Abstract
In order to metastasize, tumor cells must adapt to untoward, stressful microenvironments as they disseminate into the systemic circulation and colonize distant organ sites. Autophagy, a tightly regulated lysosomal self-digestion process that is upregulated during cellular stress, has been demonstrated to suppress primary tumor formation, but how autophagy influences metastasis remains unknown. Autophagy may inhibit metastasis by promoting anti-tumor inflammatory responses or by restricting the expansion of dormant tumor cells into macrometastases. Conversely, self-eating may promote metastasis by enhancing tumor cell fitness in response to environmental stresses, such as anoikis, during metastatic progression. Because autophagy is titratable, it may serve both pro- and anti-metastatic functions depending on the contextual demands placed on tumor cells throughout the metastatic process.
Introduction
Metastasis is the primary cause of lethality in cancer patients and consists of multiple discrete steps, including: 1) invasion of tumor cells from the primary tumor site, 2) intravasation into the vasculature or lymphatic circulation and survival in the circulation, 3) extravasation of individual tumor cells at the target organ site, and ultimately, 4) the expansion and colonization of tumor cells at the secondary site [1] (Figure 1). Despite our understanding of these basic steps, the exact mechanisms governing dissemination and metastasis remain unclear.
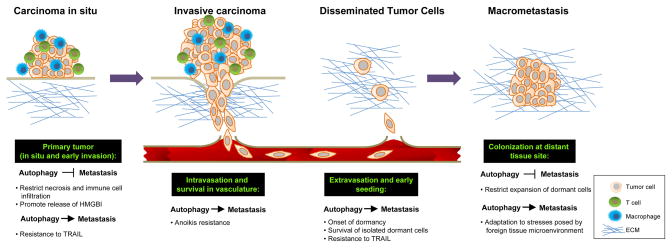
Figure 1. Potential roles of autophagy during metastasis.
Metastatic progression involves a series of established steps (black boxes with green text) during which autophagy may promote or inhibit metastasis. Primary tumor: Autophagy may inhibit metastasis by restricting necrosis and the consequent infiltration of pro-metastatic inflammatory cells or by directly promoting the release of anti-metastatic immunomodulatory factors such as HMBG1. Alternatively, it may promote metastasis by allow tumor cells to resist TRAIL-induced apoptosis. Intravasation and survival in systemic circulation: As tumor cells disseminate and enter the systemic vasculature, autophagy may protect cells from anoikis, and thus serve a pro-metastatic function. Extravasation and early seeding: Disseminated tumor cells (DTCs) often enter a period of dormancy, or quiescence, as they cope with exposure to hostile microenvironments at distant sites. Autophagy may favor survival of quiescent tumor cells during dormancy. Colonization at distant tissue site: Autophagy may restrict the development of DTCs into larger macrometastases by maintaining dormancy for extended periods of time; alternatively, it may also promote macrometastases by allowing the tumor cells to overcome the stresses of tumor growth in a foreign microenvironment.
Cancer cells face diverse environmental and cellular stresses during every step of metastatic progression. To cope, tumor cells induce adaptive pathways, such as autophagy, a tightly regulated lysosomal self-digestion process utilized by cells under duress. Indeed, autophagy has been found to be upregulated in cancer cells during many of the principal events directing metastasis, including: 1) hypoxia and metabolic stress, 2) response to inflammatory signals in the tumor microenvironment, 3) loss of cell-extracellular matrix (ECM) contact, and 4) quiescence of solitary dormant cells [2,3]. Here, we overview how autophagy can either promote or impede metastasis, focusing on selected steps of this complex process (Figure 1). Due to the diametrically opposing functions of autophagy, we speculate that self-eating is titrated during metastatic progression to best meet the needs of stressed tumor cells [4].
Autophagy, necrosis and inflammation during metastasis
The initial steps of metastasis require signals at the primary tumor that promote migration and local invasion and that facilitate the intravasation of tumor cells into the systemic circulation. Various cell types within the tumor microenvironment supply cancer cells with such signals [5]. In particular, inflammatory cells infiltrate tumor sites in response to necrosis resulting from hypoxia and metabolic stress, both of which commonly affect solid tumors. Although certain inflammatory cells, such as cytotoxic T cells and natural killer (NK) cells are anti-metastatic, chronic tumor inflammation associated with severe hypoxia and metabolic stress generally favors pro-tumor immunity[6–8]. Importantly, infiltration of pro-tumor inflammatory mediators, like macrophages, correlates with poor clinical prognosis, underscoring the importance of understanding the biological mechanisms by which tumor cells tip the balance in favor of pro-tumor immunity over tumor suppressive immunity [8].
Although autophagy primarily serves cell autonomous functions in stressed tumor cells, it may indirectly inhibit inflammation at the primary site that is required for initiation of metastasis. By promoting survival during hypoxia and metabolic stress, autophagy reduces tumor cell necrosis and consequent macrophage infiltration of the primary tumor. Indeed, this ability of autophagy to restrict necrosis and preclude macrophage associated tumor inflammation was demonstrated to suppress primary tumor growth [9]. Furthermore, in the polyoma middle T (PyMT) transgenic model of breast cancer metastasis, macrophage infiltration at the primary tumor is required for invasion and metastasis [7,10]. Taken together, this suggests that similar to its ability to suppress primary tumor formation, autophagy may attenuate the induction of metastasis by preventing macrophage infiltration [6,9].
In addition, autophagy may directly mediate tumor-associated inflammatory responses by regulating the release of immunomodulatory factors such as high-mobility group box protein 1 (HMGB1) from tumor cells. When released, HMGB1 activates dendritic cells by binding Toll-Like Receptor 4 on their surface. This induces a potent anti-tumor immune response that may kill tumor cells and prevent metastasis [11]. Interestingly, in glioblastoma cells treated with a targeted toxin, high levels of autophagy are induced during cell death, and autophagy is required for efficient HMGB1 release from dying cells [12]. This suggests that additional stresses that induce cell death with autophagy may elicit HMGB1 release and stimulate anti-tumor immunity.
Anti-tumor immune modulation of metastasis is also associated with death-ligand induced apoptosis. In particular, TNF-Related Apoptosis Inducing Ligand (TRAIL) appears to have a critical role in regulating suppression of metastasis by T cells and NK cells. TRAIL receptor mutations render cancer cells refractory to TRAIL induced apoptosis, and this positively correlates with increased metastasis [13]. Interestingly, recent work demonstrates that autophagy is upregulated in cancer cells that are resistant to TRAIL, serving a cytoprotective function [14,15]. Hence, in addition to genetic aberrations in the TRAIL-receptor pathway, tumor cells may acquire TRAIL resistance via autophagy upregulation and go on to metastasize.
Collectively, these studies highlight the contrasting roles for autophagy in mediating inflammatory signals during metastasis and emphasize the need for studies aimed at interrogating how autophagy interacts with other pathways to ultimately dictate how the primary tumor communicates with its microenvironment during the onset of metastasis.
Detachment-induced autophagy and anoikis resistance during metastasis
In order to metastasize, carcinoma cells must acquire the ability to survive and expand in absence of proper ECM contact while traversing the systemic circulation and occupying a foreign microenvironment at a distant organ site [1,16]. In normal cells, the lack of proper ECM attachment leads to apoptosis, termed anoikis [17,18]. Anoikis maintains homeostasis in developing and adult tissues; for example, anoikis promotes the clearance of epithelial cells detached from the surrounding basement membrane during ductal elongation in the mouse mammary gland [19].
Therefore, for tumor cells to survive and metastasize, they must activate mechanisms to resist anoikis [17]. For example, in a model of gastric cancer, cells that demonstrate enhanced adhesion independent growth in vitro due to anti-apoptotic factor overexpression also exhibit enhanced peritoneal dissemination in vivo [20]. Moreover, the neurotrophic tyrosine kinase receptor, TrkB, has been identified as a potent anoikis suppressor. Importantly, TrkB overexpression in non-malignant cells facilitates the robust formation of lung and heart metastases following intravenous injection, indicating that anoikis resistance is both necessary and sufficient for metastasis [21]. As exemplified by this study, the aberrant activation of growth factor pathways is a common mechanism utilized by cancerous cells to evade anoikis; in fact, oncogenes that activate key growth factor signals, such as the Ras/MAPK and PI3K/Akt pathways, protect cancer cells from death [17]. However, recent work indicates that autophagy is another mechanism that protects matrix-detached epithelial cells from anoikis [2,22].
Detachment-induced autophagy was first observed during luminal clearance in mammary epithelial acini grown in three-dimensional (3D) cultures [23]. Subsequent experiments delineated that autophagy is induced upon either substratum detachment or β1 integrin receptor blockade [22]. Although autophagy was initially hypothesized to promote the death of detached cells, these studies revealed that autophagy protects epithelial cells from anoikis. Autophagy inhibition via RNAi-mediated knockdown of autophagy regulators (ATGs) enhanced detachment-induced cell death and accelerated luminal clearance in 3D mammary epithelial cultures [22]. Collectively, these data support a role for autophagy in mediating anoikis resistance; such protection may be particularly important during the later stages of cancer dissemination and metastasis where tumor cells are required to adapt to aberrant cell-ECM interactions in the systemic circulation and at distant organ sites (Figure 1).
Currently, the precise function of detachment-induced autophagy during anoikis remains unclear. Integrin engagement amplifies signals transmitted by growth factor receptor pathways, which together coordinate proper nutrient uptake and metabolism [24–26]. Similar to its role in starvation, autophagy in ECM-detached cells may compensate for the loss of extrinsic signals promoting nutrient and energy metabolism [2]. In the context of a rapidly growing tumor with high energy and biosynthetic demands, detachment-induced autophagy may promote the fitness of cells deprived of ECM contact. Alternatively, since autophagy is initially upregulated in stressed regions of primary tumors, sustained self-eating may further enable the pro-metastatic phenotype as primary tumor cells receive microenvironmental signals stimulating ECM detachment and migration.
Indeed, protection from anoikis has been shown to facilitate both the survival and expansion of metastatic cells [17,20,21]. Nonetheless, a specific requirement for detachment-induced autophagy during metastasis has yet to be demonstrated in vivo. Interestingly, processes associated with anchorage independent growth appear to serve as a useful indicator of poor clinical outcome, as the expression profile of breast and lung tissue from metastatic cancers correlates well with that of breast cancer cell line exhibiting a strong anchorage independent growth phenotype [27]. If autophagy does in fact serve a critical role in promoting anoikis resistance, self-eating also may correlate with poor prognosis with regard to metastatic disease.
Autophagy and dormancy
Dormancy describes the remarkable ability of disseminated tumor cells (DTCs) to subsist for years to decades at distant sites without giving rise to secondary tumors. The dormant cell population may constitute only a small fraction of cells that disseminate from the primary tumor and harbor the ability to form metastases. These cells usually go undetected upon diagnosis and remain refractive to common treatments targeting proliferating cells at the primary tumor [16,28]. Hence, understanding the mechanisms governing dormancy is critical for identifying treatments to eliminate these cells.
Notably, the inability of DTCs to form strong and stable ECM contacts with a new microenvironment has been proposed to induce tumor dormancy [28]. Specifically, suppression of β1 integrin signaling, a known inducer of autophagy, induces dormancy in the MMTV-PyMT model of breast cancer [22,29]. Thus, it is possible that since DTCs cannot efficiently engage a foreign ECM, impaired integrin signaling may stimulate autophagy for survival and maintenance of dormancy. Moreover, solitary dormant cells must also resist extrinsic apoptotic stimuli. In breast cancer metastases to bone, where DTCs remain dormant in the bone marrow for extended periods of time, TRAIL is abundantly expressed in the bone marrow microenvironment and can kill dormant cells. Mechanisms involving Src mediated TRAIL resistance promote the survival of dormant cells in the bone marrow [30]. Because autophagy can protect cells from TRAIL-induced apoptosis, it may similarly promote the survival of dormant cells in the bone marrow [14,15].
Tumor cell dormancy likely reflects a mechanism of evolutionary adaptation that DTCs use to survive when exposed to an inhospitable microenvironment [28]. In C. elegans, a precedent for stress-induced dormancy has already been established during dauer formation, a stage of developmental growth arrest and quiescence that occurs when larvae are exposed to hostile environments. Importantly, autophagy has been shown to be essential for this process; RNAi against ATGs decreases dauer survival [31]. These findings lend support to a conserved mechanism by which autophagy promotes survival during quiescent states. Accordingly, pathways leading to G0 arrest induce both dormancy and autophagy; a prosurvival role for autophagy during p27Kip1 induced quiescence has been demonstrated and breast cancer cell lines that typically exhibit dormant behavior in vivo have upregulated levels of p27Kip1 [32,33]. Nonetheless, the exact biological role for autophagy during quiescence remains largely unknown. If autophagy is required for growth suppression in quiescent cells, one can alternatively hypothesize that it may also limit the outgrowth of dormant cells into macro-metastases. A direct link between autophagy and tumor cell dormancy was recently uncovered in ovarian cancer cells. The tumor suppressor aplasia Ras homolog member I (ARHI), induces autophagy and promotes in vivo survival of dormant cells in the context of a tumor microenvironment [34]. Though limited in vivo models of dormancy exist, the pharmacological or genetic manipulation of autophagy during the aforementioned studies may reveal a more defined role for autophagy during dormancy.
Conclusion
The ability of autophagy to restrict necrosis and inflammation may limit the invasion and dissemination of tumor cells from a primary site, thereby restricting metastasis at an early step. On the other hand, autophagy may promote metastasis at later stages by protecting stressed tumor cells as they travel through the vasculature and colonize at distant sites. As a titratable process, autophagy is poised to serve both pro- and anti-metastatic roles depending on contextual demands. Further work using in vivo models is critical to illuminate the precise functions of autophagy at each step of metastasis and to develop a clear rationale for if (and when) we should try to manipulate autophagy to inhibit metastasis.
Acknowledgments
J.D. is supported by grants from the NIH (RO1CA126792; CA126792-S1 ARRA), the California Tobacco Related Disease Research Program (18XT-0106), and an HHMI Physician Scientist Early Career Award. A.T. is supported by grants from the NIH (RO1 CA111421).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Candia Kenific, Email: Candia.Kenific@ucsf.edu.
Andrew Thorburn, Email: Andrew.Thorburn@ucdenver.edu.
Jayanta Debnath, Email: Jayanta.Debnath@ucsf.edu.
References
- 1.Chambers AF, Groom AC, MacDonald IC. Metastasis: Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- 2.Lock R, Debnath J. Extracellular matrix regulation of autophagy. Current Opinion in Cell Biology. 2008;20:583–588. doi: 10.1016/j.ceb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hippert MM, O’Toole PS, Thorburn A. Autophagy in cancer: good, bad, or both? Cancer Res. 2006;66:9349–9351. doi: 10.1158/0008-5472.CAN-06-1597. [DOI] [PubMed] [Google Scholar]
- 5.Tlsty TD, Coussens LM. TUMOR STROMA AND REGULATION OF CANCER DEVELOPMENT. Annual Review of Pathology: Mechanisms of Disease. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 6.DeNardo D, Johansson M, Coussens L. Immune cells as mediators of solid tumor metastasis. Cancer and Metastasis Reviews. 2008;27:11–18. doi: 10.1007/s10555-007-9100-0. [DOI] [PubMed] [Google Scholar]
- 7.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4+ T Cells Regulate Pulmonary Metastasis of Mammary Carcinomas by Enhancing Protumor Properties of Macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of Pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 9**.Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis Cancer Cell 20061051–64.This paper identifies a mechanism by which autophagy suppresses tumor formation. By inhibiting autophagy via activation of AKT or allelic disruption ofbeclin, it demonstrates that autophagy prevents necrosis of primary tumors, which inhibits infiltration of pro-tumor inflammatory mediators. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating Factor 1 Promotes Progression of Mammary Tumors to Malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 12*.Thorburn J, Horita H, Redzic J, Hansen K, Frankel AE, Thorburn A. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ. 2008;16:175–183. doi: 10.1038/cdd.2008.143. This paper demonstrates a tumor suppressive role for autophagy in promoting release of an immunomodulatory factor from dying tumor cells that stimulates anti-tumor immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin MS, Kim HS, Lee SH, Park WS, Kim SY, Park JY, Lee JH, Lee SK, Lee SN, Jung SS, et al. Mutations of Tumor Necrosis Factor-related Apoptosis-inducing Ligand Receptor 1 (TRAIL-R1) and Receptor 2 (TRAIL-R2) Genes in Metastatic Breast Cancers. Cancer Res. 2001;61:4942–4946. [PubMed] [Google Scholar]
- 14.Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin X-M, Rabinowich H. Involvement of Protective Autophagy in TRAIL Resistance of Apoptosis-defective Tumor Cells. Journal of Biological Chemistry. 2008;283:19665–19677. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero-Martin G, Hoyer-Hansen M, Garcia-Garcia C, Fumarola C, Farkas T, Lopez-Rivas A, Jaattela M. TAK1 activates AMPK-dependent cytoprotective autophagy in TRAIL-treated epithelial cells. EMBO J. 2009;28:677–685. doi: 10.1038/emboj.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 17.Chiarugi P, Giannoni E. Anoikis: A necessary death program for anchorage-dependent cells. Biochemical Pharmacology. 2008;76:1352–1364. doi: 10.1016/j.bcp.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Frisch S, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mailleux AA, Overholtzer M, Schmelzle T, Bouillet P, Strasser A, Brugge JS. BIM Regulates Apoptosis during Mammary Ductal Morphogenesis, and Its Absence Reveals Alternative Cell Death Mechanisms. Developmental Cell. 2007;12:221–234. doi: 10.1016/j.devcel.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yawata A, Adachi M, Okuda H, Naishiro Y, Takamura T, Hareyama M, Takayama S, Reed JC, Imai K. Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells. Oncogene. 1998;16:2681–2686. doi: 10.1038/sj.onc.1201792. [DOI] [PubMed] [Google Scholar]
- 21.Douma S, van Laar T, Zevenhoven J, Meuwissen R, van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034–1039. doi: 10.1038/nature02765. [DOI] [PubMed] [Google Scholar]
- 22*.Fung C, Lock R, Gao S, Salas E, Debnath J. Induction of Autophagy during Extracellular Matrix Detachment Promotes Cell Survival. Mol Biol Cell. 2008;19:797–806. doi: 10.1091/mbc.E07-10-1092. This study provides the first demonstration that interaction with the extracellular matrix regulates autophagy and that autophagy promotes resistance to anoikis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The Role of Apoptosis in Creating and Maintaining Luminal Space within Normal and Oncogene-Expressing Mammary Acini. Cell. 2002;111:29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- 24.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 25.Edinger AL, Thompson CB. Akt Maintains Cell Size and Survival by Increasing mTOR-dependent Nutrient Uptake. Mol Biol Cell. 2002;13:2276–2288. doi: 10.1091/mbc.01-12-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schafer ZT, Grassian AR, Song L, Jiang Z, Gerhart-Hines Z, Irie HY, Gao S, Puigserver P, Brugge JS. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 2009;461:109–113. doi: 10.1038/nature08268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori S, Chang JT, Andrechek ER, Matsumura N, Baba T, Yao G, Kim JW, Gatza M, Murphy S, Nevins JR. Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene. 2009;28:2796–2805. doi: 10.1038/onc.2009.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer. 2007;7:834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White DE, Kurpios NA, Zuo D, Hassell JA, Blaess S, Mueller U, Muller WJ. Targeted disruption of [beta]1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell. 2004;6:159–170. doi: 10.1016/j.ccr.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XHF, Wang Q, Gerald W, Hudis CA, Norton L, Smid M, Foekens JA, Massagué J. Latent Bone Metastasis in Breast Cancer Tied to Src-Dependent Survival Signals. Cancer Cell. 2009;16:67–78. doi: 10.1016/j.ccr.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melendez A, Talloczy Z, Seaman M, Eskelinen E-L, Hall DH, Levine B. Autophagy Genes Are Essential for Dauer Development and Life-Span Extension in C. elegans Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 32.Liang J, Shao SH, Xu Z-X, Hennessy B, Ding Z, Larrea M, Kondo S, Dumont DJ, Gutterman JU, Walker CL, et al. The energy sensing LKB1-AMPK pathway regulates p27kip1 phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 33.Barkan D, Kleinman H, Simmons JL, Asmussen H, Kamaraju AK, Hoenorhoff MJ, Liu Z-y, Costes SV, Cho EH, Lockett S, et al. Inhibition of Metastatic Outgrowth from Single Dormant Tumor Cells by Targeting the Cytoskeleton. Cancer Res. 2008;68:6241–6250. doi: 10.1158/0008-5472.CAN-07-6849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34**.Lu Z, Luo RZ, Lu Y, Zhang X, Yu Q, Khare S, Kondo S, Kondo Y, Yu Y, Mills GB, et al. The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. The Journal of Clinical Investigation. 2008;118:3917–3929. doi: 10.1172/JCI35512. This study establishes for the first time a connection between autophagy and regulation of tumor cell dormancy. It shows that inhibition of autophagy downstream of ARHI, which promotes dormancy of xenograft tumors and activates autophagy, impairs tumor outgrowth. [DOI] [PMC free article] [PubMed] [Google Scholar]