Abstract
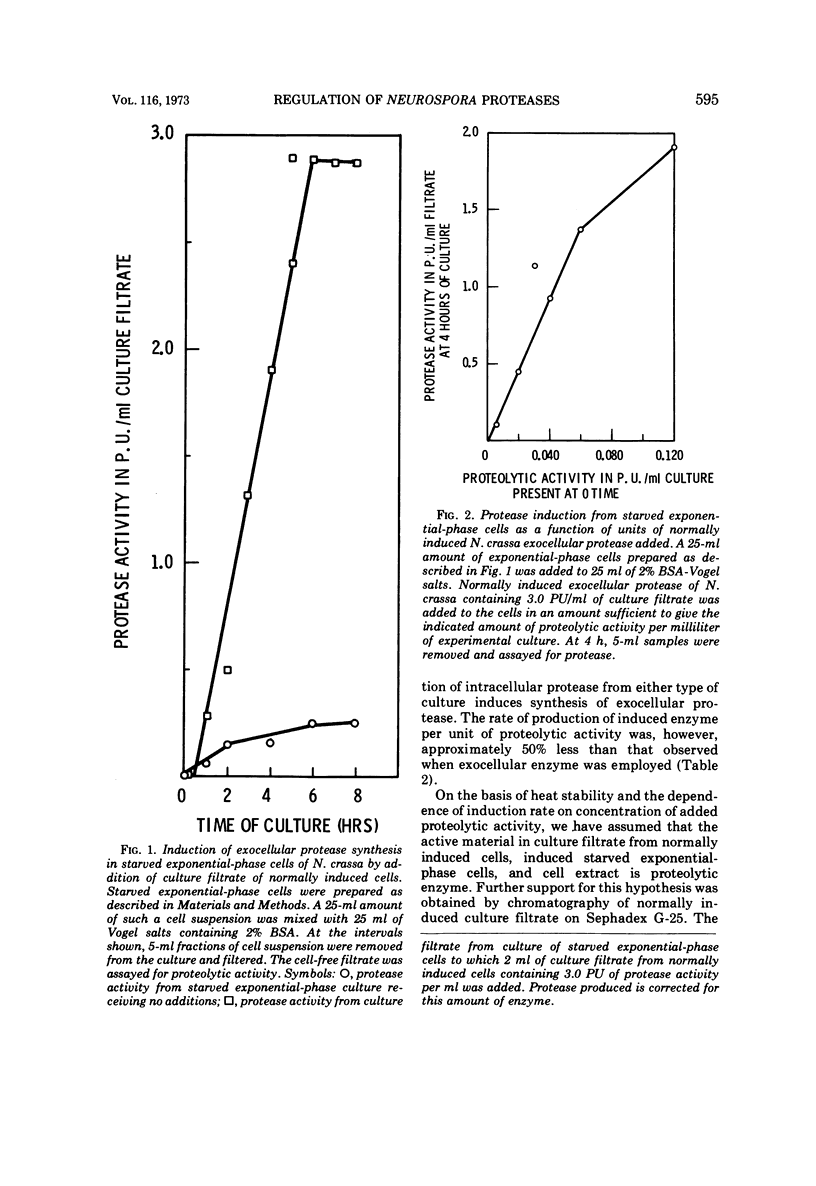
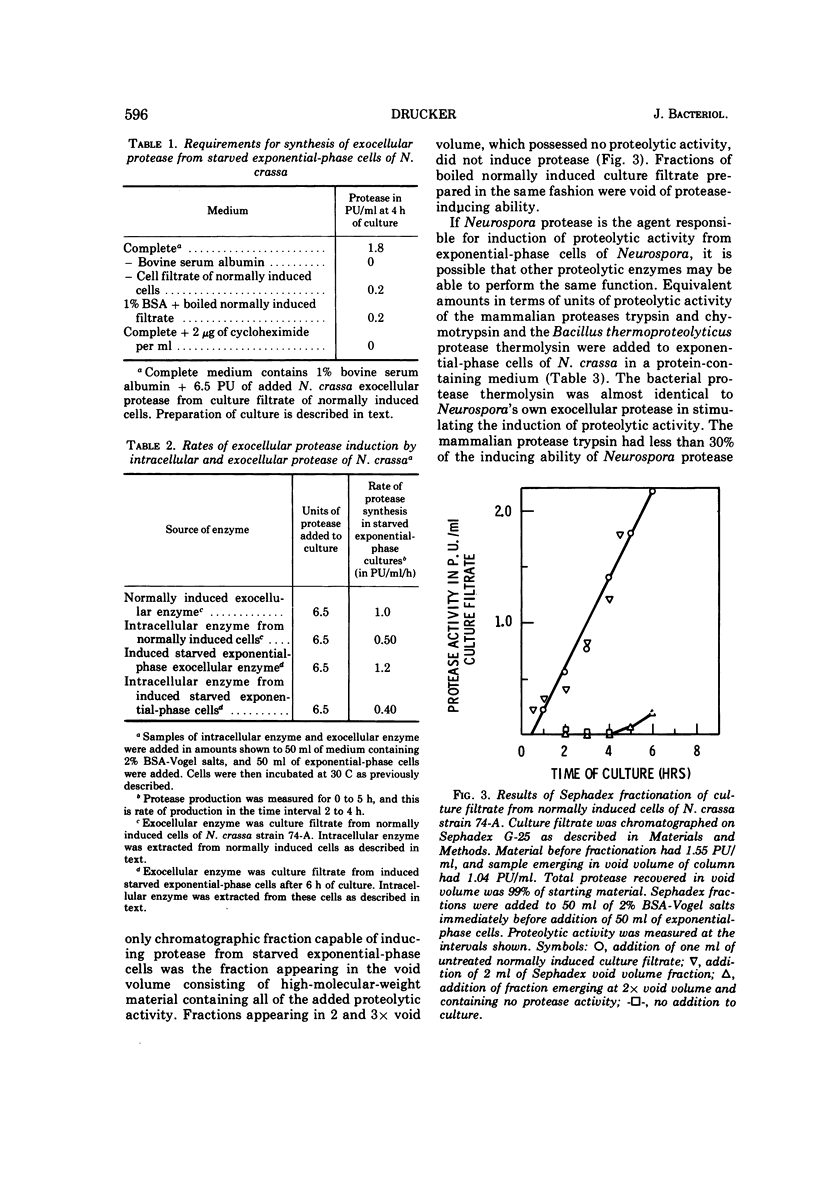
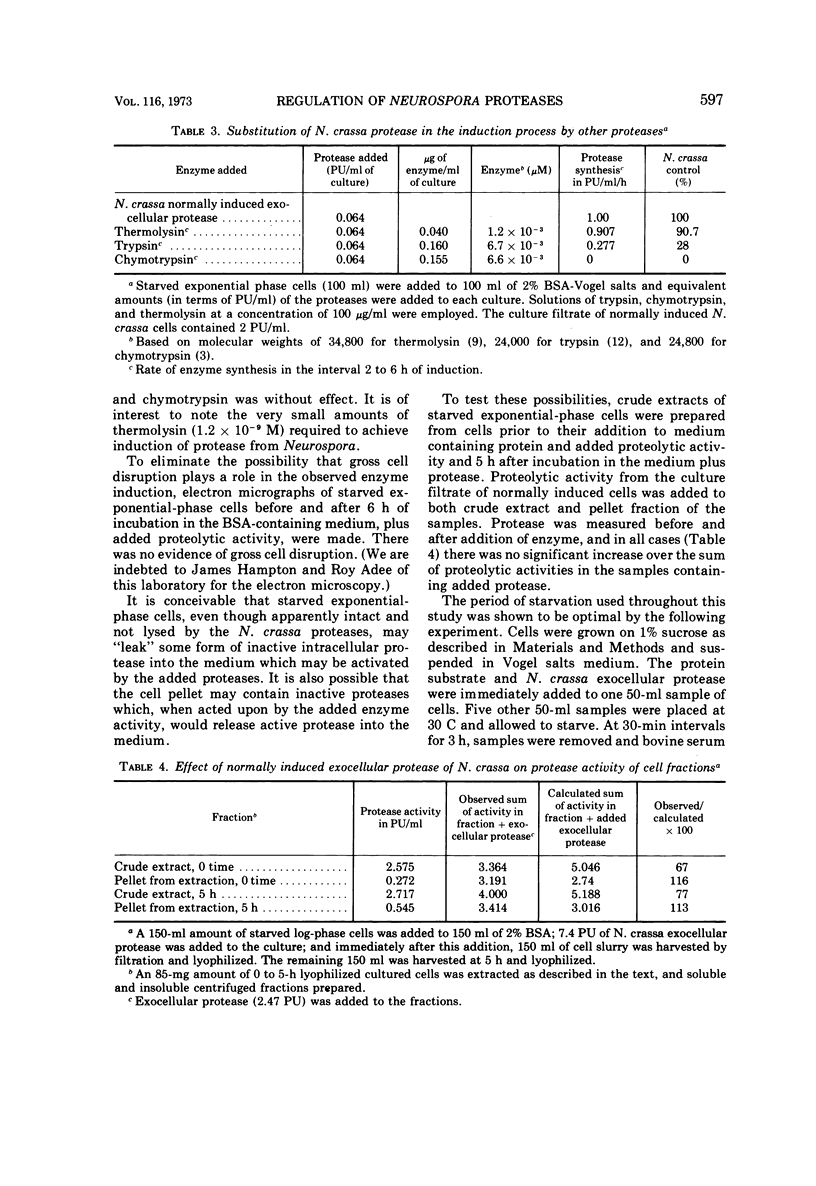
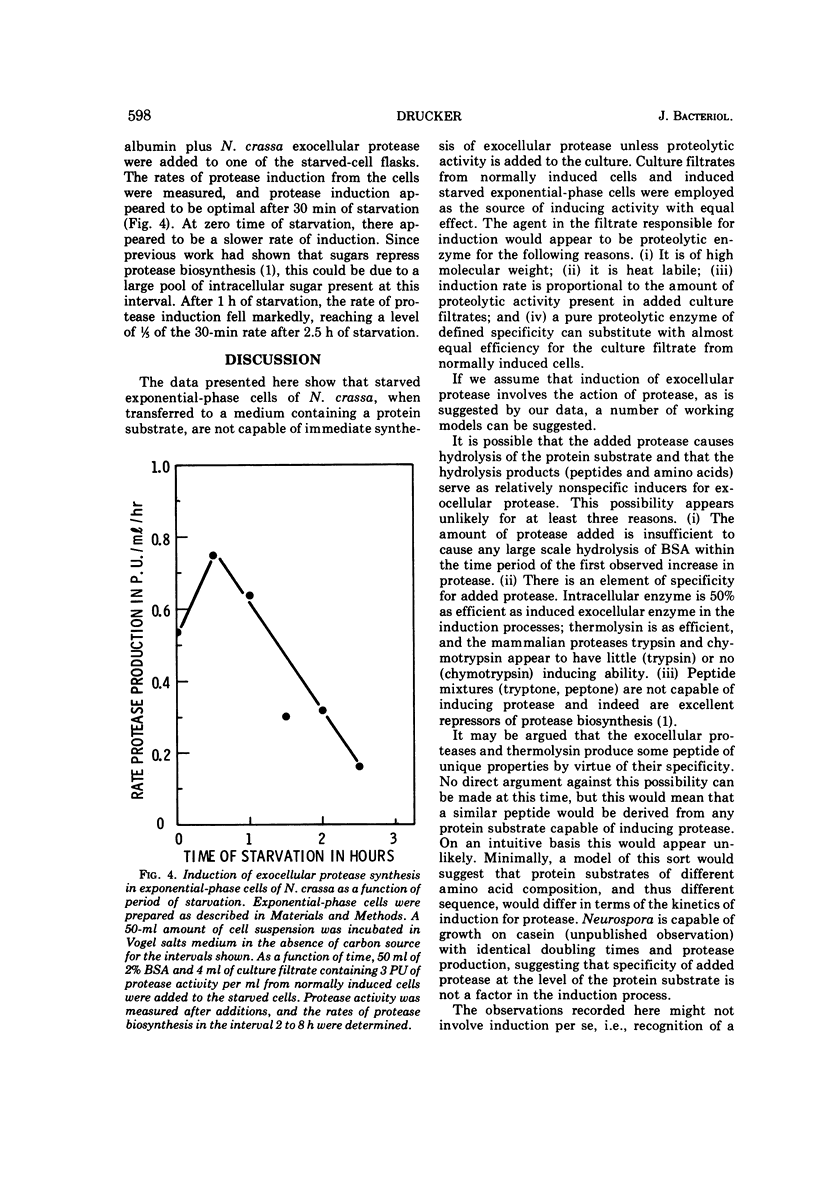
Cells of Neurospora crassa strain 74A, grown on sucrose for 12 h and transferred to a medium containing protein as sole carbon source, would not produce exocellular protease in significant amounts. When a filtrate from a culture induced to make protease by normal growth on a medium containing protein as principal carbon source was added to an exponential-phase culture in protein medium, exocellular protease was made in amounts similar to those made during normal induction. The material in the culture filtrate that participated in the induction process was identified as protease by its heat lability, molecular weight, and the dependence of induction rate on units of proteolytic activity added to the exponential-phase culture. Induction of the formation of exocellular protease by exponential-phase cells appears to require a protein substrate, added proteolytic activity, and protein synthesis. The protease produced by induced exponential-phase cells was as efficient in promoting induction as normally induced enzyme, whereas constitutive intracellular enzyme was only 50% as efficient. The bacterial protease thermolysin was able to induce exocellular protease at 90.7% of the rate observed with added N. crassa exocellular protease.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drucker H. Regulation of exocellular proteases in Neurospora crassa: induction and repression of enzyme synthesis. J Bacteriol. 1972 Jun;110(3):1041–1049. doi: 10.1128/jb.110.3.1041-1049.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S. AMINO-ACID SEQUENCE OF BOVINE CHYMOTRYPSINOGEN-A. Nature. 1964 Mar 28;201:1284–1287. doi: 10.1038/2011284a0. [DOI] [PubMed] [Google Scholar]
- MCDONALD C. E., CHEN L. L. THE LOWRY MODIFICATION OF THE FOLIN REAGENT FOR DETERMINATION OF PROTEINASE ACTIVITY. Anal Biochem. 1965 Jan;10:175–177. doi: 10.1016/0003-2697(65)90255-1. [DOI] [PubMed] [Google Scholar]
- Matile P., Jost M., Moor H. Intrazelluläre Lokalisation proteolytischer Enzyme von Neurospora crassa. Z Zellforsch Mikrosk Anat. 1965 Oct 12;68(2):205–216. [PubMed] [Google Scholar]
- Morihara K. Production of proteinase on noncarbohydrate carbon sources by Pseudomonas aeruginosa. Appl Microbiol. 1965 Sep;13(5):793–797. doi: 10.1128/am.13.5.793-797.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Hermodson M. A., Ericsson L. H., Walsh K. A., Neurath H. Amino acid sequence of thermolysin. Isolation and characterization of the fragments obtained by cleavage with cyanogen bromide. Biochemistry. 1972 Jun 20;11(13):2427–2435. doi: 10.1021/bi00763a007. [DOI] [PubMed] [Google Scholar]
- Turner J. R., Matchett W. H. Alteration of tryptophan-mediated regulation in Neurospora crassa by indoleglycerol phosphate. J Bacteriol. 1968 May;95(5):1608–1614. doi: 10.1128/jb.95.5.1608-1614.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALSH K. A., NEURATH H. TRYPSINOGEN AND CHYMOTRYPSINOGEN AS HOMOLOGOUS PROTEINS. Proc Natl Acad Sci U S A. 1964 Oct;52:884–889. doi: 10.1073/pnas.52.4.884. [DOI] [PMC free article] [PubMed] [Google Scholar]



