Abstract
Protein Kinase C-alpha (PKCα) was recently reported to increase myocardial stiffness, an effect that was proposed to be due to phosphorylation of two highly conserved sites (S11878 and S12022) within the proline-gluatamic acid-valine-lysine (PEVK) rich spring element of titin. To test this proposal we investigated the effect of PKCα on phosphorylation and passive stiffness in a mouse model lacking the titin exons that contain these two phosphorylation sites, the PEVK knockout (KO). We used skinned, gelsolin-extracted, left ventricular, myocardium from wildtype and PEVK KO mice. Consistent with previous work we found that PKCα increased passive stiffness in the WT myocardium by 27 ±6%. Importantly, this effect was completely abolished in KO myocardium. In addition, increases in the elastic and viscous moduli at a wide range of frequencies (properties important in diastolic filling) following PKCα incubation (27 ±3% and 20 ±4%, respectively) were also ablated in the KO. Back phosphorylation assays showed that titin phosphorylation following incubation with PKCα was significantly reduced by 36±12% in skinned PEVK KO myocardial tissues. The remaining phosphorylation in the KO suggests that PKCα sites exist in the titin molecule outside the PEVK region; these sites are not involved in increasing passive stiffness. Our results firmly support that the PEVK region of cardiac titin is phosphorylated by PKCα and that this increases passive tension. Thus, the PEVK spring element is the critical site of PKCα’s involvement in passive myocardial stiffness.
1. INTRODUCTION
Phosphorylation is a prominent way to rapidly adjust myofilament function[1–3]. One of the important kinases involved in this process is protein kinase C (PKC), a family of Ca2+ and/or lipid-activated serine-threonine protein kinases that function downstream of many membrane-associated signal transduction pathways[4]. Of the classical PKC isozymes (α, βI, βII, and δ) PKCα has been found to be the predominant PKC isoform expressed in the mouse, rat, and human heart and has emerged as a key player in control of myofilament function, contractile dysfunction, and development of heart failure[3, 5–14]. Studies have shown that Troponin I (TnI), Troponin T (TnT), myosin light chain 2 (MLC-2) and myosin binding protein C (MyBP-C) are all myofilament substrates for PKC[3, 15–18]. Additionally, our recent work has uncovered the sarcomeric protein titin as a novel substrate for PKCα[19].
Titin is the third most abundant protein of striated muscle (after myosin and actin) and spans the half sarcomeric distance from Z-disk to M-band[20–21]. In the I-band region of the sarcomere titin is extensible and functions as a molecular spring that develops force in sarcomeres stretched beyond their slack length (~1.9 μm)[21–23]. This force largely determines the passive stiffness of the cardiac myocyte and, together with collagen, determines myocardial passive stiffness[23–24].
Titin can be phosporylated by Protein Kinase A (PKA)[25–27], and by Protein Kinase G (PKG)[28] with both kinases resulting in a decrease in passive stiffness. Furthermore, hypophosphorylation of titin in heart failure results in reduced compliance [29], indicating the importance of titin phosphorylation in normal physiological adaptations as well as in pathophysiology. Phosphorylation of titin by PKCα, however, is the first posttranslational modification of titin shown to cause an increase in passive stiffness.
Cardiac titin’s extensible I-band is comprised of three sequence motifs: the serially-linked immunoglobulin-like (Ig) domains, the N2B element, and the PEVK region (so named because it contains primarily proline (P), glutamate (E), valine (V) and lysine (K) residues[30–32]). Only the PEVK is phosphorylated by PKCα[19], whereas the N2B element is phosphorylated by both PKA and PKG[25, 28]. Furthermore, the PEVK was shown to be specifically phosphorylated at the highly conserved amino acids S11878 and S12022 (found within PEVK exons 219 and 225, respectively, in the human genome (Swiss ProtQ8wz42)) [19]. Also, atomic force microscopy (AFM) showed that the persistence length (a measure to the bending rigidity) of the PEVK is lowered by PKCα phosphorylation[19], indicating an increase in stiffness. However, all of this evidence was obtained under in vitro conditions and does not prove that in the sarcomere PEVK phosphorylation is responsible for the increased passive stiffness measured following phosphorylation. An excellent way to examine the proposal that the PEVK region is responsible for the PKC-induced increase in passive stiffness is to study the recently developed PEVK knock-out (KO) mouse[33]. Of the 112 PEVK exons that are differentially expressed between muscle types, exons 219-225 constitute all PEVK exons in the N2B cardiac isoform, the dominant titin isoform in the left ventricle. The PEVK KO mouse is deficient in these exons, resulting in the deletion of S11878 and S12022. Thus, the objective of this study was to determine whether the deletion of titin exons encoding for the cardiac spring element of PEVK results in a loss in PCKα-induced increase in passive tension and a loss (or reduction) of PKCα-induced phosphorylation of titin.
2. MATERIALS AND METHODS
2.1. Animals
Mouse left ventricular (LV) and papillary muscle was collected from 3 month old male PEVK KO mice lacking exons 219-225 [33] and wildtype age-matched controls. Mice were anesthetized with isoflurane (Abbott Laboratories, Chicago, IL) and sacrificed by cervical dissection. The hearts were rapidly excised and the muscles dissected in oxygenated HEPES (NaCl, 133.5 mM; KCl, 5mM; NaH2PO4, 1.2mM; MgSO4, 1.2mM; HEPES, 10mM). The tissue was skinned in relaxing solution (BES 40 mM, EGTA 10 mM, MgCl2 6.56 mM, ATP 5.88 mM, DTT 1 mM, K-propionate 46.35 mM, creatine phosphate 15 mM, pH 7.0) (chemicals from Sigma-Aldrich, MO, USA) with 1% Triton-X-100 (Pierce, IL, USA) overnight at 4°C. Muscles were then washed thoroughly with relaxing solution and stored for one month or less at −20°C in relaxing solution containing 50% (v/v) glycerol. To prevent protein degradation, all solutions contained protease inhibitors (phenylmethylsulfonyl fluoride (PMSF), 0.5mM; Leupeptin, 0.04mM; E64, 0.01mM). All animal experiments were approved by the University of Arizona Institutional Animal Care and Use Committee and followed the U.S. National Institutes of Health “Using Animals in Intramural Research” guidelines for animal use.
2.2. Phosphorylation of skinned myocardium
Calcium- and phosphatidylserine-dependent PKCα, full-length human recombinant expressed in insect cells, was purchased from Enzo Life Sciences (Butler Pike, Plymouth Meeting, PA, USA). Approximately 20 μCi of 32P ATP stock solution, specific activity 3,000 Ci/mmol (PerkinElmer) was added to PKCα (0.066U/μl) in kinase solution (BES 19.2 mM pH 7.0, CaCO3-EGTA 4.8 mM, MgCl2 3.0 mM, ATP 2.9 mM, DTT 0.7 mM, K-propionate 21.7 mM, creatine phosphate 7.2 mM, NaCl 30 mM, glycerol 5%) plus lipid activator (Upstate) (phosphatidylserine 0.2 mg/ml; diacylglycerol, 0.02 mg/ml; Triton X-100 0.03%; dithiothreitol), protease inhibitors (leupeptin 0.02 mg/ml, E-64 0.01 mM, PMSF 0.25 mM), and phosphatase inhibitor (NaF 10 mM, Na3VO4 2 mM). The kinase solution was transferred to the mouse LV skinned fibers and mixed and incubated 1 hour at room temperature (RT). To reduce radioactive background of gels the fibers were washed with 1 ml of cold relaxing solution. To stop the reaction, 40 μl of solubilization buffer (750 μl of 8 M urea, 50 mM Tris pH 8.6, 300 mM 143 glycine, 5 mM DTT, and 0.001% bromophenol blue plus 250 μl of 50% glycerol containing 0.02 mg/ml leupeptin, 1 mM E-64, and 0.25 mM PMSF) was added and the tissue was then solubilized by grinding for 4 min in a microgrinder (glass/glass) (Kimble-Kontes, NJ, USA). The protein solution was incubated 10 min at 60°C, centrifuged for 10 min at 12,580×g to remove particulate fraction, and then the proteins were separated by electrophoresis on a 2–7% gel gradient SDS-PAGE and run with Fairbank’s buffer (40 mM trizma base pH 7.5, 20 mM sodium acetate, 2 mM EDTA, 0.1% SDS, 0.1 mM 2-mercaptoethanol) 1 h at 60 volts and 3 h at 90 volts with the tall mighty small vertical slab gel unit (Hoefer Inc., CA, USA). The gels were Coomassie blue stained, destained, dried, exposed to X-ray film, and scanned with an Epson Expression 1680 scanner using software v 1.01e. The dried gels were exposed to Kodak BioMax MS film with Kodak BioMax TranScreen HE at −80°C and the film developed with Kodak X-OMAT 200A processor. The autoradiographs were scanned as described above. With each gel a 21 step neutral density filter (Stouffer, IN, USA) was included to linearize optical density. The images were analyzed using ONE-DScan 2.03 (Scanalytics Inc., MD, USA). The integrated OD of the autoradiograph was normalized by the integrated OD of the Coomassie blue-stained gel, to normalize the data for differences in protein loading (loading was usually highly uniform).
2.3. Measurement of passive tension
Small muscle strips (100–200 μm in diameter, ~2mm in length) were dissected from the stored skinned preparations. A calcium independent gelsolin fragment (amino acids 1-406; dissolved in 10mM 3-(N-morpholino) propanesulfonic acid (MOPS), 1.0 mM EGTA, 1.0 mM Mg-acetate, 20 mM K-propionate, 1 mM DTT, and 20 μg/ml leupeptin, pH 7.0 at 21–23°C), was used as described previously [34] in order to extract thin filaments and prevent actomyosin interaction during PKCα treatment. Briefly, muscle preps were placed in a gelsolin (1μg/μl) cocktail (20μl gelsolin: 20μl ddH2O: 40μl 2X relaxing solution) and incubated overnight at ~3°C for ~12 hours. The extracted tissue was then rinsed at room temperature for an hour in relaxing solution. Following gelsolin extraction, non-control samples were treated with PP1 (Calbiochem; α-isoform, Rabbit Muscle, Recombinant, E. coli; 1.5U/μl) for 2 hours in order to dephosphorylate titin’s PKC target sites. Passive tension was measured with a strain gauge force transducer and fiber length was controlled by a high-speed motor. Preparations were placed in a chamber containing relaxing solution and attached to the motor arm and the force transducer via aluminum clips. The width and the height of the fiber were measured and the cross-sectional area (CSA) was calculated assuming the cross section is elliptical in shape. The measured forces were then converted to tension (force/CSA). Sarcomere length (SL) was measured on line at 1 kHz by laser diffraction [23]. At the beginning of each experiment, muscles were activated at SL 2.0 μm (pCa 4.0) to measure residual active tension. Typically maximal activate tension was between undetectable and ~1 mN/mm2, or <2 % of maximum active tension of non-extracted preparations, indicating that actin extraction by gelsolin was close to complete. To determine the effect of gelsolin extraction on titin force we ran control experiments in which we first measured the maximal calcium activated force, then stretched the muscle multiple times to SL 2.15 μm (to determine the basal passive force) and then applied gelsolin to the chamber and repeatedly measured passive force every hour for the full extraction time. At the end of the extraction, the muscle was activated one more time to ascertain the loss of crossbridge interactions. The data showed that although there was a loss in activation force following gelsolin (to ~2% of maximal) there was no significant alteration in myocardial passive tension.
2.4. Stretch-Hold Protocol
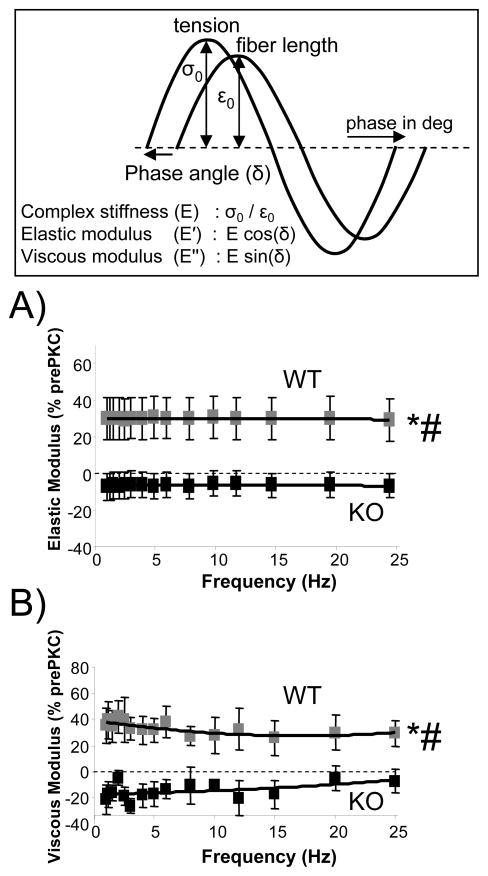
Relaxed fibers were slowly stretched (10%/sec) from their slack length (~1.85μm) to a SL of 2.3μm, followed by a 90 second hold. We then imposed a frequency sweep (FS; 1–25 Hz, amplitude 1%) and complex stiffness (E, tension amplitude/length amplitude) and the phase shift (δ) between force and length was measured. The sweep consisted of a total of 15 different frequencies with a 0.5-s rest period in between individual frequencies. The servomotor position signal and tension were digitized and their amplitudes and phases were determined with FFT (Fast Fourier Transform) analysis. Elastic and viscous moduli were determined as E (cos δ) and E (sin δ), respectively (see also top of Fig. 4). Finally, the fiber was released back to slack length and allowed to rest 12 minutes before the next stretch.
Fig. 4.
Elastic and viscous moduli determined from a sinusoidal analysis (1–25 Hz, amplitude 1%, see Fig. 3 and Materials and Methods for more details). Top panel explains how the elastic and viscous moduli were measured. Shown in A and B are the moduli following PKC treatment as a percentage of the pre-treatment values. The graph in (A) shows the percent increase in elastic moduli and (B) in viscous moduli following PKCα incubation. Elastic and viscous moduli are higher following PKCα treatment only in the PEVK WT. *Significant vs PEVK KO; # Significant vs prePKCα.
2.5. PKCα administration
First we established repeatability of the prePKCα stretch-hold-FS protocols by repeating the protocol three times (with a 20 min rest at slack length in between). If the muscles had a greater than 2% decrease in passive tension from stretch 2 to stretch 3 then additional stretch-release cycles were imposed until the change in passive tension was less than 2% (4 times was the most times that a muscle was stretched). Fibers were then stretched and held at a SL of 2.2μm. Force levels were monitored for 30 minutes, upon which the relaxing solution within the chamber was replaced by PKCα (0.066 U/μl, pCa 6.5) in mM BES 38.8 pH 7.0, EGTA 2.65 mM, CaCO3/EGTA 7.1, MgCl2 6.2, Na2-ATP 5.9, K-propionate 44.2, creatine-PO4 14.6, NaCl 27.3; glycerol 4.5%; lipid activator (PS 0.2 mg/ml, DAG 18 μg/ml), protease inhibitors (leupeptine 0.08 mg/ml, E-64 0.04 mM, PMSF 0.4 mM, pepstatin 2 μM); phosphate inhibitors (NaF 2 mM, Na3VO4 0.4 mM) for 2 hours. At the end of PKCα treatment, tissue was rinsed in relaxing solution for 15 minutes and then SL was returned to slack length and allowed to rest for 20 minutes, followed by 3 postPKCα stretch-hold-FS steps. To determine collagen’s contribution to passive force, skinned fibers were incubated with relaxing solution containing 0.6M KCl for 30 minutes followed by a 30 minute incubation in relaxing solution containing 1.0 M KI. These solutions extract thick and thin filaments, respectively, from the sarcomere, removing titin’s anchorage locations and therefore resulting in a loss of force developed by titin [24]. The resulting collagen force was then subtracted from the pre-extraction forces to determine titin-specific forces.
2.6. Statistical Analysis
Data are presented as mean ± SEM. Significant differences were probed using ANOVA. Post hoc comparisons were made using Bonferroni. Probability values <0.05 were taken as significant. Probability values shown on Figures: * p<0.05; ** p<0.01; *** p<0.001; # significantly different from prePKCα, p<0.05.
3. RESULTS
3.1. PEVK KO mouse model
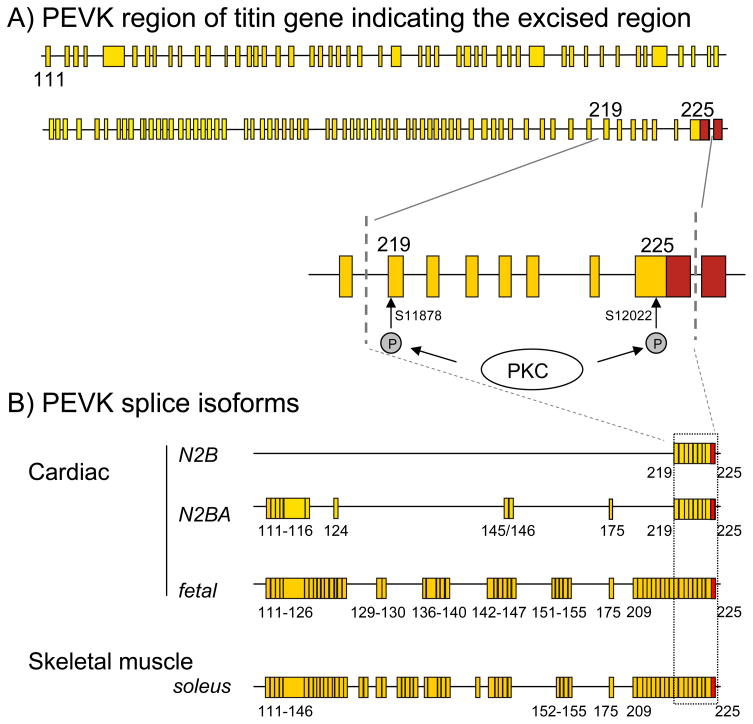
Using homologous recombination we recently generated a mouse model [33] in which titin’s PEVK exons 219 to 225 have been excised (Fig. 1A). Homozygous KO mice survive to adulthood and are fertile, without any obvious abnormalities. The reading frame 3′ of exon 225 is maintained in the mutant titin [33] and except for the targeted exons the only additional change to the wildtype allele is the residual intronic FRT site that does not interfere with gene function. The deleted exons encode 188 PEVK residues, including S11878 and S12022 which were found to be PKC sites in in vitro kinase assays [19]. Furthermore, the excised exons represent the sole PEVK exons of N2B cardiac titin, but represent only part of the PEVK exons that are found in N2BA and fetal cardiac titin isoforms, or skeletal muscle titin (Fig. 1B). Considering that N2B cardiac titin is the main titin isoform expressed in the mouse left ventricle (LV) the PEVK KO is ideal to study the role of S11878 and S12022 in the PKC-induced phosphorylation of titin and change in passive stiffness that was previously found. Because the PEVK KO mouse is not only lacking the phosphorylation sites but also the rest of the cardiac PEVK region (as found in the N2B isoform), the KO mouse will have a constitutive increase in passive stiffness. Thus, comparing the effect of basal PKCα phosphorylation levels between WT and KO mice and correlating that to passive stiffness is not possible (it would require the PEVK phosphorylation sites to be mutated and the rest of the PEVK to be kept intact).
Fig. 1.
A) Exon composition of the PEVK region of titin gene. The cardiac PEVK spring element is phosphorylated by PCKα at S11878 (exon 219) and S12022 (exon 225). Dotted lines indicate exons that are excised in the PEVK KO mouse, which eliminates both PKCα phosphorylation sites. B) PEVK exon composition of three cardiac titin isoforms and one skeletal muscle titin isoform (adult soleus muscle). Although the PEVK exon composition is highly variable, exons 219-225 are included in all isoforms (exon composition from [47–48])
3.2. PEVK KO abolishes PCKα-induced passive stiffness
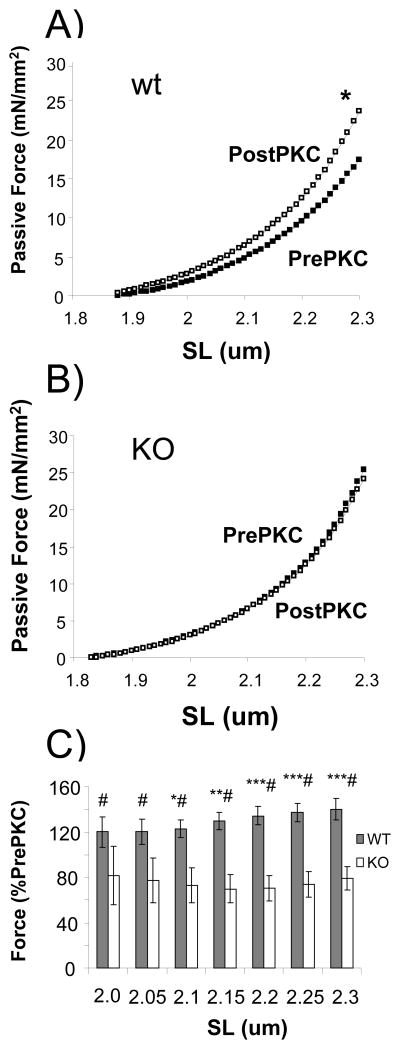
To study the effect of the deletion of exons 219 - 225 on passive tension we used skinned, thin-filament extracted (using gelsolin) LV myocardium from both PEVK KO and littermate WT mice. Removing thin filaments abolishes actomyosin interaction and ensures that the PKCα effect is a purely passive property. Following thin-filament extraction the preparations were incubated with 1.5 U/μl PP1 in order to maximally dephosphorylate titin. PKCα treatment significantly increased passive stiffness during stretch in the WT, but not in the KO (examples given in Figures 2A and 2B), and this effect occurs in a SL dependent fashion, (for summarized results see Fig. 2C). We attribute the slight decrease seen in the passive tension in KO myocardium to normal run down that occurs during repeatedly imposing mechanical stresses. Overall, these results clearly support that the increase in passive stiffness following PKCα in mouse myocardium is a result of phosphorylation of the cardiac spring element at the PEVK sequence motif.
Fig. 2.
PKCα-induced increases in passive stiffness are eliminated in PEVK KO myocardium. Example passive tension curve measured during stretch before PKCα incubation (prePKC) and after PKCα (postPKC) in (A) WT and (B) KO mice. The preparations had been pre-treated with gelsolin to extract thin filaments and prevent actomyosin interaction during and after PKCα treatment. Fibers were preincubated with PP1 to dephosphorylate titin before PKCα incubation. Passive stiffness is significantly higher following PKCα-treatment in the WT, and this effect was abolished in the PEVK KO. (C) Length-dependent changes in passive force. *Significant vs PEVK KO; # Significant vs prePKCα.
3.3. PEVK KO abolishes increases in PCKα-induced elastic and viscous stiffness
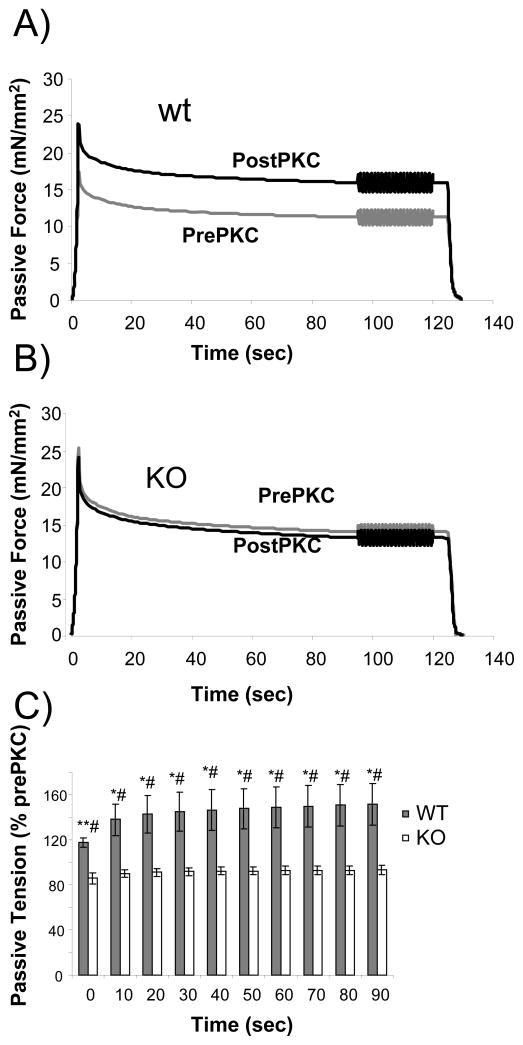
In these experiments muscles were stretched to a sarcomere length of 2.3 μm, held for 90 seconds, subjected to a sinusoidal stiffness analysis, and released back to their slack length. This protocol was repeated following PKCα incubation. Results of stretch-hold-sinusoidal oscillation experiments are shown in Fig. 3. We measured passive tension at various times (0–90 sec) during the hold phase of the protocol, to characterize the stress relaxation that occurs during this period. Interestingly, the effect of PKCα on passive tension was ~20% when the stretch was completed, but then increased during the first 20 sec of the hold to ~40%, and then stayed constant at ~40% (Figure 3C). This effect was absent in the KO mouse myocardium (Figures 3B and 3C). The sinusoidal analysis was carried out 90 sec after the stretch was completed and showed a robust increase in the elastic and the viscous moduli following PKCα treatment in the wildtype muscle, while this effect was absent in the KO (see Figure 4).
Fig. 3.
Effect of PKCα phosphorylation on titin-based passive tension in skinned myocardium held at 2.3 μm SL. Fibers were stretched from their slack length (SL ~ 1.88 μm) to a SL of 2.3 μm and were then held, followed by a sinusoidal analysis (results shown in Fig. 4) and a release back to the slack length. Passive tension was significantly higher during the hold phase following PKCα-treatment in the WT mice (A), but not in the KO mice (B). Additionally, (C) PKCα increased passive tension to a greater extent after 20sec as compared to 0 sec, an effect lost in the KO mouse. *Significant vs PEVK KO; # Significant vs prePKCα.
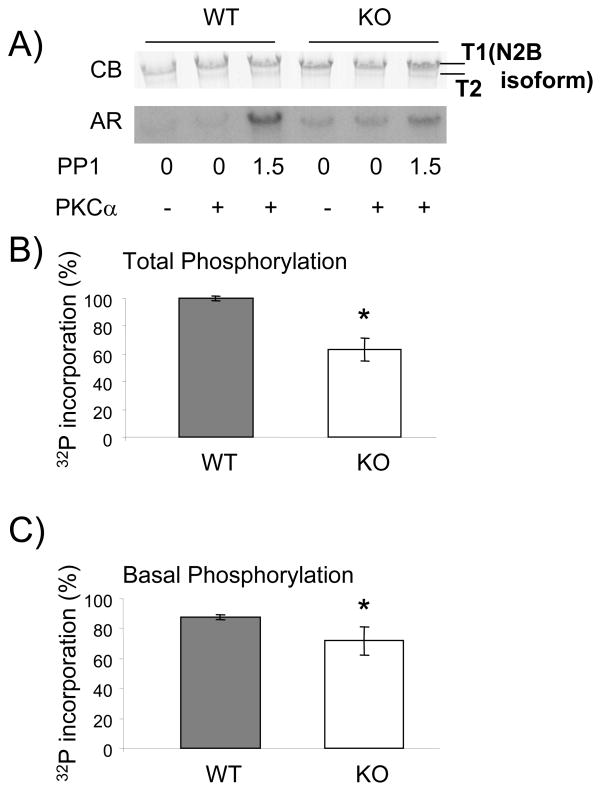
3.4. Titin phosphorylation by PKCα is significantly reduced in PEVK KO
To determine whether titin is differentially phosphorylated by PKCα in PEVK WT versus PEVK KO mice we performed 32P incorporation back-phosphorylation assays. Because baseline phosphorylation levels might be different between WT and KO specimen we pretreated the muscles with PP1 at concentrations of 1.5U/μl (which we had previously shown to maximally dephosphorylate PKC site on titin[19]. The autoradiograph (AR in Figure 5A) shows that PKCα preferentially phosphorylates the full length titin, T1 band (N2B isoform), and not T2 (the degradation product), supporting the hypothesis that PKCα primarily interacts with the cardiac spring element of titin, an effect exacerbated by pre-incubating the myocardial tissue with PP1. The measured 32P incorporation was normalized to protein levels. Total PKCα-induced phosphorylation following maximal dephosphorylation by PP1 (Figure 5B) was reduced in the KO by 36±12% (p <0.001). The remaining phosphorylation in the KO shows clearly that there are more PKCα phosphorylation sites on titin than just those found on exons 219-225. However, with 36% of the overall phosphorylation attributed to these exons, the cardiac PEVK is a major contributor to PKCα phosphorylation. Because we wished to determine the basal phosphorylation level (i.e. level that is already present), we determined the 32P incorporation following PKC phosphorylation without PP1 pre-treatment, a level that we refer to as A. Because back-phosphorylation assays only measure the amount of 32P incorporation and not the basal phosphorylation level, we calculated the basal phosphorylation level as (1-A/total) × 100%. Interestingly, as Fig. 5C shows, the basal phosphorylation was lower in the KO (n=4) as compared to the WT (n=4) (72 ± 9% vs. 88 ± 2%, respectively, p<0.05) indicating that the PKCα phosphorylation sites in the KO myocardial tissue are hypo-phosphorylated. Thus, the phosphorylation assays indicate that the cardiac PEVK is a major contributor to PKCα phosphorylation and that the PKCα sites outside the PEVK are hypophosphorylated in the PEVK KO myocardium.
Fig. 5.
PKCα-induced titin phosphorylation is reduced in PEVK KO mice. (A) Representative results on mouse skinned myocardium incubated in 32P ATP with (+) or without (−) PKC and with (1.5 units/μl) or without PP1. Top panel: Coomassie blue (CB) stained gel and bottom panel: corresponding autoradiograph (AR). (B) Maximal phosphorylation experiments of muscles pretreated with PP1 show that KO mice have fewer PKCα-inducible phosphorylation sites, revealing the loss of phosphorylation sites with the excision of exons 219-225. (C) Basal phosphorylation is significantly reduced in the KO vs. control (72% and 88%, respectively) and, thus, there are more free PKCα phorphorylation sites available in the KO vs. WT, indicating that PEVK KO cardiac myocardium is hypophosphorylated. See text for details. *Significant vs WT.
4. DISCUSSION
In this study we investigated the role of titin exons 219-225 in determining PKCα-induced myocardial stiffness. These exons encode the PEVK spring element of cardiac N2B titin and contain amino acids S11878 and S12022 (exons 219 and 225, respectively), that determine phosphorylation levels of titin following PKCα treatment as indicated by in vitro kinase experiments [19]. Thus, we hypothesized that the deletion of these exons in the PEVK KO would result in a loss of PKCα-induced passive stiffness. Our data clearly show that, in wildtype myocardium, passive stiffness is increased following PKCα phosphorylation and that this effect is absent in the PEVK KO myocardium. Thus the PKCα phosphorylation sites that regulate titin’s stiffness are likely to be located in the excised exons. Considering that mutating S11878 and S12022 to alanines effectively abolishes PEVK phosphorylation in recombinant PEVK proteins[19], it seems reasonable to conclude that the absence of a phosphorylation effect on passive stiffness in PEVK KO tissue is due to the absence of these two sites. Our PKC phosphorylation assays showed that there is still remaining 32P incorporation in titin in the PEVK KO myocardium, which suggests that there are PKC sites outside titin’s PEVK region. Considering that the T2 portion of titin is not phosphorylated by PKC (in neither WT nor KO tissues) it is likely that these non-PEVK sites are outside the A-band region of titin (which largely makes up the T2 protein) in either the Z-disk or I-band regions of the molecule. In previous in vitro experiments we did not find PKCα phosphorylation sites in the N2B element nor in the tandem Ig segments[19], therefore we consider it likely that the PKCα phosphorylation sites are located in/near the Z-disk region of the molecule, where several unique sequences are found [30] and where titin is not extensible [35–37] and, thus, no mechanical effect of phosphorylation is to be expected.
The PKCα-pathway is the first signaling pathway shown to lead to increased myocardial stiffness. In previous work it was shown that both cAMP-dependent protein kinase-A (PKA) and cGMP-dependent protein kinase-G (PKG) phosphorylate the N2B element of cardiac titin and that these two types of posttranslational modification decrease titin-based myocardial passive tension[25, 28]. The physical mechanism by which PKA/PKG phosphorylation lowers stiffness in one sequence element, N2B, and PKCα increases stiffness following phosphorylation of another sequence, PEVK, requires future study. It is likely that changes in local structure underlie the stiffness changes and that increases in contour length dominate the N2B element based reduction in stiffness and decreases in persistence length dominate the PEVK-based increase in stiffness. It is also worthwhile to note that the PKCα sites are highly conserved[19] - unlike the sequence of the N2B element with the PKA and PKG site published by Kruger et al. [28] which is only present in human. This points towards a more precise and sequence-dependent mechanism in the modulation of the PEVK’s mechanical properties. Finally, because exons 219 and 225 are found in all known titin isoforms in striated muscle (Fig. 1B) the PKC-based passive tension increase is expected in all muscle types (including skeletal muscle). The effect is predicted to be largest for the isoforms with the shortest spring region (N2B cardiac titin) and to be progressively less in isoforms with increasing lengths of the spring regions (the PEVK effect is ‘diluted’).
Our results further add to the knowledge of how titin phosphorylation events are potentially important in normal physiological adaptations and in pathophysiological processes. One pathophysiological process known to have alterations in PKCα expression is heart failure. Heart Failure (HF), characterized by impaired ability of the left ventricle to fill or eject blood, is of diverse etiology that typically results in cardiac hypertrophy. Adaptive cardiac hypertrophy is initially beneficial and is characterized by an increase in heart mass and wall thickening and is associated with improved cardiac function. However, sustained cardiac hypertrophy transitions into maladaptive hypertrophy, typically accompanied with left ventricular dilation, decreased contractile properties, reduced cardiac output and changes in myocardial passive stiffness [38].
Two major mechanisms can alter titin passive stiffness: 1) changes in titin isoform expression, and 2) phosphorylation by protein kinases (PKA, PKG, and PKC). During heart failure titin isoform switches occur either towards a longer titin isoform, as in dilated cardiomyopathy resulting in more compliant cardiac myocytes [39–40], or towards a shorter isoform, as in diastolic HF resulting in stiffer cardiac myocytes [41]. The time frame for isoform shifts, however, tends to occur slowly, from days to weeks [42]. Alternatively, alterations in the basal phosphorylation levels and phosphorylation sensitivities of many regulatory proteins, including titin, can acutely regulate passive stiffness. Both PKA and PKG have been shown to phosphorylate cardiac titin and to reduce titin-based myocardial passive tensions. Additionally, studies have demonstrated that basal phosphorylation levels of both PKA and PKG are reduced in human HF patients and the increased myocardial stiffness seen in these patients could be normalized by PKA and PKG phosphorylation (PKA > PKG) [28–29, 43]. These findings suggest that both PKA- and PKG-pathways are potentially impaired in HF and indicate a mechanism that increases passive stiffness in HF.
Concurrently, a variety of chronic cardiac diseases, acute cardiac injuries and heart failure have been shown to involve PKC isozymes [44–45] with PKCα emerging as a key player in contractile dysfunction, development of heart failure, and control of myofilament function [3, 5, 7–13, 46]. Interestingly, PKCα has been shown to directly increase PP1 activity in the heart by regulating protein phosphatase inhibitor-1 [9], an effect that will result in the hypophosphorylation of PKA and PKG sites on titin’s N2B element, and give rise to increased stiffness of this element [25]. Thus, increased PKCα levels during HF might increase myocardial stiffness by increased stiffness of both the N2B element (hypophosphorylation) and the PEVK element (hyperphosphorylation). Further work is needed to establish the role of PKCα-based signaling in alterations in passive stiffness in heart failure myocardium.
In conclusion, PKCα phosphorylation of exons 219 and 225 in the PEVK element of titin results in increased passive stiffness and excision of these sites abolished this PKCα-dependent passive stiffness. These highly conserved phosphorylation sites are found in all known titin isoforms in striated muscle, thus indicating the importance of these exons in a variety of physiological adaptations. We hypothesize that phosphorylation of the PEVK element of titin is an important mechanism for altering passive muscle stiffness during both physiological and pathophysiological adaptations of the heart.
Acknowledgments
We kindly thank Luann Wyly for her outstanding technical help. Funding by NIH grant HL62881(HG) and the DFG (MG).
Footnotes
Disclosures: none.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Solaro RJ. Multiplex kinase signaling modifies cardiac function at the level of sarcomeric proteins. J Biol Chem. 2008;283(40):26829–33. doi: 10.1074/jbc.R800037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumandea MP, et al. Molecular and integrated biology of thin filament protein phosphorylation in heart muscle. Ann N Y Acad Sci. 2004;1015:39–52. doi: 10.1196/annals.1302.004. [DOI] [PubMed] [Google Scholar]
- 3.Belin RJ, et al. Augmented protein kinase C-alpha-induced myofilament protein phosphorylation contributes to myofilament dysfunction in experimental congestive heart failure. Circ Res. 2007;101(2):195–204. doi: 10.1161/CIRCRESAHA.107.148288. [DOI] [PubMed] [Google Scholar]
- 4.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 5.Hambleton M, et al. Pharmacological- and gene therapy-based inhibition of protein kinase Calpha/beta enhances cardiac contractility and attenuates heart failure. Circulation. 2006;114(6):574–82. doi: 10.1161/CIRCULATIONAHA.105.592550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonis G, et al. Protein kinase C in the human heart: differential regulation of the isoforms in aortic stenosis or dilated cardiomyopathy. Mol Cell Biochem. 2007;305(1–2):103–11. doi: 10.1007/s11010-007-9533-3. [DOI] [PubMed] [Google Scholar]
- 7.Bayer AL, et al. Alterations in protein kinase C isoenzyme expression and autophosphorylation during the progression of pressure overload-induced left ventricular hypertrophy. Mol Cell Biochem. 2003;242(1–2):145–52. [PubMed] [Google Scholar]
- 8.Bowling N, et al. Increased protein kinase C activity and expression of Ca2+-sensitive isoforms in the failing human heart. Circulation. 1999;99(3):384–91. doi: 10.1161/01.cir.99.3.384. [DOI] [PubMed] [Google Scholar]
- 9.Braz JC, et al. PKC-alpha regulates cardiac contractility and propensity toward heart failure. Nat Med. 2004;10(3):248–54. doi: 10.1038/nm1000. [DOI] [PubMed] [Google Scholar]
- 10.Hambleton M, et al. Inducible and myocyte-specific inhibition of PKCalpha enhances cardiac contractility and protects against infarction-induced heart failure. Am J Physiol Heart Circ Physiol. 2007;293(6):H3768–71. doi: 10.1152/ajpheart.00486.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Q, et al. Protein kinase C{alpha}, but not PKC{beta} or PKC{gamma}, regulates contractility and heart failure susceptibility: implications for ruboxistaurin as a novel therapeutic approach. Circ Res. 2009;105(2):194–200. doi: 10.1161/CIRCRESAHA.109.195313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sentex E, et al. Expression of protein kinase C isoforms in cardiac hypertrophy and heart failure due to volume overload. Can J Physiol Pharmacol. 2006;84(2):227–38. doi: 10.1139/y05-120. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, et al. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am J Physiol Heart Circ Physiol. 2003;284(6):H2277–87. doi: 10.1152/ajpheart.00142.2002. [DOI] [PubMed] [Google Scholar]
- 14.Murphy S, Frishman WH. Protein kinase C in cardiac disease and as a potential therapeutic target. Cardiol Rev. 2005;13(1):3–12. doi: 10.1097/01.crd.0000124914.59755.8d. [DOI] [PubMed] [Google Scholar]
- 15.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94(2):146–58. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 16.van der Velden J, et al. Functional effects of protein kinase C-mediated myofilament phosphorylation in human myocardium. Cardiovasc Res. 2006;69(4):876–87. doi: 10.1016/j.cardiores.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, et al. Cardiac troponin I phosphorylation increases the rate of cardiac muscle relaxation. Circ Res. 1995;76(6):1028–35. doi: 10.1161/01.res.76.6.1028. [DOI] [PubMed] [Google Scholar]
- 18.Pi Y, et al. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552(Pt 3):845–57. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo C, et al. PKC phosphorylation of titin’s PEVK element: a novel and conserved pathway for modulating myocardial stiffness. Circ Res. 2009;105(7):631–8. 17. doi: 10.1161/CIRCRESAHA.109.198465. following 638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Granzier HL, Labeit S. The giant protein titin: a major player in myocardial mechanics, signaling, and disease. Circ Res. 2004;94(3):284–95. doi: 10.1161/01.RES.0000117769.88862.F8. [DOI] [PubMed] [Google Scholar]
- 21.Linke WA, et al. Towards a molecular understanding of the elasticity of titin. J Mol Biol. 1996;261(1):62–71. doi: 10.1006/jmbi.1996.0441. [DOI] [PubMed] [Google Scholar]
- 22.Labeit S, Kolmerer B, Linke WA. The giant protein titin. Emerging roles in physiology and pathophysiology. Circ Res. 1997;80(2):290–4. doi: 10.1161/01.res.80.2.290. [DOI] [PubMed] [Google Scholar]
- 23.Granzier HL, Irving TC. Passive tension in cardiac muscle: contribution of collagen, titin, microtubules, and intermediate filaments. Biophys J. 1995;68(3):1027–44. doi: 10.1016/S0006-3495(95)80278-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, et al. Changes in titin and collagen underlie diastolic stiffness diversity of cardiac muscle. J Mol Cell Cardiol. 2000;32(12):2151–62. doi: 10.1006/jmcc.2000.1281. [DOI] [PubMed] [Google Scholar]
- 25.Yamasaki R, et al. Protein kinase A phosphorylates titin’s cardiac-specific N2B domain and reduces passive tension in rat cardiac myocytes. Circ Res. 2002;90(11):1181–8. doi: 10.1161/01.res.0000021115.24712.99. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda N, et al. Phosphorylation of titin modulates passive stiffness of cardiac muscle in a titin isoform-dependent manner. J Gen Physiol. 2005;125(3):257–71. doi: 10.1085/jgp.200409177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kruger M, Linke WA. Protein kinase-A phosphorylates titin in human heart muscle and reduces myofibrillar passive tension. J Muscle Res Cell Motil. 2006;27(5–7):435–44. doi: 10.1007/s10974-006-9090-5. [DOI] [PubMed] [Google Scholar]
- 28.Kruger M, et al. Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res. 2009;104(1):87–94. doi: 10.1161/CIRCRESAHA.108.184408. [DOI] [PubMed] [Google Scholar]
- 29.Borbely A, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104(6):780–6. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 30.Labeit S, Kolmerer B. Titins: giant proteins in charge of muscle ultrastructure and elasticity. Science. 1995;270(5234):293–6. doi: 10.1126/science.270.5234.293. [DOI] [PubMed] [Google Scholar]
- 31.Linke WA, et al. I-band titin in cardiac muscle is a three-element molecular spring and is critical for maintaining thin filament structure. J Cell Biol. 1999;146(3):631–44. doi: 10.1083/jcb.146.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helmes M, et al. Mechanically driven contour-length adjustment in rat cardiac titin’s unique N2B sequence: titin is an adjustable spring. Circ Res. 1999;84(11):1339–52. doi: 10.1161/01.res.84.11.1339. [DOI] [PubMed] [Google Scholar]
- 33.Granzier HL, et al. Truncation of titin’s elastic PEVK region leads to cardiomyopathy with diastolic dysfunction. Circ Res. 2009;105(6):557–64. doi: 10.1161/CIRCRESAHA.109.200964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trombitas K, Granzier H. Actin removal from cardiac myocytes shows that near Z line titin attaches to actin while under tension. Am J Physiol. 1997;273(2 Pt 1):C662–70. doi: 10.1152/ajpcell.1997.273.2.C662. [DOI] [PubMed] [Google Scholar]
- 35.Trombitas K, Jin JP, Granzier H. The mechanically active domain of titin in cardiac muscle. Circ Res. 1995;77(4):856–61. doi: 10.1161/01.res.77.4.856. [DOI] [PubMed] [Google Scholar]
- 36.Granzier H, Helmes M, Trombitas K. Nonuniform elasticity of titin in cardiac myocytes: a study using immunoelectron microscopy and cellular mechanics. Biophys J. 1996;70(1):430–42. doi: 10.1016/S0006-3495(96)79586-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Granzier H, et al. Titin elasticity and mechanism of passive force development in rat cardiac myocytes probed by thin-filament extraction. Biophys J. 1997;73(4):2043–53. doi: 10.1016/S0006-3495(97)78234-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yip GW, et al. Hypertension and heart failure: a dysfunction of systole, diastole or both? J Hum Hypertens. 2009;23(5):295–306. doi: 10.1038/jhh.2008.141. [DOI] [PubMed] [Google Scholar]
- 39.Nagueh SF, et al. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110(2):155–62. doi: 10.1161/01.CIR.0000135591.37759.AF. [DOI] [PubMed] [Google Scholar]
- 40.Makarenko I, et al. Passive stiffness changes caused by upregulation of compliant titin isoforms in human dilated cardiomyopathy hearts. Circ Res. 2004;95(7):708–16. doi: 10.1161/01.RES.0000143901.37063.2f. [DOI] [PubMed] [Google Scholar]
- 41.van Heerebeek L, et al. Myocardial structure and function differ in systolic and diastolic heart failure. Circulation. 2006;113(16):1966–73. doi: 10.1161/CIRCULATIONAHA.105.587519. [DOI] [PubMed] [Google Scholar]
- 42.Wu Y, et al. Changes in titin isoform expression in pacing-induced cardiac failure give rise to increased passive muscle stiffness. Circulation. 2002;106(11):1384–9. doi: 10.1161/01.cir.0000029804.61510.02. [DOI] [PubMed] [Google Scholar]
- 43.Borbely A, et al. Cardiomyocyte stiffness in diastolic heart failure. Circulation. 2005;111(6):774–81. doi: 10.1161/01.CIR.0000155257.33485.6D. [DOI] [PubMed] [Google Scholar]
- 44.Palaniyandi SS, et al. Protein kinase C in heart failure: a therapeutic target? Cardiovasc Res. 2009;82(2):229–39. doi: 10.1093/cvr/cvp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Churchill E, et al. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–99. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 46.Murphy RM, Lamb GD. Endogenous calpain-3 activation is primarily governed by small increases in resting cytoplasmic [Ca2+] and is not dependent on stretch. J Biol Chem. 2009;284(12):7811–9. doi: 10.1074/jbc.M808655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Greaser ML, et al. Species variations in cDNA sequence and exon splicing patterns in the extensible I-band region of cardiac titin: relation to passive tension. J Muscle Res Cell Motil. 2002;23(5–6):473–82. doi: 10.1023/a:1023410523184. [DOI] [PubMed] [Google Scholar]
- 48.Greaser ML, et al. Developmental changes in rat cardiac titin/connectin: transitions in normal animals and in mutants with a delayed pattern of isoform transition. J Muscle Res Cell Motil. 2005;26(6–8):325–32. doi: 10.1007/s10974-005-9039-0. [DOI] [PubMed] [Google Scholar]