Abstract
An immunomodulatory role for vitamin D was first proposed more than 25 years ago, based on two salient observations. Firstly it was shown that monocytes/macrophages from patients with the granulomatous disease sarcoidosis constitutively synthesize the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) from precursor 25-hydroxyvitamin D (25OHD). Secondly, the receptor for 1,25(OH)2D (vitamin D receptor, VDR) is detectable in activated, proliferating lymphocytes. These observations suggested a mechanism whereby 1,25(OH)2D produced by monocytes could act upon adjacent T-cells or B-cells, but the impact of such a system on normal immune regulation was uncertain. Indeed, it is only in recent years that a much clearer picture of the role of vitamin D as a determinant of immune responsiveness has emerged. Two new concepts have prompted this change. Firstly studies of innate immunity have shown that intracrine induction of antimicrobial activity by vitamin D is a pivotal component of monocye/macrophage response to infection. Secondly, it is now clear that sub-optimal vitamin D status is a common feature of many populations throughout the world, with the potential to compromise monocyte/macrophage metabolism of 25OHD and subsequent actions of 1,25(OH)2D. The current review details these new developments with specific reference to the metabolic and signaling mechanisms associated with innate immune regulation by vitamin D and implications for human disease.
Keywords: vitamin D, CYP27b1, toll-like receptor, macrophage, cathelicidin, regulatory T-cells
1. Introduction
Over the last five years, our perception of what constitutes normal vitamin D status has changed dramatically. Prior to this, vitamin D-deficiency was defined by serum concentrations of the major circulating form of vitamin D (25-hydroxyvitamin D, 25OHD) associated with the bone disease rickets or its adult form, osteomalacia [1]. In the absence of rickets/osteomalacia, vitamin D status was considered to be normal. However, recent studies have defined a new category for impaired vitamin D status – vitamin D ‘insufficiency’ – referring to individuals who are not necessarily vitamin D-deficient but who nevertheless present with sub-optimal serum levels of 25OHD. There is still considerable debate concerning the absolute concentrations of serum 25OHD that define vitamin D-deficiency and –insufficiency. In a recent review of this subject Holick indicated that vitamin D-insufficiency could be characterized by circulating levels of 25OHD that were greater than vitamin D-deficiency (50 nM or 20 ng/ml) but less than 75 nM (30 ng/ml) [2].
These revised parameters for vitamin D status arose from studies showing continued suppression of serum parathyroid hormone (PTH) with circulating levels of 25OHD as high as 80 nM (32 ng/ml) [3]. However, similar observations have also been made for other vitamin D responses such as intestinal calcium uptake, and there is now general agreement with the new definitions for adequate and inadequate serum vitamin D [1,2]. At a clinical level, much of the response to this has centered on defining the best strategy for restoring and maintaining optimal vitamin D status. However, at a functional level, other key questions have arisen from this new perspective on vitamin D adequacy. Firstly, what, if any, are the physiological consequences of vitamin D (25OHD) insufficiency? Secondly, does 25OHD status influence physiology independent of the circulating levels of active 1,25-dihydroxyvitamin D (1,25(OH)2D)? Finally, if the ‘inactive’ form of vitamin D (25OHD) is able to direct physiological responses to vitamin D, what are the mechanisms that facilitate this and how do they differ from classical vitamin D endocrinology?
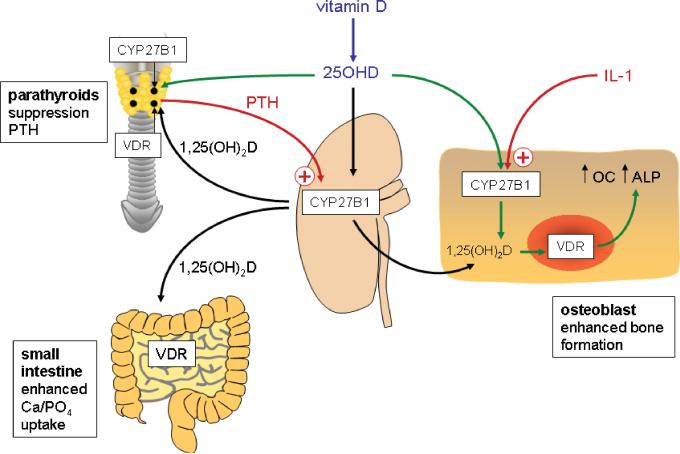
Some answers to these questions have been gleaned from new perspectives on conventional vitamin D physiology. For example, classical endocrinology defines the kidney as the major site for synthesis of systemic 1,25(OH)2D from 25OHD, with PTH stimulating the enzyme that catalyzes this conversion, 25-hydroxyvitamin D-1α-hydroxylase (1α-hydroxylase) under conditions of low extracellular calcium (see Figure 1). The 1,25(OH)2D produced by renal 1α-hydroxylase activity then enters the circulation and acts as classical steroid hormone, enhancing calcium and phosphate uptake by the gastrointestinal tract and promoting skeletal homeostasis through effects on cells such as bone-forming osteoblasts. Once calcium homeostasis has been reestablished, 1,25(OH)2D is then able to complete feedback regulation of this system by suppressing transcription of PTH in parathyroid cells. The efficacy of this system is dependent firstly on the expression of receptors for 1,25(OH)2D (vitamin D receptors, VDR) in target intestinal, bone and parathyroid cells. This, in turn, requires sufficient 1,25(OH)2D to promote VDR signaling. Within such an endocrine system, serum levels of 1,25(OH)2D are not proportional to the availability of 25OHD, the substrate for 1α-hydroxylase. Instead the key determinant of renal 1,25(OH)2D production is PTH, the pivotal activator of 1α-hydroxylase expression and activity in proximal tubule cells [4]. However, recent studies have shown that the actions of vitamin D on skeletal function and parathyroid homeostasis may not be exclusively due to the endocrine actions of 1,25(OH)2D synthesized by the kidney. As well as expressing abundant VDR, cells from the parathyroid glands [5] and bone-forming osteoblasts [6] demonstrate significant levels of 1α-hydroxylase activity. As a result, it has been proposed that some of the calciotropic actions of vitamin D are due to localized conversion of 25OHD to 1,25(OH)2D and thus intracrine, rather than endocrine, signaling by the VDR (Figure 1). In the case of the parathyroid glands, it has also been hypothesized that 25OHD exerts direct effects on the VDR, possibly utilizing the alternative binding pocket model proposed by Mizwicki et al [7]. Irrespective of the final mechanism of action, the key distinction between the endocrine and intracrine models is that the latter is not subject to regulation by PTH but is instead influenced by factors that are central to the function of the tissue in question. For example, interleukin-1 (IL-1) is a key enhancer of 1,25(OH)2D production in osteoblastic cells from bone [6] (see Figure 1). Under these conditions, extra-renal activity of 1α-hydroxylase appears to be more dependent on the level of 25OHD, in other words vitamin D status, than its renal counterpart.
Figure 1. Endocrine and intracrine mechanisms for vitamin D action.
In vitamin D endocrinology 25-hydroxyvitamin D (25OHD) generated from parental vitamin D can be activated to 1,25-dihydroxyvitamin D (1,25(OH)2D) in the kidneys catalyzed by the enzyme 1α-hydroxylase (CYP27B1). Expression of CYP27B1 is enhanced by parathyroid hormone (PTH) synthesized by the parathyroid glands under conditions of low extracellular calcium. Synthesis of 1,25(OH)2D under these conditions promotes intestinal uptake of calcium and phosphate and feedback-regulates PTH secretion in cells expressing the vitamin D receptor (VDR). Non-endocrine actions of vitamin D in tissues such as bone also involve the generation of 25OHD from parental vitamin D. However, in this case, the actions of kidney-derived 1,25(OH)2D are complemented by intracrine synthesis of this metabolite via localized expression of CYP27B1 and the VDR. Exogenous and/or endogenous 1,25(OH)2D act to promote the function of cells such as bone-forming osteoblasts (increased alkaline phosphatase (ALP) and osteocalcin (OC)). In contrast to the endocrine synthesis of 1,25(OH)2D in the kidneys, extra-renal expression of CYP27B1 is not regulated by PTH but is instead influenced by tissue-specific factors such as the cytokine interleukin-1 (IL-1).
Although localized responses to vitamin D have been described for cells involved in calcium homeostasis and bone metabolism, the vast majority of literature concerning vitamin D intracrinology has centered on so-called ‘non-classical’ systems. Prominent amongst these has been the interaction between vitamin D and the immune system, with effects extending from promotion of innate immune response to infection, and the modulation of subsequent adaptive lymphocyte activity [8]. Epidemiological studies have reported links between vitamin D-insufficiency and autoimmune diseases such as multiple sclerosis [9], type 1 diabetes [10] and Crohn's disease [11,12]. Likewise, analysis of animal models indicate that ablation of the gene for the vitamin D receptor (VDR) or the vitamin D-activating enzyme 1α-hydroxylase (CYP27B1) in mice increases their susceptibility to experimental forms of type 1 diabetes [13] and Crohn's disease [14,15]. As a consequence of these observations, much attention has focused on a possible role for the VDR as a target for autoimmune disease therapy [10]. Initial studies in this area were aimed at regulating the proliferation of T-cells and B-cells which express VDR when activated [16]. More recent studies have confirmed that T-cells are direct targets for 1,25(OH)2D, which potently modulates T-cell phenotype, promoting the development of suppressor regulatory T-cells (Tregs) [17]. In addition to this, 1,25(OH)2D is known to influence cell translocation by stimulating T-cell expression of chemokine receptor 10 (CCR10) which recognizes the chemokine CCL27 secreted by epidermal keratinocytes [18]. In this way, 1,25(OH)2D can act to promote the translocation and/or retention of T-cells cells within the skin, although studies using the VDR knockout mouse suggest that vitamin D is also involved in T-cell homing within the gastrointestinal tract [19].
Although both T-cells [18] and B-cells [20] express mRNA for 1α-hydroxylase, it is unclear whether intracrine metabolism of 25OHD has any impact on the function of these cells. In view of the central role of antigen-presenting cells (APCs) such as macrophages or dendritic cells (DCs) in directing the T- and B-cell responses that make up the adaptive immune system, a likely scenario is that the APCs themselves will act as the main site for intracrine vitamin D activity. The importance of a link between vitamin D and innate immunity has been further underlined by studies showing that monocyte/macrophage responses to bacterial infection are potently stimulated by 25OHD following localized induction of both the VDR and 1α-hydroxylase. With this in mind, the aim of the current manuscript is to review the key mechanisms by which vitamin D interacts with the innate immune system.
2. Vitamin D metabolism and monocyte/macrophage function
One of the earliest pieces of evidence describing an immunoregulatory role for vitamin D stemmed from the ability of 1,25(OH)2D to stimulate the differentiation of monocytic precursors into more mature, macrophage-like cells [21]. This highlighted the expression of VDR by monocytes/macrophages [22], and also implicated 1,25(OH)2D in the process of hematopoiesis and, in particular, myelopoiesis [23]. The latter was subsequently discounted by Koeffler and colleagues who were unable to demonstrate any myelopoietic abnormalities in the VDR knockout mouse [24]. Nevertheless, the expression of VDR by monocytes/macrophages and equivalent leukemic cells lines suggested a potential use for 1,25(OH)2D in the provision of differentiation therapy [23]. Because of the potent calcemic properties of the naturally-occurring active form of vitamin D, a wide variety of analogs of 1,25(OH)2D have been developed in an attempt to circumvent unwanted side-effects [25]. However, whilst these synthetic forms of vitamin D have demonstrated potent activity in vitro, their efficacy in vivo has been less impressive, with the exception of treatment for secondary hyperparathyroidism [26] and psoriasis [27].
Shortly after the initial report of monocyte/macrophage sensitivity to 1,25(OH)2D, a series of studies of patients with the granulomatous disease sarcoidosis provided another link between vitamin D and monocyte/macrophage function. Adams and colleagues demonstrated that macrophages isolated from sarcoid granuloma or from lung lavage fluid were capable of synthesizing 1,25(OH)2D from precursor 25OHD [28,29]. The presence in sarcoid macrophages of vitamin D-1α-hydroxylase activity provided an explanation for the elevated circulating levels of 1,25(OH)2D frequently found in patients with this disease [30]. Indeed, macrophage synthesis of 1,25(OH)2D appears to be common to granulomatous diseases in general, as well as several types of tumor involving significant macrophage infiltration [31]. However, macrophage 1α-hydroxylase activity has also been demonstrated in non-pathological settings. Monocytes isolated from normal human peripheral blood mononuclear cells are readily able to synthesize 1,25(OH)2D when treated with cytokines such as interferon-gamma (IFNγ) [32], or bacterial antigens such as lipopolysaccharide (LPS) [33]. Indeed, simple in vitro differentiation of immature human monocytes from peripheral blood mononuclear cells into mature macrophages is associated with enhanced synthesis of 1,25(OH)2D [34]. The precise benefit of elevated 1α-hydroxylase activity in macrophages versus monocytes remains unclear as, paradoxically, the latter show higher levels of VDR relative to macrophages [34]. One possibility is that abundant expression of VDR by monocytes sensitizes these cells to the differentiating effects of 1,25(OH)2D, providing a ‘fast-forward’ autocrine mechanism for subsequent maturation of cells into macrophages. If so, this would be a similar mechanism to that described for the glucocorticoid-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) during the differentiation of adipose stromal cells to adipocytes [35].
One of the most significant developments concerning the role of vitamin D and monocyte function arose from studies to identify mechanisms associated with innate immune responses to infection with Mycobacterium tuberculosis (M. tb). Modlin and colleagues used a toll-like receptor (TLR) 2 ligand to mimic M. tb interaction with the TLR2/1 complex expressed on monocytes [36]. Using DNA array analyses, they showed increased expression of the genes for VDR and CYP27B1 in monocytes following TLR2/1 activation, suggesting a potential intracrine response [36]. This was confirmed by the addition of 25OHD to TLR2/1-activated monocytes which potently upregulated expression of the antimicrobial protein cathelicidin (hCAP), a known target for transcriptional regulation by liganded VDR [37,38]. The induction of monocyte hCAP by 25OHD was inhibited by a VDR agonist and a non-specific inhibitor of 1α-hydroxylase activity, confirming the intracrine nature of this mechanism [36]. Significantly, the magnitude of TLR 2/1-mediated induction of hCAP was directly proportional to the 25OHD status of donor serum used to supplement monocyte cultures. More recent studies have expanded this observation to show that TLR-induced hCAP expression is also enhanced in patients receiving in vivo supplementation with vitamin D [39].
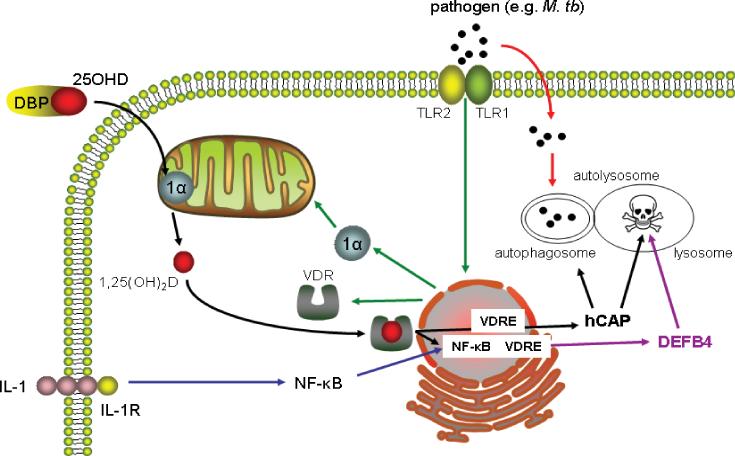
Thus, for the first time since the original sarcoidosis studies it is possible to propose a mechanism detailing the benefits of intracrine metabolism of 25OHD with respect to innate immune response to infection by monocytes/macrophages (Figure 2). In the face of an immune challenge such as infection with M. tb, pathogen-sensing receptors such as TLRs trigger enhanced expression of 1α-hydroxylase and VDR. Provided there is sufficient 25OHD available, this will then elevate local levels of 1,25(OH)2D, stimulating transcription of the hCAP gene, with the resulting antimicrobial protein being incorporated into lysosomes to promote bacterial killing. Initially hCAP was thought to act primarily by disrupting bacterial cell membranes [40]. However, recent studies indicate that 1,25(OH)2D-induced hCAP also plays a pivotal role in macrophage autophagy [41], a key mechanism in the degradation of cytsolic components that has also been implicated in host response to infection by bacteria and viruses [42]. Thus, as depicted in Figure 2, the induction of hCAP following localized synthesis of 1,25(OH)2D has the dual benefit of promoting the generation of autophagosomes whilst enhancing bacterial killing following fusion with lysosomes to form autolysosomes. It is also important to recognize that there may be other intracrine targets for vitamin D associated with antibacterial activity. Like hCAP, the gene promoter for β-defensin 4 (DEFB4), another key antimicrobial protein, is known to contain a functional vitamin D response element (VDRE) [37,38]. However, unlike hCAP, induction of DEFB4 expression by 1,25(OH)2D requires simultaneous activation of two nuclear factor-κB (NF-κB) sites adjacent to its VDRE via a mechanism involving interleukin-1 (IL-1), signaling via its cognate membrane receptor [43]. Collectively, these observations suggest that effective regulation of innate immune responses by physiological levels of 25OHD are probably not restricted to simple induction of hCAP but instead may involve several different mechanisms targeted at optimizing bacterial killing.
Figure 2. Monocyte/macrophage pathogen-sensing stimulates interaction between vitamin D and the innate immune system.
Pathogens such as Mycobaterium tuberculosis (M. tb) are phagocytosed by monocytes/macrophages but also trigger pathogen-sensing via toll-like receptors (TLR) (in the case of M. tb, the TLR2/1 complex). Activation of monocyte/macrophage TLR2/1 stimulates expression of the vitamin D receptor (VDR) and 1α-hydroxylase (1α). In this way, 25-hydroxyvitamin D (25OHD) in circulation bound to vitamin D-binding protein (DBP) is released to the monocyte/macrophage and converted to 1,25-dihydroxyvitamin D (1,25(OH)2D) in the mitochondria. The 1,25(OH)2D is then able to bind to the VDR and transcriptionally induce target genes such as antimicrobial protein cathelicidin (hCAP) via a vitamin D response element (VDRE) in the hCAP gene promoter. The antimicrobial protein β-defensin 4 (DEFB4) also exhibits a gene promoter VDRE but requires co-stimulation by activators of nuclear factor-κB (NF-κB), such as interleukin-1 (IL-1) signaling via the IL-1 receptor (IL-1R), to promote transcriptional upregulation of DEFB4. Induction of hCAP facilitates autophagosome generation and hCAP and DEFB promote bacterial killing in the resulting autolysosome.
3. Immune regulation of intracrine metabolism of vitamin D
Why is an intracrine vitamin D mechanism so central to monocyte/macrophage antimicrobial function? With respect to human monocytes, stimulation of hCAP and DEFB4 by VDR/NF-κB pathways appears to be crucial to host responses to mycobacteria such as M. tb [43]. However, the same cannot be said of mice where bacterial killing is more dependent on the localized generation of nitric oxide [44]. In the context of vitamin D activity, this appears to be due to lack of an appropriate vitamin D response element (VDRE) in the proximal promoters of hCAP-related genes for non-primate mammals [38]. The VDRE associated with vitamin D-induced hCAP expression in humans and apes, as well as New World and Old World primates, arose through incorporation of an Alu short interspersed element (SINE) which placed this gene under the control of the VDR [45]. Over the ensuing 50-60 million years this genetic modification has presumably provided an advantage as far as primate innate immunity is concerned. One explanation for this is that primates and early Homo sapiens would have existed in a state of vitamin D abundance due to relatively high levels of exposure to ultra violet light, which stimulates the production of vitamin D from 7-dehydrocholesterol in the skin. Under these conditions, vitamin D-induction of an antimicrobial protein may have conferred significant advantages in combating infectious disease. By contrast, other mammals, such as mice, with less UV-centric habitats would have experienced limited benefit from SINE incorporation of a VDRE into genes encoding antimicrobial proteins.
Another possibility is that incorporation of VDRE into the hCAP gene promoter may have helped primates to respond to specific immune challenges associated with certain pathogens. In studies comparing innate immune responses in mouse and human monocytic cell lines, we showed that in both types of cell, stimulation with a TLR2 or TLR4 ligand actively induced expression of inflammatory cytokines such as IL-1. In mouse monocytes, the TLR ligands also induced expression of cathelicidin-related antimicrobial protein (CRAMP), the murine equivalent of hCAP. This contrasted with human monocytes where the TLR ligands actually suppressed hCAP expression [39]. Co-treatment with 25OHD had no effect on TLR-induced CRAMP in mouse monocytes, but in human monocytes the presence of 25OHD ‘rescued’ TLR-mediated suppression of hCAP. Based on these observations, we have hypothesized that the presence of a VDRE in the hCAP may have provided a biological advantage in primates by counteracting TLR-driven subversion of normal antibacterial immune surveillance by pathogens such as M. tb. Corruption of innate immune responses is a well-recognized feature of many infectious organisms, and includes the suppression of antimicrobial proteins by pathogens including M. tb [46-48]. Thus, for primates living in an environment conducive to high serum levels of vitamin D, the acquisition of vitamin D responsiveness by genes such as hCAP is likely to have provided a biologically advantageous counterpoint to detrimental pathogenic effects.
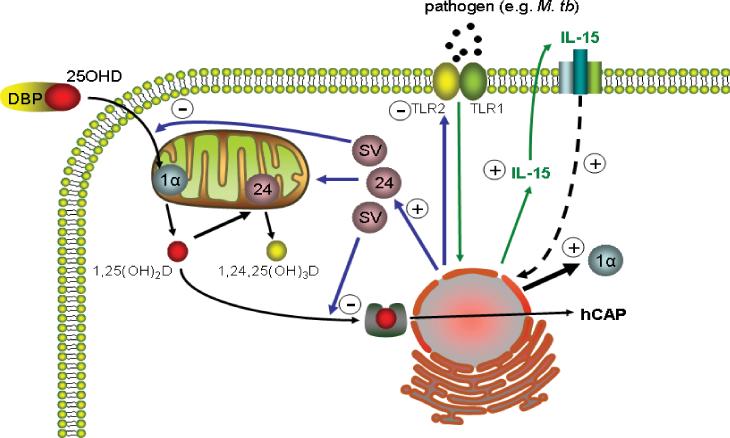
Another key benefit of incorporating vitamin D into innate immune pathways, is its ability to facilitate feedback regulation. In concert with the induction of hCAP, locally synthesized 1,25(OH)2D also potently stimulates monocyte/macrophage expression of the catabolic enzyme vitamin D-24-hydroxylase (24-hydroxylase) [36] (Figure 3). Conventional endocrinology indicates that this enhances 24-hydroxylation of 1,25(OH)2D to less active 1,24,25-trihydroxyvitamin D, thereby limiting the exposure of target cells to potentially detrimental calcemic effects of the active hormone [49]. However, monocytes/macrophages also express a truncated form of the 24-hydroxylase protein in which the N-terminal mitochondrial targeting sequence is spliced out [50]. Despite being metabolically inactive, the 24-hydroxylase splice variant retains its steroid binding pocket and is therefore still able to bind substrates such as 1,25(OH)2D or 25OHD. Furthermore, molecular modeling suggests that truncation of the 24-hydroxylase protein switches substrate preference from 1,25(OH)2D to 25OHD [51]. In this way the splice variant form of 24-hydroxylase may act primarily as a decoy to limit the availability of 25OHD to other enzymes, notably the 1α-hydroxylase. Such a mechanism would be rare in steroidogenesis but has the advantage of being metabolically economical, particularly in cells such as monoctes/macrophages where intracrine conversion of 25OHD to 1,25(OH)2D is the pivotal mechanism for vitamin D action. For more established endocrine responses involving renal synthesis of 1,25(OH)2D which then acts on peripheral tissues, it is likely that conventional 24-hydroxylase catabolism will be the preferred mode of feedback control.
Figure 3. Vitamin D and the feedback regulation of monocyte/macrophage production of hCAP.
Intracrine induction of monocyte/macrophage hCAP production by vitamin D is promoted following TLR2/1 pathogen-sensing. This mechanism involves TLR2/1 induction of interleukin-15 (IL-15), a potent stimulator of 1α-hydroxylase (1α) expression and activity. As a counterpoint, intracrine generated 1,25(OH)2D acts to suppress TLR2 expression thereby desensitizing monocytes/macrophages to further TLR2/1 activation. Locally generated 1,25(OH)2D also stimulates expression of the enzyme 24-hydroxylase (24) which catalyzes the conversion of 25OHD and/or 1,25(OH)2D to less active metabolites such as 1,24,25-trihydroxyvitamin D (1,24,25(OH)3D). Monocytes/macrophages also express a truncated splice variant (SV) form of the 24-hydroxylase protein which lacks the required mitochondrial-targeting sequence and is therefore functionally inactive and located in the cytosol. Despite this the SV protein retains its steroid-binding pocket and can thus act as a decoy for 25OHD or 1,25(OH)2D.
For almost every cell type throughout the body, including monocytes/macrophages, expression of the gene for 24-hydroxylase (CYP24A1) is regulated primarily by its main substrate, 1,25(OH)2D. Relatively little is known concerning other factors that influence the expression of this catabolic enzyme, although clearly this may be central to the efficacy of intracrine activation of vitamin D. By contrast, it is evident that the vitamin D-activating enzyme 1α-hydroxylase is profoundly influenced by a variety of factors associated with immune function. As outlined in Figure 2, expression of the CYP27B1 gene is upregulated by ligands for the TLR2/1 complex on monocytes, including the 19 kDa lipoprotein of M. tb [36]. Monocyte/macrophage expression of CYP27B1 is also induced by the TLR4 ligand lipopolysaccharide (LPS) [39], indicating that both Gram positive (TLR4) and Gram negative (TLR2) bacteria are able to promote local synthesis of 1,25(OH)2D. The precise mechanism by which this occurs has yet to be fully defined. Studies using monocytic cell lines have highlighted involvement of the JAK-STAT, p38 MAP kinase, and NF-κB pathways in the induction of CYP27B1 expression by combinations of the TLR4 ligand LPS, and interferon γ [52]. The pathways associated with CYP27B1 induction by TLR ligands alone remain unclear, although recent studies using monocytes treated with the TLR2 ligand 19 kDa lipoprotein have implicated the cytokine interleukin-15 (IL-15) as an intermediary in localized synthesis of 1,25(OH)2D [53]. This is an interesting observation given that elevated expression of IL-15 is frequently associated with inflammatory diseases, notably the granulomatous disease sarcoidosis [54]. Thus, it is possible that IL-15-mediated induction of 1α-hydroxylase activity provides a link between our current view of vitamin D as a regulator of normal innate immune responses, and the original observation of dysregulated 1,25(OH)2D production associated with granulomatous disease.
4. Vitamin D, dendritic cells and antigen presentation
Although much recent attention has focused on the role of vitamin D as an activator of antimicrobial activity, it is important to recognize that innate immunity incorporates other responses to pathogens. Prominent amongst these is antigen presentation which forms a link between the innate and adaptive arms of the immune system. Initially, this was a somewhat neglected facet of the interface between vitamin D and the immune system, due, in part, to the extensive literature concerning direct effects of 1,25(OH)2D on T-cells and B-cells. As such, early studies of the impact of vitamin D on antigen presentation were focused on the role of vitamin D and vitamin D binding protein (DBP) as potential adjuvants for immunization, particularly as DBP can be post-transcriptionally modified to form a macrophage-activating factor [55-57]. However, the advent of more recent reports documenting vitamin D metabolism and signaling in antigen-presenting cells has provided a new perspective on the role of vitamin D as a modulator of antigen presentation.
Several cells are known to mediate antigen presentation, but the most well recognized group of professional APCs are dendritic cells (DCs). Populations of purified DCs have been shown to express VDR [58], and respond to 1,25(OH)2D by attenuating antigen presentation [59]. However, it was not until the advent of monocyte-derived DC cultures that the effects of vitamin D metabolites on antigen presentation were fully defined. Experiments using 1,25(OH)2D [60] or synthetic analogs of 1,25(OH)2D [61] demonstrated inhibition DC maturation, thereby suppressing the capacity of these cells to present antigen to T-cells. Thus it was proposed that vitamin D acts to promote tolerogenic adaptive immunity via modulation of APC function. In support of this, studies of pancreatic islet transplantation in mice showed lower graft rejection rates in animals treated with 1,25(OH)2D [62]. Importantly, this response was associated with decreased DC maturation and increased numbers of Tregs [62], a key feature of the interaction between vitamin D and adaptive immunity [63].
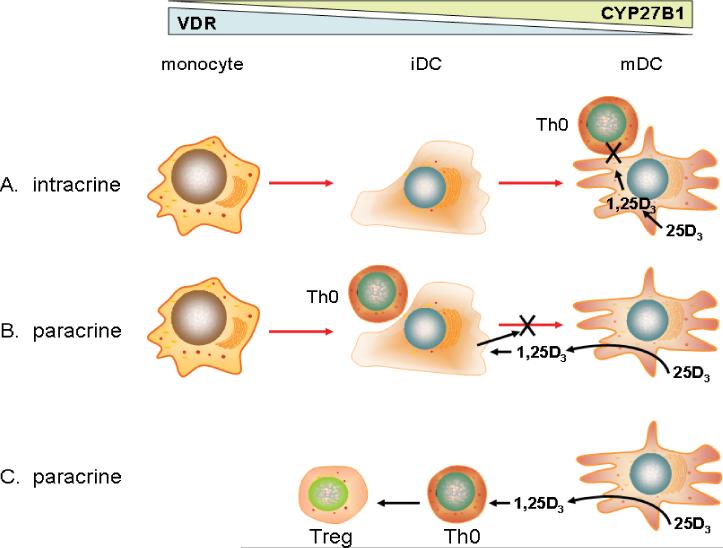
Regulation of DC maturation is a key therapeutic target for 1,25(OH)2D and its synthetic analogs as potential treatment for autoimmune disease and host-graft rejection. However, another facet of the interaction between vitamin D and antigen presentation was provided by the observation that DCs express 1α-hydroxylase in a similar fashion to macrophages [64,65]. As with macrophages, monocyte-derived DCs show increased expression and activity of 1α-hydroxylase as they differentiate towards a more mature phenotype [64]. Thus, in a similar fashion to the effects of 1,25(OH)2D outlined above, treatment with 25OHD suppresses DC maturation and inhibits T-cell proliferation, utilizing a similar intracrine pathway to that described for macrophages [64] (Figure 4A). In common with macrophages, mature DCs showed lower levels of VDR than immature DCs or monocytes [64]. This reciprocal organization of 1α-hydroxylase and VDR expression has the potential benefit of ensuring that mature antigen-presenting DCs are relatively insensitive to 1,25(OH)2D, thereby enabling an initial T-cell response and normal adaptive immunity. Instead, the 1,25(OH)2D generated by these cells will be able to act on VDR-expressing immature DCs, thus limiting further DC maturation [66] (Figure 4B). In this paracrine fashion locally synthesized 1,25(OH)2D can occur in the face of antigen-presentation and adaptive immunity whilst preventing persistence of DC maturation and over-stimulation of T-cells. Such a model is supported by studies of VDR and CYP27B1 knockout mice which exhibit lymphatic abnormalities consistent with increased numbers of mature DCs [67,68] and dysregulated DC trafficking [69].
Figure 4. Intracrine and paracrine mechanisms involved in mediating the effects of vitamin D on antigen presentation by dendritic cells.
Differentiation of monocytes to immature (iDCs) and mature dendritic cells (mDCs) is associated with the induction of 1α-hydroxylase (CYP27B1) and suppression of vitamin D receptor (VDR) expression. In this way, local conversion of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)2D) can impact on DC function in several ways. A. synthesis of 1,25(OH)2D by mDCs activates VDR signaling in an intracrine fashion despite low numbers of VDR, suppressing DC function and antigen presentation to adjacent niave T-cells (Th0). B. synthesis of 1,25(OH)2D by mDCs activates VDR signaling in an paracrine fashion, suppressing the maturation of adjacent iDCs and thereby promoting tolerogenic T-cell responses. C. synthesis of 1,25(OH)2D by mDCs acts in a paracrine fashion on VDR-expressing T-cells, promoting the generation of immunosuppressive regulatory T-cells (Treg) from Th0 cells.
In common with many other cells from the immune system DCs are heterogeneous with respect to tissue location, and cellular phenotype/function. However, broadly speaking they can be divided into two groups, myeloid (mDCs) and plasmacytoid (pDCs), which are characterized by different cytokine and chemokine profiles. mDCs and pDCs also appear to exert complementary effects on T-cells, with mDCs being efficient APCs [70] whilst pDCs are more closely associated with immune tolerance [71]. With this in mind, it is interesting to note that 1,25(OH)2D preferentially regulates mDCs, indicating that the key effect in this instance is to suppress activation of naïve T-cells. The lack of a similar response shown by pDCs does not preclude a role for vitamin D in the regulation of tolerogenic responses. One possibility is that local production of 1,25(OH)2D, rather than exogenous addition, will be more effective in achieving a pDC response. Alternatively, 1α-hydroxylase expression by pDCs may facilitate tolerance through paracrine effects of 1,25(OH)2D on VDR-expressing T-cells (Figure 4C). This, mechanism is similar to that originally proposed following the initial characterization of macrophage 1α-hydroxylase activity. At that time, 1,25(OH)2D synthesized by macrophages was thought to act in a paracrine fashion by suppressing T-cell proliferation [72]. However, more recent studies have shown that direct effects of 1,25(OH)2D on T-cells primarily involve changes in T-cell phenotype, notably the induction of tolerogenic Tregs [17].
5. Vitamin D, innate immunity and human disease
Consistent with the original studies describing a role for TLR-induced intracrine synthesis of 1,25(OH)2D following M tb. challenge of monocytes [36], clinical applications for vitamin D-regulated innate immunity have focused primarily on its effects in combating infection, in particular the disease tuberculosis (TB) [73,74]. However, serum 25OHD concentrations and circulating levels of hCAP have also been assessed in patients with sepsis, with low concentrations of the antimicrobial protein (and low serum 25OHD) being associated with increased risk of critical illness [75]. In a similar fashion, low 25OHD status has also been linked to infection and mortality in patients with end-stage kidney disease [76], and upper respiratory tract infection [77]. With respect to the latter, protective effects of vitamin D supplementation have been described for colds and influenza [78]: the ability of hCAP to exhibit antiviral as well as antibacterial properties [79] suggests that the beneficial effects of vitamin D supplementation may extend beyond bacterial infection.
Induction of hCAP by 1,25(OH)2D is not universal [80], but has nevertheless been reported for many cell types including keratinocytes [81], bronchial epithelial cells [82], myeloid cell lines [38], as well as decidual [83], and trophoblastic cells of the placenta [84]. In each case the mechanism by which 25OHD is able to regulate hCAP expression appears to be quite distinct. In the skin, normal human keratinocytes express relatively low levels of TLR2 and are therefore less sensitive to immune stimuli than macrophages. However, under conditions of epidermal wounding, transforming growth factor-beta is released and this potently stimulates 1α-hydroxylase expression and activity in keratinocytes [81]. The resulting localized accumulation of 1,25(OH)2D acts to enhance TLR expression, which in turn promotes pathogen recognition and production of antimicrobial hCAP [81]. The stimulatory effect of 1,25(OH)2D on TLR expression in keratinocytes is in direct contrast with its suppression of TLRs in monocytes [85]. The underlying mechanism(s) for these opposing actions remains to be determined, but may reflect the need for tighter feedback control of monocyte responses to prevent over-elaboration of immune activity and possible inflammatory damage.
In contrast with keratinocytes, trophoblastic cells from the placenta exhibit high levels of 1α-hydroxylase expression, facilitating constitutive production of hCAP in the presence of sufficient 25OHD [84]. This mechanism is not further enhanced by either TLR ligands or growth factors, suggesting that initial induction of the CYP27B1 gene during early gestation is alone sufficient to promote vitamin D-induced antimicrobial activity by the placenta. In the skin and placenta as well as other ‘barrier sites’ such as the lungs and gastrointestinal tract, the precise immune benefits of vitamin D-induced hCAP expression have yet to be fully defined but may include effects on respiratory infection [77] and transmission of infection during pregnancy [86].
Although autoimmune diseases are characterized by an underlying inherited predisposition, there is much current interest in environmental factors such as vitamin D that may contribute to the manifestation of these disorders [10]. Lower circulating levels of 25OHD have been linked to risk of type 1 diabetes [87], and vitamin D supplementation has been reported to protect against the disease [88]. In addition, genetic analyses have shown that gene polymorphisms for VDR [89], and CYP27b1 [90], affect susceptibility to type 1 diabetes. Other autoimmune diseases that appear to be influenced by vitamin D include Crohn's disease [91], systemic lupus erythematosus [92], and rheumatoid arthritis [10]. There is also growing evidence of a link between vitamin D and multiple sclerosis (MS) [9,93], supported by both epidemiology [94,95] and animal models [96,97]. Interestingly, studies using the experimental autoimmune encephalomyelitis model suggest that protective effects of vitamin D with respect to this murine form of MS are more effective in females [98], and involve estrogen-mediated induction of VDR and CYP27B1 [99]. In this way, future studies of vitamin D and autoimmune disease may help to identify novel mechanisms involved in mediating the actions of vitamin D at the boundary between innate and adaptive immunity.
6. Conclusions
Our new perspective on the interaction between vitamin D and human immunity has shed light on the intracrine mechanisms that are central to its immunomodulatory activity. Crucially it is now clear that these mechanisms are also common to cells from a variety of tissues outside the classical immune system, notably ‘barrier’ sites such as the skin, lungs, intestine and placenta. Irrespective of the cell type involved in mediating intracrine responses to vitamin D, these studies have underlined the potential problems that may stem from inadequate vitamin D status. The latter appears to be a prevalent condition in communities throughout the world, further emphasizing the need for new clinical studies aimed at assessing the physiological and disease consequences of vitamin D insufficiency.
Acknowledgments
This work was supported by NIH grant RO1AR050626 to M.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19:73–8. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7:439–43. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 4.Jurutka PW, Bartik L, Whitfield GK, Mathern DR, Barthel TK, Gurevich M, Hsieh JC, Kaczmarska M, Haussler CA, Haussler MR. Vitamin D receptor: key roles in bone mineral pathophysiology, molecular mechanism of action, and novel nutritional ligands. J Bone Miner Res. 2007;22(Suppl 2):V2–10. doi: 10.1359/jbmr.07s216. [DOI] [PubMed] [Google Scholar]
- 5.Ritter CS, Armbrecht HJ, Slatopolsky E, Brown AJ. 25-Hydroxyvitamin D(3) suppresses PTH synthesis and secretion by bovine parathyroid cells. Kidney Int. 2006;70:654–9. doi: 10.1038/sj.ki.5000394. [DOI] [PubMed] [Google Scholar]
- 6.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. Faseb J. 2006;20:2417–9. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 7.Mizwicki MT, Keidel D, Bula CM, Bishop JE, Zanello LP, Wurtz JM, Moras D, Norman AW. Identification of an alternative ligand-binding pocket in the nuclear vitamin D receptor and its functional importance in 1alpha,25(OH)2-vitamin D3 signaling. Proc Natl Acad Sci U S A. 2004;101:12876–81. doi: 10.1073/pnas.0403606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab. 2008;4:80–90. doi: 10.1038/ncpendmet0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66:S135–8. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adorini L, Penna G. Control of autoimmune diseases by the vitamin D endocrine system. Nat Clin Pract Rheumatol. 2008;4:404–12. doi: 10.1038/ncprheum0855. [DOI] [PubMed] [Google Scholar]
- 11.Pappa HM, Gordon CM, Saslowsky TM, Zholudev A, Horr B, Shih MC, Grand RJ. Vitamin D status in children and young adults with inflammatory bowel disease. Pediatrics. 2006;118:1950–61. doi: 10.1542/peds.2006-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vagianos K, Bector S, McConnell J, Bernstein CN. Nutrition assessment of patients with inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2007;31:311–9. doi: 10.1177/0148607107031004311. [DOI] [PubMed] [Google Scholar]
- 13.Bouillon R, Carmeliet G, Verlinden L, van Etten E, Verstuyf A, Luderer HF, Lieben L, Mathieu C, Demay M. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29:726–76. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–92. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 15.Liu N, Nguyen L, Chun RF, Lagishetty V, Ren S, Wu S, Hollis B, DeLuca HF, Adams JS, Hewison M. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008;149:4799–808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–3. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 17.Jeffery LE, Burke F, Mura M, Zheng Y, Qureshi OS, Hewison M, Walker LS, Lammas DA, Raza K, Sansom DM. 1,25-Dihydroxyvitamin D(3) and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–67. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 19.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834–9. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin d3 on human B cell differentiation. J Immunol. 2007;179:1634–47. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 21.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mangelsdorf DJ, Koeffler HP, Donaldson CA, Pike JW, Haussler MR. 1,25-Dihydroxyvitamin D3-induced differentiation in a human promyelocytic leukemia cell line (HL-60): receptor-mediated maturation to macrophage-like cells. J Cell Biol. 1984;98:391–8. doi: 10.1083/jcb.98.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunce CM BG, Hewison M. Vitamin D and haematopoiesis. Trends in Endocrinology and Metabolism. 1997;8:245–251. doi: 10.1016/s1043-2760(97)00066-0. [DOI] [PubMed] [Google Scholar]
- 24.O'Kelly J, Hisatake J, Hisatake Y, Bishop J, Norman A, Koeffler HP. Normal myelopoiesis but abnormal T lymphocyte responses in vitamin D receptor knockout mice. J Clin Invest. 2002;109:1091–9. doi: 10.1172/JCI12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adorini L, Daniel KC, Penna G. Vitamin D receptor agonists, cancer and the immune system: an intricate relationship. Curr Top Med Chem. 2006;6:1297–301. doi: 10.2174/156802606777864890. [DOI] [PubMed] [Google Scholar]
- 26.Dusso AS, Thadhani R, Slatopolsky E. Vitamin D receptor and analogs. Semin Nephrol. 2004;24:10–6. doi: 10.1053/j.semnephrol.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 27.Fogh K, Kragballe K. New vitamin D analogs in psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:199–204. doi: 10.2174/1568010043343930. [DOI] [PubMed] [Google Scholar]
- 28.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–60. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755–65. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papapoulos SE, Clemens TL, Fraher LJ, Lewin IG, Sandler LM, O'Riordan JL. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet. 1979;1:627–30. doi: 10.1016/s0140-6736(79)91076-6. [DOI] [PubMed] [Google Scholar]
- 31.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–21. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 32.Koeffler HP, Reichel H, Bishop JE, Norman AW. gamma-Interferon stimulates production of 1,25-dihydroxyvitamin D3 by normal human macrophages. Biochem Biophys Res Commun. 1985;127:596–603. doi: 10.1016/s0006-291x(85)80202-3. [DOI] [PubMed] [Google Scholar]
- 33.Reichel H, Koeffler HP, Bishop JE, Norman AW. 25-Hydroxyvitamin D3 metabolism by lipopolysaccharide-stimulated normal human macrophages. J Clin Endocrinol Metab. 1987;64:1–9. doi: 10.1210/jcem-64-1-1. [DOI] [PubMed] [Google Scholar]
- 34.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–7. [PubMed] [Google Scholar]
- 35.Bujalska IJ, Kumar S, Hewison M, Stewart PM. Differentiation of adipose stromal cells: the roles of glucocorticoids and 11beta-hydroxysteroid dehydrogenase. Endocrinology. 1999;140:3188–96. doi: 10.1210/endo.140.7.6868. [DOI] [PubMed] [Google Scholar]
- 36.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–3. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 37.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 38.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. Faseb J. 2005;19:1067–77. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 39.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–95. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nizet V, Gallo RL. Cathelicidins and innate defense against invasive bacterial infection. Scand J Infect Dis. 2003;35:670–6. doi: 10.1080/00365540310015629. [DOI] [PubMed] [Google Scholar]
- 41.Yuk JM, Shin DM, Lee HM, Yang CS, Jin HS, Kim KK, Lee ZW, Lee SH, Kim JM, Jo EK. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009;6:231–43. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Levine B, Deretic V. Unveiling the roles of autophagy in innate and adaptive immunity. Nat Rev Immunol. 2007;7:767–77. doi: 10.1038/nri2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thoma-Uszynski S, Stenger S, Takeuchi O, Ochoa MT, Engele M, Sieling PA, Barnes PF, Rollinghoff M, Bolcskei PL, Wagner M, Akira S, Norgard MV, Belisle JT, Godowski PJ, Bloom BR, Modlin RL. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–7. doi: 10.1126/science.291.5508.1544. [DOI] [PubMed] [Google Scholar]
- 45.Gombart AF, Saito T, Koeffler HP. Exaptation of an ancient Alu short interspersed element provides a highly conserved vitamin D-mediated innate immune response in humans and primates. BMC Genomics. 2009;10:321. doi: 10.1186/1471-2164-10-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, Davidson RN, Sorensen OE, Kampmann B, Griffiths CJ, Wilkinson RJ. IFN-gamma- and TNF-independent vitamin D-inducible human suppression of mycobacteria: the role of cathelicidin LL-37. J Immunol. 2007;178:7190–8. doi: 10.4049/jimmunol.178.11.7190. [DOI] [PubMed] [Google Scholar]
- 47.Islam D, Bandholtz L, Nilsson J, Wigzell H, Christensson B, Agerberth B, Gudmundsson G. Downregulation of bactericidal peptides in enteric infections: a novel immune escape mechanism with bacterial DNA as a potential regulator. Nat Med. 2001;7:180–5. doi: 10.1038/84627. [DOI] [PubMed] [Google Scholar]
- 48.Chakraborty K, Ghosh S, Kole H, Mukhopadhyay AK, Ramamurthy T, Saha DR, Mukhopadhyay D, Roychowdhury S, Hamabata T, Takeda Y, Das S. Bacterial exotoxins downregulate cathelicidin (hCAP18/LL37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol. 2008 doi: 10.1111/j.1462-5822.2008.01227.x. [DOI] [PubMed] [Google Scholar]
- 49.Sakaki T, Kagawa N, Yamamoto K, Inouye K. Metabolism of vitamin D3 by cytochromes P450. Front Biosci. 2005;10:119–34. doi: 10.2741/1514. [DOI] [PubMed] [Google Scholar]
- 50.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. J Biol Chem. 2005;280:20604–11. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 51.Adams JS, Chen H, Chun R, Ren S, Wu S, Gacad M, Nguyen L, Ride J, Liu P, Modlin R, Hewison M. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res. 2007;22(Suppl 2):V20–4. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 52.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006;21:37–47. doi: 10.1359/JBMR.050908. [DOI] [PubMed] [Google Scholar]
- 53.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–20. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agostini C, Semenzato G. Cytokines in sarcoidosis. Semin Respir Infect. 1998;13:184–96. [PubMed] [Google Scholar]
- 55.Enioutina EY, Visic D, McGee ZA, Daynes RA. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999;17:3050–64. doi: 10.1016/s0264-410x(99)00147-4. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto N, Naraparaju VR. Structurally well-defined macrophage activating factor derived from vitamin D3-binding protein has a potent adjuvant activity for immunization. Immunol Cell Biol. 1998;76:237–44. doi: 10.1046/j.1440-1711.1998.00748.x. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto N. Structural definition of a potent macrophage activating factor derived from vitamin D3-binding protein with adjuvant activity for antibody production. Mol Immunol. 1996;33:1157–64. doi: 10.1016/s0161-5890(96)00081-8. [DOI] [PubMed] [Google Scholar]
- 58.Brennan A, Katz DR, Nunn JD, Barker S, Hewison M, Fraher LJ, O'Riordan JL. Dendritic cells from human tissues express receptors for the immunoregulatory vitamin D3 metabolite, dihydroxycholecalciferol. Immunology. 1987;61:457–61. [PMC free article] [PubMed] [Google Scholar]
- 59.Dam TN, Moller B, Hindkjaer J, Kragballe K. The vitamin D3 analog calcipotriol suppresses the number and antigen-presenting function of Langerhans cells in normal human skin. J Investig Dermatol Symp Proc. 1996;1:72–7. [PubMed] [Google Scholar]
- 60.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 61.Griffin MD, Lutz WH, Phan VA, Bachman LA, McKean DJ, Kumar R. Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs. Biochem Biophys Res Commun. 2000;270:701–8. doi: 10.1006/bbrc.2000.2490. [DOI] [PubMed] [Google Scholar]
- 62.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–53. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 63.O'Garra A, Barrat FJ. In vitro generation of IL-10-producing regulatory CD4+ T cells is induced by immunosuppressive drugs and inhibited by Th1- and Th2-inducing cytokines. Immunol Lett. 2003;85:135–9. doi: 10.1016/s0165-2478(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 64.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–90. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 65.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003;102:3314–6. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 66.Hewison M, Zehnder D, Chakraverty R, Adams JS. Vitamin D and barrier function: a novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol. 2004;215:31–8. doi: 10.1016/j.mce.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–5. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha - hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enioutina EY, Bareyan D, Daynes RA. TLR-induced local metabolism of vitamin D3 plays an important role in the diversification of adaptive immune responses. J Immunol. 2009;182:4296–305. doi: 10.4049/jimmunol.0804344. [DOI] [PubMed] [Google Scholar]
- 70.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 71.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 72.Nunn JD, Katz DR, Barker S, Fraher LJ, Hewison M, Hendy GN, O'Riordan JL. Regulation of human tonsillar T-cell proliferation by the active metabolite of vitamin D3. Immunology. 1986;59:479–84. [PMC free article] [PubMed] [Google Scholar]
- 73.Martineau AR, Wilkinson RJ, Wilkinson KA, Newton SM, Kampmann B, Hall BM, Packe GE, Davidson RN, Eldridge SM, Maunsell ZJ, Rainbow SJ, Berry JL, Griffiths CJ. A single dose of vitamin d enhances immunity to mycobacteria. Am J Respir Crit Care Med. 2007;176:208–13. doi: 10.1164/rccm.200701-007OC. [DOI] [PubMed] [Google Scholar]
- 74.Nursyam EW, Amin Z, Rumende CM. The effect of vitamin D as supplementary treatment in patients with moderately advanced pulmonary tuberculous lesion. Acta Med Indones. 2006;38:3–5. [PubMed] [Google Scholar]
- 75.Jeng L, Yamshchikov AV, Judd SE, Blumberg HM, Martin GS, Ziegler TR, Tangpricha V. Alterations in vitamin D status and anti-microbial peptide levels in patients in the intensive care unit with sepsis. J Transl Med. 2009;7:28. doi: 10.1186/1479-5876-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr., Koeffler HP, Thadhani R. Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis. 2009;48:418–24. doi: 10.1086/596314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, Garland CF, Giovannucci E. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aloia JF, Li-Ng M. Correspondence. Epidemiol Infect. 2007:1–4. [Google Scholar]
- 79.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Curr HIV Res. 2007;5:410–5. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 80.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–11. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yim S, Dhawan P, Ragunath C, Christakos S, Diamond G. Induction of cathelicidin in normal and CF bronchial epithelial cells by 1,25-dihydroxyvitamin D(3). J Cyst Fibros. 2007 doi: 10.1016/j.jcf.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-Hydroxyvitamin D3 and 1,25-Dihydroxyvitamin D3 on Cytokine Production by Human Decidual Cells. Biol Reprod. 2006 doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 84.Liu N, Kaplan AT, Low J, Nguyen L, Liu GY, Equils O, Hewison M. Vitamin D Induces Innate Antibacterial Responses in Human Trophoblasts via an Intracrine Pathway. Biol Reprod. 2009;80:398–406. doi: 10.1095/biolreprod.108.073577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–70. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 86.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–30. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Littorin B, Blom P, Scholin A, Arnqvist HJ, Blohme G, Bolinder J, Ekbom-Schnell A, Eriksson JW, Gudbjornsdottir S, Nystrom L, Ostman J, Sundkvist G. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia. 2006;49:2847–52. doi: 10.1007/s00125-006-0426-x. [DOI] [PubMed] [Google Scholar]
- 88.Harris SS. Vitamin D in type 1 diabetes prevention. J Nutr. 2005;135:323–5. doi: 10.1093/jn/135.2.323. [DOI] [PubMed] [Google Scholar]
- 89.Ramos-Lopez E, Jansen T, Ivaskevicius V, Kahles H, Klepzig C, Oldenburg J, Badenhoop K. Protection from type 1 diabetes by vitamin D receptor haplotypes. Ann N Y Acad Sci. 2006;1079:327–34. doi: 10.1196/annals.1375.050. [DOI] [PubMed] [Google Scholar]
- 90.Bailey R, Cooper JD, Zeitels L, Smyth DJ, Yang JH, Walker NM, Hypponen E, Dunger DB, Ramos-Lopez E, Badenhoop K, Nejentsev S, Todd JA. Association of the vitamin D metabolism gene CYP27B1 with type 1 diabetes. Diabetes. 2007 doi: 10.2337/db07-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–4. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 92.Kamen D, Aranow C. Vitamin D in systemic lupus erythematosus. Curr Opin Rheumatol. 2008;20:532–7. doi: 10.1097/BOR.0b013e32830a991b. [DOI] [PubMed] [Google Scholar]
- 93.Raghuwanshi A, Joshi SS, Christakos S. Vitamin D and multiple sclerosis. J Cell Biochem. 2008;105:338–43. doi: 10.1002/jcb.21858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beretich B, Beretich T. Explaining multiple sclerosis prevalence by ultraviolet exposure: a geospatial analysis. Mult Scler. 2009;15:891–8. doi: 10.1177/1352458509105579. [DOI] [PubMed] [Google Scholar]
- 95.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–8. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 96.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–4. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Spach KM, Pedersen LB, Nashold FE, Kayo T, Yandell BS, Prolla TA, Hayes CE. Gene expression analysis suggests that 1,25-dihydroxyvitamin D3 reverses experimental autoimmune encephalomyelitis by stimulating inflammatory cell apoptosis. Physiol Genomics. 2004;18:141–51. doi: 10.1152/physiolgenomics.00003.2004. [DOI] [PubMed] [Google Scholar]
- 98.Spach KM, Hayes CE. Vitamin D3 confers protection from autoimmune encephalomyelitis only in female mice. J Immunol. 2005;175:4119–26. doi: 10.4049/jimmunol.175.6.4119. [DOI] [PubMed] [Google Scholar]
- 99.Nashold FE, Spach KM, Spanier JA, Hayes CE. Estrogen controls vitamin D3-mediated resistance to experimental autoimmune encephalomyelitis by controlling vitamin D3 metabolism and receptor expression. J Immunol. 2009;183:3672–81. doi: 10.4049/jimmunol.0901351. [DOI] [PubMed] [Google Scholar]