Abstract
Morphogenesis of the adult structures of holometabolous insects is regulated by ecdysteroids and juvenile hormones and involves cell-cell interactions mediated in part by the cell surface integrin receptors and their extracellular matrix (ECM) ligands. These adhesion molecules and their regulation by hormones are not well characterized. We describe the gene structure of a newly described ECM molecule, tenectin, and demonstrate that it is a hormonally regulated ECM protein required for proper morphogenesis of the adult wing and male genitalia. Tenectin’s function as a new ligand of the PS2 integrins is demonstrated by both genetic interactions in the fly and by cell spreading and cell adhesion assays in cultured cells. Its interaction with the PS2 integrins is dependent on RGD and RGD-like motifs. Tenectin’s function in looping morphogenesis in the development of the male genitalia led to experiments that demonstrate a role for PS integrins in the execution of left-right asymmetry.
Keywords: tenectin, integrin, ecdysone, metamorphosis, adhesion, looping morphogenesis, left-right asymmetry
Introduction
During development, morphogenetic movements are induced and controlled by a variety of molecules such as growth factors or hormones and their receptors. In holometabolous insects like the fruit fly, Drosophila melanogaster, and the beetle, Tenebrio molitor, ecdysteroids and juvenile hormones control development both during embryogenesis and later during larval and pupal molts. These hormones regulate gene expression patterns required for development. Following changes in gene expression, cells change shape and move by processes that require remodeling of the extracellular matrix (ECM) and alterations in the interactions of cell surface adhesion molecules with their intracellular and extracellular ligands. In both invertebrates and vertebrates, ECM proteins and their receptors are important for the anchorage of cells, cell spreading, migration, proliferation and differentiation and they have been implicated in numerous pathologies (Brown et al., 2000; Hynes 2002; Bökel and Brown, 2002; Brower 2003; Danen and Sonnenberg 2003).
Integrins are a family of heterodimeric transmembrane glycoproteins, consisting of two subunits (α and β) that serve as receptors for ECM molecules and cell surface molecules of neighboring cells. The Drosophila genome contains 5 α subunits (αPS1-αPS5) and 2 β subunits (βPS and βν) while, in vertebrates, 18 α and 8 β subunits have been identified (Brower, 2003; Takada et al., 2007, for reviews). The best characterized Drosophila integrin subunits, αPS1, αPS2 and αPS3, are encoded by the multiple edematous wings, inflated and scab loci, respectively while the βPS locus is encoded by the myospheroid locus (Brower and Jaffe, 1989; Wilcox et al., 1989; Wehrli et al., 1993; Brown, 1994; Stark et al., 1997; Grotewiel et al., 1998). A small number of integrin ligands have been identified in Drosophila: laminin α1,2 chain (Graner et al., 1998), tiggrin (Fogerty, et al., 1994; Bunch et al., 1998), tenascin-m chain (Graner et al., 1998), thrombospondin (Subramanian et al., 2007) and collagen IV (Borchiellini et al., 1996) but it is not known if tenascin or collagen IV binds integrins in vivo. αPS1 is a typical laminin-binding-type subunit while αPS2 is an RGD (arginine, glycine, aspartic acid)-binding-type subunit. Since αPS1, αPS2 and βPS subunits have clear homologs in vertebrates, the Drosophila integrin system is becoming a simple powerful tool in which to characterize integrin functions. Generally, mutants for genes involved in the integrin pathways display clear phenotypes, which facilitate in vivo studies.
Just prior to wing morphogenesis during post-embryonic development, αPS1βPS and αPS2βPS are expressed in a complementary fashion in the wing imaginal disc epithelium. αPS1βPS is expressed in the presumptive dorsal surface and αPS2βPS on the ventral surface. At metamorphosis the disc evaginates bringing in apposition αPS1βPS expressing dorsal cells with αPS2βPS expressing ventral cells (Wilcox et al., 1981; Brower et al., 1984; Leptin et al., 1987). Mutations of genes involved in the integrin pathway often cause epithelial detachment and wing blistering phenotypes. Integrins also function in muscle attachment, short-term memory, olfaction, embryonic midgut migration and axonal pathfinding (Brown et al., 2000; Bökel and Brown, 2002; Brower, 2003, for reviews). Since swapping the cytoplasmic tails between the two subunits does not detectably alter their function, crucial differences between the two subunits are located in their extracellular ligand-binding domains (Martin-Bermudo et al., 1997; Martin-Bermudo and Brown 1999). Thus, the molecular characterization of integrin ligands in Drosophila is an important step to understand integrin functions in morphogenesis.
Tenebrin was identified as a potential integrin ligand whose expression is hormonally regulated during morphogenesis in the beetle Tenebrio molitor. During the post-embryonic development of holometabolous insects, each molt is induced by a pulse of 20-hydroxyecdysone (20E), while the nature of the molt is controlled by a sesquiterpenoid hormone, the juvenile hormone (JH). 20E directly activates cascades of gene expression by binding to the 20E/receptor complex and inducing expression of early genes encoding transcription factors that coordinate the induction of large sets of secondary-response late genes, leading to the appropriate stage and tissue-specific biological responses (Russell et al., 1996; Thummel, 1996; Richards, 1992; Segraves, 1994; Henrich et al., 1999). Our screen for hormone responsive genes identified Tenebrin whose expression is regulated by 20E and JH. Tenebrin encodes a putative ECM protein with the RGD integrin-binding motif (Royer et al., 2004).
To analyze the role of tenebrin in development, we identified its homolog, tenectin, in Drosophila melanogaster and described its embryonic expression patterns (Fraichard et al., 2006). In this report we used tenectin dsRNA to generate tenectin mutants and find phenotypes in the adult wing and male genitalia. Drosophila wings originate from small clusters of undifferentiated cells constituting the imaginal discs (Oberlander, 1985), which transform from an essentially flat monolayer of epithelial cells to mature adult structures (Fristrom and Fristrom, 1993). This striking transformation is coordinated by pulses of 20E and requires genes encoding transcription factors, proteases, cytoskeletal proteins, extracellular matrix proteins and their receptors (Fristrom et al., 1993; Brower, 2003; Brabant et al., 1996; Prout et al., 1997; Walsh and Brown, 1998; D’Avino and Thummel, 2000). Ecdysone regulates integrin expression in wing morphogenesis (D’Avino and Thummel, 2000) and in the final stages of wing morphogenesis an epidermal to mesenchymal transition is regulated by the neurohormone bursicon (Kiger et al., 2007).
Looping morphogenesis of the adult male genitalia is also regulated by hormones (Ádám et al., 2003, Wilson et al., 2006). In this process, roles of multiple signaling pathways and an unconventional myosin have been reported but functions of the ECM or its integrin receptors have not (Casanova et al., 1986; Sanchez-Herrero and Crosby, 1988; Macías et al., 2004; Wassarman et al., 2000; Abbott and Lengyel, 1991; Grether et al., 1995; Ádám et al., 2003; Hozumi et al., 2006; Spéder et al., 2006; Coutelis et al., 2008).
Here we report that at metamorphosis both the wing and male genitalia require tenectin and the PS2 integrins for proper morphogenesis. We also show that ecdysone regulates tenectin expression in wing morphogenesis. Finally, we directly test the capacity of the PS2 integrins and tenectin to functionally interact in cell spreading and adhesion assays.
Materials and methods
Drosophila stocks
Flies were reared on a standard cornmeal/molasses/yeast medium at 25°C. w1118, y1 w+; P{Act5C-GAL4} 25FO1/CyO, y+ (FBst0004414), P{GawB}elavC155 (FBst0000458), w+; P{GawB}T80/CyO (FBst0001878), w+; P{GAL4-da.G32}UH1 (FBst0005460), y1 w67c23; P{EPgy2}CG31422EY16369 (FBst0021205) respectively named in this paper, WT, Act-GAL4, elav-GAL4, discs-GAL4, da-GAL4, and EY16369 were obtained from the Bloomington stock center (stock ID is indicated in parentheses). mysnj42, mysb13, mysb47, and mysb69have been described (Jannuzi et al., 2004). To obtain mysb13 males it was necessary to remove, by recombination, extraneous lethal mutations from the original chromosome.
Rapid Amplification of cDNA ends (3’ and 5’RACE)
RNAs were extracted from staged third instar larvae using Izol-RNA reagent (5 Prime). The 3’end of tenectin cDNA was obtained using 3’RACE System (Invitrogen). Total RNA was reverse-transcribed using an oligo(dT) anchor primer. Nested PCR used the anchor primer in combination with a specific primer (tnc1–3RACE ; 5’-GCAAACGAGTCCACGAGCGGTCCC-3’), followed by a second specific primer (tnc2–3RACE ; 5’-GGCCGCCGTGGTGTCGGGACG-3’). The 5’end of tenectin cDNA was obtained using 5’RACE System (Invitrogen). Reverse transcription used total RNA and an antisense tnc internal primer (tnc1–5RACE; 5’-TCATTGGTCATGATGCGG-3’). Homopolymeric oligo-dC tail was added to the 3’end of the purified cDNA using terminal deoxyribonucleotidyl transferase. A supplied sense abridged anchor primer and an antisense tnc-specific primer (tnc2–5RACE; 5’-TGTTGGATCTCCGTGTACTCC-3’) were used in a subsequent PCR. 3’ and 5’RACE products were cloned in pGEM-T (Promega) and sequenced.
Transgenic tenectin mutants
DNA from coding sequence of the tenectin gene was amplified by PCR and cloned into the pUAST vector as an inverted repeat as described (Enerly et al., 2002; Kennerdell and Carthew, 2000). Amplification used sense and antisense primers containing EcoRI and XbaI sites and BlgII and XhoI sites and were 5’-CCGGAATTCTGTTGAAATCGACACGAAGC-3’, 5’-GAAGATCTTACCTCAGGCTCCTCATGCT-3’, 5’-GCTCTAGATGTTGAAATCGACACGAAG-3’ and 5’-CCGCTCGAGTACCTCAGGCTCCTCATGCT-3’ respectively. The recombinant vector, pUAST-tnc-IR was co-injected into w1118 embryos with the helper vector pUChsπΔ2–3. Adult, G0, transformants were identified by outcrossing to w1118and chromosomes bearing tnc-IR1a (on chromosome III), tnc-IR1b (on chromosome II) were balanced over TM3, Sb or CyO balancer chromosomes.
Northern blot analysis
RNA was extracted, fractionated by formaldehyde agarose gel electrophoresis and transferred onto nylon membranes as described (D’Avino et al., 1995). 15 µg of total RNA was loaded per lane. Filters were hybridized, washed, and stripped as described (Karim and Thummel, 1991). To detect tenectin transcripts, a 1kb genomic DNA fragment from exon 5 was PCR amplified from genomic DNA. All probes were labeled with 32P by random priming (Prime-a-gene, Promega). Each blot was probed with radioactive DNA derived from E74, βFtz-F1 or tenectin. For reprobing, blots were stripped by boiling for 20–30 min in 10mM Tris-HCI (pH 7.8), 1 mM EDTA, 1% SDS.
Imaginal discs culture
Staged third instar larvae were dissected in Grace’s Insect Medium (Gibco) and the larval organs were cultured in the same at 25°C for 3 or 6 hours. In some cases 20E (5 × 10−6 M) or cycloheximide (7 × 10−5 M) was added to the medium. Cycloheximide treatment reduced incorporation of 35 S-methionine into proteins by more than 93% in a parallel experiment (data not shown). After incubation, tissues were collected and total RNA was extracted for Northern blot analysis. The levels of transcript accumulation were determined with a Molecular Dynamics 300S computing densitometer.
Quantitative real time PCR
Total RNA (1 µg) from staged third instar larvae was reverse-transcribed using the iScript cDNA Synthesis Kit (Biorad). QPCR reactions were carried out on a MyIQ (Biorad) using IQ SYBR green supermix (Biorad) using the following primer pairs; for transcript1: 5’-AGAAGCCAAATTCCCCAGTT-3’/5’-GCACTTCATGGGTTTGTTCA-3’; for transcript2: 5’-AGCGGTTGTATCTTGGTGGT-3’/5’-AGAATGGTTTTGGCCAACTG-3’. Each reaction was performed in triplicate and the mean of three independent biological replicates was calculated. All results were normalized to the RP49 and Actin 5C mRNA levels and calculated using the ΔΔCt method (Pfaffl, 2001).
Whole-mount in situ hybridization and immunohistochemistry
Whole-mount in situ hybridization was performed using a variation of the protocol described by Tautz and Pfeifle (1989). To prepare the tenectin in situ RNA probes, two distinct cDNA encoding regions were PCR-amplified using the primer pairs 5’-GACAATTCCCGAAATCTCCA-3’/5’-CAGCATCCTGAGGAGACACA-3’ and 5’-GATAACCAGGTCTCATTCTC-3’/5’-TCCGGAGAGTGGTAGGGCACG-3’ and cloned in pGEM-T easy vector (Promega). Digoxygenin-labeled sense and antisense riboprobes were synthesized by in vitro transcription using T7 and SP6 polymerase, respectively, using the Roche Dig RNA labeling system.
For immunohistochemistry, imaginal discs and larval brains were dissected in PBS and fixed with 4% paraformaldehyde in PBS for 30 min at 0°C followed by another 30 min incubation with 4% paraformaldehyde and 0.1% Triton X-100 in PBS. After washing in PBS, the imaginal discs and the larval brains were incubated overnight at 4–8°C with primary antibodies diluted in PBS containing 5% normal goat serum, 0.1% Triton X-100, and 0.02% sodium azide. Primary antibodies were mouse anti-elav (1:1000; Developmental Studies Hybridoma Center, University Iowa) and anti-tenectin (1:4000; Fraichard et al., 2006). Detection of the different primary antibodies was carried out using alkaline phosphatase anti-rabbit (1:50, Sigma), AlexaFluor594 anti-mouse (1:200, Molecular Probes), and AlexaFluor488 anti-rabbit (1:50, Molecular Probes). Immunolabeled samples were analyzed on a Leica TCS-SP2AOBS spectral confocal microscope.
Tenectin fusion proteins and purification
For the cell spreading assay, a cDNA fragment encoding 68 amino acids (residues 232–299) that includes the RGD cell attachment sequence was cloned into the pQE30 bacterial expression vector (QIA Expressionist, Invitrogen). Soluble tenectin fusion protein was purified by immobilized metal affinity chromatography with Ni-NTA resin and dialyzed into PBS.
For the cell adhesion assays, His-tagged fusion proteins were produced by cloning cDNA fragments encoding VWC#3 or VWC#5, plus 10 amino acids prior to the first cysteine and 8 amino acids following the last cysteine (residues 225–308 and 2819-2731) into pTrcHisC vector (Xpress System, Invitrogen). Mutagenic primers were used to create KpnI and EcoRI sites for cloning at these positions. Standard PCR mutagenesis was used to mutate potential integrin-binding motifs. In VWC#3 RGD was mutated to SSL. In VWC#5 RDD, RSD and RYE were mutated to ATA, SSL, and TYI respectively. Urea denatured fusion proteins were purified by affinity chromatography on Ni-NTA agarose (QIAexpresss, QIAGEN). To promote proper folding and formation of the 5 disulphide bonds in the vWFc domains, fusion proteins were dialyzed overnight in refolding buffer (1 mM EDTA, 3.5 M urea, 10 mM beta mercaptoethanol, 20 mM Tris, pH 9.1) followed by dialysis into PBS (Cardamone et al., 1995). Fusion protein concentrations were determined by optical density at 280 nm and then confirmed by polyacrylamide gel electrophoresis and staining with Coomassie Brilliant Blue.
Cell spreading and adhesion experiments
Drosophila S2/M3 cells and the same stably expressing the αPS2m8 or αPS2c and βPS integrin subunits (under the control of the Drosophila HSP70 heat shock promoter) were grown in Shields and Sang M3 medium supplemented with 12.5% heat-inactivated fetal bovine serum. The medium for the transformed cells also contained 2 × 10−7M methotrexate (Bunch and Brower, 1992; Zavortink et al., 1993).
Cell spreading assays were done as described (Jannuzi et al., 2002). Briefly, cells were first cleared of accumulated matrix and other surface proteins by dispase/collagenase treatment at 37°C. This treatment also heat shocks the cells and induces expression of the integrin transgenes. Cells were allowed to spread in coated 96 well plates for 3–4 hours before counting. Each well of a 96 well plate was coated with 50 µl of ligand in PBS overnight at 4°C. Tenectin vWFc#3a fusion protein was used at a concentration of 25 µg/ml. The wells were then washed and non-specific sites on the plate blocked by incubation with 20% dry milk for 1 hour at room temperature. Following washing with PBS, 100 µl of cells [at 2–4 × 105 cells/ml in M3 medium + 2 mg/ml bovine serum albumin (BSA)] were added. GRGDSP and GRADSP peptides (Calbiochem, San Diego, CA) or purified IgG fractions of the PS integrin function blocking antibody aBG1 (Hirano et al., 1991) and the control PS integrin binding antibody CF.6G11 (Brower et al., 1984) were added to the cells just prior to their placement onto the coated wells. The number of spread and round cells was determined by phase microscopy using a Nikon phase-contrast microscope (Nikon Diaphot-TMD). For each value, over 100 cells were scored as round or flat from each of 3 different fields and the numbers represent the average of the 3 fields.
Cell adhesion assays were done as described (Jannuzi et al., 2004). Cells were protease treated as for the cell spreading experiments and allowed to recover for 4 hours in M3 medium + 2 mg/ml BSA. 100 µl of cells (1.5 × 106 cells/ml) were added to 96 well plate wells coated with ligand and the cells were allowed to attach for 20 minutes. Nonadherent cells were washed from the wells by pipetting and the remaining cells were stained with crystal violet. Dye levels were quantified using a Synergy2 Microplate Reader (Bio-Tek Instruments) at 562 nm. Plate coating concentrations for tenectin ligands were 20 µg/ml. To determine the tenectin “specific” adhesion we subtracted nonspecific values of cells adhering to wells coated with 20 µg/ml BSA. For adhesion assays, wells were coated with ligand for 1 hour at room temperature. Adhesion assays were done in three wells for each ligand and cell line displayed. The values given are the mean ± s.e.m. for those 3 wells.
Results
tenectin encodes a putative αPS2βPS integrin ligand
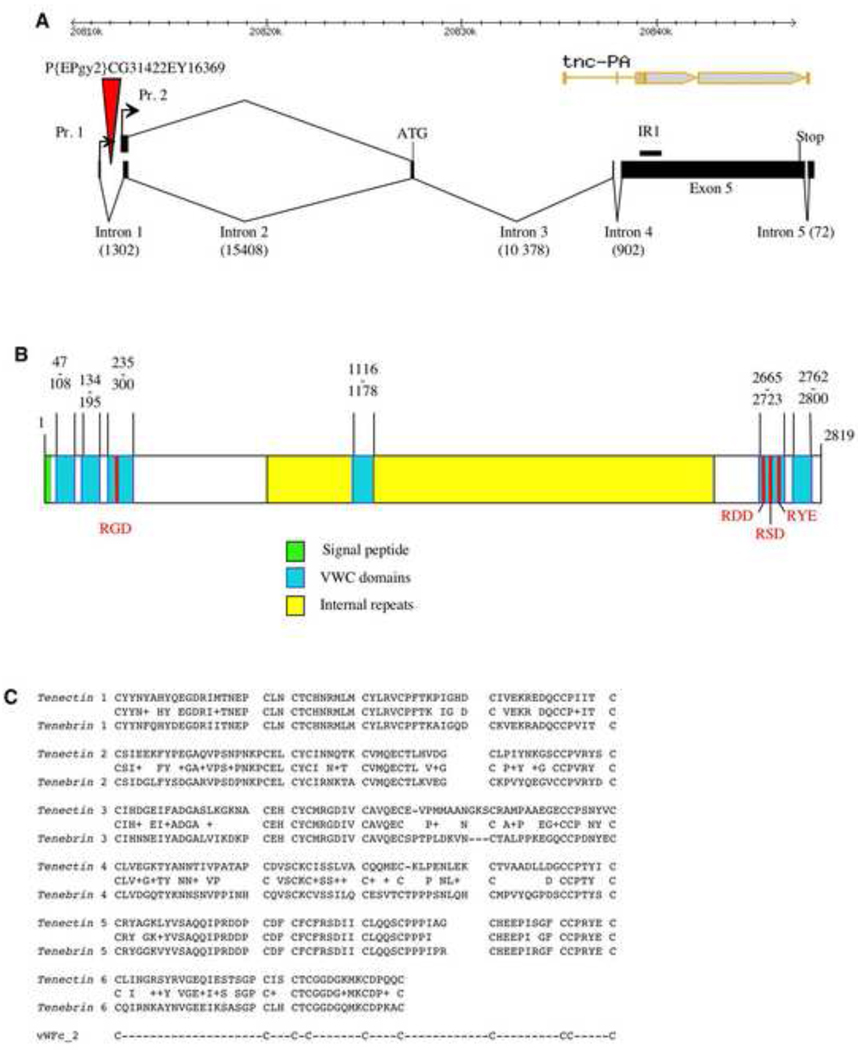
tenectin is the Drosophila melanogaster homolog of tenebrin from the beetle Tenebrio molitor. In the beetle, tenebrin was first characterized as a gene whose expression is developmentally regulated by both juvenile hormones and ecdysteroids. Its sequence and subsequent experiments in Drosophila indicated that it is a component of the extracellular matrix (ECM) (Royer et al., 2004; Fraichard et al., 2006). To begin the genetic analysis of tenectin’s function in development we have characterized its gene structure. Using a combination of 5’ and 3’RACE-PCR and the isolation of multiple cDNAs, we have determined unambiguously the gene structure of tenectin (Fig. 1). Two transcripts, differing by 95 nucleotides in their 5’ untranslated regions have been identified. The two cDNA sequences are 10,376 and 10,281 bp long and contain the same open reading frame of 2819 amino acids followed by 1461 untranslated nucleotides containing a putative polyadenylation signal. This structure for the tenectin gene differs markedly from that previously reported in flybase for CG13648 (http://flybase.org/cgi-bin/gbrowse/dmel/?Search=1;name=FBgn0039257) due to CG13648 containing incorrect predictions of transcript start sites, intron/exon positions, and the position of the AUG start codon. Our new description of the gene structure is confirmed by additional ESTs recently deposited in genbank that cover part of the 5’ end of tenectin (gb|EY199010.1|) and a region of exon 5 that was previously reported to contain an intron (gb|EC215144.1|, gb|CO309183.1|, gb|CO307535.1|, gb|EC214929.1|, gb|EC077781.1|).
Fig. 1.
tenectin gene organization. (A) Schematic of the tenectin gene showing the start and stop codons and intron splice sites for the two RNAs transcribed from 2 promoters (Pr. 1 and Pr. 2). The two transcripts contain identical translated sequences. The positions of the P{EPgy2}CG31422EY16369 element and sequence used for making the inverted repeat RNAi construct (IR1) are marked. For comparison, the flybase gene structure, with errors in intron/exon positions, is presented at the top of the figure (tnc-PA). (B) Schematic of the deduced protein showing the signal peptide, the RGD motifs, von Willebrand Factor type-c (VWC) domains and internal repeats. (C) Sequence alignments of VWC domains in tenectin and tenebrin. Consensus cysteines are also shown.
The deduced tenectin protein (Fig. 1B) is very similar to tenebrin (Royer et al., 2004). It has a molecular weight of ~300 kDa, a pI of 4, and the putative translational start site is followed by 31 hydrophobic amino acids corresponding to a signal peptide sequence (von Heijne, 1984) with a cleavage site predicted to be located between position 31 and 32 (Nielsen et al., 1997). Royer et al. (2004) described five von Willebrand type c (VWC) domains in both tenebrin and tenectin. These motifs are involved in protein-protein interactions (Verweij et al., 1986; Hunt and Barker, 1987) and have been found in ECM proteins like collagens and mucins (Sangiorgi et al., 1985; Wang and Granados, 1997), and also in signaling molecules like chordin (Sasai et al., 1994). Closer examination of the tenectin and tenebrin sequences reveals that they are of the vWFc_2 type (PROSITE, # PS50184) with a consensus sequence of C-X(18–26)-C-X(2,3)-C-X-C-X(6–14)-C-X(3,5)-C-X(1–12)-C-X(8–16)-C-C-X(2–5)-C. In addition to 5 complete VWC domains, there is one partial domain at the end of the protein that contains only the first 6 cysteines. As with tenebrin, the cell attachment sequence Arg-Gly-Asp (RGD; Ruoslahti and Pierschbacher 1987; Yamada, 1991) is found in tenectin within the third VWC domain located in the N-terminus of the protein (Royer et al., 2004). The 5th VWC domain contains 3 variants of the RGD sequence (RDD, RSD and RYE) that may serve as cell attachment motifs as well (Fig. 1B,C). The central region of the protein, excluding the 4th VWC is characterized by the presence of numerous internal repeats and is rich in Glu (19.2%), Thr (11.8%), and Pro (11.9%) as was described for tenebrin (Royer et al., 2004). Though of similar amino acid composition to tenebrin, the central region shows only 36% similarity (21% identities) in a BLASTP 2.2.18 analysis, while the VWC domains display a high degree of similarity (50–92% identities). The high Ser-Thr and Pro content (20.4% and 11.2%, respectively, for the entire protein) and the presence of the central repeats with a high Ser-Thr and Pro content (Fig. S1) suggests that tenectin may be a mucin-related-protein (Syed et al., 2008). Tenectin’s presence in the ECM of the CNS, foregut, hindgut, trachea and wing (Royer et al., 2004; Fraichard et al., 2006) and the presence of RGD motifs make it a likely ligand for the RGD-binding αPS2βPS integrins (Bunch and Brower, 1992; Fogerty et al., 1994; Graner et al., 1998; Subramanian et al., 2007).
Temporal profile of tenectin expression during metamorphosis
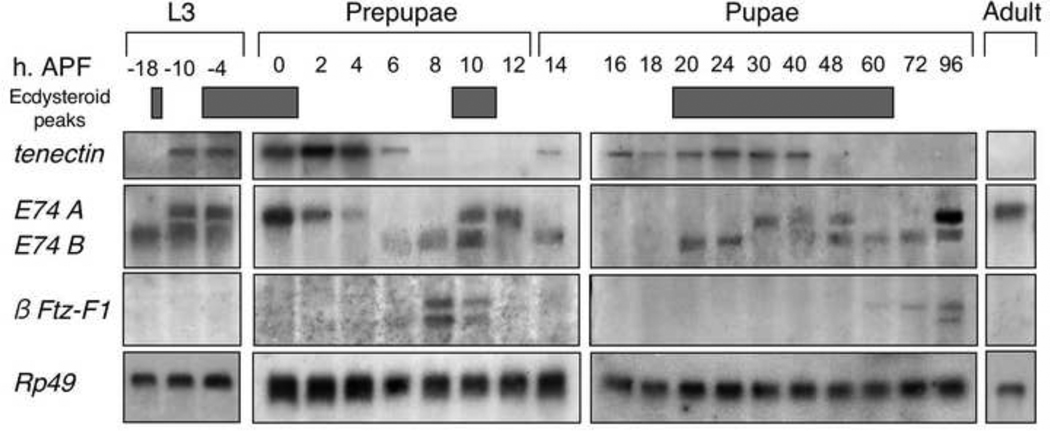
The tenectin homolog in Tenebrio molitor, tenebrin, is regulated by juvenile hormones and ecdysteroids at each molt (Royer et al., 2004). To ask if the expression of tenectin is similarly developmentally regulated during Drosophila metamorphosis, Northern blots of total mRNA from staged third instar larvae, prepupae, pupae and unstaged adults were hybridized with a probe derived from the exon 5 shared by the two tenectin transcripts. tenectin transcripts are not detectable 18 hours prior to puparium formation and then increase in the next 8 hours and reach a peak level at 2 hours after puparium formation (APF). Transcript levels then decline to a low level by 6 hours APF. Subsequently, the level of tenectin mRNA increases to a second peak during the early pupal period and to a third peak during the mid-pupal period (Fig. 2). These peaks are correlated with peaks in ecdysone titers (Fig. 2) (Richards, 1981; Handler, 1982; Warren et al., 2006). By reprobing the Northern blot we compared the expression of tenectin with that of previously described ecdysone responsive genes E74 and β-FTZ-F1 (Andres et al., 1993; Thummel, 1996; Thummel, 1997). The early expression pattern of tenectin is very similar to E74A, a class II early response gene. The second and third tenectin expression phases have some characteristics of the class I early response gene, E74B. Expression of both rises at 14 hours APF and again at 20 hours. As β-FTZ-F1 expression is repressed by ecdysone, its expression defines periods of low ecdysone. β-FTZ-F1 expression, and by extension low ecdysone, identifies the stages when tenectin is not expressed (hours APF 6–12 and 60–96). Though tenectin expression is complicated, the Northern data on whole flies are consistent with it being an ecdysone regulated gene in Drosophila just as tenebrin is in Tenebrio.
Fig. 2.
Developmental profile of tenectin expression. Northern blot hybridization of RNA isolated from staged late third instar larvae, prepupae, pupae and unstaged adults. The probe used for hybridization was prepared from the common fifth exon. The blot was reprobed to detect an early gene (E74) and a prepupal gene (β-Ftz-F1). Previously identified peaks in ecdysone titer are shown (Richards, 1981; Handler, 1982; Warren et al., 2006). Developmental times are shown on top, in hours after puparium formation (APF). Hybridization to detect rp49 mRNA (O’Connell and Rosbash, 1984) was used as a control for loading and transfer. This experiment was performed twice with very similar results (data not shown).
tenectin transcription is inducible by ecdysone
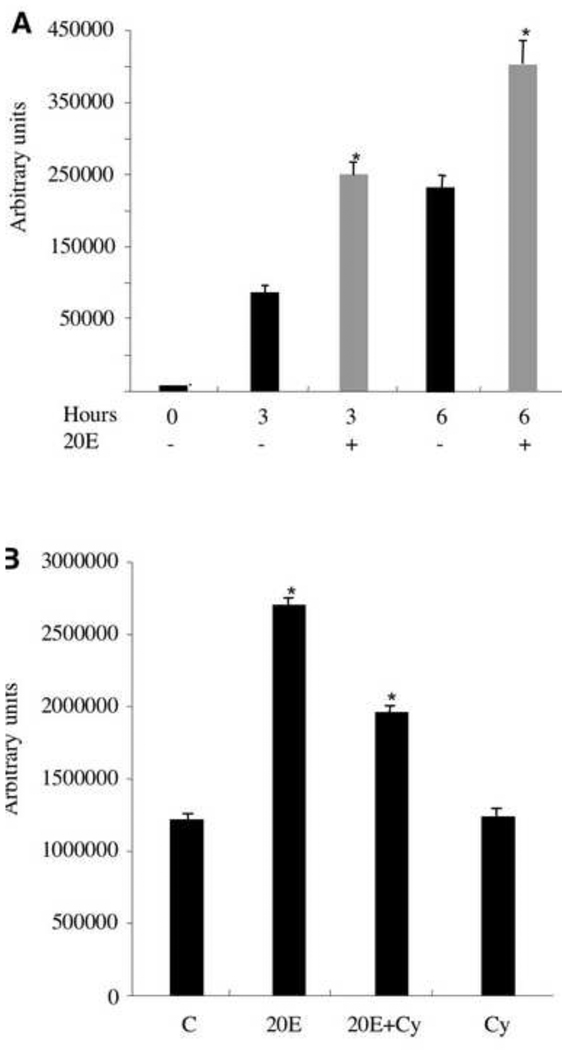
To demonstrate directly that tenectin expression is regulated by ecdysone, we analyzed the transcription of tenectin in mass-isolated third instar larval organs cultured for 0, 3 and 6 hours in the absence or presence of physiological levels of 20-hydroxyecdysone (20E) (Fig. 3A). In the absence of added 20E, tenectin mRNA levels rise dramatically at 3 and 6 hours following dissection (black bars). This increase in tenectin mRNA is likely due to the larval organs responding to the pulse of ecdysone that took place prior to dissection (at 16 hours prior to puparium formation (see Fig. 2). The addition of 20E further increases tenectin mRNA levels at both 3 and 6 hours following dissection (grey bars Fig. 3A) suggesting that the tenectin promoter is activated directly by the high titer 20E pulse that triggers puparium formation. To further address this possibility, we assessed the effect of cycloheximide on the stimulation of tenectin expression by 20E. Mass-isolated late third instar larval organs were cultured for 6 hours with 20E alone, 20E and cycloheximide, or cycloheximide alone (Fig. 3B). In the presence of physiological levels of 20E, tenectin mRNA was increased twofold by 6 hours. tenectin transcription was also induced by 20E in the presence of cycloheximide, although to reduced levels. These results indicate that tenectin expression is directly inducible by 20E but that protein synthesis is required to obtain the full induction. This would suggest that tenectin is a early late, or a type II early, ecdysone responsive gene (Stone and Thummel, 1993).
Fig. 3.
Stimulation of tenectin transcription by 20E. (A) tenectin RNA levels are shown for mass isolated third instar larval imaginal discs maintained in culture without added hormone (black bars) or treated with 5 × 10−6 M 20E (grey bars) for 0, 3 or 6 hours. (B) tenectin mRNA levels in larval organs cultured for 6 hours in the absence (C) or presence of 20E alone, 20E and cycloheximide (20E+Cy), or cycloheximide (Cy) alone. Total RNA was analyzed by Northern blot hybridization and tenectin mRNA was quantified by volume integration densitometry. Hybridization to detect rp49 mRNA (O’Connell and Rosbash, 1984) was used as a control for loading and transfer. Error bars are s.e.m. and asterisks indicate significant differences from control using Student’s t test (P<0.01).
tenectin is widely expressed in imaginal discs and brain
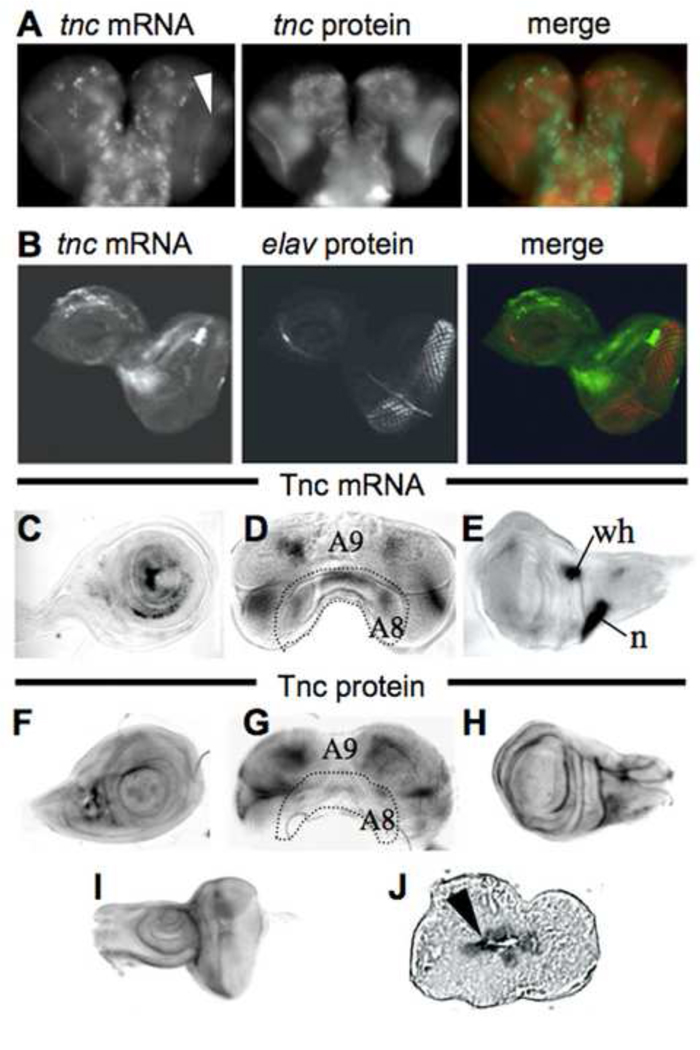
If tenectin is important to the hormonally regulated developmental processes of metamorphosis, it should be expressed in imaginal discs at this time. To determine the spatial expression of tenectin at the beginning of metamorphosis, tenectin mRNA and protein distributions were determined in a variety of imaginal discs and brains dissected from late third instar larvae. tenectin mRNA is detected in all of these structures. In the brain, tenectin transcript is expressed in voluminous cells of thoracic and abdominal ganglia and in small cells of the optic lobes corresponding to the lamina region (Fig. 4A; arrowhead). tenectin mRNA is detected in the eye-antennal (Fig. 4B), leg (Fig. 4C), male genital (Fig. 4D) and wing (Fig. 4E) discs. In the eye disc, tenectin mRNA is expressed in undifferentiated cells, anterior to the morphogenetic furrow as shown by the result of a double labeling for tenectin mRNA and Elav antibodies (Fig. 4B). In the male genital disc, tenectin mRNA is localized regions derived from A8 and A9 segments (Fig. 4D). In the wing disc, tenectin mRNA is strongly expressed in the wing hinge and in the notum (Fig. 4E). Tenectin protein is localized to the entire neuropil of the CNS (Fig. 4A) and later, it is found at the surface of the two prepupal wing layers (Fig. 4J). As expected for an ECM protein, tenectin’s distribution is wider than its mRNA expression (Fig. 4F–I). These results indicate that just prior to, or during, metamorphosis tenectin is widely expressed on most discs.
Fig. 4.
tenectin is expressed in brain and imaginal discs during post-embryonic development. tenectin nucleic acid or antibody probes were hybridized to brain and discs dissected from late third instar larvae (A–I). Brain (A) and eye-antennal disc (B) stained for tenectin transcript (green) and tenectin protein (red: A) or elav protein (red: B). Cells of the optic lobes corresponding to the lamina region expressing tenectin transcripts are indicated (arrowhead in A). Leg (C), male genital (D) and wing (E) discs probed for tenectin transcript. Leg (F), male genital (G) wing (H) and eye-antennal discs (I) probed for tenectin protein. Prepupal wing disc, transversal cut, stained for tenectin protein (J) showing its localization to the apposed surfaces of the dorsal and ventral wing layers (arrowhead). Regions of the male genital discs corresponding to different abdominal segment cells (Casares et al., 1997) are indicated (D and G). n: notum; wh: wing hinge.
RNAi tenectin knockdown mutants
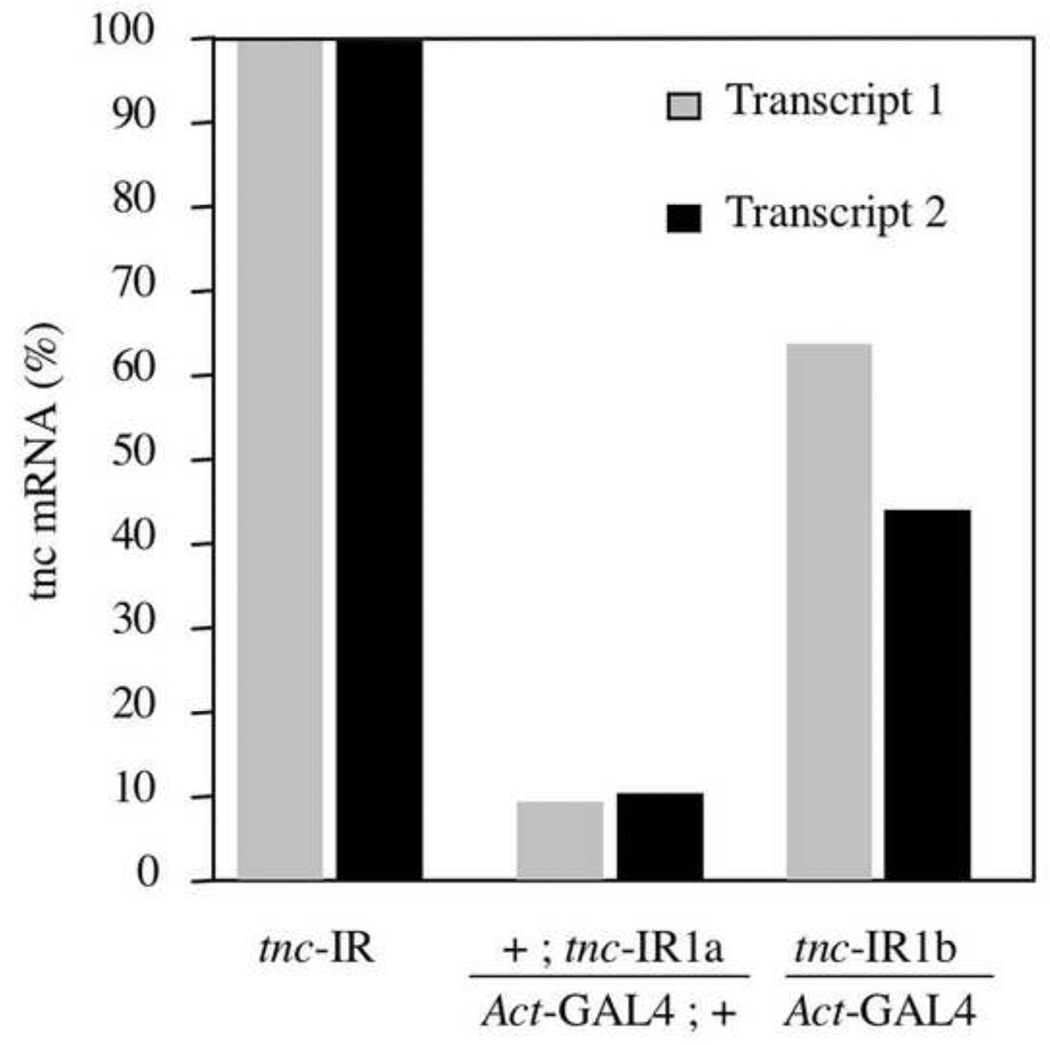
To begin a genetic analysis of tenectin’s in vivo functions, in the absence of mutations in the tenectin gene, we used RNA interference (RNAi) to reduce tenectin expression. Transgenic lines were produced, expressing a 1kb hairpin loop RNA under the control of the UAS/GAL4 system. An inverted repeat sequence was constructed from the large fifth exon of tenectin (Fig. 1A) in the region encoding the N-terminus of the protein. Two transgenic lines, tnc-IR1a, tnc-IR1b, were chosen that gave adult escapers with phenotypes when the tenectin-hairpin loop genes were driven by the expression of the ubiquitous Actin5c-GAL4 (Act-GAL4) and daughterless-GAL4 (da-GAL4) drivers. We confirmed by Northern blot analysis (data not shown) and by Q-PCR that expression of IR1 in tnc-IR1a and tnc-IR1b leads to a significant decrease of tenectin transcript. Tnc-IR1a in combination with an Act-GAL4 results in a 90% reduction in both tenectin transcripts while tnc-IR1b results in only a 50% reduction (Fig. 5).
Fig. 5.
tenectin mRNA is reduced in flies expressing tnc–IRs. tenectin transcript levels were quantified by real time PCR in staged third instar larvae heterozygous for tnc-IR1a or tnc-IR1b and these levels were set as 100% for each line (tnc-IR). Levels of both transcripts produced from the tenectin gene were measured and compared with the levels in flies carrying one copy of tnc-IR1a (or tnc-IR1b) and one copy of the Act-GAL4 driver.
Tnc-IR1 expression results in lethality, and the level of lethality correlates with the effectiveness of the hairpin loop genes. Tnc-IR1a driven by Act-GAL4 is 90% (n=205) lethal while tnc-IR1b is only 35% (n=145) lethal (Table 1). Comperable values are seen when these hairpin loop genes are driven by the da-GAL4 driver (Table 1). The reduced viability is due to death during several phases of development with a large proportion of embryonic lethality (~75% for tnc-IR1a and ~20% for tnc-IR1b). Adult escapers are lethargic and unable to jump or fly, and a large number die within one or two days after eclosion. Morphological defects of male genitalia and wings are common.
Table 1.
tnc-IR induced lethality
| Genotype | Lethality |
|---|---|
| tnc-IR1a/+ ; Act-GAL4/+ | 90% (n=205) |
| tnc-IR1a/+ ; Da-GAL4/+ | 80% (n=180) |
| tnc-IR1b/Act-GAL4 | 35% (n=145) |
| tnc-IR1b/Da-GAL4 | 36% (n=175) |
tenectin and integrin mutants display male genitalia anomalies
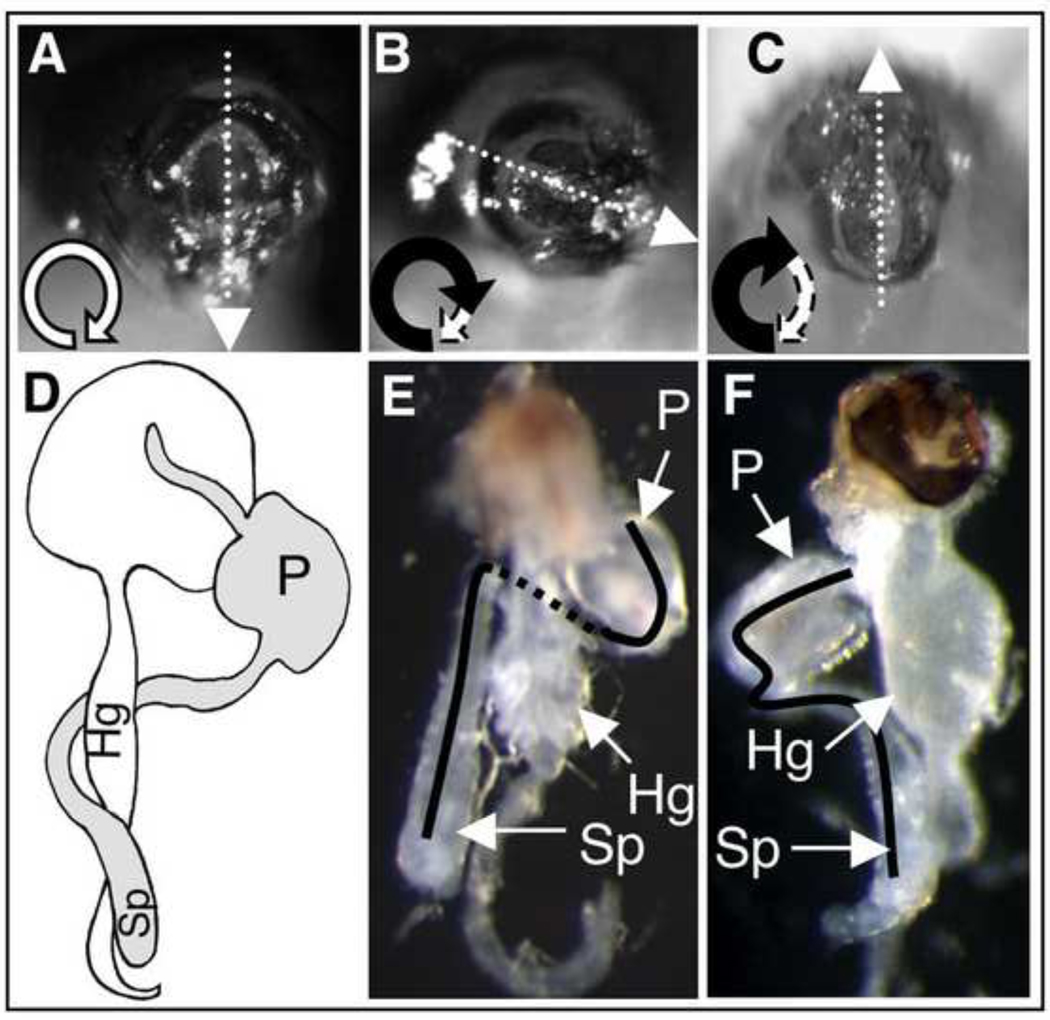
When the tnc-IR1b line was crossed to Act-GAL4, 72% of the adult male progeny showed a characteristic malrotation of genitalia (Table 2; Fig. 6B) by up to 180°. Similar experiments with tnc-IR1b crossed to da-GAL4 (Table 2), or tnc-IR1a crossed to Act-GAL4 (not shown), also resulted in adult male progeny displaying malrotation of genitalia. Correct positioning of the male genitalia takes place during metamorphosis. At this stage, the distal part of the male reproductive apparatus, the genital plate, undergoes a stereotyped 360° clockwise rotation, inducing the spermiduct to loop around the gut in a clockwise direction (Fig. 6C) (Gleichauf, 1936). Because external malrotation does not allow discrimination between under-, hyper- or counter-rotation of the genitalia, mutant males were dissected and the looping of their spermiduct analyzed. All dissected males expressing tnc-IR1 showed a clear under-rotation phenotype (Fig. 6F). Our results demonstrate that tenectin is required for the genital plate and spermiduct to undergo complete looping, but has no role in directionality.
Table 2.
Malrotated genitalia in tnc-IR and mys males
| Genotype | Rotated Genitalia |
|---|---|
| tnc-IR1b/Act-GAL4 | 72% (240) |
| tnc-IR1b/Da-GAL4 | 54% (218) |
| tnc-IR1b/discs-Gal4 | 48% (100) |
| tnc-IR1b/elav-GAL4 | 0% (95) |
| tnc-IR1b/Act-GAL4; EY16369/+ | 20% (140) |
| mysb13 28°C* | 10% (231) |
| mysb47 28°C* | 16% (219) |
| mysb69 28°C* | 20% (129) |
| mysb13 22°C | 2% (179) |
| mysb47 22°C | 0% (360) |
| mysb69 22°C | 1% (114) |
| if3 22°C | 0% (262) |
| mysb13, if3 22°C | 100% (41) |
To avoid lethality at 28°C, flies were raised at 18–22°C through first or second instar larvae and then shifted to 28°C prior to pupal development. Number of flies examined (n) is given in parentheses.
Fig. 6.
Rotation of genitalia and spermiduct looping in tnc–IR and mysb13 males. (A) Image of a wild type male external genitalia (posterior view with dorsal upwards), showing the position of the anus and penis. The direction and extent of genitalia rotation is schematized by a looping arrow (bottom left). (B and C) Images of a representative tnc–IR and mysb13 males showing genitalia malrotation. (D) Schematic representation (Ádám et al., 2003) and (E) dissected wild type male abdomen showing the rightward (when viewed from the posterior) looping of the spermiduct. (F) Dissected tnc–IR male abdomen with under-rotation phenotype. sp, spermiduct; g, gut; p, penis.
Under-rotation of the genitalia can be due to an abnormal neuroendocrine function, due to mutation of the adhesion molecule fasciclin2, leading to an elevated level of juvenile hormone. The function of fasciclin2 is required in the nervous system and mutants are rescued by ectopic expression of wild type fasciclin2 protein promoted by the neuronal-specific elav-GAL4 driver (Ádám et al., 2003). As tenectin is also expressed in the CNS (Fig. 4), we asked whether tenectin function is required in the nervous system or in the genital disc for correct genital rotation. When expression of the tnc-IR1 transgene is restricted to the imaginal discs using the discs-GAL4 driver, the genitalia undergo incomplete rotation (Table 2). In contrast, and when the tnc-IR1 transgene is expressed in neuronal cells, using the elav-GAL4 driver, the male genitalia were unaffected (Table 2). Taken together, these results suggest that the phenotype observed is due to abnormal tenectin expression in the genital disc and that tenectin expression is not required in the neuroendocrine system for correct genital rotation.
We are proposing that tenectin is a new PS2 integrin ligand and one potential integrin mutant previously displayed malrotation of male genitalia (Deak et al.,1982). Unfortunately, that allele was never molecularly characterized and no longer exists. Therefore, we assayed our collection of myospheroid (mys; βPS integrin subunit) function-altering mutations (Jannuzi et al., 2004) for their effects on male genitalia. Three alleles mysb47, mysb69 and mysb13 gave males with under-rotated genitalia by up to 180° (Fig. 6C, Table 2). The frequency of rotation defects is dependent on temperature as it is reduced when flies are reared at 22°C. To ask specifically if PS2 integrins are required for this process we combined the inflated (if; αPS2 integrin subunit) hypomorphic allele if3 (Lindsley and Grell, 1968 ; Brower and Jaffe, 1989; Wilcox et al., 1989), with mysb13. if3 males display no under-rotated genitalia and mysb13 males diplay slightly under-rotated genitalia at a frequency of just 2% at 22°C. mysb13, if3 males have under-rotated genitalia at a frequency of 100% (Table 2) and these are typically very extreme with 61% (n=41) of them being greater than 180° under-rotated. Thus, in addition to the extracellular matrix molecule tenectin, its proposed cell surface integrin receptor is required for proper looping morphogenesis of male genitalia.
tenectin mutants display wing defects and interact with integrin mutants
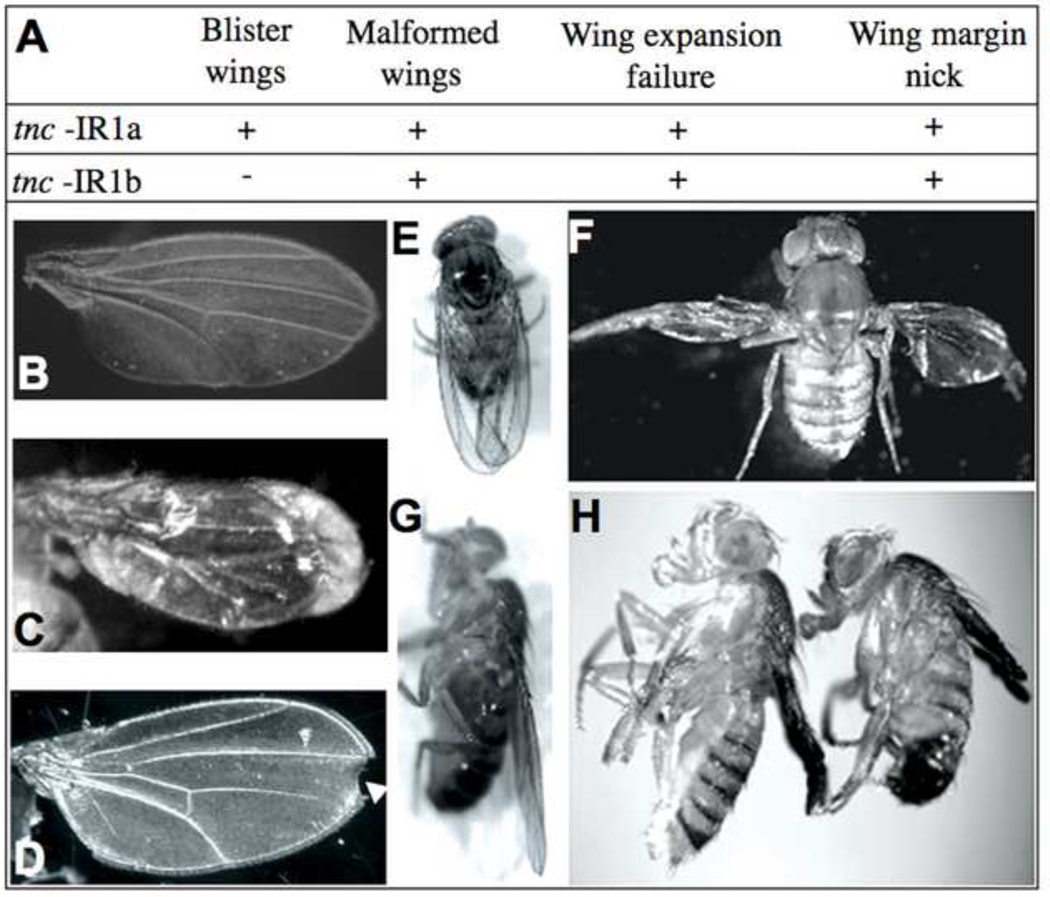
tenectin also has a role in wing morphogenesis. tnc-IR; Act-GAL4 transheterozygous adults exhibit multiple wing defects. Depending on the specific transgene used to inhibit tenectin expression the range of defects observed included wing blisters, failure of wing expansion, wing margin nicking, and malformed wings (Fig. 7). tenectin wing phenotypes resemble the phenotypes associated with mutations in integrins or their ligands (Brower and Jaffe, 1989; Brabant et al., 1993; Wilcox et al., 1989; Wehrli et al., 1993; Zusman et al., 1993; Brower et al., 1995; Bloor and Brown, 1998; Henchcliffe et al., 1993; Martin et al., 1999; Bunch et al., 1998). To assess whether tenectin and integrins function together to ensure proper wing morphogenesis, we tested whether tenectin mutations could enhance the wing blister phenotypes associated with a viable hypomorphic βPS integrin mutation, mysnj42 (Costello and Thomas, 1981). This allele has been used for several genetic interaction studies of cell adhesion (Wilcox et al., 1989; Prout et al., 1997; Schöck and Perrimon, 2003). Tnc-IR1b/Act-GAL4 flies do not produce wing blisters and hemizygous mysnj42 display a low frequency of wing blisters (10%). In mysnj42 flies, tnc-IR1b/act-Gal increased the frequency of blistering by approximately fourfold and the overall frequency of wing defects by sevenfold (Table 3). Taken together, these results suggest that tenectin and integrins function in a common pathway during wing morphogenesis.
Fig. 7.
Tnc-IRs knockdowns exhibit wing defects. Table (A) shows the different classes of wing defects exhibited by tenectin RNAi lines. Dorsal views of adult wings (B–F) either wild type (B and E) or double heterozygous for tnc-IR and Act-GAL4 (C, D and F). Lateral views of wild type (G) or transheterozygous for tnc-IR and Act-GAL4 (H). tenectin mutants exhibit wing defects: blistered wings (C), malformed wings (F), wing expansion failure (H), and wing margin nicking (D).
Table 3.
Wing defects in tnc-IR and mys males
| Genotype | BW | WD | n= |
|---|---|---|---|
| mys nj42/Y | 10% | 10% | 104 |
| tnc-IR1b/Act-GAL4 | 0% | 15% | 240 |
| mys nj42/Y; tnc-IR1b/Act-GAL4 | 45% | 68% | 29 |
BW; blistered wings, WD; wing defects
tenectin RNAi and mysnj42 rescue ectopic tenectin expression
Overexpression of tenectin results in defects that can be rescued by tenectin RNAi or mysnj42. An Epgy2 transposable element, EY16369, has been found inserted between the two tenectin transcription initiation sites (Fig. 1; Bellen et al., 2004). This transposable element contains GAL4-UAS sequences allowing tenectin to be overexpressed by GAL4 drivers. Combining the ubiquitous GAL4 driver, Act-GAL4, and EY16369 resulted in 92% lethality (Table 4). Of the adult escapers, 66% (n=33) showed wing defects characterized by an absence of wing expansion and a large number die within one or two days after hatching. No male genitalia defects were observed.
Table 4.
Rescue of tenectin overexpression
| Genotype | Lethality | WD |
|---|---|---|
| +/Act-GAL4; EY16369/+ | 92% (437) | 66% (33) |
| tnc-IR1b/Act-GAL4 | 35% (145) | 10% (462) |
|
tnc-IR1b/Act-GAL4; EY16369/+ |
23% (368) | 6% (283) |
| mys nj42/Y; Act-GAL4/+ | 24% (156) | ND |
|
mys
nj42/Y; Act-GAL4/+; EY16369/+ |
25% (201) | ND |
WD; wing defects, ND ; not determined.
Number of flies examined (n=) is given in parentheses.
Tnc-IR1b, which reduces tenectin expression, shows partial rescue of the tenectin overexpression by EY16369. Tnc-IR1b/Act-GAL4; EY16369/+ are only 23% lethal (Table 4). Also, the overexpression by EY16369 reduced the genitalia and wing defects of Tnc-IR1b (Table 2 and Table 4). To ask if the lethality of tenectin overexpression was due to integrin-mediated processes we combined EY16369 and mysnj42. The 92% lethality of males expressing EY16369 is reduced to only 25% by the presence of mysnj42 (Table 4).
Tenectin mediates cell spreading and adhesion via αPS2βPS integrins
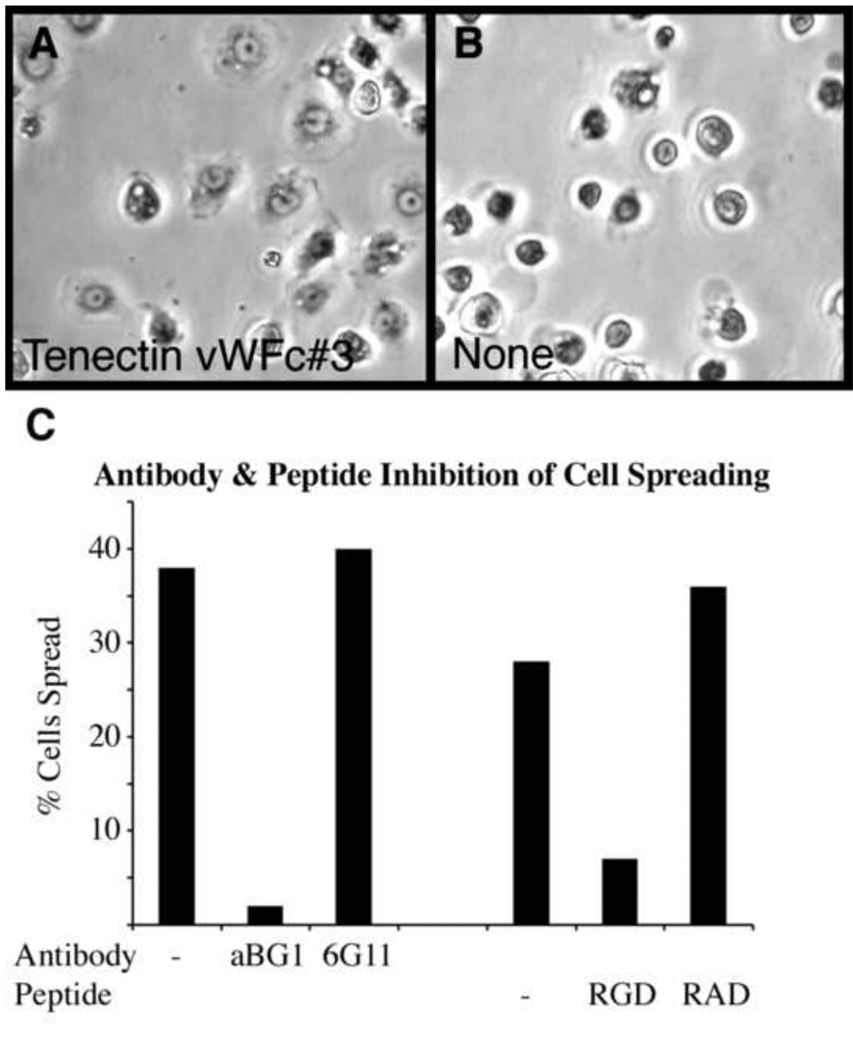
Tenectin’s RGD integrin binding motif coupled with the phenotypic and genetic interaction data strongly suggest that tenectin is an extracellular αPS2βPS integrin ligand. To confirm this, we tested the ability of αPS2βPS to mediate spreading of S2 cells on plates coated with recombinant tenectin. As reported for other RGD-containing ligands (Graner et al., 1998), tenectin supports the spreading of these cells efficiently (Fig. 8A). Cells that are not transformed with integrins displayed no spreading (not shown). To further demonstrate the integrin requirement for tenectin-mediated spreading we blocked cell spreading with a monoclonal antibody, aBG1, that is known to block the function of βPS integrins (Fig. 8C). That tenectin interacts with the RGD-binding domain of integrin is supported by the ability of a soluble competing peptide, GRGDSP, to inhibit cell spreading on tenectin (Fig. 8C).
Fig. 8.
Tenectin supports PS2 integrin-mediated cell spreading. (A) Phase contrast microscopy of cells transformed to express PS2m8 integrins cells spreading on tenectin VWC#3. (B) In the absence of ligand the cells remain round and unspread. The parental S2/M3 cells, which do not express PS2 integrins, show no spreading on tenectin VWC#3 (not shown). (C) The anti-βPS integrin function blocking antibody aBG1 inhibits cells spreading on tenectin VWC#3 while the control anti-βPS integrin CF.6G11 has no effect. Both antibodies were purified and used at a concentration of 15 µg/ml. 1.5 mg/ml of an integrin inhibitory peptide GRGDSP (RGD) inhibits cell spreading on tenectin VWC#3 while the same concentration of a control peptide GRADSP (RAD) has no effect.
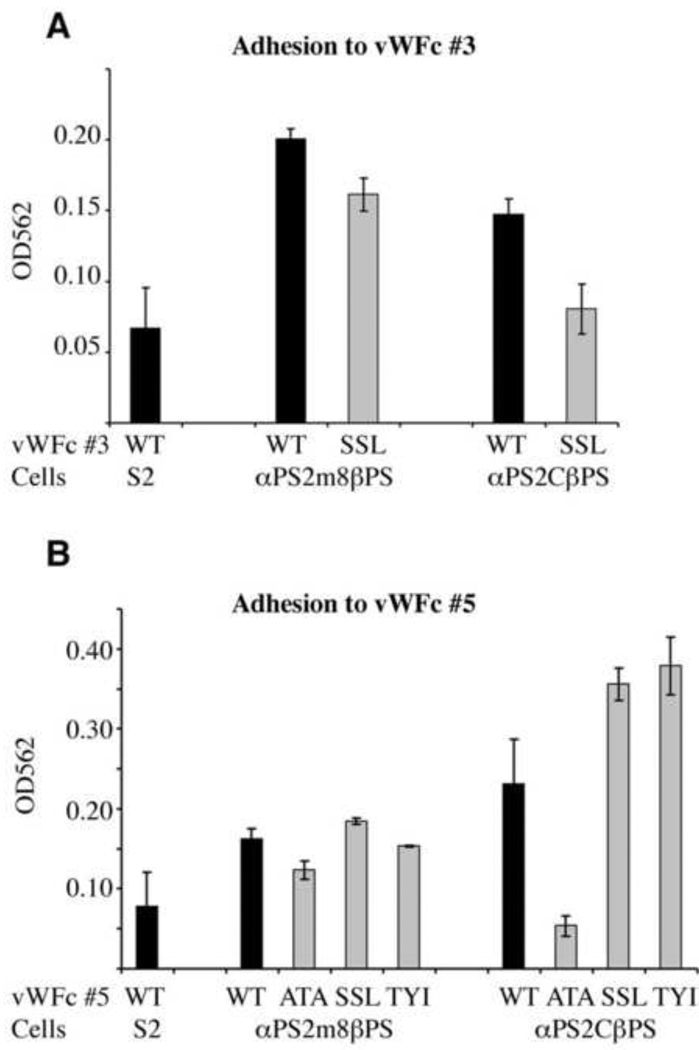
The recombinant tenectin VWC#3 used in the cell spreading studies contains the integrin binding RGD motif. VWC#5 repeat has an RSD sequence in the same location as the RGD of VWC#3. VWC#5 also contains two other potential integrin binding motifs, RDD and RYE (Fig. 1C). Additional complexity in PS2 integrin-ligand interactions arises due to the presence of two isoforms of the αPS2 integrin, αPS2m8 and αPS2c, that are present in flies and display differences in their interactions with ligands (Graner et al., 1998). To examine the importance of VWC#3 and VWC#5 and their individual binding motifs to both isoforms of PS2 integrins, we performed cell adhesion assays. Untransformed cells (S2) not expressing PS2 integrins displayed low levels of adhesion to fusion proteins containing either VWC#3 or VWC#5 and this was increased by the expression of either PS2 integrin isoform (Fig. 9A,B, black bars). Thus, two widely separated VWC domains have the ability to promote cell adhesion via both forms of PS2 integrins.
Fig. 9.
Tenectin VFC#3 andVFC#5 support PS2-mediated cell adhesion. Untransformed (S2) cells or the same expressing PS2m8 (αPS2m8βPS) and PS2c (αPS2cβPS) integrins were allowed to adhere to tenectin VWC#3 (A) or VFC#5 (B). The tenectin fusion proteins used were either wild type (WT) or the same whose potential integrin binding motifs had been mutated; RGD or RSD>SSL (SSL in A and B respectively); RDD>ATA (ATA); RYE>TYI (TYI). Adhesion was defined by the number of cells remaining attached after washing, 20 minutes after settling on the plate. The number of cells was determined by staining the cells with crystal violet and dye levels were determined using a microplate reader. To obtain tenectin dependent adhesion values, background adhesion observed in wells coated with BSA was subtracted. Three wells were scored for each ligand and the values are the mean ± s.e.m. of these three values. Differences between S2 cells and PS2m8 cells on VWF#3, between both PS2m8 cells and PS2c cells on VWF#3 wild type verses RGD>SSL, and PS2c cells on wild type VWF#5 verses RDD>ATA were significant (P< 0.05). Consistent, but less dramatic, were the differences between PS2c cells and S2 cells on VWF#3 (P=0.06), PS2c cells and S2 cells on VWF#5 (P=0.10), PS2m8 cells on wild type VWF#5 verses RDD>ATA (P=0.09), PS2m8 cells and S2 cells on VWF#5 (P=0.13).
To confirm the importance of the RGD motif in VWC#3 and to explore potential binding motifs in VWC#5, fusion proteins with mutated motifs were tested. The RGD sequence of VWC#3 was mutated to SSL. VWC#5 motifs were mutated as follows; RDD>ATA, RSD>SSL, and RYE>TYI. ATA, SSL and TYI were chosen as substitutions for the potential integrin binding motifs due to their presence at identical locations in VWC#4 (Fig. 1C). This makes it unlikely that the substitutions will cause structural changes to the VWC domains. Significantly reduced adhesiveness was found when VWC#3 RGD motif was mutated to SSL confirming a role for the RGD motif in cell adhesion (Fig. 9A). Mutation of the 3 potential motifs in VWC#5 gave varied results. Two of the potential motifs, RSD and RYE, showed no evidence for promoting adhesion, as mutating them to SSL and TYI did not decrease adhesion. For the PS2c integrin expressing cells, mutations of the RSD and RYE sequences increased adhesion (Fig. 9B) suggesting that RSD and RYE might interfere with adhesion to VWC by PS2c integrins. In contrast, RDD>ATA reduced adhesion by both PS2 integrin-expressing cell lines. Thus, RDD can serve as an integrin-binding motif in VWC#5. PS2c integrin-mediated adhesion is reduced to levels similar to untransformed S2 cells by mutations of either the RGD or the RDD motif. For cells expressing PS2m8 the effects of these mutations are significant but not as dramatic.
Discussion
tenectin mutants
Tenectin is a protein localized to the ECM during Drosophila embryonic development (Fraichard et al., 2006). The presence of an integrin binding RGD motif led us to speculate that tenectin could be a new integrin ligand. To study the function of tenectin during Drosophila development, we generated tenectin knockdowns by RNA interference (Fortier and Belote, 2000; Kennerdell and Carthew, 2000; Lam and Thummel, 2000). Two strains of tenectin knockdown flies were selected that gave visible hypomorphic phenotypes. We also characterized flies that give phenotypes due to overexpression of the endogenous tenectin gene. Lowering mRNA level by RNAi partially rescued the effects of tenectin over-expression and over-expression of tenectin partially rescues tenectin knockdown phenotypes. Thus, we are confident that our tenectin knockdown phenotypes result specifically from reduced tenectin expression.
Tenectin is a new ligand of αPS2βPS in wing epithelia
Lethality is the most prevalent phenotype displayed by ubiquitous reduction in tenectin expression but in this study we have focused on adult phenotypes to ascertain tenectin’s function in morphogenetic processes of metamorphosis. The most striking adult phenotype observed in adult flies with reduced tenectin expression is deformed wings including blisters, nicks, lack of expansion and malformation. These phenotypes resemble those associated with mutations in integrin subunits (Brower and Jaffe; 1989, Wilcox et al. 1989; Zusman et al., 1990; Brabant et al., 1993), their extracellular ligands (Henchcliffe et al., 1993; Bunch et al., 1998; Martin et al., 1999), and genes encoding intracellular proteins that interact with integrins (Bökel and Brown, 2002; Brower, 2003 for reviews). Three lines of evidence support tenectin functioning as a PS integrin ligand to facilitate wing morphogenesis. First, we find tenectin protein localized between the dorsal and ventral epithelial cell layers in pupal wings. Integrins function at this location to promote adhesion of these cell layers (see Brower, 2003 for a review). Second, a mutation of mys, encoding the βPS subunit, interacts genetically to increase the frequency of blisters in flies with reduced tenectin expression. Finally, in vitro experiments demonstrate that tenectin, through multiple RGD motifs, can function to promote αPS2βPS-mediated cell spreading and adhesion. Taken together, these genetic and biochemical data provide strong evidence that tenectin is a new ligand of αPS2βPS integrin in the wing.
Perhaps relevant to tenectin’s function in the wing, Syed et al. (2008), using a bioinformatics approach, identified tenectin as being a mucin-related-protein. In our analysis of the tenectin protein we also notice mucin like repeats. Mucins are highly hydrated O-glycosylated macromolecules that are important to the mucosal lining of mammalian organs. In addition to serving a protective function, various mucins interact with growth factors and cell surface receptors to modulate signaling. It has been shown in vertebrates that mucins also modulate cell adhesion. For example, MUC4 was found to sterically reduce the accessibility of integrins to extracellular matrix ligands and thereby interfere with adhesion (Hollingsworth and Swanson, 2004; Chaturvedi et al., 2007 and 2008). Interestingly, Zhang et al. (2008) have recently shown in Drosophila that a mucin-type glycosyltransferase, PGANT3, glycosylates another PS2 integrin ligand, tiggrin. Moreover, mutation of the pgant3 gene results in a wing-blistering phenotype. In the developing wing disc PGANT3 glycosylates tiggrin and other matrix molecules, thus potentially modulating cell adhesion through integrin-ECM interactions. Future biochemical experiments will be needed to determine if tenectin is a bona fide mucin, glycosylated by PGANT3, and whether glycosylation down- or up-regulates its adhesive function.
tenectin expression is regulated by 20E during metamorphosis
The formation of the flat bi-layered wing from a folded imaginal disc involves several steps of apposition and separation of the ventral and dorsal epidermal sheets followed ultimately by an epithelial to mesenchymal transition and migration of the cells out of the wing (Waddington, 1941; Johnson and Milner, 1987; Fristrom et al., 1993; Brabant et al., 1996; Kiger et al., 2007). The resulting wing is predominantly two layers of cuticle cemented together by ECM. These studies point out the importance of regulating the adhesive properties of the wing epidermal cells by modulating the activity of integrins and their intracellular and extracellular binding partners. One mode of regulation is at the transcriptional level and several studies have demonstrated that the hormone 20E plays an important role in regulating at least some of these morphogenetic events including integrin expression levels (Fristrom et al., 1993; D’Avino and Thummel, 2000). Consistent with tenectin’s role in wing morphogenesis we find that during metamorphosis tenectin mRNA expression correlates with the ecdysone titer profile. In vitro, imaginal disc cultures demonstrate that tenectin is a 20E target gene. The comparison of the developmental tenectin expression profile with those of early (E74A, E74B) and prepupal (β-Ftz–F1) genes defined more precisely the temporal expression pattern of tenectin. E74B is a class I transcript, induced in mid-third instar larvae in response to a low concentration of 20E and repressed at higher ecdysone concentrations. In contrast, the class II transcripts, including E74A are induced by high 20E concentration and their expressions are unaffected by higher 20E concentrations (Karim and Thummel, 1992). The temporal profile of tenectin is similar to those of E74A, with a slight delay in the peak levels of tenectin mRNA accumulation. This temporal delay in tenectin is similar to the delay observed in the early-late gene profiles. The early-late genes appear to share properties with both the early genes and late genes (Stone and Thummel, 1993). Early-late genes respond directly to ecdysone even in the presence of protein synthesis inhibitors like cycloheximide but unlike early genes their full induction requires protein synthesis due to a requirement for other ecdysone induced gene products. We propose that tenectin is an early-late gene as its expression in cultured larval organs was induced by 20E in the presence of cycloheximide but maximal induction required protein synthesis. In the wing, we propose that 20E also regulates morphogenesis by regulation of tenectin mRNA levels, suggesting that ecdysone controls wing morphogenesis and cell adhesion not only by regulating integrin expression but also their ECM ligand expression. Just as E74A and E75B do not display identical expression profiles, the tenectin expression pattern is complicated and likely involves additional modes of regulation that will need to be elucidated.
Tenectin and PS2 integrins are required for male genital disc rotation
Tenectin knockdown resulted in reduced rotation of male genitalia. Looping morphogenesis of the male genitalia occurs during the pupal stage as the genital disc undergoes a 360° dextral (clockwise) looping around the hindgut (Gleishauf, 1936). A variety of genes expressed in larval posterior abdominal segments A8, A9 and A10 have been identified that affect male genital rotation. These include genes encoding a signaling protein (Pvf1), a transcription factor (Taf1, formerly TAF250), and a pro-apoptosis gene (hid) (Casanova et al., 1986; Sanchez-Herrero and Crosby, 1988; Macías et al., 2004; Wassarman et al., 2000; Abbott and Lengyel, 1991; Grether et al., 1995). One adhesion molecule, fasciclin-2, was genetically demonstrated to be involved in genital rotation. However, the effect was indirect as Fas2spin mutant alters the synapses connecting neurosecretory cells to the organ that produces juvenile hormone (the corpora allata), and genitalia under-rotation is due to an excess of juvenile hormone (Ádám et al., 2003). These authors have demonstrated that the effects on genitalia rotation are mediated by an excess of juvenile hormone, a retinoic-like molecule, establishing a parallel between vertebrate and invertebrate left right asymmetry, since the retinoic acid is involved in the control of asymmetry in vertebrates (Spéder et al., 2007 for review). In Drosophila, excessive juvenile hormone may result in the attenuation of ecdysone regulated processes required for male genital rotation as mutations in Broad-Complex, an ecdysone early-response gene, also result in malrotation of male genitalia (Wilson et al., 2006). Mutations of the unconventional myosin 31DF gene (Myo31DF) have been shown to uniquely reverse the looping direction of genitalia (Hozumi et al., 2006; Spéder et al., 2006). Knockdown of tenectin in imaginal discs, but not in neuronal cells, resulted in incomplete rotation of the genitalia but not in direction of looping. Thus, we have for the first time identified a Drosophila ECM component required for genital looping morphogenesis.
The tenectin mutant phenotype in male genitalia prompted us to re-examine integrin hypomorphic mutations for a similar phenotype. Males bearing 3 different hypomorphic mutations in the gene encoding the βPS integrin subunit, mysb13, mysb47, and mysb69 (Jannuzi et al., 2004) displayed rotated male genitalia when raised at elevated temperatures. Deak et al. (1982) also described a mutation that was likely in myospheroid that produced rotated male genitalia when larvae and pupae were raised at elevated temperatures. Combining mysb13 with the if3 mutation in the gene encoding the αPS2 integrin subunit caused a dramatic increase in the expressivity of the rotated genitalia phenotype. Therefore, tenectin’s proposed cell surface adhesion receptor is also required for the execution of looping morphogenesis. In addition to adhesion, the PS integrins function in the regulation of intracellular signaling pathways and specifically the JNK pathway (Lee et al., 2003; James et al., 2007). JNK signaling pathway has also been suggested to function in apoptosis required for rotation of male genitalia (Macías et al., 2004). Thus, tenectin and PS integrin function in looping morphogenesis could be at the level of adhesion and/or signaling. Additional experiments are required to distinguish between these two models.
Tenectin has multiple integrin binding motifs
Tenectin’s RGD sequence in the 3rd VWC domain is conserved in the beetle homolog, tenebrin, and supported PS2 integrin mediated cell spreading. This result is expected given that RGD is a well known integrin-binding motif of the PS2 integrins. More novel is the presence in the identical location in the 5th VWC of the sequence RSD and elsewhere in this 5th repeat the occurrence of RDD and RYE sequences. The biological importance of the 5th VWC domain is supported by the extraordinary high degree of conservation in this domain between Drosophila tenectin and Tenebrio tenebrin. The two proteins share 92% (62/67) sequence identity in the 5th VWC repeat and this includes the RDD, RSD, and RYE sequences. To date, this domain is found conserved, with greater than 84% sequence identity, in mosquitoes, honey bees, crickets, wasps, the beetle, and aphids (not shown). While RGD is the best studied integrin-binding motif, experimental evidence is accumulating that variants of this sequence are also important. These variants include KQAGD, KGD, RSD, WGD, MVD and RYD found in fibrinogen, thrombospondin, tenascin-W, CD40, snake venom disintegrins, viral coat proteins, and ligand mimetic monoclonal antibodies (Springer et al., 2008; Subramanian et al., 2007; Meloty-Kapella et al., 2008; Prasad et al., 2003;Juarez et al., 2008; Van de Walle et al, 2008; Taub et al., 1989; Tomiyama et al., 1992; Hamidpour et al., 2006). Our cell adhesion assays demonstrate that VWC#5 as well as VWC#3 promotes cell adhesion mediated by PS2 integrins. Mutations of the individual RGD-variant motifs in VWF# 5 suggest that they have differing effects on different integrins. The RDD is required for strong adhesion by both the PS2m8 and PS2c integrin isoforms as mutation of this sequence reduced adhesion of cells expressing either integrin. To our knowledge, this is the first time the RDD tripeptide in an ECM protein has been found to function in integrin-mediated adhesion. It also appears that the RSD and RYE motifs may be inhibitory for adhesion mediated by the PS2c isoform as their mutations increased cell adhesion. With multiple integrin binding domains, both positive and inhibitory, tenectin potentially functions in multiple processes in development and specifically in metamorphosis.
Future experiments will be required to address the many unanswered issues regarding tenectin-PS integrin interactions including: which PS integrin(s) interact with tenectin in vivo; how the function of the motifs may be affected by the context of other ECM proteins; and how other regions of tenectin and modifications, such as glycosylation or cleavage, influence the functionality of the putative integrin binding motifs. The presence of multiple motifs also raises the possibility that tenectin can bridge integrins on neighboring cells, or on the surface of the same cell. Finally, the different motifs may be needed to bind different integrins at different times in development and this binding of different motifs may have different adhesive and/or signaling consequences.
Supplementary Material
Acknowledgments
We thank Alban Hourdry for assistance with the determination of the gene structure of tenectin. Danny Brower made comment and suggestions on early versions of the manuscript. We thank Marc Brabant for the original observation of rotated male genitalia in combinations of integrin mutants. We thank Developmental Studies Hybridoma Center (University Iowa) for providing antibodies and DrosDel project (University of Cambridge) for providing the deficiency line. This work was supported in part by the Centre National de la Recherche Scientifique (CNRS), the University of Burgundy and grants from the French ministry of Research and Education and from the FRM (Medical Research Foundation), the ARC (Cancer Research Association) and the NIH (R01GM42474).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott MK, Lengyel JA. Embryonic head involution and rotation of male terminalia require the Drosophila locus head involution defective. Genetics. 1991;129:783–789. doi: 10.1093/genetics/129.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ádám G, Perrimon N, Noselli S. The retinoic-like juvenile hormone controls the looping of left-right asymmetric organs in Drosophila. Development. 2003;130:2397–2406. doi: 10.1242/dev.00460. [DOI] [PubMed] [Google Scholar]
- Andres AJ, Fletcher JC, Karim FD, Thummel CS. Molecular analysis of the initiation of insect metamorphosis: a comparative study of Drosophila ecdysteroid-regulated transcription. Dev. Biol. 1993;160:388–404. doi: 10.1006/dbio.1993.1315. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Levis RW, Liao G, He Y, Carlson JW, Tsang G, Evans-Holm M, Hiesinger PR, Schulze KL, Rubin GM, Hoskins RA, Spradling AC. The BDGP gene disruption project: single transposon insertions associated with 40% of Drosophila genes. Genetics. 2004;167:761–781. doi: 10.1534/genetics.104.026427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloor JW, Brown NH. Genetic analysis of the Drosophila alphaPS2 integrin subunit reveals discrete adhesive, morphogenetic and sarcomeric functions. Genetics. 1998;148:1127–1142. doi: 10.1093/genetics/148.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bökel C, Brown NH. Integrins in development: moving on, responding to, and sticking to the extracellular matrix. Dev. Cell. 2002;3:311–321. doi: 10.1016/s1534-5807(02)00265-4. [DOI] [PubMed] [Google Scholar]
- Borchiellini C, Coulon J, Le Parco Y. The function of type IV collagen during Drosophila muscle development. Mech. Dev. 1996;58:179–191. doi: 10.1016/s0925-4773(96)00574-6. [DOI] [PubMed] [Google Scholar]
- Brabant MC, Brower DL. PS2 integrin requirements in Drosophila embryo and wing morphogenesis. Dev. Biol. 1993;157:49–59. doi: 10.1006/dbio.1993.1111. [DOI] [PubMed] [Google Scholar]
- Brabant MC, Fristrom D, Bunch TA, Brower DL. Distinct spatial and temporal functions for PS integrins during Drosophila wing morphogenesis. Development. 1996;122:3307–3317. doi: 10.1242/dev.122.10.3307. [DOI] [PubMed] [Google Scholar]
- Brower DL. Platelets with wings: the maturation of Drosophila integrin biology. Curr. Opin. Cell Biol. 2003;15:607–613. doi: 10.1016/s0955-0674(03)00102-9. [DOI] [PubMed] [Google Scholar]
- Brower DL, Bunch TA, Mukai L, Adamson TE, Wehrli M, Lam S, Friedlander E, Roote CE, Zusman S. Nonequivalent requirements for PS1 and PS2 integrin at cell attachments in Drosophila: genetic analysis of the alpha PS1 integrin subunit. Development. 1995;121:1311–1320. doi: 10.1242/dev.121.5.1311. [DOI] [PubMed] [Google Scholar]
- Brower DL, Jaffe SM. Requirement for integrins during Drosophila wing development. Nature. 1989;342:285–287. doi: 10.1038/342285a0. [DOI] [PubMed] [Google Scholar]
- Brower DL, Wilcox M, Piovant M, Smith RJ, Reger LA. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc. Natl. Acad. Sci. U. S. A. 1984;81:7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NH. Null mutations in the alpha PS2 and beta PS integrin subunit genes have distinct phenotypes. Development. 1994;120:1221–1231. doi: 10.1242/dev.120.5.1221. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Martin-Bermudo MD. Integrins as mediators of morphogenesis in Drosophila. Dev. Biol. 2000;223:1–16. doi: 10.1006/dbio.2000.9711. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Brower DL. Drosophila PS2 integrin mediates RGD-dependent cell-matrix interactions. Development. 1992;116:239–247. doi: 10.1242/dev.116.1.239. [DOI] [PubMed] [Google Scholar]
- Bunch TA, Graner MW, Fessler LI, Fessler JH, Schneider KD, Kerschen A, Choy LP, Burgess BW, Brower DL. The PS2 integrin ligand tiggrin is required for proper muscle function in Drosophila. Development. 1998;125:1679–1689. doi: 10.1242/dev.125.9.1679. [DOI] [PubMed] [Google Scholar]
- Cardamone M, Puri NK, Brandon MR. Comparing the refolding and reoxidation of recombinant porcine growth hormone from a urea denatured state and from Escherichia coli inclusion bodies. Biochemistry. 1995;34:5773–5794. doi: 10.1021/bi00017a009. [DOI] [PubMed] [Google Scholar]
- Casanova J, Sánchez-Herrero E, Morata G. Identification and characterization of a parasegment specific regulatory element of the abdominal-B gene of Drosophila. Cell. 1986;47:627–636. doi: 10.1016/0092-8674(86)90627-6. [DOI] [PubMed] [Google Scholar]
- Casares F, Sánchez L, Guerrero I, Sánchez-Herrero E. The geneital disc of Drosophila melanogaster. I. Segmental and compartmental organization. Dev. Genes Evol. 1997;207:216–228. doi: 10.1007/s004270050110. [DOI] [PubMed] [Google Scholar]
- Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. The FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi P, Singh AP, Moniaux N, Senapati S, Chakraborty S, Meza JL, Batra SK. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol. Cancer Res. 2007;5:309–320. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- Costello WJ, Thomas JB. Development of thoracic muscles in muscle-specific mutant and normal Drosophila melanogaster. Neuroscience Abstr. 1981;7:543. [Google Scholar]
- Coutelis JB, Petzoldt AG, Spéder P, Suzanne M, Noselli S. Left-right asymmetry in Drosophila. Semin. Cell Dev. Biol. 2008;19:252–262. doi: 10.1016/j.semcdb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- D'Avino PP, Crispi S, Polito LC, Furia M. The role of the BR-C locus on the expression of genes located at the ecdysone-regulated 3C puff of Drosophila melanogaster. Mech. Dev. 1995;49:161–171. doi: 10.1016/0925-4773(94)00313-c. [DOI] [PubMed] [Google Scholar]
- D'Avino PP, Thummel CS. The ecdysone regulatory pathway controls wing morphogenesis and integrin expression during Drosophila metamorphosis. Dev. Biol. 2000;220:211–224. doi: 10.1006/dbio.2000.9650. [DOI] [PubMed] [Google Scholar]
- Danen EH, Sonnenberg A. Integrins in regulation of tissue development and function. J. Pathol. 2003;201:632–641. doi: 10.1002/path.1472. [DOI] [PubMed] [Google Scholar]
- Deak II, Bellamy PR, Bienz M, Dubuis Y, Fenner E, Gollin M, Rähmi A, Ramp T, Reinhardt CA, Cotton B. Mutations affecting the indirect flight muscles of Drosophila melanogaster. J. Embryol. Exp. Morphol. 1982;69:61–81. [PubMed] [Google Scholar]
- Enerly E, Larsson J, Lambertsson A. Reverse genetics in Drosophila: from sequence to phenotype using UAS-RNAi transgenic flies. Genesis. 2002;34:152–155. doi: 10.1002/gene.10111. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Fessler LI, Bunch TA, Yaron Y, Parker CG, Nelson RE, Brower DL, Gullberg D, Fessler JH. Tiggrin, a novel Drosophila extracellular matrix protein that functions as a ligand for Drosophila alpha PS2 beta PS integrins. Development. 1994;120:1747–1758. doi: 10.1242/dev.120.7.1747. [DOI] [PubMed] [Google Scholar]
- Fortier E, Belote JM. Temperature-dependent gene silencing by an expressed inverted repeat in Drosophila. Genesis. 2000;26:240–244. doi: 10.1002/(sici)1526-968x(200004)26:4<240::aid-gene40>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Fraichard S, Bouge AL, Chauvel I, Bouhin H. Tenectin, a novel extracellular matrix protein expressed during Drosophila melanogaster embryonic development. Gene Expr. Patterns. 2006;6:772–776. doi: 10.1016/j.modgep.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Fristrom DR, Fristrom JW. The metamorphic development of the adult epidermis. In: Bate M, Martinez Arias A, editors. The development of Drosophila melanogaster. Vol. 2. New York: Cold Spring Harbor Laboratory Press; 1993. pp. 843–897. [Google Scholar]
- Fristrom D, Wilcox M, Fristrom J. The distribution of PS integrins, laminin A and F-actin during key stages in Drosophila wing development. Development. 1993;117:509–523. doi: 10.1242/dev.117.2.509. [DOI] [PubMed] [Google Scholar]
- Gleichauf R. Anatomie und variabilität des geschlechtapparates von Drosophila melanogaster (Meigen) Z. Wiss. Zool. 1936;148:1–66. [Google Scholar]
- Graner MW, Bunch TA, Baumgartner S, Kerschen A, Brower DL. Splice variants of the Drosophila PS2 integrins differentially interact with RGD-containing fragments of the extracellular proteins tiggrin, ten-m, and D-laminin 2. J. Biol. Chem. 1998;273:18235–18241. doi: 10.1074/jbc.273.29.18235. [DOI] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes Dev. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Grotewiel MS, Beck CD, Wu KH, Zhu XR, Davis RL. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- Hamidpour M, Behrendt M, Griffiths B, Partridge L, Lindsey N. The isolation and characterisation of antiplatelet antibodies. Eur. J. Haematol. 2006;76:331–338. doi: 10.1111/j.1600-0609.2005.00614.x. [DOI] [PubMed] [Google Scholar]
- Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Dev. Biol. 1982;93:73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- Henchcliffe C, García-Alonso L, Tang J, Goodman CS. Genetic analysis of laminin A reveals diverse functions during morphogenesis in Drosophila. Development. 1993;118:325–337. doi: 10.1242/dev.118.2.325. [DOI] [PubMed] [Google Scholar]
- Henrich VC, Rybczynski R, Gilbert LI. Peptide hormones, steroid hormones, and puffs: mechanisms and models in insect development. Vitam. Horm. 1999;55:73–125. doi: 10.1016/s0083-6729(08)60934-6. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Kando Y, Hayashi T, Goto K, Nakajima A. Synthesis and cell attachment activity of bioactive oligopeptides: RGD, RGDS, RGDV, and RGDT. J. Biomed. Mater Res. 1991;25:1523–1534. doi: 10.1002/jbm.820251209. [DOI] [PubMed] [Google Scholar]
- Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat. Rev. Cancer. 2004;4:45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- Hozumi S, Maeda R, Taniguchi K, Kanai M, Shirakabe S, Sasamura T, Spéder P, Noselli S, Aigaki T, Murakami R, Matsuno K. An unconventional myosin in Drosophila reverses the default handedness in visceral organs. Nature. 2006;440:798–802. doi: 10.1038/nature04625. [DOI] [PubMed] [Google Scholar]
- Hunt LT, Barker WC. von Willebrand factor shares a distinctive cysteine-rich domain with thrombospondin and procollagen. Biochem. Biophys. Res. Commun. 1987;144:876–882. doi: 10.1016/s0006-291x(87)80046-3. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- James BP, Bunch TA, Krishnamoorthy S, Perkins LA, Brower DL. Nuclear localization of the ERK MAP kinase mediated by Drosophila alphaPS2betaPS integrin and importin-7. Mol. Biol. Cell. 2007;18:4190–4199. doi: 10.1091/mbc.E06-07-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannuzi AL, Bunch TA, Brabant MC, Miller SW, Mukai L, Zavortink M, Brower DL. Disruption of C-terminal cytoplasmic domain of betaPS integrin subunit has dominant negative properties in developing Drosophila. Mol. Biol. Cell. 2002;13:1352–1365. doi: 10.1091/mbc.01-08-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannuzi AL, Bunch TA, West RF, Brower DL. Identification of integrin beta subunit mutations that alter heterodimer function in situ. Mol. Biol. Cell. 2004;15:3829–3840. doi: 10.1091/mbc.E04-02-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Milner MJ. The final stages of wing development in Drosophila melanogaster. Tissue Cell. 1987;19:505–513. doi: 10.1016/0040-8166(87)90044-9. [DOI] [PubMed] [Google Scholar]
- Juárez P, Comas I, González-Candelas F, Calvete JJ. Evolution of snake venom disintegrins by positive Darwinian selection. Mol. Biol. Evol. 2008;25:2391–2407. doi: 10.1093/molbev/msn179. [DOI] [PubMed] [Google Scholar]
- Karim FD, Thummel CS. Ecdysone coordinates the timing and amounts of E74A and E74B transcription in Drosophila. Genes Dev. 1991;5:1067–1079. doi: 10.1101/gad.5.6.1067. [DOI] [PubMed] [Google Scholar]
- Karim FD, Thummel CS. Temporal coordination of regulatory gene expression by the steroid hormone ecdysone. EMBO J. 1992;11:4083–4093. doi: 10.1002/j.1460-2075.1992.tb05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennerdell JR, Carthew RW. Heritable gene silencing in Drosophila using double-stranded RNA. Nat. Biotechnol. 2000;18:896–898. doi: 10.1038/78531. [DOI] [PubMed] [Google Scholar]
- Kiger JA, Jr, Natzle JE, Kimbrell DA, Paddy MR, Kleinhesselink K, Green MM. Tissue remodeling during maturation of the Drosophila wing. Dev. Biol. 2007;301:178–191. doi: 10.1016/j.ydbio.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam G, Thummel CS. Inducible expression of double-stranded RNA directs specific genetic interference in Drosophila. Curr. Biol. 2000;10:957–963. doi: 10.1016/s0960-9822(00)00631-x. [DOI] [PubMed] [Google Scholar]
- Lee SB, Cho KS, Kim E, Chung J. blistery encodes Drosophila tensin protein and interacts with integrin and the JNK signaling pathway during wing development. Development. 2003;130:4001–4010. doi: 10.1242/dev.00595. [DOI] [PubMed] [Google Scholar]
- Leptin M, Aebersold R, Wilcox M. Drosophila position-specific antigens resemble the vertebrate fibronectin-receptor family. EMBO J. 1987;6:1037–1043. doi: 10.1002/j.1460-2075.1987.tb04856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley DL, Grell EH. Genetic Variations of Drosophila melanogaster. Carnegie Inst. Wash. Publ. No. 1968:627. [Google Scholar]
- Macías A, Romero NM, Martín F, Suárez L, Rosa AL, Morata G. PVF1/PVR signaling and apoptosis promotes the rotation and dorsal closure of the Drosophila male terminalia. Int. J. Dev. Biol. 2004;48:1087–1094. doi: 10.1387/ijdb.041859am. [DOI] [PubMed] [Google Scholar]
- Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J. Cell Biol. 1999;145:191–201. doi: 10.1083/jcb.145.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Brown NH. Uncoupling integrin adhesion and signaling: the betaPS cytoplasmic domain is sufficient to regulate gene expression in the Drosophila embryo. Genes Dev. 1999;13:729–739. doi: 10.1101/gad.13.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Dunin-Borkowski OM, Brown NH. Specificity of PS integrin function during embryogenesis resides in the alpha subunit extracellular domain. EMBO J. 1997;16:4184–4193. doi: 10.1093/emboj/16.14.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloty-Kapella CV, Degen M, Chiquet-Ehrismann R, Tucker RP. Effects of tenascin-W on osteoblasts in vitro. Cell Tissue Res. 2008;334:445–455. doi: 10.1007/s00441-008-0715-4. [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- O'Connell PO, Rosbash M. Sequence, structure, and codon preference of the Drosophila ribosomal protein 49 gene. Nucleic Acids Res. 1984;12:5495–5513. doi: 10.1093/nar/12.13.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander H. The imaginal discs. In: Kerkut GA, Gilbert LI, editors. Comprehensive insect physiology, biochemistry and pharmacology. vol. 2. Oxford: Cold Spring Harbor Laboratory Press; 1985. pp. 151–182. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prout M, Damania Z, Soong J, Fristrom D, Fristrom JW. Autosomal mutations affecting adhesion between wing surfaces in Drosophila melanogaster. Genetics. 1997;146:275–285. doi: 10.1093/genetics/146.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. The radioimmune assay of ecdysteroid titres in Drosophila melanogaster. Mol. Cell Endocrinol. 1981;21:181–197. doi: 10.1016/0303-7207(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Richards G. Switching partners? Curr. Biol. 1992;2:657–659. doi: 10.1016/0960-9822(92)90123-r. [DOI] [PubMed] [Google Scholar]
- Royer V, Hourdry A, Fraichard S, Bouhin H. Characterization of a putative extracellular matrix protein from the beetle Tenebrio molitor: hormonal regulation during metamorphosis. Dev. Genes Evol. 2004;214:115–121. doi: 10.1007/s00427-004-0389-1. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Russell SR, Heimbeck G, Goddard CM, Carpenter AT, Ashburner M. The Drosophila Eip78C gene is not vital but has a role in regulating chromosome puffs. Genetics. 1996;144:159–170. doi: 10.1093/genetics/144.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Herrero E, Crosby MA. The Abdominal-B gene of Drosophila melanogaster: overlapping transcripts exhibit two different spatial distributions. EMBO J. 1988;7:2163–2173. doi: 10.1002/j.1460-2075.1988.tb03055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangiorgi FO, Benson-Chanda V, de Wet WJ, Sobel ME, Ramirez F. Analysis of cDNA and genomic clones coding for the pro alpha 1 chain of calf type II collagen. Nucleic Acids Res. 1985;13:2815–2826. doi: 10.1093/nar/13.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, Geissert D, Gont LK, De Robertis EM. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell. 1994;79:779–790. doi: 10.1016/0092-8674(94)90068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöck F, Perrimon N. Retraction of the Drosophila germ band requires cell-matrix interaction. Genes Dev. 2003;17:597–602. doi: 10.1101/gad.1068403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segraves WA. Steroid receptors and orphan receptors in Drosophila development. Semin. Cell Biol. 1994;5:105–113. doi: 10.1006/scel.1994.1014. [DOI] [PubMed] [Google Scholar]
- Spéder P, ádám G, Noselli S. Type ID unconventional myosin controls left-right asymmetry in Drosophila. Nature. 2006;440:803–807. doi: 10.1038/nature04623. [DOI] [PubMed] [Google Scholar]
- Spéder P, Petzoldt A, Suzanne M, Noselli S. Strategies to establish left/right asymmetry in vertebrates and invertebrates. Curr. Opin. Genet. Dev. 2007;17:351–358. doi: 10.1016/j.gde.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Springer TA, Zhu J, Xiao T. Structural basis for distinctive recognition of fibrinogen gammaC peptide by the platelet integrin alphaIIbbeta3. J. Cell Biol. 2008;182:791–800. doi: 10.1083/jcb.200801146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development. 1997;124:4583–4594. doi: 10.1242/dev.124.22.4583. [DOI] [PubMed] [Google Scholar]
- Stone BL, Thummel CS. The Drosophila 78C early late puff contains E78, an ecdysone-inducible gene that encodes a novel member of the nuclear hormone receptor superfamily. Cell. 1993;75:307–320. doi: 10.1016/0092-8674(93)80072-m. [DOI] [PubMed] [Google Scholar]
- Subramanian A, Wayburn B, Bunch T, Volk T. Thrombospondin-mediated adhesion is essential for the formation of the myotendinous junction in Drosophila. Development. 2007;134:1269–1278. doi: 10.1242/dev.000406. [DOI] [PubMed] [Google Scholar]
- Syed ZA, Härd T, Uv A, van Dijk-Härd IF. A potential role for Drosophila mucins in development and physiology. PLoS ONE. 2008;3:e3041. doi: 10.1371/journal.pone.0003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R, Gould RJ, Garsky VM, Ciccarone TM, Hoxie J, Friedman PA, Shattil SJ. A monoclonal antibody against the platelet fibrinogen receptor contains a sequence that mimics a receptor recognition domain in fibrinogen. J Biol. Chem. 1989;264:259–265. [PubMed] [Google Scholar]
- Tautz D, Pfeifle C. A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Files on steroids--Drosophila metamorphosis and the mechanisms of steroid hormone action. Trends Genet. 1996;12:306–310. doi: 10.1016/0168-9525(96)10032-9. [DOI] [PubMed] [Google Scholar]
- Thummel CS. Dueling orphans--interacting nuclear receptors coordinate Drosophila metamorphosis. Bioessays. 1997;19:669–672. doi: 10.1002/bies.950190806. [DOI] [PubMed] [Google Scholar]
- Tomiyama Y, Brojer E, Ruggeri ZM, Shattil SJ, Smiltneck J, Gorski J, Kumar A, Kieber-Emmons T, Kunicki TJ. A molecular model of RGD ligands. Antibody D gene segments that direct specificity for the integrin alpha IIb beta 3. J. Biol. Chem. 1992;267:18085–18092. [PubMed] [Google Scholar]
- Van de Walle GR, Peters ST, VanderVen BC, O'Callaghan DJ, Osterrieder N. Equine herpesvirus 1 entry via endocytosis is facilitated by alphaV integrins and an RSD motif in glycoprotein D. J. Virol. 2008;82:11859–11868. doi: 10.1128/JVI.00868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij CL, Diergaarde PJ, Hart M, Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986;5:1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J. Mol. Biol. 1984;173:243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]
- Walsh EP, Brown NH. A screen to identify Drosophila genes required for integrin-mediated adhesion. Genetics. 1998;150:791–805. doi: 10.1093/genetics/150.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Granados RR. Molecular cloning and sequencing of a novel invertebrate intestinal mucin cDNA. J. Biol. Chem. 1997;272:16663–16669. doi: 10.1074/jbc.272.26.16663. [DOI] [PubMed] [Google Scholar]
- Warren JT, Yerushalmi Y, Shimell MJ, O'Connor MB, Restifo LL, Gilbert LI. Discrete pulses of molting hormone, 20-hydroxyecdysone, during late larval development of Drosophila melanogaster: correlations with changes in gene activity. Dev. Dyn. 2006;235:315–326. doi: 10.1002/dvdy.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman DA, Aoyagi N, Pile LA, Schlag EM. TAF250 is required for multiple developmental events in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 2000;97:1154–1159. doi: 10.1073/pnas.97.3.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli M, DiAntonio A, Fearnley IM, Smith RJ, Wilcox M. Cloning and characterization of alpha PS1, a novel Drosophila melanogaster integrin. Mech. Dev. 1993;43:21–36. doi: 10.1016/0925-4773(93)90020-x. [DOI] [PubMed] [Google Scholar]
- Wilcox M, Brower DL, Smith RJ. A position-specific cell surface antigen in the drosophila wing imaginal disc. Cell. 1981;25:159–164. doi: 10.1016/0092-8674(81)90240-3. [DOI] [PubMed] [Google Scholar]
- Wilcox M, DiAntonio A, Leptin M. The function of PS integrins in Drosophila wing morphogenesis. Development. 1989;107:891–897. doi: 10.1242/dev.107.4.891. [DOI] [PubMed] [Google Scholar]
- Wilson TG, Yerushalmi Y, Donnell DM, Restifo LL. Interaction between hormonal signaling pathways in Drosophila melanogaster as revealed by genetic interaction between Methoprene-tolerant and Broad-Complex. Genetics. 2006;172:253–264. doi: 10.1534/genetics.105.046631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM. Adhesive recognition sequences. J. Biol. Chem. 1991;266:12809–12812. [PubMed] [Google Scholar]
- Zavortink M, Bunch TA, Brower DL. Functional properties of alternatively spliced forms of the Drosophila PS2 integrin alpha subunit. Cell Adhes. Commun. 1993;1:251–264. doi: 10.3109/15419069309097258. [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhang Y, Ten Hagen KG. A mucin-type O-glycosyltransferase modulates cell adhesion during Drosophila development. J. Biol. Chem. 2008;283:34076–34086. doi: 10.1074/jbc.M804267200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman S, Grinblat Y, Yee G, Kafatos FC, Hynes RO. Analyses of PS integrin functions during Drosophila development. Development. 1993;118:737–750. doi: 10.1242/dev.118.3.737. [DOI] [PubMed] [Google Scholar]
- Zusman S, Patel-King RS, Ffrench-Constant C, Hynes RO. Requirements for integrins during Drosophila development. Development. 1990;108:391–402. doi: 10.1242/dev.108.3.391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.