Abstract
Previously, in tightly controlled studies, using three independent, yet complementary techniques, we refuted the claim that a mitochondrial nitric oxide synthase (mtNOS) isoform exists within pure, rat liver mitochondria (MT). Of those techniques, the NOS-catalyzed [14C]-L-arginine to [14C]-L-citrulline conversion assay (NOS assay) with MT samples indicated a weak, radioactive signal that was NOS-independent [1]. Aliquots of samples from the NOS assays were then extracted with acetone, separated by high performance thin-layer chromatography (HPTLC) and exposed to autoradiography. Results obtained from these samples showed no radioactive band for L-citrulline. However, a fast-migrating, diffuse, radioactive band was observed in the TLC lanes loaded with MT samples. In this manuscript, we identify and confirm that this radioactive signal in MT samples is due to the arginase-catalyzed conversion of [14C]-L-arginine to [14C]-urea. The current results, in addition to reconfirming the absence of NOS activity in rat liver MT, also show the need to include arginase inhibitors in studies using MT samples in order to avoid confounding results when using NOS activity assays. (Supported by ES 011982 & 2G12RR008124 to RTM & UTEP, respectively).
Keywords: Nitric Oxide Synthase, Mitochondria, Arginase
Introduction
Nitric Oxide (NO•) is a highly diffusible, hydrophobic gaseous free radical [2] that influences biological functions such as blood pressure, platelet aggregation/adhesion, neurotransmission as well as cellular defense [3; 4; 5; 6; 7; 8; 9]. Physiologically, NO• is produced by three main isoforms of NOS: neuronal (nNOS), endothelial (eNOS) and inducible (iNOS) [5; 10; 11; 12]. In addition, data exist both for and against the existence of a mitochondrial NOS isoform, mtNOS. Many research groups, using a variety of approaches, have attempted to establish the existence of mtNOS in rats as well as in different species and organs [13; 14; 15; 16; 17; 18; 19; 20] as well as in different organs [13; 14; 15; 16; 17; 19; 21; 22; 23]. However, no disputable evidence exists in order to resolve this controversy. In 2004, Brookes [24] published a critical review pointing out the errors in methodologies, sample preparation, as well as the inappropriate controls used in reports from the mtNOS literature. Subsequently, Lacza et al. [25] discussed the shortcomings of the most commonly used techniques used for detection of the putative mtNOS activity.
Taking into consideration the insights provided by those authors, we initiated studies whereby pure mitochondria were isolated from rat liver, by repeated differential centrifugation and followed by Percoll-gradient purification. Using proteomic analysis with electrospray ionization linear ion-trap mass spectrometry (ESI-LIT-MS), radioactive [14C]-L-arginine to [14C]-L-citrulline conversion assays as well as immunochemical analyses, no NOS isoform could be detected in sequentially purified rat liver MT [1]. Interestingly, using the [14C]-L-arginine to [14C]-L-citrulline conversion assays, incorporating purified MT, a radioactive signal that could not be quenched by the NOS inhibitors, L- thiocitrulline or nitro-L-arginine (L-NNA), was observed. HPTLC analyses, followed by autoradiography of the acetone-extracted MT sample eluates from the L-arginine to L-citrulline conversion assay, confirmed that the signal was not due to L-citrulline. In this study, experiments were performed to isolate and identify the confounding and unidentified radioactive signal that appears in HPTLC lanes loaded with MT samples.
Materials and Methods
Chemicals and Biochemicals
4-(2-hydroxyethyl)-1 piperazineethanesulfonic acid (HEPES), L-arginine·HCl, CaCl2·4H2O, bovine brain calmodulin (CaM), (6R)-5,6,7,8-tetrahydro-L-biopterin (BH4), NADPH, L-citrulline, ethylene diamine tetraacetic acid (EDTA), ethylene glycol tetraacetic acid (EGTA), sucrose, mannitol, acetone, N-hydroxy-L-arginine and L-thiocitrulline were purchased from Sigma (St. Louis, MO). Dowex 50WX8 was obtained from Supelco (Bellefonte, PA). Percoll was obtained from GE Healthcare (Uppsala, Sweden). Complete protease inhibitor cocktail tablets were purchased from Roche (Mannheim, Germany). All other chemicals and reagents were from common commercial suppliers and were of the highest grade commercially available.
Enzymes
Recombinant nNOS (nNOSr) was over-expressed in E. coli and purified according to established methodology [26]. Urease was obtained from Sigma (St. Louis, MO). One unit of urease activity corresponds to the amount of enzyme which hydrolyzes 1 μmol urea per minute at pH 8.0 and 25 °C.
Animals
All experimental protocols involving animals were approved by University of Texas at El Paso Institutional Animal Care and Use Committee (IACUC). Male Sprague-Dawley (SD) rats (250–300 g; ~ 3 months of age) were obtained from Harlan (Houston, TX) and used in all studies.
Preparation of Mitochondria
Initially, 2–4 rats were euthanized and entire livers were excised and immersed in ice-cold mitochondrial isolation buffer (MIB) containing 215 mM mannitol, 75 mM sucrose, 1 mM EGTA, 20 mM HEPES/KOH, pH 7.2. In addition, a complete protease-inhibitor cocktail containing serine- and cysteine- protease inhibitors was included in the isolation buffer. The liver lobes were blotted, washed 2–3 times with fresh MIB and minced into small pieces with scissors. The resulting minced pieces of liver were washed with MIB to remove blood. After decanting the last wash, 6–8 ml of ice-cold MIB was added to the washed and minced tissue. The minced tissue sample was placed in a glass dounce homogenizer in portions. The apparatus was immersed in ice and a variable speed homogenizer (Glas-Col, Terre Haute, IN) was used to gently homogenize the tissue, using a loose-fitting Teflon pestle (6 strokes at 250 rpm). Following homogenization, pure mitochondria (MT) were obtained by repeated differential centrifugation followed by Percoll gradient purification as previously described [1]. The protein concentrations of MT samples were measured using the Bradford protein assay [27] with bovine serum albumin as a standard. MT samples in MIB were frozen using dimethyl sulfoxide (DMSO) 10% (v/v). MT were cooled at a uniform rate of ~ 1 °C/min. Frozen MT samples were then stored at −80 °C and thawed [28] as needed.
Assay for NOS Activity
The conversion of [14C]-L-arginine to [14C]-L-citrulline (NOS assay) was used to estimate NOS activity [29]. Reaction mixtures consisted of 50 mM HEPES (pH 7.6), 400 μM NADPH, 400 μM CaCl2, 5 μM BH4, 20 μM L-arginine containing 0.5 μCi/ml [14C]-L-arginine, and a 1.5-fold molar excess of CaM to nNOS (based on the positive control) in a total volume of 0.25 mL [30]. MT (150 μg) were included in all assays, unless otherwise stated. The potent nNOS inhibitor, L-thiocitrulline (800 μM) was used for inhibition of NOS activity. At this concentration, L-thiocitrulline will inhibit all NOS isoforms (data not shown). In other assays, the arginase inhibitor, N-hydroxy-L-arginine (40 μM), was pre-incubated with MT samples for 10 min before initiating the reactions. Reactions measuring either NOS activity or arginase activity were run for 10 min at 23°C. Reaction mixtures were quenched with an ice-cold stop solution containing 1 mM L-citrulline, 10 mM EDTA and 100 mM HEPES, pH 5.5. Eluates were then applied to 2-mL Dowex columns and [14C]-L-citrulline was eluted with two x 1-mL portions of water. The Dowex resin effectively eliminates the [14C]-L-arginine signal. Control experiments were performed as mentioned above but were not passed through Dowex columns. Except for the use of Dowex columns, the control samples were processed in the exact fashion as the experimental samples. Samples or aliquots of the eluates from the experimental and control incubations were then processed further.
High Performance-Thin Layer Chromatography Analyses of Amino Acids in MT
Aliquots of Dowex-treated (Dowex (+)) and Dowex untreated (Dowex (−)) reaction mixtures from the NOS assay were extracted with 80 % acetone. The precipitation of the proteins was enhanced with a freeze-thaw cycle (−80°C for 36 hrs). The precipitated and resuspended protein pellets were removed by centrifugation at 16,000 × g for 20 min in an Eppendorf table-top microcentrifuge at 4°C. Control experiments were performed to determine possible loss of radioactivity due to non-specific sequestration or binding within, or to, the MT protein pellet as described previously [1]. Results from control experiments indicated that there was negligible (0.01%) loss of radioactivity remaining in the pellet (data not shown). The supernatants from the 16000 × g spins, containing the amino acids and other products, were individually collected and dried under a gentle stream of nitrogen. The dried samples of supernatant were then dissolved in 100 μl methanol:water (2:1, v/v). Aliquots of the MT sample eluates were then incubated for 10 min with urease (0.05 U). HPTLC was performed in a glass tank using standard methodology. Aliquots (10 μl) of each individual MT sample were loaded on to HPTLC silica 60 plates (EMB chemicals). Samples (Dowex +/−) were loaded on separate TLC plates. Ten microliters of 2 mg/ml L-arginine, L-ornithine or L-citrulline were used as standards. A solution consisting of Butanol:acetic acid:water (60:20:20, v/v/v) was used as the mobile phase for TLC. The plates were developed with a 2 % ninhydrin solution (in acetone) and then placed on a hot plate (Model # PC-351, Corning; medium setting) for 1 min. After the visualization, non-radioactive L-arginine, L-citrulline and L-ornithine standard bands, the plates were exposed to X-ray film (Kodak) for 4.5 days (108 hrs).
Results and Discussion
Arginase-dependent signal in sample eluates of MT
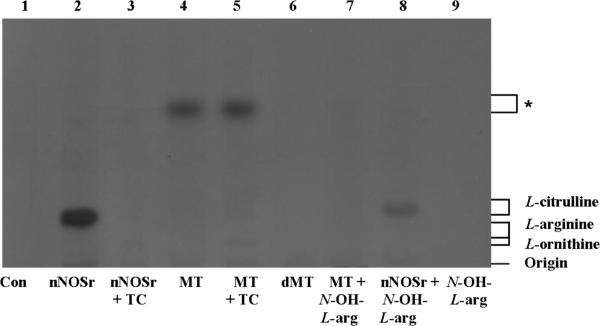
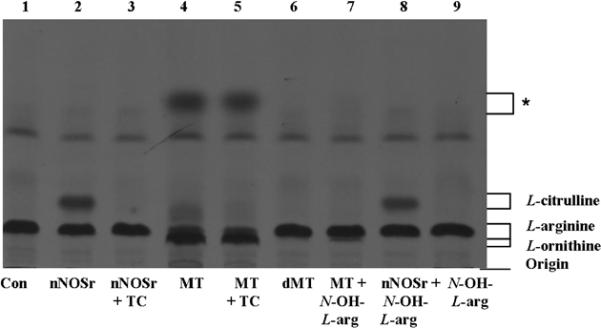
[14C]-L-arginine to [14C]-L-citrulline conversion assay samples (Dowex (+) and Dowex (−)) were extracted with acetone and subjected to HPTLC as described (Vide Infra). The products from both Dowex (+) and Dowex (−) samples were loaded exactly as in the HPTLC and both plates were analyzed in parallel for comparison. Samples (Dowex (+) and Dowex (−)) were loaded as follows: control (all ingredients except nNOSr and MT (lane 1)); nNOSr (30 nM; lane 2); nNOSr + L-thiocitrulline (800 μM; lane 3); MT (150 μg; lane 4); MT+ L-thiocitrulline (lane 5); denatured MT (dMT; 150 μg; lane 6); MT plus N-hydroxy L-arginine (N-OH L-arg; 40 μM; lane 7); nNOSr plus N-hydroxy L-arginine (N-OH L-arg; 40 μM; lane 8); N-hydroxy L-arginine (N-OH L-arg; lane 9). The standard, nonradioactive, samples, L-arginine, L-citrulline and L-ornithine were run along and used for comparison. The Dowex (+) control eluate containing all NOS substrates and cofactors but excluding nNOSr and MT (lane 1, Fig. 1) clearly showed that the positively charged [14C]-L-arginine remained bound to the Dowex resin, thus, no [14C]-L-arginine signal was observed. On the other hand, the Dowex (−) control eluates clearly display an L-arginine band (lane 1, Fig. 2).
Fig. 1. HPTLC analysis of the acetone-extracted products from the [14C]-L-arginine to [14C]-L-citrulline conversion assay.
Acetone-extracted products that were not passed through Dowex and the standards, L-arginine, L-citrulline and L-ornithine were separated by HPTLC in silica-60 gel plates and developed as described in Materials and Methods. Each sample contained all necessary substrates and cofactors for NOS activity. Samples were loaded in the following order: 1) No nNOSr/MT (negative control, Con); 2) nNOSr (30 nM); 3) nNOSr + L-thiocitrulline (800 μM; denoted as TC); 4) MT (150 μg); 5) MT (150 μg)+ L-thiocitrulline; 6) denatured MT (dMT; 150 μg); 7) MT (150 μg) + N-OH-L-arginine (40 μM); 8) nNOSr (30 nM) + N-OH-L-arginine (40 μM); 9) N-OH-L-arginine (40 μM). The migration of authentic standards of L-arginine, L-citrulline and L-ornithine, as detected by ninhydrin staining, is indicated on the right. *, unidentified compound.
Fig. 2. HPTLC analysis of the acetone-extracted products from the [14C]-L-arginine to [14C]-L-citrulline conversion assay.
Acetone-extracted products that were passed through Dowex columns and the standards, L-arginine, L-citrulline and L-ornithine were separated by HPTLC in silica-60 gel plates and developed as described in Materials and Methods. Each sample contained all necessary substrates and cofactors for NOS activity. Samples were loaded in the following order: 1) No nNOSr/MT (negative control, Con) ; 2) nNOSr (30 nM); 3) nNOSr + L-thiocitrulline (800 μM; denoted as TC); 4) MT (150 μg); 5) MT (150 μg)+ L-thiocitrulline; 6) denatured MT (dMT; 150 μg); 7) MT (150 μg) + N-OH-L-arginine (40 μM); 8) nNOSr (30 nM) + N-OH-L-arginine (40 μM); 9) N-OH-L-arginine (40 μM). The migration of authentic standards of L-arginine, L-citrulline and L-ornithine, as detected by ninhydrin staining, is indicated on the right. *, unidentified compound.
nNOSr-containing samples were used as controls for validation of the assay. The product ([14C]-L-citrulline) was present in the Dowex (+) eluates (lane 2,Fig. 1) whereas, both the reaction substrate ([14C]-L-arginine) as well as the product ([14C]-L-citrulline) were present in the Dowex (−) samples (lane 2, Fig. 2). For NOS inhibition studies, high concentrations of L-thiocitrulline (800 μM; TC) [31] were included in the conversion assays as negative controls. Inhibition of NOS using L-thiocitrulline prevented [14C]-L-citrulline production (lane 3, Figs. 1 and 2). Results from Dowex (−) and Dowex (+) MT sample eluates indicated no conversion of [14C]-L-arginine to [14C]-L-citrulline (lane 4, Figs. 1 and 2). These results were in stark contrast to the clear band from the positive control (Figs. 1 and 2) that contained L-citrulline. These results support findings from our previous studies [1], that the radioactive signal associated with MT samples does not represent NOS-catalyzed L-citrulline production.
In the lanes containing the MT samples (Dowex (+) and Dowex (−)), a fast-migrating diffuse band was observed (lane 4, Figs. 1 and 2) and this signal could not be inhibited by L-thiocitrulline (lane 5, *, Figs. 1 and 2). When denatured MT (dMT) were used (100°C, 10 min) in place of functional MT, the diffuse band did not appear, indicating that the signal was not due to the protein effect previously described [1] but due to the presence of enzymes from functional mitochondria (lane 6, Figs. 1 and 2). In order to identify the source of the weak radioactive signal emanating from the pure rat liver MT sample, N-hydroxy-L-arginine (40 μM), was added to the processed MT samples (Dowex (+) and Dowex (−)). Interestingly, the fuzzy band present in both the Dowex (+) and Dowex (−) MT samples disappeared completely in the presence of N-hydroxy-L-arginine (lane 7, Figs. 1 and 2). Control samples, wherein N-hydroxy-L-arginine (40 μM) was incubated with nNOSr and its substrates, allowed [14C]-L-citrulline formation in both Dowex (+) and Dowex (−) samples. On the other hand, when N-hydroxy-L-arginine was incubated with substrates and cofactors without nNOSr, no [14C]-L-citrulline formation was observed. Interestingly, most of the lower bands seen in the Dowex (+) and Dowex (−) samples of MT eluates matched the positive control bands produced by L-ornithine (lanes 4 and 5, Figs. 1 and 2) which appears slightly lower in the lane relative to the L-arginine band.
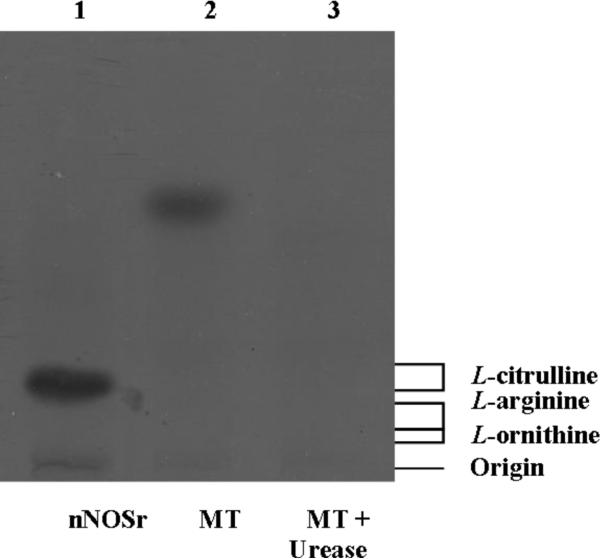
At this point, we speculated that the diffuse radioactive band was due to the arginase-catalyzed production of urea. In order to test this hypothesis, the TLC plates were sprayed with a visualization solution consisting of 5% aqueous sodium nitroprusside, 10% aqueous sodium hydroxide and 3% aqueous hydrogen peroxide (2:1:5, v/v/v) diluted 1:15 with water. However, the plates turned completely purple spontaneously, clearly indicating a false positive signal. This detection technique was not specific to detecting urea in MT samples and thus, conclusive evidence was not obtained (data not shown). Efforts made to carbonize the plates (using sulfuric acid) did not result in visualization of any band (data not shown). However, when the [14C]-L-arginine to [14C]-L-citrulline conversion assay mixtures, containing MT were treated with urease (50 mU), the diffuse band completely disappeared (lane 3, Fig. 3). This result was in stark contrast to lanes containing assay eluates of MT alone (lane 2, Fig. 3). Thus, our results strongly indicate that the diffuse radioactive band of the Dowex (+) MT sample eluates was attributable to urea.
Fig. 3. HPTLC analysis of acetone-extracted products from the [14C]-L-arginine to [14C]-L-citrulline conversion assay.
Acetone-extracted samples that were passed through Dowex columns and the standards, L-arginine, L-citrulline and L-ornithine were separated by HPTLC in silica-60 gel plates and developed as described in Materials and Methods. Each sample contained all necessary substrates and cofactors for NOS activity. Samples were loaded in the following order: 1) nNOSr (30 nM); 2) MT (150 μg); 3) MT (150 μg) + Urease (50 mU); The migration of authentic standards of L-arginine, L-citrulline and L-ornithine, as detected by ninhydrin staining, is indicated on the right.
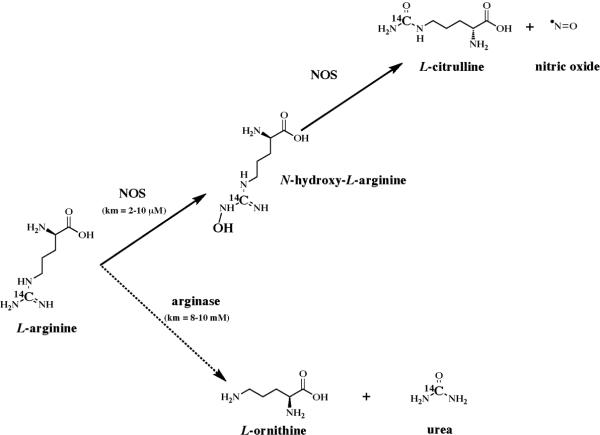
Most groups performing radioactive assays for determination of NOS activity in MT use 3H-L-arginine. We used 14C-L-arginine to measure NOS activity because it provides a more stable signal with little chance of isotope exchange, thus decreasing apparent false-positive results due to tritium exchange with water in an aqueous environment. The [14C]-L-arginine used in the conversion assays was radiolabeled at the ureido carbon. We used L-[Ureido-14C]-arginine (and not the arginine labeled at the guanidino carbon) in order to avoid any interference arising from arginase activity. Interference was decreased because only 17% (one-sixth) of the labeled carbon atoms are converted to urea [32]. Despite this precaution, we still observed an arginase-dependent conversion of [ureido-14C]-L-arginine to 14C urea and L-ornithine, which was dependent on the presence of MT. Although the arginase-catalyzed reaction produced L-ornithine and urea as products from the substrate L-arginine, the NOS reaction proceeds much faster than the arginase-catalyzed reaction due to the increased affinity of NOS (Km = 2–10 μM) for the substrate, L-arginine. This affinity is apparent when the Km (8–10 mM) of arginase for L-arginine is compared to that of NOS (Scheme 1). Possible explanations for detecting this reaction could be that rat liver MT contains enormous amounts of arginase [33; 34; 35]. Indeed, arginase 1 was already shown to be present in highly pure mitochodrium preparations by large-scale proteomic analysis [1; 36].
Scheme 1.
Lacza et al. [37] also showed a radioactive signal produced by mouse liver MT using the 14C radioactive conversion assay eluates that were passed through 0.5 ml Dowex 50 resin. Also, they observed a significant radioactive signal in liver mitochondria of nNOS- and eNOS-knockout mice as well as in iNOS inhibitor-treated mitochondria. It is to be noted that the Lacza group incubated samples of mitochondria with 1 mM L-ornithine, an arginase inhibitor, yet still were able to observe a weak radioactive signal. However, our studies using the arginase inhibitor, N-hydroxy-L-arginine (40 μM), clearly inhibited the formation of the radioactive band visualized in HPTLC assays. On the other hand, the radioactive signal observed by Lacza group could also not be quenched using the NOS inhibitor, N-nitro-L-arginine methyl esther (100 μM), clearly indicating that the signal was not NOS-dependent. Attempts to inhibit arginase by use of arginase inhibitors in the MT samples during the NOS activity assays, and/or actual removal of arginase using KCl washes during MT preparation have been suggested previously [19; 37; 38; 39]. Nevertheless, most groups studying mtNOS activity (using either the 14C or 3H labeled L-arginine assay) do not make an effort to inhibit arginase. Results from the present studies indicate that it is necessary to add arginase inhibitors when assaying MT samples, in order to eliminate the arginase-dependent signal which is due to formation of radiolabeled urea.
Conclusion
The question of whether there is NOS in mitochondria remains unresolved for several reasons. Poor reproducibility of data, inconsistent purity of mitochondria preparations, use of inappropriate controls and lack of use of complementary and confirmatory techniques are some of the factors that have caused inconsistency in determining whether there is a NOS or not in mitochondria. Our previous studies refuted the presence of any NOS in mitochondria and substantiated our claims using complementary yet independent techniques including the radioactive NOS assay [1]. However, the reason(s) behind the NOS-independent, weak radioactive signal appearing in NOS activity assays must be recognized as a confounding factor when determining mtNOS activity. Using amino-acid HPTLC analyses with sample eluates from the radioactive L-arginine to L-citrulline assay, the radioactive signal present in rat liver MT eluate samples was shown to be due to the arginase-dependent conversion of [14C]-L-arginine to 14C-urea. The studies described within this report, and thus any conclusions, pertain only to rat liver. The issues of NOS in mitochondria from other organs, or for that matter, other species, are still unresolved.
Acknowledgments
This publication was made possible by grant # ES 011982 to R.T. Miller from the National Institute of Environmental Health Sciences (NIEHS)/NIH. This project was also supported in part by grant # 5G12RR008124 to the Border Biomedical Research Center (BBRC)/University of Texas at El Paso from the National Center for Research Resources (NCRR)/NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NIH or NIEHS.
The abbreviations used are
- NOS
Nitric Oxide Synthase
- NO•
Nitric Oxide
- MT
Isolated and purified Purified Mitochondria
- HPTLC
High Performance Thin-Layer Chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Venkatakrishnan P, Nakayasu ES, Almeida IC, Miller RT. Absence of Nitric-oxide Synthase in Sequentially Purified Rat Liver Mitochondria. J Biol Chem. 2009;284:19843–55. doi: 10.1074/jbc.M109.003301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. FASEB J. 1989;3:31–6. doi: 10.1096/fasebj.3.1.2642868. [DOI] [PubMed] [Google Scholar]
- [3].Ignarro LJ, Buga GM, Byrns RE, Wood KS, Chaudhuri G. Endothelium-derived relaxing factor and nitric oxide possess identical pharmacologic properties as relaxants of bovine arterial and venous smooth muscle. J Pharmacol Exp Ther. 1988;246:218–26. [PubMed] [Google Scholar]
- [4].Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–18. [PubMed] [Google Scholar]
- [5].Forstermann U, Pollock JS, Schmidt HH, Heller M, Murad F. Calmodulin-dependent endothelium-derived relaxing factor/nitric oxide synthase activity is present in the particulate and cytosolic fractions of bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:1788–92. doi: 10.1073/pnas.88.5.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murad F, Forstermann U, Nakane M, Schmidt H, Pollock J, Sheng H, Matsumoto T, Warner T, Mitchell J, Tracey R, et al. The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol. 1992;58(Suppl 2):150P–157P. [PubMed] [Google Scholar]
- [7].Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1991;88:10480–4. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Knowles RG, Palacios M, Palmer RM, Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989;86:5159–62. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hibbs JB, Jr., Taintor RR, Vavrin Z, Rachlin EM. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988;157:87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- [10].Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990;87:682–5. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res. 1987;61:866–79. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- [12].Hibbs JB., Jr. Synthesis of nitric oxide from L-arginine: a recently discovered pathway induced by cytokines with antitumour and antimicrobial activity. Res Immunol. 1991;142:565–9. doi: 10.1016/0923-2494(91)90103-p. discussion 596–8. [DOI] [PubMed] [Google Scholar]
- [13].Tatoyan A, Giulivi C. Purification and characterization of a nitric-oxide synthase from rat liver mitochondria. J Biol Chem. 1998;273:11044–8. doi: 10.1074/jbc.273.18.11044. [DOI] [PubMed] [Google Scholar]
- [14].Kanai AJ, Pearce LL, Clemens PR, Birder LA, VanBibber MM, Choi SY, de Groat WC, Peterson J. Identification of a neuronal nitric oxide synthase in isolated cardiac mitochondria using electrochemical detection. Proc Natl Acad Sci U S A. 2001;98:14126–31. doi: 10.1073/pnas.241380298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ghafourifar P, Richter C. Nitric oxide synthase activity in mitochondria. FEBS Lett. 1997;418:291–6. doi: 10.1016/s0014-5793(97)01397-5. [DOI] [PubMed] [Google Scholar]
- [16].Rothe F, Huang PL, Wolf G. Ultrastructural localization of neuronal nitric oxide synthase in the laterodorsal tegmental nucleus of wild-type and knockout mice. Neuroscience. 1999;94:193–201. doi: 10.1016/s0306-4522(99)00263-8. [DOI] [PubMed] [Google Scholar]
- [17].Hotta Y, Otsuka-Murakami H, Fujita M, Nakagawa J, Yajima M, Liu W, Ishikawa N, Kawai N, Masumizu T, Kohno M. Protective role of nitric oxide synthase against ischemia-reperfusion injury in guinea pig myocardial mitochondria. Eur J Pharmacol. 1999;380:37–48. doi: 10.1016/s0014-2999(99)00531-2. [DOI] [PubMed] [Google Scholar]
- [18].French S, Giulivi C, Balaban RS. Nitric oxide synthase in porcine heart mitochondria: evidence for low physiological activity. Am J Physiol Heart Circ Physiol. 2001;280:H2863–7. doi: 10.1152/ajpheart.2001.280.6.H2863. [DOI] [PubMed] [Google Scholar]
- [19].Lacza Z, Puskar M, Figueroa JP, Zhang J, Rajapakse N, Busija DW. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radic Biol Med. 2001;31:1609–15. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- [20].Dennis J, Bennett JP., Jr. Interactions among nitric oxide and Bcl-family proteins after MPP+ exposure of SH-SY5Y neural cells I: MPP+ increases mitochondrial NO and Bax protein. J Neurosci Res. 2003;72:76–88. doi: 10.1002/jnr.10539. [DOI] [PubMed] [Google Scholar]
- [21].Henrich M, Hoffmann K, Konig P, Gruss M, Fischbach T, Godecke A, Hempelmann G, Kummer W. Sensory neurons respond to hypoxia with NO production associated with mitochondria. Mol Cell Neurosci. 2002;20:307–22. doi: 10.1006/mcne.2002.1111. [DOI] [PubMed] [Google Scholar]
- [22].Bustamante J, Bersier G, Badin RA, Cymeryng C, Parodi A, Boveris A. Sequential NO production by mitochondria and endoplasmic reticulum during induced apoptosis. Nitric Oxide. 2002;6:333–41. doi: 10.1006/niox.2001.0420. [DOI] [PubMed] [Google Scholar]
- [23].Escames G, Leon J, Macias M, Khaldy H, Acuna-Castroviejo D. Melatonin counteracts lipopolysaccharide-induced expression and activity of mitochondrial nitric oxide synthase in rats. Faseb J. 2003;17:932–4. doi: 10.1096/fj.02-0692fje. [DOI] [PubMed] [Google Scholar]
- [24].Brookes PS. Mitochondrial nitric oxide synthase. Mitochondrion. 2004;3:187–204. doi: 10.1016/j.mito.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [25].Lacza Z, Pankotai E, Csordas A, Gero D, Kiss L, Horvath EM, Kollai M, Busija DW, Szabo C. Mitochondrial NO and reactive nitrogen species production: does mtNOS exist? Nitric Oxide. 2006;14:162–8. doi: 10.1016/j.niox.2005.05.011. [DOI] [PubMed] [Google Scholar]
- [26].Roman LJ, Sheta EA, Martasek P, Gross SS, Liu Q, Masters BS. High-level expression of functional rat neuronal nitric oxide synthase in Escherichia coli. Proc Natl Acad Sci U S A. 1995;92:8428–32. doi: 10.1073/pnas.92.18.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- [28].Nukala VN, Singh IN, Davis LM, Sullivan PG. Cryopreservation of brain mitochondria: a novel methodology for functional studies. J Neurosci Methods. 2006;152:48–54. doi: 10.1016/j.jneumeth.2005.08.017. [DOI] [PubMed] [Google Scholar]
- [29].Nishimura JS, Narayanasami R, Miller RT, Roman LJ, Panda S, Masters BS. The stimulatory effects of Hofmeister ions on the activities of neuronal nitric-oxide synthase. Apparent substrate inhibition by l-arginine is overcome in the presence of protein-destabilizing agents. J Biol Chem. 1999;274:5399–406. doi: 10.1074/jbc.274.9.5399. [DOI] [PubMed] [Google Scholar]
- [30].Miller RT. Dinitrobenzene-mediated production of peroxynitrite by neuronal nitric oxide synthase. Chem Res Toxicol. 2002;15:927–34. doi: 10.1021/tx020016y. [DOI] [PubMed] [Google Scholar]
- [31].Narayanan K, Griffith OW. Synthesis of L-thiocitrulline, L-homothiocitrulline, and S-methyl-L-thiocitrulline: a new class of potent nitric oxide synthase inhibitors. J Med Chem. 1994;37:885–7. doi: 10.1021/jm00033a004. [DOI] [PubMed] [Google Scholar]
- [32].Leech AR, Beis I, Newsholme EA. Radiochemical assays for creatine kinase and arginine kinase using rapid ion exchange separations. Anal Biochem. 1978;90:561–75. doi: 10.1016/0003-2697(78)90150-1. [DOI] [PubMed] [Google Scholar]
- [33].Mora J, Martuscelli J, Ortiz Pineda J, Soberon G. The Regulation of Urea-Biosynthesis Enzymes in Vertebrates. Biochem J. 1965;96:28–35. doi: 10.1042/bj0960028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gasiorowska I, Porembska Z, Jachimowicz J, Mochnacka I. Isoenzymes of arginase in rat tissues. Acta Biochim Pol. 1970;17:19–30. [PubMed] [Google Scholar]
- [35].Cheung CW, Raijman L. Arginine, mitochondrial arginase, and the control of carbamyl phosphate synthesis. Arch Biochem Biophys. 1981;209:643–9. doi: 10.1016/0003-9861(81)90324-6. [DOI] [PubMed] [Google Scholar]
- [36].Forner F, Foster LJ, Campanaro S, Valle G, Mann M. Quantitative proteomic comparison of rat mitochondria from muscle, heart, and liver. Mol Cell Proteomics. 2006;5:608–19. doi: 10.1074/mcp.M500298-MCP200. [DOI] [PubMed] [Google Scholar]
- [37].Lacza Z, Snipes JA, Zhang J, Horvath EM, Figueroa JP, Szabo C, Busija DW. Mitochondrial nitric oxide synthase is not eNOS, nNOS or iNOS. Free Radic Biol Med. 2003;35:1217–28. doi: 10.1016/s0891-5849(03)00510-0. [DOI] [PubMed] [Google Scholar]
- [38].Kato K, Giulivi C. Critical overview of mitochondrial nitric-oxide synthase. Front Biosci. 2006;11:2725–38. doi: 10.2741/2002. [DOI] [PubMed] [Google Scholar]
- [39].Giraldez RR, Zweier JL. An improved assay for measurement of nitric oxide synthase activity in biological tissues. Anal Biochem. 1998;261:29–35. doi: 10.1006/abio.1998.2721. [DOI] [PubMed] [Google Scholar]