Abstract
We hypothesize that occupational exposure to PCBs is associated with a reduction in central dopamine (DA) similar to changes previously seen in PCB exposed adult non-human primates. To test that hypothesis we used [123I]β-CIT SPECT imaging to estimate basal ganglia DA transporter density in former capacitor workers. Women, but not men, showed an inverse relationship between lipid-adjusted total serum PCB concentrations and DA transporter densities in the absence of differences in serum PCB concentrations. These sex differences may reflect age-related reductions in the levels of gonadal hormones since these hormones have been shown experimentally to alter response to DA neurotoxicants. These findings may aid in better understanding the roles that sex and age play in modifying central DA function following exposure, not only to PCBs, but also to other DA neurotoxicants as well as further elucidating the role of gonadal hormones in influencing the initiation and/or progression of neurodegenerative disorders.
Keywords: polychlorinated biphenyls (PCBs), occupational exposure, striatal dopamine transporter (DAT), β-CIT Imaging, adults
Introduction
Polychlorinated biphenyls (PCBs) are widely dispersed in the environment due to leakage from electrical transformers and capacitors as well as release from factories that manufactured these components (Erickson, 1997; Horn et al., 1979; National Research Council, 1979). Epidemiological studies show that developmental exposure to foods contaminated by PCBs results in possible cognitive deficits in infants and children (Jacobson and Jacobson, 2003; Stewart et al., 2005).
Negative consequences are also seen in adults. Schantz et al. (2001) and Fitzgerald et al. (2007; 2008) demonstrate, respectively, subtle cognitive and behavioral changes in recreational fishermen from the Great Lakes and in adults who consumed PCB-contaminated fish from the upper Hudson River. In addition, Seegal et al. (1991) demonstrated that adult non-human primates exposed to PCBs, resulting in serum levels at the upper range reported in capacitor workers (Wolff et al., 1982b), significantly reduced basal ganglia dopamine (DA) concentrations—an effect that persisted for months following cessation of exposure (Seegal et al., 1994a).
These findings provide the rationale for studying adults whose occupational exposure to PCBs resulted in serum levels 10 to 20-fold higher than in the general population (Lawton et al., 1985). Furthermore, this population represents one of the most highly exposed in the United States and the majority of the workers were exposed only to PCBs. Thus, this cohort provides the opportunity to determine whether changes in central DA, as seen in adult non-human primates (Seegal et al., 1994a), also occur in occupationally exposed humans.
In this study we used in vivo molecular imaging of the dopamine transporter (DAT) to investigate the effects of occupational exposure to PCBs on striatal DAT density in male and female former capacitor workers. This imaging technique has been widely used to assess striatal DAT density in both healthy subjects and in subjects with degenerative disorders characterized by dopaminergic dysfunction, including Parkinson's disease (PD) and diffuse Lewy body disease (Colloby et al., 2005). Importantly, recent studies by Prince et al. (2006) and Steenland et al. (2006) found that women former capacitor workers had significantly higher mortality from PD than did men. The Steenland et al. study (2006) is of particular import because the subjects were from the same cohort we studied.
Because of the known and/or suggested neuroprotective effects of ovarian hormones—i.e.; (i) women have a lower incidence of idiopathic PD than do men (Baldereschi et al., 2000; Wenning et al., 2005); (ii) bilateral oophorectomy increases the incidence of PD (Benedetti et al., 2001); (iii) female rodents demonstrate smaller changes in central DA concentrations following exposure to DA neurotoxicants than do comparably exposed male animals (Dluzen, 2000; Miller et al., 1998); and (iv) ovariectomy exacerbates DA loss following exposure to DA neurotoxicants—an effect largely prevented by estrogen supplementation (Dluzen, 2000; Miller et al., 1998); we included sex as a variable in all statistical analyses. Our original hypothesis is that workers with higher serum PCB concentrations will have lower DAT densities and that the association will be stronger among men than women.
Methods
Study population
The population consisted of 6,798 former men and women capacitor workers who had been employed by General Electric Corporation (GE) at either of two capacitor factories in upstate New York (Ft. Edward and Hudson Falls) for at least 3 months between 1946 and 1977. In 1977 the use of PCBs at these sites was banned by the U.S. Environmental Protection Agency (U.S. Environmental Protection Agency, 1978). A complete description of the study population, the tracing and screening procedures, the demographics of the cohort and serum PCB concentrations is presented elsewhere by Seegal et al. (2010).
A total of 241 men and women who lived within a 100 mile radius of Albany NY participated in the study that included neurological and neuropsychological assessments, determination of bone lead concentrations, circulating thyroid hormone levels and serum PCB concentrations. 89 of those subjects (50 men and 39 women; 37% of the total study population) agreed to participate in the imaging portion of the study conducted at the Institute for Neurodegenerative Disorders in New Haven, CT. The results of the imaging portion of the study are presented here.
Human subjects protection
Prior to initiation of the study, the design and instruments, including those associated with tracing, screening, recruitment, and data collection, were reviewed and approved by Institutional Review Boards at each of the study's collaborating institutions (NYSDOH, Albany Medical Center, University at Albany, Institute for Neurodegenerative Disorders and the U.S. Army).
β-CIT imaging techniques
Subjects underwent [123I]β-CIT SPECT imaging as previously described (Laruelle et al., 1994; Seibyl et al., 1995). High specific activity [123I]β;-CIT was prepared from the corresponding trimethylstannyl precursor and subjects were injected with up to 6 mCi of [123I]β-CIT (Innis et al., 1993). Manual regions of interest (ROI) analysis of the [123I]β-CIT/SPECT scans was performed using previously described methods (Seibyl et al., 1995), by a nuclear medicine technologist who was blinded to the serum PCB concentration of all participants. The primary quantitative imaging outcome measure, the specific non-displaceable putamen uptake (V3″), was determined through a standardized analysis method (Seibyl et al., 1995).
Measurement of serum PCB concentrations
The method for the analysis of serum PCB concentrations is described elsewhere (Seegal et al.; 2009). Briefly, the analytical methods of Gammon et al. (2002) and Brock et al. (1996) were followed to determine concentrations of 27 individual PCB congeners and nine organochlorine pesticides. Total serum lipids were determined from serum cholesterol and triglycerides using the algorithm of Phillips et al. (1989).
In addition to measuring total serum PCB concentrations, serum concentrations of ‘light’, ‘heavy’ and ‘occupational’ PCB congeners were also determined. Light was defined as those PCB congeners eluting prior to DDE; heavy as those congeners eluting after DDE (Wolff et al., 1982a) and ‘occupational’ (Wolff, personal communication) defined as those congeners that were unique markers of occupational exposure—i.e., congeners whose concentrations were low in the general population and were derived from exposure to the Aroclor mixtures used in the capacitor plants. These occupational congeners included (PCB28, PCB74, PCB118, PCB105, and PCB156).
Because the distribution of lipid-adjusted serum PCB concentrations was positively skewed, the data were log-transformed and all statistical analyses used that metric. Graphic examination of the β-CIT variable revealed that it was symmetrically distributed and required no transformation.
Statistical procedures
The statistical analysis proceeded in three stages. First, the direct effects of age and sex on the relationships between PCBs and striatal DAT densities were determined using the univariate general linear model of Draper and Smith (1981). Next, we determined the correlations between log-adjusted serum PCB concentrations, age, sex and other relevant variables (see Table 3) that were associated either with exposure and/or outcome (β-CIT SPECT). In order to control for these potential confounders we evaluated the correlation of each of them to either PCBs or β-CIT for any variable whose correlation with both exposure and outcome was significant at p≤0.20. Finally, we evaluated the fitted parameter estimates both with and without potential confounder(s). If inclusion of the confounder resulted in a shift greater than 10% in the estimate for PCBs on β-CIT, it was retained in the final model; otherwise it was dropped from further consideration as a confounder. As discussed in greater detail below, the final statistical model included age, body mass index and sex.
Table 3.
Correlations between β-CIT, PCBs and classical confounders and other potential confounders in the β-CIT study cohort.
| Cofounder | β-CIT | Serum PCBs | |||
|---|---|---|---|---|---|
| Na | Correlation | p | Correlation | p | |
| Serum PCBs | 89 | -0.322 | 0.002 | ||
| β-CIT | 89 | -0.322 | 0.002 | ||
| Sex | 89 | 0.242 | 0.022 | 0.029 | 0.785 |
| Age | 89 | -0.338 | 0.001 | 0.273 | 0.010 |
| Body Mass Index | 85 | -0.192 | 0.078 | 0.148 | 0.177 |
| Serum DDE | 89 | -0.315 | 0.003 | 0.305 | 0.004 |
| Serum DDT | 89 | -0.201 | 0.059 | 0.681 | 0.001 |
| Serum Lipids | 89 | 0.261 | 0.013 | -0.218 | 0.040 |
| Use of Cardio Medications | 84 | -0.214 | 0.050 | 0.001 | 0.992 |
| Education | 85 | -0.063 | 0.566 | -0.110 | 0.314 |
| Bone Lead | 86 | -0.006 | 0.960 | -0.083 | 0.448 |
| Income | 83 | -0.093 | 0.401 | -0.012 | 0.916 |
| Caffeinated Drinks (past year) | 78 | 0.084 | 0.467 | 0.016 | 0.892 |
| Alcoholic Drinks (past year) | 85 | -0.053 | 0.629 | 0.051 | 0.643 |
| Alcoholic Drinks (past 10 years) | 85 | -0.086 | 0.431 | 0.038 | 0.727 |
| Cigarette Packs (past year) | 85 | 0.009 | 0.934 | 0.090 | 0.412 |
| Cigarette Packs (past 10 years) | 85 | 0.054 | 0.621 | 0.027 | 0.806 |
| Use of CNS Active Medications | 78 | 0.099 | 0.388 | 0.053 | 0.646 |
| Use of NSAIDS | 78 | -0.032 | 0.784 | -0.037 | 0.750 |
| TSH | 54 | -0.121 | 0.384 | 0.263 | 0.055 |
| T4 | 54 | 0.049 | 0.727 | 0.056 | 0.689 |
| FT4 | 54 | -0.041 | 0.771 | 0.058 | 0.677 |
| T3 | 54 | -0.090 | 0.519 | 0.073 | 0.598 |
| FT3 | 54 | -0.212 | 0.124 | 0.146 | 0.292 |
Number of observations varies across variables due to missing values
Results
The β-CIT cohort and comparisons with non-participants
Table 1 summarizes the means and standard deviations of demographic and background characteristics for the 89 individuals who participated in the imaging study, as well as highlighting any significant differences between them, as well as the 152 subjects who did not participate in the imaging study.
Table 1.
Demographic and background characteristics for the β-CIT cohort of study participants and non-β-CIT participants from the GE capacitor worker study (N=241).
| β-CIT Participants (N=89) | Non-β-CIT-Participants (N=152) | |||
|---|---|---|---|---|
| Characteristic | N a | Mean ± SEM or % | N a | Mean ± SEM or % |
| Age (years) | ||||
| Overall | 89 | 63.5 ± 0.8 | 152 | 64.9 ± 0.7 |
| By Sex: | ||||
| Men | 50 | 62.6 ± 0.9 | 79 | 65.1 ± 1.0 |
| Women | 39 | 64.6 ± 1.4 | 73 | 64.8 ± 1.1 |
| Sex | ||||
| Men | 50 | 56.2 % | 79 | 52.0 % |
| Women | 39 | 43.8 % | 73 | 48.0 % |
| BMI (kg/m2) | ||||
| Men | 46 | 29.81 ± 0.67 | 76 | 28.60 ± 0.51 |
| Women | 39 | 30.69 ± 1.01 | 72 | 29.60 ± 0.72 |
| Education (school years complete) | ||||
| Men | 46 | 13.07 ± 0.31 | 76 | 13.11 ± 0.27+ |
| Women | 39 | 12.50 ± 0.19 | 73 | 12.29 ± 0.23 |
| Bone Lead (μg/g) | ||||
| Men | 49 | 17.70 ± 1.11 | 74 | 17.65 ± 1.03 |
| Women | 37 | 14.32 ± 1.51 | 70 | 15.37 ± 1.28 |
| Caffeinated Beverages Past Year (drinks) | ||||
| Men | 46 | 958 ± 85 | 79 | 885 ± 61 |
| Women | 39 | 730 ± 102 | 73 | 943 ± 67 |
| Number of Packs of Cigarettes in the Last 10 Years | ||||
| Men | 46 | 600 ± 218 | 76 | 648 ± 157 |
| Women | 39 | 857 ± 375 | 73 | 872 ± 201 |
| Number of Alcoholic Drinks per week in the Last 10 Years | ||||
| Men | 46 | 5.85 ± 0.97+++ | 76 | 7.71 ± 1.23+++ |
| Women | 39 | 1.58 ± 0.61 | 73 | 0.90 ± 0.20 |
| Use of Cardiovascular Drugsb | ||||
| Men | 45 | 66.67 %++ | 73 | 61.64 % |
| Women | 39 | 33.33 % | 73 | 46.58 % |
| Use of CNS Active Medicationsc | ||||
| Men | 50 | 58.00 % | 79 | 58.23 % |
| Women | 39 | 41.03 % | 73 | 49.32 % |
Number of observations varies across characteristics due to missing values
Cardiovascular Drugs (Class 24) include: antilipemic agents, vasodilating agents, alpha- and beta-adrenergic and calcium-channel blocking agents, and renin-angiotensin-aldosterone system inhibitors.
CNS Active Medications include: antihistamines, sympathomimetic agents, beta-adrenergic blocking agents, angiotensin-converting enzyme inhibitors, COX-2 inhibitors, other non-steroidal anti-inflammatory agents, opiate agonists, miscellaneous analgesics and antipyretics, thyroid agents and antithyroid agents.
p≤0.05, significant difference between men and women within the β-CIT participants or the non-β-CIT-participants
p≤0.01, significant difference between men and women within the β-CIT participants or the non-β-CIT-participants
p≤0.001, significant difference between men and women within the β-CIT participants or the non-β-CIT-participants
There were no significant differences in age between those who underwent β-CIT imaging and those who did not. 51.2% of the subjects used cardiovascular medications; 50.6% took centrally active medications. Based on these demographic variables we conclude that selection bias is unlikely and that the B-CIT findings can be generalized to the entire cohort. Four men were removed from subsequent analyses, despite a prescreening questionnaire, because the extensive interview revealed that those subjects had suffered a brain trauma which might affect the outcomes. Additionally, any individual who reported in the initial screening interview that he or she had been diagnosed with PD were excluded from the study.
The imaging cohort consisted of 89 individuals (39 women [44%] and 50 men [56%]), ranging in age from 51 to 85 years (mean=63.5, SEM=0.9). There were no significant differences in age between the men and women who participated in the imaging study. Men, however, reported using more cardiovascular medicines (p≤0.01) than did women and drank alcoholic beverages more often than women (p≤0.001). There was no significant correlation between the consumption of alcohol and either β-CIT or serum PCBs in the cohort (Table 3).
Serum PCB concentrations in men and women former capacitor workers
The geometric means and standard errors of the mean for serum PCB concentrations (for light, heavy, total and occupational) on a lipid-adjusted basis (ppm) for men and women for the 85 subjects in the β-CIT cohort are shown in Table 2. In the β-CIT cohort, the lipid-adjusted serum total PCB concentration for men was 1.01 ppm ± 1.21 and for women 0.95 ppm ± 4.75. There were no significant differences in any of these measures of exposure between men and women in the β-CIT cohort. In addition, there were no significant differences between β-CIT participants and non- β-CIT participants for any of the PCB measures (data not shown).
Table 2.
Current lipid-adjusted serum PCB concentrations by sex in individuals that underwent β-CIT SPECT imaging and are included in the final data analysis.
| PCB Congener | β-CIT Particip ants (N=85) | |
|---|---|---|
| Grouping | Geometric Mean | SEM |
| Men (N=46) | ||
| Total PCBs | 1.01 | 0.18 |
| Light PCBs a | 0.39 | 0.12 |
| Heavy PCBs b | 0.55 | 0.08 |
| Occupational PCBs c | 0.28 | 0.13 |
| Women (N=39) | ||
| Total PCBs | 0.95 | 0.76 |
| Light PCBs a | 0.38 | 0.37 |
| Heavy PCBs b | 0.52 | 0.44 |
| Occupational PCBs c | 0.31 | 0.43 |
Elute before DDE;
Elute after DDE;
Markers for occupational exposure.
[123I]β-CIT densities decrease with age in both men and women
The relationship between the age of the subjects and striatal DAT density was determined separately for men and women. As seen in Figure 1, age was inversely associated with striatal DAT density for both men and women, i.e., as the age of the individuals increased, striatal DAT density decreased regardless of the sex of the individual. Indeed, analysis of variance detected a main effect of age (F=5.171, p=0.02) but failed to detect either a significant effect of sex (F=0.346, p=0.56) or a significant age × sex interaction (F=0.059, p=0.809).
Figure 1.
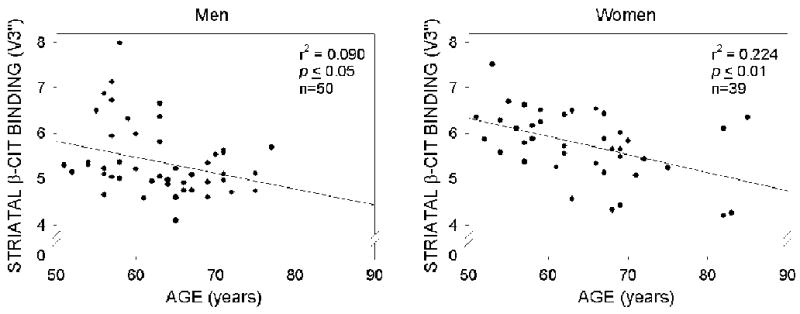
Dopamine transporter density measured by β-CIT SPECT imaging as a function of age by sex in former capacitor factory workers.
Serum total PCB concentrations are inversely associated with striatal [123I]β-CIT densities in women
The relationship between log serum total PCB concentrations on a lipid-adjusted basis and striatal β-CIT densities, by sex, without statistical adjustment for potential confounders is shown in Figure 2. Unlike the effects of age, the sex of the individual significantly influenced the relationship between log- and lipid-adjusted total serum PCB concentrations and β-CIT densities. Serum PCB concentrations were statistically significantly and inversely related to striatal β-CIT densities for women, but not for men. Indeed, the main effect of sex was significant (F=5.79, p=0.02) as was the main effect of log total serum PCB concentrations (on a lipid basis), for women (F=12.93, p≤0.001), but not for men (F=2.69, p=0.10).
Figure 2.
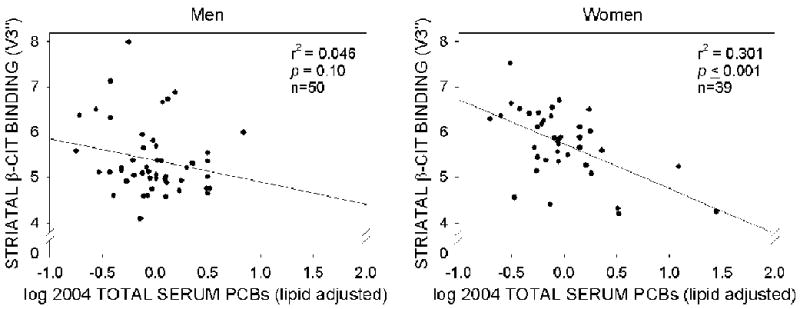
Dopamine transporter density measured by β-CIT SPECT imaging as a function of the log of current serum PCB concentrations (expressed on a lipid–adjusted basis, ppm) by sex prior to adjusting for potential confounders in former capacitor factory workers.
Correlations between PCBs, β-CIT SPECT, age, sex and other relevant variables
We next determined whether the above described relationship between log-adjusted serum PCB concentrations and striatal β-CIT densities persisted after correction for classical confounders (i.e., those statistically associated with both exposure and outcome) as well as other confounders (i.e., variables associated with either exposure or outcome). The results of these analyses are shown in Table 3. Confounders that were statistically associated with both exposure and outcome included age, body mass index (BMI), DDT levels and total serum lipids (expressed as gm/l). When these variables were included as potential confounders in a univariate analysis of variance, the relationship between log total lipid-adjusted serum PCBs for women was still significant (F=5.37, p=0.02) while the relationship between log total serum PCBs for men was not (F=0.36, p=0.55). Successive univariate analyses, where confounders shown to be non-significant were removed, resulted in a final analysis of variance that included age, BMI and sex. The resulting statistical association between log total serum PCB concentrations (on a lipid basis) and striatal β-CIT densities remained highly significant for women (F=6.16, p=0.015), but not for men (F=0.10, p=0.75) and is presented in Figure 3. In summary, the inverse relationship between serum PCB levels and DAT density, observed only in women, remained highly significant even after adjusting, for example, for the effects of age which is correlated both with a decrease in β-CIT binding as well as increased serum PCB levels.
Figure 3.
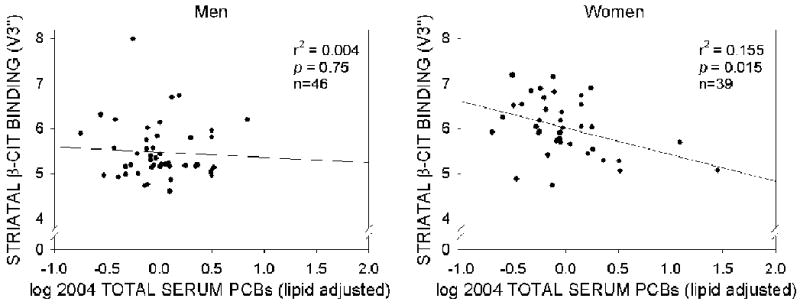
Dopamine transporter density measured by β-CIT SPECT imaging as a function of the log of current serum PCB concentrations (expressed on a lipid-adjusted basis, ppm) by sex after adjusting for age and BMI in former capacitor factory workers.
Similar findings were also seen between log- and lipid-adjusted serum total PCB concentrations and either putamen or caudate β-CIT densities. For the putamen, β-CIT densities for women were significantly and inversely related to log total serum PCB concentrations (on a lipid basis) (F=5.46, p=0.02), but not for men (F=0.19, p=0.67). For the caudate, β-CIT densities for women were significantly and inversely related to log total serum PCB concentrations (on a lipid basis) (F=5.83, p=0.02); again, that was not the case for men (F=0.75, p=0.39).
We also examined whether the class of PCBs—i.e., light, heavy or occupational—altered the statistical relationships described between total serum PCB concentrations and β-CIT densities. For each of the subclasses described above, the significant and inverse relationship between serum PCB concentrations and β-CIT densities remained for women but not for men (data not shown).
Discussion
We demonstrate that, following long-term occupational exposure to PCBs and after statistical correction for a number of confounders, including age, only women show a significant inverse relationship between lipid-adjusted total serum PCB concentrations and caudate, putamen or combined caudate and putamen β-CIT densities despite the fact that neither serum PCB levels nor age were statistically different between men and women. These findings support, in part, our previously published results obtained in non-human primates (Seegal et al., 1991) demonstrating that long-term exposure to PCBs significantly reduced basal ganglia DA concentrations.
Although the reductions in striatal and putamenal [123I]β-CIT densities in women former capacitor workers were statistically significant, the values for [123I]β-CIT striatal uptake for all subjects, on an age-corrected basis, were within the normal range based on comparisons to an existing data base of 73 healthy adults subjects at the same imaging center (Jennings et al., 2004). Having said that, the data presented in Figure 3 demonstrates, after statistically controlling for age and body mass index, a maximal reduction of between 20 and 25% in striatal β-CIT binding in PCB-exposed women. A reduction of this magnitude is unlikely to be associated with clinical signs of parkinsonism. Indeed, none of the individuals exhibited clinical signs of disease due, perhaps, to the exclusion of individuals who reported that they had been diagnosed with PD. Nevertheless, these reductions may serve as an early marker of disease and would warrant further follow-up. Finally, only a single scan was acquired in our study, hence we lack the temporal data necessary to determine whether subjects with high serum PCB levels would show a more rapid decline in [123I]β-CIT striatal uptake with increasing age than either subjects who had not been occupationally exposed to PCBs or workers exposed only to low concentrations of PCBs. Nevertheless, our present results and the observations of Steeenland et al. (2006) and Prince et al. (2006) suggest that occupationally exposed women are at a heightened risk of developing PD.
There are several possible reasons for the disparate results between men and women obtained by us and Steenland et al. (2006) and Prince et al. (2006). First, those prior studies gathered data from all former capacitor workers, including deceased individuals. We, obviously, could obtain [123I]β-CIT striatal imaging data only from living individuals. Second, our study was limited to individuals who agreed to participate; thus bias may have been introduced into our study since we could obtain imaging data only from individuals healthy enough to travel from upstate New York to New Haven, CT. Finally, as mentioned above, we excluded anyone diagnosed with PD; thereby limiting the range of changes in basal ganglia function associated with PCB exposure.
The reported alterations in basal ganglia DA function are surprising because they stand in contrast to the large number of epidemiological studies that show that the incidence of idiopathic PD is approximately twice as great in men as in women (Ragonese et al., 2004). In addition, many experimental studies have demonstrated neuroprotection in female rodents following exposure to a number of DA neurotoxicants, including 6-OHDA and MPTP (Dluzen, 2000), compared to male rodents (Miller et al., 1998). These sex differences in susceptibility are thought to be due to the actions of ovarian hormones, since supplementation of ovariectomized animals, particularly with estrogen, partially ameliorates reductions in measures of central DA integrity. Hence, based on both experimental and epidemiological findings, one would predict that men, rather than women, would show greater reductions in basal ganglia DA terminal densities following occupational exposure to PCBs.
What potential mechanisms may be responsible for the greater sensitivity of women to PCBs in this study? One potential explanation is based on the work of Bossé et al. (1997) who showed that ovariectomy reduces [3H] GBR-12935 binding in the striatum, suggesting a reduction in either the density or function of the DAT. In addition, Mariussen and Fonnum (Mariussen and Fonnum, 2001) and Bemis and Seegal (Bemis and Seegal, 2004) have shown that PCBs inhibit the DAT. Given the importance of this transporter in regulating both intra and extra-synaptosomal DA concentrations and the consequences of such inhibition (Cyr et al., 2003; Jaber et al., 1997), modulation of DAT density or function by post-menopausal reductions in central estrogen may interact with PCBs resulting in a loss of function greater than either menopause or PCBs alone.
We further suggest that age related decline in central gonadal hormones, either of ovarian origin or conversion of testosterone to estrogen (Barreto et al., 2009; Veiga et al., 2004), has different consequences in men and women following exposure to known DA neurotoxicants. This suggestion is supported by data from Murray et al. (2003) and Tamas et al. (2005) who have shown that following 6-OHDA: (i) ovariectomy increases the loss of basal ganglia DA, compared to that seen in the intact female; (ii) castration reduces the loss of basal ganglia DA, compared to that seen in the intact male; (iii) estrogen supplementation in the ovariectomized rat restores DA to levels seen in the intact female and (iv) estrogen supplementation in the castrated male reduces the protection following castration to levels seen in the intact male rat. These findings suggest that central estrogen is neuroprotective in the female while estrogen (from aromatization of testosterone) may be a risk factor in the male brain. Thus, reductions in ovarian hormones following menopause (all women in this study were postmenopausal) is posited to result in the loss of known neuroprotective factor(s) (i.e., estrogen and progesterone) while reductions in circulating testosterone and central aromatase activity in the aging male may result in the loss of a neuro-risk factor (i.e., central estrogen). For this hypothesis to provide a viable explanation for the sex differences in the effects of occupational exposure to PCBs on caudate and putamen β-CIT SPECT binding it is necessary to posit that, if we had conducted the imaging study in younger, reproductively competent workers, we would have found that men would be more affected by PCB exposure than women. In partial support of this, we have recently shown that PCB exposure leads to greater loss of striatal DA in reproductively competent male rodents than in similarly aged female rodents.
There are inherent limitations in the ability to extrapolate findings from any study using experimental animals to humans that may weaken our ability to explain the findings from this study. For example, the rats in the Murray et al. (2003) and Tamas et al. (2005) studies underwent gonadectomy prior to exposure to 6-OHDA, while the former capacity workers in our study were exposed to PCBs while they were still reproductively competent. Nevertheless, the results are intriguing and support the findings of both Steenland et al. (2006) and Prince et al. (2006) who report an increase in PD-associated mortality in highly exposed women but not in men. We must emphasize that the results presented here were obtained in a fairly small number of workers and it would be highly desirable to increase the number of observations to include other cohorts of individuals who had been either occupationally or inadvertently exposed to PCBs, as well as younger, highly exposed individuals.
In summary, we find that elevations in lipid-adjusted total serum PCB concentrations, due to long-term occupational exposure, are inversely associated with measures of DAT density in women, but not in men despite the lack of significant differences in serum PCB levels between the men and women and statistical adjustment for age. We suggest that the combination of age-related changes in ovarian hormone levels and exposure to high levels of PCBs, both known to alter DAT function (Bemis and Seegal, 2004; Bossé et al., 1997; Mariussen and Fonnum, 2001) and the viability of DA neurons (Leranth et al., 2000; Lyng et al., 2007; Seegal et al., 1994b), results in the somewhat paradoxical finding that reproductively senescent women, but not men, account for the significant relationship between current serum PCB levels and reductions in striatal DAT binding. Finally, the unexpected results of this study emphasize not only the need to consider sex as an important variable in determining the central response(s) to known and suspected neurotoxicants but, also suggest broadening the definition of gene × environment interactions to include sex.
Acknowledgments
We wish to thank Ed Bloch, the United Electrical, Radio and Machine Workers of America Union Local 332 Executive Committee and the employees of the Fort Edward and Hudson Falls General Electric plants for their participation and assistance. We acknowledge the technical support of Karen Ireland and Zhisong Lui at Mount Sinai School of Health for the laboratory analyses of serum PCB concentrations.
This study was supported in part by the United States Army Grant DAMD17-02-1-0173 and NIEHS Grant to 1R01ES014675-01 to Richard F. Seegal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baldereschi M, et al. Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. Neurology. 2000;55:1358–1363. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- Barreto G, et al. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2009;25:3039–3046. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- Bemis JC, Seegal RF. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol Sci. 2004;80:288–295. doi: 10.1093/toxsci/kfh153. [DOI] [PubMed] [Google Scholar]
- Benedetti MD, et al. Hysterectomy, menopause, and estrogen use preceding Parkinson's disease: an exploratory case-control study. Mov Disord. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- Best SE, et al. Striatal dopamine transporter availability with [123I]β-CIT SPECT is unrelated to gender or menstrual cycle. Psychopharmacology. 2005;183:181–189. doi: 10.1007/s00213-005-0158-5. [DOI] [PubMed] [Google Scholar]
- Bossé R, et al. Ovariectomy and estradiol treatment affect the dopamine transporter and its gene expression in the rat brain. Mol Brain Res. 1997;46:343–346. doi: 10.1016/s0169-328x(97)00082-x. [DOI] [PubMed] [Google Scholar]
- Brock JW, et al. An improved analysis for chlorinated pesticides and polychlorinated biphenyls (PCBs) in human and bovine sera using solid-phase extraction. J Anal Toxicol. 1996;20:528–536. doi: 10.1093/jat/20.7.528. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, et al. Progression of dopaminergic degeneration in dementia with Lewy bodies and Parkinson's disease with and without dementia assessed using 123I-FP-CIT CPECT. Eur J Nucl Med Mol Imaging. 2005;32:1176–1185. doi: 10.1007/s00259-005-1830-z. [DOI] [PubMed] [Google Scholar]
- Cyr M, et al. Sustained elevation of extracellular dopamine causes motor dysfunction and selective degeneration of striatal GABAergic neurons. Proc Natl Acad Sci U S A. 2003;100:11035–11040. doi: 10.1073/pnas.1831768100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dluzen DE. Neuroprotective effects of estrogen upon the nigrostriatal dopaminergic system. J Neurocytol. 2000;29:387–399. doi: 10.1023/a:1007117424491. [DOI] [PubMed] [Google Scholar]
- Draper NR, Smith H. Applied Regression Analysis. John Wiley & Sons; New York: 1981. [Google Scholar]
- Erickson MD. Analytical chemistry of PCBs. CRC Press; Boca Raton: 1997. [Google Scholar]
- Fitzgerald EF, et al. Polychlorinated biphenyl exposure and neuropsychological status among older residents of upper Hudson River communities. Environ Health Perspect. 2008;116:209–215. doi: 10.1289/ehp.10432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, et al. Environmental exposures to polychlorinated biphenyls (PCBs) among older residents of upper Hudson River communities. Environ Res. 2007;104:352–360. doi: 10.1016/j.envres.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Gammon MD, et al. Environmental toxins and breast cancer on Long Island. II. Organochlorine compound levels in blood. Cancer Epidemiol Biomarkers Prev. 2002;11:686–697. [PubMed] [Google Scholar]
- Horn EG, et al. The problem of PCBs in the Hudson River system. Ann NY Acad Sci. 1979;320:591–609. doi: 10.1111/j.1749-6632.1979.tb56637.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, et al. Single photon emission computed tomographic imaging demonstrates loss of striatal dopamine transporters in Parkinson's disease. Proc Natl Acad Sci U S A. 1993;90:11965–11969. doi: 10.1073/pnas.90.24.11965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber M, et al. The dopamine transporter: A crucial component regulating dopamine transmission. Mov Disord. 1997;12:629–633. doi: 10.1002/mds.870120502. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr. 2003;143:780–788. doi: 10.1067/S0022-3476(03)00577-8. [DOI] [PubMed] [Google Scholar]
- Laruelle M, et al. Graphical, kinetic and equilibrium analyses of in vivo [123I]β-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab. 1994;14:982–994. doi: 10.1038/jcbfm.1994.131. [DOI] [PubMed] [Google Scholar]
- Lawton RW, et al. Effects of PCB exposure on biochemical and hematological findings in capacitor workers. Environ Health Perspect. 1985;60:165–184. doi: 10.1289/ehp.8560165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leranth C, et al. Estrogen is essential for maintaining nigrostriatal dopamine neurons in primates: implications for parkinson's disease and memory. J Neurosci. 2000;20:8604–8609. doi: 10.1523/JNEUROSCI.20-23-08604.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyng GD, et al. Polychlorinated biphenyl-induced neurotoxicity in organotypic cocultures of developing rat ventral mesencephalon and striatum. Toxicol Sci. 2007;97:128–139. doi: 10.1093/toxsci/kfm027. [DOI] [PubMed] [Google Scholar]
- Mariussen E, Fonnum F. The effect of polychlorinated biphenyls on the high affinity uptake of the neurotransmitters dopamine, serotonin, glutamate and GABA, into rat brain synaptosomes. Toxicology. 2001;159:11–21. doi: 10.1016/s0300-483x(00)00374-7. [DOI] [PubMed] [Google Scholar]
- Miller DB, et al. The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann N Y Acad Sci. 1998;844:153–165. [PubMed] [Google Scholar]
- Murray HE, et al. Dose- and sex-dependent effects of the neurotoxin 6-hydroxydopamine on the nigrostriatal dopaminergic pathway of adult rats: differential actions of estrogen in males and females. Neuroscience. 2003;116:213–222. doi: 10.1016/s0306-4522(02)00578-x. [DOI] [PubMed] [Google Scholar]
- National Research Council. Polychlorinated Biphenyls. National Academy of Sciences; Washington, D.C.: 1979. [Google Scholar]
- Phillips DL, et al. Chlorinated hydrocarbon levels in human serum: Effects of fasting and feeding. Arch Environ Contam Toxicol. 1989;18:495–500. doi: 10.1007/BF01055015. [DOI] [PubMed] [Google Scholar]
- Prince MM, et al. Update: cohort mortality study of workers highly exposed to polychlorinated (PCBs) during the manufacture of electrical capacitors, 1940-1998. Environmental health: A global access science source. 2006;5:13. doi: 10.1186/1476-069X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragonese P, et al. Risk of Parkinson disease in women: effect of reproductive characteristics. Neurology. 2004;62:2010–2014. doi: 10.1212/wnl.62.11.2010. [DOI] [PubMed] [Google Scholar]
- Schantz SL, et al. Impairments of memory and learning in older adults exposed to polychlorinated biphenyls via consumption of Great Lakes fish. Environ Health Perspect. 2001;109:605–611. doi: 10.1289/ehp.01109605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seegal RF, et al. Comparison of effects of Aroclors 1016 and 1260 on nonhuman primate catecholamine function. Toxicology. 1991;66:145–163. doi: 10.1016/0300-483x(91)90215-m. [DOI] [PubMed] [Google Scholar]
- Seegal RF, et al. Decreases in dopamine concentrations in adult non-human primate brain persist following removal from polychlorinated biphenyls. Toxicology. 1994a;86:71–87. doi: 10.1016/0300-483x(94)90054-x. [DOI] [PubMed] [Google Scholar]
- Seegal RF, et al. PCBs reduce the number of dopaminergic neurons in rat substantia nigra determined by laser-scanning confocal microscopy. Toxicologist. 1994b;14:353. [Google Scholar]
- Seegal RF, et al. Estimating the half-lives of PCB congeners in former capacitor workers measured over a twenty-eight year interval. J Expos Sci Environ Epidemiol. 2010 doi: 10.1038/jes.2010.3. in press. [DOI] [PubMed] [Google Scholar]
- Seibyl JP, et al. Decreased single-photon emission computed tomographic [123I] β-CIT striatal uptake correlates with symptom severity in Parkinson's disease. Ann Neurol. 1995;38:589–598. doi: 10.1002/ana.410380407. [DOI] [PubMed] [Google Scholar]
- Steenland K, et al. Polychlorinated biphenyls and neurodegenerative disease mortality in an occupational cohort. Epidemiology. 2006;17:8–13. doi: 10.1097/01.ede.0000190707.51536.2b. [DOI] [PubMed] [Google Scholar]
- Stewart P, et al. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to. PCBs Neurotoxicol Teratol. 2005;27:771–780. doi: 10.1016/j.ntt.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Tamas A, et al. Age and gender differences in behavioral and morphological outcome after 6-hydoxydopamine induced lesion of the substantia nigra in rats. Behav Brain Res. 2005;158:221–229. doi: 10.1016/j.bbr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- U.S.Environmental Protection Agency. Polychlorinated biphenyls (PCBs): Manufacturing, processing, distribution in commerce, and use bans. Fed Regist. 1978;43:24802–24818. [Google Scholar]
- Veiga S, et al. Sex hormones and brain aging. Exp Gerontol. 2004;39:1623–1631. doi: 10.1016/j.exger.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Wenning GK, et al. Prevalence of movement disorders in men and women aged 50-89 years (Bruneck Study cohort): a population-based study. Lancet Neurol. 2005;4:815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- Wolff MS, et al. Body burden of polychlorinated biphenyls among persons employed in capacitor manufacturing. Int Arch Environ Health. 1982a;49:199–208. doi: 10.1007/BF00377929. [DOI] [PubMed] [Google Scholar]
- Wolff MS, et al. Disposition of polychlorinated biphenyl congeners in occupationally exposed persons. Toxicol Appl Pharmacol. 1982b;62:294–306. doi: 10.1016/0041-008x(82)90128-4. [DOI] [PubMed] [Google Scholar]


