Abstract
The adult peripheral taste system is capable of extensive functional plasticity after injury. Sectioning the chorda tympani (CT), a primary sensory afferent nerve, elicits transient changes in the uninjured, contralateral population of taste receptor cells. Remarkably, the deficits are specific to the sodium transduction pathway. Normal function is quickly restored in the intact nerve, in parallel with an influx of macrophages to both the denervated and uninjured sides of the tongue. However, changing the dietary environment by restricting sodium blocks the macrophage response and prolongs functional alterations. Since the functional deficits occur before macrophages are present in the peripheral taste system, we hypothesized that neutrophils play a role in modulating neural responses in the intact CT. First, the dynamics of the neutrophil response to nerve injury were analyzed in control-fed and sodium-deficient rats. Nerve sectioning briefly increased the number of neutrophils on both the denervated and uninjured sides of the tongue. The low-sodium diet amplified and extended the bilateral neutrophil response to injury, in parallel with the persistent changes in sodium taste function. To test the impact of neutrophils on taste function, we depleted these cells prior to nerve sectioning and recorded neural responses from the intact CT. This treatment restored normal sodium responses in the uninjured nerve. Moreover, recruiting neutrophils to the tongue induced deficits in sodium taste function in both CT nerves. Neutrophils play a critical role in ongoing inflammatory responses in the oral cavity, and may induce changes in taste perception. We also suggest that balanced neutrophil and macrophage responses enable normal neural responses after neural injury.
Keywords: neural degeneration, neuro-immune interactions, chorda tympani nerve, epithelial sodium channel (ENaC), lipopolysaccharide, taste receptor cells
The immune response to neural and receptor cell degeneration has both positive and negative effects on functional recovery (Schwartz, 2003, Scholz and Woolf, 2007, Cui et al., 2009). The taste system provides an excellent model for studying the complex role of immune activity after neural injury. Taste buds on the anterior two-thirds of the tongue are trophically maintained by the innervation of the chorda tympani nerve (CT). Upon sectioning of this nerve, taste buds degenerate (Guth, 1971). The CT nerve later regenerates, reinnervates new taste receptor cells, and normal taste function is restored in a well-defined set of steps (Cheal et al., 1977, Hill and Phillips, 1994). Thus, the immune response to neural injury can be analyzed in a system where functional recovery is routine.
Another advantage is that the adult peripheral taste system remains sensitive to environmental manipulation after nerve injury. Taste receptor cells that regenerate under altered dietary conditions exhibit selective functional deficits. A sodium deficient diet decreases neural responses to sodium stimuli in the regenerated CT (Hill and Phillips, 1994, Hendricks et al., 2002, McCluskey and Hill, 2002). These changes mimic the suppressive effect of the diet on sodium taste responses during development (Hill and Mistretta, 1990).
Taste receptor cells in the contralateral sensory field remain normally innervated by the uninjured CT nerve, but exhibit profound functional changes nonetheless. Sodium responses are first suppressed by contralateral CT sectioning in combination with a sodium-deficient diet. Taste responses to sodium progressively become hypersensitive, however, by about seven weeks after contralateral injury (Hill and Phillips, 1994). Others have also shown changes on the uninjured side of the tongue after unilateral CT sectioning. For example, short-circuit current decreases in both the contralateral, uninjured and denervated lingual epithelium in control-fed dogs (Simon et al., 1993). Intact neurons also demonstrate increased spontaneous activity after neighboring spinal or peripheral nerves are injured (Campbell and Meyer, 2006). Generally these far-reaching effects initiated by neighboring injury are not widely appreciated.
Leukocytes appear to mediate the effects of injury on nearby taste receptor cells. Activated macrophages respond to CT nerve sectioning, and spread to the adjacent, uninjured side of the tongue. Sodium deficiency blocks the macrophage response, and also results in decreased sodium responses in uninjured taste receptors (Hill and Phillips, 1994, McCluskey, 2004). However, upregulation of immune function by systemic lipopolysaccharide (LPS) restores both normal macrophage levels and taste function in the neighboring CT (Phillips and Hill, 1996, Cavallin and McCluskey, 2005). Manipulations which damage nearby taste nerves or the lingual epithelium, in combination with the dietary treatment, also result in reduced taste function in the neighboring CT (Hendricks et al., 2002).
We recently demonstrated that sodium responses are reduced at 24 hr after contralateral axotomy, regardless of dietary treatment. Normal taste function recovers by day 2 in control-fed animals, but persists indefinitely in sodium-deficient rats (Wall and McCluskey, 2008). These changes occur before the bilateral macrophage response to injury, and are inconsistent with findings that macrophage levels predict normal taste responses (McCluskey, 2004, Cavallin and McCluskey, 2005, Guagliardo et al., 2009). Thus, we hypothesized that another leukocyte population mediates early functional deficits in uninjured taste receptor cells. Neutrophils are the first leukocytes to arrive at damaged tissue, and release molecules that can affect neural function (Borregaard et al., 2007). Polymorphonuclear leukocytes, or neutrophils, have also been observed within canine taste buds after denervation (Olmsted, 1921). We tested whether neutrophils impair taste function, and if injury is a prerequisite to neural plasticity in the adult taste system.
EXPERIMENTAL PROCEDURES
Animals
The Animal Care and Use Committee at the Medical College of Georgia approved all protocols, which followed guidelines set by the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Female specified pathogen-free Sprague Dawley rats (Charles River, Wilmington, MA) were 116 to 259 g at the time of treatment. Rats were housed in cages with barrier tops and received autoclaved food, bedding, and water.
Groups and experimental manipulations
CT sectioning and sodium depletion
To determine the dynamics of the neutrophil response to CT sectioning, separate groups of rats (n=66) received the following treatments: 1) Unilateral CT section and dietary sodium restriction (“CT Section + Diet”; n=6 each at four post-sectioning periods); 2) CT sectioning alone (“CT Section”; n=6 each at four post-sectioning periods); 3) Dietary sodium restriction alone (n=6); 4) Sham section alone (control for the surgical incision; n=6); 5) No treatment (normal controls; n=6). Rats receiving nerve section were injected with atropine sulfate [0.5 mg/ml; intraperitoneal (i.p.)] and then anesthetized with a mixture of ketamine (40 mg/kg; i.p.) and xylazine (10 mg/kg; i.p.). The right CT nerve was exposed through a ventral approach and transected after its bifurcation from the lingual nerve, as in previous work (Hill and Phillips, 1994, McCluskey, 2004). After unilateral CT sectioning, rats in sodium-restricted groups received two injections of the diuretic furosemide (10 mg each within 24 hours, i.p.; Sigma), low-sodium chow (0.03% NaCl, MP Biomedicals, Solon, OH) and distilled water. Administration of furosemide ensured rapid excretion of sodium. Rats in control-fed groups were maintained on control food (0.25% NaCl; Purina, St. Louis, MO) and tap water.
Neutrophil depletion
We depleted neutrophils systemically to determine their influence on peripheral taste function. Rats were injected with a rabbit anti-polymorphonuclear (PMN) antibody (3 ml/kg; Accurate, Westbury, NY; Cut + anti-PMN; n=5) or normal rabbit serum (Cut + NRS; n=5) in 0.9% sterile saline one day before CT sectioning (Anthony et al., 1997, Bennett et al., 1998, Perkins and Tracey, 2000, McColl et al., 2007, Ryu et al., 2007). On day 1 post-sectioning, neurophysiological responses to taste stimuli were recorded.
Lingual LPS injection
To stimulate lingual neutrophil infiltration, rats received subcutaneous injections of 10 µg LPS (E. coli 026:B6, Sigma, St. Louis, MO) in 10 µl sterile PBS (n=8). Injections were given to the ventral tongue (rather than the fungiform taste bud field) approximately 1 mm from the tip. Controls received PBS alone (n=4) or no treatment (n=5). Taste responses were recorded from CT nerves ipsilateral and contralateral to the injection site. Recordings were performed at 6 or 24 hr after LPS injections. Since the timing had no effect on responses (p > 0.05), these groups were combined for further analyses.
Neutrophil immunohistochemistry
Rats were overdosed with sodium pentobarbital (80 mg/kg; i.p.) and tongues dissected at day 0.5 (i.e. 12 hrs), 1, 2, or 3 after CT sectioning and/or sodium restriction (day 0). The neutrophil response was examined in coronal frozen sections from three regions of the fungiform taste bud field: 1) anterior; 2) mid; and 3) posterior tongue as previously described (McCluskey, 2004). In some rats, spleens were collected for positive control staining of neutrophils. Neutrophil responses to lingual LPS injection were also determined using this collection scheme.
Sections were fixed in 0.2% glutaraldehyde in phosphate-buffered saline (PBS; pH 7.5) and incubated in a well-characterized rabbit anti-rat myeloperoxidase antibody (MPO; 1:100; Abcam, Cambridge, MA) (Carlson et al., 1998, Perkins and Tracey, 2000, Klebanoff, 2005, Fleming et al., 2006, Nathan, 2006). MPO is abundant in neutrophils and is used to generate bacteriocidal acids (Abbas and Lichtman, 2003). Sections were then incubated in biotinylated goat anti-rabbit IgG (1:100; Jackson Immunoresearch, West Grove, PA), avidin-biotin complex (Vector Labs, Burlingame, CA), and developed with diaminobenzidine. Incubation in purified rabbit IgG (1:100; Abcam, Cambridge, MA) was used to assess non-specific staining.
Neutrophil analysis
Neutrophil counts were performed blinded to condition with a computer imaging system equipped with MetaMorph software (MDS Analytical Technologies, Downington, PA) and a digital color camera (Cool Snap; Roper Scientific/Photometrics, Tuscon, AZ). Images captured at 50× were used to count the number of neutrophils in four regions per coronal section: 1) the denervated epithelium and lamina propria; 2) the denervated submucosa and muscle; 3) the intact, contralateral epithelium and lamina propria; and 4) the intact submucosa and muscle. Immunopositive neutrophils within vessels were not counted. For each of the four regions of the tongue, a standard-sized area (12.9 mm2) was placed in the same position for a total of 206.4 mm2 per section. Thus, the selection and placement of the region of interest remained standard for each rat.
To quantify the neutrophil response to LPS injection the same procedures were followed, except that non-overlapping regions of interest were placed in areas visually determined to contain the highest density of neutrophils. The same epithelial and mucosal subregions (on the injured and intact sides of the tongue) described above were used. This method was used to analyze the macrophage response to gustatory nerve injury in previous work (McCluskey, 2004, Cavallin and McCluskey, 2005), and allowed us to focus on areas of inflammation. Images were minimally enhanced for contrast and color.
Assessing neutrophil depletion
We could not use the rabbit MPO antibody to detect neutrophils in depletion experiments since the anti-rabbit secondary antibody cross-reacted with injected protein. Therefore, we stained neutrophils with the HIS48 monoclonal antibody (1:10; AbD Serotec, Raleigh, NC) (Panichi et al., 2001, Reckless et al., 2001, Faas et al., 2003, Schaser et al., 2006, Iqbal et al., 2008), followed by anti-mouse IgM secondary antibody (Vector Labs). The staining was visualized with DAB and neutrophil depletion confirmed by counting round HIS48+ cells with a visible nucleus in a section from the anterior tongue. The specificity of neutrophil depletion was determined by staining macrophages with mouse anti-rat ED1 (1:400; ABD Serotec). Immunohistochemistry and analyses were performed according to previously published methods (McCluskey, 2004, Cavallin and McCluskey, 2005, Guagliardo et al., 2009).
Neurophysiology
CT recordings were performed to determine the influence of neutrophils on taste function. Rats were anesthetized with chloral hydrate (525 mg/kg, i.p.). Additional injections were given as necessary to maintain anesthesia at a surgical level. Dissection of the CT nerve and multifiber recordings were conducted as described in previous work (Hill and Phillips, 1994, Wall and McCluskey, 2008). Summated electrical activity was monitored and analyzed using PowerLab software (AD Instruments, Inc., Colorado Springs, CO).
Taste stimuli included concentration series (0.05–0.50M) of NaCl, sodium acetate (NaAc), and KCl salts. Stimulation with 1M sucrose, 0.01M quinine, 0.01N HCl, and 0.10–0.50M monosodium glutamate (MSG) was used to measure non-salt responses. All stimuli were presented at room temperature. Taste responses were analyzed by normalizing to 0.50M ammonium chloride (NH4Cl) responses at the beginning and end of each concentration series (Hill and Phillips, 1994; Phillips and Hill, 1996 (Wall and McCluskey, 2008). Specifically, the magnitude of steady state integrated responses was measured at 20 sec after onset, and expressed as a ratio of the mean steady state responses to NH4Cl before and after the series. We analyzed stable series bracketed by NH4Cl responses within 10% of each other. A concentration series of NaCl in 50µM amiloride was recorded at the end of each experiment to assess epithelial sodium channel (ENaC) function in taste receptor cells. Rats were then euthanized with sodium pentobarbital (80 mg/kg, i.p.) and tissues dissected.
Statistical analyses
The mean number of lingual neutrophils from control groups receiving either sham nerve sectioning, dietary sodium restriction, or no treatment (data not shown) were compared by analyses of variance (ANOVA) followed by Newman-Keuls post-tests using Prism 3.0 (GraphPad Software, La Jolla, CA). No differences were found between groups (p > 0.05). Therefore, counts from these groups were pooled and are referred to as the “Control” group in the analysis of neutrophil dynamics. The number of neutrophils in rats receiving CT section alone or in combination with sodium restriction was compared to Control values at 12 hours, day 1, 2, and 3 post-section. Specifically, the mean number of neutrophils on the sectioned and intact sides of the tongue was compared to corresponding control values using ANOVAs, followed by Dunnett’s post-tests where appropriate.
Mean relative response ratios from the intact CT nerve were compared in Cut+NRS vs. Cut+anti-PMN antiserum treated animals by one-tailed Student’s t-tests. The effect of depletion on neutrophil numbers was compared among these groups with one-tailed Student’s t-tests. In experiments using LPS to recruit neutrophils, ANOVAs followed by Newman-Keuls post-tests were used to compare three sets of neural responses: PBS-injected, CT nerve ipsilateral to LPS injection, and CT nerve contralateral to LPS injection. The number of neutrophils in LPS-treated vs. PBS-treated groups was assessed with one-tailed Student’s t-tests. The percentage of ED1+ pixels/standard area was evaluated with two-tailed Student’s t-tests to determine whether the manipulations increased or decreased macrophage levels. The α level was set at p ≤ 0.05 for all analyses.
RESULTS
Neutrophil responses to CT nerve injury in sodium-deficient and control-fed animals
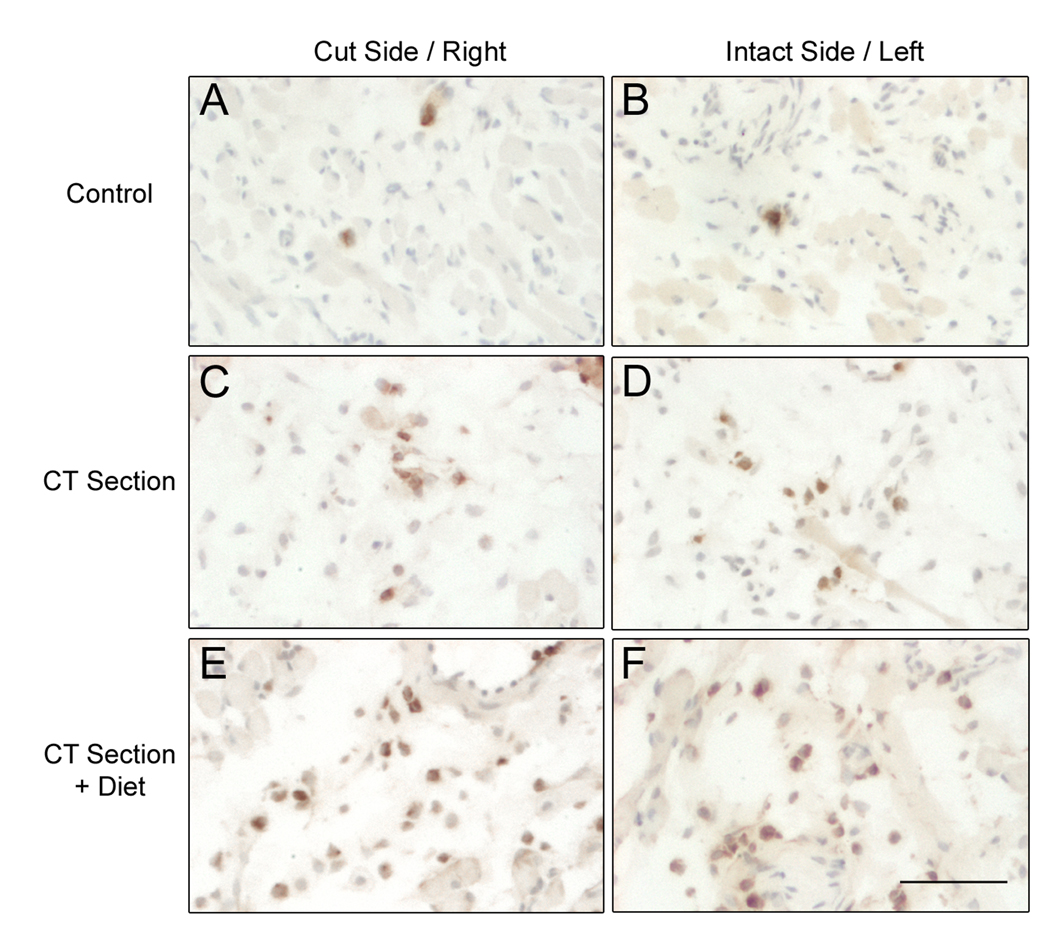
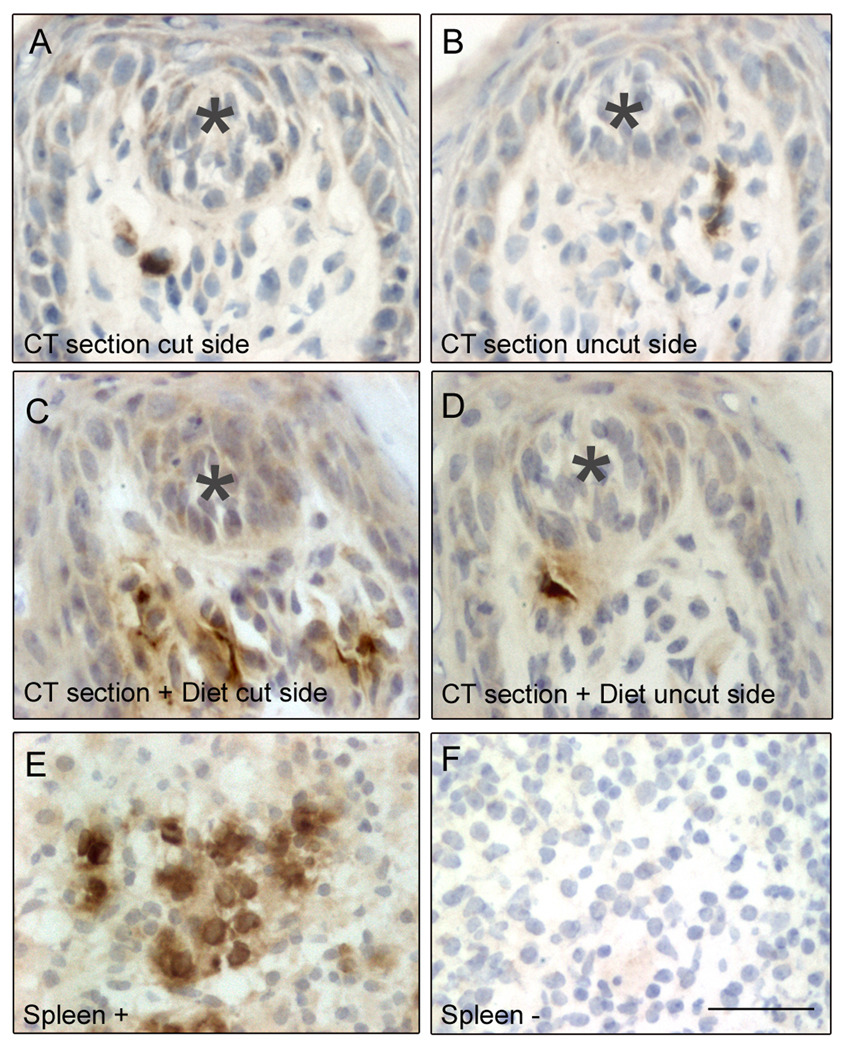
MPO+ neutrophils were present in the lingual mucosa, lamina propria, and fungiform papillae in all treatment groups (Fig. 1–2). Within fungiform papillae, neutrophils often had a polarized morphology typical of migrating cells (Hoffstein et al., 1982). Following nerve section, we observed more neutrophils in each tissue compartment. A sodium-deficient diet further increased neutrophils on both the denervated and uninjured sides of the tongue. Positive assay controls included MPO-stained spleen sections (Fig. 2). Tongue or spleen sections incubated in pre-immune rabbit IgG exhibited little non-specific staining (Fig. 2).
Figure 1.
Images of myeloperoxidase (MPO)+ neutrophils (brown) in the denervated (“Cut Side”) and uninjured (“Intact Side”) lingual mucosa at d1 after nerve sectioning. Right and left sides of a sham-sectioned control are shown for comparison. (A-B) Few neutrophils are present in control animals. CT nerve sectioning increases the number of neutrophils on the (C) injured and (D) contralateral, intact sides of the tongue. (E-F) Dietary sodium deficiency further augments the neutrophil response on both sides of the tongue. Scale bar in F = 60 µm.
Figure 2.
Myeloperoxidase (MPO)+ neutrophils (brown) in fungiform papillae at day 1 post-section. Immunopositive neutrophils are present in papillae adjacent to taste buds (indicated by grey stars) on the (A) sectioned (“cut”) and the (B) uninjured (“uncut”) sides of the tongue in control-fed animals. Neutrophils are more prominent after nerve sectioning in sodium-restricted (“diet”) rats on both the (C) denervated and (D) intact sides of the tongue. Some neutrophils display a more polarized morphology characteristic of migrating cells. (E) Positive control staining identifies neutrophils in spleen. (F) Little non-specific staining is present in negative control sections of spleen. Scale bar in F = 30 µm.
Quantitative analyses confirmed the effects of nerve sectioning and diet on neutrophils as shown in Fig. 3. At each time point, neutrophil responses were more robust in the anterior region of the fungiform sensory field. This region also has the highest density of taste buds, suggesting that neutrophils preferentially infiltrate regions near degenerating sensory cells. At 12 hr post-sectioning (Fig. 3A), neutrophils were increased on both the sectioned (p<0.05) and intact (p<0.05) sides of the anterior region of the tongue irrespective of dietary treatment. There were no significant differences in the mid and posterior regions of the tongue at this time (p>0.05).
Figure 3.
Dynamics of the lingual neutrophil response to CT nerve injury. Neutrophil responses in the “Control” group include untreated and sham-sectioned animals (day 1 post-injury). Control responses are shown for each time point for comparison with other groups. (A) Neutrophils are elevated on both the cut and intact sides of the anterior sensory field at day 0.5 post-section regardless of dietary regimen. Neutrophil responses to nerve injury are spatially restricted to the anterior region at this time. (B) At day 1 post-sectioning, the neutrophil response is significantly elevated on the intact side of the anterior tongue in control-fed animals. Sodium deficiency amplifies the bilateral neutrophil response in the anterior lingual region. On the denervated side of the tongue, neutrophils are also significantly increased in the mid and posterior regions of the fungiform taste field. (C) By day 2 post-sectioning, the number of lingual neutrophils in control-fed animals is similar to controls. Neutrophils remain significantly increased in sodium-deficient animals vs. controls in each region. (D) At day 3 post-sectioning, neutrophil responses subside, but are statistically elevated in sectioned vs. control groups. * p < 0.05; ** p < 0.001.
At day 1 after nerve section, the number of neutrophils subsided in control-fed animals, though it remained significantly elevated on the uninjured side of the anterior tongue (p<0.05; Figure 3B). The neutrophil response was even more striking in sodium-deficient animals. In this group, the number of neutrophils increased dramatically on both sides of the anterior region (p<0.001), and on the denervated side of the mid (p < 0.05) and posterior fungiform field (p < 0.001; Figure 3B).
By day 2 post-injury, the neutrophil response was still bilaterally increased in each lingual region in sodium-restricted (p < 0.05) but not control-fed rats (p > 0.05; Fig. 3C). Though the neutrophil response was statistically increased at day 3 after sectioning in the anterior lingual region of both dietary groups vs. controls (Fig. 3D; p < 0.05), the absolute number MPO+ cells approached baseline. Thus, the neutrophil response peaked at 12 hr post-injury in control-fed rats, while the altered dietary environment amplified and prolonged the response.
Neutrophil depletion restores normal sodium taste function
The neutrophil response to nerve injury is inversely predictive of sodium taste function (Wall and McCluskey, 2008). In other words, when the number of neutrophils increases, neural responses to sodium are reduced in the intact CT. Therefore, we tested the hypothesis that neutrophils mediate functional deficits in the uninjured taste receptor cells and CT nerve. We used two strategies to accomplish this. First, neutrophils were depleted by systemic injection of an anti-PMN antibody, and neurophysiological responses to taste stimuli were recorded from the uninjured CT at day 1 post-sectioning. Next, we confirmed the detrimental effect of neutrophils on sodium taste function by recruiting them to the uninjured peripheral taste system and recording taste responses.
Examples of representative CT responses to NaCl are shown in Figure 4, and mean responses to sodium stimuli in Figure 5. As expected from previous work (Wall and McCluskey, 2008), control animals treated with normal rabbit serum prior to nerve sectioning (Cut + NRS) demonstrated reduced CT responses to NaCl at day 1 post-injury (Fig. 4). In contrast, responses to NaCl were restored to normal levels when the neutrophil response was inhibited (Cut + anti-PMN; Fig. 4). Specifically, responses to 0.10 M (p=0.021), 0.25 M (p=0.003), and 0.50 M NaCl (p=0.010) were significantly elevated in anti-PMN-treated animals compared to controls (Fig. 5A). Responses to 0.10M–0.50M NaAc were also significantly increased in Cut + anti-PMN compared to Cut+NRS treated rats (p = 0.026 – 0.048; Figure 5B).
Figure 4.
Integrated responses to taste stimuli recorded from the intact chorda tympani (CT) nerve one day after contralateral nerve sectioning. (A) Responses from a rat that received a control injection of normal rabbit serum in combination with CT sectioning (Cut + NRS). Steady state responses to NaCl and NaAc (measured at 20 sec after onset) are reduced in the intact CT compared to the bracketing NH4Cl responses. (B) Responses from an animal that was injected with anti-PMN serum to deplete neutrophils prior to CT sectioning (Cut+anti-PMN). Neutrophil depletion elevates steady state responses to sodium stimuli compared to bracketing NH4Cl responses. Responses to non-sodium stimuli are similar in the two rats. The epithelial sodium channel blocker, amiloride (Amil), reduces steady state CT responses to NaCl to comparable levels (bottom) relative to NH4Cl responses in the control and neutrophil-depleted rat. Stimulus concentration is given in molarity (M) with the exception of .01 normal (N) HCl. Scale bars under 0.05M NaCl responses = 20 sec.
Figure 5.
Mean ± SEM responses to sodium in neutrophil-depleted and control rats. Responses were recorded from the intact CT at day 1 after contralateral nerve section. (A) CT responses to 0.10–0.50 NaCl are reduced in control animals receiving i.p. injection of normal rabbit serum (NRS) as expected at this early period. Depletion of neutrophils with anti-polymorphonuclear (PMN) serum restores normal neural responses to NaCl. (B) Responses to 0.10–0.50M sodium acetate (NaAc) are significantly higher (i.e. restored to normal levels) in anti-PMN vs. NRS treated groups after contralateral nerve injury. (C) The epithelial sodium channel antagonist, amiloride, reduces responses to 0.10–0.50M NaCl to the same level in neutrophil-depleted and neutrophil-replete groups. * p < 0.05.
Neutrophil depletion could elicit changes in amiloride-sensitive and/or amiloride-insensitive sodium transduction pathways in taste receptor cells (DeSimone and Lyall, 2006). Application of this ENaC antagonist to the tongue reduced mean responses to NaCl to the same magnitude in each group (p > 0.05; Fig. 5C), with the exception of the response to 0.05 M NaCl in anti-PMN treated animals. Furthermore, responses to non-sodium stimuli, including 0.05–0.50M KCl (Fig. 6A), 1M sucrose, 0.01M quinine, 0.01N HCl, and 0.3M MSG (Fig. 6B) were similar in neutrophil-depleted and control groups after contralateral CT sectioning (p>0.05). In sum, normal neural function is restored when the neutrophil response to contralateral injury is prevented. The sodium-specificity and amiloride sensitivity of the effect indicate that neutrophils impact sodium transduction through ENaC on uninjured taste receptor cells.
Figure 6.
Mean ± SEM neural responses to non-sodium stimuli in neutrophil-depleted and control groups. Responses were recorded from the intact chorda tympani (CT) nerve at 1 day after contralateral sectioning. (A) Neural responses to a concentration series of KCl did not significantly differ between anti-polymorphonuclear (PMN) and normal rabbit serum (NRS) injected groups (p>0.05). (B) Responses from the uninjured CT to sucrose, quinine, HCl, and MSG were similar in neutrophil-depleted and neutrophil-replete groups (p>0.05).
Confirmation of neutrophil depletion and specificity of anti-PMN treatment
Injection of anti-PMN serum dramatically reduced neutrophil infiltration of the tongue after CT nerve sectioning (Fig. 7). There was a significant decrease in the mean number of HIS48+ neutrophils on both the denervated (p=0.04) and intact (p=0.005) sides of the tongue (Fig. 7E). Previous work indicates that activated macrophages, another leukocyte population, respond to CT sectioning and affect taste function (McCluskey, 2004, Cavallin and McCluskey, 2005). Therefore, we tested the specificity of the anti-PMN treatment. There was no significant difference in the ED1+ macrophage response in anti-PMN and NRS treated groups on either side of the tongue (p > 0.05; not shown).
Figure 7.
Neutrophil responses to CT sectioning in neutrophil-depleted and control rats. HIS48 was used to label neutrophils following neurophysiological recordings. Neutrophils (brown) are present on the (A) sectioned and (B) intact side of the tongue in a rat receiving unilateral nerve sectioning and normal rabbit serum (NRS). Few neutrophils are present on the (C) sectioned or (D) uninjured side of the tongue in rats given CT sectioning and anti-polymorphonuclear (PMN) serum. (E) The number of neutrophils on the both sides of the tongue was significantly reduced by anti-PMN treatment. Scale bar in C = 30 µm. * p = 0.04; **p=0.005.
Lingual LPS injection impairs taste function
We also asked whether recruiting neutrophils to the tongue with an inflammatory stimulus, rather than nerve sectioning, would cause deficits in sodium taste function. Rats received a subcutaneous injection of either LPS or PBS to the ventral surface of the tongue. Local administration of LPS is commonly used to model inflammation, and is particularly effective in recruiting neutrophils (Rydell-Tormanen et al., 2006, Zhou et al., 2009). Taste responses were recorded from both CT nerves 24 hr later. Examples of CT response traces are shown in Fig. 8, and mean dose-response curves in Fig. 9.
Figure 8.
Integrated responses recorded from the chorda tympani (CT) nerve one day after subcutaneous injections of (A) PBS or (B) lipopolysaccharide (LPS; bottom) to the ventral tongue. In the rat treated with LPS to recruit neutrophils, steady state responses to NaCl and NaAc are low relative to bracketing NH4Cl responses. Steady state responses to other stimuli are comparable in the control and LPS-treated rat. Additional responses recorded in the MSG and miscellaneous (“Misc”) stimulus series were removed for presentation purposes, as seen in breaks in baseline. Stimulus concentration is given in molarity. Scale bars under 0.05M NaCl responses = 20 sec.
Figure 9.
Mean ± SEM CT responses one day after lingual lipopolysaccharide (LPS) injection to recruit neutrophils. (A) Responses to 0.10–0.50M NaCl were significantly reduced by lingual LPS injection compared control animals. CT nerves ipsilateral and contralateral to the ventral injection site exhibited the decrease in sodium taste function. (B) Responses to 0.25 and 0.50M sodium acetate (NaAc) were significantly decreased in both CT nerves in LPS-injected rats. (C) Responses to KCl were not significantly different in control compared to LPS-injected groups. (D) Responses to additional nonsodium stimuli did not significantly differ in LPS-injected compared to control groups. *p < 0.05; **p<0.01; ***p<0.001.
Neither neurophysiological responses nor neutrophil numbers were significantly different in PBS-injected vs. normal controls (p > 0.05). Thus, these groups were collapsed for further analyses. As shown in Fig. 8 and Fig. 9A, responses to 0.10–0.50M NaCl were significantly reduced in LPS-injected animals vs. PBS injected controls (p < 0.001–0.05). Neural responses to 0.25 M and 0.50M NaAc were also decreased by LPS injection (Fig. 9B; p < 0.01). Sodium taste function was inhibited in CT nerves ipsilateral and contralateral to the LPS injection site. In contrast, responses to non-sodium stimuli such as a concentration series of KCl (Fig. 9C), 0.10 and 0.50M MSG, 1.0M sucrose, and 0.01M quinine (Fig. 9D; p > 0.05) were not significantly different between groups. Thus, a lingual neutrophil response to an inflammatory stimulus or nerve injury is detrimental to peripheral taste function. The functional change is specific to one taste modality, indicating that sodium transduction in taste receptor cells is affected.
Leukocyte responses to lingual LPS injection
We analyzed leukocyte infiltration in the tongues of rats receiving ventral LPS or PBS injection after recordings were completed. MPO+ neutrophils were more numerous in the fungiform papillae and lingual mucosa at day 1 after LPS injection (Fig. 10). Quantitative analyses of neutrophils in the anterior region of the fungiform field demonstrated a significant increase ipsilateral (p = 0.005) and contralateral (p = 0.011) to the ventral LPS injection site (Fig. 10G). The number of neutrophils was also elevated bilaterally in the mid and caudal regions of the fungiform field in LPS-injected vs. control rats (Fig. 10H-I; p=0.0001–.0.006). Thus, ventral injection of LPS recruited neutrophils throughout the rostral-caudal axis of the fungiform field, while nerve injury initiated a more spatially restricted neutrophil response.
Figure 10.
MPO+ neutrophils (brown) recruited by lipopolysaccharide (LPS) injection to the ventral tongue. (A-B) Few neutrophils are present in fungiform papillae near taste buds (gray stars) on either side of the tongue in PBS-injected control rats. (C) Small numbers of neutrophils are found in the lingual mucosa in PBS-injected controls. (D-E) Many neutrophils are observed in fungiform papillae adjacent to taste buds, both ipsilateral and contralateral to LPS injections. (F) Robust neutrophil responses to LPS are observed in the lingual mucosa. (G-I) LPS induces a significant, bilateral increase in neutrophils compared to controls in each region of the fungiform field. Scale bar in E = 30 µm. Scale bar in F = 60 µm. *p<0.05; **p <0.01; ***p<0.001.
We also determined whether LPS initiated macrophage entry to the tongue, since these leukocytes are predictive of normal sodium taste function after injury (McCluskey, 2004, Cavallin and McCluskey, 2005, Guagliardo et al., 2009). ED1+ activated macrophages were elevated on the uninjected (p = 0.03) but not the injected side of the tongue (p>0.05) compared to PBS-injected controls (not shown). However, neutrophils appear to be a more prominent part of the leukocyte response to LPS. In summary, in the current work we define the dynamics of the neutrophil response to injury in the taste system, and demonstrate by recruitment and depletion that neutrophils have detrimental effects on sodium taste function.
DISCUSSION
The peripheral gustatory system is capable of extraordinary functional plasticity, even in adulthood. Converging evidence has suggested that the immune response to injury plays an important role in early functional changes in the neighboring, intact taste receptor cells (Phillips and Hill, 1996, Hendricks et al., 2002, McCluskey, 2004, Cavallin and McCluskey, 2005, Cavallin and McCluskey, 2007a, Guagliardo et al., 2009). In the current study, we identify immune cells that mediate changes in sodium taste function soon after CT nerve injury. Within 12 hr post-sectioning, neutrophils are recruited to both sides of the anterior tongue. The neutrophil response is quickly resolved in control-fed animals, in parallel with the recovery of normal taste function. Dietary sodium restriction amplifies and extends both the bilateral neutrophil response to nerve injury and abnormal sodium responses in the intact nerve. Importantly, we demonstrate that blocking the neutrophil response to injury restores normal neural responses to 0.10M–0.50M NaCl and NaAc. Neutrophils responding to a local inflammatory stimulus, in the absence of nerve injury, also induce deficits in sodium taste function.
Potential mechanisms of altered sodium transduction
One of the most intriguing aspects of this and previous work is the sodium specificity of functional changes induced by nerve injury or lingual inflammation. The sodium transduction pathway is the only modality affected by these events. Moreover, group-related differences in sodium responses are abolished by the ENaC blocker, amiloride, in nerve injury experiments. These findings suggest a specific cellular mechanism for the regulation of taste receptor cell function by neutrophils. Namely, neutrophils are proposed to release factors that modulate ENaC expression and/or function in taste receptors as they do in other epithelial cells. For example, neutrophil elastase activates a silent subset of ENaC channels in bronchial epithelial cells (Caldwell et al., 2005). Neutrophils also produce cytokines capable of regulating ENaC, including interleukin (IL)-1β and tumor necrosis factor (TNF)-α (Barmeyer et al., 2004, Dagenais et al., 2004, Roux et al., 2005, Choi et al., 2007). The molecular mechanisms of neutrophil-taste cell interactions are currently being resolved in vitro.
Dynamics and regulation of innate immune responses in the gustatory system
We demonstrate that neutrophils are detrimental to taste function. Yet macrophages, another type of leukocyte, are predictive of normal taste function after neighboring nerve injury (McCluskey, 2004, Cavallin and McCluskey, 2005). How can these opposing effects on taste responses be resolved? We suggest that the dynamics and composition of cellular infiltration are key aspects of the immune response to neural injury (Fig. 11). Neutrophils are the first inflammatory invaders after nerve injury in the peripheral taste system, as they are throughout the body (Abbas and Lichtman, 2003). The neutrophil response to CT injury is quickly diminished in control-fed animals, as macrophages invade the fungiform taste field. Normal taste function is then restored in the neighboring, intact CT (McCluskey, 2004). Dietary sodium deficiency disrupts the normal immune response to injury, in addition to its developmental effects in the gustatory system (Hill and Mistretta, 1990). The diet amplifies and extends the neutrophil response, abolishes the macrophage response to axotomy (McCluskey, 2004, Guagliardo et al., 2009), and abnormal sodium taste function persists (Phillips and Hill, 1996, Hendricks et al., 2002, Wall and McCluskey, 2008). Thus, a balanced immune response to injury is critical for the function of neighboring, intact sensory receptor cells and associated neurons.
Figure 11.
Summary of leukocyte responses to nerve injury and effects on gustatory function. (Left) The dorsal surface of the tongue is shown schematically. In this unilateral nerve injury model, one chorda tympani nerve (CT) is sectioned, and neurophysiological responses are recorded from the contralateral, uninjured CT nerve. The denervated and uninjured (shaded) taste receptor fields are distinctly innervated. (Right) Innate immune responses and their temporal relationship to neural responses in the uninjured CT nerve are depicted. (Top) After nerve sectioning, neutrophils briefly invade the tongue. CT responses to sodium also decrease transiently. Activated macrophages invade, neutrophil responses decline, and normal neural responses are restored. (Bottom) Dietary sodium deficiency perturbs innate immune responses to gustatory nerve injury. The neutrophil response is augmented and extended in sodium-restricted animals. The activated macrophage response remains at baseline levels, and abnormal sodium taste responses persist.
In the scenario depicted in Fig. 11, macrophages may play dual roles in the injured or infected peripheral taste system. First, macrophages could release factors which affect sodium transduction pathways in taste receptor cells, either directly or as part of an inflammatory cascade. This occurs in lung epithelial cells, as activated macrophages downregulate the expression of the ENaC-α, β, and γ subunits as well as amiloride-sensitive sodium transport (Dickie et al., 2000). However, in the peripheral taste system, we suggest that macrophages may play a beneficial role in restoring ENaC function and normal taste responses (Wall and McCluskey, 2008).
Macrophages also terminate the neutrophil response by initiating apoptosis in vitro (Meszaros et al., 2000). Thus, the sodium-deficient diet may act, at least in part, by blocking recruitment of the cells that halt neutrophil activity. Sodium restriction decreases the expression of vascular adhesion molecule (VCAM)-1, one of the precursors to macrophage entry, after CT nerve injury (Cavallin and McCluskey, 2007a). Recent work shows that administration of exogenous aldosterone, which is elevated by sodium restriction, also blocks macrophage responses to CT section and suppresses sodium taste responses in the neighboring, intact nerve (Guagliardo et al., 2009). If macrophages fail to respond to injury, the neutrophil response proceeds unchecked as evident in sodium-deficient animals. The result is an amplified and prolonged neutrophil response that negatively affects peripheral taste function.
Neutrophil responses in other injured neural systems
Neutrophils play an important role during injury and inflammation, as they degrade damaged tissue, generate reactive oxygen species, and kill bacteria (Nathan, 2006, Borregaard et al., 2007). These leukocytes typically infiltrate injured neural tissues within 24 hr, consistent with the dynamics of the response in denervated and uninjured taste receptor cell fields shown here (Taoka et al., 1997, Carlson et al., 1998, Perkins and Tracey, 2000, Fleming et al., 2006). While neutrophils have beneficial roles in clearing bacteria from injured tissues (Nathan, 2006), their effects on intact “bystander” neurons are generally undesirable, as we find in the peripheral taste system. The role of neutrophils in neural function has been widely explored by manipulating the inflammatory response to damage. Blocking the neutrophil response to nerve injury ameliorates pain hypersensitivity after sciatic nerve injury (Perkins and Tracey, 2000), improves motor function after spinal cord injury (Taoka et al., 1997), and protects striatal neurons from quinalone-induced degeneration (Ryu et al., 2007). Neutrophils are also cytotoxic to hippocampal neurons (Dinkel et al., 2004) and dorsal root ganglion cells (Shaw et al., 2008) in vitro. However, the mechanisms responsible for the adverse effects of neutrophils on neurons are not fully understood, and the consequences of neutrophil recruitment on other specialized sensory receptor cells are largely unknown.
Inflammation and taste function
Neutrophil recruitment to the tongue elicits functional deficits in nearby taste receptor cells, suggesting that oral or lingual infection might lead to taste disturbances. A link between infection or oral inflammation and taste disorders is well-described, though medications, age, and underlying medical conditions likely contribute to perceptual changes (Schiffman, 1997, Bromley and Doty, 2003). Experimental evidence showing that inflammation modulates taste function in the absence of injury is sparse, though a recent report suggests that LPS can affect circumvallate taste cell function. Systemic injection of LPS (i.p.) reduced the expression of the immediate early gene, c-fos, in murine circumvallate taste receptor cells, suggesting that activity is reduced (Wang et al., 2007). In contrast, systemic LPS administration had no effect on fungiform taste receptor cell function in otherwise untreated rats, as assessed by CT nerve recordings (Phillips and Hill, 1996). Rather, targeting of the immune response to the tongue by denervation of taste receptor cells (Phillips and Hill, 1996) or lingual endotoxin, as in the current work, was required to enact changes in neural signals from the anterior two-thirds of the tongue.
Local injection of LPS is often used to model inflammation and study innate immune responses. This endotoxin signals through the toll-like receptor (TLR)-4 to activate endothelial cells. Activated endothelial cells then initiate leukocyte tethering, rolling, and transmigration through vessels to the tissue (Kerfoot and Kubes, 2005, Zarbock and Ley, 2008). Taste receptor cells exist within a barrier epithelium exposed to mechanical damage and pathogens. Thus, neutrophil recruitment with subsequent modulation of gustatory function may be an ongoing event (Olmsted, 1921), though analyses of the human peripheral taste system have largely focused on lymphocytes and dendritic cells (Cruchley et al., 1989, Feng et al., 2009). While lingual LPS has effects in addition to neutrophil recruitment, complementary results in this study show that when neutrophils respond to either CT nerve injury or lingual inflammation, sodium taste function is altered in intact taste receptor cells.
These studies provide needed insight to gustatory-immune interactions, which in turn impact taste perception and nutritional choices. They also contribute to our understanding of leukocyte responses to injury and inflammation in a neural system which successfully regenerates. An emerging theme in this work is that leukocyte responses must be tightly controlled to maintain normal neural function, even in neighboring, uninjured sensory receptor cells. Finally, these findings highlight the ability of the innate immune system to elicit plasticity in the adult peripheral taste system.
Acknowledgements
Supported by National Institutes of Health grant DC005811 to L.P.M. We thank Laura McKie for her assistance with graphic illustration.
Abbreviations
- CT
Chorda tympani nerve
- ENaC
Epithelial sodium channel
- IL
Interleukin
- LPS
Lipopolysaccharide
- MPO
Myeloperoxidase
- MSG
Monosodium glutamate
- NaAc
Sodium acetate
- NH4Cl
Ammonium chloride
- NRS
Normal rabbit serum
- PBS
Phosphate-buffered saline
- QHCl
Quinine hydrochloride
- Anti-PMN
Anti-polymorphonuclear antiserum
- TNF
Tumor necrosis factor
- TLR
Toll-like receptor
- VCAM
Vascular cell adhesion molecule
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbas AK, Lichtman AH. Cellular and Molecular Immunology. Philadelphia: Saunders; 2003. [Google Scholar]
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain. 1997;120(Pt 3):435–444. doi: 10.1093/brain/120.3.435. [DOI] [PubMed] [Google Scholar]
- Barmeyer C, Amasheh S, Tavalali S, Mankertz J, Zeitz M, Fromm M, Schulzke JD. IL-1beta and TNFalpha regulate sodium absorption in rat distal colon. Biochem Biophys Res Commun. 2004;317:500–507. doi: 10.1016/j.bbrc.2004.03.072. [DOI] [PubMed] [Google Scholar]
- Bennett G, al-Rashed S, Hoult JR, Brain SD. Nerve growth factor induced hyperalgesia in the rat hind paw is dependent on circulating neutrophils. Pain. 1998;77:315–322. doi: 10.1016/S0304-3959(98)00114-6. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Bromley SM, Doty RL. Clinical disorders affecting taste: evaluation and management. In: Doty RL, editor. Handbook of Taste and Olfaction. Basel: Marcel Dekker, Inc.; 2003. pp. 935–957. [Google Scholar]
- Caldwell RA, Boucher RC, Stutts MJ. Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol. 2005;288:L813–L819. doi: 10.1152/ajplung.00435.2004. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Lipopolysaccharide-induced up-regulation of activated macrophages in the degenerating taste system. J Neurosci Res. 2005;80:75–84. doi: 10.1002/jnr.20438. [DOI] [PubMed] [Google Scholar]
- Cavallin MA, McCluskey LP. Upregulation of intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 after unilateral nerve injury in the peripheral taste system. J Neurosci Res. 2007a;85:364–372. doi: 10.1002/jnr.21128. [DOI] [PubMed] [Google Scholar]
- Cheal M, Dickey WP, Jones LB, Oakley B. Taste fiber responses during reinnervation of fungiform papillae. J Comp Neurol. 1977;172:627–646. doi: 10.1002/cne.901720406. [DOI] [PubMed] [Google Scholar]
- Choi JY, Choi YS, Kim SJ, Son EJ, Choi HS, Yoon JH. Interleukin-1beta suppresses epithelial sodium channel beta-subunit expression and ENaC-dependent fluid absorption in human middle ear epithelial cells. Eur J Pharmacol. 2007;567:19–25. doi: 10.1016/j.ejphar.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Cruchley AT, Williams DM, Farthing PM, Lesch CA, Squier CA. Regional variation in Langerhans cell distribution and density in normal human oral mucosa determined using monoclonal antibodies against CD1, HLADR, HLADQ and HLADP. J Oral Pathol Med. 1989;18:510–516. doi: 10.1111/j.1600-0714.1989.tb01353.x. [DOI] [PubMed] [Google Scholar]
- Cui Q, Yin Y, Benowitz LI. The role of macrophages in optic nerve regeneration. Neuroscience. 2009;158:1039–1048. doi: 10.1016/j.neuroscience.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagenais A, Frechette R, Yamagata Y, Yamagata T, Carmel JF, Clermont ME, Brochiero E, Masse C, Berthiaume Y. Downregulation of ENaC activity and expression by TNF-alpha in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L301–L311. doi: 10.1152/ajplung.00326.2002. [DOI] [PubMed] [Google Scholar]
- DeSimone JA, Lyall V. Taste receptors in the gastrointestinal tract III. Salty and sour taste: sensing of sodium and protons by the tongue. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1005–G1010. doi: 10.1152/ajpgi.00235.2006. [DOI] [PubMed] [Google Scholar]
- Dickie AJ, Rafii B, Piovesan J, Davreux C, Ding J, Tanswell AK, Rotstein O, O'Brodovich H. Preventing endotoxin-stimulated alveolar macrophages from decreasing epithelium Na+ channel (ENaC) mRNA levels and activity. Pediatr Res. 2000;48:304–310. doi: 10.1203/00006450-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Dinkel K, Dhabhar FS, Sapolsky RM. Neurotoxic effects of polymorphonuclear granulocytes on hippocampal primary cultures. Proc Natl Acad Sci U S A. 2004;101:331–336. doi: 10.1073/pnas.0303510101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faas MM, Van Der Schaaf G, Schipper M, Moes H. Glomerular immunoglobulin deposits induce glomerular inflammation in pregnant but not in non-pregnant rats. Am J Reprod Immunol. 2003;49:57–63. doi: 10.1034/j.1600-0897.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- Feng P, Yee KK, Rawson NE, Feldman LM, Feldman RS, Breslin PA. Immune cells of the human peripheral taste system: dominant dendritic cells and CD4 T cells. Brain Behav Immun. 2009;23:760–766. doi: 10.1016/j.bbi.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- Guagliardo NA, West KN, McCluskey LP, Hill DL. Attenuation of peripheral salt taste responses and local immune function contralateral to gustatory nerve injury: effects of aldosterone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1103–R1110. doi: 10.1152/ajpregu.00219.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L. Degeneration and regeneration of taste buds. In: Antrum H, editor. Handbook of Sensory Physiology. vol.IV. Berlin: Springer-Verlag; 1971. pp. 63–74. [Google Scholar]
- Hendricks SJ, Sollars SI, Hill DL. Injury-induced functional plasticity in the peripheral gustatory system. J Neurosci. 2002;22:8607–8613. doi: 10.1523/JNEUROSCI.22-19-08607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Mistretta CM. Developmental neurobiology of salt taste sensation. Trends Neurosci. 1990;13:188–195. doi: 10.1016/0166-2236(90)90046-d. [DOI] [PubMed] [Google Scholar]
- Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994;14:2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein ST, Friedman RS, Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982;95:234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal S, Thomas A, Bunyan K, Tiidus PM. Progesterone and estrogen influence postexercise leukocyte infiltration in overiectomized female rats. Appl Physiol Nutr Metab. 2008;33:1207–1212. doi: 10.1139/H08-108. [DOI] [PubMed] [Google Scholar]
- Kerfoot SM, Kubes P. Local coordination verses systemic disregulation: complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. J Leukoc Biol. 2005;77:862–867. doi: 10.1189/jlb.1004607. [DOI] [PubMed] [Google Scholar]
- Klebanoff SJ. Myeloperoxidase: friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- McCluskey LP. Up-regulation of activated macrophages in response to degeneration in the taste system: effects of dietary sodium restriction. J Comp Neurol. 2004;479:43–55. doi: 10.1002/cne.20307. [DOI] [PubMed] [Google Scholar]
- McCluskey LP, Hill DL. Sensitive periods for the effect of dietary sodium restriction on intact and denervated taste receptor cells. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1275–R1284. doi: 10.1152/ajpregu.00282.2002. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meszaros AJ, Reichner JS, Albina JE. Macrophage-induced neutrophil apoptosis. J Immunol. 2000;165:435–441. doi: 10.4049/jimmunol.165.1.435. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Olmsted J. Effects of cutting the lingual nerve of the dog. Journal Comp Neurol. 1921;33:149–154. [Google Scholar]
- Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G. Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int. 2001;60:87–95. doi: 10.1046/j.1523-1755.2001.00775.x. [DOI] [PubMed] [Google Scholar]
- Perkins NM, Tracey DJ. Hyperalgesia due to nerve injury: role of neutrophils. Neuroscience. 2000;101:745–757. doi: 10.1016/s0306-4522(00)00396-1. [DOI] [PubMed] [Google Scholar]
- Phillips LM, Hill DL. Novel regulation of peripheral gustatory function by the immune system. Am J Physiol. 1996;271:R857–R862. doi: 10.1152/ajpregu.1996.271.4.R857. [DOI] [PubMed] [Google Scholar]
- Reckless J, Tatalick LM, Grainger DJ. The pan-chemokine inhibitor NR58-3.14.3 abolishes tumour necrosis factor-alpha accumulation and leucocyte recruitment induced by lipopolysaccharide in vivo. Immunology. 2001;103:244–254. doi: 10.1046/j.1365-2567.2001.01228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux J, Kawakatsu H, Gartland B, Pespeni M, Sheppard D, Matthay MA, Canessa CM, Pittet JF. Interleukin-1beta decreases expression of the epithelial sodium channel alpha-subunit in alveolar epithelial cells via a p38 MAPK-dependent signaling pathway. J Biol Chem. 2005;280:18579–18589. doi: 10.1074/jbc.M410561200. [DOI] [PubMed] [Google Scholar]
- Rydell-Tormanen K, Uller L, Erjefalt JS. Neutrophil cannibalism--a back up when the macrophage clearance system is insufficient. Respir Res. 2006;7:143. doi: 10.1186/1465-9921-7-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu JK, Tran KC, McLarnon JG. Depletion of neutrophils reduces neuronal degeneration and inflammatory responses induced by quinolinic acid in vivo. Glia. 2007;55:439–451. doi: 10.1002/glia.20479. [DOI] [PubMed] [Google Scholar]
- Schaser KD, Stover JF, Melcher I, Lauffer A, Haas NP, Bail HJ, Stockle U, Puhl G, Mittlmeier TW. Local cooling restores microcirculatory hemodynamics after closed soft-tissue trauma in rats. J Trauma. 2006;61:642–649. doi: 10.1097/01.ta.0000174922.08781.2f. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Taste and smell losses in normal aging and disease. Jama. 1997;278:1357–1362. [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Owolabi SA, Bagley J, Morin N, Cheng E, LeBlanc BW, Kim M, Harty P, Waxman SG, Saab CY. Activated polymorphonuclear cells promote injury and excitability of dorsal root ganglia neurons. Exp Neurol. 2008;210:286–294. doi: 10.1016/j.expneurol.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, Elliott EJ, Erickson RP, Holland VF. Ion transport across lingual epithelium is modulated by chorda tympani nerve fibers. Brain Res. 1993;615:218–228. doi: 10.1016/0006-8993(93)90031-h. [DOI] [PubMed] [Google Scholar]
- Taoka Y, Okajima K, Uchiba M, Murakami K, Kushimoto S, Johno M, Naruo M, Okabe H, Takatsuki K. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79:1177–1182. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- Wall PL, McCluskey LP. Rapid changes in gustatory function induced by contralateral nerve injury and sodium depletion. Chem Senses. 2008;33:125–135. doi: 10.1093/chemse/bjm071. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou M, Brand J, Huang L. Inflammation Activates the Interferon Signaling Pathways in Taste Bud Cells. J Neurosci. 2007;27:10703–10713. doi: 10.1523/JNEUROSCI.3102-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Ley K. Mechanisms and consequences of neutrophil interaction with the endothelium. Am J Pathol. 2008;172:1–7. doi: 10.2353/ajpath.2008.070502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Andonegui G, Wong CH, Kubes P. Role of endothelial TLR4 for neutrophil recruitment into central nervous system microvessels in systemic inflammation. J Immunol. 2009;183:5244–5250. doi: 10.4049/jimmunol.0901309. [DOI] [PubMed] [Google Scholar]