Abstract
Steroid hormones regulate various physiological processes including development, reproduction, and metabolism. These regulatory molecules are synthesized from cholesterol in endocrine organs -such as the adrenal glands and gonads- via a multi-step enzymatic process that is catalyzed by the cytochrome P450 superfamily of monooxygenases and hydroxysteroid dehydrogenases. Steroidogenesis is induced by trophic peptide hormones primarily via the activation of a cAMP/protein kinase A (PKA)-dependent pathway. However, other signaling molecules, including cytokines and growth factors, control the steroid hormone biosynthetic pathway. More recently, sphingolipids, including ceramide, sphingosine-1-phosphate, and sphingosine, have been found to modulate steroid hormone secretion at multiple levels. In this review, we provide a brief overview of the mechanisms by which sphingolipids regulate steroidogenesis. In addition, we discuss how steroid hormones control sphingolipid metabolism. Finally, we outline evidence supporting the emerging role of bioactive sphingolipids in various nuclear processes and discuss a role for nuclear sphingolipid metabolism in the control of gene transcription.
Keywords: Steroidogenesis, sphingolipids, nuclear lipids, ceramide, S1P, sphingosine
1. Introduction
Cortisol, testosterone, progesterone, aldosterone, and estradiol are steroid hormones that regulate multiple physiological processes such as development, metabolism, secondary sex differentiation, and inflammation [1-4]. Members of the cytochrome P450 family of monooxygenases (CYPs) and hydroxysteroid dehydrogenases (HSDs) synthesize these steroid hormones from cholesterol in the adrenal gland, gonads, placenta, intestines, and in the central and peripheral nervous systems [2, 5-10] (Figure 1). Cholesterol metabolism is primarily regulated by trophic peptide hormones, including adrenocorticotropin (ACTH) in the adrenal gland and follicular stimulating hormone (FSH) and leutenizing hormone (LH) in the gonads. These peptide hormones are released from the anterior pituitary gland and activate a cAMP/PKA-dependent pathway in target tissues via binding to their cognate G-protein coupled receptors (GPCRs). Increased intracellular cAMP leads to a rapid increase in free cholesterol production and its subsequent import into mitochondria as well as the coordinate transcriptional activation of all the genes involved in steroid hormone biosynthesis.
Figure 1.
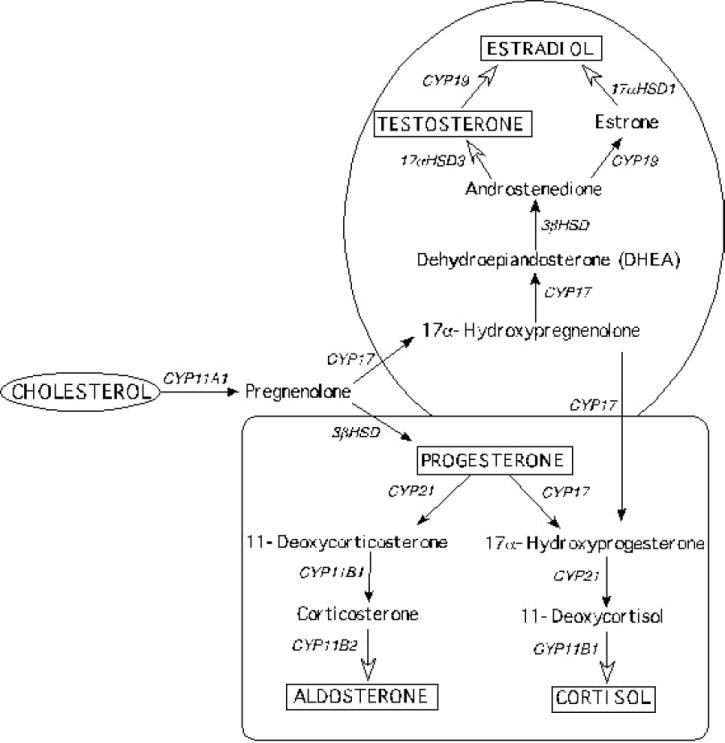
Steroid hormone biosynthetic pathways. Diagram representing the sequential metabolism of cholesterol into the major steroid hormones in the adrenal cortex (square) and gonads (circle). The steroidogenic gene responsible for each enzymatic reaction is indicated above the arrow. CYP11A1 (encodes P450scc); CYP17 (encodes P450c17α); 3β-HSD (encodes 3β hydroxysteroid dehydrogenase); CYP21 (encodes P450c21); CYP11B1 (encodes P45011β); CYP11B2 (encodes aldosterone synthase); CYP19 (encodes aromatase); 17α-HSD (encodes 17α hydroxysteroid dehydrogenase).
Although the cAMP/PKA pathway is the primary, and most extensively characterized, regulator of steroidogenesis, various other signaling molecules crosstalk with this pathway to modulate hormone production [11]. Growth factors including insulin-like growth factor (IGF)-I and IGF-II, transforming growth factor β 1 (TGFβ1), platelet-derived growth factor (PDGF), and fibroblast growth factor 9 (FGF9) have all been shown to modulate steroid hormone production [12-16]. For example, both IGF-1 and IGF-II regulate steroidogenesis by binding to IGF-I receptors and modulating steroidogenic gene expression [13, 15]. In bovine granulosa cells, TGFβ1 stimulates estradiol production by increasing CYP19 mRNA levels and aromatase activity while inhibiting progesterone production by suppressing StAR, CYP11A1, and 3β-HSD1 transcription [16]. Further, TGFβ1 decreases P450c17α activity in H295R cells by repressing CYP17 gene expression via the activation of activin receptor-like kinase 5 (ALK5) [17]. Finally, mutations in PDGF target genes result in altered hormone production in the testis and ovaries [14] while aberrant FGF9 expression stimulates testosterone production in mouse Leydig cells [12].
Sphingolipids also have multiple established regulatory roles in steroid hormone biosynthesis [18-22]. These signaling lipids belong to a large family of glycolipids and phospholipids that are characterized by a common sphingoid base backbone. A growing number of sphingolipid species, including ceramide (cer), sphingosine-1-phosphate (S1P), sphingosine (SPH), and sphingomyelin (SM), have been reported to modulate various steps of the steroidogenic pathway. They stimulate steroid hormone secretion by regulating steroidogenic gene transcription [23-26], acting as intracellular second messengers [27-32] and/or extracellular paracrine/autocrine regulators [25, 33-35], and by serving as ligands for nuclear receptors [26]. In this review, a summary of studies focused on the various roles of sphingolipids in the regulation of steroidogenesis will be presented. In addition, we highlight emerging data on the novel signaling and regulatory roles for sphingolipids in the nuclei of cells.
2. Steroid hormone biosynthesis
As mentioned previously, the biosynthesis of steroid hormones is essential for physiological homeostasis. The vital role that these molecules play in human physiology dictates the need for a complex network of regulatory mechanisms that act concertedly to maintain optimal circulating plasma hormone concentrations. CYPs, HSDs, and other accessory proteins are selectively expressed in different steroidogenic tissues to assure the production of steroid hormones in a tissue-specific manner. For example, zone-specific expression of CYP17 [9] and cytochrome b5 [36-40] allow for glucocorticoid production in the zona fasciculata and androgen synthesis in the zona reticularis, whereas, the absence of CYP17 in the zona glomerulosa allows for mineralocorticoid secretion. In addition, temporal expression of these enzymes controls proper steroid hormone production during development.
Activation of the steroid hormone biosynthetic pathway is initiated when peptide trophic hormones binds to their cognate GPCRs. As mentioned earlier, upon receptor activation, two temporally distinct phases of steroidogenesis occur: a rapid phase and a slower chronic response. In the acute phase, stored cholesterol esters are cleaved and transported to the inner mitochondrial membrane, the site of the first enzymatic step of cholesterol metabolism. De-esterification of newly imported and stored cholesterol esters facilitates its utilization in steroid hormone production and is catalyzed by hormone sensitive lipase (HSL). The movement of free cholesterol into mitochondria is the rate-limiting step in steroid hormone production and involves several proteins. One key protein is steroidogenic acute regulatory protein (StAR), the founding member of the START (StAR-related lipid transport) family of transport proteins. StAR is rapidly transcribed, translated, and localized to mitochondria upon hormonal stimulation [41-43]. In addition to StAR, a 10-kDa translocator protein (TSPO, formerly known as the peripheral-type benzodiazepine receptor- PBR) [44], PKA regulatory subunit-Iα (PKA-RIα)- associated protein, and voltage-dependent anion channel (VDAC) [41, 45, 46] facilitate the traffic of cholesterol to the inner mitochondrial membrane.
In the chronic phase of steroidogenesis, all the genes responsible for cholesterol metabolism (Figure 1) are transcriptionally activated. The activation of signaling culminates in the interaction of various transcription factors with the promoters of steroidogenic genes [11, 47-55]. In response to cAMP signaling, the nuclear receptor steroidogenic factor-1 (SF-1/Ad4BP/NR5A1) is targeted to most steroidogenic genes [54, 56]. SF-1 is also essential for gonadal and adrenal development [57, 58] as evidenced by the phenotype of targeted disruption of the receptor in mice [59, 60]. The central role of SF-1 in steroidogenesis is also evident in humans where mutations in the receptor result in various clinical pathologies including gonadal dysgenesis, adrenal failure, sex reversal, and underandrogenization [61]. Although SF-1 plays a critical role in conferring coordinate transcription of steroidogenic genes, other transcription factors including GATA4 and GATA6 [62-64], cAMP response element binding protein (CREB) [65, 66], specificity protein (Sp) family members [62, 67], and nerve growth factor 1B (NGF-1B) [68, 69] also participate in steroidogenic transcriptional regulation.
3. The sphingolipid metabolic pathway
Sphingolipids are a large family of glycolipids and phospholipids that share a common sphingoid base backbone. These once called ‘structural’ lipids are now well-established signaling molecules that play multiple roles in a vast number of cellular processes. Some of which include cell growth, differentiation, and migration, apoptosis [22, 26, 70-82], and autophagy [22, 83, 84]. Aberrant sphingolipid metabolism is linked to varied disease states including insulin resistance [85-87], cancer [72, 88, 89], and neurodegeneration [90-92]. The structural diversity of this family of lipids is vast, thus sphingolipid metabolism is a highly regulated process where a series of enzymes work concomitantly to maintain sphingolipid homeostasis.
Serine palmitoyltransferase (SPT) catalyzes the first step in sphingolipid de novo biosynthesis. This step involves the condensation of l-serine and palmitoyl-CoA to form the intermediate 3-ketodihydrosphingosine, which is further metabolized into dihydrosphingosine (sphinganine) and dihydroceramide (Figure 2). Desaturation of dihydroceramide forms cer (N-acylsphingosine), which constitutes the basic structure of higher order sphingolipids including SM, cerebrosides, and gangliosides (GM). The complex variety of different sphingolipid metabolites is formed by the combination of different head groups such as phosphocholine and carbohydrates O-linked to cer. In addition, the breakdown of cer forms SPH, which can be phosphorylated to generate S1P. Cer can also be phosphorylated to form ceramide-1-phosphate (C1P) (Figure 2). Recently, it was shown that SPT is able to utilize l-alanine [93] and shorter acyl-CoA molecules [94, 95] as alternative substrates, thus generating atypical metabolites and expanding the list of possible physiologically important sphingolipid species.
Figure 2.
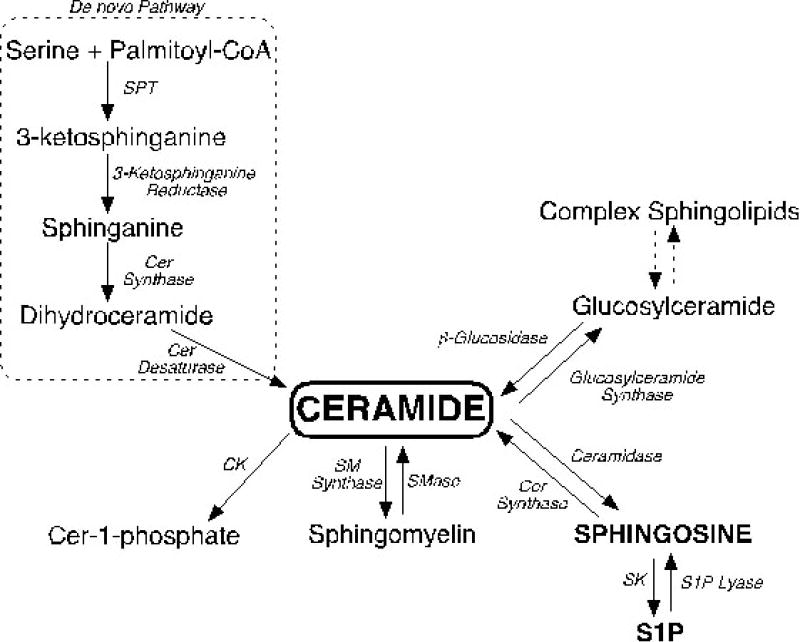
Overview of the sphingolipid metabolic pathway. Ceramide (cer) is central to sphingolipid metabolism and can be generated via de novo biosynthesis, through the degradation of complex sphingolipids, or by the recycling of sphingosine. Degradation of ceramide leads to the formation of sphingosine and sphingosine-1-phosphate (S1P). Abbreviations: serine palmitoyltransferase (SPT), ceramide (cer), ceramide kinase (CK), sphingomyelin (SM), sphingomyelinase (SMase), sphingomyelin synthase (SM synthase), sphingosine kinase (SK), sphingosine-1-phosphate (S1P).
There are a multitude of physiological and cellular roles for individual sphingolipid species, including GMs, SM, cer, C1P, SPH, and S1P [19, 26, 71, 73, 76, 77, 79, 87, 96-108]. GMs are important constituents of cell membranes and play important roles in cell growth, differentiation, and adhesion [109, 110]. SM is the most abundant sphingolipid in mammalian cells and, in addition to being an important membrane component, is the primary intracellular source of cer. Various stimuli including TNF-α, interleukin 1β (IL-1β), vitamin D3 (1,25-(OH)2D3), and cytotoxic agents can activate SM hydrolysis [31, 103, 108, 111, 112]. Cer participates as a second messenger in numerous cellular events including apoptosis, senescence, and cell cycle arrest [113-115] while its phosphorylated form, C1P, promotes cell differentiation and survival [97, 116, 117]. Similar to cer and C1P, SPH and S1P have opposing roles in cellular processes: the former acts as a pro-apoptotic agent [96, 118, 119] while the later mediates cell migration, proliferation, and survival [73, 75, 81, 120].
Because different sphingolipids have specific effects on cell function, the intracellular concentrations of each sphingolipid molecular species are tightly controlled by sphingolipid metabolizing enzymes. These include the aforementioned SPT, acid/neutral sphingomyelinase (SMase), SM synthase, acid/neutral/alkaline ceramidase (ASAH), and sphingosine kinase (SK), S1P lyase, and ceramide kinase (CK) (Figure 2). Most of these enzymes are localized to specific sub-cellular locations, where they act maintain sphingolipid homeostasis in distinct microenvironments [121]. Because sphingolipids are mainly hydrophobic and specific mechanisms for sphingolipid transport have not been extensively characterized, the subcellular location where these molecules are generated most likely dictates their site of action.
4. Sphingolipid signaling in steroidogenesis
A growing body of literature has established the integral role that distinct sphingolipid species play in steroid hormone production. As it will be discussed below, cer, SPH, and S1P have all been implicated as secondary modulators of steroidogenesis. These lipids can act at different levels of the steroidogenic signaling pathway including (I) participating in various regulatory signaling cascades as second messengers, (II) acting as paracrine/autocrine regulators, and (III) serving as ligands for nuclear receptors. As previously stated, steroid hormone production is mainly regulated by trophic peptide hormones, which activate multiple signaling cascades at target cells. In addition to the cAMP/PKA pathway, numerous other signaling systems including calcium [122], steroidogenic-inducing protein [123], interleukins (IL-3, IL-6, IL-1β), and TNF-α [23, 124, 125] regulate steroidogenesis. One of the mechanisms by which these extracellular regulators control steroidogenesis is by modulating sphingolipid metabolism. In the adrenal cortex, for example, ACTH rapidly activates sphingolipid metabolism leading to decreased levels of SM, cer, and SPH, with a concomitant increase in S1P secretion [19]. In addition, IL-1β and TNF-α signaling have been shown to regulate cellular functions, including steroid hormone production, through sphingolipid metabolism [126-128].
(I) Sphingolipids as second messengers in steroidogenic regulatory pathways
Cer modulates steroid hormone production primarily by serving as a second messenger in cytokine and growth factor signaling cascades [23, 30, 31, 126, 129-131]. These extracellular mediators, including TNF-α, interferon –γ (INF-γ), and IL-1β, activate SM hydrolysis and promote cer intracellular accumulation [126, 127, 132]. Ultimately, increased cer levels alter cellular steroidogenic output. Given that cer comprises the structural backbone for all sphingolipids, cer can modulate steroid hormone production both directly and indirectly through metabolism into other bioactive sphingolipids (Figure 2). Therefore, it is important to point out that even though cer has been implicated in the regulation of steroid hormone biosynthesis, the precise molecular mechanisms involved in its actions are mostly unknown. In addition, data must be carefully interpreted to conclude that the responses observed are indeed a result of cer accumulation and not a bioactive metabolite.
Cer has been shown to regulate progesterone and testosterone production (Figure 3). In ovarian granulosa cells, activation of SMase by IL-1β suppresses progesterone production in a cer-dependent manner [31]. Further, Budnik et al. reported that SM hydrolysis also resulted in decreased progesterone secretion in MA-10 cells by suppressing StAR protein expression [23]. Importantly, the authors discussed that although SM hydrolysis also led to S1P accumulation, the inhibitory action of this cytokine was not reversed by SK inhibition. Similarly, cer-dependent StAR protein suppression was also reported in rat Leydig cells where it resulted in decreased testosterone synthesis [24]. Significantly, other chemically similar sphingolipids, such as SPH or S1P, did not mirror the effect of cer. Notably, cer accumulation observed in these reports was triggered by TNF-α signaling, which, similar to IL-1β, activates SMase activity [133]. Santana et al. reported that TNF-α signaling represses P450 aromatase activity in granulosa cells through a mechanism involving cer production [131]. Cells treated with bacterial SMase or cell-permeable cer displayed equivalent inhibition of aromatase activity, suggesting that TNF-α mediated activation of SM hydrolysis and subsequent cer production are involved in the regulation of this P450 enzyme. In rat Leydig and luteal cells, cer suppresses human chorionic gonadotropin (hCG)-stimulated testosterone and progesterone production, respectively, in a dose-dependent manner [28, 30]. Finally, cer was shown to modulate the mRNA expression of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), the glucocorticoid reactivation enzyme, in preadipocytes [129]. Cell-permeable C2-cer (N-acetoyl-D-erythro-sphingosine) induced the expression and recruitment of CCAAT/enhancer binding protein β (C/EBPβ) to the 11β-HSD1 gene. In addition, cer treatment upregulated 11β-HSD1 activity in these cells [129], thus suggesting a role for cer in regulating active circulating steroid hormones.
Figure 3.
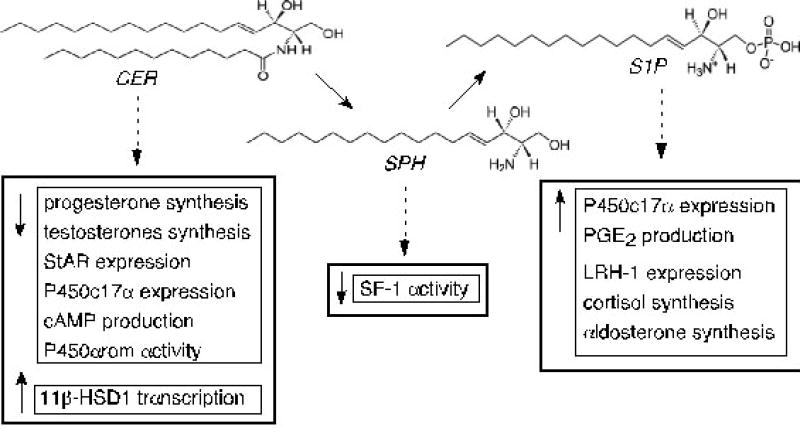
The structural similarities of ceramide (CER), sphingosine (SPH), and sphingosine-1-phosphate (S1P) and their established roles in steroid hormone production. Although these lipids are structurally similar, they have unique roles in cellular function. CER functions as an intracellular second messenger to suppress progesterone and testosterone biosynthesis while S1P acts primarily via binding to cell-membrane S1P receptors to induce cortisol and aldosterone production. SPH binds to and antagonizes the function of steroidogenic factor 1 (SF-1). Abbreviations: Steroidogenic acute regulatory protein (StAR); cytochrome P450 17α-hydroxylase (P450c17α); cytochrome P450 aromatase (P450arom); 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1); prostaglandin E2 (PGE2); liver receptor homologue-1 (LRH-1).
Although most studies demonstrate a role for cer as an inhibitor of steroidogenesis, other reports present contradicting findings. Soboloff et al. reported that different acyl-chain length cer have opposite effects on LH-induced progesterone production in hen granulosa cells [32]. C6-cer (N-hexanoyl-D-erythro-sphingosine) and C8-cer (N-octanoyl-D-eythro-sphingosine) increased intracellular [Ca2+] and progesterone secretion whereas C2-cer had no effect on intracellular Ca2+ and suppressed steroid hormone production. Further, Kwun et al. demonstrated that C2-cer increases basal and hCG-stimulated progesterone production in MA-10 Leydig cells through a mechanism not linked to the induction of apoptosis [27]. The reason for these discrepancies is unknown, but the use of cer with different acyl-chain lengths may underlie the inconsistencies observed. Of note, Soboloff et al. [32] and others [134-139] have found that different molecular species of cer exert different cellular effects. Also, because sphingolipid metabolism is a highly dynamic process, the cellular responses reported may stem from the conversion of cer into another bioactive metabolite. Nonetheless, the majority of reports collectively suggest that the role of cer in steroidogenesis is likely independent from its role in cell growth and/or apoptosis because cell death was not reported as a reason for the decrease in the net steroid hormone output [27, 140].
(II) Sphingolipids as paracrine/autocrine regulators
S1P is well-established to induce cellular responses in a paracrine and/or autocrine manner through its binding to a family of GPCRs (S1PRs). In fact, the most important functions of S1P are mediated through the activation of these cell surface receptors [141, 142]. There are 5 S1PRs with each receptor coupling to multiple heterotrimeric G proteins [143-145]. Significant work has been done to characterize the downstream signaling cascades and cellular responses associated with the activation of the different S1PRs. S1PR1 couples to Gi and activates the phospholipase C (PLC), phosphatidylinositol 3-kinase (PI3K), and extracellular-signal regulated kinase (ERK) pathways. S1PR2 and S1PR3 couple to Gi, G12/13, and Gq and active multiple downstream cascades including PLC, PI3K, ERK, and Rho GTPase. S1PR4 activates PLC and ERK whereas S1PR5 inhibits adenylyl cyclase and ERK (reviewed in [146]).
In steroidogenesis, the regulatory functions of S1P are primarily mediated through S1PRs. We have demonstrated that the S1P secreted from cAMP-stimulated human H295R adrenocortical cells induces CYP17 transcription via a paracrine mechanism that requires S1PR activation and nuclear translocation of sterol regulatory element binding protein 1 (SREBP1) [25]. In the zona fasciculata of bovine adrenal cells, S1P stimulates cortisol biosynthesis by activating protein kinase C (PKC) and phospholipase D (PLD) through a pertussis toxin-sensitive receptor-mediated mechanism [35]. S1P-induced cortisol production, unlike ACTH stimulation, did not stimulate cAMP formation but rather was mediated through intracellular [Ca2+]. Similar S1PR-dependent PLD activation by S1P was also reported in adrenal glomerulosa cells [34]. Brizuela et al. characterized S1P as an inducer of aldosterone production through an S1PR1/3-mediated mechanism involving the activation of PI3K/Akt and ERK pathways [33]. In addition, S1P activates prostaglandin-E2 (PGE2) production [147], which induces CYP19 gene expression [148, 149]. PGE2 also regulates 11β–HSD1 activity in luteinizing granulosa cells [150] (Figure 3). Interestingly, we have demonstrated that S1P induces the expression of liver receptor homologue-1 (LRH-1, NR5A2) in MCF-7 breast cancer cell [151], which regulates CYP19 transcription and estrogen production [152]. Given that aberrant estrogen production is a feature of breast cancer development and S1P induces tumorgenesis, it is likely that increased S1P concentrations in breast cells controls local estrogen production. Recently, an S1P-specific humanized monoclonal antibody called LT1009 (sonepcizumab) was developed and found to inhibit S1P-induced cell growth, migration, and angiogenesis [153-155]. This antibody is currently under Phase I trials for cancer treatment. Therefore, given the role of S1P in LRH-1 transcription and tumorgenesis, it is tempting to speculate that some of its chemopreventive effects are mediated by lowering estrogen production.
(III) Sphingolipids as ligands for nuclear receptors
Our laboratory has contributed to expanding the role of sphingolipids in steroidogenesis by demonstrating that SPH is an antagonist for SF-1 [26] (Figure 3). SPH is bound to the receptor under basal conditions and exchanged for PA, an SF-1-agonist [156], upon cAMP stimulation. SPH antagonizes cAMP-stimulated CYP17 reporter gene activity and antagonizes coactivator recruitment. Interestingly, silencing ASAH1 (acid ceramidase) expression mimicked cAMP-induced CYP17 transcription, further supporting the role of SPH as a repressor of SF-1 activity [26]. We have found that lysoSM (sphingosylphosphorylcholine) is also able to bind SF-1 in H295R cells under basal conditions and that cAMP treatment promotes its dissociation from the receptor [26]. The implications of this binding are unknown but it suggests that multiple sphingolipids can potentially regulate receptor activity. Although the mechanisms controlling ligand availability are yet to be uncovered, the recently described nuclear localization of sphingolipid metabolizing enzymes (discussed bellow) suggests that nuclear sphingolipid concentrations are locally controlled. In addition, we have found that cAMP stimulation leads to an increase in nuclear ceramidase activity (Lucki and Sewer, unpublished observations), thus demonstrating a potential link between ACTH/cAMP signaling and nuclear SPH production.
(IV) Regulation of sphingolipid enzymes by trophic hormones
As discussed above, the sphingolipid metabolic pathway is comprised of a series of enzymes that dynamically regulate sphingolipid concentrations (Figure 2). Because different sphingolipid species have unique cellular functions, these enzymes play a central role in modulating the bioactive activity of these molecules. A growing list of factors modulates the activity of sphingolipid enzymes including steroidogenic regulatory agents such as TNF-α, IL-1β, ACTH, and growth factors [19, 25, 127, 130, 157, 158].
TNF-α and IL-1β have both been show to activate SMase in multiple cell types [29, 31, 127, 130, 158]. In Jeg-3 choriocarcinoma cells, SMase activity mediates TNF-α-dependent hormone production [29]. In addition, SM degradation was linked to cholesterol mitochondrial movement and increase in steroid hormone biosynthesis in mouse Leydig cells [159]. Degnan et al. demonstrated that Leydig cells incubated with exogenous SMase produced lower levels of testosterone in both basal and hCG-stimulated cells [130]. As discussed above, cer generated by SM hydrolysis can participate in various signaling cascades to regulate steroidogenesis. Interestingly, TNF-α was also shown to activate SK and increase endogenous S1P in human umbilical vein endothelial cells [160]. In addition, S1P was also reported to accumulate in response to TNF-α treatment in MA-10 cells [23], suggesting that this cytokine not only regulates SMase function, but also stimulates SK activity in steroidogenic cells.
In H295R cells, ACTH rapidly activates sphingolipid metabolism by decreasing the intracellular amounts of SM, cer, and SPH, while increasing S1P production via SK activation [19, 25]. These findings point to a role for ACTH signaling in controlling the activity of multiple sphingolipid enzymes. We have recently reported that cAMP increases the transcription, translation, and catalytic activity of ASAH1 in H295R cells [161]. Interestingly, we have also found that SK1 is rapidly translocated into the nucleus of H295R cells in response to ACTH/cAMP stimulation (Li et al., unpublished observations), thus indicating that S1P is potentially being produced in the nucleus of these cells. Because SPH suppresses SF-1-dependent steroidogenic gene transcription [25], it is possible that SK1 nuclear import facilitates SPH phosphorylation into S1P and consequently modulates the transcription of steroidogenic genes. Further studies are required to define the physiological significance of SK1 in modulating nuclear SPH concentrations.
4. Sphingolipid-mediated actions of steroid hormones
An equally important concept in the relationship between sphingolipid metabolism and steroidogenesis is the regulation of sphingolipid metabolism by steroid hormones. Sphingolipids have been reported to mediate multiple actions of estrogens, glucocorticoids, neurosteroids, and vitamin D3 [6, 162-170]. SK has been reported to bridge crosstalk between estrogens and growth factor signaling in breast cancer cells. 17β-estradiol (E2)-dependent SK activity is essential for E2-dependent cell growth and SK overexpression mimics the mitogenic effects of ER [170]. In addition, SK mediates E2-stimulated endothelial growth factor receptor (EGFR) transactivation, Ca2+ mobilization, and ERK1/2 activation [169, 170]. The observed E2-induced SK activation appear to be at least in part, dependent on G-protein coupled receptor 30 (GPR30) because when this receptor is downregulated [169] or absent [170], E2-dependent SK activity is inhibited. In addition, SK1 has been reported to have anti-apoptotic effects in breast cancer cells in an E2-dependent manner through inhibition of caspase-7 activation and poly(ADP-ribose) polymerase (PARP) cleavage [167]. Recently, Sukocheva et al. further strengthened the link between SK and ER-dependent cancer progression by demonstrating that SK1 confers tamoxifen resistance in MCF-7 cells [171]. Further, a recent clinical study reported that ASAH1 expression strongly correlates with ER-positive tumor cells and is a significant prognostic marker [168]. Thus, it is plausible to speculate that ER-induced breast cancercell growth is, at least in part, dependent ASAH1 and SK.
Glucocorticoids have also been shown to act through sphingolipid metabolism. Dexamethasone (dex) was reported to protect human fibroblast from apoptosis by inducing S1P formation [162]. Nieuwenhuis et al. recently demonstrated that this process involves S1P export through the ATP binding cassette (ABC)-transporter ABCC1 and activation of S1PR3 [163]. In addition, dex-induced intracellular S1P formation was shown to occur via upregulation of SK1, but not SK2, transcription and protein expression [163]. Similarly, dex-induced thymocyte apoptosis was shown to involve acid SMase activation and subsequent increase in cer levels [172]. In fact, cer is becoming appreciated as an important player in glucocorticoid-induced myopathy [173] and glucocorticoids have been shown to induce cer formation in various cell types [85, 101, 174]. Cer is known to induce mitochondrial dysfunction, oxidative stress, and insulin resistance, which are characteristics of glucocorticoid-mediated myopathy [173]. However the precise molecular mechanisms through which cer mediates this process are not entirely understood.
Vitamin D3 has long been reported as an inducer of SM turnover [103] and 1,25-(OH)2D3-dependent cer formation plays a role in cell differentiation in HL-60 cells [175, 176]. In addition, the anti-apoptotic properties of S1P were also shown to mediate the cytoprotective actions of 1,25-(OH)2D3 [164, 165, 177, 178]. Vitamin D3 activates SK and inhibits cer-induced apoptosis in a time- and dose-dependent manner [164]. Further, S1P formation was shown to protect keratinocytes from apoptosis despite acute SMase activation and cer formation in these cells [165]. More recently, Sauer et al. reported that Vitamin D3-induced S1P formation protected human fibroblast from apoptosis by increasing Bcl-2 expression [177].
Finally, neurosteroids have joined the list of sphingolipid modulators. Griffin et al. [179] have demonstrated that the neurosteroid allopregnanolone stimulates the degradation of complex sphingolipids and improves neurodegeneration in the Niemann-Pick Type C-1 (NPC-1) mouse model [6]. Further, Mellon et al. reported that the neuroprotective actions of allopregnanolone are partially mediated through GABAA and pregnane-X receptors [180].
5. Nuclear Sphingolipid Metabolism
Nuclear lipid metabolism is an important mechanism through which bioactive lipids modulate cell function. Recent studies have uncovered the extensive metabolism of lipids that occurs in the nuclei of various cell types [181, 182]. These studies have pointed towards important signaling and regulatory roles for nuclear lipids, including sphingolipids [18, 183-185]. For example, nuclear cer has been shown to participate in Fas-induced apoptosis in Jurkat T-cells as a result of caspase-3-dependent activation of SMase [184]. Also, glycosphingolipids have been shown to promote cytoprotection through regulation of nuclear Ca2+[186]. The ganglioside GM1 forms a complex with a sodium-calcium exchanger in the nuclear envelope (NE) and facilitates the transfer of Ca2+ from the nucleoplasm to the endoplasmic reticulum (ER) [187]. Finally, Hait et al. linked nuclear SK2/S1P to epigenetic regulation of gene expression by demonstrating that SK2 is associated with histone deacetylase 1 and 2 (HDAC1/2) in repressor complexes. S1P inhibits HDAC1/2 activity and SK2 induces p21 and c-fos gene transcription by enhancing histone H3 acetylation [183].
To date, multiple sphingolipid enzymes have been detected in the nuclei of various cell types. SMase was reported in the nuclear matrix [188-190], NE [191], and chromatin [188] whereas SM synthase was detected in chromatin and NE [192]. In addition, nuclear ceramidase and SK activities were demonstrated in rat hepatocytes and Swiss 3T3 cells, respectively [193-195]. However, SK2 is the predominantly nuclear isoform of SK in many cells [196]. Due to the hydrophobic nature of most sphingolipids, the nuclear expression of sphingolipid enzymes suggest that these bioactive lipids may have unique roles in nuclear function that are independent of the cytoplasmic functions of these molecules.
In steroidogenesis, recent data supports a role for these lipids in the regulation of gene expression. The characterization of SPH as an antagonist for SF-1 [26] was the first clue to the role of nuclear sphingolipids in the regulation of steroidogenesis. Further, due to the predominantly nuclear localization of SK2 [196] and cAMP-induced nuclear translocation of SK1 (Li et al., unpublished observations), S1P is likely being formed in the nucleus, perhaps as a way to control SPH nuclear levels and/or activate another uncharacterized nuclear process. In addition, we have found that ASAH1 is localized to the nucleus of H295R adrenocortical cells and ASAH1 represses SF-1-dependent CYP17 reporter gene activity by a direct enzyme-receptor (Lucki et al., unpublished observations). Thus, although further experimental evidence is necessary to dissect the mechanisms through which nuclear sphingolipids modulate steroidogenic gene transcription, it is evident that these molecules are likely to play key regulatory roles in the nucleus. Mass spectrometry, coupled with cell fraction, biophysical approaches, and microscopy are likely to provide more insight into the functional role(s) of bioactive sphingolipids in regulating gene transcription and other nuclear processes.
6. Conclusion
This brief review presents a summary of the established roles for sphingolipids in steroid hormone production. These bioactive molecules modulate steroidogenesis by regulating the expression of steroidogenic genes and enzymes, functioning as second messengers in various signaling pathways, acting as paracrine/autocrine regulators, and serving as ligands for nuclear receptors. Cer, SPH, and S1P play unique modulatory roles in steroidogenesis and trophic hormones, including ACTH, TNF-α, and IL-1β, regulate the activity of sphingolipid enzymes. In addition, sphingolipids have been shown to mediate the actions of various steroid hormones.
Nonetheless, there are still gaps in our understanding of the precise molecular mechanisms involved in sphingolipid-mediated steroidogenesis. Undoubtedly, future studies will uncover novel roles for these multi-faceted molecules. In addition, as novel bioactive sphingolipid species are discovered, the list of sphingolipid steroidogenic regulators is likely to expand. Finally, the recent discovery of nuclear sphingolipid metabolism and novel functions for these molecules in nuclear processes illustrates expanding functional diversity and hint to additional cellular functions that are yet to be discovered.
Table 1.
Summary of recent data obtained for the regulation of sphingolipid enzymes by steroid hormones, their sphingolipid mediators, and cellular responses that result from this regulation. Abbreviations: sphingosine kinase (SK), sphingomyelinase (SMase), acid ceramidase (ASAH1), sphingosine-1-phosphate (S1P), sphingosine (SPH), ceramide (cer), endothelial growth factor receptor (EGFR), extracellular regulated kinase (Erk), poly(ADP-ribose) polymerase (PARP). ? Indicates sphingolipid mediator has not yet been identified.
| Hormone | Target Enzyme | Effect on Target | Sphingolipid Mediator | Cellular Response |
|---|---|---|---|---|
| Estrogen | SK | ↑Activity | S1P | EGFR activation, Ca2+ mobilization, Erk1/2 activation, caspase 7 inhibition, PAPR cleavage, Tamoxifen resistance. |
| ASAH1 | ↑Gene transcription | ? | ||
| Glucocorticoids | SK1 | ↑Gene transcription and activity | S1P | Cell survival |
| Acid SMase | ↑Activity | cer | Apoptosis | |
| Allopregnanolone | ↑Activity | ? | ↓Neurodegeneration | |
| Vitamin D3 | SMase | ↑Activity | cer | Cell differentiation |
| SK | ↑Activity | S1P | Cell survival/ cytoprotective |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Foster RH. Reciprocal influences between the signalling pathways regulating proliferation and steroidogenesis in adrenal glomerulosa cells. J Mol Endocrinol. 2004;32:893–902. doi: 10.1677/jme.0.0320893. [DOI] [PubMed] [Google Scholar]
- 2.Ghayee HK, Auchus RJ. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metab Disord. 2007;8:289–300. doi: 10.1007/s11154-007-9052-2. [DOI] [PubMed] [Google Scholar]
- 3.Newton R, Holden NS. Separating transrepression and transactivation: A distressing divorce for the glococorticoid receptor? Molecular Pharmacology. 2007;72:799–809. doi: 10.1124/mol.107.038794. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Ashman HG, Reddi AH. Actions of vertebrate sex hormones. Annu Rev Physiol. 1971;33:31–82. doi: 10.1146/annurev.ph.33.030171.000335. [DOI] [PubMed] [Google Scholar]
- 5.Abdallah MA, Lei ZM, Li X, Greenwold N, Nakajima ST, Jauniaux E, Rao Ch V. Human fetal nongonadal tissues contain human chorionic gonadotropin/luteinizing hormone receptors. J Clin Endocrinol Metab. 2004;89:952–956. doi: 10.1210/jc.2003-030917. [DOI] [PubMed] [Google Scholar]
- 6.Mellon S, Gong W, Griffin LD. Niemann pick type C disease as a model for defects in neurosteroidogenesis. Endocr Res. 2004;30:727–735. doi: 10.1081/erc-200044016. [DOI] [PubMed] [Google Scholar]
- 7.Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–124. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller M, Atanasov A, Cima I, Corazza N, Schoonjans K, Brunner T. Differential regulation of glucocorticoid synthesis in murine intestinal epithelial versus adrenocortical cell lines. Endocrinology. 2007;148:1445–1453. doi: 10.1210/en.2006-0591. [DOI] [PubMed] [Google Scholar]
- 9.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 10.Tsutsui K, Ukena K, Usui M, Sakamoto H, Takase M. Novel brain function: biosynthesis and actions of neurosteroids in neurons. Neurosci Res. 2000;36:261–273. doi: 10.1016/s0168-0102(99)00132-7. [DOI] [PubMed] [Google Scholar]
- 11.Sewer MB, Dammer EB, Jagarlapudi S. Transcriptional regulation of adrenocortical steroidogenic gene expression. Drug Metab Rev. 2007;39:371–388. doi: 10.1080/03602530701498828. [DOI] [PubMed] [Google Scholar]
- 12.Lin YM, Tsai CC, Chung CL, Chen PR, Sunny Sun H, Tsai SJ, Huang BM. Fibroblast growth factor 9 stimulates steroidogenesis in postnatal Leydig cells. Int J Androl. 2009 doi: 10.1111/j.1365-2605.2009.00966.x. [DOI] [PubMed] [Google Scholar]
- 13.Mani AM, Fenwick M, Cheng Z, Sharma M, Singh D, Wathes C. IGF1 induces up-regulation of steroidogenic and apoptotic regulatory genes via activation of phosphatidylinositol-dependent kinase/AKT in bovine granulosa cells. Reproduction. 2009 doi: 10.1530/REP-09-0050. [DOI] [PubMed] [Google Scholar]
- 14.Schmahl J, Rizzolo K, Soriano P. The PDGF signaling pathway controls multiple steroid-producing lineages. Genes Dev. 2008;22:3255–3267. doi: 10.1101/gad.1723908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spicer LJ, Aad PY. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 2007;77:18–27. doi: 10.1095/biolreprod.106.058230. [DOI] [PubMed] [Google Scholar]
- 16.Zheng X, Boerboom D, Carriere PD. Transforming growth factor-beta1 inhibits luteinization and promotes apoptosis in bovine granulosa cells. Reproduction. 2009;137:969–977. doi: 10.1530/REP-08-0365. [DOI] [PubMed] [Google Scholar]
- 17.Derebecka-Holysz N, Lehmann TP, Holysz M, Trzeciak WH. TGF-beta inhibits CYP17 transcription in H295R cells acting via activin receptor-like kinase 5. Endocr Res. 2009;34:68–79. doi: 10.1080/07435800903137050. [DOI] [PubMed] [Google Scholar]
- 18.Ledeen RW, Wu G. Sphingolipids of the nucleus and their role in nuclear signaling. Biochim Biophys Acta. 2006;1761:588–598. doi: 10.1016/j.bbalip.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Ozbay T, Merrill AH, Jr, Sewer MB. ACTH regulates steroidogenic gene expression and cortisol biosynthesis in the human adrenal cortex via sphingolipid metabolism. Endocr Res. 2004;30:787–794. doi: 10.1081/erc-200044040. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2007;282:2125–2129. doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 21.Urs AN, Dammer E, Kelly S, Wang E, Merrill AH, Jr, Sewer MB. Steroidogenic factor-1 is a sphingolipid binding protein. Mol Cell Endocrinol. 2007;265-266:174–178. doi: 10.1016/j.mce.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng W, Kollmeyer J, Symolon H, Momin A, Munter E, Wang E, Kelly S, Allegood JC, Liu Y, Peng Q, Ramaraju H, Sullards MC, Cabot M, Merrill AHJ. Ceramides and other bioactive sphingolipid backbones in health and disease: lipidomic analysis, metabolism and roles in membrane structure, dynamics, signaling and autophagy. Biochem Biophys Res Commun. 2006;1758:1864–1884. doi: 10.1016/j.bbamem.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Budnik LT, Jahner D, Mukhopadhyay AK. Inhibitory effects of TNF alpha on mouse tumor Leydig cells: possible role of ceramide in the mechanism of action. Mol Cell Endocrinol. 1999;150:39–46. doi: 10.1016/s0303-7207(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 24.Morales V, Santana P, Diaz R, Tabraue C, Gallardo G, Blanco FL, Hernandez I, Fanjul LF, Ruiz de Galarreta CM. Intratesticular Delivery of Tumor Necrosis Factor-{alpha} and Ceramide Directly Abrogates Steroidogenic Acute Regulatory Protein Expression and Leydig Cell Steroidogenesis in Adult Rats. Endocrinology. 2003;144:4763–4772. doi: 10.1210/en.2003-0569. [DOI] [PubMed] [Google Scholar]
- 25.Ozbay T, Rowan A, Leon A, Patel P, Sewer M. Cyclic adenosine 5’-monophosphate-dependent sphingosine-1-phosphate biosynthesis induces human CYP17 gene transcription by activating cleavage of sterol regulating element binding protein 1. Endocrinology. 2006;147:1427–1437. doi: 10.1210/en.2005-1091. [DOI] [PubMed] [Google Scholar]
- 26.Urs AN, Dammer E, Sewer M. Sphingosine regulates the transcription of CYP17 by binding to steroidogenic factor-1. Endocrinology. 2006;147:5249–5258. doi: 10.1210/en.2006-0355. [DOI] [PubMed] [Google Scholar]
- 27.Kwun C, Patel A, Pletcher S, Lyons B, Abdelrahim M, Nicholson D, Morris E, Salata K, Francis GL. Ceramide increases steroid hormone production in MA-10 Leydig cells. Steroids. 1999;64:499–509. doi: 10.1016/s0039-128x(99)00013-6. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Ni J, Bian S, Yao L, Zhu H, Zhang W. Inhibition of steroidogenesis and induction of apoptosis in rat luteal cells by cell-permeable ceramide in vitro. Sheng Li Xue Bao. 2001;53:142–146. [PubMed] [Google Scholar]
- 29.McClellan DR, Bourdelat-Parks B, Salata K, Francis GL. Sphingomyelinase affects hormone production in Jeg-3 choriocarcinoma cells. Cell Endocrinology Metabolism. 1997;9:19–24. [Google Scholar]
- 30.Meroni SB, Pellizzari EH, Canepa DF, Cigorraga SB. Possible involvement of ceramide in the regulation of rat Leydig cell function. J Steroid Biochem Mol Biol. 2000;75:307–313. doi: 10.1016/s0960-0760(00)00188-6. [DOI] [PubMed] [Google Scholar]
- 31.Santana P, Llanes L, Hernandez I, Gonzalez-Robayna I, Tabraue C, Gonzalez-Reyes J, Quintana J, Estevez F, Ruiz de Galarreta CM, Fanjul LF. Interleukin-1 beta stimulates sphingomyelin hydrolysis in cultured granulosa cells: evidence for a regulatory role of ceramide on progesterone and prostaglandin biosynthesis. Endocrinology. 1996;137:2480–2489. doi: 10.1210/endo.137.6.8641202. [DOI] [PubMed] [Google Scholar]
- 32.Soboloff J, Sorisky A, Desilets M, Tsang BK. Acyl chain length-specific ceramide-induced changes in intracellular Ca2+ concentration and progesterone production are not regulated by tumor necrosis factor alpha in hen granulosa cells. Biol Reprod. 1999;60:262–271. doi: 10.1095/biolreprod60.2.262. [DOI] [PubMed] [Google Scholar]
- 33.Brizuela L, Rabano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3K/PKB and MEK/ERK 1/2 pathways. J Lipid Res. 2007;48:2264–2274. doi: 10.1194/jlr.M700291-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Brizuela L, Rabano M, Pena A, Gangoiti P, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine 1-phosphate: a novel stimulator of aldosterone secretion. J Lipid Res. 2006;47:1238–1249. doi: 10.1194/jlr.M500510-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Rabano M, Pena A, Brizuela L, Marino A, Macarulla JM, Trueba M, Gomez-Munoz A. Sphingosine-1-phosphate stimulates cortisol secretion. FEBS Lett. 2003;535:101–105. doi: 10.1016/s0014-5793(02)03882-6. [DOI] [PubMed] [Google Scholar]
- 36.Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. doi: 10.1074/jbc.273.6.3158. [DOI] [PubMed] [Google Scholar]
- 37.Katagiri M, Kagawa N, Waterman MR. The role of cytochrome b5 in the biosynthesis of androgens by human P450c17. Arch Biochem Biophys. 1995;317:343–347. doi: 10.1006/abbi.1995.1173. [DOI] [PubMed] [Google Scholar]
- 38.Miller WL, Auchus RJ. Role of cytochrome b5 in the 17,20-lyase activity of P450c17. J Clin Endocrinol Metab. 2000;85:1346. doi: 10.1210/jcem.85.3.6434-3. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen AD, Corbin CJ, Pattison JC, Bird IM, Conley AJ. The developmental increase in adrenocortical 17,20-lyase activity (biochemical adrenarche) is driven primarily by increasing cytochrome b5 in neonatal rhesus macaques. Endocrinology. 2009;150:1748–1756. doi: 10.1210/en.2008-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pandey AV, Miller WL. Regulation of 17,20 lyase activity by cytochrome b5 and by serine phosphorylation of P450c17. J Biol Chem. 2005;280:13265–13271. doi: 10.1074/jbc.M414673200. [DOI] [PubMed] [Google Scholar]
- 41.Miller WL. Mechanism of StAR’s regulation of mitochondrial cholesterol import. Mol Cell Endocrinol. 2007;265-266:46–50. doi: 10.1016/j.mce.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Sewer MB, Waterman MR. Insights into the transcriptional regulation of steroidogenic enzymes and StAR. Rev Endocr Metab Disord. 2001;2:269–274. doi: 10.1023/a:1011516532335. [DOI] [PubMed] [Google Scholar]
- 43.Thomson M. Molecular and cellular mechanisms used in the acute phase of stimulated steroidogenesis. Hormone Metabolism Research. 1997;30:16–28. doi: 10.1055/s-2007-978825. [DOI] [PubMed] [Google Scholar]
- 44.Papadopoulos V. Peripheral-type benzodiazepine/diazepam binding inhibitor receptor: biological role in steroidogenic cell function. Endocr Rev. 1993;14:222–240. doi: 10.1210/edrv-14-2-222. [DOI] [PubMed] [Google Scholar]
- 45.Hauet T, Liu J, Li H, Gazouli M, Culty M, Papadopoulos V. PBR, StAR, and PKA: partners in cholesterol transport in steroidogenic cells. Endocr Res. 2002;28:395–401. doi: 10.1081/erc-120016814. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Li H, Papadopoulos V. PAP7, a PBR/PKA-RIalpha-associated protein: a new element in the relay of the hormonal induction of steroidogenesis. J Steroid Biochem Mol Biol. 2003;85:576–586. doi: 10.1016/s0960-0760(03)00213-9. [DOI] [PubMed] [Google Scholar]
- 47.Arlt W, Stewart PM. Adrenal corticosteroid biosynthesis, metabolism, and action. Endocrinol Metab Clin North Am. 2005;34:293–313. viii. doi: 10.1016/j.ecl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Bassett MH, White PC, Rainey WE. Regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Bornstein SR, Rutkowski H, Vrezas I. Cytokines and steroidogenesis. Mol Cell Endocrinol. 2004;215:135–141. doi: 10.1016/j.mce.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology. 2002;143:3651–3657. doi: 10.1210/en.2001-211359. [DOI] [PubMed] [Google Scholar]
- 51.Jamnongjit M, Hammes SR. Ovarian steroids: the good, the bad, and the signals that raise them. Cell Cycle. 2006;5:1178–1183. doi: 10.4161/cc.5.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mendelson CR, Jiang B, Shelton JM, Richardson JA, Hinshelwood MM. Transcriptional regulation of aromatase in placenta and ovary. J Steroid Biochem Mol Biol. 2005;95:25–33. doi: 10.1016/j.jsbmb.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 53.Otis M, Gallo-Payet N. Role of MAPKs in angiotensin II-induced steroidogenesis in rat glomerulosa cells. Mol Cell Endocrinol. 2007;265-266:126–130. doi: 10.1016/j.mce.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 54.Sewer MB, Waterman MR. ACTH modulation of transcription factors responsible for steroid hydroxylase gene expression in the adrenal cortex. Microsc Res Tech. 2003;61:300–307. doi: 10.1002/jemt.10339. [DOI] [PubMed] [Google Scholar]
- 55.Sirianni R, Carr BR, Ando S, Rainey WE. Inhibition of Src tyrosine kinase stimulates adrenal androgen production. J Mol Endocrinol. 2003;30:287–299. doi: 10.1677/jme.0.0300287. [DOI] [PubMed] [Google Scholar]
- 56.Bakke M, Zhao L, Hanley NA, Parker KL. SF-1: a critical mediator of steroidogenesis. Molecular and Cellular Endocrinology. 2001;171:5–7. doi: 10.1016/s0303-7207(00)00384-1. [DOI] [PubMed] [Google Scholar]
- 57.Hammer GD, Parker KL, Schimmer BP. Minireview: transcriptional regulation of adrenal development. Endocrinology. 2005;146:1018–1024. doi: 10.1210/en.2004-1385. [DOI] [PubMed] [Google Scholar]
- 58.Parker KL, Rice DA, Lala DS, Ikeda Y, Luo X, Wong M, Bakke M, Zhao L, Frigeri C, Hanley NA, Stallings N, Schimmer BP. Steroidogenic factor 1: an essential mediator of endocrine development. Recent Prog Horm Res. 2002;57:19–36. doi: 10.1210/rp.57.1.19. [DOI] [PubMed] [Google Scholar]
- 59.Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994;8:2302–2312. doi: 10.1101/gad.8.19.2302. [DOI] [PubMed] [Google Scholar]
- 60.Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 61.Ozisik G, Achermann JC, Meeks JJ, Jameson JL. SF1 in the development of the adrenal gland and gonads. Horm Res. 2003;59(Suppl 1):94–98. doi: 10.1159/000067831. [DOI] [PubMed] [Google Scholar]
- 62.Huang N, Dardis A, Miller WL. Regulation of cytochrome b5 gene transcription by Sp3, GATA-6, and steroidogenic factor 1 in human adrenal NCI-H295A cells. Mol Endocrinol. 2005;19:2020–2034. doi: 10.1210/me.2004-0411. [DOI] [PubMed] [Google Scholar]
- 63.Fluck CE, Miller WL. GATA-4 and GATA-6 modulate tissue-specific transcription of the human gene for P450c17 by direct interaction with Sp1. Mol Endocrinol. 2004;18:1144–1157. doi: 10.1210/me.2003-0342. [DOI] [PubMed] [Google Scholar]
- 64.Jimenez P, Saner K, Mayhew B, Rainey WE. GATA-6 is expressed in the human adrenal and regulates transcription of genes required for adrenal androgen biosynthesis. Endocrinology. 2003;144:4285–4288. doi: 10.1210/en.2003-0472. [DOI] [PubMed] [Google Scholar]
- 65.Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- 66.Manna PR, Eubank DW, Lalli E, Sassone-Corsi P, Stocco DM. Transcriptional regulation of the mouse steroidogenic acute regulatory protein gene by the cAMP response-element binding protein and steroidogenic factor 1. J Mol Endocrinol. 2003;30:381–397. doi: 10.1677/jme.0.0300381. [DOI] [PubMed] [Google Scholar]
- 67.Lin CJ, Martens JW, Miller WL. NF-1C, Sp1, and Sp3 are essential for transcription of the human gene for P450c17 (steroid 17alpha-hydroxylase/17,20 lyase) in human adrenal NCI-H295A cells. Mol Endocrinol. 2001;15:1277–1293. doi: 10.1210/mend.15.8.0679. [DOI] [PubMed] [Google Scholar]
- 68.Bassett MH, Suzuki T, Sasano H, De Vries CJ, Jimenez PT, Carr BR, Rainey WE. The orphan nuclear receptor NGFIB regulates transcription of 3beta-hydroxysteroid dehydrogenase. implications for the control of adrenal functional zonation. J Biol Chem. 2004;279:37622–37630. doi: 10.1074/jbc.M405431200. [DOI] [PubMed] [Google Scholar]
- 69.Bassett MH, Suzuki T, Sasano H, White PC, Rainey WE. The orphan nuclear receptors NURR1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2004;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- 70.Mao C, Obeid LM. Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phosphate. Biochim Biophys Acta. 2008;1781:424–434. doi: 10.1016/j.bbalip.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Merrill AH, Jr, Nikolova-Karakashian M, Schmelz EM, Morgan E, Stewart J. Regulation of cytochrome P450 expression by sphingolipids. Chemistry and Physics of Lipids. 1999;102:131–139. doi: 10.1016/s0009-3084(99)00081-x. [DOI] [PubMed] [Google Scholar]
- 72.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530:9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 73.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 74.Sanchez AM, Malagarie-Cazenave S, Olea N, Vara D, Cuevas C, Diaz-Laviada I. Spisulosine (ES-285) induces prostate tumor PC-3 and LNCaP cell death by de novo synthesis of ceramide and PKCzeta activation. Eur J Pharmacol. 2008;584:237–245. doi: 10.1016/j.ejphar.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 75.Spiegel S, Milstien S. Sphingosine 1-phosphate, a key cell signaling molecule. J Biol Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- 76.Suzuki E, Handa K, Toledo MS, Hakomori S. Sphingosine-dependent apoptosis: a unified concept based on multiple mechanisms operating in concert. Proc Natl Acad Sci U S A. 2004;101:14788–14793. doi: 10.1073/pnas.0406536101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine-1-phosphate metabolism on the cell surface and in the extracellular space. Cellular Signaling. 2007;19:229–237. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Thevissen K, Francois IE, Winderickx J, Pannecouque C, Cammue BP. Ceramide involvement in apoptosis and apoptotic diseases. Mini Rev Med Chem. 2006;6:699–709. doi: 10.2174/138955706777435643. [DOI] [PubMed] [Google Scholar]
- 79.Thon L, Mohlig H, Mathieu S, Lange A, Bulanova E, Winoto-Morbach S, Schutze S, Bulfone-Paus S, Adam D. Ceramide mediates caspase-independent programmed cell death. FASEB J. 2005;19:1945–1956. doi: 10.1096/fj.05-3726com. [DOI] [PubMed] [Google Scholar]
- 80.Wang F, Van Brocklyn JR, Hobson JP, Movafagh S, Zukowska-Grojec Z, Milstien S, Spiegel S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J Biol Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- 81.Yamamura S, Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate inhibits actin nucleation and pseudopodium formation to control cell motility of mouse melanoma cells. FEBS Lett. 1996;382:193–197. doi: 10.1016/0014-5793(96)00175-5. [DOI] [PubMed] [Google Scholar]
- 82.Zeidan YH, Hannun YA. Translational aspects of sphingolipid metabolism. Trends Mol Med. 2007;13:327–336. doi: 10.1016/j.molmed.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 83.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 84.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Sphingolipids in macroautophagy. Methods Mol Biol. 2008;445:159–173. doi: 10.1007/978-1-59745-157-4_11. [DOI] [PubMed] [Google Scholar]
- 85.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez-Veledo S, Hernandez R, Teruel T, Mas JA, Ros M, Lorenzo M. Ceramide mediates TNF-alpha-induced insulin resistance on GLUT4 gene expression in brown adipocytes. Arch Physiol Biochem. 2006;112:13–22. doi: 10.1080/13813450500508137. [DOI] [PubMed] [Google Scholar]
- 87.Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53:25–31. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 88.Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 89.Saddoughi SA, Song P, Ogretmen B. Roles of bioactive sphingolipids in cancer biology and therapeutics. Subcell Biochem. 2008;49:413–440. doi: 10.1007/978-1-4020-8831-5_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyn-Cook LE, Jr, Lawton M, Tong M, Silbermann E, Longato L, Jiao P, Mark P, Wands JR, Xu H, de la Monte SM. Hepatic ceramide may mediate brain insulin resistance and neurodegeneration in type 2 diabetes and non-alcoholic steatohepatitis. J Alzheimers Dis. 2009;16:715–729. doi: 10.3233/JAD-2009-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Osuchowski MF, Edwards GL, Sharma RP. Fumonisin B1-induced neurodegeneration in mice after intracerebroventricular infusion is concurrent with disruption of sphingolipid metabolism and activation of proinflammatory signaling. Neurotoxicology. 2005;26:211–221. doi: 10.1016/j.neuro.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 92.Tamboli IY, Prager K, Barth E, Heneka M, Sandhoff K, Walter J. Inhibition of glycosphingolipid biosynthesis reduces secretion of the beta-amyloid precursor protein and amyloid beta-peptide. J Biol Chem. 2005;280:28110–28117. doi: 10.1074/jbc.M414525200. [DOI] [PubMed] [Google Scholar]
- 93.Zitomer NC, Mitchell T, Voss KA, Bondy GS, Pruett ST, Garnier-Amblard EC, Liebeskind LS, Park H, Wang E, Sullards MC, Merrill AH, Jr, Riley RT. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J Biol Chem. 2009;284:4786–4795. doi: 10.1074/jbc.M808798200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Han G, Gupta SD, Gable K, Niranjanakumari S, Moitra P, Eichler F, Brown RH, Jr, Harmon JM, Dunn TM. Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc Natl Acad Sci U S A. 2009;106:8186–8191. doi: 10.1073/pnas.0811269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hornemann T, Penno A, Rutti MF, Ernst D, Kivrak-Pfiffner F, Rohrer L, von Eckardstein A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J Biol Chem. 2009;284:26322–26330. doi: 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, Igarashi Y. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66:358–366. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 97.Gomez-Munoz A, Kong JY, Salh B, Steinbrecher UP. Ceramide-1-phosphate blocks apoptosis through inhibition of acid sphingomyelinase in macrophages. J Lipid Res. 2004;45:99–105. doi: 10.1194/jlr.M300158-JLR200. [DOI] [PubMed] [Google Scholar]
- 98.Auge N, Nikolova-Karakashian M, Carpentier S, Parthasarathy S, Negre-Salvayre A, Salvayre R, Merrill AH, Jr, Levade T. Role of sphingosine 1-phosphate in the mitogenesis induced by oxidized low density lipoprotein in smooth muscle cells via activation of sphingomyelinase, ceramidase, and sphingosine kinase. J Biol Chem. 1999;274:21533–21538. doi: 10.1074/jbc.274.31.21533. [DOI] [PubMed] [Google Scholar]
- 99.Cuvillier O, Nava VE, Murthy SK, Edsall LC, Levade T, Milstien S, Spiegel S. Sphingosine generation, cytochrome c release, and activation of caspase-7 in doxorubicin-induced apoptosis of MCF7 breast adenocarcinoma cells. Cell Death Differ. 2001;8:162–171. doi: 10.1038/sj.cdd.4400793. [DOI] [PubMed] [Google Scholar]
- 100.Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lepine S, Lakatos B, Courageot MP, Le Stunff H, Sulpice JC, Giraud F. Sphingosine contributes to glucocorticoid-induced apoptosis of thymocytes independently of the mitochondrial pathway. J Immunol. 2004;173:3783–3790. doi: 10.4049/jimmunol.173.6.3783. [DOI] [PubMed] [Google Scholar]
- 102.Nikolova-Karakashian MN, Russell RW, Booth RA, Jenden DJ, Merrill AH., Jr Sphingomyelin metabolism in rat liver after chronic dietary replacement of choline by N-aminodeanol. J Lipid Res. 1997;38:1764–1770. [PubMed] [Google Scholar]
- 103.Okazaki T, Bell RM, Hannun YA. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. Role in cell differentiation. J Biol Chem. 1989;264:19076–19080. [PubMed] [Google Scholar]
- 104.Payne SG, Milstien S, Barbour SE, Spiegel S. Modulation of adaptive immune responses by sphingosine-1-phosphate. Semin Cell Dev Biol. 2004;15:521–527. doi: 10.1016/j.semcdb.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 105.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 106.Sweeney EA, Inokuchi J, Igarashi Y. Inhibition of sphingolipid induced apoptosis by caspase inhibitors indicates that sphingosine acts in an earlier part of the apoptotic pathway than ceramide. FEBS Lett. 1998;425:61–65. doi: 10.1016/s0014-5793(98)00198-7. [DOI] [PubMed] [Google Scholar]
- 107.Van Brocklyn JR, Lee MJ, Menzeleev R, Olivera A, Edsall L, Cuvillier O, Thomas DM, Coopman PJ, Thangada S, Liu CH, Hla T, Spiegel S. Dual actions of sphingosine-1-phosphate: extracellular through the Gi-coupled receptor Edg-1 and intracellular to regulate proliferation and survival. J Cell Biol. 1998;142:229–240. doi: 10.1083/jcb.142.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeidan YH, Pettus BJ, Elojeimy S, Taha T, Obeid LM, Kawamori T, Norris JS, Hannun YA. Acid ceramidase but not acid sphingomyelinase is required for tumor necrosis factor-{alpha}-induced PGE2 production. J Biol Chem. 2006;281:24695–24703. doi: 10.1074/jbc.M604713200. [DOI] [PubMed] [Google Scholar]
- 109.Hakomori S. Traveling for the glycosphingolipid path. Glycoconj J. 2000;17:627–647. doi: 10.1023/a:1011086929064. [DOI] [PubMed] [Google Scholar]
- 110.Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth. Specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986;261:2434–2440. [PubMed] [Google Scholar]
- 111.Andrieu-Abadie N, Levade T. Sphingomyelin hydrolysis during apoptosis. Biochim Biophys Acta. 2002;1585:126–134. doi: 10.1016/s1388-1981(02)00332-3. [DOI] [PubMed] [Google Scholar]
- 112.Huwiler A, Pfeilschifter J. Altering the sphingosine-1-phosphate/ceramide balance: a promising approach for tumor therapy. Curr Pharm Des. 2006;12:4625–4635. doi: 10.2174/138161206779010422. [DOI] [PubMed] [Google Scholar]
- 113.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 114.Gomez-Munoz A. Ceramide 1-phosphate/ceramide, a switch between life and death. Biochim Biophys Acta. 2006;1758:2049–2056. doi: 10.1016/j.bbamem.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 115.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gangoiti P, Granado MH, Wang SW, Kong JY, Steinbrecher UP, Gomez-Munoz A. Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell Signal. 2008;20:726–736. doi: 10.1016/j.cellsig.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 117.Gomez-Munoz A, Frago LM, Alvarez L, Varela-Nieto I. Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem J. 1997;325(Pt 2):435–440. doi: 10.1042/bj3250435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hung WC, Chang HC, Chuang LY. Activation of caspase-3-like proteases in apoptosis induced by sphingosine and other long-chain bases in Hep3B hepatoma cells. Biochem J. 1999;338(Pt 1):161–166. [PMC free article] [PubMed] [Google Scholar]
- 119.Sakakura C, Sweeney EA, Shirahama T, Hagiwara A, Yamaguchi T, Takahashi T, Hakomori S, Igarashi Y. Selectivity of sphingosine-induced apoptosis. Lack of activity of DL-erythyro-dihydrosphingosine. Biochem Biophys Res Commun. 1998;246:827–830. doi: 10.1006/bbrc.1998.8719. [DOI] [PubMed] [Google Scholar]
- 120.Olivera A, Kohama T, Edsall L, Nava V, Cuvillier O, Poulton S, Spiegel S. Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J Cell Biol. 1999;147:545–558. doi: 10.1083/jcb.147.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hannun YA, Obeid LM. Principles of bioactive lipid signaling: lessons from sphingolipids. Nature Reviews, Molecular and Cell Biology. 2009;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 122.Gallo-Payet N, Payet MD. Excitation-secretion coupling: involvement of potassium channels in ACTH-stimulated rat adrenocortical cells. J Endocrinol. 1989;120:409–421. doi: 10.1677/joe.0.1200409. [DOI] [PubMed] [Google Scholar]
- 123.Stocco DM, Khan SA. Effects of steroidogenesis inducing protein (SIP) on steroid production in MA-10 mouse Leydig tumor cells: utilization of a non-cAMP second messenger pathway. Mol Cell Endocrinol. 1992;84:185–194. doi: 10.1016/0303-7207(92)90029-6. [DOI] [PubMed] [Google Scholar]
- 124.Hedger MP. Testicular leukocytes: what are they doing? Rev Reprod. 1997;2:38–47. doi: 10.1530/ror.0.0020038. [DOI] [PubMed] [Google Scholar]
- 125.Weber MM, Michl P, Auernhammer CJ, Engelhardt D. Interleukin-3 and interleukin-6 stimulate cortisol secretion from adult human adrenocortical cells. Endocrinology. 1997;138:2207–2210. doi: 10.1210/endo.138.5.5239. [DOI] [PubMed] [Google Scholar]
- 126.Cai Z, Bettaieb A, Mahdani NE, Legres LG, Stancou R, Masliah J, Chouaib S. Alteration of the sphingomyelin/ceramide pathway is associated with resistance of human breast carcinoma MCF7 cells to tumor necrosis factor-alpha-mediated cytotoxicity. J Biol Chem. 1997;272:6918–6926. doi: 10.1074/jbc.272.11.6918. [DOI] [PubMed] [Google Scholar]
- 127.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 128.Sawai H, Okazaki T, Takeda Y, Tashima M, Sawada H, Okuma M, Kishi S, Umehara H, Domae N. Ceramide-induced translocation of protein kinase C-delta and -epsilon to the cytosol. Implications in apoptosis. J Biol Chem. 1997;272:2452–2458. doi: 10.1074/jbc.272.4.2452. [DOI] [PubMed] [Google Scholar]
- 129.Arai N, Masuzaki H, Tanaka T, Ishii T, Yasue S, Kobayashi N, Tomita T, Noguchi M, Kusakabe T, Fujikura J, Ebihara K, Hirata M, Hosoda K, Hayashi T, Sawai H, Minokoshi Y, Nakao K. Ceramide and Adenosine 5’-Monophosphate-Activated Protein Kinase Are Two Novel Regulators of 11{beta}-Hydroxysteroid Dehydrogenase Type 1 Expression and Activity in Cultured Preadipocytes. Endocrinology. 2007;148:5268–5277. doi: 10.1210/en.2007-0349. [DOI] [PubMed] [Google Scholar]
- 130.Degnan BM, Bourdelat-Parks B, Daniel A, Salata K, Francis GL. Sphingomyelinase inhibits in vitro Leydig cell function. Ann Clin Lab Sci. 1996;26:234–242. [PubMed] [Google Scholar]
- 131.Santana P, Llanes L, Hernandez I, Gallardo G, Quintana J, Gonzalez J, Estevez F, Ruiz de Galarreta C, Fanjul LF. Ceramide mediates tumor necrosis factor effects on P450-aromatase activity in cultured granulosa cells. Endocrinology. 1995;136:2345–2348. doi: 10.1210/endo.136.5.7720683. [DOI] [PubMed] [Google Scholar]
- 132.Kolesnick RN, Haimovitz-Friedman A, Fuks Z. The sphingomyelin signal transduction pathway mediates apoptosis for tumor necrosis factor, Fas, and ionizing radiation. Biochem Cell Biol. 1994;72:471–474. doi: 10.1139/o94-063. [DOI] [PubMed] [Google Scholar]
- 133.Dressler KA, Mathias S, Kolesnick RN. Tumor necrosis factor-alpha activates the sphingomyelin signal transduction pathway in a cell-free system. Science. 1992;255:1715–1718. doi: 10.1126/science.1313189. [DOI] [PubMed] [Google Scholar]
- 134.Eto M, Bennouna J, Hunter OC, Hershberger PA, Kanto T, Johnson CS, Lotze MT, Amoscato AA. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003;57:66–79. doi: 10.1002/pros.10275. [DOI] [PubMed] [Google Scholar]
- 135.Eto M, Bennouna J, Hunter OC, Lotze MT, Amoscato AA. Importance of C16 ceramide accumulation during apoptosis in prostate cancer cells. Int J Urol. 2006;13:148–156. doi: 10.1111/j.1442-2042.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 136.Kanto T, Kalinski P, Hunter OC, Lotze MT, Amoscato AA. Ceramide mediates tumor-induced dendritic cell apoptosis. J Immunol. 2001;167:3773–3784. doi: 10.4049/jimmunol.167.7.3773. [DOI] [PubMed] [Google Scholar]
- 137.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, Obeid LM, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 138.Kroesen BJ, Pettus B, Luberto C, Busman M, Sietsma H, de Leij L, Hannun YA. Induction of apoptosis through B-cell receptor cross-linking occurs via de novo generated C16-ceramide and involves mitochondria. J Biol Chem. 2001;276:13606–13614. doi: 10.1074/jbc.M009517200. [DOI] [PubMed] [Google Scholar]
- 139.Thomas RL, Jr, Matsko CM, Lotze MT, Amoscato AA. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J Biol Chem. 1999;274:30580–30588. doi: 10.1074/jbc.274.43.30580. [DOI] [PubMed] [Google Scholar]
- 140.Son DS, Arai KY, Roby KF, Terranova PF. Tumor necrosis factor alpha (TNF) increases granulosa cell proliferation: dependence on c-Jun and TNF receptor type 1. Endocrinology. 2004;145:1218–1226. doi: 10.1210/en.2003-0860. [DOI] [PubMed] [Google Scholar]
- 141.Hla T, Lee MJ, Ancellin N, Liu CH, Thangada S, Thompson BD, Kluk M. Sphingosine-1-phosphate: extracellular mediator or intracellular second messenger? Biochem Pharmacol. 1999;58:201–207. doi: 10.1016/s0006-2952(99)00086-6. [DOI] [PubMed] [Google Scholar]
- 142.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 143.Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-Out” Signaling of Sphingosine-1-Phosphate: Therapeutic Targets. Pharmacol Rev. 2008;60:181–195. doi: 10.1124/pr.107.07113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Windh RT, Lee MJ, Hla T, An S, Barr AJ, Manning DR. Differential coupling of the sphingosine 1-phosphate receptors Edg-1, Edg-3, and H218/Edg-5 to the G(i), G(q), and G(12) families of heterotrimeric G proteins. J Biol Chem. 1999;274:27351–27358. doi: 10.1074/jbc.274.39.27351. [DOI] [PubMed] [Google Scholar]
- 145.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 146.Kihara A, Mitsutake S, Mizutani Y, Igarashi Y. Metabolism and biological functions of two phosphorylated sphingolipids, sphingosine 1-phosphate and ceramide 1-phosphate. Prog Lipid Res. 2007;46:126–144. doi: 10.1016/j.plipres.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Pettus BJ, Kitatani K, Chalfant CE, Taha TA, Kawamori T, Bielawski J, Obeid LM, Hannun YA. The coordination of prostaglandin E2 production by sphingosine-1-phosphate and ceramide-1-phosphate. Mol Pharmacol. 2005;68:330–335. doi: 10.1124/mol.104.008722. [DOI] [PubMed] [Google Scholar]
- 148.Olofsson J, Leung PC. Auto/paracrine role of prostaglandins in corpus luteum function. Mol Cell Endocrinol. 1994;100:87–91. doi: 10.1016/0303-7207(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 149.Michael AE, Abayasekara DR, Webley GE. The luteotrophic actions of prostaglandins E2 and F2 alpha on dispersed marmoset luteal cells are differentially mediated via cyclic AMP and protein kinase C. J Endocrinol. 1993;138:291–298. doi: 10.1677/joe.0.1380291. [DOI] [PubMed] [Google Scholar]
- 150.Chandras C, Harris TE, Bernal AL, Abayasekara DR, Michael AE. PTGER1 and PTGER2 receptors mediate regulation of progesterone synthesis and type 1 11beta-hydroxysteroid dehydrogenase activity by prostaglandin E2 in human granulosa lutein cells. J Endocrinol. 2007;194:595–602. doi: 10.1677/JOE-07-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hadizadeh S, King DN, Shah S, Sewer MB. Sphingosine-1-phosphate regulates the expression of the liver receptor homologue-1. Mol Cell Endocrinol. 2008;283:104–113. doi: 10.1016/j.mce.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 152.Clyne CD, Speed CJ, Zhou J, Simpson ER. Liver receptor homologue-1 (LRH-1) regulates expression of aromatase in preadipocytes. J Biol Chem. 2002;277:20591–20597. doi: 10.1074/jbc.M201117200. [DOI] [PubMed] [Google Scholar]
- 153.O’Brien N, Jones ST, Williams DG, Cunningham HB, Moreno K, Visentin B, Gentile A, Vekich J, Shestowsky W, Hiraiwa M, Matteo R, Cavalli A, Grotjahn D, Grant M, Hansen G, Campbell MA, Sabbadini R. Production and characterization of monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res. 2009 doi: 10.1194/jlr.M900048-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, Kundra V, Mills GB, Sabbadini RA. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 155.Wojciak JM, Zhu N, Schuerenberg KT, Moreno K, Shestowsky WS, Hiraiwa M, Sabbadini R, Huxford T. The crystal structure of sphingosine-1-phosphate in complex with a Fab fragment reveals metal bridging of an antibody and its antigen. Proc Natl Acad Sci U S A. 2009;106:17717–17722. doi: 10.1073/pnas.0906153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li D, Urs AN, Allegood J, Leon A, Merrill AH, Jr, Sewer MB. Cyclic AMP-stimulated interaction between steroidogenic factor 1 and diacylglycerol kinase theta facilitates induction of CYP17. Mol Cell Biol. 2007;27:6669–6685. doi: 10.1128/MCB.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lucki N, Sewer MB. The cAMP-responsive element binding protein (CREB) regulates the expression of acid ceramidase (ASAH1) in H295R human adrenocortical cells. Biochim Biophys Acta. 2009;1791:706–713. doi: 10.1016/j.bbalip.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Henkes LE, Sullivan BT, Lynch MP, Kolesnick R, Arsenault D, Puder M, Davis JS, Rueda BR. Acid sphingomyelinase involvement in tumor necrosis factor alpha-regulated vascular and steroid disruption during luteolysis in vivo. Proc Natl Acad Sci U S A. 2008;105:7670–7675. doi: 10.1073/pnas.0712260105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Porn MI, Tenhunen J, Slotte JP. Increased steroid hormone secretion in mouse Leydig tumor cells after induction of cholesterol translocation by sphingomyelin degradation. Biochim Biophys Acta. 1991;1093:7–12. doi: 10.1016/0167-4889(91)90131-g. [DOI] [PubMed] [Google Scholar]
- 160.Xia P, Wang L, Gamble JR, Vadas MA. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- 161.Lucki N, Sewer MB. The cAMP-responsive element binding protein (CREB) regulates the expression of acid ceramidase (ASAH1) in H295R human adrenocortical cells. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hammer S, Sauer B, Spika I, Schraut C, Kleuser B, Schafer-Korting M. Glucocorticoids mediate differential anti-apoptotic effects in human fibroblasts and keratinocytes via sphingosine-1-phosphate formation. J Cell Biochem. 2004;91:840–851. doi: 10.1002/jcb.10766. [DOI] [PubMed] [Google Scholar]
- 163.Nieuwenhuis B, Luth A, Chun J, Huwiler A, Pfeilschifter J, Schafer-Korting M, Kleuser B. Involvement of the ABC-transporter ABCC1 and the sphingosine 1-phosphate receptor subtype S1P(3) in the cytoprotection of human fibroblasts by the glucocorticoid dexamethasone. J Mol Med. 2009;87:645–657. doi: 10.1007/s00109-009-0468-x. [DOI] [PubMed] [Google Scholar]
- 164.Kleuser B, Cuvillier O, Spiegel S. 1Alpha,25-dihydroxyvitamin D3 inhibits programmed cell death in HL-60 cells by activation of sphingosine kinase. Cancer Res. 1998;58:1817–1824. [PubMed] [Google Scholar]
- 165.Manggau M, Kim DS, Ruwisch L, Vogler R, Korting HC, Schafer-Korting M, Kleuser B. 1Alpha,25-dihydroxyvitamin D3 protects human keratinocytes from apoptosis by the formation of sphingosine-1-phosphate. J Invest Dermatol. 2001;117:1241–1249. doi: 10.1046/j.0022-202x.2001.01496.x. [DOI] [PubMed] [Google Scholar]
- 166.Anic M, Mesaric M. The influence of sex steroid hormones on ganglioside biosynthesis in rat kidney. Biol Chem. 1998;379:693–697. doi: 10.1515/bchm.1998.379.6.693. [DOI] [PubMed] [Google Scholar]
- 167.Nava VE, Hobson JP, Murthy S, Milstien S, Spiegel S. Sphingosine kinase type 1 promotes estrogen-dependent tumorigenesis of breast cancer MCF-7 cells. Exp Cell Res. 2002;281:115–127. doi: 10.1006/excr.2002.5658. [DOI] [PubMed] [Google Scholar]
- 168.Ruckhaberle E, Holtrich U, Engels K, Hanker L, Gatje R, Metzler D, Karn T, Kaufmann M, Rody A. Acid ceramidase 1 expression correlates with a better prognosis in ER-positive breast cancer. Climacteric. 2009:1–12. doi: 10.3109/13697130902939913. [DOI] [PubMed] [Google Scholar]
- 169.Sukocheva O, Wadham C, Holmes A, Albanese N, Verrier E, Feng F, Bernal A, Derian CK, Ullrich A, Vadas MA, Xia P. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sukocheva OA, Wang L, Albanese N, Pitson SM, Vadas MA, Xia P. Sphingosine kinase transmits estrogen signaling in human breast cancer cells. Mol Endocrinol. 2003;17:2002–2012. doi: 10.1210/me.2003-0119. [DOI] [PubMed] [Google Scholar]
- 171.Sukocheva O, Wang L, Verrier E, Vadas MA, Xia P. Restoring endocrine response in breast cancer cells by inhibition of the sphingosine kinase-1 signaling pathway. Endocrinology. 2009;150:4484–4492. doi: 10.1210/en.2009-0391. [DOI] [PubMed] [Google Scholar]
- 172.Cinque B, Fanini D, Di Marzio L, Palumbo P, La Torre C, Donato V, Velardi E, Bruscoli S, Riccardi C, Cifone MG. Involvement of cPLA2 inhibition in dexamethasone-induced thymocyte apoptosis. Int J Immunopathol Pharmacol. 2008;21:539–551. doi: 10.1177/039463200802100307. [DOI] [PubMed] [Google Scholar]
- 173.Dirks-Naylor AJ, Griffiths CL. Glucocorticoid-induced apoptosis and cellular mechanisms of myopathy. J Steroid Biochem Mol Biol. 2009;117:1–7. doi: 10.1016/j.jsbmb.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 174.Marchetti MC, Di Marco B, Cifone G, Migliorati G, Riccardi C. Dexamethasone-induced apoptosis of thymocytes: role of glucocorticoid receptor-associated Src kinase and caspase-8 activation. Blood. 2003;101:585–593. doi: 10.1182/blood-2002-06-1779. [DOI] [PubMed] [Google Scholar]
- 175.Bielawska A, Linardic CM, Hannun YA. Modulation of cell growth and differentiation by ceramide. FEBS Lett. 1992;307:211–214. doi: 10.1016/0014-5793(92)80769-d. [DOI] [PubMed] [Google Scholar]
- 176.Okazaki T, Bielawska A, Bell RM, Hannun YA. Role of ceramide as a lipid mediator of 1 alpha,25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J Biol Chem. 1990;265:15823–15831. [PubMed] [Google Scholar]
- 177.Sauer B, Gonska H, Manggau M, Kim DS, Schraut C, Schafer-Korting M, Kleuser B. Sphingosine 1-phosphate is involved in cytoprotective actions of calcitriol in human fibroblasts and enhances the intracellular Bcl-2/Bax rheostat. Pharmazie. 2005;60:298–304. [PubMed] [Google Scholar]
- 178.Sauer B, Ruwisch L, Kleuser B. Antiapoptotic action of 1alpha,25-dihydroxyvitamin D3 in primary human melanocytes. Melanoma Res. 2003;13:339–347. doi: 10.1097/00008390-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 179.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 180.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–420. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Albi E, Viola Magni M. The role of intranuclear lipids. Biology of the Cell. 2004;96:657–667. doi: 10.1016/j.biolcel.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 182.Tamiya-Koizumi K. Nuclear lipid metabolism and signaling. J Biochem. 2002;132:13–22. doi: 10.1093/oxfordjournals.jbchem.a003190. [DOI] [PubMed] [Google Scholar]
- 183.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325:1254–1257. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Watanabe M, Kitano T, Kondo T, Yabu T, Taguchi Y, Tashima M, Umehara H, Domae N, Uchiyama T, Okazaki T. Increase of nuclear ceramide through caspase-3-dependent regulation of the “sphingomyelin cycle” in Fas-induced apoptosis. Cancer Res. 2004;64:1000–1007. doi: 10.1158/0008-5472.can-03-1383. [DOI] [PubMed] [Google Scholar]
- 185.Ledeen RW, Wu G. Nuclear sphingolipids: metabolism and signaling. J Lipid Res. 2008;49:1176–1186. doi: 10.1194/jlr.R800009-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ledeen RW, Wu G. Gangliosides of the nuclear membrane: a crucial locus of cytoprotective modulation. J Cell Biochem. 2006;97:893–903. doi: 10.1002/jcb.20731. [DOI] [PubMed] [Google Scholar]
- 187.Wu G, Xie X, Lu ZH, Ledeen RW. Sodium-calcium exchanger complexed with GM1 ganglioside in nuclear membrane transfers calcium from nucleoplasm to endoplasmic reticulum. Proc Natl Acad Sci U S A. 2009;106:10829–10834. doi: 10.1073/pnas.0903408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Albi E, Magni MP. Chromatin neutral sphingomyelinase and its role in hepatic regeneration. Biochem Biophys Res Commun. 1997;236:29–33. doi: 10.1006/bbrc.1997.6803. [DOI] [PubMed] [Google Scholar]
- 189.Neitcheva T, Peeva D. Phospholipid composition, phospholipase A2 and sphingomyelinase activities in rat liver nuclear membrane and matrix. Int J Biochem Cell Biol. 1995;27:995–1001. doi: 10.1016/1357-2725(95)00087-6. [DOI] [PubMed] [Google Scholar]
- 190.Tamiya-Koizumi K, Umekawa H, Yoshida S, Kojima K. Existence of Mg2+-dependent, neutral sphingomyelinase in nuclei of rat ascites hepatoma cells. J Biochem. 1989;106:593–598. doi: 10.1093/oxfordjournals.jbchem.a122901. [DOI] [PubMed] [Google Scholar]
- 191.Alessenko A, Chatterjee S. Neutral sphingomyelinase: localization in rat liver nuclei and involvement in regeneration/proliferation. Mol Cell Biochem. 1995;143:169–174. doi: 10.1007/BF01816950. [DOI] [PubMed] [Google Scholar]
- 192.Albi E, Magni MV. Sphingomyelin synthase in rat liver nuclear membrane and chromatin. FEBS Lett. 1999;460:369–372. doi: 10.1016/s0014-5793(99)01378-2. [DOI] [PubMed] [Google Scholar]
- 193.Kleuser B, Maceyka M, Milstien S, Spiegel S. Stimulation of nuclear sphingosine kinase activity by platelet-derived growth factor. FEBS Lett. 2001;503:85–90. doi: 10.1016/s0014-5793(01)02697-7. [DOI] [PubMed] [Google Scholar]
- 194.Shiraishi T, Imai S, Uda Y. The presence of ceramidase activity in liver nuclear membrane. Biol Pharm Bull. 2003;26:775–779. doi: 10.1248/bpb.26.775. [DOI] [PubMed] [Google Scholar]
- 195.Tsugane K, Tamiya-Koizumi K, Nagino M, Nimura Y, Yoshida S. A possible role of nuclear ceramide and sphingosine in hepatocyte apoptosis in rat liver. J Hepatol. 1999;31:8–17. doi: 10.1016/s0168-8278(99)80158-5. [DOI] [PubMed] [Google Scholar]
- 196.Ding G, Sonoda H, Yu H, Kajimoto T, Goparaju SK, Jahangeer S, Okada T, Nakamura S. Protein kinase D-mediated phosphorylation and nuclear export of sphingosine kinase 2. J Biol Chem. 2007;282:27493–27502. doi: 10.1074/jbc.M701641200. [DOI] [PubMed] [Google Scholar]


