Abstract
Ecdysis triggering hormones (ETH) from peripheral endocrine Inka cells initiate the ecdysis sequence through action on central neurons expressing ETH receptors (ETHR) in model moth and dipteran species. We used various biochemical, molecular and blast search techniques to detect these signaling molecules in representatives of diverse arthropods. Using peptide isolation from tracheal extracts, cDNA cloning or homology search, we identified ETHs in a variety of hemimetabolous and holometabolous insects. Most insects produce two related ETHs, but only a single active peptide was isolated from the cricket and one peptide is encoded by eth gene of the honeybee, parasitic wasp and aphid. Immunohistochemical staining with antiserum to Manduca PETH revealed Inka cells on tracheal surface of diverse insects. In spite of conserved ETH sequences, comparison of the natural and ETH-induced ecdysis sequence in the honeybee and beetle revealed considerable species-specific differences in pre-ecdysis and ecdysis behaviors. DNA sequences coding for putative ETHR were deduced from available genomes of several hemimetabolous and holometabolous insects. In all examined insects the ethr gene encodes two subtypes of the receptor (ETHR-A and ETHR-B). Phylogenic analysis showed that these receptors fall into a family of closely related GPCRs. Here we report for the first time presence of putative ETHs and ETHRs in genomes of other arthropods, the tick (Arachnida) and water flea (Crustacea). Possible source of ETH in ticks was detected in paired cells located in all pedal segments. Our results provide further evidence of structural and functional conservancy of ETH-ETHR signaling.
Keywords: ecdysis triggering hormone, ETH receptor, Inka cell, ecdysis behavior, insect, tick, crustacean
1. INTRODUCTION
Ecdysis is a common innate behavior necessary for regular shedding of the old cuticle during development of many invertebrates including nematodes and arthropods. The ecdysis sequence is described only in few insects and is usually composed of three phases named pre-ecdysis, ecdysis and post-ecdysis [34]. Each of these behaviors is characterized by specific contractions of skeletal muscles and is under control of peptide hormones and transmitters [35]. The principal humoral factors activating the ecdysis sequence are peptides named pre-ecdysis and ecdysis triggering hormones (ETHs) produced and released by endocrine Inka cells [20, 30]. The Inka cell is a major component of the epitracheal gland first described by Ikeda in the silkmoth Bombyx mori (1913) [12]. Paired epitracheal glands attached to tracheal trunks near each spiracle have been found in prothoracic and abdominal segments in all representatives of Lepidoptera, Diptera, and some Coleoptera and Hymenoptera. In other Holometabola, including most beetles and bees, and all examined Hemimetabola ETH is liberated during ecdysis from numerous small Inka cells dispersed throughout the tracheal system [33].
ETHs represent a large family of peptide hormones produced by insect Inka cells [35]. Two active peptides named pre-ecdysis triggering hormone (PETH) and ecdysis triggering hormone (ETH) in moths and ETH1 and ETH2 in other insects are usually encoded by eth gene. These two peptides have been shown to exhibit different roles and/or potency in activation of the ecdysis sequence in Manduca sexta [30,31] and Drosophila melanogaster [20,22]. The blood-borne ETHs initiate the ecdysis sequence through direct actions on the central nervous system (CNS) [30,31]. Discovery of the ETH receptor genes in Drosophila [13,23] and Manduca [14] facilitated identification of target neurons within the CNS. The known ethr genes encode two splicing variants (ETHR-A and ETHR-B) of G protein-coupled receptors (GPCR), which are expressed in different sets of central neurons [14,15]. All ETHR-A neurons produce inhibitory and/or excitatory neuropeptides which are released upon ETH action to regulate different phases of the ecdysis sequence [14,15]. The expression and function of ETH and ETHR has been extensively examined only in the moths Manduca and Bombyx [14,29,31,32], Drosophila [22,23,15] and more recently in the beetle Tribolium castaneum [3] and the mosquito Aedes aegypti [6]. Direct evidence for the presence of ETH-ETHR signaling pathway and its functional analysis is lacking in other insects. In this paper we focus on identification of ETH and ETHR as crucial components for activation of the ecdysis sequence in various insects. The presence of these components in Ixodes and Daphnia genomes and PETH-like immunoreactivity in paired segmental cells in ticks indicates that similar ecdysis signaling system may be widespread in other arthropods.
2. MATERIALS AND METHODS
2.1. Experimental animals
We used the following insects in this study: the American cockroach Periplaneta americana (Blattodea), the cricket Gryllus sp. (Ensifera, Orthoptera), the locusts Locusta migratoria and Schistocerca americana (Caelifera, Orthoptera), the honeybee Apis mellifera (Hymenoptera), the mealworm beetle Tenebrio molitor and the Colorado potato beetle Leptinotarsa decemlineata (Coleoptera). Larval stages of Leptinotarsa were collected in potato fields and reared at room temperature. Honeybee pharate pupae, pupae and pharate adults were obtained from the laboratory colony of the Institute of Molecular Biology in Bratislava (Apis mellifera carnica) and kept at 30°C. Other insects were obtained from our laboratory colonies and reared at 25 °C until they developed into desired stage. Ticks Ixodes ricinus and Rhipicephalus appendiculatus were obtained from our laboratory colonies and kept after the blood meal at 25°C in glass vials.
2.2. Peptide isolation and synthesis
For isolation of ETHs, tracheal systems from ~20-30 pharate adults of Periplaneta, Schistocerca and Gryllus were dissected under saline (140 mmol l-1 NaCl; 5 mmol l-1 KCL; 5 mmol l-1 CaCl2; 1 mmol l-1 MgCl2; 4 mmol l-1 NaHCO3; 5 mmol l-1 Hepes; pH 7.2), heated at 90°C for 5 min, homogenized in a tissue grinder and centrifuged at 10 000 g for 10 min. Supernatants were fractionated by reverse-phase high performance liquid chromatography (RP-HPLC) using a Microsorb-MV™ C4 column, 4.6×250 mm (Rainin Instruments, Woburn, MA, USA) with a linear gradient of acetonitrile (3-50% in 90 min) and constant gradient of 0.1% trifluoracetic acid in water. Isolated fractions were tested by enzyme immunoassays with the rabbit antiserum against PETH (dilution 1:70,000). Conjugates were prepared by coupling synthetic PETH to horseradish peroxidase (HRP; Sigma, St. Louis, MO, USA) with glutaraldehyde [9]. PETH-HRP conjugate was used in dilution 1:4000. Enzyme immunoassays were performed as described [36]. PETH-immunoreactive peptides were sufficiently pure after a single HPLC fractionation. Sequences of these peptides were determined by liquid secondary ion mass spectrometry and MALDI-MAS-MAS. Apis ETH, Tribolium ETH1, ETH2, and Drosophila ETH1 were custom synthesized at the Suntory Institute for Bioorganic Research (Osaka, Japan).
2.3. Molecular biology and bioinformatics
For isolation of total RNA from Apis, tracheae from ~20 pharate pupae were dissected and immediately frozen on dry ice and stored at -70°C. Total RNA was prepared from these tracheae using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The first-strand cDNA was prepared from the total RNA using SMART cDNA synthesis kit (BD Biosciences, CA, USA). This cDNA and nucleotide primers designed from predicted partial genomic sequence of Apis eth gene were used for rapid amplification of cDNA ends. In the 3′RACE procedure we used ETH-F1 primer (5′-atgaagtgcctgccttcttc) or nested ETH-F2 primer (5′-tcttcctgaaaatcgccaag) and universal 3′SMART primer (5′-tcaacgcagagtacttttttttttt) in PCR (2 min at 94°C and 35 times: 40 sec at 94°C, 40 sec at 55°C, 2 min at 72°C). The 5′RACE was performed by PCR (2 min at 94°C and 35 times: 40 sec at 94°C, 40 sec at 60°C, 2 min at 72°C) in the presence of the adaptor 5′SMART primer (5′-gtatcaacgcagagtacgcg) and ETH-R1 primer (5′-aaagtgttgccacgaccaat) or nested ETH-R2 primer (5′-aggtcttgaaaagggctcgt). PCRs were performed using the DynaZyme™ II DNA Polymerase Kit (Finnzymes, Espoo, Finnland), the cDNA amplicons were purified with the Wizard SV Gel and the PCR Clean Up kit (Promega, Madison, WI, USA), TA ligated into pGEMR-T Easy vector (Promega) and at least three cDNA amplicon clones were custom sequenced.
Nucleotide and protein sequences were analyzed by free bioinformatics tools on the JustBio web site (http://www.justbio.com/, Clustal W 1.8 Aligner, Translator and Primer3), by the neural network promoter prediction software (Berkeley Drosophila Genome Project web site; Reese, 2001), by the transmembrane domain prediction software (TMHMM 2.0.; Krogh et al., 2001) and by the signal peptide prediction software (SignalP 3.0; [7]). Putative and recently annotated genes for ETH and ETHR were determined by BLAST (tblastn, blastp) at the NCBI web site. The data matrix of aligned sequences (Clustal W) was analyzed using parsimony in PAUP* software version 4.0b10 (Swofford, 2002), with a TBR heuristic search of 10,000 replicates and the option ‘save multiple trees’ activated. Node support was measured using non-parametric bootstrapping [8] using 1,000 pseudoreplicates of 50 random additions. The phylogenic tree of ETHR was constructed by a minimum evolution method in MEGA4.
Phylogenies of each conceptually translated ETHR sequences were based on the sequences aligned using CLUSTAL W. The aligned sequences were trimmed for conserved region of transmembrane domains 1 to 7 or for the coding region for alternatively spliced exons (subtype A and B) depending on the purpose of analysis. Trees were constructed in MEGA4 software by using neighbor-joining or minimum evolution method with 500 bootstrapping.
2.4. Immunohistochemistry
To describe Inka cells in different insects we used wholemount immunofluorescent procedure described by [33]. Briefly, tracheae from pharate stages of Locusta, Periplaneta, Apis, Tenebrio and Leptinotarsa were dissected under the saline described above. Pharate nymphs and pharate adults of ticks Ixodes ricinus and Rhipicephalus appendiculatus were cut and opened along lateral sides with spring scissors under the same saline and the gut and fat body were carefully removed. Insect tracheal system and cleaned tick bodies were fixed at 4% paraformaldehyde in phosphate buffered saline (PBS, pH 7.4) overnight, washed with PBS-0.3% Triton X-100 (PBST), pre-absorbed with 5% normal goat serum and incubated in the rabbit PETH antiserum for 2 days [31]. Bound antibody was visualized with Alexa Fluor 488-labeled goat anti-rabbit IgG (Invitrogen/Molecular Probes). Tissues were then washed with PBST and mounted in glycerin containing DAPI (2 μg ml-1; Sigma, St. Louis, MO, USA). Stained cells were observed under fluorescent microscope (Nikon Eclipse 600) using a triple band pass filter and photographed with a Nikon Coolpix 990 digital camera (Nikon, Tokyo, Japan).
2.5. Behavioral observations
To determine role(s) of newly identified ETHs we compared the natural and peptide-induced eclosion sequence in pharate adults of Apis and Tenebrio. Pharate adults of Apis execute the eclosion sequence in honeycomb cells. For observation of natural and peptide-induced behaviors one wall of each honeycomb cell was removed. Pharate adults of Tenebrio were placed on a filtration paper in groups of 5-10 for determination of developmental markers under stereomicroscope. Synthetic ETHs were dissolved in distilled water and injected into abdomens of these animals 3-12 hr before natural initiation of ecdysis. Distilled water was injected as a negative control. The time of initiation, duration and specific patterns of induced behavioral sequences were observed under stereomicroscope. The latency and duration of each behavioral phase were measured with a stopwatch.
3. RESULTS
3.1. Isolation and characterization of ETHs from orthopteroid insects
We used enzyme immunoassay with PETH antiserum to isolate ETHs in RP-HPLC-fractionated tracheal extracts dissected from 20-30 pharate adults of the cockroach Periplaneta americana, the locust Schistocerca americana and the cricket Gryllus sp. A single fractionation resulted in isolation of two ETHs in the cockroach and locust and one substance in the cricket. Their amino acid sequences were determined by liquid secondary ion mass spectrometry and MALDI-MAS-MAS (Fig. 1A). The Schistocerca ETH1 and ETH2 are identical with those encoded by the cDNA precursor of Locusta [5]. Comparison of ETHs identified in these orthopteroid insects with other isolated or deduced sequences revealed that -FFLKASKSVPRI-NH2 sequence motif is highly conserved in all hemimetabolous insects as well as in hymenopterans, most dipterans and Tribolium ETH2 (Fig. 1B).
Fig. 1.

Comparison of identified and characterized ETHs with those predicted from cDNA clones or genomes of diverse insects, the water flea and the tick.
A) Biochemically identified amino acid sequences of ETHs from orthopteroid insects.
B) Predicted ETH sequences from cDNA clones or genomes of the hemi- and holometabolous insects, the water flea and the tick show high homology with identified and functionally characterized ETHs from the beetle T. castaneum, the moths B. mori and M. sexta, the mosquito A. aegypti and the fruit fly D. melanogaster. Sequence identities and similarities are indicated by bold letters highlighted by green and yellow, respectively.
3.2. Structure of the eth gene and its precursor in the honeybee
We blasted the Drosophila ETH pre-propeptide sequence against the honeybee genome database to identify the eth gene. The resulting match in the genomic contig (GeneBank accession no. NW_001253067.1) contains ETH sequence, followed by amidation and restriction site, and another related peptide with no restriction site in an open reading frame (ORF).
This DNA sequence served for design of the specific nucleotide primers. Subsequent RACE-PCRs resulted in the identification of 5′and 3′ cDNA fragments with 143 bp overlaps. The entire transcript (618 bp) contains an ORF starting with ATG at bp 39 and ending with TAA at bp 507. The deduced 156 amino acid pre-propeptide starts with a signal peptide (34 amino acids) followed by a single copy of ETH and associated peptide (AP). The ETH and AP sequences are separated by G-R residues indicating the presence of amidation and cleavage sites (Fig. 2).
Fig. 2.
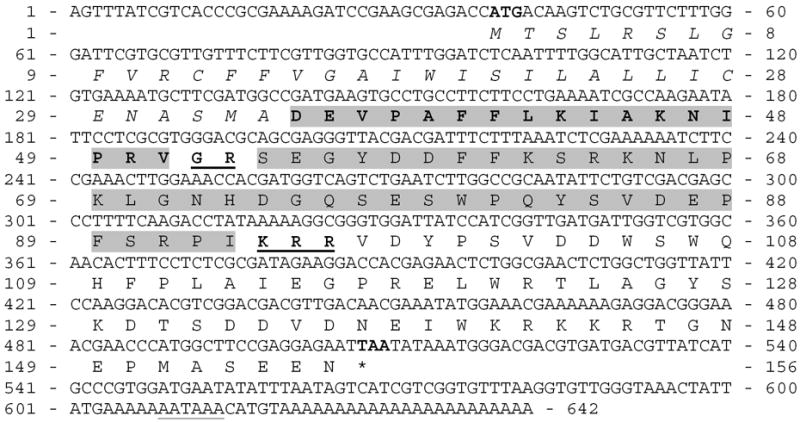
The entire cDNA encoding ETH in Apis mellifera. The predicted signal peptide is in italics, a single copy of ETH is depicted in bold and highlighted grey and putative non-amidated associated peptide is highlighted grey. The amidation and processing sites between peptides are bold and underlined. The putative translation start and stop codons are bold and polyadenylation signal is underlined in the DNA sequence.
In silico screening for the corresponding genomic sequence revealed that Apis eth gene is split into 6 exons located on the 13th chromosome. All introns contained canonical splicing acceptor (GT) and donor (AG) sites. The transcription start of the very short exon 1 (bp 1 to 23) was recognized by the promoter prediction software analysis (Score 0.88; 99% accuracy of prediction).
3.3. In silico identification of ETHs from insects and other arthropods
We compared newly identified Apis ETH pre-propeptide sequence with other ETH precursors in the genomic sequences of other arthropods (Table 1). In silico screen revealed recently annotated eth genes in the mosquitoes Aedes aegypti [6], Anophles gambiae [33], the beetle Tribolium castaneum [3] and the parasitic wasp Nasonia vitripennis (automated annotation), but also detected sequences encoding ETHs in the aphid Acyrthosiphon pisum, the lice Pediculus humanus, the mosquito Culex quinquefasciatus, the tsetse fly Glossina morsitans, eleven Drosophila species, the tick Ixodes scapularis and the water flea Daphnia pulex (Fig 1B).
Table 1.
List of identifed or predicted ETHs and their precursors in arthropods
| Species | Evidence | GeneBank accession no. DNA/Protein | Reference |
|---|---|---|---|
| Periplaneta americana | Peptide | This study | |
| Locusta migratoria | Peptide, mRNA | CO822155 | [5] |
| Schistocerca americana | Peptide | This study | |
| Gryllus sp. | Peptide | This study | |
| Acyrthosiphon pisum | mRNA | EX621344 | This study |
| Pediculus humanus | Predicted gDNA | match in gb|AAZO01005618.1 | This study |
| Aedes aegypti | Peptide, mRNA | ABI93272 | [6] |
| Anopheles gambiae | mRNA | [24,33] | |
| Culex quinquefasciatus | Predicted gDNA | match in gb|AAWU01006874.1 | This study |
| Drosophila ananassae | Predicted gDNA | XP_001960394 | This study |
| D. erecta | Predicted gDNA | XP_001976675 | This study |
| D. grimshawi | Predicted gDNA | XP_001986745 | This study |
| D. melanogaster | mRNA | AAF14282 | [20,21] |
| D. mojavensis | Predicted gDNA | XP_002004620 | This study |
| D. persimilis | Predicted gDNA | XP_002018415 | This study |
| D. pseudobscura | Predicted gDNA | XP_001361212 | This study |
| D. sechellia | Predicted gDNA | XP_002043132 | This study |
| D. simulans | Predicted gDNA | XP_002083035 | This study |
| D. virilis | Predicted gDNA | XP_002048870 | This study |
| D. willistoni | Predicted gDNA | XP_002062955 | This study |
| D. yakuba | Predicted gDNA | XP_002092896 | This study |
| Glossina morsitans | Predicted gDNA | match in Tfly_23-t4364c16.p1ka | This study |
| Apis mellifera | mRNA | BAG80963 | This study |
| Nasonia vitripennis | mRNA | NP_001136107 | Comp annotation |
| Tribolium castaneum | Peptide, mRNA | [2,3,17] | |
| Manduca sexta | Peptide, mRNA | AAD45613 | [30,31] |
| Bombyx mori | Peptide, mRNA | [1,31,32] | |
| Daphnia pulex | mRNA | WFes0080416b | This study |
| Ixodes scapularis | Predicted gDNA | match in gnl|ti|1309379169 | This study |
Abbreviations: Comp annotation - peptide sequence annotated by automated computational genome analysis; mRNA - identified whole or partial gene transcript; Peptide - amino acid sequence of biochemically identified peptides; Predicted gDNA – whole or partial prepro-peptide predicted from the genomic DNA.
These sequence data were obtained from the Wellcome Trust Sanger Institute and can be obtained by the Glossina morsitans Blast server.
These sequence data were produced by the US Department of Energy Joint Genome Institute in collaboration with the Daphnia Genomics Consortium and are available in the wFleaBase web site. Other sequences can be retrieved via Entrez, The Life Science Search Engine at NCBI web site.
All identified or anotated ETH precursors displayed moderate to high similarities with Apis ETH precursor sequence (Fig 3). As expected, the predicted precursor protein (GeneBank accession no. NP_001136107) from Nasonia encodes only a single ETH and showed the highest sequence similarity with Apis ETH precursor. Apis ETH was 88 % identical and 94% similar with Nasonia ETH, whereas Apis AP was 46 % identical and 73% similar with the Nasonia AP. In the aphid Acyrthosiphon cDNA sequence (GeneBank accession no. EX621344) we again predicted a single ETH flanked by usual processing and amidation sites (Fig 3). Other precursors containing ETHs almost identical with Drome-ETH1 and ETH2 were identified in conceptually translated hypothetical proteins from eleven Drosophila species (Table 1, Fig. 1). Some of the newly identified ETHs were synthesized and injected into pharate stages of Apis and Tenebrio to confirm their biological activity and determine eclosion behavior in these species (see below).
Fig. 3.

Identified or predicted ETH precursors in insects and crustacean. Predicted signal peptides are italicized, ETH sequences are bold and highlighted grey, C-terminal amidation sites are underlined and consensual proteolytic cleavage sites flanking active peptides are bold.
We also identified the cDNA sequence from Daphnia (wFleaBase accession no. WFes0080416) encoding the first putative ETH precursor in a representative of Crustacea (Fig 3). Similar to the insect ETH precursors the putative Daphnia ETH pre-propeptide consists of signal sequence followed immediately by two ETHs and associated peptide sequence. A fragment of genomic DNA encoding putative ETH was found in the Ixodes database (Table 1, Fig. 1), but the entire precursor sequence could not be determined.
3.4. The eclosion sequence in Apis
The following morphological markers proved useful to determine developmental stage of pharate adults before eclosion. Dark pigmentation appears in eyes (-70 h) and cuticle of the head capsule (-48 h), thorax (-30 h), legs (-24 h) and the entire pharate adult (-12 h).
Pharate adults of Apis executed the eclosion sequence in honeycomb cells. Natural pre-eclosion started ~2-2.5 h before eclosion onset and was characterized by antennal movements, short contractions of proboscis up and down, rhythmic opening and closing of mandibles, movements of the head capsule sideways and soft tarsal movements. The initial pre-eclosion movements were weak and occasionally interrupted by quiet periods, but after ~1-1.5 h contractions of all appendages became gradually stronger and regular, while tarsal movements develop to robust crossing and scratching of entire legs. This regular and obvious pre-eclosion behavior usually lasts for ~1 hr and is followed by a quiet period for ~20-25 min. Pharate adults then switch to eclosion behavior characterized by anteriorly directed peristaltic contractions of abdomen and grooming-like movements of legs. Peristaltic contractions result in rupture of the old cuticle along the dorsal thorax and abdomen, while strong grooming-like movements of legs result in pealing of the old pupal cuticle from the head and all appendages. Eclosing animals utilize sticky walls of honeycomb cells for support and successful shedding of the old cuticle (n=10). Pharate adults removed from cells showed the eclosion behavior for several hours but failed to shed the pupal cuticle (n=8).
Injection of Apis ETH (5 and 10 pmol) into pharate adults ~12 h prior to natural eclosion induced premature pre-eclosion in 9-13 min (N=12). The pre-eclosion behavior sequence lasted for 32-45 min and was followed by a quiet period for 10-15 min. Most pharate adults (9 out of 12) progressed to eclosion behavior which lasted for ~15-40 min, but only three adults completely emerged from the old pupal cuticle after prolonged eclosion movements (30-40 min). Remaining animals (n=6) shed the old cuticle only partially confirming that synthetic ETH induced the premature eclosion behavior. Animals injected with ETH (1 pmol) or saline showed no specific response. Drosophila ETH1 (5 and 10 pmol) induced similar behaviors in honeybee pharate adults (n=9), except the latency from injection to initiation of pre-eclosion was longer (12-17 min) and only 3 out of 9 animals progressed to eclosion behavior.
3.5. The eclosion sequence inTenebrio
Pharate adults displayed following markers before natural eclosion. Yellow pigmentation of eyes, mandibles and antennae appeared 2 days and turned dark brown 1 day before expected eclosion (-48 h and -24 h, respectively). The entire cuticle turned brown ~12–16 h before eclosion and the old pupal cuticle starts to shrink between all abdominal segments ~3-3.5 h before eclosion.
Natural pre-eclosion-like movements started about 7–8 h before eclosion and were characterized by up and down twitches of the entire abdomen. This behavior begun with irregular series of 3-60 twitches; each twitch contraction lasted ~0.5 sec and intervals between twitches were ~1 sec. These series of twitches were interrupted by quiet pauses lasting from several sec to 3 min. Pre-eclosion contractions were gradually intensified and more regular, developing to continuous abdominal twitches ~3-4 h before eclosion (~50-65 twitches per min). At about -2 h contractions were even stronger and include head and thorax movements up and down towards strong abdominal twitches. At this phase animals occasionally opened and close mandibles, stretched and relax claws and all tarsi. The pre-eclosion movements result in separation of the old and new cuticle, which can be observed at the tip of adult abdomen and appendages at -2 h. Before initiation of eclosion all legs were stretched laterally. The eclosion behavior was initiated by a strong abdominal rotation followed by anteriorly directed peristaltic movements combined with 3-7 occasional robust abdominal rotations (each rotation lasts for 1-2 s). These movements and rotations resulted in rupture of the old cuticle along the dorsal body midline and shedding of the old cuticle on head and thorax followed by legs and abdomen. The eclosion behavior lasted for 16-18 min from the first abdominal rotation to adult emergence.
Premature pre-eclosion behavior was induced by injection of Tribolium ETH1 (5 and 10 pmol) at ~12 h or 3-4 h before natural eclosion (n=20). Injected ETH1 induced in 19 out of 20 animals pre-eclosion (up and down twitches) in 5-12 min. This behavior ceased after 40-70 min and animals did not progress to the eclosion phase. Injection of ETH1 (1 pmol) induced premature pre-eclosion in 4 out of 7 animals, whereas pharate adults injected with 0.5 pmol (n=6) or saline (n=5) showed no response.
Injection of Tribolium ETH2 (5 and 10 pmol) into pharate adults (n=13) at -3–4 h induced the complete eclosion sequence. After ~5 min latency all tested animals displayed pre-eclosion movements lasting for ~30 min and then switched to eclosion behavior. After 18–23 min all animals successfully eclosed. Injection of synthetic ETH2 at −12 h triggers pre-eclosion (n=10) and 8 animals progressed into prolonged eclosion behavior. Four of them eclosed in 20-27 min, but remaining four pharate adults displayed peristaltic movements and ~20 abdominal rotations for 30–60 min, but they failed to rupture and shed the old cuticle. Four out of 6 animals injected at -3–4 h with ETH2 (1 pmol) showed pre-eclosion behavior in 8-12 min. This behavior lasted for 30-55 min and ceased without progression to eclosion behavior. Animals injected with ETH2 (0.5 pmol) and saline injected controls showed no responses.
3.6. Variability of Inka cells in different insects
To confirm that newly identified ETHs in tracheal extracts of various insects are produced by Inka cells, we used immunohistochemical staining with antiserum against Manduca PETH recognizing C-termini of all ETHs (PRXamide; X = I, L, V, M). In all the examined insects the antiserum reacted with Inka cells on tracheal surface within one day prior to ecdysis and no other structures were stained. Numerous and small Inka cells with short cytoplasmic processes were individually scattered on the surface of broad and narrow tracheae in pharate nymphs and pharate adults of the cockroach Periplaneta (Figs. 4A,B). Similar Inka cells were observed in pharate nymphs and pharate adults of the locust Locusta, but they were more oval with very short cytoplasmic processes (Figs. 4C,D). In both species these cells may rarely occur in couples. In pharate adults of the honey bee Apis a large number of small oval Inka cells was located around narrow segmental tracheal branches connecting the main longitudinal trachea with each spiracle (Fig. 4E).
Fig. 4.
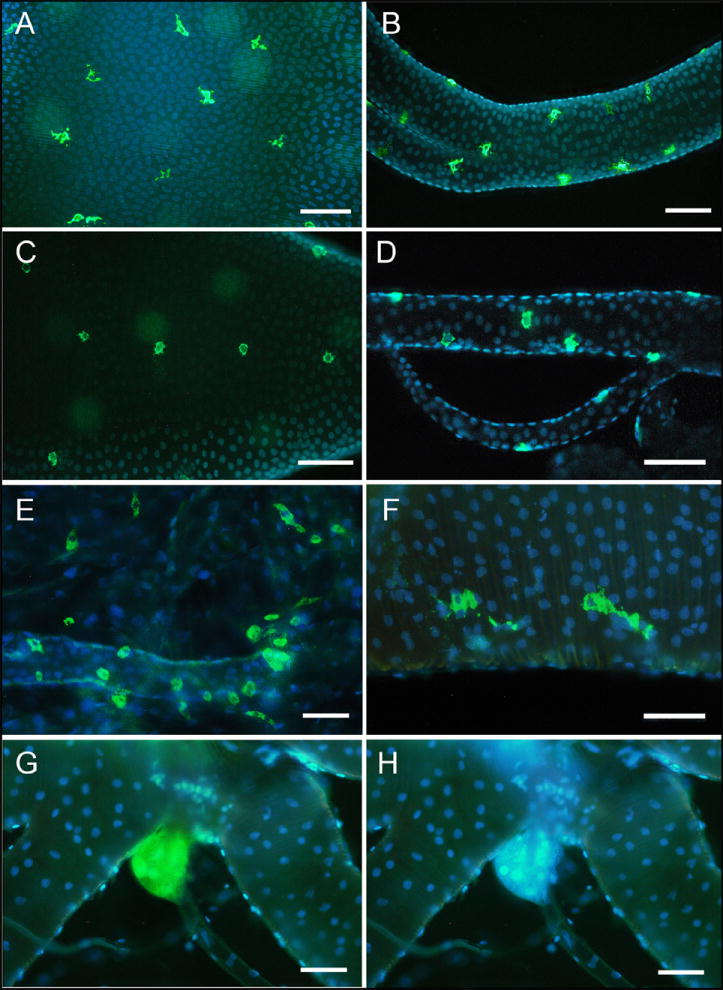
PETH-immunoreactive Inka cells in pharate stages of diverse insect species.
(A, B) Numerous small Inka cells (green color) scattered on surface of the broad segmental trachea (A) and narrow tracheal branch (B) in pharate nymph of the cockroach Periplaneta. (C, D) Similar cells are located on tracheal surface in pharate nymph of the locust Locusta. (E) A large number of small Inka cells is concentrated on narrow tracheal branch near each spiracle of pharate adult of the honeybee, Apis. (F) Paired Inka cells with prominent cytoplasmic processes in pharate adult of the beetle Tenebrio. (G, H) The beetle Leptinotarsa contains single large oval Inka cells attached to tracheal bush near each spiracle. DAPI nuclear staining (blue color) revealed multiple nuclei in these cells (H). Scale bars, 100 μm.
We also stained Inka cells in two beetle species (Tenebrio and Leptinotarsa). The former beetle was used for functional examination of Tribolium ETHs. Morphology and number of Inka cells was strikingly different in Tenebrio (closely related to Tribolium) compared to the unrelated Colorado potato beetle Leptinotarsa. In Tenebrio pharate adults a group of 4-6 or 1-2 middle-sized cells with broad cytoplasmic processes were located on broad tracheae near each prothoracic or each abdominal spiracle, respectively (Fig. 4F). In addition, numerous small oval immunoreactive cells were observed mostly on narrow tracheal branches (not shown). On the other hand, Leptinotarsa contained nine pairs of large oval and multinucleated Inka cells (Figs. 4G,H). These cells were individually and segmentally distributed in tracheal bushes attached to the prothoracic and each abdominal spiracle.
Blast search in the genome of the tick Ixodes scapularis led to identification of a peptide showing high sequence similarity with insect ETHs (Fig. 1). Therefore, we used the PETH antiserum to examine possible source of this peptide on the tracheal system or in the periphery of two available tick species - Ixodes ricinus and Rhipicephalus appendiculatus. No immunoreactive cells were observed on tracheal surface of pharate 2nd instar larvae and pharate adults, but oval paired cells were detected on lateral sides of each pedal segment in pharate larvae of both species (Figs. 5A,B). Since these peripheral cells with putative endocrine function have not been described before, we named them “pedal endocrine cells”. Although these cells show strong PETH-immunoreactivity, it is not clear that they represent Inka cell homologues in ticks.
Fig. 5.
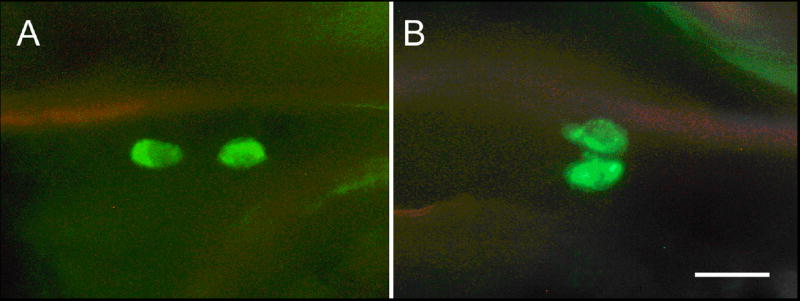
PETH-immunoreactive peripheral cells in pharate nymphs of the ticks Ixodes ricinus (A) and Rhipicephalus appendiculatus (B). These paired cells are located on lateral sides of each pedal segment. Scale bar, 5 μm.
3.7. In silico identification of ETH receptors
Important parts of the ETH signaling are two splicing variants of its receptor (ETHR-A and ETHR-B) differentially expressed in the CNS of Drosophila and Manduca [14,15]. We searched sequence databases to find related proteins in Apis and other insects. Gene encoding Apis ETHR-A composed of 421 aa was predicted by automated computational analysis (Table 2). In the corresponding genomic region (GeneBank accession no. NW_001253200) we deduced an alternative 3-prime exon (bps 149234-149956) encoding the subtype B specific sequence composed of 464 aa. The entire coding region of Apis ethr is split into 8 exons of which the last two are subtype-specific (Fig. 6).
Table 2.
Identified and predicted GPCRs in arthropods showing homology with characterized Drosophila ETHR-A. Sequence Drosophila ETHR-A was used for prediction of closely related genomic DNA (gDNA) encoding amino acid sequences in putative exons of common N-terminal and/or subtype specific sequences.
| Protein and species | GeneBank accession no. | Score (bits) | Expect Value | Citation |
|---|---|---|---|---|
| ETHR-A Acyrthosiphon pisum | XP_001944756a | 403 | 5e-117 | This study |
| ETHR-B Acyrthosiphon pisum | XP_001944816a | 416 | 8e-121 | This study |
| ETHR-A Pediculus humanus | Predicted gDNA | 319 | 7e-92 | This study |
| ETHR-B Pediculus humanus | EEB17194 | 362 | 1e-104 | This study |
| ETHR-A Rhodnius prolixus | Predicted gDNA | 434 | 2e-126 | This study |
| ETHR-B Rhodnius prolixus | Predicted gDNA | 410 | 2e-119 | This study |
| ETHR-A Aedes aegypti | ABI93273 | 471 | 3e-137 | [6] |
| ETHR-B Aedes aegypti | ABI93274 | 449 | 1e-130 | [6] |
| ETHR-A Anopheles gambiae | Predicted gDNA | This study | ||
| ETHR-B Anopheles gambiae | Predicted gDNA | This study | ||
| ETHR-A Culex quinquefasciatus | XP_001845191a | 458 | 2e-133 | This study |
| ETHR-B Culex quinquefasciatus | XP_001845190a | 437 | 3e-127 | This study |
| ETHR-A Drosophila melanogaster | NP_650960 | 100 | [13,15,23] | |
| ETHR-B Drosophila melanogaster | NP_996255 | 457 | 4e-133 | [13,15,23] |
| ETHR-A Nasonia vitripennis | Not detected | |||
| ETHR-B Nasonia vitripennis | XP_001606566 | 343 | 7e-99 | Automated annotation |
| ETHR-A Apis mellifera | XP_001120367 | 309 | 1e-88 | Automated annotation |
| ETHR-B Apis mellifera | Predicted gDNA | 414 | 3e-120 | This study |
| ETHR-A Tribolium castaneum | ABN79653 | 413 | 7e-120 | [3] |
| ETHR-B Tribolium castaneum | ABN79654 | 416 | 6e-121 | [3] |
| ETHR-A Manduca sexta | AAX19163 | 398 | 3e-115 | [14] |
| ETHR-B Manduca sexta | AAX19164 | 440 | 5e-128 | [14] |
| ETHR-A Bombyx mori | AB330426 | 387 | 5e-112 | [29] |
| ETHR-B Bombyx mori | AB330427 | 427 | 4e-124 | [29] |
| ETHR-A Daphnia pulex | NCBI_GNO_62774b | 315 | 2e-90 | Automated annotation |
| ETHR-B Daphnia pulex | Predicted gDNA | 285 | 2e-81 | This study |
| ETHR-A Ixodes scapularis | XP_002399688 | 100 | 5e-26 | This study |
| ETHR-B Ixodes scapularis | XP_002434887 | 83.6; 50.8 | 1e-20; 1e-10 | This study |
| ETHR-A Strongylocentrotus purpuratus | XP_786973 | 129 | 2e-34 | Automated annotation |
| ETHR-B Strongylocentrotus purpuratus | XP_781583 | 137; 89 | 1e-36; 2e-22 | Automated annotation |
Referenced hypothetical proteins predicted by automated computational analysis contain only part of ETHR related sequence. The functional ETHRs containing all transmembrane domains were predicted manually in this study.
Daphnia ETHRs are based on sequence data produced by the US Department of Energy Joint Genome Institute in collaboration with the Daphnia Genomics Consortium (wFleaBase web site). Other sequences can be retrieved via Entrez, The Life Science Search Engine at NCBI web site.
Fig. 6.
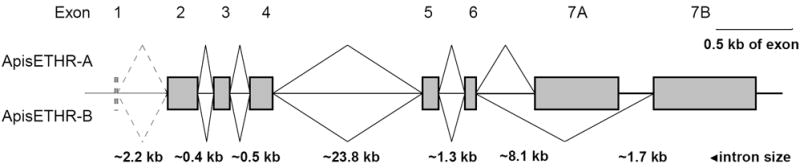
Organization of the predicted Apis-ethr. The putative Apis ETHR encodes two receptor subtypes produced by alternative splicing of mutually exclusive exons 7A and 7B.
Both subtypes of Apis ETHR contain seven putative transmembrane domains (Fig. 7) and share N-terminal sequences up to the 4th transmembrane region domain as described in Drosophila and Manduca ETHRs. The BLAST search revealed that Apis ETHRs are similar to already characterized ETHRs as well as to GPCRs recently inferred from genomic sequences (Table 2).
Fig. 7.
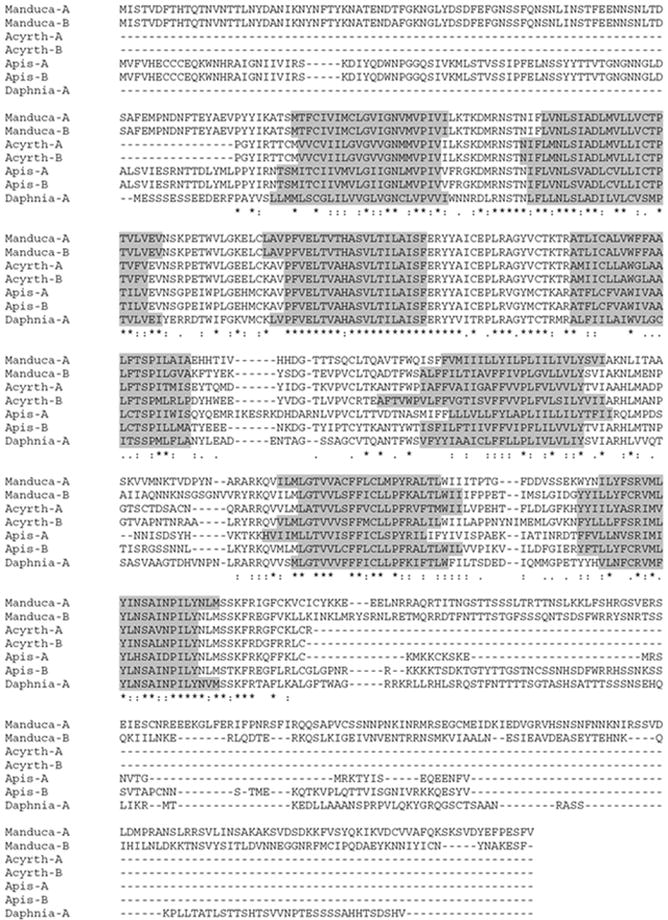
Amino acid sequence alignment (CLUSTAL W 1.8) of the predicted honeybee Apis ETHR-A and -B, the pea aphid Acyrthosiphon ETHR-A and-B, the water flea Daphnia ETHR-A and the characterized moth Manduca ETHR-A and -B. Sequences of Acyrthosiphon ETHRs are incomplete. Seven predicted transmembrane domains are grey highlighted. Sequence identities are indicated by asterisks, gaps by dashes and similarities are denoted by periods and colons.
The first Hemimetabola ETHRs have been recently predicted in the aphid Acyrthosiphon (Table 2). However, the inferred aphid ETHR-A and ETHR-B sequences were incomplete and did not contain all transmembrane domains. Therefore we manually predicted the aphid ETHRs using Drosophila ETHR-A as a BLAST probe. We deduced one ETHR common exon, as well as subtype A and B specific exons located in a single genomic contig (NW_001918139.1). An additional upstream common exon was found in the contig NW_001924517.1. Our predicted Acyrthosiphon ETHRs still lack N′ and C′ termini, but contain all 7 transmembrane domains and display striking similarities with all known ETHRs (Fig. 7). Using similar BLAST based approach we predicted ETHRs in the lice Pediculus, the bug Rhodnius, the mosquito Culex and more interestingly, in the water flea Daphnia and the tick Ixodes (Table 2, Supplement 1). Similar to the insect ETHRs, two hypothetical Daphnia ETHRs contain a common N-terminal part and subtype-specific C-terminal part. On the other hand, two different genes encoding GPCRs were predicted as putative ETHR-A and ETHR-B in Ixodes. Screening for ETHR-related sequences in other invertebrate databases revealed two putative 7-transmembrane receptors, which have been annotated by automated computational analysis in the purple urchin (Strongylocentrotus purpuratus; Echinodermata) (Table 2). These putative GPCRs displayed only weak sequence similarity with conserved domains of insect ETHRs (see Fig 7 for conserved domains) and therefore were not used for subsequent cladistic analysis.
In the phylogenic analysis of entire ETHRs (transmembrane domains 1 to7) all insect ETHRs were grouped into a monophyletic clade together with the predicted GPCRs from Daphnia and Ixodes. As expected, related insect taxa were grouped together (e.g. hemimetabolous insects, moths, dipterans and hymenopterans). Note strong support for insect, Daphnia and Ixodes ETHRs when related pyrokinin and CAPA receptors are used as the outgroup. The entire clade containing all Arthropoda ETHRs is apparently related to the outgroup of Drosophila receptors for pyrokinins and CAPA (Fig. 8).
Fig. 8.
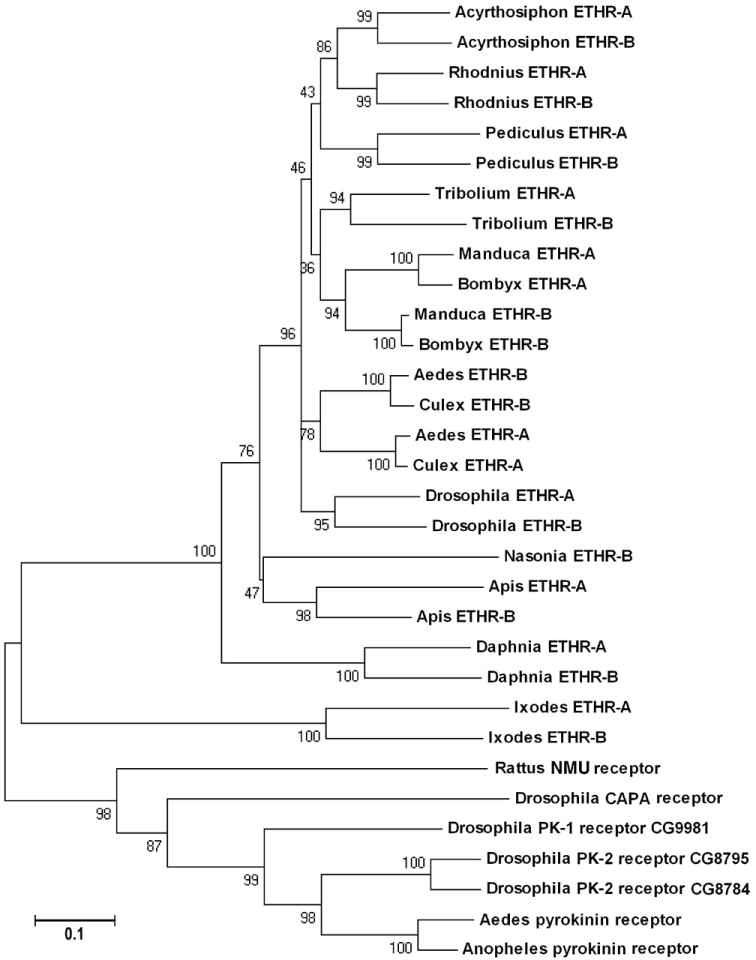
The phylogenic relationship of ETHRs and related GPCRs using all transmembrane domains 1 to 7. Note that all insect ETHRs are grouped with predicted Daphnia and Ixodes ETHRs when related pyrokinin receptors are used as the outgroup. The tree was constructed by a minimum neighbor-joining method (Gaps/Missing Data-Parwise deletion) in MEGA4. Numbers are for percent supports in 500 bootstrapping. PK-1, pyrokinin-1; PK-2, pyrokinin-2; NMU, neuromedin U-25.
Phylogenic analysis of subtype-specific sequences (transmembrane domains 4 to 7) clustered ETHR-A and ETHR-B into clearly separated groups (Fig. 9). This suggests that subtype-specific sequences of all insects are closely related and distinct ETHR-A and ETHR-B exons probably originated before the diversification of insects into different families.
Fig. 9.
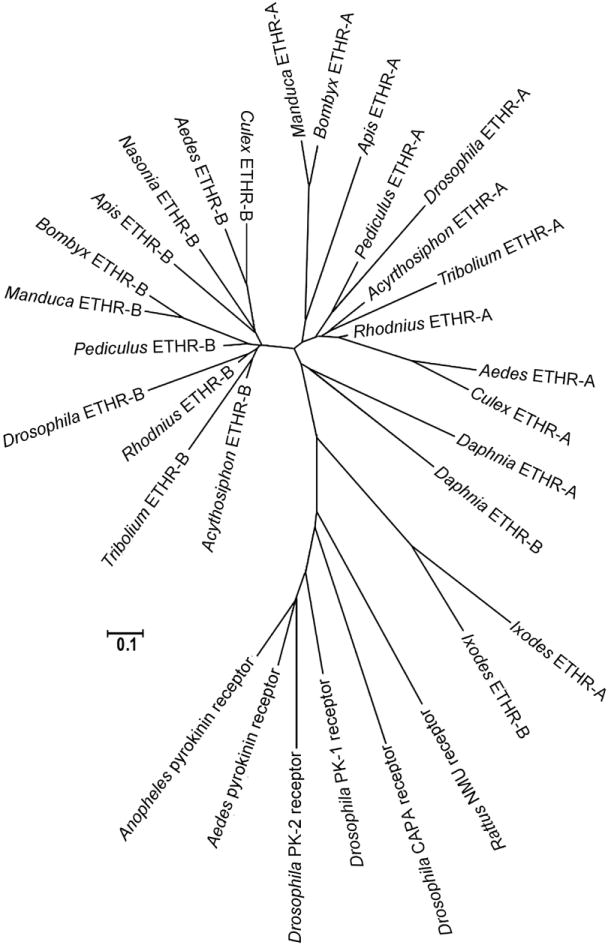
The phylogenic relationship of the alternatively spliced exons encoding a subtype A and B regions of the ETHR and related GPCRs (transmembrane domains 4 to 7).
ETHR-A- and ETHR-B-specific sequences form two apparently separate groups in all insects. Note that branches for alternatively spliced exons (A and B) are dated before the diversification of insect taxa. The tree was constructed by a minimum evolution method (Gaps/Missing Data-Parwise deletion) in MEGA4.
4. DISCUSSION
4.1. Structural similarity of ETHs and their receptors
After the first identification of ETH in Manduca [30], related peptides have been isolated from epitracheal gland extracts, cloned from the cDNA or deduced in silico from available genomes and databases of several species belonging to diverse insect orders - Orthoptera, Coleoptera, Hymenoptera, Lepidoptera and Diptera [2,5,11,20,31,32,33,35]. So far, the locust Locusta is a single representative of Hemimetabola in which two almost identical sequences of ETHs have been deduced from the whole body cDNA library. Presence of the fully processed amidated peptides was confirmed in tracheal extracts using the nanoflow liquid chromatography and MSMS fragmentation analysis [5]. However, it was not clear if related peptides are produced by Inka cells of other hemimetabolous insects. Therefore, we isolated and identified ETHs from tracheal extracts of other three hemimetabolous orthopteroid insects, the cockroach Periplaneta, the cricket Gryllus sp. and the locust Schistocerca, and deduced closely related peptides from putative eth genes of the aphid Acyrthosiphon and the lice Pediculus. Recent progress in insect genomics has resulted in prediction of ETHs from several holometabolous insects including Apis [11], Tribolium [2,35], and mosquitoes Anopheles [33] and Aedes [6]. In this study we cloned Apis cDNA encoding ETH and predicted closely related peptides in the mosquito Culex quinquefasciatus, tsetse fly Glossina palpalis and numerous Drosophila spp. (Table 1). Primary structures of these peptides are highly conserved especially at their C-termini.
ETH are known to act via two splicing variants of GPCRs differentially expressed in the CNS of Drosophila and Manduca [14,15]. Orthologs of two ETHR subtypes have been recently identified and characterized in several holometabolous insects including Tribolium, Bombyx and Aedes [3,6,29]. In this study we inferred both subtypes of ETHR from genomic sequences of Rhodnius, Acyrthosiphon, Pediculus, Apis, Anopheles and Culex. These data demonstrate conserved structures and organization of ETHRs in all examined representatives of Hemimetabola and Holometabola.
We also identified orthologs of eth and ethr genes in other arthropods including the tick Ixodes (Arachnida) and water flea Daphnia (Crustacea) indicating that ETH-ETHR signaling is widespread in ecdysing animals. Curiously, two proteins structurally related to ETHRs have been predicted from the genome of the urchin Strongylogaster (Echinodermata) (Table 2). Possible role(s) of ETHR-like proteins in the non-ecdysing deuterostomian invertebrate are elusive, but suggest functional modification of these GPCRs during evolution. Functional shift of ecdysis-signaling molecules in primitive deuterostomians is also indicated by bursicon-like proteins deduced from the sea urchin genome. This heterodimeric neurohormone required for plasticization and hardening of the cuticle in insect post-ecdysis processes is missing in more advanced deuterostomians [27].
ETHs belong to a group of insect -PRXamide peptides evolutionary related to the vertebrate neuromedin U [21]. In our analysis receptors for selected Drosophila -PRXamide neuropeptides (CAPA and pyrokinins) constitute a clade well separated from a clade of all mentioned ETHRs. These data provide further evidence for ligand-receptor co-evolution [21] in insects and other invertebrates.
4.2. Redundant function of ETHs in most insects
Most insects produce two active ETHs encoded by a single eth gene [5,20,31,32,33,35]. Inka cells of Manduca and Drosophila produce shorter and longer peptide hormones which exert different behavioral responses. Shorter peptides (Manduca PETH and Drosophila ETH2) elicit only a subset of behaviors, whereas longer peptides (Manduca ETH and Drosophila ETH1) induce the entire ecdysis sequence [22,31]. Therefore, shorter peptides seem to be redundant. Bombyx PETH (11 aa) and ETH (23 aa) also differ considerably in length, but their potency to induce the entire ecdysis sequence in all stages is very similar [32]. Recent studies also showed that Aedes ETH1 and ETH2 are equally potent in inducing the ecdysis sequence of pharate larvae, pupae and adults of this mosquito species [6].
In this report we show that Apis cDNA encodes a single ETH located immediately after the signal sequence and predicted eth genes from the wasp Nasonia and aphid Acyrthosiphon also encode a single ETH. Apis ETH elicits the entire eclosion sequence in pharate adults of honeybees, similarly as longer ETHs elicit complete ecdysis sequences in different stages of Manduca and Drosophila [22,31]. This clearly indicates that the longer ETH can substitute role(s) of the second shorter peptide and two similar peptides do not seem to be important for binding to different receptor subtypes and regulation of distinct functions. Instead, duplication of ETHs may serve for rapid increase of their concentration in the hemolymph and faster activation of target cells.
Alternative splicing of the ETHR precursor results in production of ETHR-A and ETHR-B subtype each expressed in different networks of central neurons producing multiple neuropeptides in Manduca and Drosophila [14,15]. Ca2+ imaging showed that ETH1 application to the isolated CNS of Drosophila results in subsequent activation of these peptidergic ETHR-A networks corresponding to pre-ecdysis, ecdysis and post-ecdysis [15]. Specific roles of various neuropeptides in ETHR neurons were further supported by electrophysiological experiments in Manduca. For example, a mixture of kinins and diuretic hormones produced by ETHR-A neurons control pre-ecdysis I, while CCAP and myoinhibitory peptides regulate ecdysis behavior [14]. Molecular approaches in Drosophila and Tenebrio showed that targeted killing of specific peptidergic ETHR neurons or suppressed expression of certain peptides or their receptors results in specific behavioral deficits corresponding to expected functions of each peptide and its receptor [3,15,19]. Curiously, beetles with suppressed ETHR-B expression display no apparent behavioral phenotype suggesting different roles of each ETHR subtype in the ecdysis sequence [3] Since the structure and organization of all functionally characterized and putative ETHR-A and ETHR-B are conserved in all examined invertebrates (except the tick) they probably play different roles in activation or inhibition of pre-ecdysis, ecdysis and post-ecdysis processes. We can speculate that Ixodes ETHR-A and ETHR-B encoded by two different genes also control different functions during ecdysis processes. These observations also support previous conclusions and show that a single ETH is sufficient for activation of both receptor subtypes and subsequent execution of the entire ecdysis sequence.
4.3. Comparison of ETH action during the ecdysis sequence in various insects
The role of ETH in ecdysis sequence has been described in detail only in few insects including two moths [30,31,32], Drosophila [15,18,22], Tribolium [3] and Aedes [6]. The ecdysis sequence was also described in the cricket Teleogryllus oceanicus [4], the locust Schistocerca gregaria [10] and saturniid moths [28], but ETH role in these species has not been examined. Since we identified ETHs in Apis and Tribolium, we wanted to confirm their biological activity in the honeybee and Tenebrio, a beetle species closely related to Tribolium. Previously published observations and our recent data show that all studied insects display species- and stage-specific differences in larval, pupal and adult ecdysis/eclosion behavioral sequences. The pre-ecdysis and pre-eclosion motor programs appear to be the most diverse and each examined species displays a very characteristic set of movements for loosening and splitting of the old cuticle. For example; pre-eclosion behavior of Tenebrio is characterized by up and down twitches of the entire body, whereas pharate adults of Apis exhibit various movements of head and body appendages. Pre-ecdysis is followed immediately by ecdysis, but adult eclosion is delayed in some species by a quiescent period, e.g. in Drosophila [18] and Bombyx [32]. Pharate adults of Apis also exhibit this quiescent period for ~15-20 min while Tenebrio switch from pre-eclosion to eclosion behavior immediately.
Behavioral sequences of most studied animals show some common features. Ecdysis and eclosion behaviors involve in most insects peristaltic abdominal movements necessary for complete shedding of the old cuticle. However, abdominal rotations or grooming-like leg movements have been observed along peristaltic eclosion contractions in Apis and Tenebrio, respectively. This suggests that ETHs act via different networks of central neurons in various insects and these networks may be modified during metamorphosis to control shedding of new adult organs.
In summary, our data provide evidence that Inka cells, ETHs and their receptors occur in a wide range of insects. In addition, we have shown here for the first time that peptides closely related to ETHs and ETHRs occur in representatives of ticks and crustaceans. Although there is remarkable variability in number and morphology of Inka cells, as well as in behavioral displays during the ecdysis/eclosion sequences, our data indicate that the conserved ETH-ETHR signaling may be widespread in arthropods and perhaps in other invertebrates.
Supplementary Material
ETHR sequences predicted in this study. Missing parts of amino acid sequences are indicated by a question mark.
Acknowledgments
This study was supported by a grant from the National Institute of Health, USA (GM 67310), and Slovak grant agencies, Agentúra na podporu výskumu a vývoja (APVV-51-039105) and Vedecká grantová agentúra (VEGA 2-6090-26, VEGA2/0132/09). We thank Dr. Fedor Čiampor for advice with the phylogenic analysis of ETHRs, Dr. Mirko Slovák for providing pharate nymphs and adults of the ticks Ixodes ricinus and Rhipicephalus appendiculatus, and Dr. Jozef Šimúth for providing honeybee pharate pupae and pharate adults.
Footnotes
The sequence reported in this study for the first time has been deposited in the GeneBank database (Apis ETH: GeneBank accession no. AB469047).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adams ME, Žitňan D. Identification of ecdysis-triggering hormone in the silkworm Bombyx mori. Biochem Biophys Res Comm. 1997;230:188–91. doi: 10.1006/bbrc.1996.5915. [DOI] [PubMed] [Google Scholar]
- 2.Amare A, Sweedler JV. Neuropeptide precursors in Tribolium castaneum. Peptides. 2007;28:1282–91. doi: 10.1016/j.peptides.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakane Y, Li B, Muthukrishnan S, Beeman RW, Kramer KJ, Park Y. Functional analysis of four neuropeptides, EH, ETH, CCAP and bursicon, and their receptors in adult ecdysis behavior of the red flour beetle, Tribolium castaneum. Mech Develop. 2008;125:984–95. doi: 10.1016/j.mod.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Carlson JR, Bentley D. Ecdysis: neural orchestration of a complex behavioral performance. Science. 1977;195:1006–8. doi: 10.1126/science.841322. [DOI] [PubMed] [Google Scholar]
- 5.Clynen E, Huybrechts J, Verleyen P, De Loof A, Schoofs L. Annotation of novel neuropeptide precursors in the migratory locust based on transcript screening of a public EST database and mass spectrometry. BMC Genomics. 2006;7:201. doi: 10.1186/1471-2164-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai L, Adams ME. Ecdysis triggering hormone signaling in the yellow fever mosquito Aedes aegypti. Gen Comp Endocrinol. 2009;162:43–51. doi: 10.1016/j.ygcen.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyrløv JB, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 8.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–91. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 9.Harlow E, Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Press; 1988. [Google Scholar]
- 10.Hughes TD. The imaginal ecdysis of the desert locust Schistocerca gregaria 1. Description of the behavior. Physiol Entomol. 1980;5:47–54. [Google Scholar]
- 11.Hummon AB, Richmond TA, Verleyen P, Baggerman G, Huybrechts J, Ewing MA, et al. From the genome to the proteome: uncovering peptides in the Apis brain. Science. 2006;314:647–9. doi: 10.1126/science.1124128. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda E. Rimensen Kimon. In: Ikeda E, editor. Experimental Anatomy and Physiology of Bombyx mori. Tokyo, Japan: Meibundo; 1913. pp. 242–3. [Google Scholar]
- 13.Iversen A, Cazzamali G, Williamson M, Hauser F, Grimmelikhuijzen CJ. Molecular identification of the first insect ecdysis triggering hormone receptors. Biochem Biophys Res Comm. 2002;299:924–31. doi: 10.1016/s0006-291x(02)02798-5. [DOI] [PubMed] [Google Scholar]
- 14.Kim YJ, Žitňan D, Cho KH, Mizoguchi A, Schooley D, Adams ME. Central peptidergic ensembles associated with organization of an innate behavior. Proc Natl Acad Sci USA. 2006;103:14211–6. doi: 10.1073/pnas.0603459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, Žitňan D, Galizia G, Cho KH, Adams ME. A command chemical triggers an innate behavior by sequential activation of multiple peptidergic ensembles. Curr Biol. 2006;16:1395–1407. doi: 10.1016/j.cub.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Krogh A, Larsson B, von Heijne G, Sonnhammer ELL. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol. 2001;305:567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Predel R, Neupert S, Hauser F, Tanaka Y, Cazzamali G, et al. Genomics, transcriptomics, and peptidomics of neuropeptides and protein hormones in the red flour beetle Tribolium castaneum. Genome Res. 2008;18:113–22. doi: 10.1101/gr.6714008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNabb SL, Baker JD, Agapite J, Steller H, Riddiford LM, Truman JW. Disruption of a behavioral sequence by targeted death of peptidergic neurons in Drosophila. Neuron. 1997;19:813–23. doi: 10.1016/s0896-6273(00)80963-0. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Schroeder AJ, Helfrich-Forster C, Jackson FR, Ewer J. Targeted ablation of CCAP neuropeptide-containing neurons of Drosophila causes specific defects in execution and circadian timing of ecdysis behavior. Development. 2003;130:2645–56. doi: 10.1242/dev.00503. [DOI] [PubMed] [Google Scholar]
- 20.Park Y, Žitňan D, Gill SS, Adams ME. Molecular cloning and biological activity of ecdysis-triggering hormones in Drosophila melanogaster. FEBS Letters. 1999;463:133–8. doi: 10.1016/s0014-5793(99)01622-1. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, Kim YJ, Adams ME. Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proc Natl Acad Sci USA. 2002;99:11423–8. doi: 10.1073/pnas.162276199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park Y, Filippov V, Gill SS, Adams ME. Deletion of the ecdysis-triggering hormone gene leads to lethal ecdysis deficiency. Development. 2002;129:493–503. doi: 10.1242/dev.129.2.493. [DOI] [PubMed] [Google Scholar]
- 23.Park Y, Kim YJ, Dupriez V, Adams ME. Two subtypes of ecdysis-triggering hormone receptor in Drosophila melanogaster. J Biol Chem. 2003;278:17710–5. doi: 10.1074/jbc.M301119200. [DOI] [PubMed] [Google Scholar]
- 24.Riehle MA, Garczynski SF, Crim JW, Hill CA, Brown MR. Neuropeptides and peptide hormones in Anopheles gambiae. Science. 2002;298:172–5. doi: 10.1126/science.1076827. [DOI] [PubMed] [Google Scholar]
- 25.Reese MG. Application of a time-delay neural network to promoter annotation in the Drosophila melanogaster genome. Comput Chem. 2001;26:51–6. doi: 10.1016/s0097-8485(01)00099-7. [DOI] [PubMed] [Google Scholar]
- 26.Swofford DL. PAUP* Phylogenetic analysis using parsimony (* and other methods), version 4.0b10. Sunderland, MA: Sinauer and Associates; 2002. [Google Scholar]
- 27.Van Loy T, Van Hiel M, Vandersmissen P, Poels J, Mendive F, Vassart G, et al. Evolutionary conservation of bursicon in the animal kingdom. Gen Comp Endocrinol. 2007;153:59–63. doi: 10.1016/j.ygcen.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 28.Truman JW. Physiology of insect ecdysis Part 1: the eclosion behavior of saturniid moths and its hormonal release. J Exp Biol. 1971;54:805–14. [Google Scholar]
- 29.Yamanaka N, Yamamoto S, Žitňan D, Watanabe K, Kawada T, Satake H, et al. Neuropeptide receptor transcriptome reveals unidentified neuroendocrine pathways. PLoS ONE. 2008;3:e3048. doi: 10.1371/journal.pone.0003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Žitňan D, Kingan TG, Hermesman J, Adams ME. Identification of ecdysis-triggering hormone from an epitracheal endocrine system. Science. 1996;271:88–91. doi: 10.1126/science.271.5245.88. [DOI] [PubMed] [Google Scholar]
- 31.Žitňan D, Ross LS, Žitňanová I, Hermesman JL, Gill SS, Adams ME. Steroid induction of a peptide hormone gene leads to orchestration of a defined behavioral sequence. Neuron. 1999;23:523–35. doi: 10.1016/s0896-6273(00)80805-3. [DOI] [PubMed] [Google Scholar]
- 32.Žitňan D, Hollar L, Spalovska I, Takac P, Žitňanová I, Gill SS. Molecular cloning and function of ecdysis-triggering hormones in the silkworm Bombyx mori. J Exp Biol. 2002;205:3459–73. doi: 10.1242/jeb.205.22.3459. [DOI] [PubMed] [Google Scholar]
- 33.Žitňan D, Žitňanová I, Spálovská I, Takáč P, Park Y, Adams ME. Conservation of ecdysis-triggering hormone signalling in insects. J Exp Biol. 2003;206:1275–89. doi: 10.1242/jeb.00261. [DOI] [PubMed] [Google Scholar]
- 34.Žitňan D, Adams ME. Neuroendocrine regulation of insect ecdysis. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 3. 2005. pp. 1–60. [Google Scholar]
- 35.Žitňan D, Kim YJ, Žitňanová I, Roller L, Adams ME. Complex steroid-peptide-receptor cascade controls insect ecdysis. Gen Comp Endocrinol. 2007;153:88–96. doi: 10.1016/j.ygcen.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Žitňanová I, Adams ME, Žitňan D. Dual ecdysteroid action on epitracheal glands and the central nervous system preceding ecdysis of Manduca sexta. J Exp Biol. 2001;204:3483–95. doi: 10.1242/jeb.204.20.3483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ETHR sequences predicted in this study. Missing parts of amino acid sequences are indicated by a question mark.


