Abstract
Plasmacytoid dendritic cells (PDC) represent a distinct immune cell type specialized in direct virus recognition and rapid secretion of type I interferon. The origin and lineage affiliation of PDC have been controversial, in part because PDC show features of both lymphocytes and dendritic cells (DC). Recent studies helped elucidate the cellular and molecular basis of PDC development. In particular, the common developmental origin and genetic similarity of PDC and classical antigen-presenting DC have been established. In addition, E protein transcription factor E2-2 was shown to control lineage commitment and gene expression program of PDC. Because E proteins are essential regulators of lymphocyte development, E2-2 activity may underlie the distinct “lymphoid” features of PDC.
Introduction
In the decade since their seminal description by the Liu and Colonna labs in 1999, PDC were recognized as a separate immune cell lineage with specific functions. Studies in humans and in mice helped define the hallmark property of PDC: direct recognition of viruses through the endosomal nucleic acid-sensing Toll-like receptors TLR7 and TLR9, and the ensuing secretion of type I interferon (interferon α/β, IFN) and other cytokines. The IFN secretion by PDC is distinguished by rapid kinetics, high level (up to 1000-fold higher than most cells) and broad spectrum of IFN types. Accordingly, PDC appear critical for innate immune responses against many viruses including HIV. Conversely, aberrant activation of PDC by self nucleic acids is thought to cause elevated interferon levels in autoimmune diseases such as lupus and psoriasis [1,2].
The PDC show distinct molecular and cellular features that underlie their unique IFN-producing capacity. These include: i) prolonged endosomal retention of TLR ligands; ii) high basal expression of transcription factor IRF7, the master regulator of IFN response; iii) “plasmacytoid” secretory morphology resembling antibody-secreting plasma cells; iv) expression of specific surface receptors that modulate IFN secretion, such as human ILT7 and BDCA-2 and murine Siglec-H [3,4]. Remarkable progress has been achieved in elucidating the molecular underpinnings of PDC function, including the positive [5•,6•,7•] and negative [8••] control of IFN secretion. Here we review recent advances in our understanding of PDC development, transcriptional regulation and lineage identity.
Developmental origins of PDC
PDC develop in the bone marrow (BM), where they are relatively abundant (1-2% in the mouse). Experimentally, PDC can be derived from multiple progenitor types, including committed lymphoid or myeloid progenitors [9-11]. Notably, estrogen treatment that depletes lymphoid progenitors does not affect PDC, suggesting that the latter develop largely from non-lymphoid cell sources [12]. However, the identity of the committed steady-state PDC progenitor in the BM remained unclear.
The development of both PDC and classical or conventional DC (cDC) depends on the cytokine Flt3 ligand (Flt3L) and its receptor Flt3 [9,10,13,14•]. Furthermore, PDC and cDC are the only cell types generated in Flt3L-supplemented BM cultures [15], suggesting the existence of a common progenitor for both lineages. In elegant studies, the Shortman and Manz labs have identified such common dendritic cell progenitor (CDP, or pro-DC) in Flt3L cultures and in vivo [16••,17••]. The CDP in the murine BM express Flt3L receptor Flt3/CD135 and M-CSF receptor M-CSFR/CD115, consistent with the ability of M-CSF to drive cDC and PDC development [18]. Furthermore, even the more upstream macrophage/DC progenitor (MDP) that gives rise to monocytes, macrophages and cDC was recently shown to have PDC potential [19,20]. Thus, the progressive differentiation of early myeloid progenitors to MDP and then to CDP likely represents a major cellular source of PDC development.
PDC heterogeneity
PDC show heterogeneous expression of several genes such as CD2 in humans [21] and CD4 [22] and Rag1 [23] in mice. Indeed, recent lineage tracing studies confirmed that up to 30% of PDC have a history of Rag1 expression [24]. Because CDP are uniformly Rag1-negative, these results suggest the possibility of partial lymphoid contribution to PDC development. In addition, differences in inflammatory cytokine production and T cell stimulation in vitro were observed between Rag1+/Rag1- or CD2+/CD2- PDC [21,23]. Thus, a certain degree of developmental and/or functional heterogeneity may exist within the PDC population in mice and in humans.
A recent study described heterogeneous expression of chemokine receptor Ccr9 on murine PDC, and proposed that the Ccr9+ PDC subset has a unique tolerogenic capacity [25]. However, other studies found that PDC are uniformly Ccr9+ [26•,27], whereas Ccr9- cells appear to be cDC precursors rather than bona fide PDC [27].
The question of PDC lineage affiliation
Ever since the identification of PDC as a distinct cell type, their lineage affiliation has been difficult to define. True to their name, PDC appear closely related to cDC in several key aspects, including common developmental origin (see above) and functional properties such as efficient TLR-mediated pathogen recognition, cytokine secretion and antigen presentation. Furthermore, human PDC in vitro [28] and murine immature BM PDC in vivo [29•] can differentiate into cDC after stimulation. Finally, recent genome-wide analysis revealed that the gene expression profile of PDC is closer to cDC than to lymphocytes or myeloid cells [30•].
Despite these similarities, PDC are clearly distinct from cDC and instead display many features of lymphocytes. Most obviously, steady-state PDC have the morphology of a secretory lymphocyte and thus lack the hallmark of dendritic cells, i.e. the dendritic morphology. Second, the localization and recirculation/homing patterns of PDC appear different from cDC but similar to lymphocytes [31]. Third, PDC are quiescent and relatively long-lived, whereas cDC turn over rapidly within days and proliferate to a certain extent [22,32]. Fourth, PDC share many essential molecular features of B lymphocytes, including:
specific molecular markers such as CD45RA/B220;
expression of pIII transcript of the MHC class II transactivator (CIITA) [33] and continuous MHC class II synthesis after activation [34,35];
a B cell receptor-like signal transduction pathway downstream of their inhibitory receptors such as BDCA-2 [36•,37•]
Finally, a truly baffling feature of PDC is the expression of many genes involved in early lymphocyte development, such as Dntt (Tdt), VpreB, PTCRA (pre-Tα) and Rag1/2; the latter correlates wiht the presence of D-J rearrangements of the IgH locus in PDC but not in cDC [11,12,23,38]. All these genes are unlikely to be functional outside of lymphocyte development; furthermore, their expression in PDC is species-specific (e.g. only human but not murine PTCRA can be expressed in PDC [11]). Such expression occurs in PDC derived from either lymphoid or myeloid progenitors, suggesting that it is not a simple reflection of their developmental history [11]. Thus, PDC appear to be affiliated with DC lineage, but show a substantial genetic and functional overlap with lymphocytes, particularly B cells. Recent data on the transcriptional control of PDC may provide insight into this unique genetic makeup, as described below.
Transcriptional control of PDC development
Here we summarize several transcription factors implicated in PDC development, with additional factors described in recent reviews [39,40].
Irf8
Mice deficient in the interferon regulatory factor (Irf) family member Irf8/ICSBP lack PDC in the periphery and in Flt3L-supplemented BM cultures [41,42]. These animals also lack certain cDC subsets and show multiple functional defects, suggesting that Irf8 is broadly required for DC development and innate immunity.
Stat proteins
Stat3, a common transcriptional effector of many cytokine receptors, was shown to be activated by Flt3 signaling and mediate Flt3L-driven development of human and murine PDC in vitro [13,43,44•]. A major effect on cDC development was also noted, suggesting that Stat3 signal is not specific for PDC, and may occur at the common progenitor stage. In contrast, Stat5 is activated by myeloid cytokine GM-CSF and mediates its inhibitory effect on Flt3L-driven PDC development, in part through direct repression of Irf8 promoter [44•].
Ikaros
Ikaros and related zinc finger transcription factors are essential for many aspects of hematopoiesis and lymphoid development. Ikaros family members are required for cDC development in vivo and in vitro, likely due to their essential role in Flt3 expression [45,46]. Allman and colleagues showed that animals with a hypomorphic allele of Ikaros have normal cDC compartment but lack PDC in the periphery, whereas PDC in the BM have an aberrant “hyperactivated” phenotype [47]. Thus, PDC appear particularly sensitive to reduced levels of Ikaros, highlighting their distinct genetic requirements from cDC.
SpiB
SpiB is an ETS family transcription factor expressed predominantly in B cells, where it is required for optimal germinal center reaction. Blom and colleagues described prominent SpiB expression in PDC, and subsequently showed its contribution to the human PDC development in vitro [48]. Although SpiB is similarly enriched in murine PDC, SpiB-deficient mice showed normal splenic PDC content (B. Blom, pers. communication). Thus, SpiB is more likely to regulate only certain aspects of PDC development and/or function, possibly due to partial redundancy with a related factor, PU.1. Indeed, PU.1 is expressed in PDC at moderate levels (compared to high-level expression in cDC) [49], and its role in PDC development remains to be established.
E2-2
E proteins are a family of basic helix-loop-helix (bHLH) transcription factors with critical roles in lymphocyte development and function (for a recent review, see [50] and articles by Kee and Goldrath in this issue). The three mammalian E proteins (E2a, HEB and E2-2) form homo- or heterodimers to bind similar target sites termed E boxes (CANNTG), and their activity is antagonized by repressors of the Id family (Id1-4). In 2000, Spits et al. demonstrated that the development of human lymphocytes and PDC (but not of cDC) in vitro was inhibited by Id protein overexpression [51], thus implicating E proteins in PDC development.
Recently, E2-2 (gene symbol Tcf4) has been identified as the E protein family member that specifically controls PDC development. E2-2 is expressed abundantly in murine and human PDC, at lower levels in B cells and scarcely in cDC and other cell types. Germ line or conditional deletion of E2-2 resulted in the loss of PDC population and of PDC-mediated IFN secretion, whereas other cell types (including cDC and B cells) developed normally. As in the case of other E proteins, E2-2 displayed haploinsufficiency in its target cell type: the otherwise normal E2-2+/- animals contained reduced number of functionally impaired PDC. Furthermore, human patients with Pitt-Hopkins syndrome, a rare condition caused by monoallelic loss of E2-2, harbored PDC with aberrant surface phenotype and impaired interferon secretion capacity. Thus, genetic evidence from mice and humans revealed an essential and specific role of E2-2 in PDC development [26•]. Consistent with this, E2-2 knockdown or overexpression in the human hematopoietic progenitors reduced or increased PDC development in vitro, respectively [52•].
E2-2 was shown to directly control several PDC-specific genes including BDCA-2, ILT7 and IRF7, likely accounting for the unique baseline expression of the latter in the PDC. In addition, E2-2 showed binding to the promoters of Irf8 and SpiB, and their expression was reduced in E2-2+/- PDC [26•]. The expression patterns of E2-2 and SpiB are very similar, suggesting that SpiB is indeed a major direct target of E2-2; in turn, SpiB may enhance E2-2 expression [52•]. Thus, E2-2 initiates a transcriptional network whose components (SpiB, Irf8, Irf7 and others) would collectively induce PDC-specific gene expression program.
A model of PDC development and lineage identity
The evidence reviewed above suggests the following possible scenario of PDC development. The majority of PDC arise along the common PDC/cDC developmental pathway, i.e. from CDP in the BM; the induction of E2-2 expression by yet unknown signals would divert CDP and/or their immediate progeny from the “default” cDC fate towards the PDC lineage. The high expression level (as opposed to mere presence) of E2-2 appears critical, as even a two-fold reduction in E2-2+/- mice impairs PDC development. A certain fraction of PDC may arise from Flt3+ lymphoid progenitors (which may partially overlap with CDP/pre-DC), and show subtle genetic and functional differences. However, all PDC appear to require E2-2 for their development, and share the hallmark IFN-secreting capacity and core gene expression profile. The induced E2-2 would enhance the expression of other key transcription factors including Irf8 and SpiB, launching a self-sustaining genetic network that controls PDC-specific gene expression program.
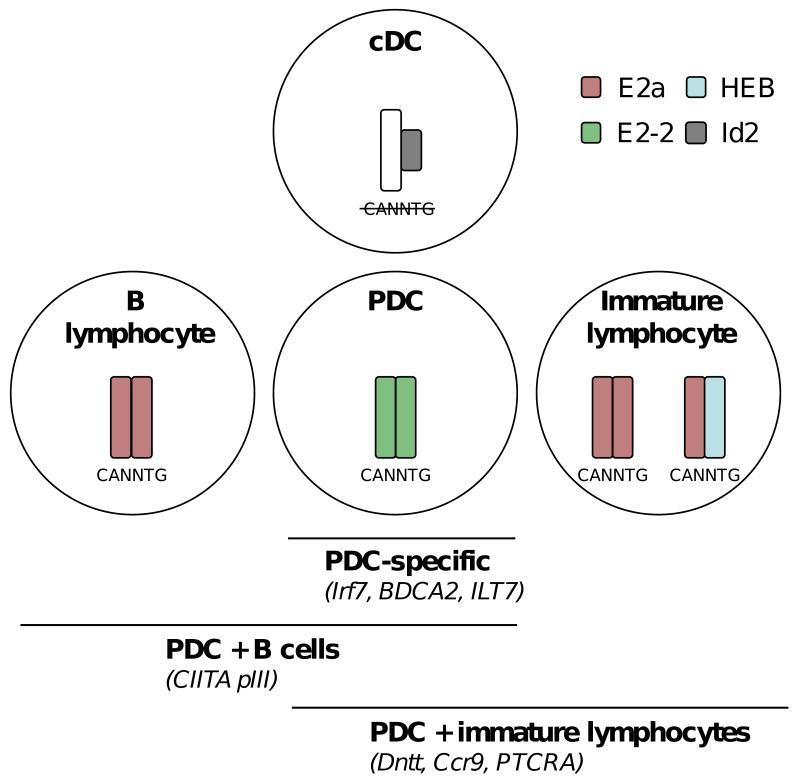
Both T and B lymphocyte development critically depends on E protein activity; in particular, B cells require E2a for almost every step of their development, homeostasis and function. In contrast, most innate immune cell types do not require E proteins, and indeed some of them (such as NK cells and certain cDC subsets) depend on E protein inhibition by Id2 for their development [50]. Therefore, the identification of an E protein as a key determinant of PDC development may help explain their “lymphoid” features, particularly the similarity with B cells. In this scenario, the shared gene expression program of PDC and cDC would be controlled by factors generally implicated in myeloid/DC development, such as PU.1 and Stat3. On the other hand, E2-2 (likely in cooperation with other PDC-enriched transcription factors such as SpiB and Irf8) would control several distinct gene sets (Fig. 1):
Figure 1. E proteins and their targets in dendritic cells.
Shown is the schematic composition of E protein complexes and their cognate E box sites (CANNTG) in B cells, immature T or B lymphocytes, cDC and PDC. The homodimeric E2-2 complex in PDC is inferred because E2a and HEB are dispensable for PDC development in vivo [26]. The proposed classes of E protein target genes in PDC are indicated, including PDC-specific, shared PDC/B cell-specific, and shared PDC/immature lymphocyte-specific targets.
PDC-specific genes (e.g. basal IRF7, BDCA-2/CLEC4A, ILT7/LILRA4)
genes commonly expressed in PDC and B cells, controlled by E2a in the latter. One example would be CIITA pIII, a well-defined E2a target in B cells [53] that is also prominently bound by E2-2 in PDC (our unpublished observations).
genes commonly expressed in PDC and developing lymphocytes, and controlled by E2a or E2a/HEB in the latter. The examples would include Dntt and Ccr9, which are highly E2-2-dependent in PDC [26•] and also represent major targets of E2a in early lymphopoiesis [54]. Such expression therefore may be considered a “by-product” of E protein activity in PDC, consistent with its apparent lack of functionality or evolutionary conservation.
Thus, steady-state PDC may be viewed as dendritic cells diverted into a B lymphocyte-like state by E protein activity. Given that PDC may differentiate into cDC in some circumstances [28,29•], the E protein-controlled lymphoid features of PDC might be reversible by activation and other stimuli, thus allowing PDC to reveal their “true dendritic nature”.
Conclusions
The characterization of PDC lineage has presented a considerable challenge, as PDC display molecular markers and features of several cell types and can be derived from multiple progenitors. Nevertheless, recent advances firmly established their common developmental origin and genetic relationship with the dendritic cell lineage. On the other hand, prominent lymphoid features of PDC may be largely due to the activity of the hallmark lymphoid transcription factors, E proteins. In particular, E protein factor E2-2 orchestrates a transcriptional network that controls PDC-specific gene expression, as well as the genes shared with B cells and with immature lymphocytes. Important questions to be addressed in the future include the nature of early signals that induce PDC lineage commitment and E2-2 expression; the additional components, relative hierarchy and direct targets of the PDC-specific transcriptional circuitry; and the regulation of mature PDC maintenance in the periphery.
Acknowledgments
I am grateful to members of my lab for their contribution to our study of PDC, and to Bianca Blom and Paul Kincade for communicating unpublished results. Supported by the SPAR/American Asthma Foundation award and NIH grant AI072571.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 2.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 3.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–260. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat Rev Immunol. 2008 doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 5.Tai LH, Goulet ML, Belanger S, Toyama-Sorimachi N, Fodil-Cornu N, Vidal SM, Troke AD, McVicar DW, Makrigiannis AP. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Murine Ly49Q, a C-type lectin-like receptor specific for MHC class I molecules, is expressed specifically on PDC. This study shows an essential role for this PDC-specific receptor in IFN secretion and antiviral immunity.
- 6.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Together with Guiducci et al., this study demonstrated an essential role for the PI3K signaling pathway in TLR-induced IFN secretion by PDC.
- 7.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, Liu YJ, Barrat FJ, Soumelis V. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J Exp Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]; • See above
- 8.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang YH, Shaw JL, Du Q, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J Exp Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• ILT7 (LILRA4) is a human PDC-specific receptor that inhibits IFN secretion. This study identified interferon-inducible surface molecule Bst2 as the endogenous ILT7 ligand, revealing a PDC-specific negative feedback mechanism for IFN secretion. Notably, mice lack ILT7 but instead express Bst2 specifically on PDC, possibly showing a “shortcut” form of the same feedback mechanism.
- 9.D'Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karsunky H, Merad M, Cozzio A, Weissman IL, Manz MG. Flt3 ligand regulates dendritic cell development from Flt3+ lymphoid and myeloid-committed progenitors to Flt3+ dendritic cells in vivo. J Exp Med. 2003;198:305–313. doi: 10.1084/jem.20030323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, Traver D, Leder P, Sakaguchi N, Akashi K. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Harman BC, Miller JP, Nikbakht N, Gerstein R, Allman D. Mouse plasmacytoid dendritic cells derive exclusively from estrogen-resistant myeloid progenitors. Blood. 2006;108:878–885. doi: 10.1182/blood-2005-11-4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onai N, Obata-Onai A, Tussiwand R, Lanzavecchia A, Manz MG. Activation of the Flt3 signal transduction cascade rescues and enhances type I interferon-producing and dendritic cell development. J Exp Med. 2006;203:227–238. doi: 10.1084/jem.20051645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, Merad M, Shengelia T, Yao K, Nussenzweig M. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Among other results, this study documented for the first time a profound depletion of PDC in Flt3-deficient animals.
- 15.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, Trinchieri G, O'Garra A, Liu YJ. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J Exp Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, Dakic A, Carotta S, O'Keeffe M, Bahlo M, Papenfuss A, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]; •• This and the following study have identified a common dendritic cell progenitor (CDP, or pro-DC) in the murine BM Unbiased prospective isolation and clonogenic assays were used to define the Flt3+ bipotential progenitor of both cDC and PDC, providing the first direct evidence that the two cell types arise in the same developmental pathway.
- 17.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clonogenic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]; •• See above.
- 18.Fancke B, Suter M, Hochrein H, O'Keeffe M. M-CSF: a novel plasmacytoid and conventional dendritic cell poietin. Blood. 2008;111:150–159. doi: 10.1182/blood-2007-05-089292. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, Yao K, Chu FF, Randolph GJ, Rudensky AY, Nussenzweig M. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Auffray C, Fogg DK, Narni-Mancinelli E, Senechal B, Trouillet C, Saederup N, Leemput J, Bigot K, Campisi L, Abitbol M, et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J Exp Med. 2009;206:595–606. doi: 10.1084/jem.20081385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsui T, Connolly JE, Michnevitz M, Chaussabel D, Yu CI, Glaser C, Tindle S, Pypaert M, Freitas H, Piqueras B, et al. CD2 distinguishes two subsets of human plasmacytoid dendritic cells with distinct phenotype and functions. J Immunol. 2009;182:6815–6823. doi: 10.4049/jimmunol.0802008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, Anders EM, Wu L, Lahoud MH, Henri S, Scott B, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8(+) dendritic cells only after microbial stimulus. J Exp Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, Kincade PW. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, Fehling HJ, Kincade PW. Asynchronous RAG-1 expression during B lymphopoiesis. J Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, Locksley R, Holmberg D, Zweier C, den Hollander NS, Kant SG, et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study has identified E protein E2-2 as a regulator of PDC development and of PDC-specific gene expression in mice and in humans.
- 27.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9- dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 28.Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid pre-dendritic cell differentiation. Eur J Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- 29.Liou LY, Blasius AL, Welch MJ, Colonna M, Oldstone MB, Zuniga EI. In vivo conversion of BM plasmacytoid DC into CD11b+ conventional DC during virus infection. Eur J Immunol. 2008;38:3388–3394. doi: 10.1002/eji.200838282. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Building on their previous work, the authors documented virus-induced, IFN-dependent differentiation of immature PDC into cDC in the murine BM. This study confirms that the initial lineage commitment in immature PDC can be reversed to cDC, which may represent the “default” cell fate of DC development.
- 30.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, Bessou G, Xu H, Vivier E, Sellars M, Pierre P, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study summarizes a tour-de-force effort by the authors to build a genome-wide expression atlas of murine and human immune cell types Among other conclusions, it provides unbiased evidence for the common gene expression profile of cDC and PDC, and for the close similarity of these cell types between mice and humans.
- 31.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 32.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 33.LeibundGut-Landmann S, Waldburger JM, Reis e Sousa C, Acha-Orbea H, Reith W. MHC class II expression is differentially regulated in plasmacytoid and conventional dendritic cells. Nat Immunol. 2004;5:899–908. doi: 10.1038/ni1109. [DOI] [PubMed] [Google Scholar]
- 34.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 35.Sadaka C, Marloie-Provost MA, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood. 2009;113:2127–2135. doi: 10.1182/blood-2008-10-178152. [DOI] [PubMed] [Google Scholar]
- 36.Cao W, Zhang L, Rosen DB, Bover L, Watanabe G, Bao M, Lanier LL, Liu YJ. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 2007;5:e248. doi: 10.1371/journal.pbio.0050248. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study delineates a signaing pathway utilized by human PDC-specific inhibitory receptors BDCA2 and ILT7, which associate with FcεRIγ. Strikingly, the pathway is very similar to the one downstream of antigen receptor in B cells, and involves B cell adaptors such as BLNK/SLP-65. The results reveal specific molecular mechanisms shared by PDC and B cells.
- 37.Rock J, Schneider E, Grun JR, Grutzkau A, Kuppers R, Schmitz J, Winkels G. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2. Eur J Immunol. 2007;37:3564–3575. doi: 10.1002/eji.200737711. [DOI] [PubMed] [Google Scholar]; • See Cao et al. above.
- 38.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, O'Keeffe M, Wu L, Wilson A, Shortman K. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 39.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Merad M, Manz MG. Dendritic cell homeostasis. Blood. 2009;113:3418–3427. doi: 10.1182/blood-2008-12-180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, 3rd, Belardelli F, Gabriele L. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8alpha(+) dendritic cells. J Exp Med. 2002;196:1415–1425. doi: 10.1084/jem.20021263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170:1131–1135. doi: 10.4049/jimmunol.170.3.1131. [DOI] [PubMed] [Google Scholar]
- 43.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 44.Esashi E, Wang YH, Perng O, Qin XF, Liu YJ, Watowich SS. The Signal Transducer STAT5 Inhibits Plasmacytoid Dendritic Cell Development by Suppressing Transcription Factor IRF8. Immunity. 2008;28:509–520. doi: 10.1016/j.immuni.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study confirmed the essential role of Stat3 in Flt3L-induced PDC and cDC development, and revealed an inhibitory role of Stat5 in the process. The activation of Stat5 by GM-CSF opposed PDC development, possibly through the direct repression of Irf8 expression.
- 45.Wu L, Nichogiannopoulou A, Shortman K, Georgopoulos K. Cell-autonomous defects in dendritic cell populations of Ikaros mutant mice point to a developmental relationship with the lymphoid lineage. Immunity. 1997;7:483–492. doi: 10.1016/s1074-7613(00)80370-2. [DOI] [PubMed] [Google Scholar]
- 46.Galy A, Christopherson I, Ferlazzo G, Liu G, Spits H, Georgopoulos K. Distinct signals control the hematopoiesis of lymphoid-related dendritic cells. Blood. 2000;95:128–137. [PubMed] [Google Scholar]
- 47.Allman D, Dalod M, Asselin-Paturel C, Delale T, Robbins SH, Trinchieri G, Biron CA, Kastner P, Chan S. Ikaros is required for plasmacytoid dendritic cell differentiation. Blood. 2006;108:4025–4034. doi: 10.1182/blood-2006-03-007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schotte R, Nagasawa M, Weijer K, Spits H, Blom B. The ETS transcription factor Spi-B is required for human plasmacytoid dendritic cell development. J Exp Med. 2004;200:1503–1509. doi: 10.1084/jem.20041231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nutt SL, Metcalf D, D'Amico A, Polli M, Wu L. Dynamic regulation of PU. 1 expression in multipotent hematopoietic progenitors J Exp Med. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kee BL. E and ID proteins branch out. Nat Rev Immunol. 2009;9:175–184. doi: 10.1038/nri2507. [DOI] [PubMed] [Google Scholar]
- 51.Spits H, Couwenberg F, Bakker AQ, Weijer K, Uittenbogaart CH. Id2 and Id3 inhibit development of CD34(+) stem cells into predendritic cell (pre-DC)2 but not into pre-DC1. Evidence for a lymphoid origin of pre-DC2. J Exp Med. 2000;192:1775–1784. doi: 10.1084/jem.192.12.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagasawa M, Schmidlin H, Hazekamp MG, Schotte R, Blom B. Development of human plasmacytoid dendritic cells depends on the combined action of the basic helix-loop-helix factor E2-2 and the Ets factor Spi-B. Eur J Immunol. 2008;38:2389–2400. doi: 10.1002/eji.200838470. [DOI] [PubMed] [Google Scholar]; • The authors demonstrated abundant expression of E2-2 in human PDC, and documented its contribution to PDC development from hematopoietic progenitors in vitro. Together with Cisse et al., this study supports a conserved role of E2-2 in PDC development.
- 53.van der Stoep N, Quinten E, Marcondes Rezende M, van den Elsen PJ. E47, IRF-4, and PU.1 synergize to induce B-cell-specific activation of the class II transactivator promoter III (CIITA-PIII) Blood. 2004;104:2849–2857. doi: 10.1182/blood-2004-03-0790. [DOI] [PubMed] [Google Scholar]
- 54.Dias S, Mansson R, Gurbuxani S, Sigvardsson M, Kee BL. E2A proteins promote development of lymphoid-primed multipotent progenitors. Immunity. 2008;29:217–227. doi: 10.1016/j.immuni.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]