Abstract
Here, we report a novel mechanism regulating migration of the anterior visceral endoderm (AVE) by BMP signaling through BMPRIA. In Bmpr1a-deficient (Bmpr-null) embryos, the AVE does not migrate at all. In embryos with an epiblast-specific deletion of Bmpr1a (Bmpr1anull/flox; Sox2Cre embryos), the AVE cells migrate randomly from the distal end of embryos, resulting in an expansion of the AVE. Dkk1, which is normally expressed in the anterior proximal visceral endoderm (PxVE), is downregulated in Bmpr-null embryos, whereas it is circumferentially expressed in Bmpr1anull/flox; Sox2Cre embryos at E5.75–6.5. These results demonstrate an association of the position of Dkk1 expressing cells with direction of the migration of AVE. In Bmpr1anull/flox; Sox2Cre embryos, a drastic decrease of WNT signaling is observed at E6.0. Addition of WNT3A to the culture of Bmpr1anull/flox; Sox2Cre embryos at E5.5 restores expression patterns of Dkk1 and Cer1. These data indicate that BMP signaling in the epiblast induces Wnt3 and Wnt3a expression to maintain WNT signaling in the VE, resulting in downregulation of Dkk1 to establish the anterior expression domain. Thus, our results suggest that BMP signaling regulates the expression patterns of Dkk1 for anterior migration of the AVE.
Keywords: BMP signaling, AVE, Dkk1, Wnt3, Wnt3a
The process by which the anterior-posterior (A-P) body axis is determined during early mouse embryogenesis is of great interest not only because this step is the first body plan of post-implantation mouse embryos, but also because of the complicated nature of the molecular mechanism involved in the development of the anterior visceral endoderm (AVE) (Beddington and Robertson, 1999; Lu et al., 2001; Rossant and Tam, 2004). The AVE is initially formed at the distal end of the visceral endoderm at E5.5 and migrates toward the anterior side of the embryo by E6.0, when the A-P axis becomes morphologically evident (Rivera-Perez et al., 2003; Srinivas et al., 2004). NODAL signaling plays important roles in the formation and migration of the AVE (Brennan et al., 2001; Chen et al., 2006; Ding et al., 1998; Norris et al., 2002; Yamamoto et al., 2004). WNT signaling also affects the migration of the AVE; to enhance or lower the WNT signaling asymmetrically induces unilateral migration of the AVE (Kimura-Yoshida et al., 2005). However, there are many unanswered questions including whether other signaling pathway(s) function in the formation and migration of the AVE and how such pathways relate to NODAL signaling or WNT signaling in the process. For example, Bone Morphogenetic Protein (BMP) signaling is required for the maintenance of Nodal expression at E5.5 in the visceral endoderm (VE) (Di-Gregorio et al., 2007). BMP signaling is also required for the expression of Wnt3 (Ben-Haim et al., 2006), which elicits the majority of the WNT signaling that plays a critical role in migration of the AVE (Kimura-Yoshida et al., 2005).
BMPs comprise a large subgroup within the TGF-beta superfamily. BMP signaling is involved in a variety of functions during developmental process (Kishigami and Mishina, 2005; Zhao, 2003). Bmpr1a, which encodes a type I receptor for BMP2 and BMP4, is expressed in all tissues derived from the inner cell mass and trophoblast (Dewulf et al., 1995) and Bmp4 shows restricted expression in the extraembryonic ectoderm (Lawson et al., 1999). Bmpr1a and Bmp4 constitute the major genes that define BMP signaling in early post-implantation development of mice. Mosaic inactivation of Bmpr1a in the epiblast revealed that Bmpr1a is required for proper recruitment of epiblast cells during gastrulation (Miura et al., 2006). Deficiency of Bmpr1a in the epiblast may also affects VE development (Davis et al., 2004). However, if and how BMP signaling participates in these critical functions during the development of the AVE is not well understood.
In this study, we investigated Bmpr1a deficient embryos (Bmpr-null embryos) and embryos that lack Bmpr1a in an epiblast-specific manner (Bmpr1anull/flox; Sox2Cre embryos) for a potential involvement of BMP signaling in the AVE development. The inactivation of Bmpr1a in the epiblast was carried out by recombination of a floxed allele for Bmpr1a (Mishina et al., 2002) with Cre recombinase expressed in Sox2-Cre transgenic mice (Hayashi et al., 2002). Sox2-Cre drives more efficient Cre-mediated recombination from earlier stage of mouse development compared to Mox2-Cre (Hayashi et al., 2002). We found that Bmpr-null embryos show no migration of the AVE, but Bmpr1anull/flox; Sox2Cre embryos exhibit random migration of the AVE. Bmpr1a in the VE is required for Dkk1 expression in the proximal VE (PxVE). On the other hand, BMP signaling in the epiblast positively regulates the expression of Wnt3 and Wnt3a in the presumptive posterior epiblast, which leads to a downregulation of Dkk1 in the overlying VE and the migration of AVE cells towards Dkk1-expressing cells in the presumptive anterior proximal VE.
Results
The AVE does not migrate in Bmpr-null embryos
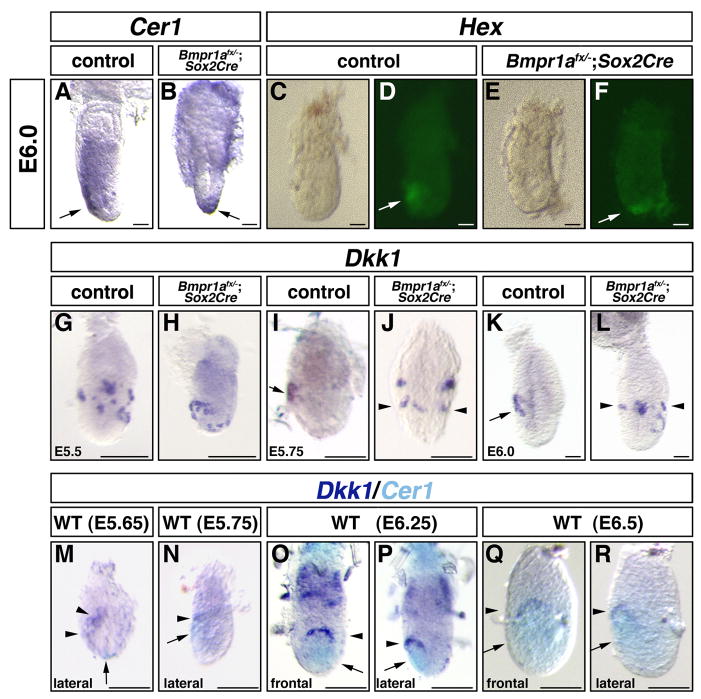
Establishment of the A-P axis of the mouse embryo becomes apparent by the migration of the AVE that starts around E5.75 (Rivera-Perez et al., 2003; Srinivas et al., 2004). Although the AVE was formed in Bmpr-null embryos as indicated by the expression of Hex and Cer1, migration of the AVE was not observed in Bmpr-null embryos (Fig. 1A–D) (n=6/6 for Hex, 5/5 for Cer1). Dkk1, expressed in a circular pattern in the PxVE in control embryos, was not expressed in Bmpr-null embryos at E5.5, indicating that Bmpr1a is required for its expression (Fig. 1E, F) (n=5/5). These data indicate that Bmpr1a is required for the migration of the AVE.
Figure 1.
The AVE does not migrate in Bmpr-null embryos
Whole mount in situ analysis for Cer1 (A, B) or Dkk1 (E, F) and the expression of Hex-GFP (C, D). Cer1 or Hex-GFP was expressed in the AVE of control embryos (A, C arrow). In Bmpr-null embryos, Cer1 and Hex expression is localized to the distal tip (B, D, arrow). Dkk1 is expressed in the PxVE at E5.5 control embryos (E, arrows), but not in Bmpr-null embryos (F). Bars=100 μm.
A-P axis defect is observed in Bmpr1anull/flox; Sox2Cre embryos
To address the role of BMP signaling in the epiblast for migration of the AVE, we next analyzed Bmpr1anull/flox; Sox2Cre embryos that lack Bmpr1a in an epiblast-specific manner (Di-Gregorio et al., 2007). Unlike Bmpr-null embryos, Bmpr1anull/flox; Sox2Cre embryos initiate gastrulation to form germ layers (Di-Gregorio et al., 2007) (Fig. 2A). Among forty Bmpr1anull/flox; Sox2Cre embryos examined at E8.5, about half of them showed apparent A-P axis evidenced by the expression of Otx2 and histological analyses (Fig. 2B and data not shown). These embryos were morphologically similar to Bmpr-MORE embryos (mosaic inactivation of Bmpr1a in the epiblast) that we previously described (Miura et al., 2006). The remaining Bmpr1anull/flox; Sox2Cre embryos had a poorly extended body axis (Fig. 2C). Such variability of the phenotypes may be due to a mixed background of the floxed allele for Bmpr1a. These embryos expressed germ layer markers such as Mox1, Foxa2 and Otx2 (data not shown), suggesting that all three germ layers are formed in Bmpr1anull/flox; Sox2Cre embryos. Expressions of posterior markers, Brachyury, Cripto, and Lefty2, also showed two distinct patterns during gastrulation (Fig. 2D–L). Comparable expression patterns with control embryos were observed in half of the Bmpr1anull/flox; Sox2Cre embryos (Fig. 2E, H, K, 9/17, 8/17, 4/8, respectively). However, in the rest of the Bmpr1anull/flox; Sox2Cre embryos, posterior markers that are normally detected at the primitive streak were expressed at the proximal potion of the epiblast (Fig. 2F, I, L, 8/17, 9/17, 4/8, respectively). Di-Gregorio et al. reported downregulation of Cripto in the Bmpr1anull/flox; Sox2Cre embryos (3/9) (Di-Gregorio et al., 2007), but all of the mutant embryos examined here showed comparable levels of expression despite the differences of expression domains Fig. 2H–I, 17/17). This may be due to the differences of genetic background, because we maintained the floxed allele for Bmpr1a in a mixed background. These results indicate that there was an A-P axis defect of the epiblast development in half of the Bmpr1anull/flox; Sox2Cre embryos during gastrulation, leading to poor extension of the body axis by E8.5 (Fig. 2C).
Figure 2.
Bmpr1anull/flox; Sox2Cre embryos develop defects in A-P axis development (A–C) Whole view at E8.5. A-P shows anterior and posterior side of the embryo. (A) Control embryo. (B) About half (22/40) of Bmpr1anull/flox; Sox2Cre embryos developed an apparent A-P axis (left panel, lateral view, arrow indicates a head) and expanded somites (data not shown). A Bmpr1anull/flox; Sox2Cre embryo in the right panel (dorsal view) shows the expression of Otx2, a forebrain marker, indicating anterior development (arrow). (C) The rest of the Bmpr1anull/flox; Sox2Cre embryos showed less extended body axis. Bars=250 μm. (D–L) Whole mount in situ analyses of posterior markers. Brachyury, Cripto and Lefty2 are mesoderm markers at E6.5. In control embryos, these markers were observed at the primitive streak (D, G, J). Nine out of seventeen Bmpr1anull/flox; Sox2Cre embryos expressed Brachyury normally (E), but eight out of seventeen Bmpr1anull/flox; Sox2Cre embryos expressed Brachyury proximally (F). Four out of eight Bmpr1anull/flox; Sox2Cre embryos expressed Cripto normally (H), but four out of eight Bmpr1anull/flox; Sox2Cre embryos expressed Cripto proximally (I). Two out of four Bmpr1anull/flox; Sox2Cre embryos expressed Lefy2 normally (K), but two out of four Bmpr1anull/flox; Sox2Cre embryos expressed Lefy2 proximally (L). Bars=100 μm.
Next, we examined expressions of AVE markers such as Cer1, Hex at E6.0, since the AVE normally migrates to the anterior side of the embryo by this stage, as well as Dkk1 expression. Expression patterns of Cer1 and Hex showed that the AVE does not migrate in half of the Bmpr1anull/flox; Sox2Cre embryos by E6.0 (3/5 for Cer1 and 7/15 for Hex) (Fig. 3B, F). Dkk1 was expressed in the anterior PxVE of control embryos at E6.0 (Fig. 3K). In Bmpr1anull/flox; Sox2Cre embryos, Dkk1 was expressed circumferentially in the PxVE (3/6) (Fig. 3L). During E5.5 and 5.75, the Dkk1 expression pattern shows a dramatic change in normal development (Kimura-Yoshida et al., 2005). In control embryos, Dkk1 expression was observed in a circular pattern in the PxVE at E5.5 (3/3) (Fig. 3G). However, Dkk1 was strongly downregulated from most of the PxVE, leaving a small expression domain at the most anterior part of the PxVE at E5.75 (7/7) (Fig. 3I). In contrast, Dkk1 was still expressed circularly in the PxVE in Bmpr1anull/flox; Sox2Cre embryos (2/4 at E5.5, Fig. 3H, 4/8 at E5.75, Fig. 3J), suggesting that downregulation of the Dkk1 did not occur at E5.75.
Figure 3.
The expression of Dkk1 is not downregulated in Bmpr1anull/flox; Sox2Cre embryos (A–B) Whole mount in situ analysis of Cer1 at E6.0. (C–F) Whole mount observation of Hex-GFP expression at E6.0. Cer1 (A, arrow) and Hex (D, arrow) are expressed in the AVE at E6.0 in control embryos. In Bmpr1anull/flox; Sox2Cre embryos, the expression of Cer1 or Hex showed that the AVE had not migrated at E6.0 (arrow in B, F). (G–L) Whole mount in situ analyses of Dkk1. Dkk1 is expressed in a circular pattern in the PxVE of E5.5 control embryos (G). In Bmpr1anull/flox; Sox2Cre embryos, the expression of Dkk1 is observed circumferentially (H). By E5.75, Dkk1 expression is strongly downregulated but remains in the most anterior part of the PxVE (I, arrow). In Bmpr1anull/flox; Sox2Cre embryos, the expression of Dkk1 persists circumferentially in the PxVE (J, arrowheads). At E6.0, Dkk1 is expressed in the anterior PxVE (K, arrow). In Bmpr1anull/flox; Sox2Cre embryos, the expression of Dkk1 was observed circumferentially at E6.0 (L, arrowheads). (M–R) Double in situ analysis for Dkk1 (purple, arrowhead) and Cer1 (light blue, arrow) at E5.65 (M), E5.75 (N), E6.25 (O, P) and E6.5 (Q, R) of CD1 mouse embryos. Note that anterior shift of the expression domain of Dkk1 is observed before anterior migration of the Cer1 expression domain. Bars=50 μm in A–F, K, L, 100 μm in G–J, M, 150 μm in N and 200 μm in O–R.
We further compared the changes in expression domains of Dkk1 along with migration of the AVE. At E5.65, 3 embryos out of 4 showed anteriorly shifted expression of Dkk1 (Fig. 3M). In these embryos, the AVE marked by Cer1 was localized at the distal tip of the embryos (Fig. 3M, 2/3). At E5.75, the embryos showed anterior movement of the AVE (3/3) and such embryos also showed anteriorly shifted expression of Dkk1 (Fig. 3N). At E6.25 and E6.5, Dkk1 was expressed as a horseshoe shape in a domain adjacent to upper part of the AVE of control embryos (Fig. 3O–R). These results indicate that anterior-shift of the expression domains of Dkk1 preceded migration of the AVE.
Expansion of the AVE caused by ectopic migration of the AVE cells in Bmpr1anull/flox; Sox2Cre embryos
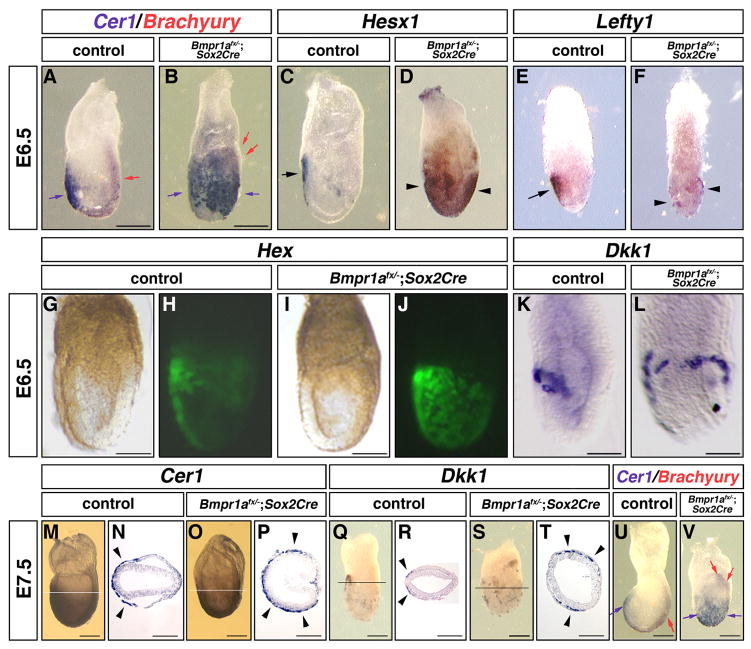
By E6.5, the AVE further migrated anteriorly in control embryos (Fig. 4A, C, E, H). However, Cer1, Hesx1, Lefty1 and Hex expression domains were expanded proximally and laterally in half of the Bmpr1anull/flox; Sox2Cre embryos (Cer1 3/6, Hesx1 2/4, Lefty1 2/4, Hex 12/23) (Fig. 4B, D, F, J). Similarly to E6.0, Dkk1 was expressed circumferentially in the PxVE in Bmpr1anull/flox; Sox2Cre embryos (4/7) (Fig. 4L). Expansions of Cer1 and Dkk1 expression were also observed at E7.5 (Cer1 8/15, Dkk1 2/4) (Fig. 4P, T). Proximal expression of posterior markers was observed only in those embryos that showed expansion of the Cer1 expression domain among Bmpr1anull/flox; Sox2Cre embryos (E6.5, 2/2, E7.5, 6/6) (Fig. 4B, V). Bmpr1anull/flox; Sox2Cre embryos that showed normal expression of Cer1 showed posterior expression of the posterior markers like control embryos (not shown). These results indicate that expansion of the AVE caused A-P axis defect of the epiblast in Bmpr1anull/flox; Sox2Cre embryos.
Figure 4.
The AVE expands in Bmpr1anull/flox; Sox2Cre embryos
Whole mount in situ analysis (A–F, K–V) and the expression of Hex-GFP (G–J). The expression of Cer1, Hesx1, Lefty1 and Hex-GFP indicated expansion of the AVE occurred in Bmpr1anull/flox; Sox2Cre embryos (B, D, F, J). The expression of Dkk1 was observed circumferentially in Bmpr1anull/flox; Sox2Cre embryos (L). Horizontal lines in M, O, Q and S indicate approximate position of sections in N, P, R, T. Cer1 or Dkk1 expression was observed only in the anterior side of the VE of control embryos (arrowheads, N, R). Expanded expression of Cer1 or Dkk1 was observed in the VE of Bmpr1anull/flox; Sox2Cre embryos (arrowheads, P, T). (U, V) Double in situ analysis for Brachyury (red) and Cer1 (blue). Blue arrow is Cer1 expression at the AVE and red arrow is Brachyury expression at the primitive streak (U). Blue arrows indicate expanded expression of Cer1. Red arrows indicate proximal expression of Brachyury (V).
Bars=100 μm in A–L, 50 μm in M–V.
Expansion of the AVE can be due to aberrant expression of the AVE markers in the posterior side of the VE or ectopic migration of the AVE cells. To distinguish these two possibilities, lineage analysis of the AVE cells of both control and Bmpr1anull/flox; Sox2Cre embryos was performed. The distal VE of control and Bmpr1anull/flox; Sox2Cre embryos, which harbored Hex-GFP transgene, were labeled with DiI at E5.5 and cultured with DR50. In control embryos, DiI labeled cells were observed unilaterally after 16 and 40-hour cultures (Fig. 5B, D, 4/4). Hex expressing cells were found in a similar location to that of DiI labeled cells, indicating that the AVE migrated anteriorly (4/4) (Fig. 5C, E–F) (Thomas et al., 1998). In Bmpr1anull/flox; Sox2Cre embryos with an expanded Hex expression domain (Fig. 5I, K) (n=3), DiI labeled cells, which were initially located at the distal end of the VE (Fig. 5G), started to migrate randomly at 16 hours (Fig. 5H) and were found scattered in embryonic VE at 40 hours (Fig. 5J). Hex expressing cells occupied a similar domain (Fig. 5I, K–L, 3/3). These results indicate that the AVE migrated randomly in the mutant embryos. Bmpr1anull/flox; Sox2Cre embryos with a normal Hex expression domain (n=2) showed anterior movement of DiI labeled cells like the control embryos (not shown). Thus, BMP signaling in the epiblast is required for proper and restricted migration of the cells in the AVE.
Figure 5.

The AVE cells migrate randomly to expand the AVE in Bmpr1anull/flox; Sox2Cre embryos
DiI fluorescence (A–B, D, G–H, J), Hex-GFP expression (C, E, I, K) and merged image (F, L). The distal VE of control (n=4) and Bmpr1anull/flox; Sox2Cre embryos (n=5) at E5.5 were successfully labeled with DiI (A, G, arrowhead) and cultured with DR50 for approximately 16 hours (B–C, H–I) and 40 hours (D–F, J–L). In control embryos, anterior migration of the AVE cells was observed at 16 and 40 hours after culture (C, E, arrowhead) and the Hex-GFP expressing cells show co-localization of DiI labeled cells (B, D, F, arrowhead). In three Bmpr1anull/flox; Sox2Cre embryos out of five, a subtle, yet random movement of the DiI labeled cells and the AVE were observed at 16 hours (H–I). At 40 hours, the AVE cells migrate randomly from distal end of the VE and were found scattered in embryonic VE (J, arrowheads). Hex-expressing cells (K, arrowheads) show co-localization with DiI labeled cells (L, arrowheads). Note that the size of the embryonic portion of cultured embryos tends to be smaller than the extraembryonic portion when E5.5 embryos are cultured to gastrulation stage (Miura and Mishina, 2003). Bars=100 μm in A–L.
WNT signaling is decreased in Bmpr1anull/flox; Sox2Cre embryos
In Bmpr1anull/flox; Sox2Cre embryos, Bmpr1a is disrupted only in the epiblast, but they show abnormal expression of Dkk1 in the PxVE. These facts prompted us to hypothesize that secreted factors whose expression is regulated by BMP signaling could be directly involved in the regulation of Dkk1 expression in the PxVE. We found that Wnt3 and Wnt3a were dramatically reduced in half of Bmpr1anull/flox; Sox2Cre embryos at E5.5 (6/11) or E5.75 (2/4), respectively (Fig. 6B, D). To determine the status of WNT signaling, we first observed the expression of Axin2, one of target genes of WNT signaling, in E6.0 embryos (Lustig et al., 2002). Axin2 expression was largely decreased in half of Bmpr1anull/flox; Sox2Cre embryos (5/10) (Fig. 6F). When cells received WNT signaling, beta-catenin is activated by inhibition of its phosphorylation (active beta-catenin). Presence of active beta-catenin was examined using an antibody recognizing only the non-phosphorylated (active) form of beta-catenin in E6.75 embryos (Kimura-Yoshida et al., 2005). In control embryos, active beta-catenin was observed in the posterior side of the embryo, but it was downregulated in the AVE (Fig. 6G). The levels of active beta-catenin were drastically decreased in half of Bmpr1anull/flox; Sox2Cre embryos (4/7) (Fig. 6H).
Figure 6.
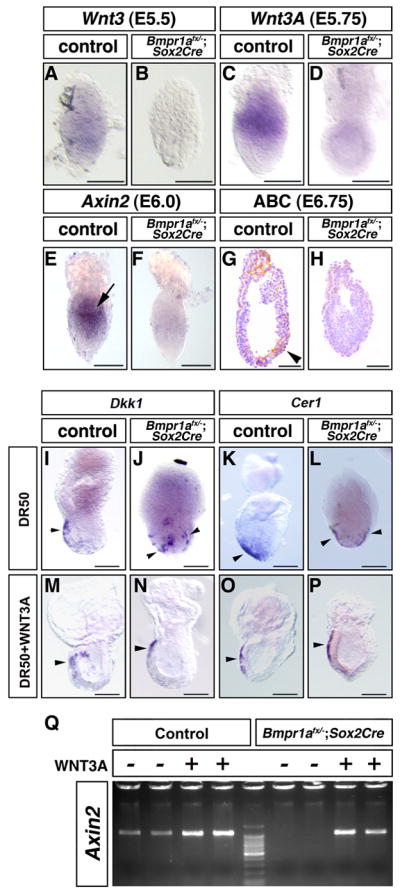
WNT signaling is required for the anteriorly restricted expression of Dkk1 and AVE migration in Bmpr1anull/flox; Sox2Cre embryos
(A–F, I–P) Whole mount in situ analysis. The expression of Wnt3 in E5.5 embryos (A) was lost in six out of eleven Bmpr1anull/flox; Sox2Cre embryos at E5.5 (B). The expression of Wnt3a in E5.75 embryos (C) was lost in four out of eight Bmpr1anull/flox; Sox2Cre embryos at E5.75 (D).
Expression for Axin2 was observed in the proximal-posterior embryonic portion of E6.0 embryos (E, arrow). Axin2 expression was significantly reduced in five out of ten Bmpr1anull/flox; Sox2Cre embryos at E6.0 (F). (G, H) Immunohistochemistry for the non-phosphorylated form of beta-catenin (active beta-catenin). Active beta-catenin was detected as brown staining in the posterior side of the control E6.75 embryo (G, arrow), including the VE, but not in the AVE. Signal for active beta-catenin was strongly down-regulated in Bmpr1anull/flox; Sox2Cre embryos (4/10) (H). Control or Bmpr1anull/flox; Sox2Cre embryos were cultured with DR50 alone or DR50 supplemented with WNT3A protein (50 ng/ml) for 40–48hrs. Dkk1 was expressed anteriorly in control embryos (I, M, arrowhead) and in Bmpr1anull/flox; Sox2Cre embryos treated with WNT3A (N, arrowhead), but half of Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 showed circumferential expression (J, arrowheads). The AVE migrated anteriorly in control embryos (K, O, arrowhead) and in Bmpr1anull/flox; Sox2Cre embryos treated with WNT3A (P, arrowhead), but half of Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 showed bilateral migration of the AVE cells (L, arrowheads). Embryo culture was performed on three different occasions and embryos were pooled for analysis. (Q) Semi-quantitative RT-PCR analysis for Axin2 expression of cultured embryos. Total RNA was extracted from individual embryo. RT-PCR was performed twice and a representative result was shown. Bars=100 μm.
Increased Wnt signaling rescues anteriorly restricted Dkk1 expression and AVE migration in Bmpr1anull/flox; Sox2Cre embryos
We next tested if addition of WNT protein rescues observed phenotypes in Bmpr1anull/flox; Sox2Cre embryos. Control or Bmpr1anull/flox; Sox2Cre embryos at E5.5 were cultured for approximately 48 hours in DR50 alone or DR50 supplemented with WNT3A protein. Since 50 ng/ml of WNT3A protein shows maximal ability to differentiate osteoblastic cells (Fischer et al., 2002), we used this concentration throughout this experiment. In both control and Bmpr1anull/flox; Sox2Cre embryos, about 70% of embryos grew well regardless of being cultured with or without WNT3A protein and these embryos were subjected to further analysis. First, cultured embryos were tested for Dkk1 expression. In control embryos cultured with DR50 alone, Dkk1 was anteriorly expressed (8/8) (Fig. 6I). However, in 50 % of Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 alone, Dkk1 was expressed circumferentially (4/8) (Fig. 6J, Supplement Fig. 1). In cultured embryos with WNT3A protein, Axin2 expression was augmented in both control and Bmpr1anull/flox; Sox2Cre embryos showing that WNT signaling was increased (Fig. 6Q). Control embryos cultured with DR50 supplemented with WNT3A protein also expressed Dkk1 anteriorly (13/13) (Fig. 6M). When WNT3A protein was added to the culture of Bmpr1anull/flox; Sox2Cre embryos, Dkk1 was expressed anteriorly in 10/11 Bmpr1anull/flox; Sox2Cre embryos (Fig. 6N, Supplement Fig. 1). Cultured embryos were also tested for Cer1 expression. Expansion of the AVE was observed in 50% of cultured Bmpr1anull/flox; Sox2Cre embryos with DR50 alone (2/4) (Fig. 6L). However when cultured with WNT3A protein, 5/5 Bmpr1anull/flox; Sox2Cre embryos displayed anterior migration of the AVE (Fig. 6P, Supplement Fig. 2). These results indicate that increased WNT signaling can restore anteriorly restricted expression of Dkk1 and migration of the AVE in Bmpr1anull/flox; Sox2Cre embryos.
Discussion
In this paper, we showed that the expression of Dkk1 is significantly downregulated and the AVE does not migrate in Bmpr-null embryos. In contrast, Dkk1 is circumferentially expressed in the PxVE and the AVE migrates proximally and bilaterally in Bmpr1anull/flox; Sox2Cre embryos that lost BMPRIA signaling in an epiblast-specific manner. Wnt3 and Wnt3a expression is strongly downregulated in Bmpr1anull/flox; Sox2Cre embryos and exogenously added WNT3A protein restores the normal expressions of Dkk1 and Cer1 in Bmpr1anull/flox; Sox2Cre embryos.
Here, we present a model describing the function of BMP signaling in regulating AVE migration (Fig. 7). In wild type embryos (WT), BMP signaling in the VE mediated by BMPRIA induces Dkk1 expression in the PxVE circumferentially. On the other hand, BMP signaling in the epiblast mediated by BMPRIA induces Wnt3 and Wnt3a expression in the epiblast to inhibit Dkk1 expression in the majority of the PxVE, except for the most anterior domain. The resulting anterior expression of the Dkk1 induces anterior migration of the AVE. Thus, Dkk1 is expressed in a domain adjacent to upper part of the AVE in wild type embryos. In Bmpr-null embryos (Bmpr-null), Dkk1expression is downregulated in the PxVE, resulting in lack of AVE migration. In Bmpr1anull/flox; Sox2Cre embryos (Bmpr1anull/flox; Sox2Cre), Wnt3 and Wnt3a are both downregulated and circumferential Dkk1 expression persists in the PxVE. Thus, the AVE cells migrate randomly toward PxVE resulting in an expansion of the AVE.
Figure 7.
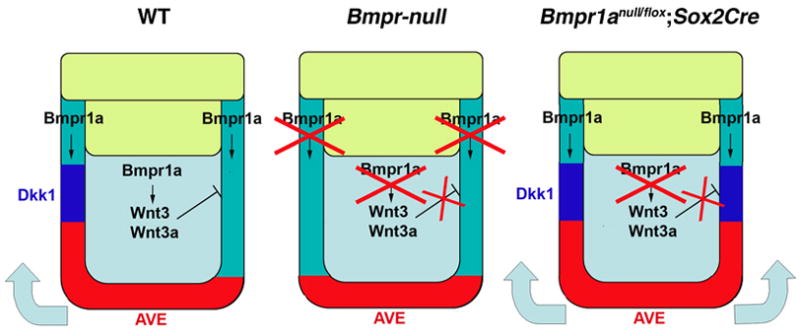
Models of how BMP signaling through BMPR1A regulates anterior movement of the AVE through Dkk1 expression
Our model describing the function of BMP signaling through BMPR1A in the early mouse embryo in regulating AVE migration. (WT) BMP signaling in the VE induces Dkk1 expression in the PxVE circumferentially. On the other hand, BMP signaling in the epiblast induces Wnt3 and Wnt3a expression in the epiblast to inhibit Dkk1 expression in majority of the PxVE except for a most anterior domain. The resulting anterior expression of Dkk1 induces anterior migration of the AVE. (Bmpr-null) In Bmpr-null embryos, Dkk1expression is downregulated (or not induced) in PxVE, resulting in lack of AVE migration. (Bmpr1anull/flox; Sox2Cre) In the embryos lacking Bmpr1a in an epiblast-specific manner, Wnt3 and Wnt3a are both downregulated and circumferential Dkk1 expression remains in the PxVE. Thus, the AVE cells migrate bilaterally toward PxVE. In both cases, embryos fail to establish a normal A-P axis due to the abnormal migration of the AVE (no migration in Bmpr-null embryos, random migration in Bmpr1anull/flox; Sox2Cre embryos).
Similar to Bmpr-null embryos, migration of the AVE was not observed in half of the Bmpr1anull/flox; Sox2Cre embryos by E6.0. However, different from Bmpr-null embryos, Dkk1 is circumferentially expressed in the PxVE. Then by E6.5, we observed abnormal migration of AVE cells towards the proximal portion of the embryo. In normal embryos, we showed that an anterior shift of the Dkk1 expression domain precedes migration of the AVE (Fig. 3). All embryos that showed anterior movement of the AVE also showed anterior-restricted expression of Dkk1. Thus, it appears that AVE cells are attracted by DKK1 protein or regions that produce DKK1. This is in good agreement with the recent report that the AVE tends to migrate toward the side of the embryo where DKK1 coated beads are attached (Kimura-Yoshida et al., 2005). The delayed migration of the AVE observed at E6.0 in Bmpr1anull/flox; Sox2Cre embryos might seem to be inconsistent with our hypothesis above, considering that Dkk1 is already expressed circumferentially in the PxVE at E6.0. A possibility is that the amount of DKK1 protein is less than what is required to attract the AVE cells when Dkk1 expression domain is not restricted in the most anterior part of the PxVE. At E6.5, more numbers of PxVE cells show Dkk1 expression to induce migration of the AVE in Bmpr1anull/flox; Sox2Cre embryos. Whether DKK1 protein directly attracts AVE cells or different levels of canonical WNT signaling control this process needs to be addressed in the future experiments.
Dkk1 is normally expressed circumferentially in the PxVE at E5.25–E5.5 and then at E5.75, its expression is largely downregulated and only observed in a small domain in the anterior PxVE (Fig. 1, 3, 4) (Kimura-Yoshida et al., 2005). In Bmpr1anull/flox; Sox2Cre embryos, Dkk1 is still expressed circumferentially in the PxVE at E5.75 and thereafter. This indicates that BMP signaling through BMPRIA is required for the downregulation of Dkk1 expression in the posterior PxVE. Because Bmpr1a is disrupted only in the epiblast, a secreted factor(s) that is a downstream target of BMP signaling in the epiblast is a suitable candidate that might directly regulate Dkk1 expression in the PxVE. Wnt3 is reported as a downstream target of BMP signaling during early mouse development (Ben-Haim et al., 2006). We showed that Wnt3 and Wnt3a expression was strongly downregulated in Bmpr1anull/flox; Sox2Cre embryos at E5.5 and E5.75, respectively. Further, we observed that addition of WNT3A protein to E5.5 Bmpr1anull/flox; Sox2Cre embryos recovered anterior expression of Dkk1 and migration of the AVE. Therefore, we hypothesize that in normal development, WNT signaling is required to downregulate Dkk1 expression in the posterior PxVE by E5.75 and that this is a prerequisite for anterior migration of the AVE. This model is in good agreement with the fact that strong WNT signaling is detected in the VE of normal embryos at E5.5 and later (Kimura-Yoshida et al., 2005). However, as Dkk1 mutant embryos develop the AVE normally (Mukhopadhyay et al., 2001), we acknowledge that there should be other molecules, which function similar to Dkk1, expressing in the anterior PxVE at E5.75 to 6.0.
A recent report suggests that Wnt3 is required to maintain Dkk1 expression in the anterior PxVE. In Wnt3−/− embryos, expression of Dkk1 is not detected when examined at E6.5–E7.25 (Lewis et al., 2008). Wnt3 seems to directly regulate Dkk1 expression since the Dkk1 promoter contains a T cell factor site through which WNT signaling up-regulates Dkk1 expression in vitro (Niida et al., 2004). However, in Bmpr1anull/flox; Sox2Cre embryos, despite the decreased WNT signaling at E5.5–6.0, Dkk1 is expressed at comparable levels to those of controls. One possible explanation for this discrepancy is that initiation of Dkk1 expression at E5.5 (or earlier) is Wnt-independent or requires a very limited amount of WNT proteins. It is also possible that Bmpr1anull/flox; Sox2Cre embryos may produce levels of WNT signaling that are not detectable, but are enough to induce Dkk1. Dkk1 expression in the Wnt3 null embryos at E5.5 is not reported, but these experiments should shed light on this matter.
It is noteworthy that Dkk1 expression is still maintained in the anterior PxVE after shifting the expression of Wnt3 to the posterior side of the embryo at E5.75–6.0 (Rivera-Perez and Magnuson, 2005). Similarly, Dkk1 is expressed in the anterior PxVE during E6.5–E7.5, when WNT signaling is not observed in the anterior side of the embryo (Kimura-Yoshida et al., 2005). Together these observations suggest that WNT signaling is not required (or a very limited amount is required) to maintain Dkk1 expression at E5.75 and thereafter, and this may explain the discrepancy of Dkk1 expression despite undetectable levels of WNT signaling found in the VE of Bmpr1anull/flox; Sox2Cre embryos. As shown in Fig. 1, Dkk1 is not expressed in the VE of Bmpr1a null embryos. Because Bmpr1a in the VE of Bmpr1a fx/-; Sox2-Cre embryos is functional, it is an interesting possibility that BMP signaling in the VE is involved in induction and/or maintenance of Dkk1 expression in the VE.
Our results strongly suggest that Wnt3 and Wnt3a contribute to the anterior restriction of the expression of Dkk1 before anterior migration of the AVE. Then, how Dkk1 expression is maintained in the anterior PxVE? One possibility is that there is an unknown factor that positively regulates Dkk1 expression in the anterior PxVE at E5.75 and thereafter. The positive regulation of Dkk1 expression in the anterior PxVE probably overcomes the inhibitory effect exerted by the expression of Wnt3 and Wnt3a or exogenous WNT3A protein on Dkk1 expression. Although Otx2 induces Dkk1 expression, its expression is not limited to anterior side of the embryo in the pre-streak stage. Therefore, Otx2 alone may not be a regulator for the anterior shift of Dkk1 expression. The identification of such regulator(s) of Dkk1 expression would be a great success to solve one of the most challenging questions in developmental biology, the mechanism of AVE movement.
Material and Methods
Mouse
Mouse strains and genotyping were described elsewhere (Hayashi et al., 2002; Mishina et al., 2002; Mishina et al., 1995; Rodriguez et al., 2001). Floxed allele for Bmpr1a was used as a mixed background of 129SV, C57BL/6 and Swiss strains. Timed mating was set up between males heterozygous null for Bmpr1a carrying Sox2-Cre and females homozygous for the floxed allele for Bmpr1a. Compound homozygous females for the floxed allele for Bmpr1a and Hex-GFP transgene were used for some of the experiments. Embryos were genotyped for the presence of the null allele and Sox2-Cre. Cre-dependent deletion of Bmpr1a was confirmed by PCR using appropriate primers (Mishina et al., 2002). All mouse experiments were performed in accordance with NIEHS/NIH guidelines covering the humane care and use of animals in research.
Whole mount in situ hybridization
Whole mount in situ hybridization was performed as described previously (Belo et al., 1997). Probes used were Otx2 (Ang et al., 1996), Brachyury (Wilkinson et al., 1990), Cripto (Ding et al., 1998), Lefty2 (Meno et al., 1999), Cer1 (Shawlot et al., 1998), Dkk1 (Glinka et al., 1998), Wnt3 (Liu et al., 1999), Wnt3a (Yamaguchi et al., 1999) and Axin2 (Lustig et al., 2002).
Immunohistochemistry
E6.75 embryos were fixed in 4% paraformaldehyde, dehydrated through graded alcohol and embedded in paraffin. Sections (5 μm) were deparaffinized, and stained with biotinylated anti-active beta-catenin antibody (Millipore, ×100 dilution) using Mouse on Mouse Biotinylation Double Stain kit (Biocare) according to manufacture’s protocols.
Embryo culture and cell lineage analysis
Embryos were harvested in dissecting medium (Miura and Mishina, 2003) and cultured in DMEM with 50% rat serum (DR50) or DR50 supplied with WNT3A (R&D) at a concentration of 50 ng/ml, respectively in a 4-well dish (Nunc) under 5% CO2 at 37 °C. Cell lineage analysis was done as described before (Wilson and Beddington 1996). Briefly, 0.5% stock solution of 1,1′-dioctadecyl-3,3,3′,3′-tetramethy-indocarbocyanine perchlorate (DiI) (Molecular Probes, Eugene, OR) in 100% ethanol was diluted tenfold with 0.3M sucrose before use. Then, 0.05% DiI solution was introduced into a 5 μm diameter glass needle. The needle was attached to the distal VE of the E5.5 embryo and DiI was released to label the tissue. Then, these embryos were cultured with DR50 in a 4-well set (Nunc) in a 5% CO2 incubator at 37°C. After culture, embryos were placed in phosphate buffered saline (PBS) and observed with an inverted microscope (Leica) under light or Ultra Violet and pictured with appropriate filters.
RT-PCR analysis
Total RNA was extracted from each cultured embryo using PicoPure RNA isolation kit (Arkus). Total RNA harvested was measured with Nanodrop 1000 (ThemoScientific) and the same amount of total RNA was used for each RT-PCR reaction. RT-PCR was performed using One step RT-PCR kit (Qiagen) according to manufacture’s protocol. Axin2 primers are Axin2F (5′-ACCAGCACCACCACCATCAGCAGT-3′) and Axin2R (5′-ACTGTCTCGTCGTCCCAGATCTCC-3′) that yield 1221bp product.
Supplementary Material
Supplement figure 1. Increased WNT signaling rescues anterior Dkk1 expression in Bmpr1anull/flox; Sox2Cre embryos (Whole mount in situ for Dkk1).
(A, B) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50. Four Bmpr1anull/flox; Sox2Cre embryos showing circumferential Dkk1 expression (A). Four Bmpr1anull/flox; Sox2Cre embryos showing unilateral Dkk1 expression (B). One Bmpr1anull/flox; Sox2Cre embryo shown as the most left embryos in (A) is also shown in Fig. 5(5J) for a typical example.
(C) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 containing 50ng/ml of WNT3A. Ten Bmpr1anull/flox; Sox2Cre embryos showed unilateral Dkk1 expression (arrowheads). One Bmpr1anull/flox; Sox2Cre embryo (asterisk) showed ectopic expression of Dkk1. One Bmpr1anull/flox; Sox2Cre embryo shown at the upper most right is also shown in Fig. 5(5N) for a typical example. Bars=100 μm.
Supplement figure 2. Increased WNT signaling rescues anterior AVE migration of Bmpr1anull/flox; Sox2Cre embryos (Whole mount in situ for Cer1).
(A) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50. Two Bmpr1anull/flox; Sox2Cre embryos showing circumferential Cer1 expression (arrows). Two Bmpr1anull/flox; Sox2Cre embryos showing unilateral Cer1 expression (arrow).
(B) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 containing 50ng/ml of WNT3A. Five Bmpr1anull/flox; Sox2Cre embryos showed unilateral Cer1 expression (arrow).
Embryos shown at the most left panels are also shown in Fig. 5(5L, 5P) as a typical example. Bars=100 μm.
Acknowledgments
We thank Drs. T. A. Rodriguez for Hex-GFP mice; A. McMahon for Sox2-Cre mice, B.G. Herrmann, B. Shawlot, M. Shen, J. Klingensmith, H. Sasaki, Y. Saga and M. Kuehn for in situ probes. We also thank Drs. M. Yamamoto, H. Hamada, and T. A. Rodriguez for exchanging unpublished results, Ucchii Uchimura, Tonya Simmons, and the Knock Out Core at NIEHS for mouse activity, L. E. Davis and I. Nwosu for technical assistance, Y. P. Young, A. Shimono, C. Kimura-Yoshida, T. A. Rodriguez, K. McCann, M. K. Ray and W. Shawlot for comments on the manuscript. This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences (ES071003-11) and a conditional gift from RIKEN Brain Science Institute to Y.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ang SL, Jin O, Rhinn M, Daigle N, Stevenson L, Rossant J. A targeted mouse Otx2 mutation leads to severe defects in gastrulation and formation of axial mesoderm and to deletion of rostral brain. Development. 1996;122:243–252. doi: 10.1242/dev.122.1.243. [DOI] [PubMed] [Google Scholar]
- Beddington RS, Robertson EJ. Axis development and early asymmetry in mammals. Cell. 1999;96:195–209. doi: 10.1016/s0092-8674(00)80560-7. [DOI] [PubMed] [Google Scholar]
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. doi: 10.1016/s0925-4773(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Ben-Haim N, Lu C, Guzman-Ayala M, Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ, Constam DB. The nodal precursor acting via activin receptors induces mesoderm by maintaining a source of its convertases and BMP4. Dev Cell. 2006;11:313–323. doi: 10.1016/j.devcel.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Brennan J, Lu CC, Norris DP, Rodriguez TA, Beddington RS, Robertson EJ. Nodal signalling in the epiblast patterns the early mouse embryo. Nature. 2001;411:965–969. doi: 10.1038/35082103. [DOI] [PubMed] [Google Scholar]
- Chen C, Ware SM, Sato A, Houston-Hawkins DE, Habas R, Matzuk MM, Shen MM, Brown CW. The Vg1-related protein Gdf3 acts in a Nodal signaling pathway in the pre-gastrulation mouse embryo. Development. 2006;133:319–329. doi: 10.1242/dev.02210. [DOI] [PubMed] [Google Scholar]
- Davis S, Miura S, Hill C, Mishina Y, Klingensmith J. BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol. 2004;270:47–63. doi: 10.1016/j.ydbio.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A, Sancho M, Stuckey DW, Crompton LA, Godwin J, Mishina Y, Rodriguez TA. BMP signalling inhibits premature neural differentiation in the mouse embryo. Development. 2007 doi: 10.1242/dev.005967. [DOI] [PubMed] [Google Scholar]
- Ding J, Yang L, Yan YT, Chen A, Desai N, Wynshaw-Boris A, Shen MM. Cripto is required for correct orientation of the anterior-posterior axis in the mouse embryo. Nature. 1998;395:702–707. doi: 10.1038/27215. [DOI] [PubMed] [Google Scholar]
- Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391:357–362. doi: 10.1038/34848. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Lewis P, Pevny L, McMahon AP. Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev. 2002;119(Suppl 1):S97–S101. doi: 10.1016/s0925-4773(03)00099-6. [DOI] [PubMed] [Google Scholar]
- Kimura-Yoshida C, Nakano H, Okamura D, Nakao K, Yonemura S, Belo JA, Aizawa S, Matsui Y, Matsuo I. Canonical Wnt signaling and its antagonist regulate anterior-posterior axis polarization by guiding cell migration in mouse visceral endoderm. Dev Cell. 2005;9:639–650. doi: 10.1016/j.devcel.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kishigami S, Mishina Y. BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev. 2005;16:265–278. doi: 10.1016/j.cytogfr.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, Korving JP, Hogan BL. Bmp4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SL, Khoo PL, De Young RA, Steiner K, Wilcock C, Mukhopadhyay M, Westphal H, Jamieson RV, Robb L, Tam PP. Dkk1 and Wnt3 interact to control head morphogenesis in the mouse. Development. 2008;135:1791–1801. doi: 10.1242/dev.018853. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lu CC, Brennan J, Robertson EJ. From fertilization to gastrulation: axis formation in the mouse embryo. Curr Opin Genet Dev. 2001;11:384–392. doi: 10.1016/s0959-437x(00)00208-2. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol Cell Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meno C, Gritsman K, Ohishi S, Ohfuji Y, Heckscher E, Mochida K, Shimono A, Kondoh H, Talbot WS, Robertson EJ, Schier AF, Hamada H. Mouse Lefty2 and zebrafish antivin are feedback inhibitors of nodal signaling during vertebrate gastrulation. Mol Cell. 1999;4:287–298. doi: 10.1016/s1097-2765(00)80331-7. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Hanks MC, Miura S, Tallquist MD, Behringer RR. Generation of Bmpr/Alk3 conditional knockout mice. Genesis. 2002;32:69–72. doi: 10.1002/gene.10038. [DOI] [PubMed] [Google Scholar]
- Mishina Y, Suzuki A, Ueno N, Behringer RR. Bmpr encodes a type I bone morphogenetic protein receptor that is essential for gastrulation during mouse embryogenesis. Genes Dev. 1995;9:3027–3037. doi: 10.1101/gad.9.24.3027. [DOI] [PubMed] [Google Scholar]
- Miura S, Davis S, Klingensmith J, Mishina Y. BMP signaling in the epiblast is required for proper recruitment of the prospective paraxial mesoderm and development of the somites. Development. 2006;133:3767–3775. doi: 10.1242/dev.02552. [DOI] [PubMed] [Google Scholar]
- Miura S, Mishina Y. Whole-embryo culture of E5.5 mouse embryos: development to the gastrulation stage. Genesis. 2003;37:38–43. doi: 10.1002/gene.10229. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, Niehrs C, Belmonte JC, Westphal H. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Niida A, Hiroko T, Kasai M, Furukawa Y, Nakamura Y, Suzuki Y, Sugano S, Akiyama T. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23:8520–8526. doi: 10.1038/sj.onc.1207892. [DOI] [PubMed] [Google Scholar]
- Norris DP, Brennan J, Bikoff EK, Robertson EJ. The Foxh1-dependent autoregulatory enhancer controls the level of Nodal signals in the mouse embryo. Development. 2002;129:3455–3468. doi: 10.1242/dev.129.14.3455. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Mager J, Magnuson T. Dynamic morphogenetic events characterize the mouse visceral endoderm. Dev Biol. 2003;261:470–487. doi: 10.1016/s0012-1606(03)00302-6. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Magnuson T. Primitive streak formation in mice is preceded by localized activation of Brachyury and Wnt3. Dev Biol. 2005;288:363–371. doi: 10.1016/j.ydbio.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Rodriguez TA, Casey ES, Harland RM, Smith JC, Beddington RS. Distinct enhancer elements control Hex expression during gastrulation and early organogenesis. Dev Biol. 2001;234:304–316. doi: 10.1006/dbio.2001.0265. [DOI] [PubMed] [Google Scholar]
- Rossant J, Tam PP. Emerging asymmetry and embryonic patterning in early mouse development. Dev Cell. 2004;7:155–164. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Shawlot W, Deng JM, Behringer RR. Expression of the mouse cerberus-related gene, Cerr1, suggests a role in anterior neural induction and somitogenesis. Proc Natl Acad Sci U S A. 1998;95:6198–6203. doi: 10.1073/pnas.95.11.6198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Rodriguez T, Clements M, Smith JC, Beddington RS. Active cell migration drives the unilateral movements of the anterior visceral endoderm. Development. 2004;131:1157–1164. doi: 10.1242/dev.01005. [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Brown A, Beddington RS. Hex: a homeobox gene revealing peri-implantation asymmetry in the mouse embryo and an early transient marker of endothelial cell precursors. Development. 1998;125:85–94. doi: 10.1242/dev.125.1.85. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Takada S, Yoshikawa Y, Wu N, McMahon AP. T (Brachyury) is a direct target of Wnt3a during paraxial mesoderm specification. Genes Dev. 1999;13:3185–3190. doi: 10.1101/gad.13.24.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Saijoh Y, Perea-Gomez A, Shawlot W, Behringer RR, Ang SL, Hamada H, Meno C. Nodal antagonists regulate formation of the anteroposterior axis of the mouse embryo. Nature. 2004;428:387–392. doi: 10.1038/nature02418. [DOI] [PubMed] [Google Scholar]
- Zhao GQ. Consequences of knocking out BMP signaling in the mouse. Genesis. 2003;35:43–56. doi: 10.1002/gene.10167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement figure 1. Increased WNT signaling rescues anterior Dkk1 expression in Bmpr1anull/flox; Sox2Cre embryos (Whole mount in situ for Dkk1).
(A, B) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50. Four Bmpr1anull/flox; Sox2Cre embryos showing circumferential Dkk1 expression (A). Four Bmpr1anull/flox; Sox2Cre embryos showing unilateral Dkk1 expression (B). One Bmpr1anull/flox; Sox2Cre embryo shown as the most left embryos in (A) is also shown in Fig. 5(5J) for a typical example.
(C) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 containing 50ng/ml of WNT3A. Ten Bmpr1anull/flox; Sox2Cre embryos showed unilateral Dkk1 expression (arrowheads). One Bmpr1anull/flox; Sox2Cre embryo (asterisk) showed ectopic expression of Dkk1. One Bmpr1anull/flox; Sox2Cre embryo shown at the upper most right is also shown in Fig. 5(5N) for a typical example. Bars=100 μm.
Supplement figure 2. Increased WNT signaling rescues anterior AVE migration of Bmpr1anull/flox; Sox2Cre embryos (Whole mount in situ for Cer1).
(A) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50. Two Bmpr1anull/flox; Sox2Cre embryos showing circumferential Cer1 expression (arrows). Two Bmpr1anull/flox; Sox2Cre embryos showing unilateral Cer1 expression (arrow).
(B) Bmpr1anull/flox; Sox2Cre embryos cultured with DR50 containing 50ng/ml of WNT3A. Five Bmpr1anull/flox; Sox2Cre embryos showed unilateral Cer1 expression (arrow).
Embryos shown at the most left panels are also shown in Fig. 5(5L, 5P) as a typical example. Bars=100 μm.