Abstract
The essential role of the medial temporal lobe (MTL) in long-term memory for individual events is well established, yet important questions remain regarding the mnemonic functions of the component structures that constitute the region. Within the hippocampus, recent functional neuroimaging findings suggest that formation of new memories depends on the den tate gyrus and the CA3 field, whereas the contribution of the subiculum may be limited to retrieval. During encoding, it has been further hypothesized that structures within MTL cortex contribute to encoding in a content-sensitive manner, whereas hippocampal structures may contribute to encoding in a more domain-general manner. In the current experiment, high-resolution fMRI techniques were utilized to assess novelty and subsequent memory effects in MTL subregions for two classes of stimuli—faces and scenes. During scanning, participants performed an incidental encoding (target detection) task with novel and repeated faces and scenes. Subsequent recognition memory was indexed for the novel stimuli encountered during scanning. Analyses revealed voxels sensitive to both novel faces and novel scenes in all MTL regions. However, similar percentages of voxels were sensitive to novel faces and scenes in perirhinal cortex, entorhinal cortex, and a combined region comprising the dentate gyrus, CA2, and CA3, whereas parahippocampal cortex, CA1, and subiculum demonstrated greater sensitivity to novel scene stimuli. Paralleling these findings, subsequent memory effects in perirhinal cortex were observed for both faces and scenes, with the magnitude of encoding activation being related to later memory strength, as indexed by a graded response tracking recognition confidence, whereas subsequent memory effects were scene-selective in parahippocampal cortex. Within the hippocampus, encoding activation in the subiculum correlated with subsequent memory for both stimulus classes, with the magnitude of encoding activation varying in a graded manner with later memory strength. Collectively, these findings suggest a gradient of content sensitivity from posterior (parahippocampal) to anterior (perirhinal) MTL cortex, with MTL cortical regions differentially contributing to successful encoding based on event content. In contrast to recent suggestions, the present data further indicate that the subiculum may contribute to successful encoding irrespective of event content.
INTRODUCTION
Memory is central to our cognitive lives, enabling us to discriminate novel people, places, and things from stimuli that are familiar due to previous experience. The medial temporal lobe (MTL) plays an essential role in the acquisition and retrieval of episodic memories—long-term memories for specific events (Preston & Wagner, 2007; Squire, Stark, & Clark, 2004; Eichenbaum & Cohen, 2001; Eichenbaum, 2000; Tulving, 1983; Cohen & Squire, 1980). The ability to subsequently distinguish novel and familiar stimuli is thought to partially depend on novelty encoding processes in the MTL that support successful memory formation (Tulving, Markowitch, Craik, Habib, & Houle, 1996; Tulving & Kroll, 1995). For example, neuropsychological data demonstrate that MTL lesions impair novelty detection (Knight, 1996), and numerous neuroimaging studies in humans indicate that functional activation in MTL structures is greater when encountering novel relative to repeated stimuli (Henson, Cansino, Herron, Robb, & Rugg, 2003; Zeineh, Engel, & Bookheimer, 2000; Stern et al., 1996), with the magnitude of the MTL novelty response correlating with subsequent memory outcome (Kirchhoff, Wagner, Maril, & Stern, 2000; see also Dudukovic & Wagner, 2007).
The MTL circuit is composed of the hippocampal formation (the dentate gyrus, CA fields, and subiculum) and the surrounding entorhinal, perirhinal, and parahippocampal cortical areas. The anatomical organization of the MTL suggests that its component regions mediate the acquisition, retention, and retrieval of memory in different ways. For example, perirhinal cortex and parahippocampal cortex receive inputs from unimodal and polymodal association cortices in the lateral temporal, frontal, and parietal lobes via distinct pathways (Suzuki & Amaral, 1994; Tranel, Brady, Van Hoesen, & Damasio, 1988; Van Hoesen & Pandya, 1975; Van Hoesen, Pandya, & Butters, 1975; Jones & Powell, 1970). In nonhuman primates, the predominant inputs to perirhinal cortex are from unimodal visual association areas in the adjacent inferior temporal cortex, a region important for visual object processing, whereas parahippocampal cortex receives its predominant inputs from posterior visual association areas and posterior parietal cortex, whose functions are more visuospatial in nature (Suzuki, 2009; Suzuki & Amaral, 1994). Perirhinal and parahippocampal cortex provide the major inputs to entorhinal cortex, which, in turn, provides the major inputs to the hippocampus (Witter & Amaral, 1991; Suzuki & Amaral, 1990; Witter, Van Hoesen, & Amaral, 1989; Amaral, Insausti, & Cowan, 1987; Van Hoesen et al., 1975). Given this convergence of inputs, the hippocampus is hypothesized to bind the distinct elements of an event into an integrated memory representation (Diana, Yonelinas, & Ranganath, 2007; Eichenbaum, Yonelinas, & Ranganath, 2007; Mayes, Montaldi, & Migo, 2007; Davachi, 2006; Manns & Eichenbaum, 2006; Davachi, Mitchell, & Wagner, 2003; O’Reilly & Rudy, 2001). Within the hippocampus, inputs to the dentate gyrus arrive from entorhinal cortex, with the dentate gyrus then projecting to the CA3 field (Lavenex & Amaral, 2000; Amaral & Insausti, 1990). Projections from CA3 provide the primary input to CA1 (Duvernoy, 1998; Amaral & Insausti, 1990), which, in turn, projects to the subiculum, the major output structure of the hippocampus.
Guided by this neuroanatomical knowledge, a central goal of current research is to understand how the mnemonic functions of specific MTL subregions are constrained by intrinsic organization and extrinsic connectivity with neocortex. One possibility is that the unidirectional nature of the connections in the hippocampal formation leads to functional differences in the early and late components of the circuit based on mnemonic stage. Indeed, recent fMRI evidence suggests that hippocampal subfields early in the trisynaptic pathway—namely, voxels encompassing the dentate gyrus, CA2, and CA3—are differentially involved in the successful encoding of episodic memories (Eldridge, Engel, Zeineh, Bookheimer, & Knowlton, 2005; Zeineh, Engel, Thompson, & Bookheimer, 2003), whereas the late component of the trisynaptic pathway—the subiculum—is engaged during episodic retrieval but does not contribute to episodic encoding (Eldridge et al., 2005; Zeineh et al., 2003; Gabrieli, Brewer, Desmond, & Glover, 1997). Other evidence, however, suggests that the subiculum may be sensitive to stimulus novelty (Bakker, Kirwan, Miller, & Stark, 2008; Zeineh et al., 2000), raising the possibility that the subiculum also contributes to or is modulated by episodic encoding.1 Because it is unknown whether such novelty responses in the subiculum correlate with behavioral evidence of successful memory formation, at present, the relation between subiculum activity and encoding is uncertain.
Another factor that is thought to differentiate MTL subfield function is the nature of an event’s content. The extrinsic connectivity between MTL cortical areas and sensory neocortex suggests that MTL subregional contributions to encoding are constrained by the neocortical inputs to each region. In the case of MTL cortical areas, the predominance of inputs from ventral visual cortical areas to perirhinal cortex and dorsal visual cortical areas to parahippocampal cortex suggests that these regions may be differentially sensitive to the encoding of visual objects and visuospatial information, respectively (Diana et al., 2007; Eichenbaum et al., 2007; Davachi, 2006; Manns & Eichenbaum, 2006). Consistent with this hypothesis, MTL damage inclusive of human perirhinal cortex results in recognition memory deficits for objects (Buffalo, Reber, & Squire, 1998) and is also posited to impair visual discrimination of complex objects and faces (Barense, Gaffan, & Graham, 2007; Lee, Buckley, et al., 2006; Barense et al., 2005; Lee, Buckley, et al., 2005; Lee, Bussey, et al., 2005; cf. Shrager, Gold, Hopkins, & Squire, 2006; Levy, Shrager, & Squire, 2005), whereas damage to human parahippocampal cortex results in impaired performance on spatial memory tasks (Epstein, DeYoe, Press, Rosen, & Kanwisher, 2001; Bohbot et al., 1998).
Functional neuroimaging studies offer a mixed picture regarding the nature of content sensitivity in human MTL cortex. On the one hand, some studies have demonstrated differential sensitivity to visual object and visuospatial stimuli along the anterior–posterior axis of the parahippocampal gyrus, with (a) perirhinal cortex demonstrating greater activation when subjects view object stimuli, such as novel objects (Pihlajamaki et al., 2003), encode associations between intraobject features (Staresina & Davachi, 2006, 2008), and encode objects in context (Awipi & Davachi, 2008; Lee, Scahill, & Graham, 2008; Lee, Bandelow, Schwarzbauer, Henson, & Graham, 2006; Pihlajamaki et al., 2004), and (b) parahippocampal cortex demonstrating greater activation when subjects view visuospatial stimuli, such as scenes, houses, spatial configurations, and known landmarks (Awipi & Davachi, 2008; Sommer, Rose, Glascher, Wolbers, & Buchel, 2005; Pihlajamaki et al., 2004; Epstein, Harris, Stanley, & Kanwisher, 1999; Epstein & Kanwisher, 1998; Maguire, Frackowiak, & Frith, 1997). By contrast, a recent study that directly compared activation during the encoding stage of a short-delay recognition task revealed activation in perirhinal cortex during the encoding of both objects and locations, but activation in anterior parahippocampal cortex selective to the encoding of locations (Buffalo, Bellgowan, & Martin, 2006). Multivoxel pattern analysis also suggests that perirhinal activation may not afford discrimination between visual stimulus categories (e.g., objects vs. scenes), whereas parahippocampal activation affords discrimination not only between objects and scenes but also between different classes of visual objects (e.g., faces vs. toys vs. abstract shapes) (Diana, Yonelinas, & Ranganath, 2008). Thus, it remains unclear whether human perirhinal cortex differentially responds to novel visual objects or whether perirhinal function generalizes across novel objects and novel visuospatial stimuli. Moreover, because prior studies have not examined whether content-sensitive MTL cortical activation relates to subsequent memory performance, it remains uncertain whether such MTL cortical responses reflect processes that vary with encoding success (Paller & Wagner, 2002; Brewer, Zhao, Desmond, Glover, & Gabrieli, 1998; Wagner et al., 1998).
Relative to MTL cortex, the greater convergence of neocortical inputs in the hippocampus suggests that hippocampal subfields may contribute to encoding regardless of event content (Diana et al., 2007; Eichenbaum et al., 2007; Davachi, 2006; Manns & Eichenbaum, 2006). Indeed, neuropsychological studies of patients with MTL lesions suggest a distinction between hippocampal and MTL cortical structures based on event content. For example, focal hippocampal damage can result in intact recognition memory for single items and for associations between items of the same domain (e.g., pairs of faces, pairs of words), but marked impairment of memory for across-domain associations (e.g., objects and their locations, faces and spoken names) (Mayes et al., 2004). Neuroimaging data also suggest that the hippocampus supports encoding that generalizes across event content (Awipi & Davachi, 2008; Diana et al., 2008; Staresina & Davachi, 2008; Prince, Daselaar, & Cabeza, 2005). For example, the viewing of faces and hearing of names in isolation activates the hippocampus, whereas face–name pairings may result in a redistributed pattern of hippocampal activation suggestive of the integration of across-domain event elements (Small et al., 2001).
Other evidence, however, suggests that the hippocampus may play a differential role in the processing and encoding of visuospatial information (for a review, see Bird & Burgess, 2008). For example, patients with focal hippocampal lesions demonstrate impaired recognition memory for spatial scenes but intact recognition for faces, whereas patients with larger MTL lesions that also encompassed perirhinal cortex demonstrate impaired recognition for both scenes and faces (Taylor, Henson, & Graham, 2007). Patients with selective hippocampal lesions also demonstrate impaired recognition (Bird, Vargha-Khadem, & Burgess, 2008; Bird, Shallice, & Cipolotti, 2007; Cipolotti et al., 2006) and visual discrimination of visuospatial information (Lee, Buckley, et al., 2005; Lee, Bussey, et al., 2005), with preserved recognition and visual discrimination of novel faces. Moreover, patients with Alzheimer’s disease, which is thought to involve predominant hippocampal atrophy, demonstrate impaired visual discrimination selective for visual scenes, whereas patients with semantic dementia, which is associated with predominant perirhinal damage, demonstrate impaired visual discrimination selective for faces (Lee, Levi, Davies, Hodges, & Graham, 2007; Lee, Buckley, et al., 2006). These observations have led some to posit that the human hippocampus differentially mediates visuospatial encoding, rather than contributing to encoding in a domain-general manner.
The present study sought to address outstanding questions about MTL function, utilizing high-resolution fMRI to examine the role of MTL subregions in mnemonic encoding of visual object (face) and visuospatial (scene) stimuli. High-resolution fMRI techniques provide superior localization of MTL structures, including subfields of the hippocampus, enabling more precision when characterizing the mnemonic function of specific MTL subregions. Few studies have directly compared content-sensitive encoding responses between MTL cortical regions and the hippocampus, and none have examined whether content-sensitive encoding responses exist in subfields of the human hippocampus. Within anatomically defined MTL subregions, the present study characterized (1) each region’s sensitivity to novel faces and scenes, (2) how the response in each region is related to subsequent recognition memory for each stimulus class, and (3) how the magnitude of activation in each region relates to later memory confidence. We were particularly interested in testing whether novelty responses in the subiculum are associated with subsequent memory performance, and whether novelty and subsequent memory effects in each MTL subregion are sensitive to event content (faces and scenes).
METHODS
Participants
Twenty-six healthy, right-handed volunteers participated after giving informed consent in accordance with a protocol approved by the Stanford Institutional Review Board. Participants received $20/hr for their involvement. Data from 20 participants were included in the analyses (age 18–22 years, mean = 19.9 ± 1.5 years; 11 women), with data from the other six being excluded due to poor behavioral performance (4 participants), difficulty with stimulus presentation (1 participant), and excessive motion (1 participant).
Behavioral Procedures
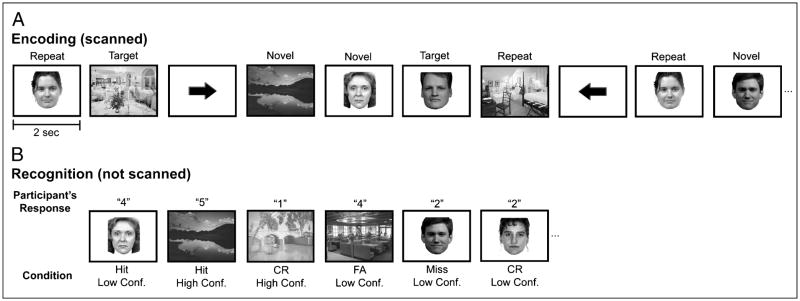
During functional scanning, participants performed a target detection task on pictures of scenes and faces (Figure 1A). Stimuli were generated using Matlab (The MathWorks, Natick, MA) on an iMac laptop computer and back-projected via a magnet-compatible projector onto a screen that could be viewed through a mirror mounted above the participant’s head. Participants responded with a button pad held in their right hand. Across 10 event-related functional runs, participants viewed a total of 200 novel scenes and 200 novel faces, 2 repeated scenes and 2 repeated faces (each seen 25 times across the runs), and 1 target scene and 1 target face (each seen 50 times across the runs). On each trial, a stimulus would appear for 2 sec, and participants indicated with a yes/no keypress whether the stimulus was one of the two target stimuli. Participants viewed both repeated and target stimuli 20 times each prior to scanning to ensure the familiarity of these items. A baseline task was intermixed with the target detection trials. During baseline trials, an arrow was presented for 2 sec; participants were instructed to indicate by key-press whether the arrow pointed to the left or to the right. The order of conditions was determined by a sequencing program that optimizes the efficiency of event-related fMRI designs (Dale, 1999).
Figure 1.
Materials and task design. (A) The scanned encoding task required participants to perform target detection on novel and repeated face and scene stimuli. (B) During an unscanned recognition memory task, participants viewed the novel stimuli from the target detection task as well as unstudied faces and scenes. Participants provided recognition responses based on a 5-point scale (1 = absolutely sure an item was new, 2 = somewhat sure an item was new, 3 = very unsure an item was old or new, 4 = somewhat sure an item was old, and 5 = absolutely sure an item was old).
The experiment consisted of a study–test, study–test design, such that after the first five study runs there was a recognition memory test, and then the second five study runs were followed by a second recognition memory test. During each test, memory for the novel stimuli presented during the immediately preceding five study runs was assessed via a recognition test (not scanned) consisting of the 200 studied items (100 novel scenes, 100 novel faces) intermixed with 100 foils (50 unstudied scenes, 50 unstudied faces). Participants viewed each stimulus and made recognition memory judgments along a 5-point confidence scale: 1 = absolutely sure an item was new, 2 = somewhat sure an item was new, 3 = very unsure an item was old or new, 4 = somewhat sure an item was old, and 5 = absolutely sure an item was old (Figure 1B). Participants were instructed to minimize “3” responses and to use that category only when they were very uncertain about the status of the test probe. Each recognition memory test was self-paced and lasted approximately 15 min. Items were counterbalanced across conditions, functional runs, and participants.
Imaging Procedures
Imaging data were acquired on a 3.0-T Signa whole-body MRI system (GE Medical Systems, Milwaukee, WI, USA) with a custom-made transmit/receive head coil. Head movement was minimized using a “bite bar” and additional foam padding. Prior to functional imaging, high-resolution, T2-weighted, flow-compensated spin-echo structural images [repetition time (TR) = 3000 msec; echo time (TE) = 68 msec; 0.43 × 0.43 mm in-plane resolution] were acquired in twenty-two 3-mm-thick slices perpendicular to the main axis of the hippocampus, allowing for the segmentation of hippocampal subfields (CA2/3/DG, CA1, and subiculum) and MTL cortices (perirhinal, parahippocampal, and entorhinal cortices).
Functional images were acquired using a high-resolution T2*-sensitive gradient-echo spiral in/out pulse sequence (Glover & Law, 2001) with the same slice locations as the structural images (TR = 4000 msec; TE = 34 msec; flip angle = 90°; FOV = 22 cm; 1.7 × 1.7 × 3.0 mm resolution). Prior to functional scanning, a high-order shimming procedure, based on spiral acquisitions, was utilized to reduce B0 heterogeneity (Kim, Adalsteinsson, Glover, & Spielman, 2002). Critically, spiral in/out methods are optimized to increase signal-to-noise ratio and BOLD contrast-to-noise ratio in uniform brain regions while reducing signal loss in regions compromised by susceptibility-induced field gradients (SFG) (Glover & Law, 2001), including the anterior MTL. Compared to other imaging techniques (Glover & Lai, 1998), spiral in/out methods result in less signal dropout and greater task-related activation in the MTL (Preston, Thomason, Ochsner, Cooper, & Glover, 2004), allowing targeting of structures that have previously proven difficult to image due to SFG (e.g., perirhinal cortex).
A total of 630 functional volumes were acquired for each participant over 10 scanning runs. In order to obtain a field map for correction of magnetic field heterogeneity, the first time frame of the functional time series was collected with an echo time 2 msec longer than all subsequent frames. For each slice, the map was calculated from the phase of the first two time frames and applied as a first-order correction during reconstruction of the functional images. In this way, blurring and geometric distortion were minimized on a per-slice basis. In addition, correction for off-resonance due to breathing was applied on a per-time-frame basis using phase navigation (Pfeuffer, Van de Moortele, Ugurbil, Hu, & Glover, 2002). This initial volume was then discarded as well as the following two volumes of each scan (a total of 12 sec) to allow for T1 stabilization.
Imaging Analyses
Data were preprocessed using SPM2 (Wellcome Department of Imaging Neuroscience, London, UK) and custom Matlab routines. Functional images were corrected to account for the differences in slice acquisition times by interpolating the voxel time series using sinc interpolation and resampling the time series using the center slice as a reference point. Functional volumes were then realigned to the first volume in the time series to correct for motion. A mean T2*-weighted volume was computed during realignment, and the T2-weighted anatomical volume was coregistered to this mean functional volume. To preserve spatial resolution, data were not spatially smoothed or normalized.
Voxel-based statistical analyses were conducted at the individual participant level, treating each voxel according to a general linear model and accounting for the intrinsic autocorrelation in fMRI data. A statistical model was constructed to assess the relationship between encoding activation and subsequent memory performance for each class of stimuli. In this model, novel trials were categorized based on responses on the subsequent recognition memory test. Separate regressors for each stimulus class were constructed for items that were later recognized with high confidence (5 responses) and low confidence (4 responses), and for forgotten items (1 and 2 responses). Very unsure responses (3 responses) were included as a regressor in the model when an individual participant had more than 10 responses of this type. Otherwise, these responses were modeled as a covariate of no interest. Additional regressors for repeated and target trials for each stimulus class were also included in this model. In this model, each trial was treated as an impulse, and those events were convolved with a canonical hemodynamic response function. Linear contrasts were performed to generate SPM(t) maps representing differences in brain activation between conditions.
Group analyses were performed using region-of-interest (ROI) analyses targeting MTL subregions. Anatomically defined ROIs were demarcated on the high-resolution structural images for hippocampal subfields (CA2/3/DG, CA1, and subiculum) and MTL cortices (perirhinal, parahippocampal, and entorhinal cortices), using techniques adapted for analysis and visualization of MTL subregions (Zeineh et al., 2000, 2003; Pruessner et al., 2000, 2002; Insausti et al., 1998; Amaral & Insausti, 1990). In addition, because hippocampal subfields cannot be delineated in the most anterior and posterior extents of the hippocampus at the resolution employed, anterior hippocampal and posterior hippocampal ROIs (inclusive all subfields) were also demarcated (Zeineh et al., 2003); these regions correspond approximately to MNI coordinates of y = 0 to y = −6 for the anterior hippocampus and y = −33 to y = −40 for the posterior hippocampus. For each anatomical ROI, percent signal change was extracted separately for each stimulus class for the following four conditions: (1) high-confidence hits, (2) low-confidence hits, (3) misses, and (4) repeated items. Percent signal change was calculated using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002) as the peak amplitude of the observed hemodynamic response averaged across all voxels in each region, which, for all conditions, was the second TR representing the period of time 4 to 8 sec poststimulus onset. For each MTL region, data were submitted to a mixed-effects ANOVA with hemisphere, stimulus class, and condition as repeated factors and participants as a random effect. Planned comparisons were subsequently performed to assess pairwise differences between stimulus class and memory condition effects.
Anatomically defined ROIs average activation across all voxels within a region, whose response may be heterogeneous in nature or nonresponsive to the task. To extend the anatomically defined ROI analyses, additional functionally defined ROIs focused on voxels that demonstrated sensitivity to novel face and scene stimuli relative to baseline. A statistical model was calculated with regressors for novel, repeated, and target trials for each stimulus class. For individual participants, contrast images were defined for (1) novel faces relative to baseline and (2) novel scenes relative to baseline. These contrasts were then masked with the anatomically defined ROIs for that participant. To ensure inclusion of all voxels modulated by the encoding task, a liberal threshold of p < .025 (uncorrected) was used to isolate active voxels within each anatomical region.
For these content-sensitive voxels in each MTL region, we assessed the functional overlap within an individual participant to determine the percentage of voxels that were sensitive to (1) novel face stimuli, (2) novel scene stimuli, or (3) both novel face and scene stimuli. Differences in content sensitivity were compared across MTL regions by repeated-measures ANOVAs. These three populations of voxels were treated as distinct functional ROIs. To assess subsequent memory effects in these novelty sensitive voxels, similar procedures to those used in the anatomically defined ROI analyses were employed for data extraction and statistical analyses for these functionally defined ROIs, wherein encoding activation was compared across novel stimuli based on subsequent recognition. Data are reported for ROIs with an average of four or more activated voxels.
RESULTS
Target Detection Performance
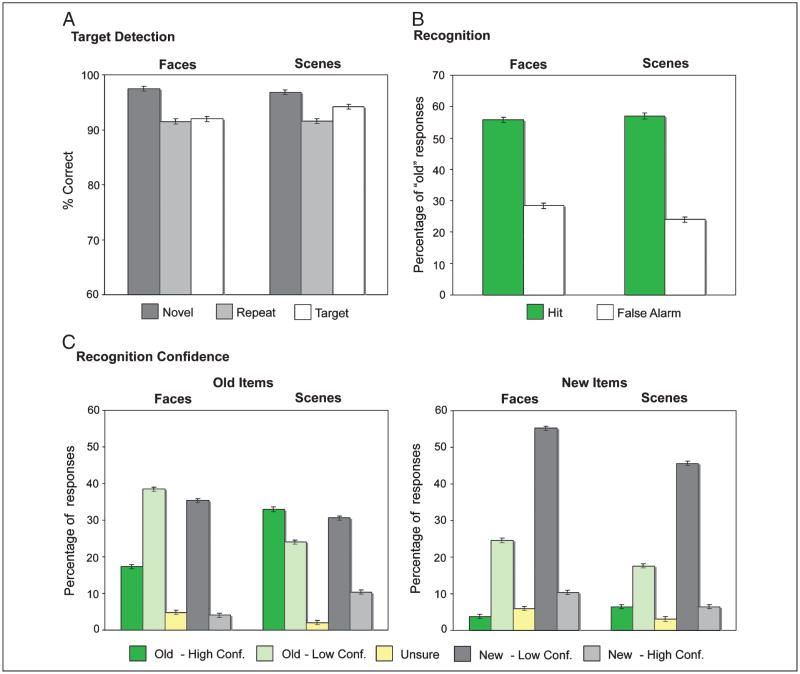
Percent correct performance on the target detection task averaged 93.7 (SE = 1.5) for face and 94.2 (SE = 1.4) for scene stimuli (Figure 2A). A repeated-measures ANOVA revealed a trend for an effect of trial type [novel, repeated, target: F(2, 38) = 3.13, p = .055], but no effect of stimulus class (face, scene: F < 1) nor an interaction [F(2, 38) = 1.03, p > .35]. Pairwise comparisons revealed superior performance on novel trials (97.2, SE = 0.5) relative to repeated [91.6, 3.0; t(19) = 2.08, p = .05] and target trials [93.1, 1.1; t(19) = 3.79, p = .001]. Analyses of reaction times on correct trials revealed an effect of stimulus class [F(1, 19) = 23.09, p < .001], but no effect of trial type (F < 1). Reaction times for faces (748 msec, SE = 19 msec) were faster than those for scenes (791 msec, 20 msec). A Stimulus class × Trial type interaction [F(2, 38) = 5.51, p < .01] revealed that reaction times decreased from target to novel face stimuli, but increased from target to novel scene stimuli. Pairwise comparisons between trial types for both faces and scenes, however, revealed no significant differences (ts < 1).
Figure 2.
Behavioral performance. (A) Target detection accuracy during scanned encoding for novel (dark gray), repeated (light gray), and target (white) stimuli. (B) Percentage of “old” responses on the recognition memory task collapsed across confidence for face and scene stimuli. Hits (green) and false alarms (white) are plotted separately for each stimulus class. (C) Percentage of responses on the recognition memory task for old and new items by level of confidence for face and scene stimuli. High-confidence “old” responses (dark green), low-confidence “old” responses (light green), “unsure” responses (yellow), low-confidence “new” responses (dark gray), and high-confidence “new” responses (light gray) are plotted separately for each stimulus class.
Recognition Memory Performance
Hit and false alarm rates on the subsequent recognition memory test are depicted in Figure 2B. Corrected recognition performance (hits – false alarms) was significantly above chance for faces [t(19) = 9.59, p < .001] and scenes [t(19) = 9.31, p < .001]; corrected recognition was modestly but significantly greater for scenes than faces [t(19) = 2.74, p < .05]. Additional analysis of performance by response confidence revealed more high-confidence “old” responses for scene relative to face stimuli, as evidenced by a significant interaction between stimulus class and confidence level [F(4, 76) = 25.20, p < .001; Figure 2C]. Moreover, the ability to confidently differentiate between old and new stimuli was superior for scene relative to face stimuli, as revealed by a three-way interaction of stimulus class, trial type (old vs. new), and recognition response (high confidence–old, low confidence–old, low confidence–new, high confidence–new) [F(4, 76) = 15.79, p < .001].
Anatomical ROI Analyses
For each anatomically defined MTL subregion (ROI), a repeated-measures ANOVA was performed to assess the effects of hemisphere, stimulus class (faces and scenes), and condition (high confidence [HC] recognized, low confidence [LC] recognized, forgotten [F], and repeated items) on encoding activation (Table 1). Overall, hemisphere did not interact with either stimulus class or condition, except where noted below. As detailed in Table 1, effects of stimulus class and condition were predominantly observed in MTL cortex.
Table 1.
Anatomically Defined ROIs
| Stimulus |
Condition |
Stimulus × Condition |
|
|---|---|---|---|
| Region | F(1, 19) (p) | F(3, 57) (p) | F(3, 57) (p) |
| MTL Cortex | |||
| Perirhinal | – | 3.04 (.04) | – |
| Parahippocampal | 59.26 (<.001) | – | – |
| Entorhinal | 5.14 (.04) | 2.19 (.10) | – |
| Hippocampus | |||
| CA2,3/Dentate gyrus | 3.46 (.08) | – | 2.29 (.10) |
| CA1 | – | – | – |
| Subiculum | – | – | – |
| Anterior hippocampus | 5.86 (.03) | – | – |
| Posterior hippocampus | – | – | – |
Values in bold indicate significance at p < .05; – indicates p > .10.
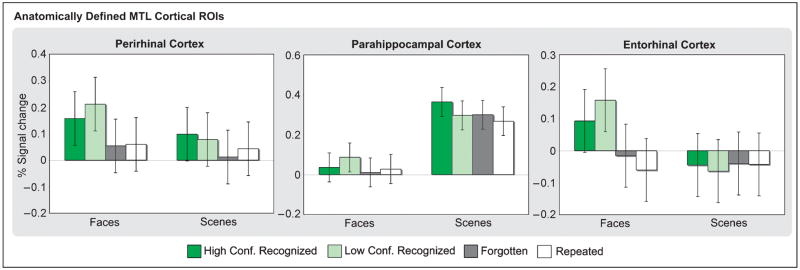
The effect of stimulus class differed across MTL cortical areas (Figure 3; Table 1). In particular, parahippocampal cortex was more active during the processing of scenes relative to faces, whereas activation was greater during face relative to scene processing in perirhinal and entorhinal cortex, with this effect being significant in the latter region. These across-region differences were supported by Region × Stimulus class interactions [parahippocampal vs. perirhinal: F(1, 19) = 29.09, p < .001; parahippocampal vs. entorhinal: F(1, 19) = 33.93, p < .001].
Figure 3.
Encoding activation in anatomically defined MTL cortical regions based on subsequent memory. Percent signal change in perirhinal, parahippocampal, and entorhinal cortices is plotted for stimuli recognized with high confidence (dark green), stimuli recognized with low confidence (light green), forgotten (dark gray), and repeated (white) stimuli for faces and scenes.
The effect of condition was modest in these anatomically based ROIs, reaching significance only in perirhinal cortex (Figure 3; Table 1). Pairwise comparisons revealed greater perirhinal activation for HC and LC recognized stimuli relative to F stimuli [t(19) = 2.44, p < .05 and t(19) = 2.33, p < .05, respectively] and to repeated stimuli [t(19) = 1.84, p = .08 and t(19) = 2.31, p < .05, respectively]. Similarly, whereas entorhinal cortex did not demonstrate an effect of condition or a Stimulus class × Condition interaction, a separate ANOVA examining condition during face encoding revealed greater activation during LC recognized faces relative to F faces [t(19) = 2.81, p = .01] as well as HC and LC recognized relative to repeated faces [t(19) = 2.12, p < .05 and t(19) = 4.12, p = .001, respectively].
Within the hippocampus, the only ROI to demonstrate a main effect across the factors of interest was the anterior hippocampus (Table 1), with activation being greater for face relative to scene stimuli. Within specific hippocampal subfields, the subiculum demonstrated a Hemisphere × Condition interaction [F(1, 19) = 4.20, p < .01]. In the left subiculum, activation was greater during HC and LC recognized stimuli relative to repeated stimuli [t(19) = 1.88, p = .08 and t(19) = 2.67, p < .05, respectively], with a trend for greater activation during LC recognized relative to F stimuli [t(19) = 1.78, p = .09]. By contrast, right subiculum activation did not differ between novel and repeated stimuli (ts < 1), although there was a trend for greater activation during LC recognized relative to F stimuli [t(19) = 2.02, p = .06].
Content-sensitive Voxels
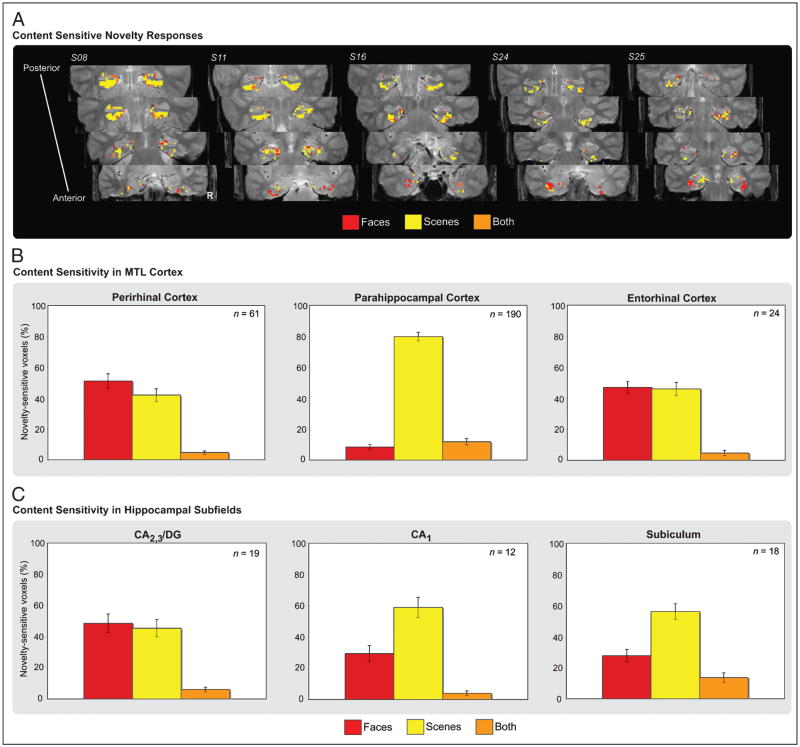
The preceding anatomically based analyses are maximally sensitive when all voxels within a region respond to task conditions in a homogenous manner. However, this is often not the case, as distinct populations of voxels within a region may be unresponsive to task conditions or different populations of voxels within a region may demonstrate different patterns of sensitivity. Accordingly, we explored the potential for heterogeneity of responses within MTL subregions by identifying voxels demonstrating sensitivity to novel stimuli of each class (regardless of memory outcome) relative to baseline (p < .025; see Methods). Given the modest effect of hemisphere in our anatomical ROI analyses, we considered voxels from each MTL subregion collapsed across hemisphere. For content-sensitive voxels in each region, we then assessed the functional overlap of effects to determine the proportion of active voxels sensitive to face stimuli, scene stimuli, or both face and scene stimuli (Figure 4A). As with the anatomical ROI analyses, these functional procedures were performed on individual subject data and then pooled for group-level analysis.
Figure 4.
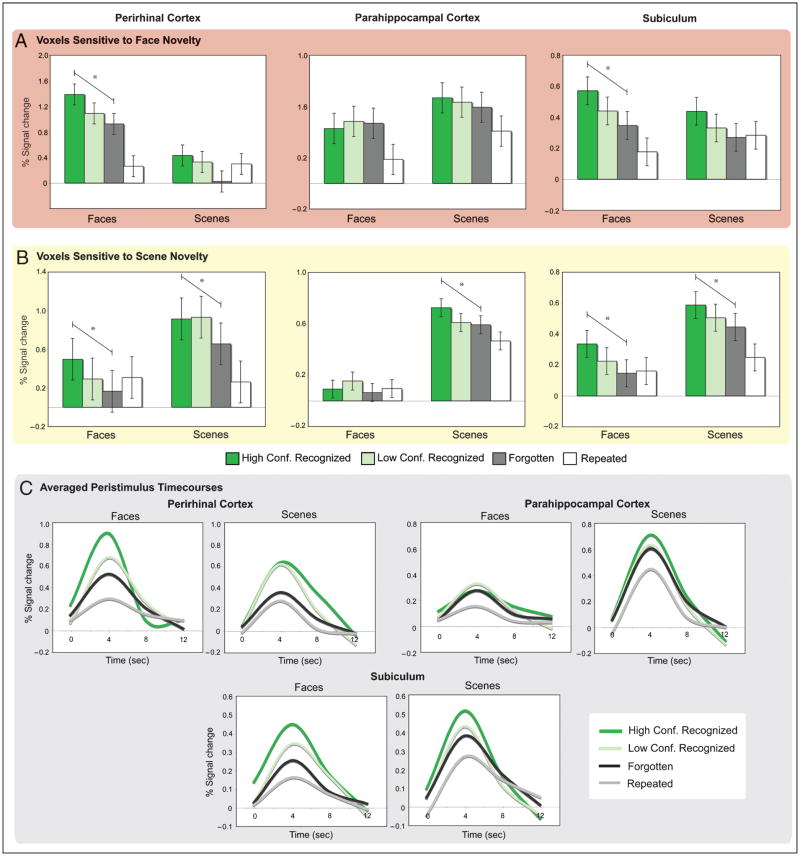
(A) MTL novelty responses sensitive to stimulus content displayed for five individual participants. Voxels sensitive to face (red), scene (yellow), and both face and scene (orange) novelty are displayed along the anterior–posterior axis of the MTL. The region of the parahippocampal gyrus depicted in the two most anterior slices corresponds to perirhinal cortex, whereas the region of the parahippocampal gyrus depicted in the two most posterior slices corresponds to parahippocampal cortex. (B) Pattern of content sensitivity in anatomically defined MTL cortical regions. For voxels demonstrating sensitivity to novel stimuli relative to baseline, the percentage of novelty-sensitive voxels response to faces (red), scenes (yellow), and both classes of stimuli (orange) are plotted for perirhinal, parahippocampal, and entorhinal cortices, and similarly in (C) for the hippocampal subfields (CA2,3/DG, CA1, and subiculum). The mean number of content-sensitive voxels within a region is represented in the upper right-hand corner of the graph for each ROI.
Analysis of stimulus class effects revealed predominant functional homogeneity in parahippocampal cortex and functional heterogeneity in perirhinal cortex and entorhinal cortex. Specifically, in parahippocampal cortex, the percentage of active voxels selective for scene stimuli (79.8%) was greater than that selective for face stimuli [8.4%; t(19) = 16.90, p < .001], indicating a relative homogeneity of response (Figure 4B). A chi-square test of content-sensitive voxel counts performed on individual participants revealed that all 20 of our participants demonstrated more scene-sensitive than face-sensitive voxels in parahippocampal cortex (all χ2 > 4.26, p < .05). By contrast, the percentage of active voxels demonstrating selectivity to face or to scene stimuli did not significantly differ in perirhinal cortex [51.0% vs. 41.9%; t(19) = 1.09, p > .10] nor in entorhinal cortex (47.0% vs. 45.9%; t < 1). In perirhinal cortex, a chi-square test of novelty responsive voxels revealed that five participants demonstrated more face-sensitive than scene-sensitive voxels (all χ2 > 4.55, p < .05), whereas five participants demonstrated the opposite pattern of content sensitivity (all χ2 > 6.90, p < .01); the remaining 10 participants demonstrated no significant difference (all χ2 < 4). Chi-square tests of content sensitivity in entorhinal cortex revealed no significant effects in any of the 16 participants with a sufficient number of voxels to permit analysis (all χ2 < 3). The across-region differences in content sensitivity between parahippocampal and perirhinal cortex were supported by Region × Stimulus class interactions based on voxel percentages [F(2, 38) = 63.13, p < .001], as well as a Region × Stimulus class interaction based on individual chi-square tests of voxel frequencies (all χ2 > 4, p < .05).
Functional differences were also observed between hippocampal ROIs (Figure 4C). Whereas the percentage of active voxels selective for faces (48.5%) or for scenes (43.4%) did not differ in CA2,3/DG (t < 1), the percentage of scene- versus face-selective voxels was greater in CA1 [59.0% vs. 29.4%, respectively; t(19) = 2.89, p < .01] and in the subiculum [56.1% vs. 27.7%; t(19) = 3.37, p < .005]. Region × Stimulus class interactions confirmed that content sensitivity in CA2,3/DG differed from that in CA1 [F(2, 38) = 4.82, p < .05] and the subiculum [F(2, 38) = 8.57, p = .001]. Individually based chi-square tests of content-sensitive voxel frequencies revealed that seven participants demonstrated greater sensitivity to scene novelty relative to face novelty in CA1 (all χ2 > 4, p < .05), with the remaining participants demonstrating no difference between stimulus class (all χ2 < 3). A similar pattern was observed in the subiculum, with eight participants demonstrating greater sensitivity to scene novelty relative to face novelty (all χ2 > 4, p < .05). Although six participants demonstrated content preferences in CA2,3/DG, two demonstrated a greater number of novelty-sensitive voxels for faces relative to scenes (all χ2 > 5.4, p < .05) and four demonstrated a preference for scenes (all χ2 > 4.5, p < .05).
Subsequent Memory Effects in Content-sensitive Voxels: Perirhinal Cortex
To assess whether encoding activation varied as a function of later memory performance in content-sensitive voxels, a repeated-measures ANOVA was performed with subsequent memory condition (HC recognized, LC recognized, and F) and stimulus class (face and scene) as factors. Separate ANOVAs were performed on the face- and scene-sensitive voxels in each ROI.
In perirhinal cortex, voxels sensitive to faces demonstrated an effect of memory condition that did not interact with stimulus class (Figure 5A; Table 2). These face-sensitive voxels demonstrated graded activation according to subsequent memory for novel stimuli [HC recognized, LC recognized, and F; linear trend: F(1, 19) = 6.43, p = .02]. Pairwise comparisons revealed greater activation during HC recognized relative to F stimuli [t(19) = 2.54, p < .05] as well as LC recognized relative to F stimuli [t(19) = 2.84, p = .01]. When encoding effects were considered separately for each stimulus class, perirhinal face-sensitive voxels demonstrated a graded subsequent memory effect for faces, as evidenced by a significant linear trend across the HC recognized, LC recognized, and F conditions [F(1, 19) = 13.70, p < .005]. Pairwise comparisons revealed greater activation during the encoding of HC recognized faces relative to LC recognized [t(19) = 2.11, p < .05] and F faces [t(19) = 3.70, p < .01], and a trend for greater activation during LC recognized relative to F faces [t(19) = 1.90, p = .07]. In contrast, perirhinal face-sensitive voxels did not demonstrate an effect of memory for scenes when indexed by either linear contrasts or pairwise comparisons (ps > .10).
Figure 5.
Encoding activation in content-sensitive (A) face and (B) scene voxels based on subsequent memory. Percent signal change in perirhinal and parahippocampal cortices as well as the subiculum is plotted for stimuli recognized with high confidence (dark green), stimuli recognized with low confidence (light green), forgotten (dark gray), and repeated (white) stimuli for faces and scenes. Asterisks indicated a significant linear trend across subsequent memory performance for novel stimuli. (C) Peristimulus time courses averaged across face- and scene-sensitive voxels in functionally defined ROIs for face and scene stimuli recognized with high confidence (dark green), stimuli recognized with low confidence (light green), forgotten (black), and repeated (gray).
Table 2.
Functionally Defined Face-sensitive Voxels
| Stimulus |
Memory Condition |
Stimulus × Memory Condition |
|
|---|---|---|---|
| Region (n) | F (p) | F (p) | F (p) |
| MTL Cortex | |||
| Perirhinal (20) | 52.29 (<.001) | 5.14 (.02) | – |
| Parahippocampal (20) | 3.55 (.08) | – | – |
| Entorhinal (20) | 35.29 (<.001) | – | – |
| Hippocampus | |||
| CA2,3/Dentate gyrus (20) | 56.57 (<.001) | – | – |
| CA1 (18) | 32.95 (<.001) | 2.96 (.07) | – |
| Subiculum (19) | 16.44 (.001) | 4.75 (.02) | – |
| Anterior hippocampus (18) | 25.16 (<.001) | – | – |
| Posterior hippocampus (19) | 33.45 (<.001) | – | – |
Values in bold indicate significance at p < .05; – indicates p > .10; n = number of participants included in the analyses.
Perirhinal scene-sensitive voxels demonstrated an effect of memory condition across stimulus class (Figure 5B; Table 3). In these perirhinal voxels, a significant linear trend was observed for novel stimuli based on subsequent memory [F(1, 19) = 12.52, p < .01]. Pairwise comparisons revealed greater activation during HC and LC recognized relative to F stimuli [t(19) = 3.54, p < .01 and t(19) = 2.61, p < .05, respectively]. When considered separately for each stimulus class, encoding activation demonstrated a significant linear trend for novel scenes based on subsequent memory [F(1, 19) = 5.94, p < .05]. However, paired comparisons revealed that although activation was greater during the processing of HC and LC recognized scenes relative to F scenes [t(19) = 2.44, p < .05, and t(19) = 2.23, p < .05, respectively], activation did not differ between the HC and LC conditions (t < 1). Moreover, a subsequent memory effect for the nonpreferred face stimuli was observed in scene-sensitive voxels [linear trend: F(1, 19) = 8.27, p = .01], with paired comparisons revealing greater activation for HC recognized than for F faces [t(19) = 2.88, p = .01].
Table 3.
Functionally Defined Scene-sensitive Voxels
| Stimulus |
Memory Condition |
Stimulus × Memory Condition |
|
|---|---|---|---|
| Region (n) | F (p) | F (p) | F (p) |
| MTL Cortex | |||
| Perirhinal (20) | 71.25 (<.001) | 7.60 (.002) | – |
| Parahippocampal (20) | 271.09 (<.001) | 3.19 (.05) | 3.95 (.04) |
| Entorhinal (20) | 69.49 (<.001) | – | – |
| Hippocampus | |||
| CA2,3/Dentate gyrus (19) | 28.16 (<.001) | – | – |
| CA1 (18) | 107.21 (<.001) | – | – |
| Subiculum (20) | 47.03 (<.001) | 10.72 (<.001) | – |
| Anterior hippocampus (17) | 17.15 (.001) | – | – |
| Posterior hippocampus (19) | 50.75 (<.001) | – | – |
Values in bold indicate significance at p < .05; – indicates p > .10; n = number of participants included in the analyses.
Finally, perirhinal voxels that were sensitive to both novel faces and novel scenes demonstrated an effect of memory condition [F(2, 26) = 5.90, p < .01] that did not differ based on stimulus class [F(2, 26) = 2.69, p = .09]. Specifically, subsequent memory effects were observed in these perirhinal voxels for faces [linear trend: F(1, 13) = 10.22, p < .01] and scenes [linear trend: F(1, 13) = 7.15, p < .05]. HC and LC recognized faces elicited greater activation than F faces [t(13) = 3.20, p < .005, and t(13) = 2.29, p < .05]. Similarly, HC recognized scenes elicited greater activation than F scenes [t(13) = 2.67, p < .005]. However, activation did not differ between stimuli recognized with HC and LC for either faces or scenes (ps > .40).
Subsequent Memory Effects in Content-sensitive Voxels: Parahippocampal Cortex
In contrast to perirhinal cortex, activation in parahippocampal face-sensitive voxels did not differentiate based on subsequent memory (Figure 5A; Table 2). This difference between parahippocampal and perirhinal cortex was supported by a two-way interaction between region (parahippocampal vs. perirhinal) and stimulus class [F(1, 19) = 47.81, p < .001], and by a two-way interaction between region and memory condition [F(2, 38) = 3.65, p < .05].
Parahippocampal scene-sensitive voxels showed an effect of memory and a Memory × Stimulus class interaction (Figure 5B; Table 3). Specifically, parahippocampal scene-sensitive voxels demonstrated a subsequent memory effect for scenes, as evidenced by a significant linear trend across HC recognized, LC recognized, and F scenes [F(1, 19) = 10.16, p < .01]. Pairwise comparisons revealed greater activation during HC recognized relative to LC recognized [t(19) = 2.07, p < .05] and F scenes [t(19) = 3.19, p < .01]. By contrast, although these voxels demonstrated greater activation during LC recognized than during F faces [t(19) = 2.96, p < .01], there were no other significant differences across face stimuli as indexed by either a linear contrast or pairwise comparisons (ps > .15). Parahippocampal and perirhinal differences were again supported by Region × Memory condition [F(3, 57) = 4.78, p < .05] and Region × Stimulus class × Memory condition [F(2, 36) = 3.28, p < .05] interactions.
Parahippocampal voxels sensitive to both novel faces and novel scenes demonstrated an effect of stimulus [scene > face: F(1, 18) = 24.23, p < .001]. However, these parahippocampal voxels did not show a significant effect of memory condition [F(2, 36) = 2.80, p = .08], or a linear trend across the subsequent memory conditions [F(1, 18) = 2.51, p = .13].
Subsequent Memory Effects in Content-sensitive Voxels: Hippocampal Subfields
Memory effects were further interrogated within content-sensitive voxels in the subfields of the hippocampus, where subsequent memory effects were primarily observed in the subiculum (Tables 2 and 3). Specifically, face-sensitive subiculum voxels demonstrated an effect of memory condition that did not interact with stimulus class (Figure 5A; Table 2). These face-sensitive voxels demonstrated graded activation according to subsequent memory for novel stimuli [HC recognized, LC recognized, and F; linear trend: F(1, 18) = 6.71, p < .02], with pairwise comparisons revealing greater activation during HC recognized relative to F stimuli [t(18) = 2.59, p < .05] and a trend for greater activation relative to LC recognized stimuli [t(18) = 1.95, p = .07]. When examining encoding activation separately for each stimulus class, a significant linear trend was observed for novel faces based on subsequent memory [F(1, 18) = 8.77, p < .01], where activation was greater during HC recognized faces relative to F faces [t(19) = 2.96, p < .01]; activation for LC recognized faces was intermediate to that for HC recognized and F faces [t(19) = 1.83, p = .08 and t(19) = 1.79, p = .09, respectively]. Graded subsequent memory effects were not observed when separately considering scene stimuli (ps > .15).
Scene-sensitive subiculum voxels also demonstrated an effect of memory condition across stimulus class (Figure 5B; Table 3). In these voxels, a significant linear trend was observed for novel stimuli based on subsequent memory [F(1, 19) = 18.71, p < .001], with HC recognized stimuli eliciting greater activation than LC recognized [t(19) = 2.49, p < .05] and F stimuli [t(19) = 4.33, p < .001]. LC recognized stimuli further differed from F stimuli [t(19) = 2.29, p < .05] This graded subsequent memory response was observed for both novel faces [F(1, 18) = 9.26, p < .01] and scenes [F(1, 18) = 6.41, p < .05], where encoding activation was greater for HC recognized faces relative to F faces [t(18) = 3.04, p < .01] and for HC recognized scenes relative to F scenes [t(18) = 2.53, p < .05]. Encoding responses for LC recognized items were intermediate to and did not differ from HC recognized or F items for either face or scene stimuli (ts < 1.9).
DISCUSSION
The present high-resolution fMRI study of content-sensitive encoding responses in MTL cortical regions and hippocampal subfields revealed two main findings. First, we observed a dissociation along the anterior–posterior axis of the parahippocampal gyrus, with the magnitude of encoding activation in perirhinal cortex being related to later memory for both faces and scenes, whereas subsequent memory effects in parahippocampal cortex were scene selective. This observation builds on prior reports of differential MTL cortical sensitivity to event content by linking these differential responses to encoding success (as indexed by subsequent memory). Second, the present data extend views of hippocampal encoding mechanisms, providing evidence that encoding responses in the human subiculum correlate with later successful recognition. Moreover, in contrast to parahippocampal cortex, encoding activation in the subiculum predicted subsequent memory strength for both face and scene stimuli.
Content-sensitive Encoding in MTL Cortex
Prior studies that directly compared the processing of visual object and visuospatial information in perirhinal cortex alternately observed specificity for visual object information (Awipi & Davachi, 2008; Lee et al., 2008; Pihlajamaki et al., 2004) or content-general patterns of responding (Buffalo et al., 2006). Consistent with the latter pattern, our data revealed activation in perirhinal cortex that generalized across visual object and visuospatial information, and extended these prior studies by demonstrating that the magnitude of this perirhinal response is related to mnemonic encoding (as indexed by later recognition confidence). The content-general nature of perirhinal responding was supported by three observations. First, across the entire anatomically defined perirhinal region, subsequent memory effects were observed that did not differentiate between stimulus classes. Second, the percentage of perirhinal voxels sensitive to novel faces and the percentage sensitive to novel scenes did not significantly differ. Third, subsequent memory effects were observed for the preferred stimulus class in face- and scene-sensitive voxels, again demonstrating that encoding activation in perirhinal cortex was related to memory outcome for both types of stimuli. Moreover, whereas encoding activation in face-sensitive perirhinal voxels was not predictive of memory for the nonpreferred scene stimuli, activation in scene-sensitive voxels demonstrated a subsequent memory response for the nonpreferred face stimuli, suggesting that the mnemonic function of these scene-sensitive voxels generalizes to support encoding of other stimulus domains such as faces.
The present perirhinal face encoding response is consistent with neuropsychological data demonstrating that MTL damage inclusive of human perirhinal cortex results in recognition memory impairments for objects (Buffalo et al., 1998) and impaired visual discrimination of complex objects and faces (Barense et al., 2005, 2007; Lee, Buckley, et al., 2005, 2006; Lee, Bussey, et al., 2005; cf. Shrager et al., 2006; Levy et al., 2005). By contrast, the present perirhinal subsequent memory effects for visual scenes diverge from studies of semantic dementia patients with damage inclusive of perirhinal cortex (but largely sparing parahippocampal cortex) that have not revealed corresponding deficits in visual discrimination for spatial information (Lee et al., 2007; Lee, Buckley, et al., 2006). One possible account of this discrepancy is that the perirhinal encoding response to visuospatial information reflects the input from parahippocampal cortex to perirhinal cortex (Suzuki, 2009; Suzuki & Amaral, 1994). Another possibility is that complex scene stimuli—such as those used here and in prior studies of visuospatial memory and perception (Kirchhoff et al., 2000; Epstein et al., 1999; Epstein & Kanwisher, 1998; Maguire et al., 1997)—entail images of objects in space. Accordingly, perirhinal activation could reflect encoding of the visual objects in scenes, rather than of visuospatial information per se. Future studies that directly explore functional interactions between perirhinal and parahippocampal cortex and that manipulate the nature of the visuospatial information to be encoded are required to adjudicate between these accounts.
In contrast to perirhinal cortex, our data support a specific role for the parahippocampal cortex in the successful encoding of visuospatial information. Indeed, the anatomically defined parahippocampal region demonstrated greater activation to scene relative to face stimuli, and the vast majority of parahippocampal voxels sensitive to novel stimuli were specifically sensitive to novel scenes. Moreover, subsequent memory effects were only observed in parahippocampal scene-sensitive voxels for the preferred stimulus class, where encoding activation in these voxels was greatest for scenes later recognized with high confidence relative to scenes recognized with low confidence and forgotten scenes. Collectively, the pattern of response in parahippocampal cortex converges with previous neuropsychological (Epstein et al., 2001; Bohbot et al., 1998) and neuroimaging studies (Awipi & Davachi, 2008; Sommer et al., 2005; Pihlajamaki et al., 2004; Epstein et al., 1999; Epstein & Kanwisher, 1998; Maguire et al., 1997) that suggest a specialized role for parahippocampal cortex in the encoding of visuospatial information.
Application of fMRI multivoxel pattern analysis, however, has recently raised questions about the selectivity of human parahippocampal cortical function (Diana et al., 2008). In particular, Diana et al. (2008) demonstrated that parahippocampal activation not only affords classification of whether subjects are viewing objects or scenes but also carries sufficient information to discriminate between different classes of visual objects (e.g., faces vs. toys vs. abstract shapes), suggesting that nonspatial information is processed within the parahippocampal region. Moreover, Bar, Aminoff, and Ishai (2008), Bar, Aminoff, and Schacter (2008), Aminoff, Gronau, and Bar (2007), Bar (2004), and Bar and Aminoff (2003) have hypothesized that parahippocampal cortex is generally involved in processing both spatial and nonspatial contextual information. Consistent with these findings, the current study revealed a minority of voxels in parahippocampal cortex that were sensitive to novel faces. However, encoding activation in these face-sensitive parahippocampal voxels did not predict later memory for faces, suggesting that parahippocampal processing is not reliably related to face mnemonic encoding.2 As such, the present data suggest that the core MTL cortical representation critical to successful encoding of faces may be based on mnemonic processing in perirhinal cortex.
Content-sensitive Encoding in Hippocampal Subfields
Our results indicate a domain-general role for the hippocampus in the successful formation of new memories, extending current evidence on hippocampal function by demonstrating that subiculum activation relates to successful event encoding. Interestingly, although the proportion of voxels in the subiculum sensitive to novel scenes was greater than that sensitive to novel faces, subsequent memory effects were observed in both the face- and scene-sensitive voxels. Moreover, subsequent memory effects were observed for both the preferred and nonpreferred stimuli in the scene-sensitive subiculum voxels.
The observation of significant subsequent memory effects in the human subiculum diverge from two prior high-resolution fMRI studies that demonstrated a dissociation between hippocampal subregions based on the stage of episodic memory processing (Eldridge et al., 2005; Zeineh et al., 2003). Specifically, in a blocked-design study, Zeineh et al. (2003) revealed that encoding activation in a region encompassing CA2/3/DG correlated with associative learning, whereas activation in the subiculum predominantly related to episodic retrieval. Complementing these findings, Eldridge et al. (2005) assessed subsequent memory and retrieval effects in hippocampal subfields using a paired associate task, where later recognition of one member of a study pair was probed using the remember–know (R/K) paradigm. Eldridge et al. observed greater encoding activation in CA2/3/DG associated with subsequent R and K hits relative to later forgotten items, whereas retrieval activity in the subiculum was greater during R hits relative to K hits, misses, and correct rejections. Interestingly, Eldridge et al. also observed that encoding activity in the left subiculum was greater for subsequent K hits relative to both subsequent R hits and forgotten items (R hits and forgotten items did not differ). Interpretation of this encoding pattern is complex, however, as one would expect that a region involved in successful memory formation would demonstrate similar encoding responses for subsequent R and K hits or greater activation for R hits than K hits, rather than the reverse. By contrast, the current study replicated prior observations of novelty-related responses in the subiculum (Bakker et al., 2008; Zeineh et al., 2000), and extended these findings by demonstrating that encoding activation in the subiculum was graded based on later recognition memory strength. Accordingly, the present data challenge the notion that subiculum encoding activation is unrelated to successful memory formation.
It is worth noting that although we observed voxels in every hippocampal region that was sensitive to novel stimuli, activation in CA1 and in CA2/3/DG was not associated with later memory performance. This finding differs from the encoding responses observed in CA2/3/DG when using associative memory paradigms (Eldridge et al., 2005; Zeineh et al., 2003), raising the possibility that variance in CA field activation during encoding is not as strongly related to later item recognition memory as it is to later performance on memory tasks that test memory for the conjunction or relation between multiple event elements. Alternatively, the present absence of clear subsequent memory effects in CA1 and in CA2/3/DG may reflect a null result that should be interpreted with caution. Indeed, given the trisynaptic circuit, it is unclear how the subiculum could mediate subsequent memory in the absence of associated processes within CA1 and in CA2/3/DG based on its hypothesized role in the amplification of CA field responses (O’Mara, 2006). On the other hand, it has also been suggested that, through direct projections from MTL cortical regions (including perirhinal cortex), the subiculum may be important for object–space associative memory that combines input from MTL cortical regions with input from the CA fields (O’Mara, 2006). Accordingly, although our findings argue against a restricted role of the subiculum to retrieval, future studies are required to more fully understand how the subiculum interacts with earlier components of the hippocampal circuit to support episodic encoding.
The current findings support theories that suggest that the hippocampus contributes to mnemonic encoding regardless of event content (Diana et al., 2007; Eichenbaum et al., 2007; Davachi, 2006; Manns & Eichenbaum, 2006). Consistent with our findings, recent neuroimaging work has demonstrated sensitivity to novel faces and scenes within the hippocampus (Lee et al., 2008), and additional studies have demonstrated content-general encoding responses in the anterior hippocampus (Awipi & Davachi, 2008) that are related to the amount and not to the type of information successfully encoded (Staresina & Davachi, 2008). Our data extend these findings by demonstrating that encoding responses in the subiculum are directly related to later recognition confidence across stimulus classes. Moreover, our findings bear on current arguments about whether the hippocampus subserves domain-general or domain-sensitive encoding. Specifically, whereas some neuropsychological findings suggest that damage limited to the hippocampus selectively impairs recognition memory for visuospatial information (Bird et al., 2007, 2008; Lee et al., 2007; Taylor et al., 2007; Cipolotti et al., 2006; Lee, Buckley, et al., 2005, 2006; Lee, Bussey, et al., 2005), other findings reveal that recognition impairments for visual words, objects, and faces can result from selective hippocampal damage (Bayley, Wixted, Hopkins, & Squire, 2008; Gold, Hopkins, & Squire, 2006; Manns, Hopkins, Reed, Kitchener, & Squire, 2003; Stark & Squire, 2003). The present subsequent memory effects for both preferred and nonpreferred stimuli in content-sensitive subiculum voxels are compatible with the domain-general view of mnemonic processing in the human hippocampus.
MTL Encoding Activation and Later Memory Strength
The present data may also bear on recent efforts to understand the relation between MTL activation at encoding and gradations in later memory performance, such as gradations in subsequent memory confidence (Shrager, Kirwan, & Squire, 2008; Ranganath et al., 2003). In particular, a recent study demonstrated encoding activation in left perirhinal cortex and bilateral hippocampus that positively correlated with three levels of subsequent recognition confidence for hits, suggesting that the magnitude of perirhinal and hippocampal activation during learning is correlated with the strength of the memory formed (Shrager et al., 2008). However, in the Shrager et al. (2008) study, when perirhinal and hippocampal activation was considered across five levels of confidence that also included the low- and high-confidence forgotten items, a U-shaped function was observed such that activation for subsequently recognized items did not differ from that for forgotten items. This U-shaped pattern may call into question the relationship between encoding activation in these regions and later memory strength. By contrast, the present data demonstrated clear graded subsequent memory effects in perirhinal cortex and in the subiculum that differ based on later recognition confidence, as activation was greatest for items later recognized with high confidence, intermediate for items later recognized with low confidence, and lowest for items later forgotten. This graded subsequent memory effect in perirhinal cortex is consistent with Ranganath et al. (2003), who demonstrated encoding activation in perirhinal cortex that varied in a continuous manner across recognition confidence, and extends this pattern to the subiculum. However, because the present subsequent memory test did not differentiate conjunctive memory strength from item memory strength,3 future studies are needed to determine whether this qualitatively similar pattern in perirhinal cortex and the subiculum reflect qualitatively similar or distinct mnemonic functions.
Conclusions
The present study documents a gradient of content sensitivity from posterior (parahippocampal) to anterior (perirhinal) MTL cortex, with MTL cortical regions differentially contributing to successful mnemonic encoding based on event content. In contrast to recent suggestions, the present data further indicate that the subiculum may contribute to successful encoding irrespective of event content. The current findings highlight the need for consideration of event content when evaluating encoding processes in MTL regions and support theories that suggest that, in contrast to the hippocampus, the nature of an event’s content is an important organizing principle within MTL cortical regions (Diana et al., 2007; Eichenbaum et al., 2007; Davachi, 2006; Manns & Eichenbaum, 2006). Finally, our results demonstrate that encoding responses in the subiculum and perirhinal cortex vary with later recognition confidence, providing evidence that the magnitude of encoding activation in these regions correlates with the strength of the memory formed.
Acknowledgments
Supported by the National Institute of Mental Health (5R01-MH076932 to A. D. W. and F32-MH071092 to A. R. P.), the National Alliance for Research on Schizophrenia and Depression, and the Alfred P. Sloan Foundation.
Footnotes
Following the neuroimaging literature on encoding correlates of subsequent recognition memory, the term episodic memory is used here to encompass memory for individual event elements (items) as well as memory for item–context conjunctions.
Although the failure to observe subsequent memory effects for face stimuli in parahippocampal cortex may be due to low power because of the relatively small number of voxels in this region that demonstrated face-sensitive novelty responses, we note that similar numbers of content-sensitive voxels were isolated in the subiculum that demonstrated clear subsequent memory effects. This latter finding may argue against a power interpretation of the null face encoding effects in parahippocampal cortex.
We note that some recognition models have posited that item familiarity and recollection can both vary in a continuous manner (Rotello, Macmillan, & Reeder, 2004; Wixted & Stretch, 2004; cf. Yonelinas, 1994). As with the present study, the data from Shrager et al. (2008) do not provide leverage on whether the gradation in memory strength was for item information, recollective information, or some combination of the two, as the nature of the recognition memory test did not distinguish subsequent memory responses related to item versus recollective (conjunctive) processes.
References
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The human nervous system. San Diego, CA: Academic Press; 1990. pp. 711–755. [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. Journal of Comparative Neurology. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cerebral Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Awipi T, Davachi L. Content-specific source encoding in the human medial temporal lobe. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2008;34:769–779. doi: 10.1037/0278-7393.34.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller M, Stark CE. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar M. Visual objects in context. Nature Reviews Neuroscience. 2004;5:617–629. doi: 10.1038/nrn1476. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Ishai A. Famous faces activate contextual associations in the parahippocampal cortex. Cerebral Cortex. 2008;18:1233–1238. doi: 10.1093/cercor/bhm170. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E, Schacter DL. Scenes unseen: The parahippocampal cortex intrinsically subserves contextual associations, not scenes or places per se. Journal of Neuroscience. 2008;28:8539–8544. doi: 10.1523/JNEUROSCI.0987-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Bussey TJ, Lee AC, Rogers TT, Davies RR, Saksida LM, et al. Functional specialization in the human medial temporal lobe. Journal of Neuroscience. 2005;25:10239–10246. doi: 10.1523/JNEUROSCI.2704-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Gaffan D, Graham KS. The human medial temporal lobe processes online representations of complex objects. Neuropsychologia. 2007;45:2963–2974. doi: 10.1016/j.neuropsychologia.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Bayley PJ, Wixted JT, Hopkins RO, Squire LR. Yes/no recognition, forced-choice recognition, and the human hippocampus. Journal of Cognitive Neuroscience. 2008;20:505–512. doi: 10.1162/jocn.2008.20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: Insights from spatial processing. Nature Reviews Neuroscience. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bird CM, Shallice T, Cipolotti L. Fractionation of memory in medial temporal lobe amnesia. Neuropsychologia. 2007;45:1160–1171. doi: 10.1016/j.neuropsychologia.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Bird CM, Vargha-Khadem F, Burgess N. Impaired memory for scenes but not faces in developmental hippocampal amnesia: A case study. Neuropsychologia. 2008;46:1050–1059. doi: 10.1016/j.neuropsychologia.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Bohbot VD, Kalina M, Stepankova K, Spackova N, Petrides M, Nadel L. Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia. 1998;36:1217–1238. doi: 10.1016/s0028-3932(97)00161-9. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: Brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PS, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learning and Memory. 2006;13:638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Reber PJ, Squire LR. The human perirhinal cortex and recognition memory. Hippocampus. 1998;8:330–339. doi: 10.1002/(SICI)1098-1063(1998)8:4<330::AID-HIPO3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cipolotti L, Bird C, Good T, Macmanus D, Rudge P, Shallice T. Recollection and familiarity in dense hippocampal amnesia: A case study. Neuropsychologia. 2006;44:489–506. doi: 10.1016/j.neuropsychologia.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Squire LR. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Current Opinion in Neurobiology. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell J, Wagner AD. Multiple routes to memory: Distinct medial temporal lobe processes build item and source memories. Proceedings of the National Academy of Sciences; U.S.A. 2003. pp. 2157–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18:536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudukovic NM, Wagner AD. Goal-dependent modulation of declarative memory: Neural correlates of temporal recency decisions and novelty detection. Neuropsychologia. 2007;45:2608–2620. doi: 10.1016/j.neuropsychologia.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Duvernoy HM. The human hippocampus. New York: Springer; 1998. [Google Scholar]
- Eichenbaum H. A cortical–hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Cohen NJ. From conditioning to conscious recollection: Memory systems of the brain. New York: Oxford University Press; 2001. [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton BJ. A dissociation of encoding and retrieval processes in the human hippocampus. Journal of Neuroscience. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, DeYoe EA, Press DZ, Rosen AC, Kanwisher N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cognitive Neuropsychology. 2001;18:481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: Recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Brewer JB, Desmond JE, Glover GH. Separate neural bases of two fundamental memory processes in the human medial temporal lobe. Science. 1997;276:264–266. doi: 10.1126/science.276.5310.264. [DOI] [PubMed] [Google Scholar]
- Glover GH, Lai S. Self-navigated spiral fMRI: Interleaved versus single-shot. Magnetic Resonance in Medicine. 1998;39:361–368. doi: 10.1002/mrm.1910390305. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gold JJ, Hopkins RO, Squire LR. Single-item memory, associative memory, and the human hippocampus. Learning and Memory. 2006;13:644–649. doi: 10.1101/lm.258406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Cansino S, Herron JE, Robb WG, Rugg MD. A familiarity signal in human anterior medial temporal cortex? Hippocampus. 2003;13:301–304. doi: 10.1002/hipo.10117. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR, American Journal of Neuroradiology. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kim DH, Adalsteinsson E, Glover GH, Spielman DM. Regularized higher-order in vivo shimming. Magnetic Resonance in Medicine. 2002;48:715–722. doi: 10.1002/mrm.10267. [DOI] [PubMed] [Google Scholar]
- Kirchhoff BA, Wagner AD, Maril A, Stern CE. Prefrontal–temporal circuitry for novelty encoding and subsequent memory. Journal of Neuroscience. 2000;20:6173–6180. doi: 10.1523/JNEUROSCI.20-16-06173.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Amaral DG. Hippocampal–neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lee AC, Bandelow S, Schwarzbauer C, Henson RN, Graham KS. Perirhinal cortex activity during visual object discrimination: An event-related fMRI study. Neuroimage. 2006;33:362–373. doi: 10.1016/j.neuroimage.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Gaffan D, Emery T, Hodges JR, Graham KS. Differentiating the roles of the hippocampus and perirhinal cortex in processes beyond long-term declarative memory: A double dissociation in dementia. Journal of Neuroscience. 2006;26:5198–5203. doi: 10.1523/JNEUROSCI.3157-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, et al. Specialization in the medial temporal lobe for processing of objects and scenes. Hippocampus. 2005;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Lee AC, Bussey TJ, Murray EA, Saksida LM, Epstein RA, Kapur N, et al. Perceptual deficits in amnesia: Challenging the medial temporal lobe “mnemonic” view. Neuropsychologia. 2005;43:1–11. doi: 10.1016/j.neuropsychologia.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Lee AC, Levi N, Davies RR, Hodges JR, Graham KS. Differing profiles of face and scene discrimination deficits in semantic dementia and Alzheimer’s disease. Neuropsychologia. 2007;45:2135–2146. doi: 10.1016/j.neuropsychologia.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Lee AC, Scahill VL, Graham KS. Activating the medial temporal lobe during oddity judgment for faces and scenes. Cerebral Cortex. 2008;18:683–696. doi: 10.1093/cercor/bhm104. [DOI] [PubMed] [Google Scholar]
- Levy DA, Shrager Y, Squire LR. Intact visual discrimination of complex and feature-ambiguous stimuli in the absence of perirhinal cortex. Learning and Memory. 2005;12:61–66. doi: 10.1101/lm.84405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RS, Frith CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers. Journal of Neuroscience. 1997;17:7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Recognition memory and the human hippocampus. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, Gummer A, et al. Associative recognition in a patient with selective hippocampal lesions and relatively normal item recognition. Hippocampus. 2004;14:763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- Mayes AR, Montaldi D, Migo E. Associative memory and the medial temporal lobes. Trends in Cognitive Sciences. 2007;11:126–135. doi: 10.1016/j.tics.2006.12.003. [DOI] [PubMed] [Google Scholar]
- O’Mara S. Controlling hippocampal output: The central role of subiculum in hippocampal information processing. Behavioural Brain Research. 2006;174:304–312. doi: 10.1016/j.bbr.2006.08.018. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, Rudy JW. Conjunctive representations in learning and memory: Principles of cortical and hippocampal function. Psychological Review. 2001;108:311–345. doi: 10.1037/0033-295x.108.2.311. [DOI] [PubMed] [Google Scholar]
- Paller KA, Wagner AD. Observing the transformation of experience into memory. Trends in Cognitive Sciences. 2002;6:93–102. doi: 10.1016/s1364-6613(00)01845-3. [DOI] [PubMed] [Google Scholar]
- Pfeuffer J, Van de Moortele PF, Ugurbil K, Hu X, Glover GH. Correction of physiologically induced global off-resonance effects in dynamic echo-planar and spiral functional imaging. Magnetic Resonance in Medicine. 2002;47:344–353. doi: 10.1002/mrm.10065. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Hanninen T, Kononen M, Mikkonen M, Jalkanen V, et al. Encoding of novel picture pairs activates the perirhinal cortex: An fMRI study. Hippocampus. 2003;13:67–80. doi: 10.1002/hipo.10049. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, et al. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. European Journal of Neuroscience. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Preston AR, Thomason ME, Ochsner KN, Cooper JC, Glover GH. Comparison of spiral-in/out and spiral-out BOLD fMRI at 1.5 and 3 T. Neuroimage. 2004;21:291–301. doi: 10.1016/j.neuroimage.2003.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Wagner AD. The medial temporal lobe and memory. In: Kesner RP, Martinez JL, editors. Neurobiology of learning and memory. 2. Oxford: Elsevier; 2007. pp. 305–337. [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kohler S, Crane J, Pruessner M, Lord C, Byrne A, et al. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: Considering the variability of the collateral sulcus. Cerebral Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, et al. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: Minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D’Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2003;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA. Sum–difference theory of remembering and knowing: A two-dimensional signal-detection model. Psychological Review. 2004;111:588–616. doi: 10.1037/0033-295X.111.3.588. [DOI] [PubMed] [Google Scholar]
- Shrager Y, Gold JJ, Hopkins RO, Squire LR. Intact visual perception in memory-impaired patients with medial temporal lobe lesions. Journal of Neuroscience. 2006;26:2235–2240. doi: 10.1523/JNEUROSCI.4792-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrager Y, Kirwan CB, Squire LR. Activity in both hippocampus and perirhinal cortex predicts the memory strength of subsequent remembered information. Neuron. 2008;59:547–553. doi: 10.1016/j.neuron.2008.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nature Neuroscience. 2001;4:442–449. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- Sommer T, Rose M, Glascher J, Wolbers T, Buchel C. Dissociable contributions within the medial temporal lobe to encoding of object–location associations. Learning and Memory. 2005;12:343–351. doi: 10.1101/lm.90405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. Journal of Neuroscience. 2006;26:9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Selective and shared contributions of the hippocampus and perirhinal cortex to episodic item and associative encoding. Journal of Cognitive Neuroscience. 2008;20:1478–1489. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark CE, Squire LR. Hippocampal damage equally impairs memory for single items and memory for conjunctions. Hippocampus. 2003;13:281–292. doi: 10.1002/hipo.10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, et al. The hippocampal formation participates in novel picture encoding: Evidence from functional magnetic resonance imaging; Proceedings of the National Academy of Sciences; U.S.A. 1996. pp. 8660–8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA. Comparative analysis of the cortical afferents, intrinsic projections and interconnections of the parahippocampal region in monkeys and rats. In: Gazzaniga MS, editor. The cognitive neurosciences. IV. Cambridge, MA: MIT Press; 2009. [Google Scholar]
- Suzuki WA, Amaral DG. Cortical inputs to the CA1 field of the monkey hippocampus originate from the perirhinal and parahippocampal cortex but not from area TE. Neuroscience Letters. 1990;115:43–48. doi: 10.1016/0304-3940(90)90515-b. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Taylor KJ, Henson RN, Graham KS. Recognition memory for faces and scenes in amnesia: Dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2428–2438. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Tranel D, Brady DR, Van Hoesen GW, Damasio AR. Parahippocampal projections to posterior auditory association cortex (area Tpt) in Old-World monkeys. Experimental Brain Research. 1988;70:406–416. doi: 10.1007/BF00248365. [DOI] [PubMed] [Google Scholar]
- Tulving E. Elements of episodic memory. Cambridge: Cambridge University Press; 1983. [Google Scholar]
- Tulving E, Kroll N. Novelty assessment in the brain and long-term memory encoding. Psychonomic Bulletin & Review. 1995;2:387–390. doi: 10.3758/BF03210977. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitch HJ, Craik FIM, Habib R, Houle S. Novelty and familiarity activations in pet studies of memory encoding and retrieval. Cerebral Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Research. 1975;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey: II. Frontal lobe afferents. Brain Research. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, et al. Building memories: Remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. Journal of Comparative Neurology. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. Journal of Neuroscience. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wixted JT, Stretch V. In defense of the signal detection interpretation of remember/know judgments. Psychonomic Bulletin & Review. 2004;11:616–641. doi: 10.3758/bf03196616. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver–operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Bookheimer SY. Application of cortical unfolding techniques to functional MRI of the human hippocampal region. Neuroimage. 2000;11:668–683. doi: 10.1006/nimg.2000.0561. [DOI] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face–name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]