Abstract
Opioid signaling has been strongly implicated in driving palatable food consumption. The nucleus accumbens (NAcc) is one important site of this effect; hyperphagia elicited by administration of exogenous mu opioid receptor (MOR) ligands in this brain region has been well documented. However, the role that endogenous opioid ligands in the NAcc play in controlling food intake remains poorly understood. Enkephalins, which signal through both the MOR and delta opioid receptor (DOR), are highly expressed within a subset of NAcc neurons, and have been shown to be sensitive to manipulations of diet and motivation. To investigate a potential role for these signaling molecules in regulating palatability-driven consumption, we measured high fat chow intake in rats following a series of pharmacological manipulations of NAcc opioid signaling. NAcc infusion of the MOR agonist [D-Ala2, N-MePHe4, Gly-ol]-enkephalin (DAMGO) robustly increased palatable food intake, as has previously been demonstrated. In contrast, neither infusion of met-enkephalin, its synthetic analogue [D-ala2] Met-enkephalin (DALA) nor the DOR-specific ligand [D-Pen2, Pen5]-enkephalin (DPDPE) had significant effects on food intake. However, when administered in combination with DAMGO, DPDPE significantly suppressed the magnitude of DAMGO-evoked feeding. Further analysis of DPDPE effects revealed that the drug strongly increased locomotor activity. Suppressive effects on feeding, then, may have occurred through competition between feeding and locomotion for behavioral expression.
Keywords: Palatability, food intake, consumption, enkephalin, nucleus accumbens
1. Introduction
Opioid signaling has long been implicated in controlling short term food intake, particularly of highly reinforcing food items. Several lines of evidence suggest that this behavioral effect, which is dependent on a CNS site of action (Marks-Kaufman et al., 1985), occurs through opioid-induced potentiation of the palatability of the food items. First, opioid-induced hyperphagia is more robustly expressed during consumption of highly reinforcing (usually sweet and/or fatty) foods (Cooper and Turkish, 1989). Second, opioid agonists increase (Doyle et al., 1993, Pecina and Berridge, 1995, Rideout and Parker, 1996) while opioid antagonists decrease measures of positive taste reactivity (Parker et al., 1992, Ferraro et al., 2002), stereotyped orofacial behaviors that are tightly correlated with the reinforcing value of tastants (Berridge, 2000). Third, post-ingestive signaling is not necessary for opioid-induced hyperphagia, as opioid effects are expressed in sham feeding animals (Kirkham and Cooper, 1988), as well as in animals consuming non-caloric tastants (Beczkowska et al., 1993). These latter data are consistent with the notion that opioids alter neural representation of palatability, rather than acting through direct effects on satiety mechanisms. Fourth and finally, pharmacological blockade of opioid signaling in human subjects diminishes reports of the hedonic impact of a palatable test meal (Yeomans and Wright, 1991, Yeomans and Gray, 1996). Importantly, opioid manipulations have no impact on taste discrimination in rodents nor taste recognition in humans, arguing against the possibility that opioid effects on feeding are the consequence of changes in neural processing of taste quality (O’Hare et al., 1997, Yeomans and Gray, 2002).
Multiple neural sites of action have been identified for opioid-induced hyperphagia (Glass et al., 1999). Opioid agonists have particularly potent effects when administered into the nucleus accumbens (NAcc), the ventral striatal region which plays a critical role in reward-directed behaviors (Mucha and Iversen, 1986, Bakshi and Kelley, 1993b, Pecina and Berridge, 2000, Ragnauth et al., 2000, Kim et al., 2004, Taha et al., 2009). Opioid signaling in the NAcc elicits an increase in feeding similar to that occurring after systemically administered opioids – the effect is independent of caloric content (Zhang and Kelley, 2002); alters taste reactivity measures (Pecina and Berridge, 2000); and is more pronounced for palatable tastants (Zhang and Kelley, 2002). In addition, direct administration of the nonspecific opioid antagonist naltrexone into the NAcc suppresses consumption of sucrose, implicating an endogenous signaling system in this brain region in promoting palatable food intake (Kelley et al., 1996).
In the NAcc, signaling through the mu opioid receptor (MOR) most potently elicits consumption, with administration of the MOR-specific agonist [D-Ala2, N-MePHe4, Gly-ol]-enkephalin (DAMGO) resulting in uninterrupted hyperphagia that can be sustained for hours (Zhang et al., 1998). Signaling through the delta opioid receptor (DOR) has also been reported to increase consumption, usually in a more transient fashion (Majeed et al., 1986, Bakshi and Kelley, 1993a, Zhang and Kelley, 1997, Ragnauth et al., 2000). However, the role of DOR signaling in modulating feeding is not fully understood, as administration of a DOR antagonist in the NAcc also causes an increase in consumption (Kelley et al., 1996). Finally, though kappa opioid receptor signaling has well described aversive effects – eliciting conditioned place aversion (Bals-Kubik et al., 1993) and suppressing NAcc dopamine release (Di Chiara and Imperato, 1988) – studies of food intake suggest NAcc KOR signaling has limited effects on consumption. NAcc infusion of KOR agonists does not alter food intake (Bakshi and Kelley, 1993a; Zhang and Kelley, 1997), while NAcc infusion of KOR antagonists has been reported to have no effect (Kelley et al., 1996), or to suppress intake in certain behavioral contexts (Bodnar et al., 1995).
These pharmacological studies provide a departure point for understanding mechanisms contributing to opioid-induced hyperphagia, importantly identifying the MOR as a critical site of action. They do not, however, clarify the role of endogenous opioid ligands in driving food intake. Anatomical and functional data provide evidence suggestive of a role for NAcc enkephalinergic signaling in modulating food intake. Within the NAcc, enkephalins are abundant, and are expressed within roughly half of medium spiny neurons (Meredith, 1999). Enkephalins bind to and activate the MOR (as well as the DOR) (Raynor et al., 1994), and might thus, like DAMGO, activate MOR-dependent signaling pathways leading to hyperphagia. Medium spiny neurons have abundant local collaterals, which provide the anatomical substrate for local enkephalin release (Meredith, 1999). In addition, expression levels of preproenkephalin (PPENK), the precursor gene for enkephalinergic ligands, are sensitive to manipulations of diet and motivation (Schiltz et al., 2007, Will et al., 2007).
In the present study, we evaluated consumption of a palatable food after direct NAcc infusion of pharmacological agents manipulating opioidergic, particularly enkephalinergic, signaling. While MOR-selective ligands elicited robust hyperphagia, as previously demonstrated, neither DOR-selective nor mixed MOR/DOR ligands had significant effects on consumption. Unexpectedly, administration of a DOR-selective agonist suppressed feeding elicited by MOR stimulation; this suppressive effect on feeding appears to have been secondary to the vigorous locomotion induced by DOR-stimulation.
2. Materials and Methods
2.1 Animals and experimental overview
All procedures used were approved by the University of Utah Medical School, Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley rats (n=21; 400–500 grams) were used in all experiments. Rats were allowed ad libitum access to food and water throughout the experimental period. Two groups of rats were used in these experiments. Rats in group 1 (n=10) were implanted bilaterally with cannulae directed at the NAcc core and shell (a total of 4 cannulae/rat). Rats in group 2 (n=11) were implanted bilaterally with cannulae directed at anterior and posterior sites within the ventrolateral striatum (4 cannulae/rat). In Experiment 1, both groups of rats were used to assess the effects of met-enkephalin and DAMGO infusions into these four distinct sites (NAcc core and shell; anterior and posterior ventrolateral striatum) on high fat chow intake. In subsequent experiments (Experiments 2–5), group 1 rats were used to assess the effects of pharmacological manipulations of opioid signaling specifically in the NAcc core, the site at which DAMGO-induced feeding was most robust. In Experiment 2, the effects of infusion of the enkephalinase inhibitor thiorphan on high fat chow intake were assessed. In Experiment 3, the effects of [D-Ala2] Met-enkephalin (DALA), a synthetic and protease-resistant analogue of enkephalin, on high fat chow intake were evaluated. In Experiment 4, the effects of DAMGO and DPDPE (a DOR-specific agonist) on high fat chow intake - administered singly and in combination - were measured. Finally, in Experiment 5, the effects of DAMGO, DALA, and DPDPE on locomotor activity were evaluated. Experimental treatments are summarized in Table 1.
Table 1.
Summary of experimental groups and drug treatments. Drug concentrations for met-enkephalin and DALA were chose to match and exceed a behaviorally effective dose of DAMGO. Thiorphan and DPDPE concentrations were chosen from previous reports of behaviorally effective concentrations (Kalivas and Richardson-Carlson, 1986, Bakshi and Kelley, 1993a). All drugs were infused bilaterally in 0.5 microliters/NAcc.
| Exp’t | Subjects (group) | N (# rats) | Infusion site(s) | Drug(s) infused (nmole/NAcc) | Experimental measure |
|---|---|---|---|---|---|
| 1 | Group 1 | 10 | NAcc core & shell | DAMGO: 0.25 met-enk: 0.025, 0.25, 2.5 |
High fat chow consumption |
| Group 2 | 11 | Ant. & post. VL striatum | “ | “ | |
| 2 | Group 1 | 10 | NAcc core | Thiorphan: 4, 13, 40 | “ |
| 3 | Group 1* | 7 | NAcc core | DALA: 0.025, 0.25, 2.5, 12.5 | “ |
| 4 | Group 1 | 10 | NAcc core | DAMGO: 0.25 DPDPE: 4 DAMGO + DPDPE: 0.25 + 4 |
“ |
| 5 | Group 1 | 10 | NAcc core | DAMGO: 0.25 DPDPE: 4 DALA: 12.5 |
Locomotor activity |
A subset of 7 rats from Group 1 was used in this experiment.
2.2 Cannula implantation
In initial surgeries (n = 6 rats) anesthesia was induced and maintained with a cocktail of ketamine and xylazine (induction, 75–100 + 0.5–1mg/kg IP; maintenance, 40–50 mg/kg ketamine hourly). In subsequent surgeries (n = 15 rats), anesthesia was induced and maintained with isoflurane (induction, 5%; maintenance, 2% in O2). 24 gauge stainless steel cannulae (Small Parts, Miramar, FL) were targeted to four different sites in the ventral striatum: the NAcc core and shell (Group 1) or anterior and posterior sites within the ventrolateral striatum (Group 2). Stereotaxic coordinates for each site were: NAcc shell: anterioposterior (AP) +1.4, mediolateral (ML) +/− 0.85, and dorsoventral (DV) −6.5; NAcc core: AP +1.4, ML +/− 1.85, DV −6.5; anterior VL striatum: AP +1.6, ML +/− 3.4, and DV −6.2; posterior VL striatum: AP +0.6, ML +/− 3.4, and DV −6.2. These coordinates describe the cannula placements, which were 1 mm dorsal to the targeted injection site. All animals received 3 mLs lactated ringers solution (SC), access to an ibuprofen solution in their home cage water bottle, and one week of recovery post surgery.
2.3 Drug infusions and high fat chow intake
Following a one week recovery period, animals were habituated to a high fat diet (Bio-Serv, Frenchtown, NJ) in daily one-hour exposures over 2–3 days, followed by 2 days of sham injections, in which the animals were handled and injectors were placed within cannulae but no drug was infused. Thereafter, drug injection commenced. For each experiment, drug infusions took place on alternate days, allowing a rest day between infusions. During infusions, injectors extended 1mm beyond the tip of the cannulae; thus final injection sites were 1 mm ventral to the cannula target coordinates. All drugs were infused in a total volume of 0.5 μl/injection site at 0.25 μl/minute, and injectors were left in place for an additional minute following the 2 minute infusion period. All measures of high fat chow intake were made in animals’ home cages (pre-measured high fat chow was presented in a ceramic bowl) during 1 hour sessions. For feeding experiments, drug concentrations for met-enkephalin (0.025, 0.25, 2.5 nmoles/NAcc) and the enkephalin analogue DALA (0.025, 0.25, 2.5, 12.5 nmoles/NAcc) were chosen to bracket a dose of DAMGO that elicits robust feeding (0.25 nanomoles/NAcc). Thiorphan doses (4, 13, 40 nmoles/NAcc) were guided by previous reports that concentrations as low as 1–10 nmoles were sufficient to elicit significant behavioral effects (Kalivas and Richardson-Carlson, 1986, Cador et al., 2002). Finally, the DPDPE dose (4 nmoles/NAcc) was chosen to match the concentration identified in a previous report as the dose maximally effective in eliciting food intake when infused into the NAcc (Bakshi and Kelley, 1993a). Experimental treatments, including drug concentrations, are summarized in Table 1. For each experiment, drugs and doses were randomized across days.
2.4 Locomotion
In Experiment 5 the effects of DAMGO, DALA, and DPDPE infusions into the NAcc core on locomotor activity were measured. For each drug, the highest concentration tested in feeding experiments was used (DAMGO, 0.25; DALA, 12.5; DPDPE, 4 nmoles/NAcc). During the first three infusion sessions, randomized infusions of control saline, DAMGO, or DALA were administered. All DPDPE infusions occurred on the fourth and final infusion day to conserve a limited supply of the drug. Immediately after each infusion, rats were placed into an 18″ × 24″ locomotor chamber, the floor of which was marked with regular spaced gridlines. Locomotor activity was recorded on digital video for one hour immediately following infusion. Subsequently, rearing and locomotion (line crossings in the x and y dimensions) were quantified by an observer blind to drug treatment.
2.5 Statistical analysis
One way repeated measures (RM) ANOVA was used to compare drug effects on high fat chow consumption in Experiments 1–4. Two-way RM ANOVA was used to analyze locomotor activity in Experiment 5 (comparing drug effects across time).
2.6 Histology
Rats were deeply anesthetized with sodium pentobarbital (Euthasol, 40 mg/kg) and perfused with physiological saline and 4% formaldehyde. Brains were extracted, cryoprotected overnight, and cryostat sectioned. Sections were stained with methyl red to anatomically localize cannula placements, which were identified through visible inspection for cannula tracks.
3. Results
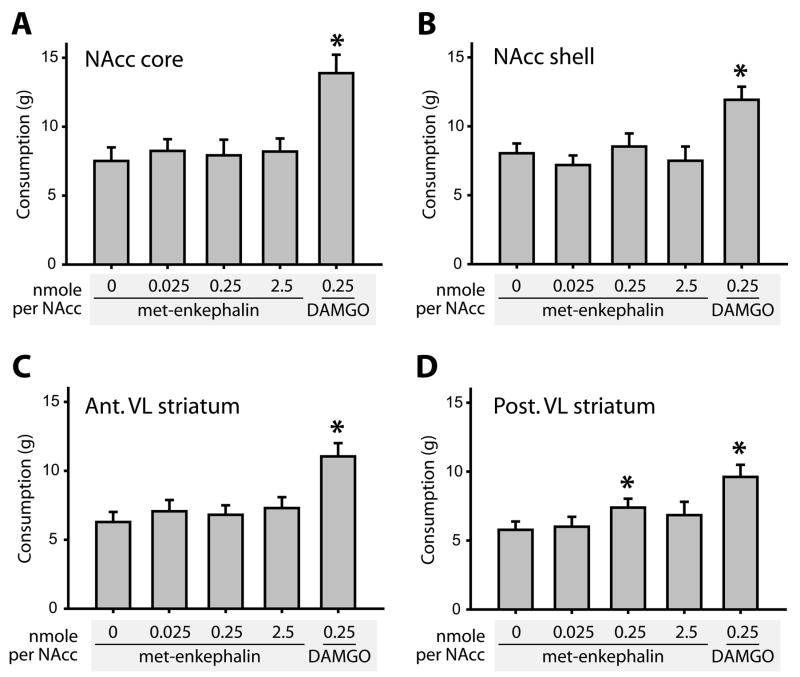
In Experiment 1, we tested the effects of met-enkephalin infusion into the NAcc, measuring high fat chow consumption after three doses of met-enkephalin in comparison to control saline or DAMGO (Figure 1A–D). At each anatomical site tested, there was a significant main effect of drug infusion (NAcc core: F(9,36) = 12.5; NAcc shell: F(9,36) = 13.9; anterior VL striatum: F(9,36) = 10.3; posterior VL striatum: F(9,36) = 8.1; all p<0.001). However, post-hoc tests revealed that in each case, this was the result of a significant elevation of consumption after DAMGO infusion (p<0.001 relative to saline infusion). For posterior VL striatum infusions only, post-hoc tests revealed a marginally significant effect of met-enkephalin infusions (0.25 ng/NAcc dose, p = 0. 05). This effect was modest (27% increase over baseline, versus 66% increase following DAMGO infusion), and no dose-dependence was evident.
Figure 1.
Met-enkephalin infusion into the ventral striatum does not alter high fat chow consumption. Infusion sites in the NAcc core (A), NAcc shell (B), anterior ventrolateral striatum (C), and posterior ventrolateral striatum (D), are shown. In each site, the effects of control saline, met-enkephalin (3 concentrations), and DAMGO (1 concentration) on food intake were assessed. DAMGO reliably elicited hyperphagia (p<0.001 in all sites), while met-enkephalin increased consumption only after the 0.25 nmoles/NAcc dose in the posterior ventrolateral striatum, at a marginally significant level (p=0.049). In this and other bar graphs, bars represent mean value ± SEM. For figures 1–4, consumption is presented as grams of high fat chow consumed over a one hour session.
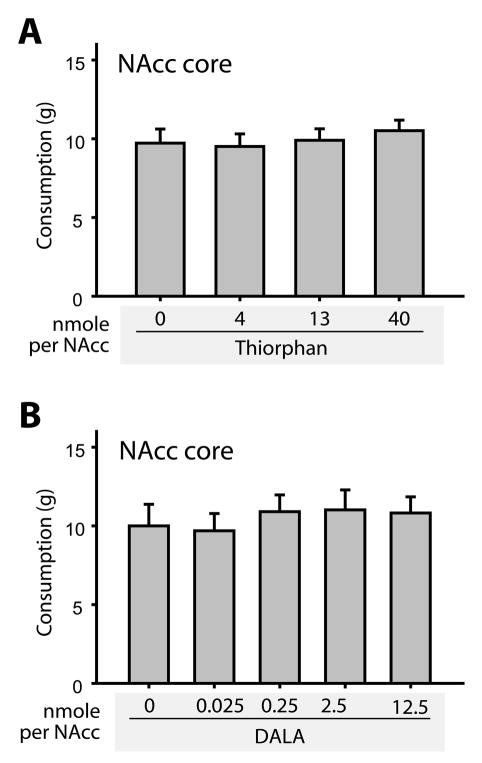
In Experiment 2, we tested the effects of the enkephalinase inhibitor thiorphan on high fat chow intake (Figure 2A). In this and subsequent experiments, we confined our study to the NAcc core, the brain region in which DAMGO elicited the largest increase in consumption (Figure 1). In Experiment 3 (Figure 2B), we infused DALA, a long-lasting synthetic analogue of met-enkephalin, into the NAcc core (Pert et al., 1976). As for Experiment 1, however, neither infusion had significant effects on consumption (thiorphan: F(9, 26) = 0.36, p=0.78; DALA, F(4, 30) = 0.26, p = 0.9). Even after infusion of DALA levels 50-fold higher than the effective dose of DAMGO (12.5 nmoles DALA/NAcc vs. 0.25 nmoles DAMGO/NAcc), food intake did not differ from baseline levels (Figure 2B).
Figure 2.
Neither the enkephalinase inhibitor thiorphan (A), nor the long-lasting synthetic enkephalin analogue DALA (B) alter food intake when injected into the NAcc core. Food intake did not differ from baseline levels after infusion of any concentration of either drug.
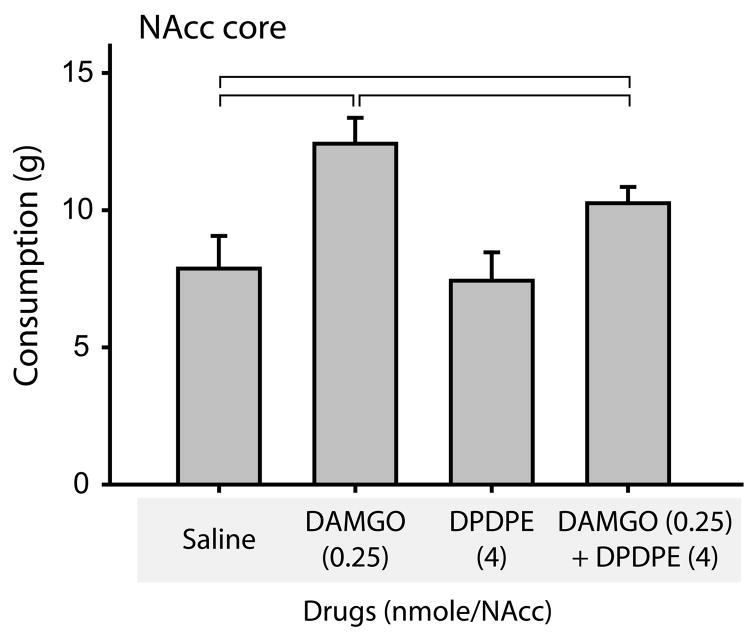
Neither met-enkephalin nor DALA significantly affected high fat chow intake. Because these peptides signal at both the MOR and DOR, we hypothesized that signaling through the DOR might attenuate MOR-mediated hyperphagia. To study this directly, we investigated the effects of DAMGO, the MOR-specific ligand, in combination with DPDPE, a DOR-specific ligand, both singly and in combination (Figure 3). In this experiment, there was a main effect of drug treatment (F(9,27)=11.0, p<0.001 relative to saline control). Post-hoc comparisons showed that DAMGO, as expected, significantly elevated consumption (p<0.001). DPDPE alone did not alter consumption (p=0.66 relative to saline control). Interestingly, however, DPDPE, when administered in combination with DAMGO, significantly attenuated the resulting hyperphagia (a 47% reduction in the magnitude of the DAMGO-induced hyperphagia; p<0.05, comparing DAMGO to DAMGO + DPDPE treatment).
Figure 3.
The DOR-specific agonist DPDPE attenuates DAMGO-induced hyperphagia. When infused alone, DPDPE had no effects on consumption (third bar). However, when co-infused with DAMGO (fourth bar), DPDPE significantly decreased DAMGO induced consumption (compare with DAMGO alone, second bar). DAMGO was infused at 0.25 nmoles/NAcc in these experiments; DPDPE was infused at 4 nmoles/NAcc. Brackets indicate significantly different levels of high fat chow intake (p< 0.01).
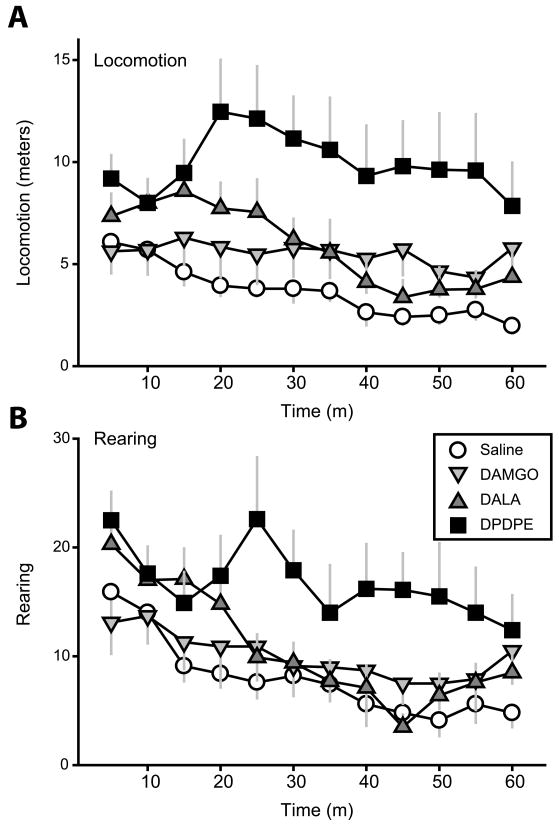
To probe the mechanism underlying DOR-induced attenuation of MOR-elicited hyperphagia, we assessed locomotor activity in an open field test following DAMGO, DALA, and DPDPE infusion into the NAcc core (Figure 4). For both locomotion and rearing, there were significant main effects of drug (locomotion, F(3,27)=6.5, p=0.002; rearing, F(3,27)=4.4, p=0.01) and time (locomotion, F(11,99)=3.5, p<0.001; rearing, F(11,99)=9.8, p<0.001) and a significant interaction between the two (locomotion, F(33,297)=1.7, p=0.01; rearing, F(33,297)=1.7, p=0.02). Notably, DPDPE infusion resulted in a highly significant, sustained elevation of locomotion and rearing (both p<0.05, post-hoc test comparison to saline effects). Though DALA infusion caused a transient and modest trend toward elevated locomotion and rearing (e.g., p=0.07 analyzing locomotion over first 20 minutes), neither DALA nor DAMGO effects differed significantly from those measured following saline control when analyzed over the full hour (all p≫0.05).
Figure 4.
Locomotor activity following manipulation of NAcc core opioid signaling. (A) Locomotion and (B) rearing were significantly elevated relative to baseline levels only after infusion of the DOR-specific agonist DPDPE. DALA caused a transient trend toward increased behavioral activation over the first 20 minutes only.
4. Discussion
We found no significant effects of NAcc infusion of met-enkephalin; DALA, a long-lasting enkephalin analogue; or thiorphan, an enkephalinase inhibitor, on consumption of a highly palatable high fat chow (Figures 1–2). Because opioid receptor levels vary across the ventral striatum (Mansour et al., 1987), the initial test of met-enkephalin and DAMGO effects were made through infusions at four distinct sites spanning this region. Infusion of the MOR-specific agonist DAMGO resulted in robust hyperphagia, with the largest effects occurring following administration into the NAcc core, confirming the initial mapping study of this effect (Zhang and Kelley, 2000). Together, our data provide little support for the notion that enkephalinergic signaling in the NAcc has substantial effects on food intake.
These results were surprising, given that enkephalinergic signaling occurs through the DOR and the MOR; signaling through both receptors has previously been shown to result in elevated food intake (shown for example, in (Bakshi and Kelley, 1993a)). To investigate MOR/DOR interactions further, we extended our investigation to study the effects of DAMGO and DPDPE, when infused singly or together, on food intake. Only under conditions of DAMGO+DPDPE infusion did we find a significant effect of DOR signaling on food intake, as DPDPE infusion significantly suppressed consumption stimulated by DAMGO (Figure 4). Further studies revealed that this suppression was likely the consequence of the robust locomotor activity elicited by DPDPE, which, given its magnitude and sustained duration (Figure 5), would be expected to compete with DAMGO-induced feeding for behavioral expression. Consistent with this idea, DPDPE alone did not suppress high fat chow intake relative to baseline consumption levels. The suppressive effects of DPDPE on consumption were only apparent after DAMGO-induced hyperphagia – a condition in which consummatory behaviors typically occupied much of the experimental session.
Figure 5.
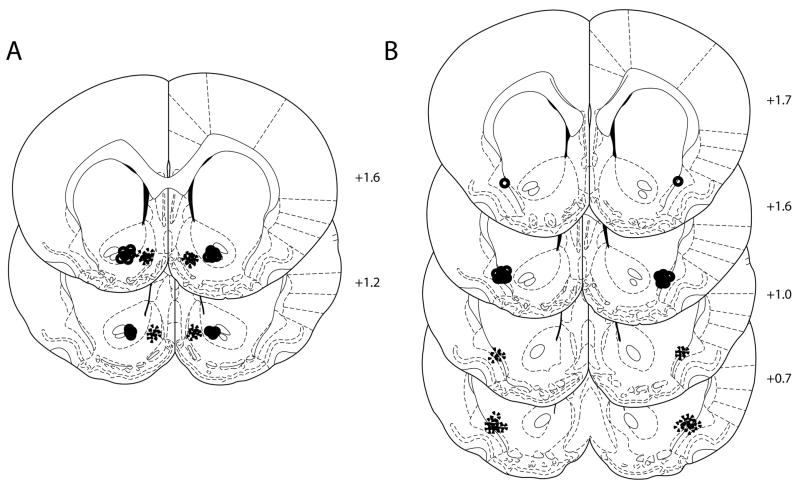
Cannula placements. (A) Cannula placements in Group 1 were tightly clustered in the medial shell (dashed lines) and central NAcc core (solid lines) near the anterior commissure. Placements in Group 2 were clustered at the border of the ventrolateral striatum and the dorsal endopiriform nucleus for anterior (solid lines) and posterior (dashed lines). Group 1 rats were used in Experiments 1–5, while Group 2 rats were used only in Experiment 1 (Figures 1C–D).
The role of DOR signaling in the NAcc in modulating food intake remains puzzling. Though we found no effects of DOR signaling on feeding in this study, many previous investigators have reported a transient but significant elevation of food intake following administration of DOR ligands (Majeed et al., 1986, Bakshi and Kelley, 1993a, Zhang and Kelley, 1997, Ragnauth et al., 2000). Typically this effect has a rapid onset and fleeting duration, in contrast to the delayed onset and prolonged duration of feeding following MOR stimulation (Majeed et al., 1986, Bakshi and Kelley, 1993a, Zhang and Kelley, 1997, Ragnauth et al., 2000). These results are consistent with a stimulatory role for DOR signaling in the control of consumption. Intriguingly, however, Kelley and colleagues (Kelley et al., 1996) showed that antagonizing NAcc DORs also results in increased food intake. Administration of the DOR-specific antagonist naltrindole resulted in a near doubling of sucrose intake in this experiment - surprisingly, this effect could be blocked by systemic administration of the nonspecific opioid antagonist naltrexone. This suggests that the suppressive effects of DOR signaling on food intake are dependent on an interaction with a second, undefined opioidergic signaling pathway that promotes feeding. The authors hypothesized that the latter pathway may be mediated through MOR signaling. Our own results are consistent with a suppressive effect of DOR signaling on food intake, but as an indirect consequence of the potent increase in locomotion caused by DOR stimulation. Notably, Bodnar and colleagues (Ragnauth et al., 2000) also investigated MOR/DOR interactions, but arrived at conclusions opposite ours – they found that feeding elicited by DAMGO infusion could be blocked by co-administration of the DOR antagonist naltrindole, suggesting a cooperative effect of MOR and DOR signaling in mediating food intake. Cooperative interactions of MOR and DOR signaling are also supported by studies of nociception in MOR knockout mice, as the analgesic effects of DOR agonists are absent or attenuated in these animals (Matthes et al., 1996, Sora et al., 1999, Gaveriaux-Ruff and Kieffer, 2002).
To place our results into context, and to consolidate a more comprehensive view of the pharmacology of NAcc opioidergic feeding, we summarize the relevant literature in Table 2. In this table, we include the magnitude and direction of pharmacological manipulations of NAcc opioids on feeding. Four clear trends are evident. First, unsurprisingly, there is strong evidence that NAcc MOR signaling has pronounced and consistent effects in promoting food intake. Second, despite our negative results following DPDPE infusion, previous studies report consistent effects of DOR stimulation in increasing food intake. Third, DOR signaling clearly and consistently increases locomotion, including in our present results. Fourth and finally, NAcc KOR signaling has few consistent effects effect on feeding, with most experiments (six of nine) suggesting minimal effects on intake.
Table 2. NAcc feeding pharmacology: summary of the literature.
Summary of studies of the pharmacology of NAcc opioid-induced feeding. Changes in feeding following drug infusion were calculated from the primary literature (rounded to 10%); for each drug, the concentration eliciting the maximal feeding effect is shown. This dose is indicated to the right of each drug; when results were not significant, the maximum concentration that was tested is indicated. Also summarized (final column) are the effects of each drug on locomotor behavior. The direction of the arrowhead indicates the valence of the change in behavior (▴, increase; ▾, decrease). NS indicates results that were not significantly different from baseline. A dash indicates the study did not measure the specified behavior. Bodnar and colleagues (Bodnar et al., 1995) measured drug effects on glucoprivic, deprivation, and palatability-induced consumption.
| Reference | Drug | Conc (nm/NAcc) | Feeding | Locomotion | |
|---|---|---|---|---|---|
| Nonspecific antagonists | Kelley et al., 1996 | Naloxone | 83 | 40% ▾ | 30% ▾ |
| Majeed et al., 1986 | Naloxone | 10 | NS | - | |
| Bodnar et al., 1995 | Naltrexone | 55 | 30% ▾1 | - | |
| Kelley et al., 1996 | Naltrexone | 55 | 30% ▾ | NS | |
| MOR agonists | Bakshi and Kelley, 1993 | DAMGO | 4.0 | 180% ▴ | 280% ▴ |
| Ragnauth et al., 2000 | DAMGO | 4.0 | 150% ▴ | - | |
| Zhang and Kelley, 1997 | DAMGO | 0.04 | 60% ▴ | NS | |
| This MS | DAMGO | 0.25 | 90% ▴ | NS | |
| Cunningham and Kelley, 1992 | DAMGO | 1.6 | - | 150% ▴ | |
| Mucha and Iversen, 1986 | Morphine | 10 | 290% ▴ | - | |
| Zhang and Kelley, 1997 | Morphine | 7.5 | 70% ▴ | NS | |
| Majeed et al., 1986 | Morphine | 10 | 80% ▴ | - | |
| Cunningham and Kelley, 1992 | Morphine | 8.8 | - | 150% ▴ | |
| MOR antagonists | Bodnar et al., 1995 | β-FNA | 8.3 | 40% ▾ | - |
| Kelley et al., 1996 | β-FNA | 31 | 50% ▾ | 30% ▾ | |
| Majeed et al., 1986 | β-FNA | 5.0 | NS | - | |
| Kelley et al., 1996 | Naloxonazine | 15 | NS | 70% ▾ | |
| DOR agonists | Majeed et al., 1986 | DADLE | 10 | 50% ▴ | - |
| Ragnauth et al., 2000 | Deltorphin | 6.4 | 60% ▴ | - | |
| Bakshi and Kelley, 1993 | DPDPE | 4.0 | 200% ▴ | 395% ▴ | |
| Majeed et al., 1986 | DPDPE | 10 | 40% ▴ | - | |
| Ragnauth et al., 2000 | DPDPE | 3.2 | 200% ▴ | - | |
| Zhang and Kelley, 1997 | DPDPE | 4.0 | 50% ▴ | 180% ▴ | |
| This MS | DPDPE | 4.0 | NS | 170% ▴ | |
| Cunningham and Kelley, 1992 | DPDPE | 2.6 | - | 290% ▴ | |
| DOR antagonists | Kelley et al., 1996 | Naltrindole | 44 | 80% ▴ | 60% ▾ |
| KOR agonists | Majeed et al., 1986 | α-neoendorphin | 10 | 40% ▴ | - |
| Majeed et al., 1986 | Bremacozine | 100 | NS | - | |
| Majeed et al., 1986 | Dynorphin | 10 | 30% ▴ | - | |
| Zhang and Kelley, 1997 | Dynorphin | 3.5 | NS | NS | |
| Bakshi and Kelley, 1993 | U50, 488H | 4.0 | NS | - | |
| Majeed et al., 1986 | U50, 488H | 100 | NS | - | |
| Zhang and Kelley, 1997 | U50, 488H | 4.0 | NS | NS | |
| KOR antagonists | Bodnar et al., 1995 | Nor-BNI | 5.6 | NS1 | - |
| Bodnar et al., 1995 | Nor-BNI | 5.6 | 30% ▾2 | - | |
| Bodnar et al., 1995 | Nor-BNI | 5.6 | 30% ▾3 | - | |
| Kelley et al., 1996 | Nor-BNI | 14 | NS | NS | |
| Mixed MOR/DOR signaling | This MS | met-enkephalin | 2.5 | NS | - |
| This MS | DALA | 12.5 | NS | NS | |
| Mucha and Iversen, 1986 | BW768c | 0.1 | 290% ▴ | - | |
| Majeed et al., 1986 | β-endorphin | 10 | 40% ▴ | - | |
| This MS | Thiorphan | 40 | NS | - | |
| This MS | DAMGO+DPDPE | 0.25+4 | 50% ▾ of DAMGO ▴ | - | |
| Ragnauth et al., 2000 | DAMGO+naltrindole | 4+8.8 | 100% ▾ of DAMGO ▴ | - | |
| Ragnauth et al., 2000 | DAMGO+DALCE | 4+ 6 | 40% ▾ of DAMGO ▴ | - | |
| Cunningham and Kelley, 1992 | DALA | 3.4 | - | 170% ▴ |
Palatability-induced intake
Deprivation induced intake
Glucoprivic intake
It is unclear why we DPDPE administration did not increase food intake in our experiments. It seems unlikely that technical difficulties with drug activity or infusion procedure were responsible, however, as DPDPE did substantially elevate locomotion in our experiments (Figure 4), as reported by other investigators (Cunningham and Kelley, 1992, Bakshi and Kelley, 1993a, Zhang and Kelley, 1997). Drug concentration is also unlikely to have been an issue, as the concentration we used was identical to that effectively used to elicit increased consumption in a previous study (Bakshi and Kelley, 1993a). One speculative possibility is that DPDPE-induced feeding arises as a consequence of a general increase in arousal, rather than through recruitment of neural circuits with more specific relevance to food intake (such as neural circuits underlying palatability, as appear to be recruited by MOR agonists). Consistent with this possibility, Kelley et al noted in a previous study that measures of behavioral activation (locomotion, rearing) increased in parallel with and were correlated with levels of sucrose consumption (drinking bouts, drinking duration) following DPDPE infusion into the NAcc (Zhang and Kelley, 1997). Thus, in our experiments, increases in arousal may have been primarily expressed through locomotion, rather than food intake, particularly given that our sessions were relatively short (1 hour, rather than 4 hours, as used in other experiments (Majeed et al., 1986, Bakshi and Kelley, 1993a, Ragnauth et al., 2000), and given that DOR-induced changes in locomotion are generally much larger than changes in food intake. In a short experimental session, DOR-induced behavioral changes may thus be principally evident as changes in locomotion. Consistent with this interpretation, locomotor behavior is generally more profoundly elevated by DOR stimulation than food intake (Table 2).
Though the effects of DOR signaling on feeding are unsettled, effects on locomotion appear to be reliable – agonists increase, and antagonists decrease, locomotion (Table 2). This result is intriguing for several reasons. First, PPENK levels are altered by manipulations of motivational state. Short term hunger is correlated with increased PPENK levels (Will et al., 2007), as is exposure to contextual cues associated with a highly palatable food (Schiltz et al., 2007). These are both behavioral contexts in which food-seeking behaviors would be expected to be elevated, as a natural prelude to consumption itself – indeed, the Schiltz (2007) study demonstrated increased locomotion as a consequence of exposure to food-associated contexts. Thus, these studies suggest a possible role for enkephalin in food-seeking behaviors preceding consumption itself. Second, recent studies show that drug-seeking behaviors can be attenuated with intra-NAcc infusion of naltrindole, the specific DOR antagonist (Ciccocioppo et al., 2002, Ward and Roberts, 2007). The results of the Ciccocioppo et al (2002) study are particularly intriguing in considering food-seeking behaviors, as animals in these experiments were tested under extinction conditions, in which their behavior would not have been influenced by acute pharmacological effects of drug administration in this study. Given extensive overlap between drug and food-seeking circuits (Thiele et al., 2003), then, these studies raise the possibility that DOR signaling may play an important role in food-seeking behaviors, perhaps activated by endogenous enkephalin release. To our knowledge this hypothesis has not investigated explicitly in the context of food seeking. Future studies focusing on the role of endogenous DOR signaling in the context of foraging behaviors, rather than consumption itself, will be interesting in addressing this point.
Acknowledgments
This work was supported by grants from the University of Utah Research Foundation, NARSAD and NIMH.
Abbreviations
- β-FNA
Beta-funaltrexamine
- DADLE
[D-Ala2, D-Leu5]-enkephalin
- DALA
[D-Ala2] Met-enkephalin
- DALCE
[D-Ala, Leu5, Cys6]-enkephalin
- DAMGO
[D-Ala2, N-MePHe4, Gly-ol]-enkephalin
- DOR
Delta opioid receptor
- DPDPE
[D-Pen2, Pen5]-enkephalin
- KOR
Kappa opioid receptor
- MOR
Mu opioid receptor
- NAcc
Nucleus accumbens
- Nor-BNI
Nor-binaltorphimine
- PPENK
Preproenkephalin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakshi VP, Kelley AE. Feeding induced by opioid stimulation of the ventral striatum: role of opiate receptor subtypes. J Pharmacol Exp Ther. 1993a;265:1253–1260. [PubMed] [Google Scholar]
- Bakshi VP, Kelley AE. Striatal regulation of morphine-induced hyperphagia: an anatomical mapping study. Psychopharmacology (Berl) 1993b;111:207–214. doi: 10.1007/BF02245525. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264:489–495. [PubMed] [Google Scholar]
- Beczkowska IW, Koch JE, Bostock ME, Leibowitz SF, Bodnar RJ. Central opioid receptor subtype antagonists differentially reduce intake of saccharin and maltose dextrin solutions in rats. Brain Res. 1993;618:261–270. doi: 10.1016/0006-8993(93)91274-v. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Bodnar RJ, Glass MJ, Ragnauth A, Cooper ML. General, mu and kappa opioid antagonists in the nucleus accumbens alter food intake under deprivation, glucoprivic and palatable conditions. Brain Res. 1995;700:205–212. doi: 10.1016/0006-8993(95)00957-r. [DOI] [PubMed] [Google Scholar]
- Cador M, Marco N, Stinus L, Simonnet G. Interaction between neuropeptide FF and opioids in the ventral tegmental area in the behavioral response to novelty. Neuroscience. 2002;110:309–318. doi: 10.1016/s0306-4522(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, Weiss F. Effect of selective blockade of mu(1) or delta opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Cooper SJ, Turkish S. Effects of naltrexone on food preference and concurrent behavioral responses in food-deprived rats. Pharmacol Biochem Behav. 1989;33:17–20. doi: 10.1016/0091-3057(89)90422-x. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res. 1992;588:104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle TG, Berridge KC, Gosnell BA. Morphine enhances hedonic taste palatability in rats. Pharmacol Biochem Behav. 1993;46:745–749. doi: 10.1016/0091-3057(93)90572-b. [DOI] [PubMed] [Google Scholar]
- Ferraro FM, 3rd, Hill KG, Kaczmarek HJ, Coonfield DL, Kiefer SW. Naltrexone modifies the palatability of basic tastes and alcohol in outbred male rats. Alcohol. 2002;27:107–114. doi: 10.1016/s0741-8329(02)00220-3. [DOI] [PubMed] [Google Scholar]
- Gaveriaux-Ruff C, Kieffer BL. Opioid receptor genes inactivated in mice: the highlights. Neuropeptides. 2002;36:62–71. doi: 10.1054/npep.2002.0900. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Billington CJ, Levine AS. Opioids and food intake: distributed functional neural pathways? Neuropeptides. 1999;33:360–368. doi: 10.1054/npep.1999.0050. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Richardson-Carlson R. Endogenous enkephalin modulation of dopamine neurons in ventral tegmental area. Am J Physiol. 1986;251:R243–249. doi: 10.1152/ajpregu.1986.251.2.R243. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Bless EP, Swanson CJ. Investigation of the effects of opiate antagonists infused into the nucleus accumbens on feeding and sucrose drinking in rats. J Pharmacol Exp Ther. 1996;278:1499–1507. [PubMed] [Google Scholar]
- Kim EM, Quinn JG, Levine AS, O’Hare E. A bi-directional mu-opioid-opioid connection between the nucleus of the accumbens shell and the central nucleus of the amygdala in the rat. Brain Res. 2004;1029:135–139. doi: 10.1016/j.brainres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Cooper SJ. Naloxone attenuation of sham feeding is modified by manipulation of sucrose concentration. Physiol Behav. 1988;44:491–494. doi: 10.1016/0031-9384(88)90310-1. [DOI] [PubMed] [Google Scholar]
- Majeed NH, Przewlocka B, Wedzony K, Przewlocki R. Stimulation of food intake following opioid microinjection into the nucleus accumbens septi in rats. Peptides. 1986;7:711–716. doi: 10.1016/0196-9781(86)90083-5. [DOI] [PubMed] [Google Scholar]
- Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- Marks-Kaufman R, Plager A, Kanarek RB. Central and peripheral contributions of endogenous opioid systems to nutrient selection in rats. Psychopharmacology (Berl) 1985;85:414–418. doi: 10.1007/BF00429656. [DOI] [PubMed] [Google Scholar]
- Matthes HW, Maldonado R, Simonin F, Valverde O, Slowe S, Kitchen I, Befort K, Dierich A, Le Meur M, Dolle P, Tzavara E, Hanoune J, Roques BP, Kieffer BL. Loss of morphine-induced analgesia, reward effect and withdrawal symptoms in mice lacking the mu-opioid-receptor gene. Nature. 1996;383:819–823. doi: 10.1038/383819a0. [DOI] [PubMed] [Google Scholar]
- Meredith GE. The synaptic framework for chemical signaling in nucleus accumbens. Ann N Y Acad Sci. 1999;877:140–156. doi: 10.1111/j.1749-6632.1999.tb09266.x. [DOI] [PubMed] [Google Scholar]
- Mucha RF, Iversen SD. Increased food intake after opioid microinjections into nucleus accumbens and ventral tegmental area of rat. Brain Res. 1986;397:214–224. doi: 10.1016/0006-8993(86)90622-0. [DOI] [PubMed] [Google Scholar]
- O’Hare EO, Cleary J, Bartz PJ, Weldon DT, Billington CJ, Levine AS. Naloxone administration following operant training of sucrose/water discrimination in the rat. Psychopharmacology (Berl) 1997;129:289–294. doi: 10.1007/s002130050193. [DOI] [PubMed] [Google Scholar]
- Parker LA, Maier S, Rennie M, Crebolder J. Morphine- and naltrexone-induced modification of palatability: analysis by the taste reactivity test. Behav Neurosci. 1992;106:999–1010. doi: 10.1037//0735-7044.106.6.999. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Central enhancement of taste pleasure by intraventricular morphine. Neurobiology (Bp) 1995;3:269–280. [PubMed] [Google Scholar]
- Pecina S, Berridge KC. Opioid site in nucleus accumbens shell mediates eating and hedonic ‘liking’ for food: map based on microinjection Fos plumes. Brain Res. 2000;863:71–86. doi: 10.1016/s0006-8993(00)02102-8. [DOI] [PubMed] [Google Scholar]
- Pert CB, Pert A, Chang JK, Fong BT. (D-Ala2)-Met-enkephalinamide: a potent, long-lasting synthetic pentapeptide analgesic. Science. 1976;194:330–332. doi: 10.1126/science.968485. [DOI] [PubMed] [Google Scholar]
- Ragnauth A, Moroz M, Bodnar RJ. Multiple opioid receptors mediate feeding elicited by mu and delta opioid receptor subtype agonists in the nucleus accumbens shell in rats. Brain Res. 2000;876:76–87. doi: 10.1016/s0006-8993(00)02631-7. [DOI] [PubMed] [Google Scholar]
- Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T. Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol Pharmacol. 1994;45:330–334. [PubMed] [Google Scholar]
- Rideout HJ, Parker LA. Morphine enhancement of sucrose palatability: analysis by the taste reactivity test. Pharmacol Biochem Behav. 1996;53:731–734. doi: 10.1016/0091-3057(95)02077-2. [DOI] [PubMed] [Google Scholar]
- Schiltz CA, Bremer QZ, Landry CF, Kelley AE. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 2007;5:16. doi: 10.1186/1741-7007-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sora I, Li XF, Funada M, Kinsey S, Uhl GR. Visceral chemical nociception in mice lacking mu-opioid receptors: effects of morphine, SNC80 and U-50,488. Eur J Pharmacol. 1999;366:R3–5. doi: 10.1016/s0014-2999(98)00933-9. [DOI] [PubMed] [Google Scholar]
- Taha SA, Katsuura Y, Noorvash D, Seroussi A, Fields HL. Convergent, not serial, striatal and pallidal circuits regulate opioid-induced food intake. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Navarro M, Sparta DR, Fee JR, Knapp DJ, Cubero I. Alcoholism and obesity: overlapping neuropeptide pathways? Neuropeptides. 2003;37:321–337. doi: 10.1016/j.npep.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Roberts DC. Microinjection of the delta-opioid receptor selective antagonist naltrindole 5′-isothiocyanate site specifically affects cocaine self-administration in rats responding under a progressive ratio schedule of reinforcement. Behav Brain Res. 2007;182:140–144. doi: 10.1016/j.bbr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will MJ, Vanderheyden WM, Kelley AE. Striatal opioid peptide gene expression differentially tracks short-term satiety but does not vary with negative energy balance in a manner opposite to hypothalamic NPY. Am J Physiol Regul Integr Comp Physiol. 2007;292:R217–226. doi: 10.1152/ajpregu.00852.2005. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Selective effects of naltrexone on food pleasantness and intake. Physiol Behav. 1996;60:439–446. doi: 10.1016/s0031-9384(96)80017-5. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- Yeomans MR, Wright P. Lower pleasantness of palatable foods in nalmefene-treated human volunteers. Appetite. 1991;16:249–259. doi: 10.1016/0195-6663(91)90062-w. [DOI] [PubMed] [Google Scholar]
- Zhang M, Gosnell BA, Kelley AE. Intake of high-fat food is selectively enhanced by mu opioid receptor stimulation within the nucleus accumbens. J Pharmacol Exp Ther. 1998;285:908–914. [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Opiate agonists microinjected into the nucleus accumbens enhance sucrose drinking in rats. Psychopharmacology (Berl) 1997;132:350–360. doi: 10.1007/s002130050355. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Enhanced intake of high-fat food following striatal mu-opioid stimulation: microinjection mapping and fos expression. Neuroscience. 2000;99:267–277. doi: 10.1016/s0306-4522(00)00198-6. [DOI] [PubMed] [Google Scholar]
- Zhang M, Kelley AE. Intake of saccharin, salt, and ethanol solutions is increased by infusion of a mu opioid agonist into the nucleus accumbens. Psychopharmacology (Berl) 2002;159:415–423. doi: 10.1007/s00213-001-0932-y. [DOI] [PubMed] [Google Scholar]