Abstract
Protease inhibitors (PIs) have been implicated in the development of HIV-associated lipodystrophy through a reduction in the differentiation of preadipocytes. While atazanavir (ATV) is associated with fewer clinical metabolic abnormalities in the short-term, the effects of long-term exposure are not known. ATV effects on preadipocyte replication or differentiation would indicate the potential for long-term problems. This study compared ritonavir (RTV) and ATV effects on preadipocyte replication and differentiation in human primary cultures. Preadipocytes from subcutaneous fat were studied in the presence of therapeutic concentrations of RTV and ATV for replication, differentiation, and adipokine secretion. The effects of the drugs on the expression of PPARγ and related genes during differentiation were also assessed by real time quantitative PCR. RTV induced a significant inhibition of preadipocyte proliferation, differentiation and adiponectin secretion. ATV at concentrations within the range of therapeutic levels did not affect differentiation or adiponectin secretion, but did have inhibitory effects on preadipocyte proliferation. Inhibition of differentiation by PIs was associated with a decrease expression of PPARγ, C/EBPα, and aP2 genes.
In summary, although ATV at therapeutic levels has a smaller impact on adipogenesis, alterations in preadipocyte proliferation suggest the potential for adverse effects with long-term use.
Keywords: Adipogenesis, adiponectin, HIV protease inhibitors, preadipocyte, PPARγ
1. INTRODUCTION
Protease inhibitors (PIs) have been linked to the HIV-associated lipodystrophy (LD), characterized by loss of peripheral fat (lipoatrophy), dyslipidemia and insulin resistance (Jain et al., 2001; Koutkia and Grinspoon, 2004; Miller et al., 2003; Rudich et al., 2005) and in particular to a reduced ability of preadipocytes to differentiate (Dowell et al., 2000; Grigem et al., 2005; Jones et al., 2008; Lenhard et al., 2000). However, not all PI have the same propensity to induce metabolic complications or cause the same degree of adverse effects (Jain et al., 2001). Among the more currently used PIs, ritonavir (RTV), a very effective drug often used as a booster agent, is often associated with clinical LD. In contrast, atazanavir (ATV), a relatively newer PI, has been shown to be associated with fewer metabolic abnormalities and improve dyslipidemia and metabolic profile in patients previously treated with other PIs (Becker, 2003; Jemsek et al., 2006; Möbius et al., 2005; Sension et al., 2009). However, it is still not completely clear whether the use of ATV, in addition to reducing metabolic abnormalities, may also prevent or minimize the development of lipoatrophy. A better understanding of the effects of a PI on adipocyte metabolism is an important step in ensuring that PIs are both effective and not associated with side effects with long-term use.
At the cellular level, loss of body fat associated with PI treatment has been shown to be mainly related to inhibition of adipocyte differentiation with lower accumulation of lipids (Dowell et al., 2000; Rudich et al., 2005). Even in the absence of HIV infection, PIs have been shown to inhibit adipocyte differentiation and lipid accumulation, impair insulin signaling, and adipokine secretion (e.g. (Dowell et al., 2000; Grigem et al., 2005; Kim et al., 2006; Roche et al., 2002; Rudich et al., 2005; Zhang et al., 1999)). Most studies on the effect of PIs on adipogenesis have only addressed aspects of differentiation and have not assessed proliferation of preadipocytes. Preadipocyte proliferation is important since cells undergo clonal expansion prior to differentiation (Otto and Lane, 2005). Inhibition of preadipocyte proliferation may represent an additional mechanism by which PIs contribute to lipoatrophy, i.e. by reducing the number of precursor cells available for differentiation. Little is known about the effect of ATV on this important pathway. Therefore, this study was undertaken to compare the effects of RTV and ATV on preadipocyte replication. The study was performed in human primary cell cultures rather than rodent or human cell lines as used by others (e.g. (Dowell et al., 2000; Grigem et al., 2005; Kim et al., 2006; Lenhard et al., 2000; Roche et al., 2002; Vernochet et al., 2005; Zhang et al., 1999)) in order to reflect more nearly conditions in patients. Preadipocytes from subcutaneous fat, the depot generally affected by lipoatrophy, were studied in the presence of therapeutic concentrations of both RTV and ATV. Cells were assessed for replication, differentiation, and adipokine secretion. The molecular mechanism of the effects of PI on adipogenesis was also explored by observing changes in the expression of genes involved in the PPARγ pathway (i.e., PPARγ C/EBPα, SRBP-1 and aP2) during differentiation.
2. METHODS
2.1. Patients and study design
Subcutaneous fat tissue samples were obtained from healthy kidney donors undergoing nephrectomy at the Stony Brook University Medical Center. 15 Subjects (11 females, 4 males,) with an average age of 42 ± 12 y and BMI of 30 ± 5, all HIV-seronegative, participated in the study. Subcutaneous fat was removed from the mid-abdominal wall area near the ombelicus, after induction of standard general anesthesia and immediately placed in a sterile Hank’s Buffered Salt Solution (HBSS) containing antibiotics and amphotericin, pH 7.4. Preadipocytes were isolated from the adipose tissue and submitted in vitro to antiretroviral medications RTV at 0, 5 and 10 µmol/L and ATV at 0, 10 and 30 µmol/L on both the preadipocyte replication and preadipocyte differentiation. The effects of antiretroviral medications were compared with control samples which were preadipocytes cultured and stimulated to differentiate as described below but in the absence of antiretroviral medications.
The protocol was approved by the Stony Brook University Institutional Review Board and all subjects gave a written informed consent before enrollment in the study.
2.2. Pre-adipocyte isolation and culture
Fat biopsies were immediately transported to the lab, and processed within one hour from collection. Preadipocytes were isolated in aseptic conditions, with a modification of a previously described method (Hauner et al., 1989). Briefly, fat specimens were washed, freed from any visible blood vessel and connective tissue, and digested for 45 min at 37°C in phosphate-buffered saline (PBS) containing 3 mg/ mL collagenase (Type II, Worthington, Lakewood, NJ), and 3.5 % bovine serum albumin, pH 7.4. Following digestion, stromal cells were separated from mature adipocytes by centrifugation and incubated in erythrocyte lysing buffer (154 mmol/L NH4Cl, 10 mmol/L K2HPO4, 1 mmol/L EDTA, pH 7.4) for 10 min at room temperature to eliminate contaminating red blood cells. Cell suspension was then filtered through a 70 µm nylon filter to remove debris and then centrifuged. Pelleted preadipocytes were resuspended and plated in medium consisting of DMEM/F-12 (Gibco, Carlsbad, CA) supplemented with 10 % Fetal Calf Serum (FCS), 2 mmol/L glutamine, 100 IU/mL penicillin and 100 µg/mL streptomycin (Gibco). After incubation at 37°C in a humidified atmosphere with 5% CO2 for 16–18 h, attached cells were extensively washed with warm PBS, trypsinized and counted. Preadipocytes were then plated out in the same medium at a density of 5 × 103/ cm2.
For the proliferation experiments, cells were incubated for 48 hours to allow an optimal attachment. After 48 hours the medium was aspirated off and replaced with fresh medium containing RTV and ATV at varying concentrations. Cell growth (see below) was then assessed after 24 and 72 hours.
For the assessment of differentiation and gene expression, cells were grown in medium until reaching confluence. After reaching confluence, differentiation was induced by replacing the basal medium with a chemically defined serum-free medium containing varying concentrations of RTV (0, 5 and 10 µmol/L) and ATV (0, 10 and 30 µmol/L). The differentiation medium consisted of DMEM/F-12 medium supplemented with 15 mmol/L HEPES, 10 mg/L transferrin, 33 µmol/L biotin, 17 µmol/L pantothenate, 0.5 µmol/L insulin, 0.1 µmol/L dexamethasone, 0.5 nmol/L triiodothyronine (T3), 0.5 µmol/L rosiglitazone, 540 µmol/L 3-isobutyl-l-methylxanthine (IBMX) (Tchkonia et al., 2002). In all experiments medium was replaced every 2–3 days and differentiation assessed after 12 days.
Since RTV and ATV were dissolved in DMSO, all control cultures contained a comparable amount of vehicle alone (< 0.1 %).
2.3. Assessment of preadipocyte proliferation
The effect of antiretroviral drugs RTV and ATV on preadipocyte cell number was assessed with a 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) based assay, as we have previously described (Caso et al., 2004). After addition of a 5 mg/mL MTT solution (Sigma, St. Louis, MO), cells were incubated for 3 hours at 37°C, 5% CO2. Insoluble formazan crystals from the conversion of MTT by mitochondrial dehydrogenases of viable cells were then dissolved in DSMO and measured spectrophotometrically at 570 nm with background subtraction at 690 nm (Carmichael et al., 1987).
Preliminary experiments demonstrated the validity of the MTT assay to assess cell number. Preadipocytes plated at densities of 1, 2, 4, 6, 8, 12, 16, 32 × 103 /0.3 cm2 for 24 hours indicated a linear relationship between absorbance and cell density (R = 0.996).
Since the cultures were all plated at the same initial cell density, the cell number after 24–72 hours is taken as a measure of cell replication, with a reduction in cell number taken to represent a decrease in the rate at which cells were proliferating.
2.4. Assessment of preadipocyte differentiation
Adipocyte differentiation was quantified after 12 day by assessing the activity of the lipogenic marker enzyme glycerol-3-phosphate dehydrogenase (GPDH), by measuring the intracellular lipid accumulation after Oil Red O staining and by directly counting the proportion of differentiated cells.
GPDH assay
GPDH activity was determined spectrophotometrically by monitoring the oxidation of NADH, as previously described (Wise and Green, 1979). Cells were harvested in cold 50 mmol/L TRIS buffer (pH 7.5) containing 1 mmol/L EDTA and 1 mmol/L 2-mercaptoethanol. The enzymatic assay mixture consisted of 50 mmol/L triethanolamine, 0.16 mmol/L NADH, 5 mmol/L EDTA and 0.1 mmol/L 2-mercaptoethanol, pH 7.5. The reaction was started by addition of 0.2 mmol/L dihydroxyacetone phosphate and the oxidation of NADH was assessed from the change in absorbance at 340 nm using a Spectra Max M5 spectophotometer (Molecular Devices, Sunnyvale, CA). GPDH activity was standardized to the amount of cell protein and expressed as mU/ml/mg of protein, with 1 mU corresponding to the amount of enzyme oxidizing 1 nmol of NADH/min.
Oil Red O staining
After fixing the cells in a 10 % formaldehyde solution in PBS, neutral lipids were stained with Oil Red O, as described previously (Janderová et al., 2003). The intracellular lipid accumulation was then quantified by extracting the dye in isopropanol and assessing the absorbance spectrophotometrically at 500 nm.
Cell counting
Adipocyte differentiation was also assessed by directly counting the number of differentiated and undifferentiared cells. Cultures stained with Oil Red O were counterstained with hematoxylin to visualize nuclei/cell contours. Differentiated cells were easily discriminated because of the characteristic multiple cytoplasmic lipid droplets, which stained bright red with Oil Red O. Five areas from each double-stained culture were randomly selected and photographed at a 40× magnification. All the differentiated and un-differentiated cells in each picture (≥ 200 cells per picture, ≥ 1000 cells in total) were counted by a person blinded to the treatment groups. The percent of differentiated cells in each treatment was obtained by averaging the results of the 5 replicates. The average coefficient of variation for the 5 replicates from a sample was approximately 12 %.
Assessment of gene expression
Expression of peroxisome proliferator-activated receptor γ (PPARγ), CCAT-enhancer binding protein α (C/EBP1α), the sterol-regulatory-element-binding protein-1 (SREBP1c), the adipocyte fatty acid binding protein (aP2), and interleukin 6 (IL-6) mRNA was assessed before and 2, 4, 8 and 12 days after addition of the differentiation medium by quantitative real-time reverse transcriptase-polymerase chain reaction (qRT-PCR). Total cell RNA was extracted with RNeasy lipid tissue mini kit (Qiagen, Inc., Valencia, CA), according to the Manufacturer's instructions. cDNA was prepared using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA). All PCR reactions were carried out using Tagman Master Mix and commercially available primers (all from Applied Biosystems) with an Opticon Real Time Detection System (Bio-Rad Laboratories, Hercules, CA). The expression of multiple genes in multiple cultures from the same individual were calculated with the 2−ΔΔCT method (Livak and Schmittgen, 2001) and normalized to the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene as internal control. Data are presented as the fold change in expression relative to expression of the target gene in undifferentiated pre-adipocytes.
Adiponectin production
The ability of cultures to secrete the adipokine, adiponectin, was determined after 12 days in differentiation medium containing varying concentrations of RTV or ATV. After incubation for 2 hours in fresh medium, the amount of adiponectin released in the incubation medium was measured by ELISA, with a commercially available kit (Linco Research, St. Charles, MO). Adiponectin production was normalized to the cell protein and expressed as ng/mg protein.
2.5. Statistics
Results are expressed as means ± SD. Differences between control and treated cultures were analyzed using a randomized block ANOVA with post-hoc Bonferroni’s multiple comparison test. P values ≤ 0.05 were considered statistically significant.
3. RESULTS
3.1. Effect of PIs on preadipocyte proliferation
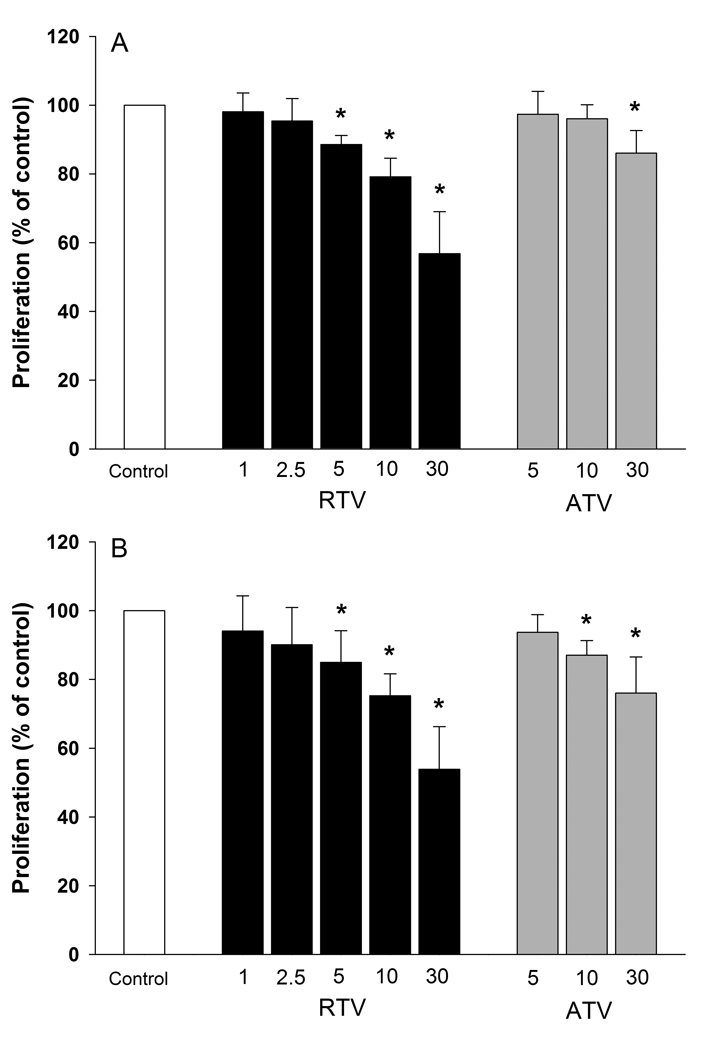
Both RTV and ATV inhibited preadipocyte proliferation in a concentration-dependent manner. As shown in Figure 1, the effect of RTV was evident at drug concentrations ≥ 5 µmol/L. In cultures with 5 µmol/L RTV, cell number was reduced by 11 % at 24 hours (P < 0.01) and by 15 % at 72 hours (P = 0.05) compared to controls. Inhibition of replication increased progressively with increasing concentrations of RTV. In the presence of 30 µmol/L RTV, the cell number was reduced by 43 % at 24 hours (P < 0.001) and 46 % at 72 hours (P < 0.001) compared to controls (Fig. 1). ATV also depressed preadipocyte proliferation, although at concentrations higher than RTV. At a concentration of 5 µmol/L, ATV did not have a detectable effect on cell number. The cell number of cultures incubated with 10 µmol/L ATV was comparable to that of controls at 24 hours, but significantly lower after 72 hours (−13%, P < 0.005). Increasing ATV concentrations to 30 µmol/L resulted in a depression of cell number by 14 % at 24 hours and 24 % (P < 0.001) at 72 hours (Fig. 1).
Figure 1. Pre-adipocyte proliferation in the presence of varying concentrations (µmol/L) of ATV and RTV for 24 (Panel A) and 72 hours (Panel B) (MTT test).
The results are expressed as percent values of control cultures (mean ± SD, n = 6). * P ≤ 0.05.
3.2. Effect of PIs on preadipocyte differentiation
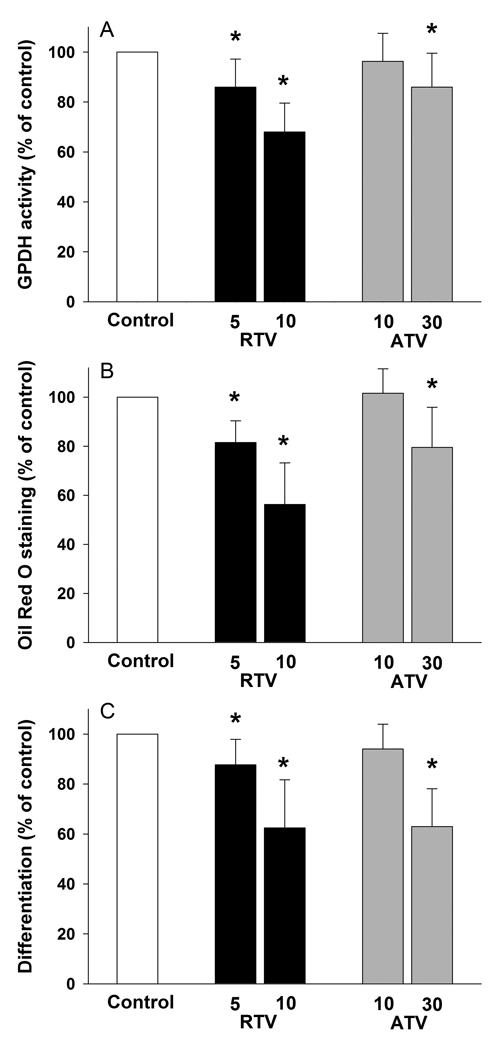
Assessment of GPDH enzyme activity showed a significant reduction of preadipocyte differentiation by RTV at both 5 and 10 µmol/L (Fig. 2A). GPDH activity was reduced by 14 % (P < 0.005) in the presence of 5 µmol/L RTV. Inhibition of differentiation was more prominent when RTV concentration was increased to 10 µmol/L (−32%, P < 0.001; Fig. 2A). ATV showed no effect on GPDH activity at a concentration of 10 µmol/L. However, a significant reduction of GPDH activity was observed when ATV concentration was increased to 30 µmol/L (− 28%, P < 0.05; Fig. 2A).
Figure 2. Effect of RTV (5 and 10 µmol/L) and ATV (10 and 30 µmol/L) on differentiation of human preadipocytes.
Differentiation was assessed after 12 days by measuring the GPDH activity (panel A), Oil Red O staining (Panel B) and by direct count of the proportion of differentiated cells (Panel C). All results are expressed as percent values of control cultures. mean ± SD, (RTV groups, n = 10; ATV groups n = 11). * P ≤ 0.05.
Measurement of lipid accumulation by quantification of eluted Oil Red O stain also revealed an inhibition of differentiation by RTV. RTV at concentration of 5 and 10 µmol/L inhibited lipid accumulation by 18 (P = 0.05) and 44 % (P < 0.001) respectively (Fig. 2B). Similarly to the assessment of GPDH activity, ATV only affected lipid accumulation at a concentration of 30 µmol/L (− 20%, P < 0.005) (Fig. 2B).
Direct observation of the cell cultures confirmed that RTV not only inhibited lipid accumulation but also reduced the number of differentiating cells. The percent of differentiated cells in cultures with 5 and 10 µmol/L RTV were respectively 12 % (P = 0.05) and 36 % (P = 0.001) lower than controls (Fig. 2C). Similar concentrations of ATV had no effect on the proportion of differentiated cells (Fig. 2C). However, a 20 % reduction in the proportion of differentiated cells was observed at 30 µmol/L ATV concentrations (P < 0.001) (Fig. 2C).
3.3. Effect of PIs on adiponectin secretion
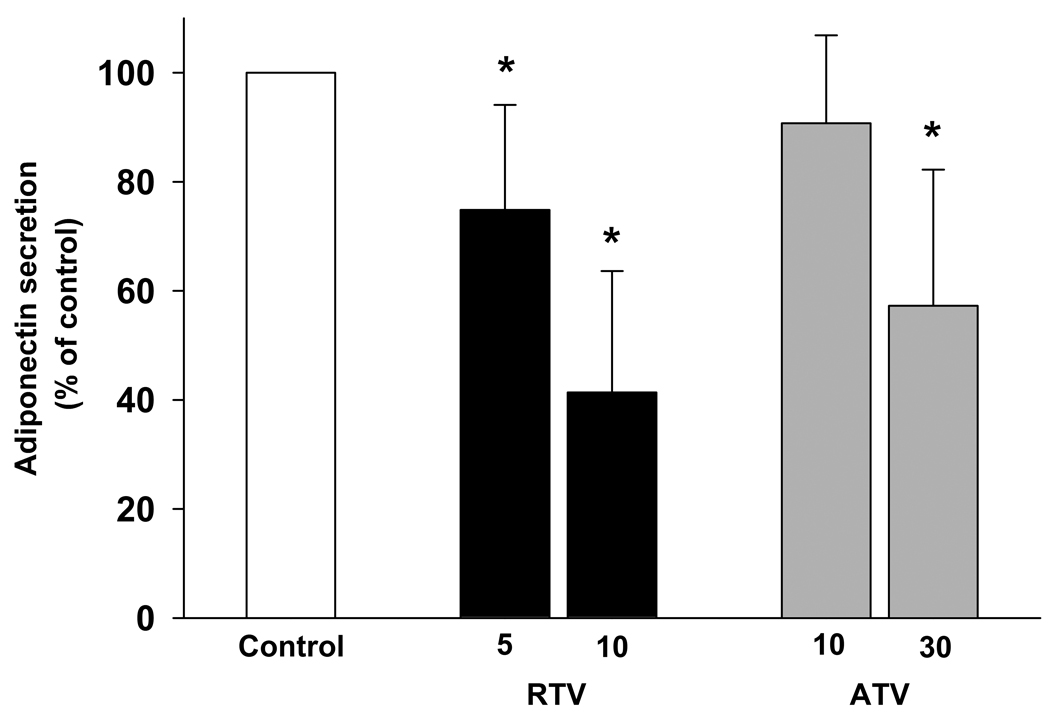
A significant depression in adiponectin secretion was observed in the presence of RTV. Adiponectin secretion was reduced by 25 % (P < 0.01) at 5 µmol/L RTV and by 59 % (P < 0.001) at 10 µmol/L RTV (Fig. 3). A reduction in the secretion of adiponectin was also detected in the presence of ATV, but only at a concentration of 30 µmol/L (−43%, P < 0.05; Fig. 3).
Figure 3. Effect of RTV (5 and 10 µmol/L) and ATV (10 and 30 µmol/L) on adiponectin secretion.
Adiponectin secretion was assessed after 12 days differentiation. Results were calculated as ng/µg of cell protein and expressed as percent values of control cultures. mean ± SD, (RTV group, n = 9; ATV group n = 10). * P < 0.05.
3.4. Gene expression during preadipocyte differentiation in the presence of PIs
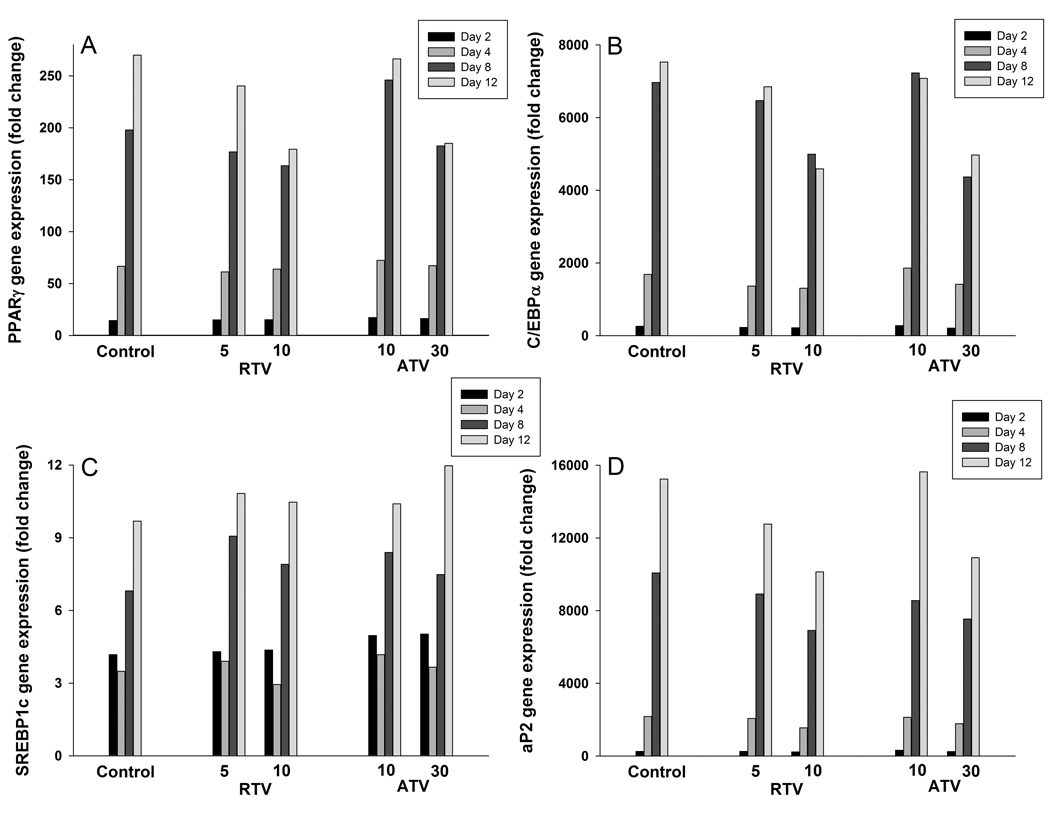
The expression of SREBP1c, PPARγ, C/EBP1α, and aP2 genes progressively increased over the differentiation period (Fig. 4, Control). The expression of C/EBP1α was maximal on day 8, while the expression of the other 3 genes was highest on day 12 (Fig. 4). Although the expression of SREBP1c also increased with length of exposure to the differentiation medium, SREBP1c expression was higher at day 2 (43% of the final value) than expression of PPARγ, C/EBP1α, and aP2 . Incubation with 5 and 10 µmol/L RTV progressively inhibited the increase of PPARγ, C/EBP1α, and aP2 gene expression on day 8 and 12. ATV also showed a similar effect, but only at 30 µmol/L concentration (Fig. 4). There was no evident effect of both PIs on the expression of the SREBP1c gene.
Figure 4. Effect of RTV (5 and 10 µmol/L) and ATV (10 and 30 µmol/L) on the time course expression of PPARγ (Panel A), C/EBP1α (Panel B), SREBP1c (Panel C), and a2P (Panel D) genes during differentiation.
Gene expression was assessed by qRT-PCR in control and treated cultures on day 2, 4, 8 and 12. Data are expressed as fold changes from undifferentiated preadipocytes and normalized to GAPDH mRNA expression.
The expression of the IL-6 gene did not show any change over the differentiation period, and was not affected by either PI (data not shown).
4. DISCUSSION
In assessing the potential for the PIs RTV and ATV to affect adipocyte phenotype, there are three possible ways in which to reduce the formation of mature adipose tissue; one, is to reduce the number of cells available for differentiation (reduced predipocyte proliferation, i.e. MTT assay), a second is to reduce the proportion of preadipocytes which differentiate (i.e, cell counting), and a third is to reduce the amount of lipid which accumulates per differentiated adipocyte (i.e. Oil Red O staining). In fact, in this study there is evidence that both protease inhibitors RTV and ATV exhibit all three mechanisms. There was an inhibition of proliferation and both aspects of differentiation in primary cultures of human preadipocytes treated with RTV and ATV. Although both drugs showed a concentration-dependent effect on both proliferation and differentiation, higher concentrations of ATV were needed than the concentrations of RTV eliciting negative effects. The inhibition of preadipocyte differentiation by either PI was associated with an inhibition of the production of the adipokine, adiponectin, and with lower expression of genes controlling the differentiation process (i.e. PPARγ and C/EBP1α) or coding for markers of the mature adipocyte phenotype (i.e. aP2).
RTV depressed both proliferation of preadipocytes and preadipocyte differentiation at concentrations within the range of those observed in the plasma of HIV patients on HAART (Gatti et al., 1999). At a concentration of 10 µmol/L, which is approximately 3–4 fold lower than the maximal plasma concentration of the drug (i.e. Cmax) (Gatti et al., 1999), RTV inhibited lipid accumulation by 46% (Fig. 2B). The Negative effects of RTV on total adipocyte formation resulted from both a reduction of the number of cells differentiating into mature adipocytes (Fig. 2C) and the amount of fat synthesized in each cell (Fig. 2B). At the same concentrations inhibiting differentiation, RTV also showed a depressive effect on preadipocyte proliferation (Fig. 1).
Incubation of preadipocytes with ATV within the normal therapeutic range inhibited preadipocyte replication (10 µmol/L, Fig.1B). A decrease in the proliferative capacity of preadipocytes could potentially affect lipogenesis by resulting in a smaller pool of cells with ability to differentiate to mature adipocytes. However, the inhibitory effect of ATV on preadipocyte differentiation was only observed at 30 µmol/L concentration, which is generally higher than maximal plasma levels observed during HAART (i.e. Cmax ~ 10 µmol/L) (Solas et al., 2008). The lack of effects of ATV on adipogenesis in the range of concentrations observed in the plasma of treated HIV patients is in line with the clinical findings that treatment with ATV is associated with fewer lipid abnormalities and alterations in body fat composition generally associated with lipodystrophy (Haerter et al., 2004; Jemsek et al., 2006; Möbius et al., 2005; Sension et al., 2009), and in keeping with in vitro data showing that differentiation of preadipocytes was significantly inhibited by RTV, but not by ATV a concentration of 10 µmol/L (Jones et al., 2008). However, although ATV may overall have less negative impact on fat differentiation than RTV, the effect on preadipocyte replication suggests the potential for a reduction in overall adipose tissue formation.
The mechanisms responsible for the depression of adipocyte differentiation by PI are not completely clear, although these may include a disturbance in the function of PPARγ-dependent pathways (Caron et al., 2009), perhaps mediated by SREBP1c (Caron et al., 2001; Caron et al., 2009; Fajas et al., 1999). In the present study, the expression of SREBP1c, PPARγ and C/EBP1α genes progressively increased during differentiation, as did that of the PPARγ-dependent and adipocyte specific gene, aP2, but were depressed in the culture treated with 5 and 10 µmol/L RTV and 30 µmol/L ATV (Fig. 4). The depressive effects of PI on PPARγ and C/EBP1α gene expression are in line with evidence of reduced expression of PPARγ and C/EBP1α mRNA in fat samples of lipodystrophic HIV-infected patients treated with PIs (Bastard et al., 2002) and support the view that alterations of the PPARγ pathway may play a primary role in the pathogenesis of the disturbances of adipocyte differentiation caused by PIs (Caron et al., 2009; Dowell et al., 2000). Interestingly, the blunting effect of PIs on PPARγ and C/EBP1α gene expressions was not evident early in the differentiation process, but was only observed after 4 d (Fig. 4), suggesting that the effects of PIs on the PPARγ pathway may not be part of the early events of differentiation. Differently from PPARγ and C/EBP1α, SREBP1c gene expression was not affected by RTV and ATV during differentiation (Fig. 4).
Several alterations in the production of cytokines and adipokines have been observed in HIV-treated patients. In particular, increased plasma levels of pro-inflammatory cytokines and reduced production of adiponectin by the fat tissue have been related to alterations in glucose metabolism and the development of insulin resistance and other metabolic abnormalities associated with lipodystrophy (Jan et al., 2004; Lagathu et al., 2005; Lihn et al., 2003; Mynarcik et al., 2002; Sutinen et al., 2003). Previous studies using murine and human preadipocyte cell lines have shown that PIs can directly stimulate IL-6 and suppress adiponectin gene expressions (Grigem et al., 2005; Kim et al., 2006; Lagathu et al., 2004), suggesting that the inhibitory effect of PIs on adiponectin may be mediated by the induction of IL-6 (Grigem et al., 2005). Unlike studies using human preadipocyte cell lines (Grigem et al., 2005; Kim et al., 2006), the present study did not find an alteration in IL-6 gene expression with PIs. An inhibition of adiponectin production was observed in the primary cultures in the present study with 5 and 10 µmol/L RTV and 30 µmol/L ATV, findings in contrast to Kim et al. (Kim et al., 2006), who did not observe inhibition of adiponectin production in a differentiated human adipocyte cell line treated with 50 µmol/L ATV for 18 h. The current study would suggest that the decrease in adiponectin production with PIs is not mediated by IL-6.
In summary, although the study is limited by being an in vitro assessment, it does suggest that PIs may contribute to lipoatrophy by affecting fat cell differentiation and metabolism. In particular, RTV within therapeutic plasma concentrations inhibited both preadipocyte proliferation and ability of preadipocytes to differentiate into mature adipocytes, lipid accumulation and adiponectin secretion. In contrast, ATV at concentrations close to those observed in treated patients did not affect differentiation or adiponectin secretion but showed an inhibitory effect on predipocyte proliferation. Although the clinical observations with ATV suggest fewer metabolic and fat distribution abnormalities, the finding that ATV may hinder preadipocyte replication suggests that there is still potential for deleterious effects on the accumulation of adipose tissue especially with long term exposure to the drug.
ACKNOWLEDGMENTS
The authors would like to thank Mr. Stephen Knapik for his invaluable help in recruiting, Dr. Louise Stuart for her assistance in cell counting, and all the patients who kindly agreed to participate in the study.
The study was supported in part by NIH grant DK049316, NIH General Clinical Research Grant 5-MO1-RR-10710, and New York Empire Clinical Research Investigator Program (ECRIP) fellowship to GC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bastard JP, Caron M, Vidal H, Jan V, Auclair M, Vigouroux C, Luboinski J, Laville M, Maachi M, Girard PM, Rozenbaum W, Levan P, Capeau J. Association between altered expression of adipogenic factor SREBP1 in lipoatrophic adipose tissue from HIV-1-infected patients and abnormal adipocyte differentiation and insulin resistance. Lancet. 2002;359:1026–1031. doi: 10.1016/S0140-6736(02)08094-7. [DOI] [PubMed] [Google Scholar]
- Becker S. Atazanavir: improving the HIV protease inhibitor class. Expert Rev. Anti. Infect. Ther. 2003;1:403–413. doi: 10.1586/14787210.1.3.403. [DOI] [PubMed] [Google Scholar]
- Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 1987;47:936–942. [PubMed] [Google Scholar]
- Caron M, Auclair M, Vigouroux C, Glorian M, Forest C, Capeau J. The HIV protease inhibitor indinavir impairs sterol regulatory element-binding protein-1 intranuclear localization, inhibits preadipocyte differentiation, and induces insulin resistance. Diabetes. 2001;50:1378–1388. doi: 10.2337/diabetes.50.6.1378. [DOI] [PubMed] [Google Scholar]
- Caron M, Vigouroux C, Bastard JP, Capeau J. Antiretroviral-related adipocyte dysfunction and lipodystrophy in HIV-infected patients: alteration of the PPARgamma-dependent pathways. PPAR Res. 2009;2009:507141. doi: 10.1155/2009/507141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caso G, McNurlan MA, McMillan ND, Eremin O, Garlick PJ. Tumour cell growth in culture: dependence on arginine. Clin. Sci. (Lond) 2004;107:371–379. doi: 10.1042/CS20040096. [DOI] [PubMed] [Google Scholar]
- Dowell P, Flexner C, Kwiterovich PO, Lane MD. Suppression of preadipocyte differentiation and promotion of adipocyte death by HIV protease inhibitors. J. Biol. Chem. 2000;275:41325–41332. doi: 10.1074/jbc.M006474200. [DOI] [PubMed] [Google Scholar]
- Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol. Cell. Biol. 1999;19:5495–5503. doi: 10.1128/mcb.19.8.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti G, Di Biagio A, Casazza R, De Pascalis C, Bassetti M, Cruciani M, Vella S, Bassetti D. The relationship between ritonavir plasma levels and side-effects: implications for therapeutic drug monitoring. AIDS. 1999;13:2083–2089. doi: 10.1097/00002030-199910220-00011. [DOI] [PubMed] [Google Scholar]
- Grigem S, Fischer-Posovszky P, Debatin KM, Loizon E, Vidal H, Wabitsch M. The effect of the HIV protease inhibitor ritonavir on proliferation, differentiation, lipogenesis, gene expression and apoptosis of human preadipocytes and adipocytes. Horm. Metab. Res. 2005;37:602–609. doi: 10.1055/s-2005-870526. [DOI] [PubMed] [Google Scholar]
- Haerter G, Manfras BJ, Mueller M, Kern P, Trein A. Regression of lipodystrophy in HIV-infected patients under therapy with the new protease inhibitor atazanavir. AIDS. 2004;18:952–955. doi: 10.1097/00002030-200404090-00016. [DOI] [PubMed] [Google Scholar]
- Hauner H, Entenmann G, Wabitsch M, Gaillard D, Ailhaud G, Negrel R, Pfeiffer EF. Promoting effect of glucocorticoids on the differentiation of human adipocyte precursor cells cultured in a chemically defined medium. J. Clin. Invest. 1989;84:1663–1670. doi: 10.1172/JCI114345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RG, Furfine ES, Pedneault L, White AJ, Lenhard JM. Metabolic complications associated with antiretroviral therapy. Antiviral Res. 2001;51:151–177. doi: 10.1016/s0166-3542(01)00148-6. [DOI] [PubMed] [Google Scholar]
- Jan V, Cervera P, Maachi M, Baudrimont M, Kim M, Vidal H, Girard PM, Levan P, Rozenbaum W, Lombès A, Capeau J, Bastard JP. Altered fat differentiation and adipocytokine expression are inter-related and linked to morphological changes and insulin resistance in HIV-1-infected lipodystrophic patients. Antivir. Ther. 2004;9:555–564. [PubMed] [Google Scholar]
- Janderová L, McNeil M, Murrell AN, Mynatt RL, Smith SR. Human mesenchymal stem cells as an in vitro model for human adipogenesis. Obes. Res. 2003;11:65–74. doi: 10.1038/oby.2003.11. [DOI] [PubMed] [Google Scholar]
- Jemsek JG, Arathoon E, Arlotti M, Perez C, Sosa N, Pokrovskiy V, Thiry A, Soccodato M, Noor MA, Giordano M. Body fat and other metabolic effects of atazanavir and efavirenz, each administered in combination with zidovudine plus lamivudine, in antiretroviral-naive HIV-infected patients. Clin. Infect. Dis. 2006;42:273–280. doi: 10.1086/498505. [DOI] [PubMed] [Google Scholar]
- Jones SP, Waitt C, Sutton R, Back DJ, Pirmohamed M. Effect of atazanavir and ritonavir on the differentiation and adipokine secretion of human subcutaneous and omental preadipocytes. AIDS. 2008;22:1293–1298. doi: 10.1097/QAD.0b013e3283021a4f. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Wilson CG, Wabitsch M, Lazar MA, Steppan CM. HIV protease inhibitor-specific alterations in human adipocyte differentiation and metabolism. Obesity (Silver Spring) 2006;14:994–1002. doi: 10.1038/oby.2006.114. [DOI] [PubMed] [Google Scholar]
- Koutkia P, Grinspoon S. HIV-associated lipodystrophy: pathogenesis, prognosis, treatment, and controversies. Annu. Rev. Med. 2004;55:303–317. doi: 10.1146/annurev.med.55.091902.104412. [DOI] [PubMed] [Google Scholar]
- Lagathu C, Bastard JP, Auclair M, Maachi M, Kornprobst M, Capeau J, Caron M. Antiretroviral drugs with adverse effects on adipocyte lipid metabolism and survival alter the expression and secretion of proinflammatory cytokines and adiponectin in vitro. Antivir. Ther. 2004;9:911–920. [PubMed] [Google Scholar]
- Lagathu C, Kim M, Maachi M, Vigouroux C, Cervera P, Capeau J, Caron M, Bastard JP. HIV antiretroviral treatment alters adipokine expression and insulin sensitivity of adipose tissue in vitro and in vivo. Biochimie. 2005;87:65–71. doi: 10.1016/j.biochi.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Lenhard JM, Furfine ES, Jain RG, Ittoop O, Orband-Miller LA, Blanchard SG, Paulik MA, Weiel JE. HIV protease inhibitors block adipogenesis and increase lipolysis in vitro. Antiviral Res. 2000;47:121–129. doi: 10.1016/s0166-3542(00)00102-9. [DOI] [PubMed] [Google Scholar]
- Lihn AS, Richelsen B, Pedersen SB, Haugaard SB, Rathje GS, Madsbad S, Andersen O. Increased expression of TNF-alpha, IL-6, and IL-8 in HALS: implications for reduced adiponectin expression and plasma levels. Am. J. Physiol. Endocrinol. Metab. 2003;285:E1072–1080. doi: 10.1152/ajpendo.00206.2003. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Miller J, Carr A, Emery S, Law M, Mallal S, Baker D, Smith D, Kaldor J, Cooper DA. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003;4:293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- Möbius U, Lubach-Ruitman M, Castro-Frenzel B, Stoll M, Esser S, Voigt E, Christensen S, Rump JA, Fätkenheuer G, Behrens GM, Schmidt RE. Switching to atazanavir improves metabolic disorders in antiretroviral-experienced patients with severe hyperlipidemia. J. Acquir. Immune Defic. Syndr. 2005;39:174–180. [PubMed] [Google Scholar]
- Mynarcik DC, Combs T, McNurlan MA, Scherer PE, Komaroff E, Gelato MC. Adiponectin and leptin levels in HIV-infected subjects with insulin resistance and body fat redistribution. J. Acquir. Immune Defic. Syndr. 2002;31:514–520. doi: 10.1097/00126334-200212150-00009. [DOI] [PubMed] [Google Scholar]
- Otto TC, Lane MD. Adipose development: from stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005;40:229–242. doi: 10.1080/10409230591008189. [DOI] [PubMed] [Google Scholar]
- Roche R, Poizot-Martin I, Yazidi CM, Compe E, Gastaut JA, Torresani J, Planells R. Effects of antiretroviral drug combinations on the differentiation of adipocytes. AIDS. 2002;16:13–20. doi: 10.1097/00002030-200201040-00003. [DOI] [PubMed] [Google Scholar]
- Rudich A, Ben-Romano R, Etzion S, Bashan N. Cellular mechanisms of insulin resistance, lipodystrophy and atherosclerosis induced by HIV protease inhibitors. Acta Physiol. Scand. 2005;183:75–88. doi: 10.1111/j.1365-201X.2004.01383.x. [DOI] [PubMed] [Google Scholar]
- Sension M, Andrade Neto JL, Grinsztejn B, Molina JM, Zavala I, González-Garciá J, Donnelly A, Phiri P, Ledesma E, McGrath D. Improvement in lipid profiles in antiretroviral-experienced HIV-positive patients with hyperlipidemia after a switch to unboosted atazanavir. J. Acquir. Immune Defic. Syndr. 2009;51:153–162. doi: 10.1097/QAI.0b013e3181a5701c. [DOI] [PubMed] [Google Scholar]
- Solas C, Gagnieu MC, Ravaux I, Drogoul MP, Lafeuillade A, Mokhtari S, Lacarelle B, Simon N. Population pharmacokinetics of atazanavir in human immunodeficiency virus-infected patients. Ther. Drug Monit. 2008;30:670–673. doi: 10.1097/FTD.0b013e3181897bff. [DOI] [PubMed] [Google Scholar]
- Sutinen J, Korsheninnikova E, Funahashi T, Matsuzawa Y, Nyman T, Yki-Järvinen H. Circulating concentration of adiponectin and its expression in subcutaneous adipose tissue in patients with highly active antiretroviral therapy-associated lipodystrophy. J. Clin. Endocrinol. Metab. 2003;88:1907–1910. doi: 10.1210/jc.2002-021922. [DOI] [PubMed] [Google Scholar]
- Tchkonia T, Giorgadze N, Pirtskhalava T, Tchoukalova Y, Karagiannides I, Forse RA, DePonte M, Stevenson M, Guo W, Han J, Waloga G, Lash TL, Jensen MD, Kirkland JL. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- Vernochet C, Azoulay S, Duval D, Guedj R, Cottrez F, Vidal H, Ailhaud G, Dani C. Human immunodeficiency virus protease inhibitors accumulate into cultured human adipocytes and alter expression of adipocytokines. J. Biol. Chem. 2005;280:2238–2243. doi: 10.1074/jbc.M408687200. [DOI] [PubMed] [Google Scholar]
- Wise LS, Green H. Participation of one isozyme of cytosolic glycerophosphate dehydrogenase in the adipose conversion of 3T3 cells. J. Biol. Chem. 1979;254:273–275. [PubMed] [Google Scholar]
- Zhang B, MacNaul K, Szalkowski D, Li Z, Berger J, Moller DE. Inhibition of adipocyte differentiation by HIV protease inhibitors. J. Clin. Endocrinol. Metab. 1999;84:4274–4277. doi: 10.1210/jcem.84.11.6234. [DOI] [PubMed] [Google Scholar]