2C), and punctate CRFR2 staining was observed in the region containing ganglion cell processes at the base of the IHCs, previously shown to also contain urocortin-positive efferent fibers (Vetter et al., 2002), and in which the synaptic ribbon structures, immunolabeled with anti-CtBP2 antibodies, are located (Fig. 2B, dashed circle). No primary control immunolabeling revealed no labeling under identical conditions to the primary included immunolabeling. Thus, expression of CRFR2 is widespread within the cochlea and is well positioned for activation by urocortin and CRF.
Figure 2.
Immunolocalization of CRFR2 in the mouse cochlea. 2A- CRFR2 immunolocalization was carried out using antibodies labeled with the green fluorphore, while calbindin, a marker for hair cells, was co-localized using antibodies labeled with a red fluorphore. The tunnel of Corti (ToC) spans the area between the inner and outer hair cells, labeled in red. CRFR2 expression is localized to cells lining the lumen of the endolymphatic space. These include the inner sulcus cells (IS) the lateral support cells (asterisks) up to the spiral prominence (SP), and marginal cells of the stria vascularis (arrows). Cells within the spiral ligament (SLg) behind the stria vascularis, and cells within the spiral limbus (SLm) are also immunopositive. 2B- Higher magnification of the inner hair cell region reveals punctate profiles of CRFR2 immunolabeling (red fluorphore) in close apposition to synaptic ribbons, labeled with anti-CTBP2 (green puncta). This region, outlined by the dashed circle, is the afferent synaptic zone of the inner hair cells. CTBP2 also stains the nuclei of cells due to its dual role as a nuclear protein. Note also that the inner border cell (IBC) expresses punctate CRFR2 immunolabeling. ToC-tunnel of Corti. 2C- CRFR2 is highly expressed in the cell bodies of the spiral ganglion cells.
CRFR2 activity modulates auditory thresholds and protects against noise-induced trauma
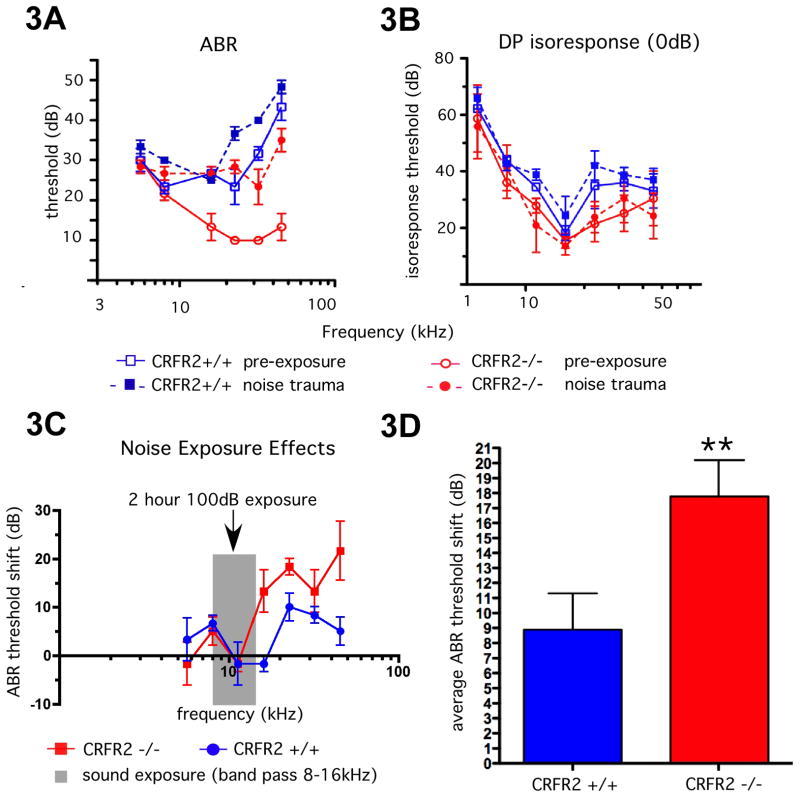
The expression patterns for CRF and CRFR2 suggested that CRF signaling could play a functional role in hearing. We used auditory brainstem response (ABR) threshold measures to examine hearing sensitivity in wild type and CRFR2-/- mice. We also examined outer hair cell function by measuring distortion product otoacoustic emissions (DPOAEs), an assessment of the mechanical outer hair cell-based cochlear amplifier important for establishing normal hearing thresholds. Mice were born and raised in either the standard vivarium in which ventilated cage housing delivered a constant moderate intensity noise between 50-60dB, or in an acoustically isolated sound attenuation chamber. ABR thresholds for tone pip frequencies up to 8kHz were identical in wild type and CRFR2-/- mice raised in either environment (see Supplementary Fig. 1). From 16-45.26 kHz, the chamber reared CRFR2-/- mice exhibited thresholds that were on average approximately 20dB more sensitive than the chamber reared wild type mice, reaching a maximal threshold difference of 40dB at 45kHz. However, when raised in the moderately noisy environment, ABR thresholds between 16-45.26kHz of CRFR2-/- mice were no longer more sensitive, and were similar to wild type mice, suggesting a substantial permanent threshold shift (PTS) in response to moderate ambient noise (Supplementary Fig. 1). DPOAE thresholds were not statistically different between CRFR2-/- and wild type mice.
To test whether CRFR2-/- mice are more susceptible to noise-induced hearing loss in a controlled manner, acoustic chamber-reared mice of both genotypes were subjected to high intensity (100dB) band-passed (8-16kHz) sound for two hours in a sound reverberant box. ABR thresholds and DPOAE isoresponse thresholds were tested for each mouse prior to, and two weeks after exposure (Fig. 3A, B). Wild type mice demonstrated a modest but statistically significant average rise (9dB) in ABR thresholds. The CRFR2-/- mice, however, exhibited a PTS that was approximately twice as great as wild type mice (Fig. 3A, C, D), indicating a greater sensitivity to noise-induced hearing loss following ablation of the CRFR2 gene. DPOAE thresholds did not deviate significantly from baseline (Fig. 3B), indicating that the threshold deficit did not occur at the level of the cochlear amplifier.
Figure 3.
Analysis of auditory function of CRFR2-/- mice. CRFR2-/- and wild type mice were born and raised in an acoustic attenuation chamber in cages on standard wire rack shelving. 3A, B- At approximately two months of age, baseline ABR and DPOAE thresholds were identified and plotted as a function of stimulus frequency (solid lines). Mice were then exposed within 24hrs to the 8-16kHz high intensity (100dB) sound for 2hrs. Two weeks post-exposure, mice were again tested for ABR and DPOAE thresholds, and post-trauma results (dashed lines) were plotted over baseline results obtained prior to exposure (solid lines). Note that ABR thresholds were altered following exposure, (3A), while DPOAE thresholds were not (3B). 3C- ABR threshold shifts were calculated and plotted as a function of stimulus frequency. Gray region represents bandwidth of traumatizing sound. CRFR2-/- mice underwent significantly greater permanent ABR threshold shifts than wild type mice. 3D- the average ABR threshold shift was computed, and a repeated measure ANOVA used to calculate statistical significance. On average, wild type mice underwent a 9dB threshold shift, while the CRFR2-/- mice underwent an 18dB threshold shift (p<0.01).
Exposure to moderate ambient noise does not elicit structural pathology in CRFR2-/- cochleae
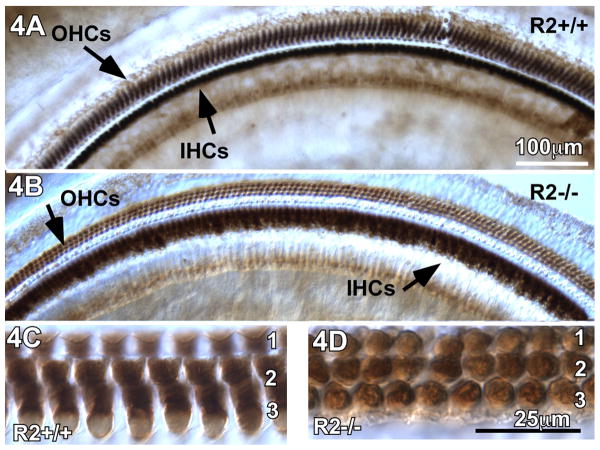
Because the CRFR2-/- mice exhibited a greater sensitivity to noise-induced hearing loss, we examined their cochleae for structural defects following exposure to constant, moderate noise. Adult wild type and CRFR2-/- mice were moved from the acoustic chamber (quiet environment) to the standard housing facility (moderate noise environment) and maintained there for 9 days. After this period of noise exposure, all cochleae were collected, processed for whole mount immunostaining, and probed for calbindin, a calcium buffering protein normally expressed by inner and outer hair cells (IHCs and OHCs). Calbindin immunostaining of whole mount cochlear sections from wild type and CRFR2-/- noise-exposed mice revealed no loss of IHCs or OHCs (Fig. 4), and no structural abnormalities were detectable at the light microscopic level under differential interference contrast illumination. Although no structural pathology was observed, the intensity of calbindin immunostaining in both IHCs and OHCs qualitatively appeared lower in CRFR2-/- mice compared to wild type mice (Fig. 4C, D). In the wild type OHC population, calbindin labeling was found throughout the cytoplasm, while in CRFR2-/- OHCs, calbindin was only observed at the nuclear level (4D), indicating a potential diminution of calcium buffering capability following loss of CRFR2 expression.
Figure 4.
Loss of CRFR2 activity does not result in structural pathology of the organ of Corti in mice that were born and raised in quiet ambient sound environment and moved to a moderately noisy ambient sound environment. 4A- Calbindin immunostaining of wild type cochleae reveal intense labeling of the OHCs and IHCs along the entire length of the cochlea. 4B- Calbindin immunostaining of CRFR2-/- cochleae also revealed immunostaining of OHCs and IHCs. While the IHCs were stained, they were not as intensely immunoreactive as observed in wild types. Additionally, the OHCs were only lightly immunoreactive compared to wild types. However, there was no evidence of OHC or IHC loss in the CRFR2-/- mice. 4C,D- Higher magnification of the outer hair cells reveals differences in the cellular localization of calbindin between wild type (C) and CRFR2-/- (D) mice. In wild type mice, immunostaining is observed throughout the cytoplasm of the hair cells, leaving the nuclei blank. In mice lacking CRFR2, the majority of calbindin immunostaining in OHCs is observed at the nuclear level.
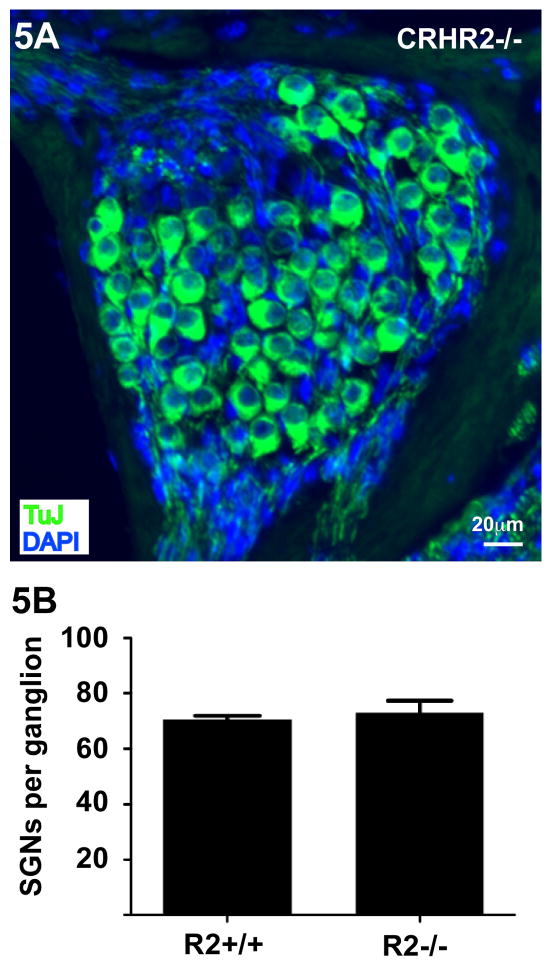
The auditory physiology data indicates that the permanent hearing threshold shift in CRFR2-/- mice results from a neural dysfunction as no shift occurs in the mechanically-based DPOAE threshold. Therefore, we also examined whether exposure to constant moderate noise produces loss of spiral ganglion neurons (the afferent output of the cochlea) in CRFR2-/- mice. Following 9 days of noise exposure, TUJ1 immunofluorescent labeling of CRFR2-/- cochleae revealed no apparent structural abnormalities or loss of the spiral ganglion cells (Figure 5A) at the light microscopic level. Spiral ganglion cell counts from the middle turn of the cochlea were similar between noise-exposed CRFR2-/- and wild type mice (average 71 cells per ganglion for wild type and 73 cells per ganglion for CRFR2-/- mice, p = 0.5949, Fig. 5B)
Figure 5.
Noise exposure does not cause a loss of spiral ganglion cells in mice lacking CRFR2. 5A- Immunofluorescent labeling for the neuronal marker TUJ1 (β-tubulin class III, green) reveals normal spiral ganglion cell populations in CRFR2-/- mice. 5B- Cell counts from a middle frequency region of three wild type and three CRFR2-/- cochleae reveal similar numbers of spiral ganglion neurons, expressed here as spiral ganglion neurons (SGNs) per ganglion. Mean values are 70.5 ± 1.3 and 73.1 ± 4.2 for wild type and CRFR2-/- mice respectively (p = 0.5949, two tailed Student's t test).
Loss of CRFR2 results in abnormal AMPA receptor GluR2/3 and GluR4 subunit expression
While DPOAE isoresponse thresholds are slightly lower in CRFR2-/- mice, the lack of significant change indicates marginal mechanical/amplifier enhancement. Thus, the full extent of enhanced acoustic sensitivity observed in CRFR2-/- mice is likely not due to these potentially small mechanical changes, but rather may originate from changes to afferent neurotransmission between inner hair cells and the apposed spiral ganglion cell processes. Glutamate, the afferent neurotransmitter used by hair cells of the inner ear, is released from inner hair cells during mechanotransduction and binds to AMPA class glutamate receptors (GluR) on the post-synaptic spiral ganglion cell dendrites. Previous work demonstrated that AMPA receptors are rapidly withdrawn from the cell surface in response to sound, suggesting a homeostatic response that limits glutamatergic transmission (Chen et al., 2007) during noise exposure. Given that CRFR2 is expressed in spiral ganglion cells and is localized to the synaptic region where it could locally modulate AMPA receptor expression, aberrant GluR expression in CRFR2-/- mice may cause their hyperacusis under quiet conditions and their enhanced susceptibility to PTS (potentially via glutamate-induced excitotoxicity) under moderately noisy conditions. Expression levels of total GluR2/3 and GluR4 subunits were examined by western blot of cochlear lysates isolated from adult mice raised and maintained under quiet conditions (acoustic chamber) or under constant moderate level ambient sound (standard vivarium).
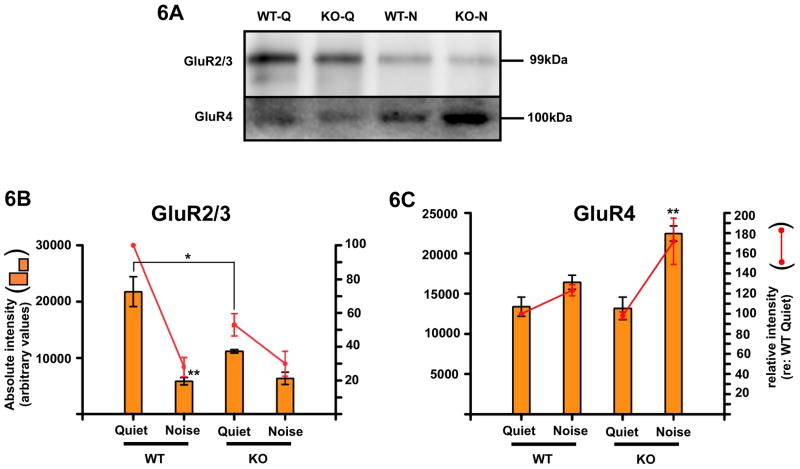
Significant differences were found in GluR subunit expression under both quiet and constant noise conditions. Under quiet conditions, CRFR2-/- mice expressed significantly less GluR2/3 than wild type mice (p=0.0168). Under noise conditions, both groups expressed similar levels of GluR2/3. However, for wild type mice, GluR2/3 levels under noise decreased significantly from quiet levels (p=0.0044, Fig. 6A, B) whereas for CRFR2-/- mice, GluR2/3 expression showed no statistical change from quiet to noise (two-way ANOVA and Bonferroni's post-hoc test) as their levels were already low under quiet conditions. GluR4 expression was not significantly different between wild type and CRFR2-/- mice under quiet conditions. Under constant noise conditions, wild type mice showed no statistically significant change in GluR4 expression (p=0.1109), but GluR4 expression increased in CRFR2-/- mice approximately 70% (Fig. 6C) over quiet condition levels (p=0.0053), suggesting misregulation of AMPA receptor expression in response to noise.
Figure 6.
Glutamate receptor subunit expression levels are altered in CRFR2-/- mice. 6A- GluR2/3 and GluR4 western blotting was performed to quantify expression levels in wild type and knockout quiet reared (WT-Q, KO-Q respectively) and wild type and knockout noise exposure reared (WT-N, KO-N respectively) mice. 6B- Absolute intensity (left ordinate and bar graph) and relative intensity normalized to the wild type quiet expression level (right ordinate and line graph) were plotted for GluR2/3 expression. Under quiet conditions, CRFR2-/- mice expressed roughly half the level of wild type mice for GluR2/3 (p=0.0168). Following constant exposure to noise, wild type mice down-regulated GluR2/3 expression approximately 80% (p=0.0044). However, no statistical difference (2-way ANOVA with Bonferroni's post-hoc test) was found in GluR2/3 expression between wild type mice under noise conditions and CRFR2-/- mice under quiet or noise conditions. 6C- Expression levels of GluR4 were similarly probed and plotted. No significant difference was observed in wild type versus CRFR2-/- GluR4 levels under quiet conditions. GluR4 expression did not change significantly with sound exposure in wild type mice. However, in CRFR2-/- mice GluR4 levels increased significantly, reaching levels approximately 70% above quiet conditions (p=0.0053). Thus, GluR2/3 expression is down regulated in CRFR2-/- mice under both quiet and noise conditions, while GluR4 becomes over-expressed under noise.
Loss of CRFR2 alters expression of proteins involved in ATP signaling in the cochlea
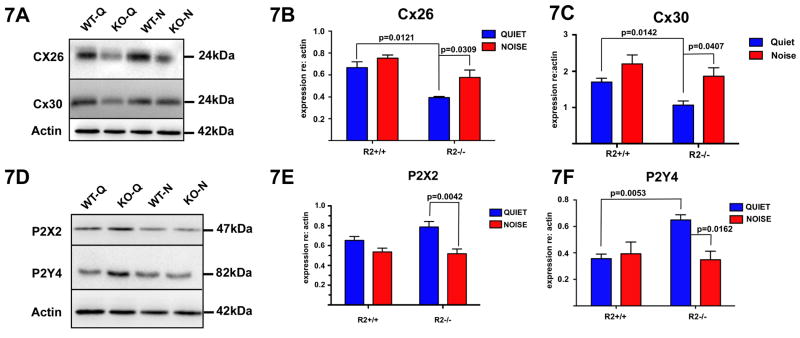
CRFR2 is highly expressed in support cells lining the lumen of the scala media. These cells communicate extensively via purinergic signaling, which has been suggested to increase with sound to protect the cochlea against damage (Housley et al., 1999; Lee et al., 2001; Zhao et al., 2005). In response to sound, support cells release ATP, stimulating purinergic receptors that regulate potassium recycling into and out of the scala media. Thus, by adjusting the charge of the endolymph, purinergic signaling can alter hair cell sensory transduction and auditory sensitivity. Because CRFR2 is expressed not only in cells that release ATP, but also in cells that transduce purinergic signals, we hypothesized that elimination of CRFR2 disrupts purinergic signaling in the cochlea. To investigate this hypothesis, we examined expression levels of ATP release machinery as well as the purinergic receptors P2X2 and P2Y4. ATP is released from support cells via connexin hemichannels composed of Connexins 26 and 30, both of which were down regulated in CRFR2-/- mice under quiet conditions compared to wild type mice (p=0.0121 and p=0.0142, respectively; Fig. 7A-C). Expression levels of neither connexin significantly changed in wild type mice under the moderate ambient noise conditions. In the CRFR2-/- mice, expression levels increased significantly with noise (Connexin 26, p=0.0309; Connexin 30 p=0.0407), but were still less than those of the wild type mice (Fig. 7B,C). Examination of purinergic receptor expression revealed a significant upregulation of P2Y4 in CRFR2-/- mice under quiet conditions compared to wild type mice (Fig. 7F), perhaps as compensation for reduced ATP output through connexin hemichannels. However, the expression of both P2X2 and P2Y4 decreased significantly in the CRFR2-/- mice (p=0.0042 and p=0.0162 respectively) from quiet to noise conditions, suggesting a reduction of purinergic signaling in the face of constant noise.
Figure 7.
Expression of proteins involved in cochlear ATP signaling is abnormal in CRFR2-/- mice. 7A- Connexin 26 and 30 were probed on western blots of wild type and CRFR2-/- cochlear lysates generated from mice raised in either quiet or noise conditions. Resultant band intensities were quantified and plotted. 7B- In wild type mice, Connexin 26 expression did not change between quiet and noise conditions. However, CRFR2-/- mice exhibited a down-regulation of Connexin 26 under quiet conditions compared to wild type mice (p=0.0121). When CRFR2-/- mice were raised in a noisy environment, Connexin 26 expression increased approximately 50% relative to quiet levels (p=0.0309). 7C- Similarly, Connexin 30 expression levels did not change in a statistically significant manner between quiet and noise conditions in wild type mice, but CRFR2-/- mice expressed lower levels of Connexin 30 under quiet conditions relative to the wild type quiet mice (p=0.0142), and up-regulated Connexin 30 approximately 50% in the presence of noise (p=0.0407). 7D- The purinergic receptors P2X2 and P2Y4 were probed on western blots of wild type and CRFR2-/- cochlear lysates generated from mice raised in either quiet or noise conditions. Resultant bands were quantified and plotted similarly to connexin data (7B, C). 7E- Wild type mice showed no statistically significant change in P2X2 expression between quiet and noisy environments. However, CRFR2-/- mice displayed a significant difference in P2X2 expression between the sound conditions with P2X2 levels down-regulated approximately 30% in noise compared to quiet (p=0.0042). 7F- Wild type mice showed no significant difference in P2Y4 expression between quiet or noisy conditions. Under quiet conditions, CRFR2-/- exhibited an approximately 50% increase in P2Y4 expression relative to wild type mice (p=0.0053). Under noise conditions, P2Y4 expression fell significantly, reaching levels similar to those detected in wild type mice (p=0.0162). Thus, under quiet conditions, the connexin channels (by which ATP release occurs) are down-regulated in CRFR2-/- mice, while ATP receptors are up-regulated, perhaps in a compensatory manner reflecting less ATP availability.
Loss of CRFR2 expression results in aberrant Akt1 pathway activation induced by sound
CRFR2 is a G-protein coupled receptor linked to PKC-mediated signaling. Changes in intracellular signaling following loss of CRFR2 activity may render cells less able to fully activate stress-related signaling cascades, and thus be more susceptible to damage. The serine/threonine kinase Akt is modulated by upstream proteins involved in classic PKC signaling, and downstream targets of Akt are involved in numerous cellular processes, including the regulation of metabolism, protein translation, and cell survival. Thus, we assessed expression levels of Akt1 and its phosphorylated isoforms (Akt1 pThr308 and Akt1 pSer473) using immunolabeling methods to reveal localized qualitative expression levels in CRFR2-/- mice and wild type mice under quiet and noisy environments. We further performed quantitative western blot analyses to determine the degree to which disruptions in Akt signaling alter Akt1 expression in CRFR2-/- mice compared to wild type mice under the different auditory environments. Finally, two canonical phosphorylation sites indicative of activated Akt1 are pThr308 and pSer473. We therefore also probed for differential phosphorylation states of these sites to begin addressing questions concerning potential changes to upstream signaling cascades feeding into Akt1 activation that might occur with loss of CRHR2 signaling.
Total Akt1
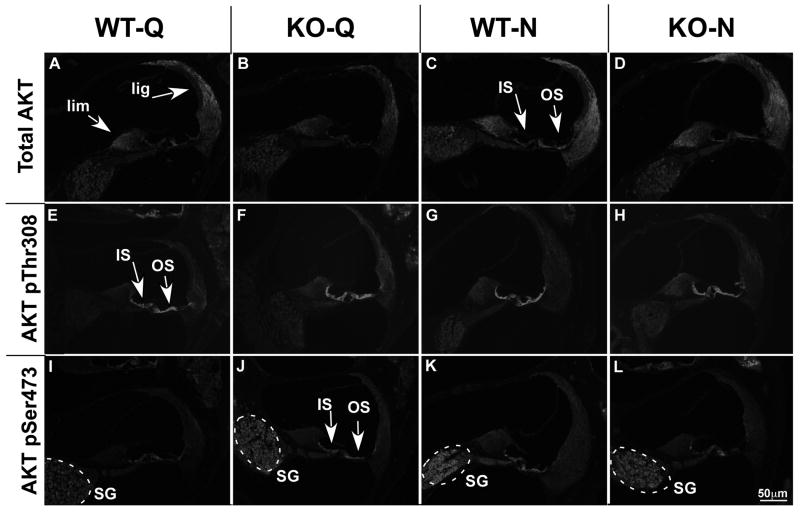
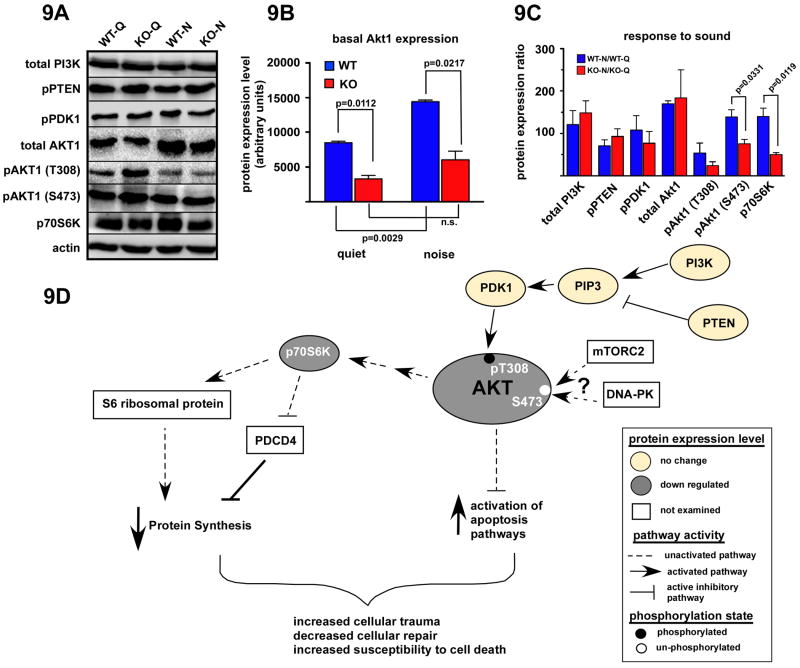
Immunolabeling for Akt1 revealed abundant expression in the wild type cochleae under quiet conditions (Fig. 8A). In particular, intense labeling was observed in the spiral ganglion cells, the spiral limbus, Deiter's cells, and the spiral ligament, especially regions containing Type I and Type III fibrocytes. While a similar expression pattern was observed in CRFR2-/- cochleae under quiet conditions, labeling of Deiter's cells and spiral ligament fibrocytes appeared less intense (despite identical exposure times) than in wild type cochleae (Fig.8B). Noise exposure increased the intensity of Akt1 labeling in both wild type and CRFR2-/- cochleae. Additionally, noise exposure induced expression of Akt1 in inner and outer sulcus cells and lateral support cells that were minimally immunopositive for Akt1 under quiet conditions (Fig. 8C,D). These immunolabeling results correlate with results from western blot experiments demonstrating significantly less Akt1 in CRFR2-/- mice compared to wild type mice under both quiet and noise conditions (p = 0.0112 and 0.0217 respectively, Fig. 9A, B). Furthermore, western blot analysis revealed a significant increase in wild type Akt1 expression with noise exposure (p = 0.0029), but a non-significant increase in Akt1 expression in CRFR2-/- mice.
Figure 8.
The expression patterns of total Akt1 and its phosphorylated isoforms, Akt1 pSer473 and Akt1 pThr308, change depending on the presence or absence of CRFR2 activity and ambient sound condition. 8A-D- Immunofluorescent labeling for total Akt1. 8A- Abundant expression is found in wild type mice under quiet conditions. Labeling is particularly intense in the spiral ganglion cells, the spiral limbus (lim) and the spiral ligament (lig). 8B- Labeling in these regions appears less intense in CRFR2-/- mice under quiet conditions. 8C, D- In both wild type and CRFR2-/- mice under noise conditions, Akt1 expression appears to increase and expand into cell populations not previously expressing Akt1, including cells of the inner sulcus and outer sulcus regions (IS and OS, respectively, arrows). 8E-H- Immunofluorescent labeling for Akt1 pThr308. 8E- Heavy expression is localized along the inner and outer sulcus regions under quiet conditions (IS and OS respectively, arrows). 8F- An increase in Akt1 pThr308 immunofluorescent intensity appears in CRFR2-/- mice compared to wild types under quiet conditions, occurring primarily in the spiral ligament inner and outer sulcus cells. 8G, H- Little difference in immunofluorescence labeling is detectable between wild type and CRFR2-/- mice following moderate noise exposure. 8I-L- Immunofluorescent labeling for Akt1 pSer473. 8I- Under quiet conditions, expression of Akt1 pSer473 is low, with the most prominent expression occurring in the spiral ganglion cells (SG, dashed circle). 8J- Akt1 pSer473 labeling appears more intense in CRFR2-/- mice under quiet conditions and includes inner and outer sulcus cells (IS and OS, respectively, arrows). 8K, L- Under noise conditions, wild type mice and CRFR2-/- mice exhibit opposite trends in label intensity; Akt1 pSer473 labeling increases with noise in wild type mice, but decreases with noise in CRFR2-/- mice.
Figure 9.
CRFR2-/- mice are defective in Akt signaling. 9A- Western blot of proteins involved in the Akt signaling cascade was performed on cochlear lysates generated from wild type and CRFR2-/- mice raised in quiet or noisy conditions. 9B- Basal Akt expression was determined for wild type and CRFR2-/- mice raised in quiet or noisy conditions. Wild type mice increased Akt expression approximately 70% when raised under noisy conditions (p=0.0029). However, CRFR2-/- mice did not increase Akt expression in a statistically significant manner in response to noise. CRFR2-/- mice expressed less Akt under both quiet (61% less) and noise (56% less) conditions than wild type mice (p=0.0112, p=0.0217, respectively). 9C- Expression levels of proteins involved in Akt signaling were quantified and plotted as a percent expression relative to quiet levels (ratio of noise to quiet levels multiplied by 100; a value of 100 represents no change in expression relative to quiet). Upstream inducers of Akt (T308) phosphorylation include PI3Kinase, PIP3, and PDK1. PTEN inhibits PIP3 formation. None of these proteins was altered in expression level in the CRFR2-/- mice, nor was their expression stimulated or inhibited by noise over the quiet condition levels. While expression of total Akt1 was lower in the CRFR2-/- mice as demonstrated in 6B, the effect of noise in inducing an up regulation of expression was approximately equal for wild type and CRFR2-/- mice. Phosphorylation of Akt at the T308 site was apparently less in the CRFR2-/- mice, but the change did not reach statistical significance. However, phosphorylation at the S473 site was significantly suppressed in the CRFR2-/- mice under noise conditions (p=0.0331). Finally, phosphorylation of p70S6Kinase was also suppressed in CRFR2-/- mice under noise control (p=0.0119). Both the pAkt (Ser473) and p70S6K phosphorylation levels were lower than that found under quiet conditions. While wild type mice increased phosphorylation of Akt (S473) 39%, CRFR2-/- mice exhibited a decrease from quiet levels of 24%. Similarly, while wild type mice increased p70S6K expression 40% over quiet levels when raised in noisy conditions, CRFR2-/- mice decreased p70S6K expression 50% from quiet expression levels when raised in noise. 9D- A signaling pathway demonstrates the key proteins assayed, their relative position along the signaling cascade, and the potential outcome of misregulated Akt activity as it occurs in the CRFR2-/- mice. Two main functional branches of the Akt pathway involve protein synthesis and cell death via apoptosis mechanisms. A misregulated Akt pathway in CRFR2-/- mice can lead to decreased p70S6K activation and/or expression as demonstrated, which then does not activate stimulatory paths involved in protein synthesis while at the same time also does not inhibit protein synthesis inhibitors such as PDCD4 as depicted. The final outcome would be lower protein synthesis, rendering cells more susceptible to degradation in the face of cellular stress. Added to this is the loss of anti-apoptotic signaling normally in place with fully activated Akt, and the affected cells may be more susceptible to damage or even loss, as demonstrated by the greater sensitivity to permanent ABR threshold shifts described in Fig. 3.
Phosphorylation of Thr308
Immunolabeling for Akt1 pThr308 revealed intense expression in both wild type and CRFR2-/- cochleae under quiet conditions, particularly in support cells lining the organ of Corti, the inner sulcus, and outer sulcus (Fig. 8E, F). In CRFR2-/- cochleae, Akt1 pThr308 expression appeared in some cell populations that did not exhibit detectable expression over background in wild type cochleae. These regions included the spiral ligament, most notably regions previously shown to contain Type II and Type III fibrocytes, and to a lesser extent the spiral ganglion cells. These data suggested a more widespread activation of Akt1 in CRFR2-/- mice under quiet conditions than occurs in wild type mice under identical conditions. The more expansive Akt pThr308 expression pattern in CRFR2-/- mice was reflected by an increase in Akt1 pThr308 signal intensity assessed by western blot analysis (Fig. 9A). Noise exposure elicited only subtle changes in Akt pThr308 expression pattern (Fig. 8G,H). Labeling intensity in the spiral ganglion cells of wild type mice increased marginally, but labeling intensity decreased in the spiral ligament of CRFR2-/- mice. Overall expression of Akt1 pThr308 in the cochleae assessed by western blot suggested a down-regulation with noise that was not significantly different between wild type and CRHR2-/- mice (Fig. 9C).
Phosphorylation of Ser473
Finally, immunolabeling for Akt1 pSer473 revealed low expression in wild type cochleae under quiet conditions, with the most prominent labeling in spiral ganglion cells and inner sulcus cells and the region of the spiral ligament containing Type III fibrocytes (Fig. 8I). CRFR2-/- cochleae showed intense Akt1 pSer473 labeling under quiet conditions in the spiral ganglion cells, the inner and outer sulcus regions, and a more expansive population of spiral ligament fibrocytes (Fig. 8J). The increase in immunolabeling in the CRFR2-/- mice under quiet conditions correlates with a 23% up-regulation of Akt1 pSer473 assessed by western blot analysis (Fig, 9A). Noise exposure yielded opposite effects on AKT pSer473 expression in wild type and CRFR2-/- cochleae (Fig. 8K,L). Noise exposure induced an increase in Akt pSer473 labeling in wild type cochleae, leading to an expression pattern resembling that observed in CRFR2-/- mice under quiet conditions. Conversely, noise exposure decreased labeling in CRFR2-/- cochleae, particularly in the inner and outer sulcus cells and the spiral ligament, inducing an expression pattern closer to that observed in wild type mice under quiet conditions. These results were reflected in western blot analysis as a significant difference in the effect of noise on AKT1 phosphorylation at Ser473. Whereas wild type mice showed an increase in Akt1 pSer473 from quiet to noise conditions, CRFR2-/- mice showed a decrease (Fig. 9A, C).
Expression of other Akt1 pathway proteins
In addition to our analysis of Akt1 and phospho-Akt1 expression, we examined a small cadre of upstream regulators (PI3K, PTEN, PIP3, and PDK1) and a downstream effector (p70S6K) (Fig. 9). To evaluate noise-induced activation of the Akt1 pathway, we determined the ratio of protein expression levels under noise conditions to protein expression levels in quiet, and compared these ratios between wild type and CRFR2-/- mice. Noise/quiet ratios for total PI3K and pPTEN, involved in activation and suppression of Akt respectively, were not significantly changed in CRFR2-/- mice compared to wild type mice. Noise/quiet ratios for pPDK1, the main kinase targeting the Thr308 residue on Akt1, were consistently reduced in CRFR2-/- mice, as were the ratios for Thr308 phosphorylation, although neither reached statistical significance due to observed variation in expression levels. Finally, p70S6K, a downstream target of Akt1 involved in protein synthesis, was significantly under-expressed in the CRFR2-/- mice compared to controls in response to sound (p=0.0119, Fig. 9C) indicating insufficient activation of Akt1 and its ability to modify downstream targets in the absence of CRFR2-mediated activity.
Discussion
Noise-induced hearing loss cuts across age, gender, ethnicity and profession, and is one of the most prevalent occupational injuries reported. It is especially prevalent in military veterans (Humes et al., 2005), and some estimates indicate that more than 30 million workers in the US alone are exposed to hazardous noise levels (DHHS NIOSH pub. 96-115). Yet significant gaps exist in our understanding of the normal biochemical mechanisms underlying the ability of the cochlea to protect itself against traumatizing sound. A full understanding of potential therapeutic targets is therefore lacking. Given the irreversible nature of cochlear injury, natural mechanisms must exist that preemptively dampen cochlear responses to protect against noise-induced damage, and unraveling such mechanisms could promote discovery of novel therapeutic agents.
Mice deficient for CRFR2 have previously been shown to be hypersensitive to stress (Bale et al., 2000). Although CRF is best known for its role in initiating systemic stress response via the HPA axis, studies have demonstrated local CRF systems (ligand and receptor) in skin (Slominski et al., 2000), brain (Bruijnzeel and Gold, 2005; Hauger et al., 2006), gut (Tache and Bonaz, 2007), and inner ear (Vetter et al., 2002). Our data reveal for the first time a complex biochemical signaling system in the inner ear that is set in motion by exposure to moderate sound and altered following loss of CRFR2 activity. The full CRF system (ligand and receptors) is expressed locally within the cochlea to modulate signaling systems involved in setting cochlear sensitivity, as well as cellular responses to physically stressful and potentially damaging stimuli. Thus, while CRFR2-/- mice have significantly better hearing sensitivity, they are also more susceptible to noise-induced hearing loss compared to wild type mice.
Typical models of PTS involve structural damage and/or loss to cochlear hair cells and ganglion cells that diminishes both afferent transmission and cochlear amplification. However, the PTS observed in CRFR2-/- mice is not characterized by elevated hearing thresholds that were worse than wild type control levels, but rather brought auditory sensitivity from a hyperacute state to the wild type normal state. Therefore, structural pathology was not expected in mice lacking CRFR2. Indeed, immunolabeling for calbindin and TUJ1 revealed intact hair cell and ganglion cell populations in noise-exposed CRFR2-/- mice. However, calbindin labeling of the hair cell populations in these mice appeared less intense, potentially indicating disturbances in compartmentalization of calcium buffering. Given that recent evidence reveals a delayed and prolonged loss of ganglion cells following a single noise trauma, with cell loss continuing even 64 weeks following the initial insult (Kujawa and Liberman, 2009), it is possible that experiments that include longer term survival of CRFR2-/- mice following noise exposure could reveal ensuing functional and/or structural damage.
While numerous biochemical cascades are undoubtedly activated by sound exposure, activation of Akt mediated pathways is of special interest, given the versatility of this kinase in controlling downstream cellular processes that include cell metabolism and survival. The anti-apoptotic role of Akt makes it an especially intriguing candidate for mediating the protective effects of CRFR2 in the cochlea. Our immunofluorescent labeling data revealed an overlap in cells expressing Akt and cells expressing CRFR2, particularly the ganglion cells, inner and outer sulcus cells, and regions containing specific spiral ligament fibrocyte populations. Also, CRFR2-/- mice show changes in the expression patterns of Akt1, Akt1 pSer473, and Akt1 pThr308 that are different from wild type mice, suggesting coordinated activity between CRFR2 and Akt signaling. Our western data demonstrated that loss of CRFR2 resulted in a loss of Akt1 expression and reduced activation of Akt1 with noise. A deficiency in Akt1 activation may lead to a rise in pro-apoptotic signals and a decrease in activation of protein synthetic pathways, some of which may be critical for recovery from, or protection against, noise-induced trauma. Interestingly, the opposite phenomenon occurs under quiet conditions in which there is increased phosphorylation (i.e. activation) of Akt1 in CRFR2-/- mice compared to wild type mice. These data suggest an increased demand for anti-apoptotic signaling in the absence of noise, which correlates with greater acoustic sensitivity and presumably a higher than normal metabolic activity in CRFR2-/- under quiet conditions. These data suggest that the auditory system of the CRFR2-/- mice is been tipped to a stressed condition even before exposure to noise.
Our data suggest that CRFR2 plays a role in purinergic signaling which could influence both IHC mediated afferent transduction and OHC mediated cochlear amplification via its effects on endocochlear potential. While the ABR threshold shifts are clearly altered in the CRFR2-/- mice, it is important to recognize that the DPOAE isoresponse thresholds are also altered, although to a considerably lesser degree, in the nulls. Sound exposure opens connexin hemichannels on support cells lining the cochlear duct, thereby releasing ATP (Zhao et al., 2005). ATP release reduces cochlear sensitivity by reducing the endocochlear potential (Housley et al., 2006). Activation of ATP sensitive P2Y4 receptors (Marcus et al., 2005) leads to phosphorylation and de-activation of the KCNQ1/KCNE complex (Marcus et al., 1998; Jentsch, 2000), thus decreasing potassium secretion into scala media and consequently lowering the hair cell sensory transduction driving force. P2X2 channels expressed along the luminal surface of the hair cells and laterally located support cells (Housley et al., 1998; Järlebark et al., 2000), and has been suggested to act as a potassium sink (Thorne et al., 2004) to further decrease the endocochlear potential. These investigators have hypothesized that this choreographed response of P2X2 and P2Y4 to ATP release may protect hair cells from damage as a result of over activation. Under both quiet and noise conditions, Connexin 26 and 30 are expressed at lower levels in CRFR2-/- mice compared to wild type mice, suggesting reduced ATP release. The mechanisms by which connexin expression decreases following loss of CRFR2 have not been addressed in this work, but it has been demonstrated that various connexins are regulated by post-translational modifications induced by kinase activity (Locke et al., 2006). Both connexin 26 and 30 possess multiple PKC substrates in their N- and C-termini tails (assessed using NetPhosK 1.0 software). The canonical signaling pathway activated by normal CRFR2 stimulation is the phospholipase C/protein kinase C pathway (Sheng et al., 2008), arguing for potential functional interactions between the CRFR2 and connexin proteins. Loss of connexin expression may drive the observed over-expression of P2X2 and P2Y4 in CRFR2-/- mice under quiet conditions as compensation for lower than normal ATP levels. Interestingly, in the presence of constant moderate noise, the purinergic receptors decrease in CRFR2-/- mice but not in controls, suggesting a paradoxical scaling down of potentially protective purinergic signaling in the face of noise in the null mice. Abnormal signaling of potentially harmful sound, coupled with accumulation of potassium in the endolymph, may underlie a second mechanism explaining the greater noise-induced ABR threshold shifts observed in the CRFR2-/- mice.
Our work shows that CRFR2 and its ligands, urocortin and CRF, are well-positioned to modulate afferent transmission in the inner ear. CRF and urocortin have been shown to modulate AMPA-class receptor subunit expression (Gounko et al., 2005), play a role in cerebellar long-term depression (Miyata et al., 1999), and to facilitate glutamatergic synaptic transmission (Liu et al., 2004; Liu et al., 2005; Pollandt et al., 2006). The main neurotransmitter used in the cochlear afferent system is glutamate, and the fast ionotropic-based glutamatergic signal is mediated through AMPA class glutamate receptors composed of GluR2, 3, and 4 subunits. While GluR1 is expressed, its expression is very low in the adult cochlea (Kuriyama et al., 1994; Glowatzki and Fuchs, 2002). The presence of GluR2 within a GluR complex dictates low calcium permeability (Hume et al., 1991). Under quiet conditions, GluR2/3 expression is low in CRFR2-/- mice compared to wild type mice, whereas GluR4 expression is similar between both groups. Therefore, one explanation for a neural basis of the observed hyperacusis in CRFR2-/- mice under quiet (i.e. normal tonic glutamate release) conditions is that fewer receptors contain the GluR2 subunit, rendering the remaining receptors more calcium permeable than normal. Under noise conditions, GluR2/3 levels drop significantly (80%) in wild type mice, presumably as a protective measure to decrease potential glutamate-mediated excitotoxicity in the face of increased (noise-induced) glutamate release. In contrast, GluR4 levels show a small (20%) increase in wild type mice under noise conditions (presumably to decrease overall glutamate-induced activity), while in CRFR2-/- mice, GluR4 levels dramatically increase (70%) with noise. These data may indicate a switch to a greater proportion of receptors potentially composed of GluR4 homomers, given the simultaneous decrease in GluR2/3 expression. Increased expression of GluR4 homomers may represent a normal, adaptive response that is misregulated or unchecked in the CRFR2-/- mice. Experiments have demonstrated that GluR4 homomers are functional, and are critical elements of synapses characterized by tonic activity such as those between photoreceptors and bipolar cells of the retina (Pang et al., 2008). Furthermore, spinal cord neurons, in which GluR4 homomers represent at least half of all GluR complexes, reveal a rapid desensitization of AMPA-induced currents compared to cortical neurons that express comparatively little GluR4 (Dai et al., 2001). Because GluR4 homomers are highly desensitizing, spinal cord neurons expressing these receptors flux less current in response to low concentrations of AMPA than cells expressing heteromeric GluRs (Dai et al., 2001). Thus, a switch to a higher proportion of GluR4 homomers in the cochlea may attenuate post-synaptic responses in the face of constant noise-induced glutamate release. The exaggerated GluR4 increase in CRFR2-/- mice could yield a more extreme attenuation, causing the 20 dB shift in ABR threshold under noise conditions. Interestingly, the combination of increased GluR4 and decreased GluR2/3 in CRFR2-/- mice under noise conditions seems to elicit a similar ganglion cell response as GluR combinations expressed in wild type mice under quiet conditions. Therefore, modifications to the composition of spiral ganglion cell GluR complexes may explain the exclusively afferent origins of the CRFR2-/- ABR shifts in response to noise.
Finally, a convergence between the GluR data and perturbations of the Akt pathways also exists to further implicate excitotoxicity as a mechanism for increased susceptibility to noise-induced hearing loss in the CRFR2-/- mice. It has been shown that activated Akt1 functions as a protective signaling cascade against glutamate-induced excitotoxicity in hippocampal cultures by inhibiting JNK activation that normally occurs during excitotoxicity (Kim et al., 2002). Perturbation of the Akt pathway due to lost CRFR2 activity may therefore lead to a loss of regulation of JNK signaling, exacerbating excitotoxic responses in the cochlea. Indeed, block of pJNK formation inhibits auditory hair cell death following both aminoglycoside treatments and noise-induced trauma (Pirvola et al., 2000).
In summary, the widespread expression of CRFR2 allows it to act on many signaling systems in numerous cells of the cochlea. Here we have demonstrated that loss of CRFR2 activity increases auditory sensitivity, while also increasing susceptibility to noise-induced threshold shifts. Thus, activation of CRFR2 in the wild type state must act to suppress both auditory sensitivity and susceptibility to cochlear changes such as PTS following exposure to damaging noise. Mechanisms explaining the results observed following loss of CRFR2 expression may include regulation of GluR expression in the afferent spiral ganglion cells, purinergic signaling in support cells lining the cochlear duct, and regulation of the Akt signaling cascade in the cochlea. Our data demonstrate the multi-faceted nature of CRF signaling in the cochlea, and suggest that CRFR2 and the Akt signaling cascade may prove to be important targets for intervention against noise-induced hearing loss.
Supplementary Material
Table 1.
| Spiral Ganglion Neurons | Inner/Outer Sulcus Cells | Spiral Ligament | Spiral Limbus | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WTQ | KOQ | WTN | KON | WTQ | KOQ | WTN | KON | WTQ | KOQ | WTN | KON | WTQ | KOQ | WTN | KON | |
| Total Akt1 | + | + | ++ | ++ | - | - | ++ | + | ++ | + | +++ | ++ | + | + | ++ | ++ |
| Akt1 pThr308 | - | + | + | + | +++ | +++ | +++ | +++ | + | ++ | + | + | - | + | + | + |
| Akt1 pSer473 | ++ | +++ | +++ | +++ | - | ++ | ++ | - | - | + | + | - | - | + | + | - |
- No immunolabel, + Minimal Label, ++ Moderate Label, +++ High Label
WTQ wild type, quiet, KOQ knockout quiet, WTN wild type noise, KON knockout noise
Acknowledgments
This research was supported by NIHR01DC006258 (DEV) and The Tufts Center for Neuroscience Research Imaging Core, P30 NS047243. The authors are also indebted to Dr. M.C. Liberman of Mass. Eye and Ear Infirmary for granting access to his auditory physiology (ABR/DP) set-up. Drs. Wylie Vale and Kuo-Fen Lee of the Salk Institute for Biological Studies (La Jolla, CA) provided initial CRFR2 null and wild type mice from which the colony was generated at Tufts Univ. School of Medicine.
Footnotes
Author contributions: CEG performed experiments on GluR and connexin channel expression and all immunolabeling, JB performed experiments defining Akt expression and associated signaling cascades, and DEV performed auditory physiology experiments. DEV and CEG wrote the manuscript. All authors reviewed the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bale T, Vale W. Increased depression-like behaviors in corticotropin-releasing factor receptor-2-deficient mice: sexually dichotomous responses. J Neurosci. 2003;23:5295–5301. doi: 10.1523/JNEUROSCI.23-12-05295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T, Vale W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Bale T, Giordano F, Vale W. A new role for corticotropin-releasing factor receptor-2: suppression of vascularization. Trends Cardiovasc Med. 2003;13:68–71. doi: 10.1016/s1050-1738(02)00214-1. [DOI] [PubMed] [Google Scholar]
- Bale T, Picetti R, Contarino A, Koob G, Vale W, Lee K. Mice deficient for both corticotropin-releasing factor receptor 1 (CRFR1) and CRFR2 have an impaired stress response and display sexually dichotomous anxiety-like behavior. J Neurosci. 2002;22:193–199. doi: 10.1523/JNEUROSCI.22-01-00193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale T, Contarino A, Smith G, Chan R, Gold L, Sawchenko P, Koob G, Vale W, Lee K. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000;24:410–414. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- Brown MR, Gray TS, Fisher LA. Corticotropin-releasing factor receptor antagonist: effects on the autonomic nervous system and cardiovascular function. Regul Pept. 1986;16:321–329. doi: 10.1016/0167-0115(86)90032-7. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005;49:505–528. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bender R, Brunson K, Pomper J, Grigoriadis D, Wurst W, Baram T. Modulation of dendritic differentiation by corticotropin-releasing factor in the developing hippocampus. Proc Natl Acad Sci USA. 2004;101:15782–15787. doi: 10.1073/pnas.0403975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nature neuroscience. 2007;10:1238–1240. doi: 10.1038/nn1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai WM, Egebjerg J, Lambert JD. Characteristics of AMPA receptor-mediated responses of cultured cortical and spinal cord neurones and their correlation to the expression of glutamate receptor subunits, GluR1-4. Br J Pharmacol. 2001;132:1859–1875. doi: 10.1038/sj.bjp.0703993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs P. Transmitter release at the hair cell ribbon synapse. Nature neuroscience. 2002;5:147–154. doi: 10.1038/nn796. [DOI] [PubMed] [Google Scholar]
- Gounko N, Rybakin V, Kalicharan D, Siskova Z, Gramsbergen A, van der Want J. CRF and urocortin differentially modulate GluRdelta2 expression and distribution in parallel fiber-Purkinje cell synapses. Mol Cell Neurosci. 2005;30:513–522. doi: 10.1016/j.mcn.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos D, Chrousos G. Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab. 2002;13:436–444. doi: 10.1016/s1043-2760(02)00670-7. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Brauns O, Dautzenberg FM. Corticotropin releasing factor (CRF) receptor signaling in the central nervous system: new molecular targets. CNS Neurol Disord Drug Targets. 2006;5:453–479. doi: 10.2174/187152706777950684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Luo L, Ryan AF. Localization of mRNA encoding the P2X2 receptor subunit of the adenosine 5′-triphosphate-gated ion channel in the adult and developing rat inner ear by in situ hybridization. The Journal of comparative neurology. 1998;393:403–414. [PubMed] [Google Scholar]
- Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells--beyond the transducer. The Journal of membrane biology. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume RI, Dingledine R, Heinemann SF. Identification of a site in glutamate receptor subunits that controls calcium permeability. Science (New York, NY. 1991;253:1028–1031. doi: 10.1126/science.1653450. [DOI] [PubMed] [Google Scholar]
- Humes LE, Joellenbeck LM, Durch JS. Noise and Military Service: Implications for Hearing Loss and Tinnitus. Institute of Medicine of the National Academies; 2005. [Google Scholar]
- Järlebark L, Housley G, Thorne P. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea. The Journal of comparative neurology. 2000;421:289–301. doi: 10.1002/(sici)1096-9861(20000605)421:3<289::aid-cne1>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Kim AH, Yano H, Cho H, Meyer D, Monks B, Margolis B, Birnbaum MJ, Chao MV. Akt1 regulates a JNK scaffold during excitotoxic apoptosis. Neuron. 2002;35:697–709. doi: 10.1016/s0896-6273(02)00821-8. [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Radulovic J, Radulovic M, Lin C, Schrick C, Hooshmand F, Hermanson O, Rosenfeld M, Spiess J. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000;24:415–419. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H, Jenkins O, Altschuler RA. Immunocytochemical localization of AMPA selective glutamate receptor subunits in the rat cochlea. Hearing research. 1994;80:233–240. doi: 10.1016/0378-5955(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci. 2001;21:9168–9174. doi: 10.1523/JNEUROSCI.21-23-09168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Neugebauer V, Grigoriadis D, Rivier J, Vale W, Shinnick-Gallagher P, Gallagher J. Corticotropin-releasing factor and Urocortin I modulate excitatory glutamatergic synaptic transmission. J Neurosci. 2004;24:4020–4029. doi: 10.1523/JNEUROSCI.5531-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke D, Koreen IV, Harris AL. Isoelectric points and post-translational modifications of connexin26 and connexin32. FASEB J. 2006;20:1221–1223. doi: 10.1096/fj.05-5309fje. [DOI] [PubMed] [Google Scholar]
- Marcus DC, Liu J, Lee JH, Scherer EQ, Scofield MA, Wangemann P. Apical membrane P2Y4 purinergic receptor controls K+ secretion by strial marginal cell epithelium. Cell Commun Signal. 2005;3:13. doi: 10.1186/1478-811X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DC, Sunose H, Liu J, Bennett T, Shen Z, Scofield MA, Ryan AF. Protein kinase C mediates P2U purinergic receptor inhibition of K+ channel in apical membrane of strial marginal cells. Hearing research. 1998;115:82–92. doi: 10.1016/s0378-5955(97)00180-9. [DOI] [PubMed] [Google Scholar]
- Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22:763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. The role of corticotropin-releasing factor in the pathogenesis of major depression. Pharmacopsychiatry. 1988;21:76–82. doi: 10.1055/s-2007-1014652. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. New vistas in neuropeptide research in neuropsychiatry: focus on corticotropin-releasing factor. Neuropsychopharmacology. 1992;6:69–75. [PubMed] [Google Scholar]
- Nemeroff CB, Owens MJ, Bissette G, Andorn AC, Stanley M. Reduced corticotropin releasing factor binding sites in the frontal cortex of suicide victims. Arch Gen Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Barrow A, Jacoby RA, Wu SM. How do tonic glutamatergic synapses evade receptor desensitization? The Journal of physiology. 2008;586:2889–2902. doi: 10.1113/jphysiol.2008.151050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter MA, Joppa M, Carmouche M, Cullen MJ, Brown B, Murphy B, Grigoriadis DE, Ling N, Foster AC. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollandt S, Liu J, Orozco-Cabal L, Grigoriadis DE, Vale WW, Gallagher JP, Shinnick-Gallagher P. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. The European journal of neuroscience. 2006;24:1733–1743. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. In: Chadwick DJ, Marsh J, Ackrill K, editors. Corticotropin-releasing Factor. London: John Wiley and Sons; 1993. pp. 5–29. [DOI] [PubMed] [Google Scholar]
- Sheng H, Zhang Y, Sun J, Gao L, Ma B, Lu J, Ni X. Corticotropin-releasing hormone (CRH) depresses n-methyl-D-aspartate receptor-mediated current in cultured rat hippocampal neurons via CRH receptor type 1. Endocrinology. 2008;149:1389–1398. doi: 10.1210/en.2007-1378. [DOI] [PubMed] [Google Scholar]
- Slominski A, Ermak G, Mazurkiewicz JE, Baker J, Wortsman J. Characterization of corticotropin-releasing hormone (CRH) in human skin. J Clin Endocrinol Metab. 1998;83:1020–1024. doi: 10.1210/jcem.83.3.4650. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Luger T, Paus R, Solomon S. Corticotropin releasing hormone and proopiomelanocortin involvement in the cutaneous response to stress. Physiological reviews. 2000;80:979–1020. doi: 10.1152/physrev.2000.80.3.979. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Pisarchik A, Zbytek B, Linton E, Mazurkiewicz J, Wei E. Cutaneous expression of corticotropin-releasing hormone (CRH), urocortin, and CRH receptors. FASEB J. 2001;15:1678–1693. doi: 10.1096/fj.00-0850rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Botchkarev V, Choudhry M, Fazal N, Fechner K, Furkert J, Krause E, Roloff B, Sayeed M, Wei E, Zbytek B, Zipper J, Wortsman J, Paus R. Cutaneous expression of CRH and CRH-R. Is there a “skin stress response system?”. Annals of the New York Academy of Sciences. 1999;885:287–311. doi: 10.1111/j.1749-6632.1999.tb08686.x. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Tache Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. The Journal of clinical investigation. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PR, Munoz DJ, Housley GD. Purinergic modulation of cochlear partition resistance and its effect on the endocochlear potential in the Guinea pig. J Assoc Res Otolaryngol. 2004;5:58–65. doi: 10.1007/s10162-003-4003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science (New York, NY. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. The Journal of comparative neurology. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Vetter D, Li C, Zhao L, Contarino A, Liberman M, Smith G, Marchuk Y, Koob G, Heinemann S, Vale W, Lee K. Urocortin-deficient mice show hearing impairment and increased anxiety-like behavior. Nat Genet. 2002;31:363–369. doi: 10.1038/ng914. [DOI] [PubMed] [Google Scholar]
- Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.