Abstract
It has been reported that dietary energy restriction, including intermittent fasting (IF), can protect heart and brain cells against injury and improve functional outcome in animal models of myocardial infarction and stroke. Here we report that IF improves glycemic control and protects the myocardium against ischemia-induced cell damage and inflammation in rats. Echocardiographic analysis of heart structural and functional variables revealed that IF attenuates the growth-related increase in posterior ventricular wall thickness, , end systolic and diastolic volumes, and reduces the ejection fraction. The size of the ischemic infarct 24 hours following permanent ligation of a coronary artery was significantly smaller, and markers of inflammation (infiltration of leukocytes in the area at risk and plasma IL-6 levels) were less, in IF rats compared to rats on the control diet. IF resulted in increased levels of circulating adiponectin prior to and after myocardial infarction. Because recent studies have shown that adiponectin can protect the heart against ischemic injury, our findings suggest a potential role for adiponectin as a mediator of the cardioprotective effect of IF.
Keywords: apoptosis, dietary energy restriction, echocardiography, inflammation, myocardial infarction
INTRODUCTION
Myocardial infarction is a major cause of morbidity and mortality [1]. Myocardial ischemia results in damage to heart cells and an inflammatory response that can impair heart function. Recent studies have shown that dietary factors can attenuate the extent of heart damage and impaired function in animal models of myocardial infarction [2]. Previous studies have shown that rats maintained on a low calorie diet exhibit reduced damage to heart cells and improved functional outcome in models of myocardial ischemia [3, 4]. Another study showed that when young rats are maintained for several months on an energy restricted intermittent fasting (IF) diet, damage to the heart due to coronary artery ligation is reduced. [5]. The mechanism by which dietary energy restriction protects the heart is not known, but may involve an enhanced ability of cells to cope with oxidative and metabolic stress [6].
Emerging evidence suggests that periodic fasting can improve various measures of health and longevity. Rats maintained on an alternate day food deprivation intermittent fasting (IF) diet exhibit decreased body weight, increased insulin sensitivity, reduced resting heart rate and blood pressure, and improved cardiovascular adaptation to stress [7]. A recent study showed that alternate day caloric restriction can reduce systemic markers of inflammation and oxidative stress and can reduce symptoms in subjects with asthma [8]. Although the mechanisms by which IF benefits the cardiovascular system are unknown, studies of cellular and molecular changes induced by IF in the brain suggest an enhancement of adaptive stress response mechanisms including upregulation of heat-shock proteins [9], growth factors [10, 11] and mitochondrial uncoupling proteins [12]. Such changes are similar to those induced by ischemic preconditioning [13], suggesting that IF imposes a mild beneficial stress on cells.
Recently, a novel cardioprotective signaling pathway involving adiponectin has been described. Adiponectin can protect myocardial cells against ischemic injury by activating the cyclic AMP-dependent protein kinase – Akt pathway [14]. The latter pathway may mediate, in part, the cardioprotective effect of caloric restriction [15]. Adiponectin induces the expression of Hif-1, suggesting a role for adiponectin in activating a major adaptive cellular response to ischemia [16]. In the present study we show that IF protects the heart against ischemic injury by a mechanism involving reduced inflammation and apoptosis of myocardial cells. These beneficial effects of IF are associated with an elevation of adiponectin levels, suggesting a role for adiponectin in IF-mediated cardioprotection.
MATERIALS AND METHODS
Animals and Dietary and Blood Collection Procedures
Male Wistar rats 2.5 months old (n=30) were purchased from Charles River (Wilmington, MA) and maintained for 1 month prior to initiation of experimental diets. Upon arrival, all rats were maintained under temperature- and light-controlled conditions with access to food (standard NIH-07 rat diet, Harlan Teklad, Indianapolis, IN) and water ad libitum (AL). The photoperiod in the colony and testing rooms was maintained on a 12-hour light/dark cycle with lights on from 6:00 AM to 6:00 PM daily. Prior to the start of 3 month experimental diet period a blood sample was collected from rats under isoflurane anesthesia using a six-station anesthesia system (SurgiVet, Waukesha, WI). Blood was collected from rats in both diet groups after a 16 hour overnight fast. Approximately 2 ml of blood was withdrawn from the tail vein of each rat into an EDTA-containing tube (BD Vacutainer, Franklin Lakes, NJ). Plasma was isolated from the blood and stored at −80° C.
Rats were randomly assigned to either ad libitum (AL) or intermittent fasting (IF) diets (15 rats in each diet group). Each of the rats was individually housed with continuous access to water. Rats in the IF group were deprived of food for 24 hours every other day. The rats were maintained under either AL or IF regimens for 3 months. Body weights were measured once per week for AL rats and twice per week (on consecutive fasting and feeding days) for the IF rats. One day prior to the myocardial infarction procedure, a blood sample was collected from each rat; a 5 µl aliquot was used for blood glucose determination and plasma was isolated from the remaining blood and stored at −80°C for measurements of insulin and adiponectin levels.
Echocardiography
Echocardiography (Echo) was performed on each of the rats prior to and three months after maintenance on either AL or IF diet to evaluate the effects of the diets on cardiovascular morphology and function. Under light anesthesia with sodium pentobarbital (30 mg/kg, i.p.), a 12-MHz transducer (HP SONOS 5500; Hewlett Packard Inc., Andover, Mass) was used to obtain 2D images of the left ventricle (LV) at long and short axes. LV mass (LVM) and LV posterior wall thickness (PWth) were measured from M-mode LV tracings. LV end-systolic volume (ESV) and LV end-diastolic volume (EDV) were calculated from 2D images using Modified Simpson's rule. LV ejection fraction (EF) was calculated from EDV and ESV. Cardiac index (CI) was calculated as cardiac output adjusted for body weight.
Myocardial Infarction and Histological Analyses
The coronary artery ligation surgery was performed as described previously; the left coronary artery was permanently ligated at 2 mm below its origin [5]. Twenty-four hours after surgery, the rats were intubated and anesthetized with isoflurane. The chest was opened, blood samples were collected from the left ventricle, and the heart was rapidly excised. Using a 16G tube, 5% Evans blue (3 ml) was rapidly injected into the aorta to distinguish the perfused area from the under-perfused area. The atria and great vessels were dissected away from the heart. The heart was cut transversely into five slices from the base to apex. One section from mid-papillary muscle level was immediately stored in liquid nitrogen for subsequent histological analyses. The second section was immediately frozen on dry ice and stored at −80°C for biochemical analyses. The remaining sections were incubated at 37°C with 4% triphenyltetrazolium chloride (TTC) for 30 min to distinguish the infarct area from the area at risk (AAR) in the under-perfused area. All images were analyzed using NIH Image software. Myocardial infarction (MI) size was expressed as a percent of the under-perfused area. Myocardial tissue sections (5 µm) from frozen samples were subjected to hematoxylin and eosin (H&E) and TUNEL staining. Inflammatory cells (neutrophils and macrophages) were counted and averaged from the five different microscopic fields of the AAR in H&E stained sections. Myocardial apoptosis in the AAR was assessed from TUNEL stained (ApopTag, Chemicon, Billerica, MA) sections.
Biochemical Analyses of Blood Samples
The level of blood glucose was assessed each time when blood was sampled using a glucose meter (FreeStyle, TheraSense, Alameda, CA). The glucose concentration was expressed as mg glucose/dL whole blood. The levels of insulin and adiponectin in plasma were assessed using ultrasensitive ELISA kits (ALPCO Dianostics, NH).
Statistical Analyses
Depending on the experimental design and data collection, several general linear model (GLM) procedures including multivariate, repeated-measures, one-way or two-way ANOVA were applied to the data analyses in which appropriate models were applicable. Post hoc assessments included the Student Newman Keuls test for the data in Table 1 and the adiponectin data (Fig. 4), and Student’s t-test for AL versus IF comparisons (Figure 1, Figure 2 and Figure 3).
Table 1.
The effect of AL/IF on Cardiac Function in Rats (Echo Readings)
| AL | IF | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| PWth(mm) | 1.58±0.04 | 1.80±0.04*** | 1.55±0.03 | 1.62±0.03# † |
| LVM(g) | 1.07±0.01 | 1.14±0.01 | 1.09±0.01 | 1.12±0.01 |
| AoD(mm) | 3.49±0.04 | 3.74±0.06* | 3.58±0.04 | 3.69±0.05 |
| LAD(mm) | 3.21±0.10 | 3.34±0.11 | 3.35±0.11 | 4.19±0.16**# †‡ |
| LAD/AoD | 0.92±0.03 | 0.90±0.03 | 0.94±0.04 | 1.14±0.05*# †‡ |
| ESV(µl) | 149±5.0 | 193±7.0*** | 156±7.0 | 207±10.0*** |
| EDV(µl) | 356±12.0 | 455±12.0*** | 362±11.0 | 413±14.0*# |
| EF (%) | 58.0±1.5 | 57.5±1.1 | 57.0±1.1 | 49.7±1.7***# †‡ |
| CI (µl/min/g) | 170±6.0 | 150±7.0 | 172±6.0 | 144±6.0** |
The numbers are mean ± SEM.
p< 0.05, 0.01, 0.001, respectively, on the comparison of Pre vs. Post
p<0.05 on the comparison of Post IF vs. Post AL
Significant difference on the Diet factor (AL vs. IF)
Significant difference on interaction of Prepost × Diet
Figure 1.
Rats maintained for 3 months on an intermittent fasting (IF) diet exhibit lower body weights, and reduced levels of circulating glucose and insulin, compared to rats maintained on an ad libitum (AL) diet. A. Body weights of AL and IF rats prior to and during the 3 month diet period. Body weights of rats on the IF diet were measured on consecutive fasting and feeding days (IF fed, IF rats on a feeding day; IF fast, IF rats on a fasting day). Values are the mean and SEM (n=15). **p<0.01 compared to the value for the AL group (for all time points from 2 through 12 weeks). B. Levels of blood glucose and plasma insulin in rats that had been maintained for 3 months on either AL or IF diets. Values are the mean and SEM (n=15). ** p<0.01 compared to AL value.
Figure 2.
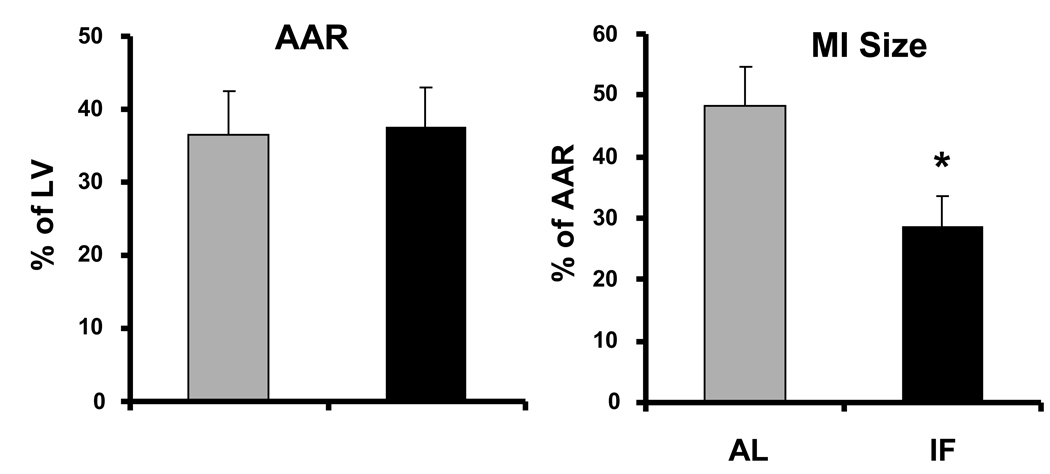
An IF diet protects the heart against ischemic injury in a rat myocardial infarction model. The area at risk (AAR) and MI size in rats 24 hr after coronary artery ligation surgery. The AAR was expressed as a percentage of the left ventricle, and the MI size was expressed as a percentage of AAR. Values are the mean and SEM (n=15). * p<0.05 compared to AL value.
Figure 3.
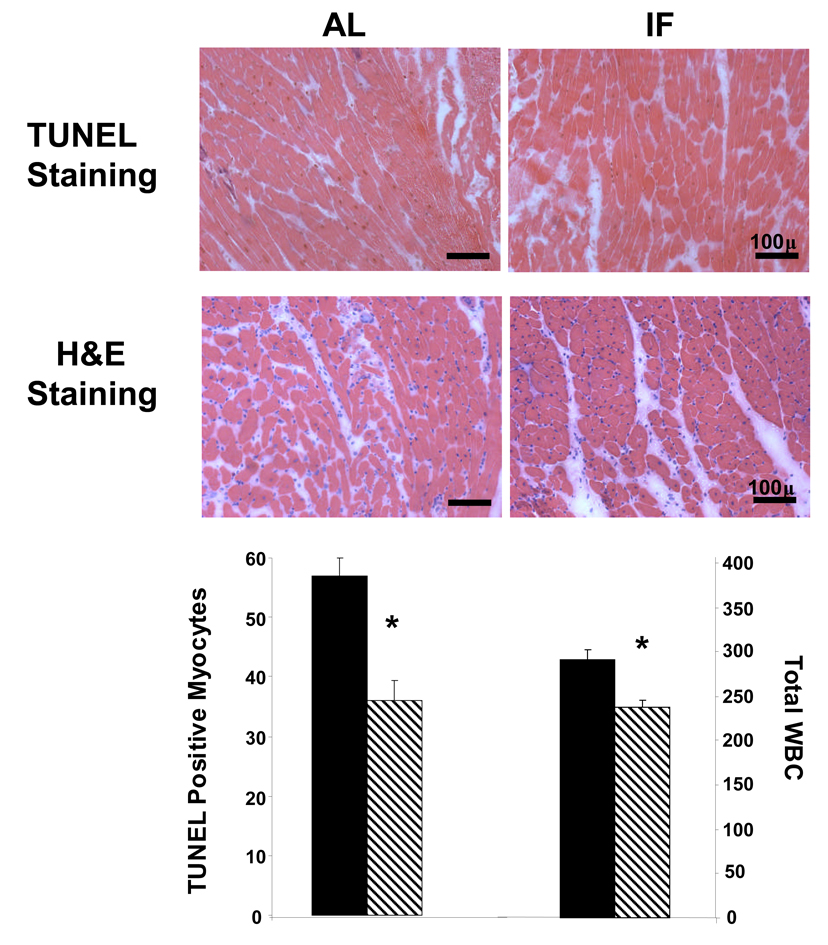
An IF diet reduces the number of cardiomyocytes with TUNEL–positive staining and decreases numbers of infiltrating pro-inflammatory cells in a rat MI model. A. Representative heart tissue sections from the AAR from rats that had been maintained for 3 months on either AL or IF diets and then subjected to myocardial ischemia for 24 hours; the sections were stained with either TUNEL or H&E (bar = 100 µm). B. Numbers of TUNEL positive myocytes (left) and inflammatory cells (right) in the AAR. Values are the mean and SEM (n = 15). *p<0.05.
RESULTS
Body Weight and Blood Glucose and Insulin Levels are Reduced by Intermittent Fasting
The IF regimen significantly attenuated body weight gain (Fig. 1A). After three months on the diets, rats on the IF diet had significantly lower body weights on both feeding and fasting days compared to rats on the AL diet (p<0.01). The body weights of IF rats were significantly lower on fasting days compared to feeding days (p<0.01). Plasma glucose and insulin levels were each significantly lower in rats on the IF diet compared to those on the AL diet (p<0.01) (Fig 1B).
Intermittent Fasting Modifies Echocardiographic Indicators of Cardiac Morphometry and Function
The echocardiographic examination was performed prior to experimental diet initiation and 3 months after diet initiation (prior to coronary artery ligation). The results of echocardiographic analysis are shown in Table 1. Prior to diet initiation there were no differences between rats assigned to the two different groups (AL and IF) in any of the echocardiographic measures. The thickness of the posterior wall of the left ventricle and the left ventricular mass increased significantly during the three month diet period in the rats on the AL diet, but not in the rats on the IF diet (Table 1). End systolic volume increased significantly during the three month diet period in rats on either the AL or IF diets, with no quantitative difference between the two diets. End diastolic volume increased significantly during the three month diet period in rats on either the AL or IF diets, with the magnitude of the increase being significantly less in rats on the IF diet. This disproportionate effect of diet on end diastolic volume resulted in significant reduction of ejection fraction during the three month period in rats on the IF diet, while the ejection fraction was unchanged during this time period in rats on the AL diet (Table 1). The cardiac index was decreased significantly during the three month diet period in rats on either the AL or IF diets; however, no quantitative difference was observed between the two diets.
Intermittent Fasting Lessens Myocardial Tissue Damage, Apoptosis and Inflammation in a Model of Myocardial Infarction
The size of the area at risk (AAR) and myocardial infarction (MI) (see Methods) were evaluated 24 hr after coronary artery ligation in all rats. The total AAR did not differ between two dietary regimen; however, the MI infarct size was significantly less in rats on the IF diet compared to those on the AL diet (Fig. 2). The numbers of cardiomyocytes with TUNEL-positive nuclei in the AAR was approximately 40% lower in the hearts of rats in the IF group compared to rats in the AL group (Fig. 3). The number of inflammatory cells (neutrophils and macrophages) was significantly lower in the AAR of the hearts of rats in the IF group compared to rats in the AL group (Fig. 3). As a molecular marker of inflammation we measured levels of interleukin-6 in the plasma and heart tissue at 24 hours after MI. Plasma IL-6 levels were significantly lower following MI in the rats on the IF diet (588.6 ± 35.2 ng/ml) compared to those on the control diet (734.1 ± 55.5 ng/ml; p<0.05).
Adiponectin Levels are Elevated in Rats Maintained on the IF Diet
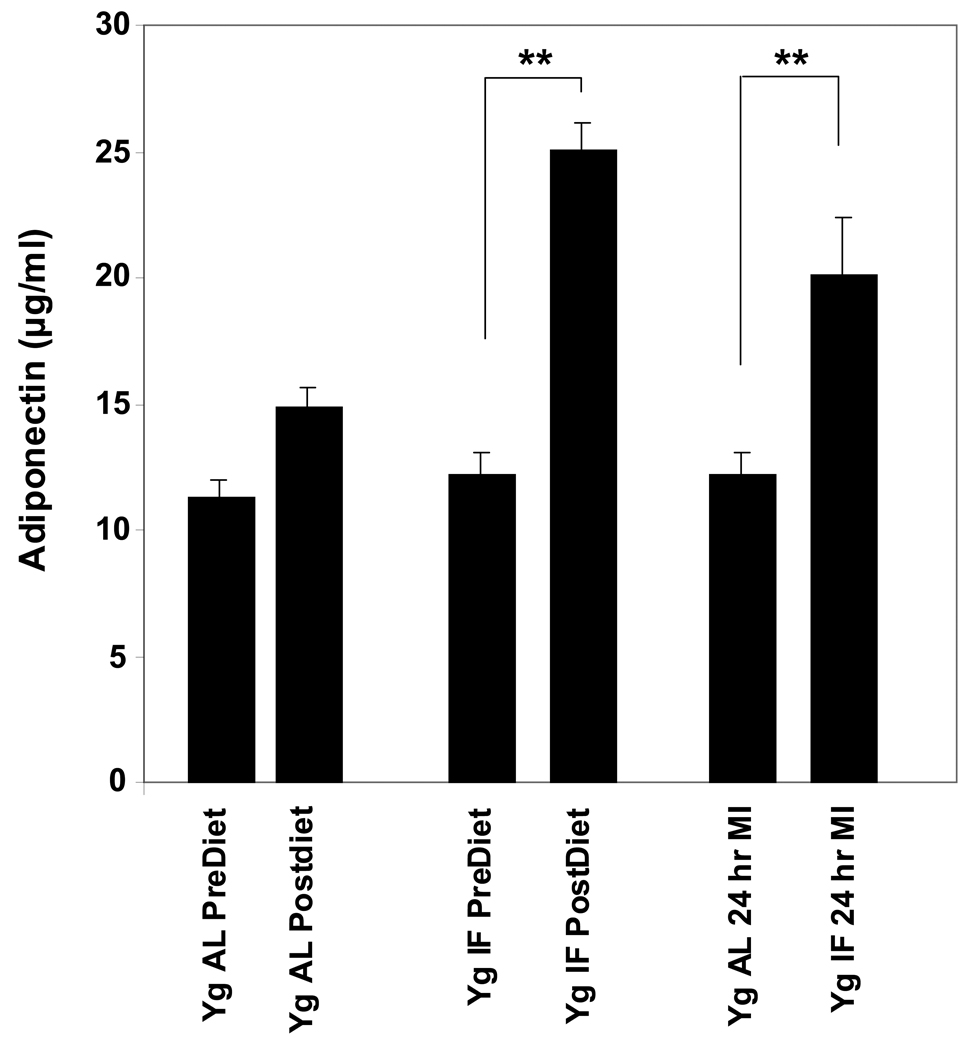
Recent findings have provided evidence that adiponectin can protect cardiac myocytes against ischemic injury [14, 17]. We therefore measured the concentration of adiponectin in plasma samples from rats in the AL and IF diet groups prior to and 24 hours after myocardial infarction. The adiponectin concentration was two-fold greater in the plasma of rats that had been maintained for 3 months on the IF diet (Fig. 4). In rats on the AL diet, adiponectin levels were unchanged 24 hours after MI. In rats on the IF diet, adiponectin levels remained significantly elevated after MI compared to adiponectin levels in the rats on the AL diet.
Figure 4.
Plasma levels of adiponectin are elevated in rats maintained on an IF diet before and after myocardial infarction. Values are the mean and SEM (n = 15). **p<0.01.
DISCUSSION
Three major findings of the present study are: 1) Rats maintained for 3 months on an IF diet exhibit a lower ejection fraction compared to rats on the AL diet, but a similar cardiac index; 2) The amount of myocardial and systemic inflammation and damage to heart cells in the AAR were reduced in rats on the IF diet 24 hours following coronary artery ligation compared to those on the AL diet; and 3) IF resulted in elevation of circulating adiponectin levels which was maintained 24 hours following MI. As expected, the size of the ventricular walls increased in all rats as they grew during the time period of the study (from 2.5 to 5.5 months of age). During this time period the body weights of rats on the AL diet increased by more than 100 grams, whereas rats on the IF diet gained less than 20 grams. However, although the ventricular wall thickness in rats on the IF diet increased by as much than in rats on the AL diet, the end diastolic volume and ejection fraction were significantly less in IF compared to AL rats. Nevertheless, the cardiac index was similar in rats on both diets suggesting that the cardiac output of rats on the IF diet is appropriate for their reduced body size.
Similar to the results of previous studies that evaluated the effects of caloric restriction [18, 19] or IF [5] on heart damage in animal models of myocardial infarction, we found that 24 hours following a permanent ligation of a descending coronary artery the MI size was significantly smaller in rats on the IF diet compared to rats on the AL diet. Using the same model of myocardial infarction, we previously found that IF confers long-lasting structural and functional benefits for the heart for up to at least 10 weeks post-infarction [5]. Previous studies have shown that many cardiac myocytes undergo apoptosis in response to myocardial ischemia. For example, mice overexpressing the anti-apoptotic protein Bcl-2 in cardiac myocytes exhibit reduced cytochrome c-mediated caspase-9-dependent cardiomyocyte apoptosis and local inflammation (neutrophil infiltration) in cardiac allografts during ischemia-reperfusion injury [20].Consistent with an anti-apoptotic mechanism of action of IF, we found that the number of myocardial cells with TUNEL positive nuclei was significantly less in the hearts of rats in the IF diet group compared to the AL group suggesting that IF protects cardiac myocytes against ischemia-induced apoptosis. Similarly, inhibition of c-Jun N-terminal kinase protects cardiac myocytes against apoptosis in a rat model of ischemia and reperfusion injury [21]. Inasmuch as inflammation (macrophage and leukocyte infiltration and pro-inflammatory cytokine production) contribute to ischemic damage following myocardial infarction [22, 23], the inhibitory effect of IF on inflammatory cell infiltration may mediate, in part, the cardioprotective effect of IF. On the other, hand IF may also act directly on cardiac myocytes to increase their resistance to apoptosis. Such a preconditioning or “hormesis” mechanism of action of IF has been proposed based on studies of the cytoprotective effects of IF in models of stroke and neurodegenerative disorders [24].
Adiponectin has previously been shown to have cardioprotective and anti-inflammatory actions. For example, Tao et al. [17] reported that adiponectin deficient mice exhibit increased myocardial damage and increased levels of markers of oxidative stress in a mouse myocardial ischemia and reperfusion model. Shibata et al. [25] observed impaired left ventricular function in adiponectin deficient mice which was associated with myocyte hypertrophy and increased cardiac cell apoptosis. Numerous studies, reviewed by Takemura [26] have documented anti-inflammatory actions of adiponectin in a variety of tissues including the cardiovascular system. The correlation between elevated plasma adiponectin levels and reduced cardiac myocyte damage and inflammation in the MI model in the present study, suggest a role for adiponectin in the cardioprotective and anti-inflammatory effects of IF. However, further experiments in which adiponectin or its receptors are selectively blocked in animals on an IF diet will be required to establish whether adiponectin signaling is a key mediator of the cardioprotective effects of IF. IF results in changes in levels of several circulating factors including decreased levels of insulin, leptin and cholesterol, and increased levels of testosterone [27]. It will therefore be important to elucidate the roles for, and interactions, of these different factors in cardiovascular responses to IF.
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging
References
- 1.Rich MW. Epidemiology, clinical features, and prognosis of acute myocardial infarction in the elderly. Am J Geriatr Cardiol. 2006;15:7–11. doi: 10.1111/j.1076-7460.2006.05273.x. [DOI] [PubMed] [Google Scholar]
- 2.de Lorgeril M, Salen P, Paillard F. Diet and medication for heart protection in secondary prevention of coronary heart disease. New concepts. Nutr Metab Cardiovasc Dis. 2000;10:216–222. [PubMed] [Google Scholar]
- 3.Long P, Nguyen Q, Thurow C, Broderick TL. Caloric restriction restores the cardioprotective effect of preconditioning in the rat heart. Mech Ageing Dev. 2002;123:1411–1413. doi: 10.1016/s0047-6374(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 4.Shinmura K, Tamaki K, Bolli R. Short-term caloric restriction improves ischemic tolerance independent of opening of ATP-sensitive K+ channels in both young and aged hearts. J Mol Cell Cardiol. 2005;39:285–296. doi: 10.1016/j.yjmcc.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP, Wan R. Beneficial effects of intermittent fasting and caloric restriction on the cardiovascular and cerebrovascular systems. J Nutr Biochem. 2005;16:129–137. doi: 10.1016/j.jnutbio.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Wan R, Camandola S, Mattson MP. Intermittent food deprivation improves cardiovascular and neuroendocrine responses to stress in rats. J Nutr. 2003;133:1921–1929. doi: 10.1093/jn/133.6.1921. [DOI] [PubMed] [Google Scholar]
- 8.Johnson JB, Summer W, Cutler RG, Martin B, Hyun DH, Dixit VD, Pearson M, Nassar M, Telljohann R, Maudsley S, Carlson O, John S, Laub DR, Mattson MP. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42:665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- 10.Lee J, Duan W, Mattson MP. Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J Neurochem. 2002;82:1367–1375. doi: 10.1046/j.1471-4159.2002.01085.x. [DOI] [PubMed] [Google Scholar]
- 11.Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu D, Chan SL, de Souza-Pinto NC, Slevin JR, Wersto RP, Zhan M, Mustafa K, de Cabo R, Mattson MP. Mitochondrial UCP4 mediates an adaptive shift in energy metabolism and increases the resistance of neurons to metabolic and oxidative stress. Neuromolecular Med. 2006;8:389–414. doi: 10.1385/NMM:8:3:389. [DOI] [PubMed] [Google Scholar]
- 13.Calabrese EJ, Bachmann KA, Bailer AJ, Bolger PM, Borak J, Cai L, Cedergreen N, Cherian MG, Chiueh CC, Clarkson TW, Cook RR, Diamond DM, Doolittle DJ, Dorato MA, Duke SO, Feinendegen L, Gardner DE, Hart RW, Hastings KL, Hayes AW, Hoffmann GR, Ives JA, Jaworowski Z, Johnson TE, Jonas WB, Kaminski NE, Keller JG, Klaunig JE, Knudsen TB, Kozumbo WJ, Lettieri T, Liu SZ, Maisseu A, Maynard KI, Masoro EJ, McClellan RO, Mehendale HM, Mothersill C, Newlin DB, Nigg HN, Oehme FW, Phalen RF, Philbert MA, Rattan SI, Riviere JE, Rodricks J, Sapolsky RM, Scott BR, Seymour C, Sinclair DA, Smith-Sonneborn J, Snow ET, Spear L, Stevenson DE, Thomas Y, Tubiana M, Williams GM, Mattson MP. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222:122–128. doi: 10.1016/j.taap.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjöquist PO, Pernow J. Adiponectin protects against myocardial ischaemia-reperfusion injury via AMPactivated protein kinase, Akt, and nitric oxide. Cardiovasc Res. 2008;78:116–122. doi: 10.1093/cvr/cvn017. [DOI] [PubMed] [Google Scholar]
- 15.Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective effects of short-term caloric restriction are mediated by adiponectin via activation of AMP-activated protein kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- 16.Natarajan R, Salloum FN, Fisher BJ, Kukreja RC, Fowler AA., 3rd Hypoxia inducible factor-1 upregulates adiponectin in diabetic mouse hearts and attenuates post-ischemic injury. J Cardiovasc Pharmacol. 2008;51:178–187. doi: 10.1097/FJC.0b013e31815f248d. [DOI] [PubMed] [Google Scholar]
- 17.Tao L, Gao E, Jiao X, Yuan Y, Li S, Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ, Ma XL. Adiponectin cardioprotection after myocardial ischemia/reperfusion involves the reduction of oxidative/nitrative stress. Circulation. 2007;115:1408–14316. doi: 10.1161/CIRCULATIONAHA.106.666941. [DOI] [PubMed] [Google Scholar]
- 18.Crandall DL, Feirer RP, Griffith DR, Beitz DC. Relative role of caloric restriction and exercise training upon susceptibility to isoproterenol-induced myocardial infarction in male rats. Am J Clin Nutr. 1981;34:841–847. doi: 10.1093/ajcn/34.5.841. [DOI] [PubMed] [Google Scholar]
- 19.Broderick TL, Belke T, Driedzic WR. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol Cell Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Nakae S, Terry RD, Mokhtari GK, Gunawan F, Balsam LB, Kaneda H, Kofidis T, Tsao PS, Robbins RC. Cardiomyocyte-specific Bcl-2 overexpression attenuates ischemia-reperfusion injury, immune response during acute rejection, and graft coronary artery disease. Blood. 2004;104:3789–3796. doi: 10.1182/blood-2004-02-0666. [DOI] [PubMed] [Google Scholar]
- 21.Ferrandi C, Ballerio R, Gaillard P, Giachetti C, Carboni S, Vitte PA, Gotteland JP, Cirillo R. Inhibition of c-Jun N-terminal kinase decreases cardiomyocyte apoptosis and infarct size after myocardial ischemia and reperfusion in anaesthetized rats. Br J Pharmacol. 2004;142:953–960. doi: 10.1038/sj.bjp.0705873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren G, Dewald O, Frangogiannis NG. Inflammatory mechanisms in myocardial infarction. Curr Drug Targets Inflamm Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 23.Sugano M, Koyanagi M, Tsuchida K, Hata T, Makino N. In vivo gene transfer of soluble TNF-alpha receptor 1 alleviates myocardial infarction. FASEB J. 2002;16:1421–1422. doi: 10.1096/fj.01-0894fje. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP. Dietary factors, hormesis and health. Ageing Res Rev. 2008;7:43–48. doi: 10.1016/j.arr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shibata R, Izumiya Y, Sato K, Papanicolaou K, Kihara S, Colucci WS, Sam F, Ouchi N, Walsh K. Adiponectin protects against the development of systolic dysfunction following myocardial infarction. J Mol Cell Cardiol. 2007;42:1065–1074. doi: 10.1016/j.yjmcc.2007.03.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takemura Y, Walsh K, Ouchi N. Adiponectin and cardiovascular inflammatory responses. Curr Atheroscler Rep. 2007;9:238–243. doi: 10.1007/s11883-007-0025-4. [DOI] [PubMed] [Google Scholar]
- 27.Martin B, Pearson M, Kebejian L, Golden E, Keselman A, Bender M, Carlson O, Egan J, Ladenheim B, Cadet JL, Becker KG, Wood W, Duffy K, Vinayakumar P, Maudsley S, Mattson MP. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology. 2007;148:4318–4333. doi: 10.1210/en.2007-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]