Abstract
Current evidence indicates that G protein-coupled receptors form dimers that may affect biogenesis and membrane targeting of the complexed receptors. We here analyzed whether expression-deficient follicle-stimulating hormone receptor (FSHR) mutants exert dominant negative actions on wild-type FSHR cell surface membrane expression. Co-transfection of constant amounts of wild-type receptor cDNA and increasing quantities of mutant (R556A or R618A) FSHR cDNAs progressively decreased agonist-stimulated cAMP accumulation, [125I]-FSH binding, and plasma membrane expression of the mature wild-type FSHR species. Co-transfection of wild-type FSHR fragments involving transmembrane domains 5–6, or transmembrane domain 7 and/or the carboxyl-terminus specifically rescued wild-type FSHR expression from the transdominant inhibition by the mutants. Mutant FSHRs also inhibited function of the luteinizing hormone receptor but not that of the thyrotropin receptor or non-related receptors. Defective intracellular transport and/or interference with proper maturation due to formation of misfolded mutant:wild-type receptor complexes may explain the negative effects provoked by the altered FSHRs.
Keywords: Follicle-stimulating hormone receptor, dominant negative receptor, dimerization, intracellular trafficking, endoplasmic reticulum, decoy
Introduction
It is well recognized that mutations of G protein-coupled receptors (GPCRs) may cause receptor retention in intracellular compartments (Sairam et al., 1996; Benkirane et al., 1997; Lee et al., 2000; Ulloa-Aguirre et al., 2004; Brothers et al., 2004; Bulenger et al., 2005; Calebiro et al., 2005; McElvaine et al., 2006; Beaumont et al., 2007; Milligan 2007). Further, some GPCRs mutants or variants may behave as dominant negative conformers blocking the intracellular trafficking of wild type (Wt) receptor species to the cell surface membrane (Zhu et al., 1998; Lee et al., 2000; Brothers et al., 2004; Guan et al., 2009), which may be an indication of intracellular receptor oligomerization or aggregation of Wt and mutant receptors. Intracellular association of GPCRs as homo- or heterodimers could lead, in principle, to cell surface targeting (Jones et al., 1998; Kaupmann et al., 1998; White et al., 1998); although the exact purpose of GPCR oligomerization at the endoplasmic reticulum (ER) or Golgi is currently unknown, one possibility is that receptor chaperoning plays a regulatory role in post-translational control of cell surface expression of these, and possibly other proteins (Lopez-Gimenez et al., 2007; Milligan 2007; Pin et al., 2007). This is the case, for example, of the GABA-B receptor, the α1D-adrenergic receptor, and the β2-adrenergic receptor, in which obligatory homodimerization or heterodimerization with related receptors seems crucial for proper folding, maturation, trafficking, surface expression, and cross-talk (Balasubramanian et al., 2004; Salahpour et al., 2004; Uberti et al., 2005; Pin et al., 2007; Thomas et al., 2007). Alternatively, oligomerization may lead to intracellular retention of the complex (Benkirane et al., 1997; Zhu et al., 1998; Le Gouill et al., 1999; Brothers et al., 2004; Ulloa-Aguirre et al., 2004) and its eventual degradation. In some cases, particularly in disease states with an autosomal dominant modes of inheritance, defective plasma membrane expression has been attributed to the dominant negative effect of the misfolded receptor on its Wt counterpart; this can limit or even abrogate plasma membrane expression of the normal receptor and result in loss-of-function disease (Zhu et al., 1998; Le Gouill et al., 1999; Lee et al., 2000; Leanos-Miranda et al., 2003; Brothers et al., 2004; Ulloa-Aguirre et al., 2004; Leanos-Miranda et al., 2005; Gehret et al., 2006).
The follicle-stimulating hormone (FSH), luteinizing hormone (LH) and thyroid-stimulating hormone (TSH) receptors are glycoproteins that belong to the superfamily of G protein-coupled receptors (GPCR), specifically the family of rhodopsin-like receptors (Ulloa-Aguirre et al., 1998; Dias et al., 2002). Glycoprotein hormone receptors are related to each other by the presence of a large extracellular domain containing several leucine-rich repeats as well as by the homologous structure of their corresponding ligands, which are noncovalently bound heterodimeric glycoproteins (Ulloa-Aguirre et al., 1998; Vassart et al., 2004; Bogerd 2007) . The human (h) FSH receptor (R) (FSHR) consists of 695 amino acids (the first 17 amino acids encoding the signal sequence) (Simoni et al., 1997; Ulloa-Aguirre et al., 1998; Dias et al., 2002). Upon agonist binding, the activated receptor stimulates a number of intracellular signaling pathways. In the classical, linear signaling cascade, occupancy of the FSHR causes activation of the heterotrimeric Gs protein and stimulation of the effector adenylyl cyclase with the consequent increase in the synthesis of the second messenger cAMP, activation of protein kinase A, phosphorylation of cAMP response element-binding protein, and activation of transcription (Ulloa-Aguirre et al., 2007b). Nonetheless, increasing evidence indicates that in addition to the adenylyl cyclase/cAMP/protein kinase-A signaling pathway, activation of the FSH receptor by its cognate ligand also triggers activation of other intracellular signaling cascades, including the MAPK and phosphoinositol-3-kinase/Akt pathways (Cameron et al., 1996; Maizels et al., 1998; Gonzalez-Robayna et al., 2000; Seger et al., 2001; Richards et al., 2002; Ulloa-Aguirre et al., 2007b).
As with many other GPCRs, the FSHR may form dimers or oligomers early during receptor biosynthesis (Thomas et al., 2007, Guan et al., 2010), which may be important to allow correct intracellular trafficking of the complex to the plasma membrane (Thomas et al., 2007) as well as for coupling to multiple G proteins (Nechamen et al., 2007). Although as in other GPCRs, formation of FSHR-FSHR complexes represents a means to enable not only positive cooperativity among receptor molecules, the opposite may also exist, that is, the occurrence of negative cooperativity as a quality control checkpoint at the ER (reviewed in Ulloa-Aguirre et al., 2004 and Bulenger et al., 2005). This may be the case of the functionally inactive alternately spliced variant of the sheep testicular FSHR, which when coexpressed with the active Wt receptor leads to cessation of agonist-stimulated cAMP accumulation (Sairam et al., 1996).
We have recently studied the function of hFSHRs with point mutations in regions bearing the BBXXB motif reversed (BXXBB, where B is a basic amino acid) involved in G protein activation of several cell surface membrane receptors (Cheung et al., 1991; Okamoto et al., 1992; Wu et al., 1995; Murthy et al., 1999; Pauwels et al., 2001) . Overexpression of the R556A mutant (in the BBXXB motif located in the carboxyl-terminal end of the third intracellular loop) profoundly altered agonist-stimulated intracellular signaling without apparently affecting the cell surface expression of the receptor (Timossi et al., 2004). However, further studies in which lower amounts of this particular mutant cDNA were transfected revealed that this altered receptor is in fact an expression- and signaling-defective mutant as both functions are markedly reduced compared with the Wt receptor species. A second mutant, in which the sequence of the BBXXB motif reversed located in the amino-terminal end of the carboxyl-terminal domain (Ctail) of the receptor was modified by replacing R618 with alanine, exhibited considerably reduced cell surface membrane expression and agonist-stimulated intracellular signaling upon overexpression in HEK-293 cells; since R618 is located within the highly conserved F(X)6LL motif required for receptor transport to the cell membrane (Duvernay et al., 2004; Dong et al., 2007) we concluded that this particular mutant is deficient in its ability to couple to G proteins as well as to normally traffic to the cell surface membrane (Timossi et al., 2004; Ulloa-Aguirre et al., 2007a).
In the present study, we describe the results from co-transfection experiments showing that the R556A and R618A expression-defective hFSHR mutants modulate Wt receptor function. We also documented that co-transfection of Wt receptor fragments, reverted the dominant negative effects of the mutants on Wt receptor expression and function, providing further evidence that FSHRs may form intracellular complexes as a quality control mechanism for regulating receptor expression and function.
Material and Methods
Construction of hFSHR mutants and truncated fragments
Construction of the hFSHR mutants was performed employing the full-length Wt hFSHR cDNA (Kelton et al., 1992) [GenBank Accession Number S59900] cloned into the mammalian expression vector pSG-5 or pcDNA3.1 (Stratagene, La Jolla, CA). Site-directed mutagenesis was performed using the QuikChange site-directed mutagenesis kit (Stratagene), as previously described (Timossi et al., 2002; Timossi et al., 2004; Uribe et al., 2008). Residues at positions 556 [in the intracellular loop 3 (IL3)] and 618 (in the Ctail) (Fig. 1) were individually replaced with Ala (hFSHR R556A and hFSHR R618A, respectively) employing the same mutagenesis procedure. Forward and reverse mutagenic oligonucleotide primers (Life Technologies, Grand Island, NY) (Timossi et al., 2004) were designed according to the cDNA sequence reported for the testicular hFSHR (Kelton et al., 1992). A chimeric hFSHR/rat (r) LHR Ctail cDNA (hFSHR/rLHR-Ctail) was constructed as previously described (Uribe et al., 2008). This chimera is conformed by amino acid residues 1–611 of the hFSHR and residues 604–674 of the rLHR [GenBank Accession Number NM_012978], is highly expressed at the plasma membrane, efficiently binds agonist, and promotes cAMP accumulation on exposure to agonist (Uribe et al., 2008). The human gonadotropin-releasing hormone receptor (GnRHR)-catfish Ctail chimera (hGnRHR-cfCtail) in pcDNA 3.1 was constructed as previously described (Maya-Nunez et al., 2000).
Figure 1.
Schematic of the third intracellular loop (IL3) (A) and a portion of the carboxyl-terminal segment (Ctail) (B) of the Wt hFSHR, indicating the localization of the mutation within the BXXBB motif of the IL3 and the Ctail of the receptor. The discontinuous line square in B indicates the position of the F(X)6LL motif.
Construction of the minigenes encoding for Wt hFSHR fragments was performed employing eigth specific oligonucleotides complementary to the Wt hFSHR cDNA (see Table in Supplementary information). Four Wt hFSHR cDNA segments encoding receptor fragments of different lengths derived from residues L509 to N678 of the Wt hFSHR were amplified by PCR. Sense oligonucleotides were synthesized with a translation start codon (ATG) in the 5’ end, while the antisense oligonucleotides were generated with a stop codon (TAA). Each oligonucleotide was phosphorylated in the 5’ end through the polynucleotidecinase (Roche Applied Science, Penzberg, Germany) reaction with ATP as substrate. Each PCR used Taq DNA polymerase (Roche Applied Science) and the expression vector pcDNA3.1(+)/hFSHR as template. For all rounds of amplification, DNA was first denatured at 94° C for 30 seconds, followed by 30 cycles that included 30 seconds of denaturation at 94° C, primer annealing for 35 seconds at 58° C, and product extension at 72° C for 12 min. To optimize the amount of full-length product, a final extension was carried out at 72° C for 10 min. The final PCR products were analyzed on an ethidium bromide-stained 10% polyacrylamide gel and bands within the expected molecular weights were cut and purified by electroelution in TBE buffer. Each DNA fragment was then precipitated on 3.0 M ammonium acetate, pH 5.2 and 100% ethanol at −20 °C. After 12 hours, the fragments were centrifuged at 14,000 rpm for 20 min and washed with 75% ethanol. All the fragments were eluted in 20 µl of Tris-EDTA, pH 8.0 and ligated into the expression vector pcDNA3.1 (Stratagene).
The identity of all cDNA constructs and the correctness of the PCR-derived sequences were verified by DNA sequencing using an automated sequencer 377 (Applied Biosystems, Foster City, Ca). For transfection, large scale plasmid DNAs were prepared using an Endofree maxiprep kit (Qiagen, Valencia CA).
The full-length LHR (kindly provided by Dr. Juan Pablo Méndez, Instituto Nacional de Ciencias Médicas y Nutrición SZ, Mexico City, Mexico), TSHR (provided by Dr. Gilbert Vassart, Université Libre de Bruxelles, Brussels, Belgium), dopamine D1 receptor (clone I.D. DRD0100000), and β2-adrenergic receptor (clone I.D. AROB200000) [obtained from the Missouri S&T cDNA Resource Center (www.cdna.org)] cDNAs were all cloned in pcDNA3.1.
Cell culture and transfection of Wt and mutant hFSHR cDNAs
Human embryonic kidney (HEK)-293 cells were maintained in an humidified atmosphere of 5% CO2 at 37° C in low-glucose Dulbecco Modified Eagle Medium (DMEM) (Life Technologies Inc., Grand Island, NY), supplemented with 5% fetal calf serum (FCS), 5 µg/ml geneticin and antibiotic-antimycotic reagent (Life Technologies). Cells were grown to 70–80% in 75 cm2 flasks (Costar, Cambridge, MA), replated at an initial density of 7.5 × 104 cells on 15.6 mm diameter wells (in 24-well cell culture plates) or 60 mm diameter culture wells (Corning Life Sciences, Lowell, Ma, USA), and cultured for 24 h at 37° C. Cells were then washed with unsupplemented DMEM and co-transfected with constant amounts of Wt hFSHR cDNA (25 or 50 ng/well for functional studies and 500 ng/well for immunoblotting) and mutant receptors cDNAs in increasing amounts to attain Wt to mutant receptor ratios of 1:1 to 1:7 by liposome-mediated endocytosis in OPTIMEM (Life Technologies). In separate experiments, different amounts of Wt hFSHR fragments cDNAs were additionally included in the co-transfections. Where necessary, the total amount of DNA used for each transfection was completed to a maximal of 53 ng/1.0 × 104 cells by the addition of an appropriate amount of empty vector DNA, so that the total amount of DNA transfected into all wells was kept constant. Under these conditions, cotransfection of two or three different DNA constructs did not lead to any detectable toxicity or cell death as assessed by the yellow tetrazolium salt XTT colorimetric asay (Roche Diagnostics GmbH, Mannheim, Germany).
In vitro bioassay and measurement of cAMP production and inositol phosphate production
Forty-eight hours after the start of transfection the medium was removed and the cells were washed twice with DMEM-5% FCS and then stimulated with increasing (0 to 75 ng/ml) or maximal (75 ng/ml) doses of recombinant FSH (Serono de México S.A. de C.V., México), recombinant human LH (Organon International BV, Oss, Holland), isoproterenol, bromocriptine, or bovine TSH (Sigma-Aldrich, St. Louis, MO, USA) in DMEM-5% FCS supplemented with 0.125 mM 3-isobutyl-methyl-xanthine (Sigma-Aldrich). At the end of the incubation period (18 h), the medium was removed and total (extracellular plus intracellular) cAMP accumulation was measured in acetylated samples by radioimmunoassay, as described previously (Zambrano et al., 1996).
For induction of inositol phosphate production to assess hGnRHR-cfCtail function, HEK-293 cells were co-transfected with the hGnRH-cfCtail chimera and mutant hFSHR cDNAs as described above. Forty-eight hours after transfection the medium was removed, the cells were washed with DMEM/0.1% bovine serum albumin (Life Technologies), and cellular inositol lipids were labeled in DMEM (inositol-free) supplemented with 4 µCi [3H]myoinositol (PerkinElmer Life Sciences Inc., Boston, MA) for 18 hours at 37° C. After preloading, the cells were washed twice in DMEM (inositol-free) containing 5 mM LiCl and stimulated with increasing concentrations of the GnRH agonist, Buserelin (Sigma, St. Louis, MO) for 2 h. Quantification of inositol phosphate production by Dowex anion exchange chromatography and liquid scintillation spectroscopy was performed as described previously (Huckle et al., 1987).
Receptor binding assay
Human pituitary FSH (specific activity 24 µCi/ug protein) was radiolabeled as described previously (Weiner et al., 1992). HEK-293 cells were transfected with Wt or mutant hFSHR cDNAs as described above. Forty-eight hours after transfection, the medium was removed, replaced with fresh medium and allowed to continue incubation at 37° C for 1 h. After the preincubation period, the medium was removed and serum-free DMEM containing 20 ng/ml [125I]-FSH was added to each well in the presence or absence of 1 µg/ml unlabeled recombinant single-chain FSH to assess for non-specific binding (Uribe et al., 2008). Hormone was allowed to bind for 1 h at 37° C before the cultures were placed on ice and washed twice with ice-cold PBS. Cell-surface radiolabeled FSH was eluted with ice-cold 50 mM glycine/100 mM NaCl, pH 3.0, for 10 min on ice and the eluate was removed to a glass tube and analyzed for radioactivity content.
SDS-PAGE and Western blot analysis
Human embryonic kidney 293 cells cultured in 60 mm diameter culture wells were co-transfected with hFSHR Wt and mutant plasmid DNAs at different Wt to mutant ratios (see above). After 48 h of the start of transfection, cells were lysed with Passive Lysis Buffer (Promega), containing 1% Igepal, 0.4% DOC, 10 mM Tris pH 7.5, and 6.6 mM EDTA, and protein extracts (15 µg/lane) were resolved by 7.5% SDS-PAGE. Following electrophoreses, proteins were transferred to Immobilon-P membranes, probed with primary antibody [5 µg mAb 106.105 (Nechamen and Dias, 2003)], and then incubated with secondary anti-mouse IgG horseradish peroxidase conjugate (Biosource International, Armadillo, CA, USA), as previously described (Lindau-Shepard et al., 1994; Nechamen et al., 2003). Signal was developed using the Pierce ECL Western Blotting detection kit (Rockford, IL, USA). Equal protein loading was confirmed in a stripped, washed and reprobed membrane with a 1:2000 anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Sigma) and 1:10,000 goat-anti-mouse IgG conjugated with horseradish peroxidase (Biosource).
Statistical analysis
Data were analyzed with one-way analysis of variance and then Student T test. (SigmaStat 3.1; Jandel Scientific Software). P<0.05 was considered significant. The results are expressed as means ± SEM from 3–4 independent experiments in triplicate incubations, unless indicated.
Results
Dominant negative effects of hFSHR mutants on agonist-stimulated cAMP production, [125I]-FSHbinding and cell surface membrane expression of the hFSHR
Human embryonic kidney-293 cells were co-transfected with the same amount of Wt receptor cDNA and increasing concentrations of each hFSHR cDNA mutant (R556A or R618A). Co-transfection of hFSHR mutants and Wt receptor cDNA at 1:1 to 1:7 Wt:mutant ratios progressively reduced maximal FSH-stimulated cAMP responses compared with cells co-transfected with the Wt receptor and pSG5 empty vector (Figs. 2A and 2D). The inhibitory effect of the mutants on Wt receptor function ocurred at increasing FSH concentrations; maximal responses declined by 60–80% in cells expressing a relative excess (7-fold) of mutant cDNAs. Human FSHR mutants co-transfected with the empty vector (1:7 mutant/vector ratio) exhibited a 4–16% the activity presented by the Wt receptor transfected under the same conditions.
Figure 2.
Concentration-dependent basal and FSH-stimulated total (extra- plus intracellular) cAMP accumulation (A and D), specific [125I]-FSH binding (B and E), and relevant portions of autoradiograms from Western blots showing a ~80 kDa band representing the mature, cell surface membrane-expressed, fully glycosylated hFSHR (C and F) in HEK-293 cells co-transfected with constant amounts of Wt hFSHR cDNA (50 ng/well in A, B, D, E; 500 ng/well in C and F) plus empty pSG5 vector (1:7 Wt:vector ratio) or plus hFSHR R556A (A-C) or R618A (D–F) cDNAs at increasing (1:1 to 1:7) Wt:mutant ratios. Wild-type and mutant FSHRs cDNAs were in pSG5. Where necessary, an appropriate amount of empty vector was added, so that the total amount of DNA transfected into each well was kept constant [400 ng/well for functional studies (A, B, D, E) or 4000 ng/well for Western blots (C and F)]. Maximal FSH-stimulated cAMP accumulation was set at 100% for each experiment, and all other values are expressed relative to this. Densitometric analyses (normalized for GAPDH expression) of three immunoblots are also shown (lower panels in C and F). The results are the mean ± SEM from 3–4 independent experiments in triplicate incubations. *p<0.05 vs all other conditions; §p<0.05 vs 1:7 (in B and E) and p<0.01 vs 1:6 (in C and F).
Specific cell surface [125I]-FSH binding was measured in cells coexpressing the Wt and each hFSHR mutant. As shown in Figs. 2B and 2E, compared with cells co-transfected with the Wt hFSHR cDNA and empty vector, HEK-293 cells coexpressing the Wt and increasing concentrations of the different mutant receptors exhibited a significantly reduced capacity to bind labelled agonist. Similar results were obtained by Western blot analysis; a ~80 kDa band representing the mature, fully glycosylated hFSHR was clearly identified in cells transiently expressing the Wt but not the mutant receptors (Figs. 2C and 2F). The intensity of this band progressively decreased in cells co-transfected with the Wt and increasing concentrations of each mutant hFSHR. These results were further corroborated by densitometric analysis of Western blots data for relative mature ~80 kDa hFSHR levels normalized to GAPDH expression. The finding that GAPDH expression did not change, ruled out the possibility that the dominant negative effects of the mutant receptors on Wt hFSHR expression was due to saturation or dysfunction of the protein synthesis machinery in cotransfected cells.
In the experiments shown in Figs. 2, 3, and 5–7 the Wt and mutant hFSHR cDNAs were hosted by the expression vector pSG5. Since the transcription efficiency of the pSG5 expression vector (driven by the Simian Virus 6 promoter) is lower than that exhibited by pcDNA3.1 vector (driven by the Cytomegalovirus promoter) (Zhou et al., 2004) (Fig. S1, inset), and this may affect the rate of Wt and mutant hFSHR synthesis and association (Milligan 2007), we performed coexpression experiments employing Wt and mutant receptors cloned in the pcDNA3.1 expression vector. The results showed that the dominant negative effects of the expression-deficient hFSHR mutants persisted even when the pcDNA3.1 was used as the expression vector, although the magnitude of the inhibition was lower than that provoked by mutant receptors cloned in the pSG5 expression vector as would be expected due to the higher level of expression of the Wt receptor (Fig. S1).
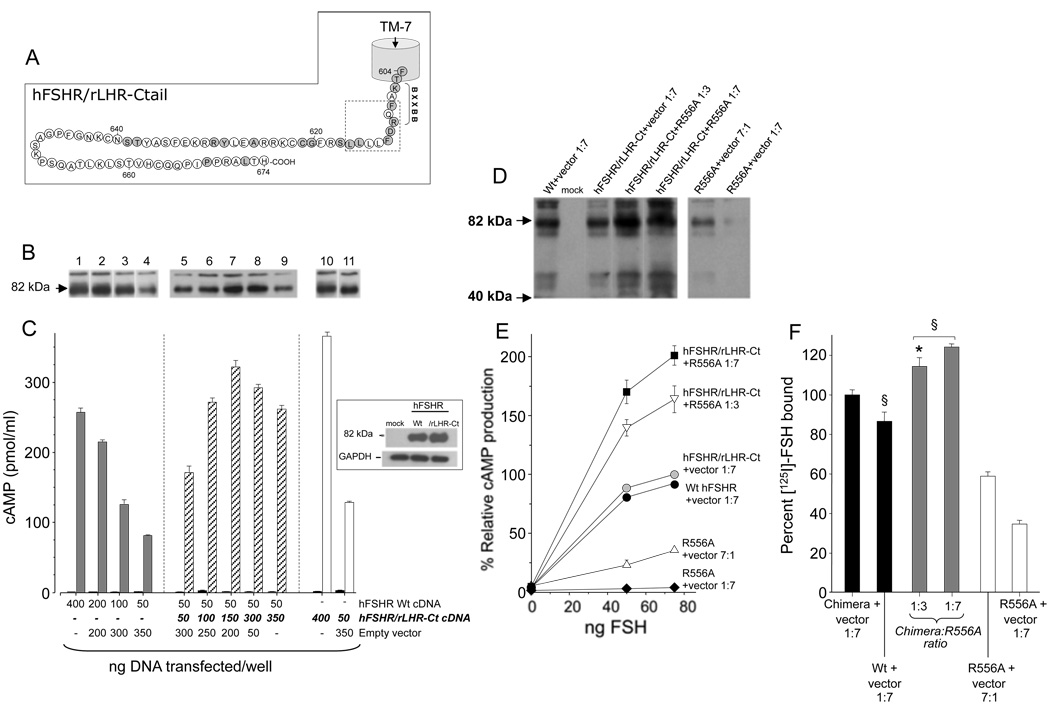
Figure 3.
A: Schematic of the carboxyl-terminal segment (Ctail) of the hFSHR/rLHR-Ctail chimera; the amino acid residues shared by the Wt hFSHR and the rLHR-Ctail are shaded in grey. B: Western blot analysis of the Wt hFSHR and hFSHR/rLHR-Ctail chimera from HEK-293 cells co-transfected with the Wt hFSHR cDNA (4000, 2000, 1000, and 500 ng/well) plus empty vector (lanes 1 to 4), constant amounts of the Wt hFSHR cDNA (500 ng/well) plus hFSHR/rLHR-Ctail chimera cDNA at increasing (1:1, 1:2, 1:3, 1:6, and 1:7) Wt:chimera ratios (lanes 5 to 9) or the hFSHR/rLHR-Ctail chimera cDNA (4000 ng and 500 ng/well) plus empty vector (lanes 10 and 11, respectively). C: cAMP accumulation in cells transfected or co-transfected as in B with the amounts of cDNA transfected adjusted to 24-well plates (see Material and Methods) and incubated in the absence (left bar in each pair) or presence (75 ng/ml; right bar in each pair) of human recombinant FSH. The results are representative from 3 independent experiments. Inset: Western blotting of the Wt hFSHR and the hFSHR/rLHR Ctail. Upper panel, relevant portion of an autoradiogram showing a ~80 kDa band representing the mature, cell surface membrane-expressed, fully glycosylated hFSHR and the hFSHR/rLHR-Ctail chimera; lower panel: immunoblot of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same gel. D: Western blot analysis of lysates from HEK-293 cells co-transfected with the Wt hFSHR (500 ng/well) or hFSHR/rLHR-Ctail chimera cDNA (500 ng/well) plus empty vector (at 1:7 Wt:vector and chimera:vector ratios), constant amounts of the hFSHR/rLHR-Ctail chimera (500 ng/well) plus the R556A expression-deficient hFSHR mutant cDNA at 1:3 and 1:7 chimera:mutant ratios, or the R556A mutant cDNA (3500 or 500 ng cDNA/well) plus empty vector (7:1 and 1:7 mutant:vector ratios, respectively). Equal protein loading was confirmed in a stripped, washed, and reprobed membrane with a 1:2000 anti-GAPDH antibody and 1:10000 goat-anti-mouse IgG conjugated with horseradish peroxidase (not shown). E and F: Maximal FSH-stimulated cAMP accumulation and specific [125I]-FSH binding in cells co-transfected as in D with the amounts of cDNA transfected adjusted to 24-well plates (See Material and Methods). The Wt hFSHR, hFSHR/rLHR-Ctail chimera and mutant R556A hFSHR cDNAs were all hosted by the pSG5 expression vector. Appropriate amounts of empty vector was added so that the total amount of DNA transfected into each well was kept constant (400 ng/well for functional studies and 4000 ng/well for immunoblots). The immunoblot shown in D is representative from 3 independent experiments; the results shown in E and F are the mean ± SEM from 3 independent experiments. §p<0.01 vs hFSHR/rLHR-Ctail plus empty vector 1:7; *p<0.05 vs chimera:hFSHR R556A 1:7.
Figure 5.

Effect of co-transfecting the Wt hFSHR L509-V582 fragment cDNA on the dominant negative effects of the R556A hFSHR mutant. A: Maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R556A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R556A cDNA, plus increasing amounts of the Wt hFSHR L509-V582 cDNA fragment. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the Wt hFSHR; black circles indicate the region of the Wt hFSHR encoded by the cDNA fragment co-transfected. B: Representative autoradiogram from an immunoblot of the hFSHR present in protein extracts from HEK-293 cells co-transfected with the Wt hFSHR cDNA, the Wt receptor cDNA plus the R556A hFSHR mutant cDNA (ratio 1:4), or the Wt hFSHR cDNA plus the R556A mutant cDNA plus the L509-V582 hFSHR fragment cDNA. Only relevant portions of a representative scanned autoradiograph are shown. The autoradiograph was overexposed in order to show the expression levels of the mutant receptor species and the immature forms of the hFSHR. C: Specific [125I]-FSH binding to HEK cells co-transfected with the cDNAs shown at the bottom of the graph. Addition of the Wt hFSHR A590-N678 fragment cDNA to co-transfections with Wt hFSHR plus R556A mutant cDNA did not modify the percent specific [125I]-FSH binding. The Wt and mutant hFSHR cDNAs were hosted by the pSG5 vector whereas the hFSHR fragments were in pcDNA3.1. The results shown in 5A and C are the mean ± SEM from 3 independent experiments. *p<0.05 vs all other conditions; ¶p<0.01 vs all other conditions; §p<0.05 vs Wt + L509-V582 fragment + empty vector. (m)FSHR, mature form of the hFSHR; (i)FSHR, immature form of the hFSHR.
Figure 7.
Effect of co-transfecting the Wt hFSHR A590-N678 fragment cDNA on the dominant negative effects of the R618A hFSHR mutant. A: Maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R618A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R618A cDNA plus increasing amounts of the Wt hFSHR A590-N678 fragment cDNA. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the Wt hFSHR indicating in black circles the region of the Wt hFSHR encoded by the cDNA fragment transfected. B: Representative autoradiogram from an immunoblot of the hFSHR present in protein extracts from HEK-293 cells co-transfected with the Wt hFSHR cDNA, the Wt receptor cDNA plus the R618A hFSHR mutant cDNA (ratio 1:5), or the Wt hFSHR cDNA plus the R618A mutant cDNA plus the Wt hFSHR A590-N678 fragment cDNA. Only relevant portions of a representative scanned autoradiograph are shown. The autoradiograph was overexposed in order to show the low expression levels of the mutant receptor species and the immature forms of the hFSHR. C: Specific [125I]-FSH binding to HEK-293 cells co-transfected with the cDNAs as indicated at the bottom of the graph. The Wt and mutant hFSHR cDNAs were hosted by the pSG5 vector whereas the hFSHR cDNA fragment was in pcDNA3.1. The results shown in 7A and C are the mean ± SEM from 3 independent experiments. *p<0.01 vs Wt + hFSHR R618A + empty vector and vs hFSHR R618A + A590–N678 fragment + empty vector. ¶p<0.01 vs Wt + emtpy vector; §p<0.01 vs Wt + A590-N678 fragment + empty vector. (m)FSHR, mature form of the hFSHR; (i)FSHR, immature form of the hFSHR.
To determine that coexpression of another modified hFSHR does not affect expression of the test receptor, we coexpressed the Wt hFSHR with a chimera composed of amino acid residues 1–611 of the hFSHR and residues 604–674 of the rLHR (Fig. 3A). As shown previously (Uribe et al., 2008), this chimeric receptor is highly expressed at the plasma membrane, efficiently binds radiolabelled ligand, and promotes cAMP production on exposure to agonist (Fig. 3). The results showed that coexpression of constant amounts of Wt receptor with increasing quantities of the hFSHR/rLHR-Ctail chimera did not perturb agonist-stimulated cAMP production or the intensity of the ~80 kDa band, but rather increased both measurements in an additive manner (Fig. 3B and C). Further, cotransfection of the hFSHR/rLHR-Ctail chimera cDNA with increasing amounts of the R556A mutant cDNA, led to a very interesting effect which confirmed that the observed dominant negative effects of the mutants on Wt hFSHR expression and cAMP responses were neither due to different amounts of proteins expressed or toxic effects of the transfection procedure nor to saturation/dysfunction of the protein synthesis and/or transport machinery of transfected cells. Specifically, the signal intensity corresponding to the mature form of the FSHR on immunoblotting was stronger as the amount of the R556A mutant receptor cDNA cotransfected progressively increased (Fig. 3D). In these particular cotransfection experiments, the presence of increasing amounts of the <80 kDa bands, which represent immature underglycosylated forms of the hFSHR, were also evident. The increase in cell surface plasma membrane expression of the hFSHR in co-transfections at 1:3 and 1:7 chimeric receptor:mutant receptor ratios, correlated with significantly higher amounts of agonist-stimulated cAMP accumulation (relative cAMP production 164±11% and 200±8%, respectively) (Fig. 3E), which cannot be solely explained by increased R556A hFSHR expression (Fig. 3F), since this particular mutant is marginally active (relative FSH-stimulated cAMP production, 36 ± 0.2% at maximal mutant receptor overexpression levels): Fig. 3E.
Dominant negative effects of hFSHR mutants on agonist-stimulated cAMP production by closely related and non-related GPCRs
We then analyzed the specificity of the dominant negative effects of the hFSHR expression mutants on the function of the Wt hFSHR species. To this end, we performed a series of experiments in which other glycoprotein hormone receptors (LHR and TSHR) or non-related receptors (i.e. the β2-adrenergic receptor, the dopamine D1 receptor, and the GnRHR) were co-transfected with each hFSHR mutant. Coexpression of the Wt LHR with either the R556A or R618A hFSHR mutants, led to attenuation of maximal agonist-stimulated cAMP (Fig. 4). No dominant negative effects on agonist-stimulated cAMP production or inositol phosphate production was observed when increasing amounts of the hFSHR mutants were co-transfected with the Wt TSHR, the catecholamine receptors tested (Gs-coupled), or the GnRHR-cfCtail chimera (Gq/11-coupled) (Figs. S2 and S3). Here again, these observations demonstrate that the dominant negative effects of the mutant FSHRs on Wt hFSHR are not due to squelching of protein synthesis, because it is clear that the expression of the mutant hFSHRs had no effect on maximal responses with these unrelated receptors expressed simultaneously.
Figure 4.
Concentration dependent cAMP accumulation in HEK-293 cells co-transfected with the Wt LHR cDNA (50 ng/well) plus empty vector (1:7 Wt:empty vector ratio), the Wt LHR cDNA (50 ng/well) plus the R556A or R618A hFSHR mutants cDNA at 1:3 to 1:7 Wt LHR:mutant ratios or the hFSHR cDNA mutants (50 ng/well) plus empty vector (1:7 mutant:vector ratio). All receptors cDNAs transfected were in pcDNA3.1. Appropriate amounts of empty vector was added so that the total amount of DNA transfected into each well was kept constant (400 ng/well). Cells were stimulated with increasing concentrations of recombinant LH (when co-transfected with the Wt LHR cDNA plus empty vector or hFSHR mutants) or recombinant FSH (when transfected with the mutant hFSHRs cDNA plus empty vector). The results are the mean ± SEM from 3 independent experiments.
Attenuation of the dominant negative effects of hFSHR mutants by Wt hFSHR fragments
Minigenes coding for distinct Wt hFSHR fragments [regions comprising L509-V582 (TM-5 and −6 and IL3), H534-V582 (IL3 and TM−6), A590-N678 (TM-7 and Ctail), and F612-N678 (Ctail only) of the full-length Wt hFSHR] were constructed and cloned in the pcDNA3.1 expression vector. The ability of these minigenes coding for small fragments of the Wt hFSHR to be expressed by HEK-293 cells has been previously documented (Timossi et al., 2002). Addition of increasing amounts of the L509-V582 fragment cDNA to co-transfections of the Wt and R556A mutant cDNAs reverted the dominant-negative effects of the latter on maximal agonist-stimulated cAMP (Fig. 5A), cell surface membrane receptor expression Fig. 5B), and [125I]-FSH binding (Fig. 5C) in a dose-depended fashion. Reversion of the dominant negative effect of the R556A mutant was only partial when a shorter fragment (H534-V582) was included in the co-transfection DNA mixture (Figs. 6A and S4A), thus involving both transmembrane regions V and VI and/or the third intracellular loop of the Wt receptor as potential sites for possible protein-protein interactions. By contrast, inclusion of the A590-N678 cDNA fragment (which encodes the seventh transmembrane domain and the Ctail of the Wt hFSHR) with co-transfections of the Wt with the R556A mutant cDNAs, did not perturb the dominant negative effect of the mutant receptor on Wt hFSHR function (Fig. 6B) or specific [125I]-FSH binding (Fig. S4B). However, transfection of this latter fragment as well as that encoding the entire Ctail of the Wt receptor (fragment F612-N678), led to almost complete reversion of the dominant negative effect of the R618A mutant on Wt receptor expression and function (Figs. 7 and S5), implying the participation of this latter domain on the dominant negative effect provoked by the R618A mutant. Co-transfection of high amounts of Wt receptor fragments with either the full-length Wt hFSHR or the hFSHR mutants, did not modify significantly the expression levels or agonist-stimulated cAMP production mediated by these receptors.
Figure 6.
Effect of co-transfecting the Wt hFSHR H534-V582 and A590-N678 fragments cDNAs on the dominant negative effects of the R556A hFSHR mutant. A: Maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R556A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R556A cDNA plus increasing amounts of the Wt hFSHR H534-V582 fragment cDNA. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the Wt hFSHR; black circles indicate the region of the Wt hFSHR encoded by the H534-V582 cDNA fragment transfected. B: Maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R556A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R556A cDNA, plus the Wt hFSHR A590-N678 fragment cDNA. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the Wt hFSHR showing in black circles the region of the Wt hFSHR encoded by the cDNA fragment co-transfected. The Wt and mutant hFSHR cDNAs were hosted by the pSG5 vector whereas the hFSHR cDNA fragments were in pcDNA3.1.The results shown are the mean ± SEM from 3 independent experiments. *p<0.01 vs all other conditions.
Since it has already been shown that the hFSHR forms dimers during biosynthesis (Thomas et al., 2007; Guan et al.,, 2010), it was reasoned that the fragments may bind to the mutant receptors and or Wt receptors preventing their association with each other. Yet it has not been demonstrated that the mutant hFSHRs can associate with the Wt hFSHR. To test this possibility, coexpression of mutant hFSHR-Flag with Wt hFSHR-myc fusion protein was carried out followed by co-immunoprecipiation with anti-myc and detection with anti-flag antibodies. The results demonstrated that the mutant forms of hFSHR co-immunoprecipitate with Wt FSHR (Fig. S6).
Discussion
A large body of evidence supports the notion that GPCRs form self-associated dimers during the processing of synthesis in the ER (Bulenger et al., 2005; Dalrymple et al., 2008; Milligan 2007; Milligan 2008). In the particular case of the gonadotropin receptors, it has been shown that dimerization/oligomerization of both the FSH and LH receptors is an obligatory requisite for expression and that it occurs constitutively early in the biosynthetic pathway (Tao et al., 2004; Thomas et al., 2007; Guan et al., 2009, 2010). Further, misfolded human LHR mutants that are retained in the ER may not only homodimerize but also form heterodimers with the Wt receptor and evoke dominant negative effects on Wt receptor expression (Guan et al., 2009).
In the present study, we have shown that co-transfection of expression-deficient mutant hFSHRs with the Wt hFSHR attenuates cell surface membrane expression of the Wt receptor species. The dominant negative effects of the receptor mutants were documented using functional and receptor-binding assays as well as Western blot analysis of the mature form of the hFSHR (m.w. ~80 kDa). In each case, receptor expression, binding capacity, and FSH-stimulated cAMP accumulation were all inversely proportional to the quantity of mutant (R556A and R618A) cDNA co-transfected with the Wt receptor. The magnitude of the dominant negative effects provoked by the mutant hFSHRs was clearly lower than those previously reported for completely inactive mutant or truncated variants of other GPCRs ( Okuda-Ashitaka et al., 1996; Benkirane et al., 1997; Grosse et al., 1997; Zhu et al., 1998; Le Gouill et al., 1999; Lee et al., 2000; Scarselli et al., 2001; McElvaine et al., 2006; Beaumont et al., 2007), including the FSHR (Sairam et al., 1996) and the LHR (Nakamura et al., 2004; Minegishi et al., 2007), but similar to that observed for point mutants of the GnRHR (Leanos-Miranda et al., 2003). This suggests that the ability of a given mutant receptor to associate with the Wt receptor species and exert differing magnitude of negative effects are both related to the distinct configuration adopted by the altered receptor and the resulting final conformation of the associated (i.e. Wt-mutant) proteins, which in turn eventually determine the functional fate of the formed complexes. In this regard, it was interesting to find that the relative decrease in FSH-stimulated signaling activity in cells co-transfected with the Wt and R556A mutant receptor (particularly at low mutant:Wt ratios), was comparatively higher than the reduction observed in [125I]-FSH binding and receptor protein expression. These disproportionate responses may be provoked by the fraction of mutant receptors that reached the cell surface plasma membrane, bound the agonist but failed to signal properly. Another possibility might be that the fraction of mutant receptors that reached the membrane in complex with the Wt receptor, allowed binding to agonist but blocked transactivation of the Wt species and/or promoted internalization and degradation of the complex, as it has recently been shown for the human LHR (Zhang et al., 2009).
Another interesting observation was that the dominant negative effect of the mutants was strikingly higher when the expression of the receptors was controlled via a less efficient expression plasmid (pSG5). This finding is consonant with previous data showing that poorly expressed GPCRs are more engaged to form hetero-oligomers, particularly when the receptor partner is expressed at higher levels (Breit et al., 2004). Certainly, the level of receptor expression with the pSG5 plasmid is closer to the naturally occurring level of receptor suggesting that even more severe effects would be expected in vivo. Alternatively, both the expression in heterologous systems (which is frequently accompanied by crowding of the quality control system) and the slow rate of transcription of the pSG5 expression plasmid, could slow down export of the newly synthesized proteins from the endoplasmic reticulum, allowing enough time for specific heterodimerization to occur.
The specificity of the inhibitory effects of the hFSHR mutants on Wt receptor function was also analyzed. The results showed that the expression deficient hFSHR mutants also attenuated LHR function, but had no effect on the TSHR and other not related GPCRs. This observation was anticipated considering the relatively high homology (~70%) between the transmembrane domains of the gonadotropin receptors (Dias et al., 2002) as well as previous data documenting dominant negative effects of a LHR splice variant lacking exon 9 on Wt FSHR expression (Minegishi et al., 2007).
Direct interactions between the Wt and mutant receptors and formation of hetero-oligomers might explain the loss of Wt receptor function and/or reduced efficiency in trafficking of the Wt receptor species to the cell surface membrane. Formation of intracellular complexes between variant or mutant receptors and the Wt receptor, with intracellular trapping of the complexed receptors, has been documented for a number of GPCRs, including the GnRHR (Brothers et al., 2004; Ulloa-Aguirre et al., 2004; Pawson et al., 2005), the dopamine D3 receptor (Karpa et al., 2000), the TSHR (Calebiro et al., 2005), the α1b- and β2-adrenergic receptors (Lopez-Gimenez et al., 2007), and the V2-vassopressin receptor (Zhu et al., 1998), among others. Further, it has been shown that interactions between receptors occur during protein synthesis and maturation, prior to cell surface delivery (Salahpour et al., 2004; Wilson et al., 2005). Our failure to detect by immunoblotting with the anti-hFSHR antibody employed high-molecular-weight (≥175,000 kDa) hFSHR forms that presumably may represent oligomers of mutant-Wt receptor complexes (Thomas et al., 2007), may reflect the inability of this antibody to recognize such particular heteromers or, less likely, the occurrence of rapid degradation of the associated receptors into fragments that cannot be recognized by the anti-hFSHR antibody employed. Both possibilities are supported by the detection in protein immunoblots of lysates from cells cotransfected with the hFSR/LHR-Ctail chimera and the R556A hFSHR mutant of immature, underglycosylated precursors of the mature hFSHR, which are the forms that may constitutively dimerize during synthesis (Thomas et al., 2007). Interestingly, these latter cotransfection experiments consistently revealed that the R556A mutant acted rather as a chaperone for the hFSHR/rLHR Ctail chimera, favoring its traffic to the cell surface membrane. Since the Ctail of the rLHR binds p38-JAB1, which promotes degradation of the receptor in the ER (Li et al., 2000), it is conceivable to consider the association of the mutant hFSHR Ctail as one of the mechanisms involved in the increased expression of the chimera. Another possibility that may explain the disproportionate increase in agonist-stimulated cAMP production over specific [125I]-FSH binding in cells co-transfected with the hFSHR/rLHR Ctail chimera and the R556A mutant (Figs. 3E and F) is that rescue of the mutant receptor (which is marginally active) by the chimera, produced a functional, fully active heterodimer via allosterically-induced conformational rearrangements between receptor protomers (reviewed in Szidonya et al., 2008), illustrating how heterozygosity of mutations in the hFSHR could go undetected in vivo. In ensemble, the overall data are consistent with the possibility that the dominant negative effects evoked by the hFSHR mutants on Wt receptor function were due to formation of intracellular mutant-Wt complexes, unable to pass quality control checkpoints and reach the plasma membrane. In this regard, it is important to note that the R618A mutation may potentially alter the conformation of the F(X)6LL export motif and thereby compromise further the export competence of the Wt-R618A mutant receptor complex. Alternatively, the improperly arranged hetero-oligomer complex may unmask endoplasmic reticulum retention signals and perturb the export of the complex to the plasma membrane.
Given that the dominant negative effects of the hFSHR mutants on Wt receptor function probably resulted from the association and degradation of mutant and Wt FSHRs complexes, we performed a series of co-transfection experiments including minigenes coding for distinct fragments of the Wt receptor, in an attempt to interfere with heterodimer formation and attenuate the dominant negative effects of the mutants via competing with full-length Wt receptor molecules to associate with the mutant species. The results showed that co-transfection of Wt receptor fragments involving transmembrane domains 5 and 6, specifically rescued expression of Wt hFSH functional receptors from the trans-dominant inhibition by the R556A mutant in a dose dependent manner, whereas fragments comprising transmembrane domain 7 and/or the Ctail rescued Wt receptor expression perturbed by the presence of the R618A mutant. These findings, in addition to showing that the minigenes co-transfected were efficiently expressed when transfected to HEK-293 cells (Timossi et al., 2002), strongly suggest that receptor sequences present in the co-transfected fragments may potentially be involved in the formation of mutant-Wt receptor hetero-oligomers. Of two possibilities, each posits a decoy effect. The fragments may bind either to the mutant receptors and/or Wt receptor preventing their association with each other or to endoplasmic reticulum surveillance proteins which detect misfolded or conformationally altered receptors, and thereby allow the passage of the heteromers to the cell surface. This of course assumes that dimerization of the Wt and mutant hFSHRs was possible and indeed this seemed to be the case. In fact, evidence has been provided showing that dimer formation is important for transport of the gonadotropin receptors from the ER to the cell surface plasma membrane (Thomas et al., 2007; Guan et al., 2009), and we have determined that the mutant hFSHR receptors can co-immunoprecipitate with the Wt receptor (Fig. S6). Nevertheless, no data is available on how the point contacts between monomers of this receptor are established. In this vein, it is interesting to note that different domains, particularly the transmembrane α-helices, can contribute to the dimerization interface between GPCRs, including the hFSHR (Guan et al., 2010). More specifically, it has been demonstrated, for example, that transmembrane 6 of the β2-adrenergic receptor participates in the receptor homodimerization interface (Hebert et al., 1996), that homodimerization of the dopamine D2-receptor includes intermolecular interactions involving transmembrane domains 4 to 7 (Ng et al., 1996; Lee et al., 2003), and that the Ctail participates in the formation of μ- and δ-opioid receptor hetero-oligomerization (Fan et al., 2005). The finding that an altered FSHR bearing a Ctail from a different receptor (hFSHR/rLH-Ctail chimera) did not provoke any negative dominant effect on Wt receptor expression or function, strongly suggests that mutations at the BXXBB motifs of the receptor changed the spatial arrangement of the IL3 and the proximal region of the Ctail (and indirectly the configuration of the adjacent transmembrane domains), leading to receptor misfolding and formation of improperly assembled hetero-oligomers more prone to be trapped and eventually destroyed in the ER.
The observation that receptor fragments specifically interfere with the formation of mutant-Wt receptor complexes allowing rescue of Wt receptor function, may have potential implications on the development of therapeutic strategies than can selectively disrupt heterodimer pairs. Such strategy would be particularly useful in patients bearing simple heterozygous mutations in which the physical interactions between Wt and mutant receptors results in intracellular entrapment of the functional receptor and impairment of cell responsiveness to agonists (Leanos-Miranda et al., 2003; Beaumont et al., 2007; Calebiro et al., 2005).
It was interesting to find that the Wt receptor fragments did not perturb agonist-stimulated Wt hFSHR signaling, particularly considering their modest but apparent effect on Wt receptor plasma membrane expression (see Figs. 5B and 7B) as well as recent structural data showing that transmembrane domains 5–7 of GPCRs appear to be involved in receptor activation and interaction with cognate G proteins (Park et al., 2008; Scheerer et al., 2008). The virtual inability of the decoys tested to affect Wt receptor signaling could be due to their failure to strongly associate with properly complexed homo-oligomers of the Wt receptor (Thomas et al., 2007) or alternatively to their dissociation from the complexed receptors upon insertion into the cell surface plasma membrane. Additionally, the decoys may affect recycling of the Wt hFSHR but not synthesis and traffic to the membrane so that sufficient amounts of receptor may still reach the plasma membrane to become fully activated by agonist.
We conclude that expression-deficient mutants of the human FSHR that maintain the capability to associate with the Wt receptor, may impair cell surface membrane expression of the latter via formation of intracellular heterocomplexes that cannot escape the ER quality control mechanisms. In vivo, the dominant negative effects of mutant hFSHRs may manifest as subfertility or hyporesponsiveness to exogenous gonadotropin stimulation in simple heterozygous subjects; in this scenario, the “decoy” approach described in the present study might be useful to rescue Wt receptor function and eventually override the defect.
Supplementary Material
Figure S1. Maximal FSH-stimulated cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA (50 ng/well) plus empty vector (1:7 Wt:empty vector ratio) or the Wt hFSHR cDNA (50 ng/well) plus the hFSHR R556A or R618 mutant cDNAs (at 1:3 or 1:7 Wt:mutant hFSHR ratios) cloned in pSG5 or pcDNA3.1. Maximal FSH-stimulated cAMP accumulation mediated by the Wt receptor cDNA cloned either in the pSG5 or pcDNA3.1 expression vectors, was set at 100% for each experiment, and all other corresponding values are expressed relative to this. The results are the mean ± SEM from 3 independent experiments in triplicate incubations. Inset: Agonist-stimulated cAMP accumulation in cells transfected with the Wt hFSHR cDNA (400 ng/well) in pcDNA3.1 or pSG5 vectors. *p<0.01 vs all other conditions; §p<0.05 vs 1:7 with the same vector; ¶p<0.01 vs 1:7 with the same vector; †p<0.01 vs 1:7 Wt:mut with the same vector.
Figure S2. Thyroid-stimulating hormone (bTSH)-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hTSHR cDNA (50 ng/well) plus empty vector (Wt:vector ratio 1:7), the Wt hTSHR cDNA (50 ng/well) plus the hFSHR R556A mutant cDNA (Wt:mutant ratios 1:6 and 1:7) or the Wt hTSHR cDNA (50 ng/well) plus the R618A hFSHR mutant cDNA (ratios 1:4 and 1:7). Inset: GnRH agonist (Buserelin)-stimulated inositol phosphate (IP) production in HEK-293 co-transfected with constant amounts (25 ng/well) of the chimeric hGnRHR-cfCtail cDNA and the hFSHR R556A or R618A mutant cDNAs at the chimera:mutant cDNA ratios indicated. Wild-type, chimeric and mutant receptors cDNAs were in pcDNA3.1.
Figure S3. Total cAMP accumulation in HEK-293 cells co-transfected with constant amounts of the Wt β2-adrenergic receptor (β2AR) (A and B) or the dopamine D1 receptor (D1R) (C and D) cDNA (50 ng/well) plus empty vector (Wt:vector 1:7) or plus the hFSHR R556A (A and C) or R618A (B and D) mutant cDNAs at the indicated Wt:mutant receptor cDNA ratios, and stimulated with increasing doses of agonist (isoproterenol or bromocriptine). Agonist-stimulated cAMP accumulation was not altered when the Wt receptors were coexpressed with the hFSHR mutants. Wild-type and mutant receptors cDNAs were in pcDNA3.1.
Figure S4. Specific [125I]-FSH binding to HEK-293 cells co-transfected with the Wt hFSHR H534-V582 (A) or A590-N678 (B) fragment cDNAs, the Wt hFSHR cDNA and the mutant R556A hFSHR cDNA. The insets show the corresponding schematics of the transmembrane domains 5 to 7, the IL3 and the Ctail of the hFSHR, with the region of the receptor encoded by the cDNA fragment co-transfected in black circles. Wild-type and mutant receptors cDNAs were in pSG5, whereas the hFSHR fragments cDNAs were in pcDNA3.1. *p<0.05 vs Wt FSHR co-transfected with the R556A mutant and the empty vector. The results shown represent the mean ± SEM from 3 independent experiments.
Figure S5. Effect of co-transfecting the Wt hFSHR F612-N678 fragment cDNA on the dominant negative effects of the R618A hFSHR mutant. A: The graph shows maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R618A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R618A cDNA, plus increasing amounts of the Wt hFSHR F612-N678 fragment cDNA. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the hFSHR showing in black circles the region of the receptor encoded by the cDNA fragment transfected. B: Specific [125I]-FSH binding to HEK-293 cells co-transfected with the cDNAs indicated at the bottom of the graph. Wild-type and mutant receptors cDNAs were in pSG5, whereas the hFSHR fragments cDNAs were in pcDNA3.1. The results shown represent the mean ± SEM from 3 independent experiments. *p<0.05 vs all other conditions; ¶p<0.01 vs Wt hFSHR + hFSHR R618A + empty vector.
Figure S6. Co-immunoprecipitation of Wt hFSHR with expression-deficient mutants. To address whether mutant hFSHRs can dimerize/oligomerize with Wt hFSHR, HEK-293T cells were co-transfected with (A) Wt myc epitope-tagged hFSHR or (B) pShuttle to balance DNA. Also, either hFSHR R556A-FLAG, hFSHR R618A4-FLAG or pShuttle was cotransfected in either case. Cell lysates were generated 24 hours later and immunoprecipitations (IPs) were performed with anti-myc mAb or isotype control mAb IgG1. Detection was with anti-FLAG M2 HRP conjugate (1:1000). After substrate development, the blots were subjected to a 30 min exposure. Expression of the mutants was quite low in these experiments; visualization of the FLAG-tagged epitope was likely only possible because of the exquisite sensitivity of the M4 anti-FLAG antibody. Immunoblot analysis of immunoprecipitated samples with anti-FLAG mAb HRP conjugate revealed only high molecular weight species (≥175 kDa) which is likely a nondissociable immature form of FLAG-tagged hFSHR (62 kDa) associated with a myc-tagged hFSHR and probably a molecular chaperone as well (Thomas et al., 2007). Although no myc-tagged hFSHR was detected when the blots were reprobed with anti-myc mAb, this band specifically co-immunoprecipitated with myc-tagged Wt hFSHR. Immunoblot analysis of the whole cell lysates (pre-IP) with anti-FSHR extracellular domain mAb 106.105, showed that Wt hFSHR-myc protein levels were quite low, but higher than FSHR R556A–FLAG. The hFSHR R618A–FLAG mutant was virtually undetectable.
Acknowledgments
Supported by grants 2006/1A/I/008 from the FOFOI-IMSS, México, and 86881 from CONACyT, México (to A.U.-A), and grants HD-19899, RR-00163, HD-18185, TW/HD-00668 (to P.M.C.), and HD-18407 (to J.A.D.) from the National Institutes of Health, Bethesda, MD, USA. Alfredo Ulloa-Aguirre is recipient of a Research Career Development Award from the Fundación-IMSS, México.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between GABAA and GABAB receptors regulates GABAB receptor trafficking. J. Biol. Chem. 2004;279:18840–18850. doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- Beaumont KA, Shekar SN, Newton RA, James MR, Stow JL, Duffy DL, Sturm RA. Receptor function, dominant negative activity and phenotype correlations for MC1R variant alleles. Hum. Mol. Genet. 2007;16:2249–2260. doi: 10.1093/hmg/ddm177. [DOI] [PubMed] [Google Scholar]
- Benkirane M, Jin DY, Chun RF, Koup RA, Jeang KT. Mechanism of transdominant inhibition of CCR5-mediated HIV-1 infection by ccr5delta32. J. Biol. Chem. 1997;272:30603–30606. doi: 10.1074/jbc.272.49.30603. [DOI] [PubMed] [Google Scholar]
- Bogerd J. Ligand-selective determinants in gonadotropin receptors. Mol. Cell. Endocrinol. 2007;260–262:144–152. doi: 10.1016/j.mce.2006.01.019. [DOI] [PubMed] [Google Scholar]
- Breit A, Lagace M, Bouvier M. Hetero-oligomerization between beta2-and beta3-adrenergic receptors generates a beta-adrenergic signaling unit with distinct functional properties. J. Biol. Chem. 2004;279:28756–28765. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- Brothers SP, Cornea A, Janovick JA, Conn PM. Human loss-of-function gonadotropin-releasing hormone receptor mutants retain wild-type receptors in the endoplasmic reticulum: molecular basis of the dominant-negative effect. Mol. Endocrinol. 2004;18:1787–1797. doi: 10.1210/me.2004-0091. [DOI] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 2005;26:131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Calebiro D, de Filippis T, Lucchi S, Covino C, Panigone S, Beck-Peccoz P, Dunlap D, Persani L. Intracellular entrapment of wild-type TSH receptor by oligomerization with mutants linked to dominant TSH resistance. Hum. Mol. Genet. 2005;14:2991–3002. doi: 10.1093/hmg/ddi329. [DOI] [PubMed] [Google Scholar]
- Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5‧-monophosphates in porcine granulosa cells. Biol. Reprod. 1996;55:111–119. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- Cheung AH, Huang RR, Graziano MP, Strader CD. Specific activation of Gs by synthetic peptides corresponding to an intracellular loop of the beta-adrenergic receptor. FEBS Lett. 1991;279:277–280. doi: 10.1016/0014-5793(91)80167-2. [DOI] [PubMed] [Google Scholar]
- Dalrymple MB, Pfleger KD, Eidne KA. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol. Ther. 2008;118:359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Dias JA, Cohen BD, Lindau-Shepard B, Nechamen CA, Peterson AJ, Schmidt A. Molecular, structural, and cellular biology of follitropin and follitropin receptor. Vitam. Horm. 2002;64:249–322. doi: 10.1016/s0083-6729(02)64008-7. [DOI] [PubMed] [Google Scholar]
- Dong Ch, Filipeanu CM, Duvernay MT, Wu W. Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta. 1768:853–870. doi: 10.1016/j.bbamem.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvernay MT, Zhou F, Wu G. A conserved motif for the transport of G protein-coupled receptors from the endoplasmic reticulum to the cell surface. J. Biol. Chem. 2004;279:30741–30750. doi: 10.1074/jbc.M313881200. [DOI] [PubMed] [Google Scholar]
- Fan T, Varghese G, Nguyen T, Tse R, O'Dowd BF, George SR. A role for the distal carboxyl tails in generating the novel pharmacology and G protein activation profile of mu and delta opioid receptor hetero-oligomers. J. Biol. Chem. 2005;280:38478–38488. doi: 10.1074/jbc.M505644200. [DOI] [PubMed] [Google Scholar]
- Gehret AU, Bajaj A, Naider F, Dumont ME. Oligomerization of the yeast alpha-factor receptor: implications for dominant negative effects of mutant receptors. J. Biol. Chem. 2006;281:20698–20714. doi: 10.1074/jbc.M513642200. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol. Endocrinol. 2000;14:1283–1300. doi: 10.1210/mend.14.8.0500. [DOI] [PubMed] [Google Scholar]
- Grosse R, Schoneberg T, Schultz G, Gudermann T. Inhibition of gonadotropin-releasing hormone receptor signaling by expression of a splice variant of the human receptor. Mol. Endocrinol. 1997;11:1305–1318. doi: 10.1210/mend.11.9.9966. [DOI] [PubMed] [Google Scholar]
- Guan R, Feng X, Wu X, Zhang M, Zhang X, Hebert TE, Segaloff DL. Bioluminescence resonance energy transfer studies reveal constitutive dimerization of the human lutropin receptor and a lack of correlation between receptor activation and the propensity for dimerization. J. Biol. Chem. 2009;284:7483–7494. doi: 10.1074/jbc.M809150200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan R, Wu X, Feng X, Zhang M, Hébert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cell. Signal. 2010;22:247–256. doi: 10.1016/j.cellsig.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert TE, Moffett S, Morello JP, Loisel TP, Bichet DG, Barret C, Bouvier M. A peptide derived from a beta2-adrenergic receptor transmembrane domain inhibits both receptor dimerization and activation. J. Biol. Chem. 1996;271:16384–16392. doi: 10.1074/jbc.271.27.16384. [DOI] [PubMed] [Google Scholar]
- Huckle WR, Conn PM. Use of lithium ion in measurement of stimulated pituitary inositol phospholipid turnover. Methods Enzymol. 1987;141:149–155. doi: 10.1016/0076-6879(87)41063-x. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Karpa KD, Lin R, Kabbani N, Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol. Pharmacol. 2000;58:677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- Kelton CA, Cheng SV, Nugent NP, Schweickhardt RL, Rosenthal JL, Overton SA, Wands GD, Kuzeja JB, Luchette CA, Chappel SC. The cloning of the human follicle stimulating hormone receptor and its expression in COS-7, CHO, and Y-1 cells. Mol. Cell. Endocrinol. 1992;89:141–151. doi: 10.1016/0303-7207(92)90220-z. [DOI] [PubMed] [Google Scholar]
- Le Gouill C, Parent JL, Caron CA, Gaudreau R, Volkov L, Rola-Pleszczynski M, Stankova J. Selective modulation of wild type receptor functions by mutants of G-protein-coupled receptors. J. Biol. Chem. 1999;274:12548–12554. doi: 10.1074/jbc.274.18.12548. [DOI] [PubMed] [Google Scholar]
- Leaños-Miranda A, Ulloa-Aguirre A, Ji TH, Janovick JA, Conn PM. Dominant-negative action of disease-causing gonadotropin-releasing hormone receptor (GnRHR) mutants: a trait that potentially coevolved with decreased plasma membrane expression of GnRHR in humans. J. Clin. Endocrinol. Metab. 2003;88:3360–3367. doi: 10.1210/jc.2003-030084. [DOI] [PubMed] [Google Scholar]
- Leaños-Miranda A, Ulloa-Aguirre A, Janovick JA, Conn PM. In vitro coexpression and pharmacological rescue of mutant gonadotropin-releasing hormone receptors causing hypogonadotropic hypogonadism in humans expressing compound heterozygous alleles. J. Clin. Endocrinol. Metab. 2005;90:3001–3008. doi: 10.1210/jc.2004-2071. [DOI] [PubMed] [Google Scholar]
- Lee SP, O'Dowd BF, Ng GY, Varghese G, Akil H, Mansour A, Nguyen T, George SR. Inhibition of cell surface expression by mutant receptors demonstrates that D2 dopamine receptors exist as oligomers in the cell. Mol. Pharmacol. 2000;58:120–128. doi: 10.1124/mol.58.1.120. [DOI] [PubMed] [Google Scholar]
- Lee SP, O'Dowd BF, Rajaram RD, Nguyen T, George SR. D2 dopamine receptor homodimerization is mediated by multiple sites of interaction, including an intermolecular interaction involving transmembrane domain 4. Biochemistry. 2003;42:11023–11031. doi: 10.1021/bi0345539. [DOI] [PubMed] [Google Scholar]
- Li S, Liu X, Ascoli M. p38JAB1 binds to the intracellular precursor of the lutropin/choriogonadotropin receptor and promotes Its degradation. J. Biol. Chem. 2000;275:13386–13393. doi: 10.1074/jbc.275.18.13386. [DOI] [PubMed] [Google Scholar]
- Lindau-Shepard B, Roth KE, Dias JA. Identification of amino acids in the C-terminal region of human follicle-stimulating hormone (FSH) beta-subunit involved in binding to human FSH receptor. Endocrinology. 1994;135:1235–1240. doi: 10.1210/endo.135.3.8070368. [DOI] [PubMed] [Google Scholar]
- Lopez-Gimenez JF, Canals M, Pediani JD, Milligan G. The alpha1b–adrenoceptor exists as a higher-order oligomer: effective oligomerization is required for receptor maturation, surface delivery, and function. Mol. Pharmacol. 2007;71:1015–1029. doi: 10.1124/mol.106.033035. [DOI] [PubMed] [Google Scholar]
- Maizels ET, Cottom J, Jones JC, Hunzicker-Dunn M. Follicle stimulating hormone (FSH) activates the p38 mitogen-activated protein kinase pathway, inducing small heat shock protein phosphorylation and cell rounding in immature rat ovarian granulosa cells. Endocrinology. 1998;139:3353–3356. doi: 10.1210/endo.139.7.6188. [DOI] [PubMed] [Google Scholar]
- Maya-Nunez G, Janovick JA, Conn PM. Combined modification of intracellular and extracellular loci on human gonadotropin-releasing hormone receptor provides a mechanism for enhanced expression. Endocrine. 2000;13:401–407. doi: 10.1385/ENDO:13:3:401. [DOI] [PubMed] [Google Scholar]
- McElvaine AT, Mayo KE. A dominant-negative human growth hormone-releasing hormone (GHRH) receptor splice variant inhibits GHRH binding. Endocrinology. 2006;147:1884–1894. doi: 10.1210/en.2005-1488. [DOI] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerisation: molecular basis and relevance to function. Biochim. Biophys. Acta. 2007;1768:825–835. doi: 10.1016/j.bbamem.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Milligan G. A day in the life of a G protein-coupled receptor: the contribution to function of G protein-coupled receptor dimerization. Br. J. Pharmacol. 2008;153 Suppl 1:S216–S229. doi: 10.1038/sj.bjp.0707490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minegishi T, Nakamura K, Yamashita S, Omori Y. The effect of splice variant of the human luteinizing hormone (LH) receptor on the expression of gonadotropin receptor. Mol. Cell. Endocrinol. 2007;260–262:117–125. doi: 10.1016/j.mce.2005.11.051. [DOI] [PubMed] [Google Scholar]
- Murthy KS, Makhlouf GM. Identification of the G protein-activating domain of the natriuretic peptide clearance receptor (NPR-C) J. Biol. Chem. 1999;274:17587–17592. doi: 10.1074/jbc.274.25.17587. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Yamashita S, Omori Y, Minegishi T. A splice variant of the human luteinizing hormone (LH) receptor modulates the expression of wild-type human LH receptor. Mol. Endocrinol. 2004;18:1461–1470. doi: 10.1210/me.2003-0489. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Dias JA. Point mutations in follitropin receptor result in ER retention. Mol. Cell. Endocrinol. 2003;201:123–131. doi: 10.1016/s0303-7207(02)00424-0. [DOI] [PubMed] [Google Scholar]
- Nechamen CA, Thomas RM, Dias JA. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol. Cell. Endocrinol. 2007;260–262:93–99. doi: 10.1016/j.mce.2006.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng GY, O’Dowd BF, Lee SP, Chung HT, Brann MR, Seeman P, George SR. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem. Biophys. Res. Commun. 1996;227:200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and alpha 2-adrenergic receptors based upon characteristics in primary structure. J. Biol. Chem. 1992;267:8342–8346. [PubMed] [Google Scholar]
- Okuda-Ashitaka E, Sakamoto K, Ezashi T, Miwa K, Ito S, Hayaishi O. Suppression of prostaglandin E receptor signaling by the variant form of EP1 subtype. J. Biol. Chem. 1996;271:31255–31261. doi: 10.1074/jbc.271.49.31255. [DOI] [PubMed] [Google Scholar]
- Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature. 2008;454:183–187. doi: 10.1038/nature07063. [DOI] [PubMed] [Google Scholar]
- Pauwels PJ, Tardif S, Colpaert FC. Differential signalling of both wild-type and Thr(343)Arg dopamine D(2short) receptor by partial agonists in a G-protein-dependent manner. Biochem. Pharmacol. 2001;62:723–732. doi: 10.1016/s0006-2952(01)00717-1. [DOI] [PubMed] [Google Scholar]
- Pawson AJ, Maudsley S, Morgan K, Davidson L, Naor Z, Millar RP. Inhibition of human type i gonadotropin-releasing hormone receptor (GnRHR) function by expression of a human type II GnRHR gene fragment. Endocrinology. 2005;146:2639–2649. doi: 10.1210/en.2005-0133. [DOI] [PubMed] [Google Scholar]
- Pin JP, Neubig R, Bouvier M, Devi L, Filizola M, Javitch JA, Lohse MJ, Milligan G, Palczewski K, Parmentier M, Spedding M. International Union of Basic and Clinical Pharmacology. LXVII. Recommendations for the recognition and nomenclature of G protein-coupled receptor heteromultimers. Pharmacol. Rev. 2007;59:5–13. doi: 10.1124/pr.59.1.5. [DOI] [PubMed] [Google Scholar]
- Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, Lo YK, Sharma SC. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent. Prog. Horm. Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- Sairam MR, Jiang LG, Yarney TA, Khan H. Follitropin signal transduction: alternative splicing of the FSH receptor gene produces a dominant negative form of receptor which inhibits hormone action. Biochem. Biophys. Res. Commun. 1996;226:717–722. doi: 10.1006/bbrc.1996.1419. [DOI] [PubMed] [Google Scholar]
- Salahpour A, Angers S, Mercier JF, Lagace M, Marullo S, Bouvier M. Homodimerization of the beta2-adrenergic receptor as a prerequisite for cell surface targeting. J. Biol. Chem. 2004;279:33390–33397. doi: 10.1074/jbc.M403363200. [DOI] [PubMed] [Google Scholar]
- Scarselli M, Novi F, Schallmach E, Lin R, Baragli A, Colzi A, Griffon N, Corsini GU, Sokoloff P, Levenson R, Vogel Z, Maggio R. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001;276:30308–30314. doi: 10.1074/jbc.M102297200. [DOI] [PubMed] [Google Scholar]
- Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. Crystal structure of opsin in its G-protein-interacting conformation. Nature. 2008;455:497–502. doi: 10.1038/nature07330. [DOI] [PubMed] [Google Scholar]
- Seger R, Hanoch T, Rosenberg R, Dantes A, Merz WE, Strauss JF, 3rd, Amsterdam A. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J. Biol. Chem. 2001;276:13957–13964. doi: 10.1074/jbc.M006852200. [DOI] [PubMed] [Google Scholar]
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr. Rev. 1997;18:739–773. doi: 10.1210/edrv.18.6.0320. [DOI] [PubMed] [Google Scholar]
- Szidonya L, Cserzó M, Hunyady L. Dimerization and oligomerization of G-protein-coupled receptors: debated structures with established and emerging functions. J. Endocrinol. 2008;196:435–453. doi: 10.1677/JOE-07-0573. [DOI] [PubMed] [Google Scholar]
- Tao YX, Johnson NB, Segaloff DL. Constitutive and agonist-dependent self-association of the cell surface human lutropin receptor. J. Biol. Chem. 2004;279:5904–5914. doi: 10.1074/jbc.M311162200. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Nechamen CA, Mazurkiewicz JE, Muda M, Palmer S, Dias JA. Follice-stimulating hormone receptor forms oligomers and shows evidence of carboxyl-terminal proteolytic processing. Endocrinology. 2007;148:1987–1995. doi: 10.1210/en.2006-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timossi C, Maldonado D, Vizcaino A, Lindau-Shepard B, Conn PM, Ulloa-Aguirre A. Structural determinants in the second intracellular loop of the human follicle-stimulating hormone receptor are involved in G(s) protein activation. Mol. Cell. Endocrinol. 2002;189:157–168. doi: 10.1016/s0303-7207(01)00720-1. [DOI] [PubMed] [Google Scholar]
- Timossi C, Ortiz-Elizondo C, Pineda DB, Dias JA, Conn PM, Ulloa-Aguirre A. Functional significance of the BBXXB motif reversed present in the cytoplasmic domains of the human follicle-stimulating hormone receptor. Mol. Cell. Endocrinol. 2004;223:17–26. doi: 10.1016/j.mce.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Uberti MA, Hague C, Oller H, Minneman KP, Hall RA. Heterodimerization with beta2-adrenergic receptors promotes surface expression and functional activity of alpha1D-;adrenergic receptors. J. Pharmacol. Exp. Ther. 2005;313:16–23. doi: 10.1124/jpet.104.079541. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Conn PM. G protein-coupled receptors and the G protein family. In: Conn PM, editor. Handbook of Physiology-Endocrinology: Section 7, Cellular Endocrinology. New York: Oxford University Press; 1998. pp. 87–141. [Google Scholar]
- Ulloa-Aguirre A, Timossi C. Structure-function relationship of follicle-stimulating hormone and its receptor. Hum. Reprod. Update. 1998;4:260–283. doi: 10.1093/humupd/4.3.260. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Janovick JA, Brothers SP, Conn PM. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 2004;5:821–837. doi: 10.1111/j.1600-0854.2004.00232.x. [DOI] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Uribe A, Zarinan T, Bustos-Jaimes I, Perez-Solis MA, Dias JA. Role of the intracellular domains of the human FSH receptor in G(alphaS) protein coupling and receptor expression. Mol. Cell. Endocrinol. 2007a;260–262:153–162. doi: 10.1016/j.mce.2005.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulloa-Aguirre A, Zarinan T, Pasapera AM, Casas-Gonzalez P, Dias JA. Multiple facets of follicle-stimulating hormone receptor function. Endocrine. 2007b;32:251–263. doi: 10.1007/s12020-008-9041-6. [DOI] [PubMed] [Google Scholar]
- Uribe A, Zarinan T, Perez-Solis MA, Gutierrez-Sagal R, Jardon-Valadez E, Pineiro A, Dias JA, Ulloa-Aguirre A. Functional and structural roles of conserved cysteine residues in the carboxyl-terminal domain of the follicle-stimulating hormone receptor in human embryonic kidney 293 cells. Biol. Reprod. 2008;78:869–882. doi: 10.1095/biolreprod.107.063925. [DOI] [PubMed] [Google Scholar]
- Vassart G, Pardo L, Costagliola S. A molecular dissection of the glycoprotein hormone receptors. Trends Biochem. Sci. 2004;29:119–126. doi: 10.1016/j.tibs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Weiner RS, Dias JA. Biochemical analyses of proteolytic nicking of the human glycoprotein hormone alpha-subunit and its effect on conformational epitopes. Endocrinology. 1992;131:1026–1036. doi: 10.1210/endo.131.3.1380433. [DOI] [PubMed] [Google Scholar]
- White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- Wilson S, Wilkinson G, Milligan G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J. Biol. Chem. 2005;280:28663–28674. doi: 10.1074/jbc.M413475200. [DOI] [PubMed] [Google Scholar]
- Wu D, Jiang H, Simon MI. Different alpha 1-adrenergic receptor sequences required for activating different G alpha subunits of Gq class of G proteins. J. Biol. Chem. 1995;270:9828–9832. doi: 10.1074/jbc.270.17.9828. [DOI] [PubMed] [Google Scholar]
- Zambrano E, Barrios-de-Tomasi J, Cardenas M, Ulloa-Aguirre A. Studies on the relative in-vitro biological potency of the naturally-occurring isoforms of intrapituitary follicle stimulating hormone. Mol. Hum. Reprod. 1996;2:563–571. doi: 10.1093/molehr/2.8.563. [DOI] [PubMed] [Google Scholar]
- Zhang M, Feng X, Guan R, Hébert TE, Segaloff DL. Structural determinants underlying constitutive dimerization of unoccupied human follitropin receptors. Cell. Signal. 2009;21:1663–1671. doi: 10.1016/j.cellsig.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, You Y, Zabner J, Ryan AJ, Mallampalli RK. The CCT promoter directs high-level transgene expression in distal lung epithelial cell lines. Am. J. Respir. Cell. Mol. Biol. 2004;30:61–68. doi: 10.1165/rcmb.2003-0020OC. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wess J. Truncated V2 vasopressin receptors as negative regulators of wild-type V2 receptor function. Biochemistry. 1998;37:15773–15784. doi: 10.1021/bi981162z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Maximal FSH-stimulated cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA (50 ng/well) plus empty vector (1:7 Wt:empty vector ratio) or the Wt hFSHR cDNA (50 ng/well) plus the hFSHR R556A or R618 mutant cDNAs (at 1:3 or 1:7 Wt:mutant hFSHR ratios) cloned in pSG5 or pcDNA3.1. Maximal FSH-stimulated cAMP accumulation mediated by the Wt receptor cDNA cloned either in the pSG5 or pcDNA3.1 expression vectors, was set at 100% for each experiment, and all other corresponding values are expressed relative to this. The results are the mean ± SEM from 3 independent experiments in triplicate incubations. Inset: Agonist-stimulated cAMP accumulation in cells transfected with the Wt hFSHR cDNA (400 ng/well) in pcDNA3.1 or pSG5 vectors. *p<0.01 vs all other conditions; §p<0.05 vs 1:7 with the same vector; ¶p<0.01 vs 1:7 with the same vector; †p<0.01 vs 1:7 Wt:mut with the same vector.
Figure S2. Thyroid-stimulating hormone (bTSH)-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hTSHR cDNA (50 ng/well) plus empty vector (Wt:vector ratio 1:7), the Wt hTSHR cDNA (50 ng/well) plus the hFSHR R556A mutant cDNA (Wt:mutant ratios 1:6 and 1:7) or the Wt hTSHR cDNA (50 ng/well) plus the R618A hFSHR mutant cDNA (ratios 1:4 and 1:7). Inset: GnRH agonist (Buserelin)-stimulated inositol phosphate (IP) production in HEK-293 co-transfected with constant amounts (25 ng/well) of the chimeric hGnRHR-cfCtail cDNA and the hFSHR R556A or R618A mutant cDNAs at the chimera:mutant cDNA ratios indicated. Wild-type, chimeric and mutant receptors cDNAs were in pcDNA3.1.
Figure S3. Total cAMP accumulation in HEK-293 cells co-transfected with constant amounts of the Wt β2-adrenergic receptor (β2AR) (A and B) or the dopamine D1 receptor (D1R) (C and D) cDNA (50 ng/well) plus empty vector (Wt:vector 1:7) or plus the hFSHR R556A (A and C) or R618A (B and D) mutant cDNAs at the indicated Wt:mutant receptor cDNA ratios, and stimulated with increasing doses of agonist (isoproterenol or bromocriptine). Agonist-stimulated cAMP accumulation was not altered when the Wt receptors were coexpressed with the hFSHR mutants. Wild-type and mutant receptors cDNAs were in pcDNA3.1.
Figure S4. Specific [125I]-FSH binding to HEK-293 cells co-transfected with the Wt hFSHR H534-V582 (A) or A590-N678 (B) fragment cDNAs, the Wt hFSHR cDNA and the mutant R556A hFSHR cDNA. The insets show the corresponding schematics of the transmembrane domains 5 to 7, the IL3 and the Ctail of the hFSHR, with the region of the receptor encoded by the cDNA fragment co-transfected in black circles. Wild-type and mutant receptors cDNAs were in pSG5, whereas the hFSHR fragments cDNAs were in pcDNA3.1. *p<0.05 vs Wt FSHR co-transfected with the R556A mutant and the empty vector. The results shown represent the mean ± SEM from 3 independent experiments.
Figure S5. Effect of co-transfecting the Wt hFSHR F612-N678 fragment cDNA on the dominant negative effects of the R618A hFSHR mutant. A: The graph shows maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the Wt hFSHR cDNA plus empty vector, the Wt hFSHR cDNA plus the R618A hFSHR mutant cDNA (ratio 1:7), or the Wt hFSHR cDNA plus the hFSHR R618A cDNA, plus increasing amounts of the Wt hFSHR F612-N678 fragment cDNA. Inset: Schematic of the transmembrane domains 5 to 7, the IL3, and the Ctail of the hFSHR showing in black circles the region of the receptor encoded by the cDNA fragment transfected. B: Specific [125I]-FSH binding to HEK-293 cells co-transfected with the cDNAs indicated at the bottom of the graph. Wild-type and mutant receptors cDNAs were in pSG5, whereas the hFSHR fragments cDNAs were in pcDNA3.1. The results shown represent the mean ± SEM from 3 independent experiments. *p<0.05 vs all other conditions; ¶p<0.01 vs Wt hFSHR + hFSHR R618A + empty vector.
Figure S6. Co-immunoprecipitation of Wt hFSHR with expression-deficient mutants. To address whether mutant hFSHRs can dimerize/oligomerize with Wt hFSHR, HEK-293T cells were co-transfected with (A) Wt myc epitope-tagged hFSHR or (B) pShuttle to balance DNA. Also, either hFSHR R556A-FLAG, hFSHR R618A4-FLAG or pShuttle was cotransfected in either case. Cell lysates were generated 24 hours later and immunoprecipitations (IPs) were performed with anti-myc mAb or isotype control mAb IgG1. Detection was with anti-FLAG M2 HRP conjugate (1:1000). After substrate development, the blots were subjected to a 30 min exposure. Expression of the mutants was quite low in these experiments; visualization of the FLAG-tagged epitope was likely only possible because of the exquisite sensitivity of the M4 anti-FLAG antibody. Immunoblot analysis of immunoprecipitated samples with anti-FLAG mAb HRP conjugate revealed only high molecular weight species (≥175 kDa) which is likely a nondissociable immature form of FLAG-tagged hFSHR (62 kDa) associated with a myc-tagged hFSHR and probably a molecular chaperone as well (Thomas et al., 2007). Although no myc-tagged hFSHR was detected when the blots were reprobed with anti-myc mAb, this band specifically co-immunoprecipitated with myc-tagged Wt hFSHR. Immunoblot analysis of the whole cell lysates (pre-IP) with anti-FSHR extracellular domain mAb 106.105, showed that Wt hFSHR-myc protein levels were quite low, but higher than FSHR R556A–FLAG. The hFSHR R618A–FLAG mutant was virtually undetectable.