Abstract
Background
To evaluate the immunological impact of the 23-valent pneumococcal polysaccharide vaccine (23vPPS) at 12 months, for children who have received zero to three infant doses of seven-valent pneumococcal conjugate vaccine (PCV), on responses to a subsequent exposure to a small dose of 23vPPS (mPPS).
Methods
Five hundred and fifty-two Fijian infants were stratified by ethnicity and randomized into eight groups to receive zero, one, two, or three PCV doses at 14 weeks, six and 14 weeks, or six, ten, and 14 weeks. Within each group, half received 23vPPS at 12 months and all received mPPS at 17 months. Sera were taken prior and one month post-mPPS.
Findings
By 17 months, geometric mean antibody concentrations (GMC) to all 23 serotypes in 23vPPS were significantly higher in children who had received 23vPPS at 12 months compared to those who had not. Post-mPPS, children who had not received the 12 month 23vPPS had a significantly higher GMC for all PCV serotypes compared with those who had (each p<0.02). For the non-PCV serotypes, children who had not received the 12 month 23vPPS had significantly higher GMC for six of 16 non-PCV serotypes (7F, 9N, 12F, 19A, 22F, 33F) than those who did (each p<0.02). After adjusting for the pre-mPPS level, exposure to 23vPPS was associated with a lower response to mPPS for all serotypes (each p<0.001).
Interpretation
Despite higher antibody concentrations at 17 months in children who had received 23vPPS at 12 months, the response to a re-challenge was poor for all 23 serotypes compared to children who had not received the 12 month 23vPPS.
Introduction
Pneumococcal disease is estimated to cause 1.6 million deaths each year, primarily in children and the elderly. The majority of these deaths occur in low income countries [1]. Over 90 serotypes in 48 serogroups of pneumococcus have been identified [2]. Most serious pneumococcal disease is caused by a relatively small number of serotypes. However these vary by age, geography, and clinical presentation [3]. The range of serotypes causing disease in affluent societies is largely confined to the serotypes found in the seven-valent pneumococcal conjugate vaccine (PCV, Prevenar™, Wyeth Vaccines). In contrast, the range of serotypes causing disease in low income countries is wider [4]. The 10-valent pneumococcal conjugate vaccine has recently been licensed in some countries, and a 13-valent vaccine is likely to be licensed by 2010.
The use of the 23-valent pneumococcal polysaccharide vaccine (23vPPS) as a booster following PCV in infancy (PCV/23vPPS) has the theoretical advantage of boosting the seven serotypes shared between PCV and 23vPPS, while broadening the serotype coverage with the addition of 16 non-PCV serotypes. For this reason it has been routinely given to Australian Indigenous children as a booster at 18 months of age following three doses of PCV in infancy. The majority of immunological studies have shown PCV/23vPPS to produce at least similar or higher antibody levels for all shared serotypes compared with a PCV boost [5-12]. Studies describing qualitative function such as opsonophagocytic activity and avidity are limited and have shown inconsistent results [8, 9]. A T-cell independent response, which is immature in infancy, is required for an immunological response to the non-PCV serotypes using the combined PCV/23vPPS approach. However the immunogenicity of 23vPPS varies by age and serotype [13-19] with poor responses demonstrated in most infant studies for serogroups 6 [13-19], 19 [13, 14, 17-19], and 23 [14-19], and inconsistent responses in other studies for serotypes/groups 1 [14, 19], 12 [13, 16], 14 [16, 19], and 18 [18].
A particular concern relating to the administration of pneumococcal polysaccharide vaccine (PPS) to unprimed young children is the theoretical risk that hyporesponsiveness may occur following re-challenge or subsequent pneumococcal exposure following PPS [20]. This phenomenon has been demonstrated in studies with Group A and C meningococcal polysaccharide vaccine [21]. Studies in young children using different valencies and formulations ranging from five to100μg/serotype of PPS have shown inconsistent results including reduced responses to some serotypes following revaccination [15, 22]. Conversely, one infant study showed no evidence of hyporesponsiveness on revaccination with PPS [16]. The assays used in these studies were less specific than techniques currently in use, and the clinical relevance of these immunological findings remains unknown.
The seven serotypes included in PCV are responsible for 55% of IPD episodes in children aged under 5 in Fiji [23]. This potential serotype coverage would increase to 83% if the 23vPPS, which does not contain serotype 6A, was used, and 87%, if the new 13-valent pneumococcal conjugate vaccine produced by Wyeth Vaccines (which includes serotypes 1, 3, 5, 6A, 7F and 19A) was used [23]. The aim of this study was to find an optimal vaccination strategy for resource poor countries in terms of serotype coverage, flexibility, and affordability. We undertook a Phase II vaccine trial in Fiji to document the safety and immunogenicity of various pneumococcal vaccination regimens combining one, two, or three doses of PCV in infancy. To broaden serotype coverage, the additional benefit of a booster of 23vPPS at 12 months of age was also assessed. To address the concerns of hyporesponsiveness to PPS following re-challenge, this paper presents the immunological response at 17 months of age to a small challenge dose of 20% of the 23vPPS (mPPS) in infants who had or had not received the 23vPPS at 12 months of age.
Methods
Study participants
The study was a single blind, open-label randomized Phase II vaccine trial undertaken in Suva, the capital of Fiji. Healthy infants aged between six and eight weeks were eligible for enrolment. Details of the selection criteria and the randomisation procedure have been reported elsewhere [24].
Study procedures and vaccines
Infants were stratified by ethnicity and randomised into one of eight groups The seven-valent CRM197 protein-polysaccharide conjugate vaccine containing polysaccharide antigen from pneumococcal serotypes 4, 6B, 9V, 14, 18C, 19F, 23F (Prevenar ®, Wyeth Vaccines) was used. The vaccine contains 2 μg/serotype, except serotype 6B which is 4μg. The three dose group received PCV at six, ten, and 14 weeks of age, the two dose group received PCV at six and 14 weeks of age and the one dose group received PCV at 14 weeks of age. Routine vaccines (Hiberix™ mixed with Tritanrix™-HepB™, GlaxoSmithKline) and oral polio were given with the primary series. Hiberix™ contains 10μg of purified Hib capsular polysaccharide covalently bound to approximately 30μg tetanus toxoid mixed with Tritanrix™-HepB™ which contains not less than 30 IU of adsorbed D toxoid, not less than 60 IU of adsorbed T toxoid, not less than 4 IU of wP, and 10μg of recombinant HBsAg protein. The children in all primary series groups were further randomized to receive a dose of 23vPPS (Pneumovax™, Merck & Co., Inc., which consists of a purified mixture of 25μg of capsular polysaccharide from 23 pneumococcal serotypes) or no vaccine at 12 months of age (window: 12 months plus four weeks). In addition, all children received Measles-Rubella vaccine at 12 months of age co-administered with 23vPPS. All children received 20% of the 23vPPS (mPPS) at 17 months of age (window: 17 months plus eight weeks). The children randomised to receive 0 or 1 PCV dose in infancy, had a single dose of PCV administered at 2 years of age.
Children were followed up for serious adverse events (SAE’s) to any of the study vaccines throughout the two year study period. The occurrence of SAE’s were sourced from parent interviews at each visit and by searching the national computerised hospital discharge records every quarter. Causality of any SAE was assigned by the study doctor and assessed by an independent safety monitor. All SAE’s were periodically reviewed by an independent Data Safety and Monitoring Board.
Laboratory procedures
Children who received the 12 month 23vPPS had bloods drawn prior to and 14 days post 23vPPS. All children had blood taken before and four weeks following the 17 month mPPS. Blood was separated by centrifugation at the health centre, kept chilled and transported to the Colonial War Memorial Hospital laboratory, Suva, where it was divided into aliquots and stored at −20°C on the same day, until transported to the Pneumococcal Laboratory, Murdoch Childrens Research Institute, Melbourne on dry ice for analysis.
Anticapsular pneumococcal antibody levels were assayed for all 23vPPS serotypes (1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F), using a modified 3rd generation ELISA based on current WHO recommendations[25]. Briefly 96-well medium binding polystyrene plates (Greiner microlon, Germany) were coated with pneumococcal polysaccharides (ATCC, USA) and incubated overnight at room temperature. Non-specific, non-opsonic antibodies were absorbed from sera by incubation overnight at 4°C with PBS containing 10% foetal bovine serum (PBS/FCS), cell wall polysaccharide (C-PS 10 μg/ml) and serotype 22F (30 μg/ml). The reference serum 89SF [26, 27] (Dr Milan Blake, FDA, USA) and samples for anti serotype 22F IgG quantitation were absorbed with PBS/FCS and C-PS. Plates were blocked with PBS/FCS and patient samples and standards added. The reaction was detected with a secondary antibody HRP conjugated anti-human IgG (Chemicon, Australia) and enzyme substrate solution, TMB (3,3′,5,5′-tetramethylbenzidine, KPL, USA) followed by a 1M H3PO4 stop solution. The absorbance (OD) was measured at 450 nm (reference filter 630 nm) on a Bio-Tek Elx808 (Bio-Tek Instruments, USA). OD was converted to antibody concentrations (μg/ml) using KCJunior software (Bio-Tek Instruments, USA). Sample dilutions were analyzed in duplicate and three controls (low, medium and high) were included on each plate to assess assay performance and inter-assay variation. Results from an inter-laboratory comparison between Wyeth Vaccines and the KTL Finland laboratory demonstrated a good correlation in measurement of serotype-specific antibody concentrations [28]. Laboratory staff members were blinded to the group allocation of each serum sample.
Data management and statistical analysis
Cleaned data were exported to Stata version 9.0 (Stata Corporation, College Station, Texas) for analysis. Serotype-specific antibody concentrations by ELISA were log transformed (to base e) to calculate GMC. Comparisons of pre- and post-mPPS GMC and between group comparisons were performed using a paired t-test and two sample t-test respectively. Simple and multi-variable regression analyses were undertaken to adjust for both the pre-mPPS log antibody concentration for all 23 serotypes, and the number of PCV doses administered for all seven PCV serotypes. A p-value of <0.05 was considered statistically significant.
The primary endpoint was serotype-specific GMC response to mPPS at 18 months of age in children who had received the 12 months 23vPPS compared to children who had not received the 23vPPS. We defined hyporesponsiveness to a particular serotype as a significantly lower GMC observed post-mPPS, in the 12 month 23vPPS group compared to the no 12 month 23vPPS group, controlling for pre-mPPS antibody levels, using multivariable regression analysis. To prevent an inflated type 1 error due to multiple comparisons, and obtain a single p-value for the null hypothesis of mPPS having no impact on the antibody response to any of the 23 serotypes, a joint test of all the regression coefficients from the aforementioned multivariable regression analysis was performed [29].
Ethical approval
The study was approved by the Fiji National Research Ethics Review Committee and the University of Melbourne Human Research Ethics Committee.
Results
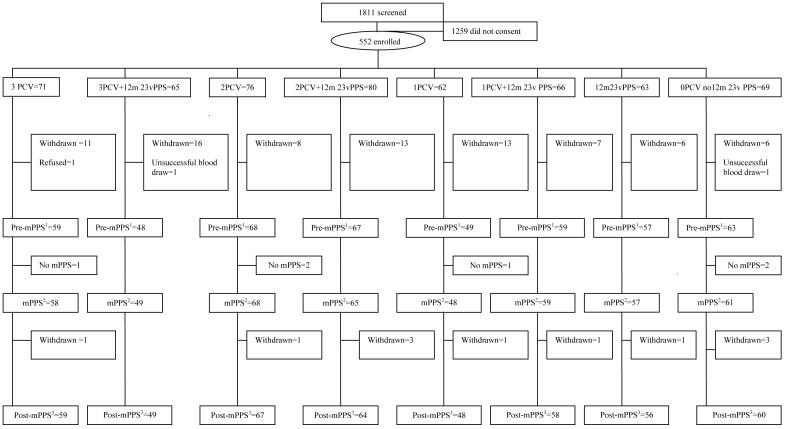
There were 552 children enrolled in the study (Figure 1) which represents a consent rate of 30.5%. There were 90 (16.3%) withdrawals and no child was withdrawn due to an adverse event resulting from administration of any of the vaccines. Characteristics and the number of children randomized to the eight groups are shown in Table 1.
Figure 1. CONSORT chart of the screened and enrolled children to 18 months of age, showing the number having the pre-mPPS blood test1, mPPS at 17 months of age2, and blood test one month post-mPPS3.
Withdrawals: 34 moved out of the study area, 16 failed to return for follow up, 13 refused a study procedure, nine developed an exclusion criterion, nine were withdrawn due to a protocol amendment, for six no reason was given, two had protocol violations, and one died from causes unrelated to receipt of the study vaccine.
Table 1. Baseline characteristics of infants at enrolment and on randomization to one of eight groups.
| Characteristics | 3 PCV | 2 PCV | 1 PCV | 0 PCV | ||||
|---|---|---|---|---|---|---|---|---|
| No 12m 23vPPS (n=71) |
12m 23vPPS (n=65) |
No 12m 23vPPS (n=76) |
12m 23vPPS (n=80) |
No 12m 23vPPS (n=62) |
12m 23vPPS (n=66) |
12m 23vPPS (n=63) |
No 12m 23vPPS (n=69) |
|
| Gender | ||||||||
| Male | 38 (53.5%) | 33 (50.8%) | 31 (40.8%) | 39 (48.8%) | 31 (50%) | 28 (42.4%) | 32 (50.8%) | 32 (46.4%) |
| Female | 33 (46.5%) | 32 (49.2%) | 45 (59.2%) | 41 (51.2%) | 31 (50%) | 38 (57.6%) | 31 (49.2%) | 37 (53.6%) |
|
Median age in
weeks |
6.7 | 6.5 | 6.5 | 6.4 | 6.5 | 6.5 | 6.5 | 6.4 |
| Ethnicity | ||||||||
| Indigenous Fijian |
39 (54.9%) | 43 (66.1%) | 48 (63.1%) | 58 (72.5%) | 41 (66.1%) | 42 (63.7%) | 35 (55.6%) | 45 (65.2%) |
| Indo-Fijian | 29 (40.9%) | 17 (26.2%) | 24 (31.6%) | 21 (26.3%) | 17 (27.4%) | 22 (33.3%) | 20 (31.7%) | 20 (29.0%) |
| Other | 3 (4.2%) | 5 (7.7%) | 4 (5.3%) | 1 (1.2%) | 4 (6.5%) | 2 (3.0%) | 8 (12.7%) | 4 (5.8%) |
|
Median weight
in grams |
4850 | 5000 | 4775 | 4800 | 4900 | 4750 | 4750 | 4800 |
Following the 12 month 23vPPS, there were significantly higher GMC (each p<0.001) for all PCV serotypes. For four of seven PCV serotypes (4, 9V, 18C, 19F) this response was most profound in the group that had received only a single dose of PCV (each p<0.001). Children who received the 23vPPS at 12 months showed significant higher GMC (each p<0.001) for all non-PCV serotypes in the 23vPPS.
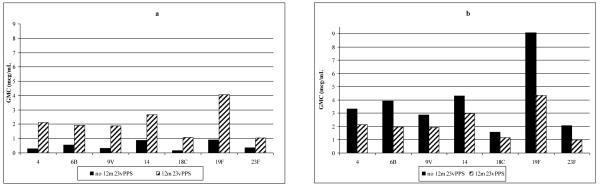
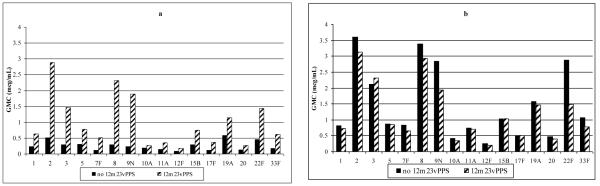
Five months following the 12 month 23vPPS and prior to the administration of the re-challenge dose of mPPS at 17 months of age, the group that had received 23vPPS at 12 months had significantly higher GMC for all the PCV and non-PCV serotypes compared with the groups that had not received the 12 month 23vPPS (Figure 2a and 3a respectively; each p<0.001).
Figure 2. Serotype-specific IgG GMC (μg/mL) to PCV serotypes at 17 months of age pre- (Figure 2a) and one month post-mPPS (Figure 2b) in children who did or did not receive the 12 month 23vPPS. Data from infants receiving 0,1,2,or 3 PCV doses have been combined in these graphs.
Figure 3. Serotype-specific IgG GMC (μg/mL) to non-PCV serotypes at 17 months of age pre- (Figure 3a) and one month post-mPPS (Figure 3b) in children who did or did not receive the 12 month 23vPPS. Data from infants receiving 0,1,2,or 3 PCV doses have been combined in these graphs.
GMC to the PCV serotypes following the re-challenge dose of mPPS at 17 months are shown in Figure 2b. The groups that did not receive the 12 month 23vPPS had better responses and significantly higher GMC for all PCV serotypes than those groups that had received the 12 month 23vPPS (Figure 2b). Response to mPPS for the non-PCV serotypes are shown in Figure 3b. The groups that did not receive the 12 month 23vPPS had significantly higher GMC for six of 16 non-PCV serotypes (7F, 9N, 12F, 19A, 22F, 33F) compared with those groups that did have the 12 month 23vPPS (Figure 3b).
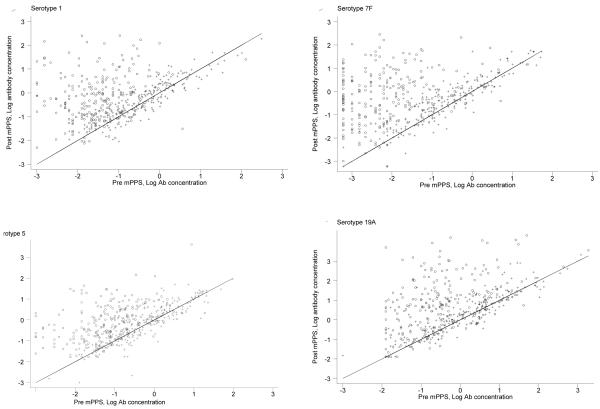
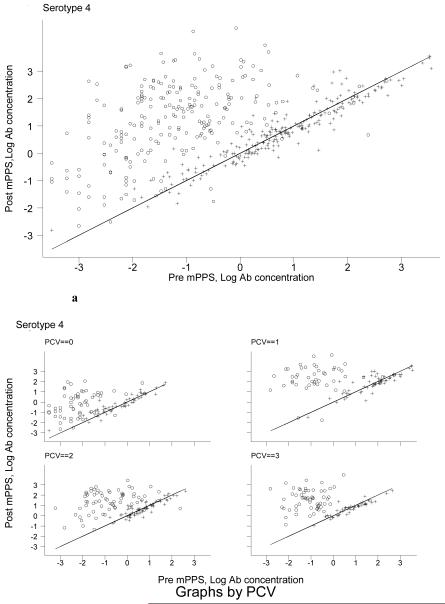
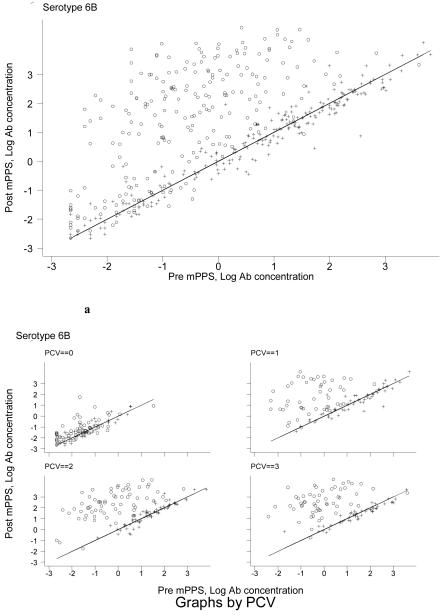
To examine the effect of 23vPPS at 12 months and the number of PCV doses in early infancy, we performed graphical examination to assess whether the poor response to mPPS in the 12 month 23vPPS recipients was due to the higher pre-mPPS antibody concentrations. Figure 4 shows the post-mPPS log antibody concentration (y-axis) against the pre-mPPS log antibody concentration (x-axis) for the non-PCV serotypes 1, 5, 7F, and 19A. For any given log antibody concentration pre-mPPS, children who had not received the 23vPPS at 12 months had higher log antibody concentrations one month post-mPPS. A similar pattern is seen for all other non-PCV serotypes (data not shown but available upon request). For PCV serotypes, a similar pattern was demonstrated. Figure 5 and Figure 6 show the post-mPPS log antibody concentration for serotypes 4 and 6B respectively, against the pre-mPPS concentration.
Figure 4. Pre- and one month post-mPPS log antibody concentrations for non-PCV serotypes 1, 5, 7F, and 19A in those that did (+) and did not (o) receive 23vPPS at 12 months of age. Data from infants receiving 0,1,2,or 3 PCV doses have been combined in these graphs.
Footnote: A 45-degree line, which indicates no change before and after the re-challenge, is super-imposed. The pre- and post-mPPS log antibody concentrations of those who received 23vPPS at 12 months mostly fell along the 45-degree line, indicating no response to mPPS. In contrast, most children who did not receive 23vPPS had an increase in antibody concentration, as indicated by the data points falling above the 45-degree line. The distribution of pre-mPPS log antibody concentrations of those that did and did not receive the 12 month 23vPPS mainly overlapped in the range between −2 to 0 for serotype 1. Within this range, it is clear that at any given log antibody concentration pre-mPPS, children who had not received the 23vPPS at 12 months had higher log antibody concentrations one month post-mPPS.
Figure 5. Pre- and one month post-mPPS log antibody concentrations for serotype 4 for children who did (+) or did not (o) receive 23vPPS at 12 months of age and by the number of PCV doses in the primary series (Figure 5a).
Figure 6. Pre- and one month post-mPPS log antibody concentrations for serotype 6B for children who did (+) or did not (o) receive 23vPPS at 12 months of age and by the number of PCV doses in the primary series (Figure 6a).
For the PCV serotypes further adjustment for prior receipt of one, two or three PCV doses in addition to 23vPPS exposure and pre-mPPS antibody concentration was undertaken. Adjustment for the number of PCV dosages had limited impact on the overall effect of prior receipt of 23vPPS on the response to mPPS. For each of the PCV dosage groups and any given pre-mPPS antibody concentration, those who did not receive 23vPPS at 12 months of age had a higher log antibody concentration post-mPPS, shown in Figures 5a and 6a for serotypes 4 and 6B respectively.
To quantify the above graphical examination, simple and multi-variable regression analyses were undertaken to adjust for the pre-mPPS log antibody concentration for each serotype, and then by number of PCV doses administered for the PCV serotypes. Without adjusting for the pre-mPPS log antibody concentration, the log antibody concentrations for the non-PCV serotypes one month post-mPPS were not significantly different between those who had or had not received 23vPPS at 12 months of age for ten of 16 non-PCV serotypes (1, 2, 3, 5, 8, 10F, 11A, 15B, 17F and 20) but were significantly lower for the remaining six of 16 serotypes in those who had received the 12 month 23vPPS. The log antibody concentrations one month post-mPPS are significantly associated with the pre-mPPS antibody concentration for all 16 non-PCV serotypes (each p<0.001). Having adjusted for the pre-mPPS log antibody concentration, exposure to 23vPPS was associated with a lower response to mPPS for all 16 non-PCV serotypes (each p<0.001). For PCV serotypes, a similar response was demonstrated. The response one month post-mPPS was significantly associated with the pre-mPPS antibody concentration for all seven PCV serotypes (p<0.001) and having adjusted for the pre-mPPS concentration, prior exposure to 23vPPS was associated with a lower response to mPPS (each p<0.001). In contrast, most children who had not received 23vPPS had an increase in antibody concentration. A joint test rejected the null hypothesis of mPPS having no impact on the antibody response to any of the 23 serotypes, having adjusted for the pre-mPPS antibody concentrations (p<0.001).
There were 101 SAE’s throughout the study period with none attributable to receipt of any of the study vaccines. In children over 12 months of age, there were 14 SAE’s in the 12 month 23vPPS group and 22 SAE’s in the group that did not receive the 23vPPS. There were four cases of inpatient pneumonia in children who had received the 12 month 23vPPS compared to seven cases in those that had not, in infants aged over 12 months of age. There were no cases of IPD throughout the study period.
Discussion
This is the first study in children, using the third generation WHO ELISA assay to measure antibody responses to all 23vPPS serotypes following receipt of that vaccine. The results show that prior receipt of 23vPPS causes immune hyporesponsiveness to a subsequent 23vPPS challenge. Despite those children who received the 12 month 23vPPS having higher circulating antibody concentrations at 17 months of age, their responses to a re-challenge with a small dose of 23vPPS demonstrated a profound lack of response to all 23 serotypes after adjusting for the pre-existing antibody concentration. In contrast, those children who had not received the 12 month 23vPPS could clearly mount a satisfactory response to mPPS.
There are a number of potential immunological mechanisms that may explain these findings. In vitro studies have suggested that polysaccharides antigens may be able to down regulate B cells [30], and that newly formed antibody via IgG, IgM, or immune complexes can bind to inhibitory Fc receptors and prevent antibody production [31]. The critical role of pneumococcal-specific memory B cells in first line of defense against pneumococcal infection has recently become an important area of research. IgM+CD27+ and ‘switched’ IgG+CD27+ memory B cells are considered to play a role in the immune response to 23vPPS since these cell populations are deficient in patients with primary immunodeficiency syndromes who are susceptible to recurrent infections with encapsulated bacteria [32, 33]. Both plasma and memory B cells are stimulated following exposure to PPS. In contrast to T-independent immune responses, priming by either PCV, previous encounter with S. pneumoniae or a cross-reacting antigen prior to 23vPPS vaccination, could stimulate immunological memory by presentation of polysaccharide-protein conjugate antigens to the immune system (T-dependent) [34]. Given the T-independent nature of PPS antigens, 23vPPS may stimulate the existing pool of memory B cells to differentiate into plasma cells and secrete antibody without replenishment of the memory B cell pool. This has been proposed as one mechanism for the hyporesponsiveness observed following polysaccharide vaccine administration [35]. Upon subsequent booster with 23vPPS or a natural infection, immune hyporesponsiveness could be induced as a result of a decreased memory B cell population and result in the reduced antibody concentrations observed in this study.
In addition, the development of immune hyporesponsiveness may also be the result of immune regulation via the establishment of pneumococcal-specific tolerogenic immune responses. Increased expression of the immunosuppressive cytokine interleukin 10 [19, 36] and suppressor T cell activity may suppress the response to PPS [37]. Recent evidence also suggests a role for CD4+ T-lymphocytes in the immune response to pneumococcal antigens [38]. Studies have demonstrated the importance of co-stimulatory signals (CD40-CD40L) for a robust immune response to pneumococcal antigens and that CD4+ T-lymphocytes can protect mice against pneumococcal colonization independent of specific antibody. These findings strongly suggest a role for cellular immunity in protection against pneumococcal infection [39-43]. Furthermore it is possible that regulatory T-lymphocytes (Treg) may suppress antibody production and other immune responses in the context of chronic antigen exposure. Hyporesponsiveness induced by Treg has been described during bacterial, viral and parasitic infections with up-regulation of CD4+CD25+ Treg and IL-10 and TGF-β secretion [37, 44]. Limited data is available on the role of Treg in the attenuation immune response to pneumococcal antigens. However a high level of exposure to pneumococci, particularly in early life, could induce Treg activity that suppresses serotype-specific IgG, thereby increasing IPD risk following 23vPPS immunization.
The clinical relevance of this immunological finding in this study are not known. There is one case report documenting immunological paralysis for four years to the causative pneumococcal serotype in a nine month old infant who had pneumococcal meningitis, despite demonstrating normal immune responses to other protein and polysaccharide antigens [45]. Most studies evaluating the impact of PPS immunization in the absence of additional PCV in infants or children have not shown any impact on pneumococcal disease or carriage [17, 46, 47]. In contrast, a study in Papua New Guinea, where children aged six months to five years of age were given either the 14 or 23vPPS in one or two doses according to age, there was a (non-significant) 19% reduction in mortality from any cause, and a 50% reduction in pneumonia mortality (95%CI 1-75%) [48]. Natural exposure in a population with a high incidence of pneumococcal infections, resulting in regular antigenic stimulation may explain this finding [20]. However, a Finnish study of the 14-valent PPS in infants aged three months to six years showed significant efficacy against vaccine type recurrent otitis media was 52% for children less than two years of age if serogroup 6 was excluded [13].
A study documenting immunological memory five years after meningococcal A/C conjugate vaccination in infancy showed that challenge with the meningococcal polysaccharide or conjugate at two years of age induced immunological memory [21]. However subsequent challenge with polysaccharide at five years of age failed to induce a similar memory response in the polysaccharide group. The authors concluded that the initial polysaccharide immunization at two years of age interfered with the immune response to subsequent polysaccharide vaccination, a finding similar to our current results with 23vPPS [21]. No adverse clinical effects have ever been documented from repeated exposure to the meningococcal polysaccharide vaccine and in this study we demonstrated no increase in clinical adverse effects to the 23vPPS, although the numbers were small and the study was not designed to study this. There was no increase in nasopharyngeal carriage of non-PCV serotypes five months after receipt of the 12 month 23vPPS (FMR, JRC, EKM). We intend to follow the children from this study to assess nasopharyngeal carriage as an increase in carriage of non-PCV types in the 12 month 23vPPS group would indicate that this immunological finding may have a biological effect. This would provide the first indication that these children may have increased susceptibility to pneumococcal disease. Further results documenting the avidity and opsonophagocytic activity post 23vPPS and mPPS, and the impact on nasopharyngeal carriage will follow. In addition, immunological assays to assess B cell subsets will enable a more comprehensive assessment of the impact of 23vPPS on immunological functioning. However, our findings suggest that additional immunization with the 23vPPS following a primary series of PCV does not provide added benefit for antibody production and instead results in impaired immune responses following a subsequent PPS antigen challenge. Whether this observation is associated with adverse clinical effects remains to be determined. Nevertheless, our findings raise some doubt over the value of the combined PCV/23vPPS schedule.
Acknowledgements
The authors wish to sincerely thank all the FiPP staff and families participating in the study, and the many other people who contributed to the study including: Amanda O’Brien, Kathryn Bright, Samantha Colquhoun, Amy Bin Chen, Timothy Gemetzis, Amy Auge, Katherine Gilbert, Evan Willis, Philip Greenwood, Beth Temple, Vanessa Johnston, Loretta Thorn, Porter Anderson, Brian Greenwood, George Siber, David Klein, Elizabeth Horigan, and Farukh Khambaty. The authors wish to thank the DSMB members. Pneumovax™ was kindly donated by CSL Biotherapies, Australia. The co-administered Tritanrix™-HepB™ and Hiberix™ vaccines were kindly donated by GlaxoSmithKline.
Funding
Funding was provided by the U.S. NIAID and the Australian National Health and Medical Research Council.
Trials Registration
Clinical Trial Registry, National Library of Medicine, USA
Clinical trial number: NCT00170612
Footnotes
Conflicts of interest
MLT has been a consultant/advisor for Wyeth. The other authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec. 2007 Mar 23;82(12):93–104. [PubMed] [Google Scholar]
- [2].Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007 Apr;45(4):1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cherian T. WHO expert consultation on serotype composition of pneumococcal conjugate vaccines for use in resource-poor developing countries, 26-27 October 2006, Geneva. Vaccine. 2007 Sep 4;25(36):6557–64. doi: 10.1016/j.vaccine.2007.06.044. [DOI] [PubMed] [Google Scholar]
- [4].Hausdorff WP, Bryant J, Kloek C, Paradiso PR, Siber GR. The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin Infect Dis. 2000 Jan;30(1):122–40. doi: 10.1086/313609. [DOI] [PubMed] [Google Scholar]
- [5].Zangwill KM, Greenberg DP, Chiu CY, Mendelman P, Wong VK, Chang SJ, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine. 2003 May 16;21(17-18):1894–900. doi: 10.1016/s0264-410x(03)00013-6. [DOI] [PubMed] [Google Scholar]
- [6].Ahman H, Kayhty H, Lehtonen H, Leroy O, Froeschle J, Eskola J. Streptococcus pneumoniae capsular polysaccharide-diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998 Mar;17(3):211–6. doi: 10.1097/00006454-199803000-00008. [DOI] [PubMed] [Google Scholar]
- [7].Kilpi T, Ahman H, Jokinen J, Lankinen KS, Palmu A, Savolainen H, et al. Protective efficacy of a second pneumococcal conjugate vaccine against pneumococcal acute otitis media in infants and children: randomized, controlled trial of a 7-valent pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine in 1666 children. Clin Infect Dis. 2003 Nov 1;37(9):1155–64. doi: 10.1086/378744. [DOI] [PubMed] [Google Scholar]
- [8].Anttila M, Eskola J, Ahman H, Kayhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998 Jun;177(6):1614–21. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- [9].Goldblatt D, Southern J, Ashton L, Richmond P, Burbidge P, Tasevska J, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006 Apr;25(4):312–9. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- [10].Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Long-term antibody levels and booster responses in South African children immunized with nonavalent pneumococcal conjugate vaccine. Vaccine. 2004 Jul 29;22(21-22):2696–700. doi: 10.1016/j.vaccine.2003.03.001. [DOI] [PubMed] [Google Scholar]
- [11].Obaro SK, Huo Z, Banya WA, Henderson DC, Monteil MA, Leach A, et al. A glycoprotein pneumococcal conjugate vaccine primes for antibody responses to a pneumococcal polysaccharide vaccine in Gambian children. Pediatr Infect Dis J. 1997 Dec;16(12):1135–40. doi: 10.1097/00006454-199712000-00007. [DOI] [PubMed] [Google Scholar]
- [12].Veenhoven R, Bogaert D, Uiterwaal C, Brouwer C, Kiezebrink H, Bruin J, et al. Effect of conjugate pneumococcal vaccine followed by polysaccharide pneumococcal vaccine on recurrent acute otitis media: a randomised study. Lancet. 2003 Jun 28;361(9376):2189–95. doi: 10.1016/S0140-6736(03)13772-5. [DOI] [PubMed] [Google Scholar]
- [13].Makela PH, Sibakov M, Herva E, Henrichsen J, Luotonen J, Timonen M, et al. Pneumococcal vaccine and otitis media. Lancet. 1980 Sep 13;2(8194):547–51. doi: 10.1016/s0140-6736(80)91989-3. [DOI] [PubMed] [Google Scholar]
- [14].Temple K, Greenwood B, Inskip H, Hall A, Koskela M, Leinonen M. Antibody response to pneumococcal capsular polysaccharide vaccine in African children. Pediatr Infect Dis J. 1991 May;10(5):386–90. doi: 10.1097/00006454-199105000-00008. [DOI] [PubMed] [Google Scholar]
- [15].Koskela M, Leinonen M, Haiva VM, Timonen M, Makela PH. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr Infect Dis. 1986 Jan-Feb;5(1):45–50. doi: 10.1097/00006454-198601000-00009. [DOI] [PubMed] [Google Scholar]
- [16].Borgono JM, McLean AA, Vella PP, Woodhour AF, Canepa I, Davidson WL, et al. Vaccination and revaccination with polyvalent pneumococcal polysaccharide vaccines in adults and infants. Proc Soc Exp Biol Med. 1978 Jan;157(1):148–54. doi: 10.3181/00379727-157-40010. [DOI] [PubMed] [Google Scholar]
- [17].Sloyer JL, Jr., Ploussard JH, Howie VM. Efficacy of pneumococcal polysaccharide vaccine in preventing acute otitis media in infants in Huntsville, Alabama. Rev Infect Dis. 1981 Mar-Apr;3(Suppl):S119–23. doi: 10.1093/clinids/3.supplement_1.s119. [DOI] [PubMed] [Google Scholar]
- [18].Leinonen M, Sakkinen A, Kalliokoski R, Luotonen J, Timonen M, Makela PH. Antibody response to 14-valent pneumococcal capsular polysaccharide vaccine in pre-school age children. Pediatr Infect Dis. 1986 Jan-Feb;5(1):39–44. doi: 10.1097/00006454-198601000-00008. [DOI] [PubMed] [Google Scholar]
- [19].Douglas RM, Paton JC, Duncan SJ, Hansman DJ. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983 Jul;148(1):131–7. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- [20].O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007 Sep;7(9):597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- [21].MacLennan J, Obaro S, Deeks J, Lake D, Elie C, Carlone G, et al. Immunologic memory 5 years after meningococcal A/C conjugate vaccination in infancy. J Infect Dis. 2001 Jan 1;183(1):97–104. doi: 10.1086/317667. [DOI] [PubMed] [Google Scholar]
- [22].Lawrence EM, Edwards KM, Schiffman G, Thompson JM, Vaughn WK, Wright PF. Pneumococcal vaccine in normal children. Primary and secondary vaccination. Am J Dis Child. 1983 Sep;137(9):846–50. doi: 10.1001/archpedi.1983.02140350024007. [DOI] [PubMed] [Google Scholar]
- [23].Russell F, Chandra R, Carapetis J, Seduadua A, Tikoduadua L, Buadromo E, et al. Epidemiology and Serotypes of Invasive Pneumococcal Disease in all ages in Fiji; 6th International Symposium of Pneumococci and Pneumococal Diseases; Reykjavik, Iceland. 2008 June 8-12; 2008. 2008. [Google Scholar]
- [24].Russell FM, Balloch A, Tang ML, Carapetis JR, Licciardi P, Nelson J, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009 Jul 17; doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nahm MH, Goldblatt D. Training manual for enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA) 2006 http://www.vaccine.uab.edu/ELISA%20Protocol.pdf.
- [26].Quataert SA, Kirch CS, Wiedl LJ, Phipps DC, Strohmeyer S, Cimino CO, et al. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995 Sep;2(5):590–7. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Concepcion N, Frasch CE. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin Diagn Lab Immunol. 1998 Mar;5(2):199–204. doi: 10.1128/cdli.5.2.199-204.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Balloch A, Mininni T, Nurkka A, Mackenzie G, Leach A, Kayhty H. Interlaboratory comparison of the specific IgG response to serotypes in Prevenar; 5th International Symposium on Pneumococci and Pneumococcal Diseases; Alice Springs, Australia. 2006 April 2-6; 2006. 2006. [Google Scholar]
- [29].Zellner A. Estimators for seemingly unrelated regression equations. J American Statistical Association. 1963;58:977–92. [Google Scholar]
- [30].Yokochi T, Kato Y, Sugiyama T, Koide N, Morikawa A, Jiang GZ, et al. Lipopolysaccharide induces apoptotic cell death of B memory cells and regulates B cell memory in antigen-nonspecific manner. FEMS Immunol Med Microbiol. 1996 Aug;15(1):1–8. doi: 10.1111/j.1574-695X.1996.tb00351.x. [DOI] [PubMed] [Google Scholar]
- [31].Heyman B. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu Rev Immunol. 2000;18:709–37. doi: 10.1146/annurev.immunol.18.1.709. [DOI] [PubMed] [Google Scholar]
- [32].Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells? J Immunol. 2007 Jul 1;179(1):13–9. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- [33].Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol. 2008 Oct 15;181(8):5306–12. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- [34].Baxendale HE, Davis Z, White HN, Spellerberg MB, Stevenson FK, Goldblatt D. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur J Immunol. 2000 Apr;30(4):1214–23. doi: 10.1002/(SICI)1521-4141(200004)30:4<1214::AID-IMMU1214>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- [35].Granoff DM, Pollard AJ. Reconsideration of the use of meningococcal polysaccharide vaccine. Pediatr Infect Dis J. 2007 Aug;26(8):716–22. doi: 10.1097/INF.0b013e3180cc2c25. [DOI] [PubMed] [Google Scholar]
- [36].Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002 Sep;17(3):341–52. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- [37].Noel C, Florquin S, Goldman M, Braun MY. Chronic exposure to superantigen induces regulatory CD4(+) T cells with IL-10-mediated suppressive activity. Int Immunol. 2001 Apr;13(4):431–9. doi: 10.1093/intimm/13.4.431. [DOI] [PubMed] [Google Scholar]
- [38].Wuorimaa T, Kayhty H, Eskola J, Bloigu A, Leroy O, Surcel HM. Activation of cell-mediated immunity following immunization with pneumococcal conjugate or polysaccharide vaccine. Scand J Immunol. 2001 Apr;53(4):422–8. doi: 10.1046/j.1365-3083.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- [39].Khan AQ, Lees A, Snapper CM. Differential regulation of IgG anti-capsular polysaccharide and antiprotein responses to intact Streptococcus pneumoniae in the presence of cognate CD4+ T cell help. J Immunol. 2004 Jan 1;172(1):532–9. doi: 10.4049/jimmunol.172.1.532. [DOI] [PubMed] [Google Scholar]
- [40].McCool TL, Harding CV, Greenspan NS, Schreiber JR. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect Immun. 1999 Sep;67(9):4862–9. doi: 10.1128/iai.67.9.4862-4869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Guttormsen HK, Sharpe AH, Chandraker AK, Brigtsen AK, Sayegh MH, Kasper DL. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect Immun. 1999 Dec;67(12):6375–84. doi: 10.1128/iai.67.12.6375-6384.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Malley R, Trzcinski K, Srivastava A, Thompson CM, Anderson PW, Lipsitch M. CD4+ T cells mediate antibody-independent acquired immunity to pneumococcal colonization. Proc Natl Acad Sci U S A. 2005 Mar 29;102(13):4848–53. doi: 10.1073/pnas.0501254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dullforce P, Sutton DC, Heath AW. Enhancement of T cell-independent immune responses in vivo by CD40 antibodies. Nat Med. 1998 Jan;4(1):88–91. doi: 10.1038/nm0198-088. [DOI] [PubMed] [Google Scholar]
- [44].Snapper CM. Differential regulation of protein- and polysaccharide-specific Ig isotype production in vivo in response to intact Streptococcus pneumoniae. Curr Protein Pept Sci. 2006 Aug;7(4):295–305. doi: 10.2174/138920306778017972. [DOI] [PubMed] [Google Scholar]
- [45].Pichichero ME. Immunological paralysis to pneumococcal polysaccharide in man. Lancet. 1985 Aug 31;2(8453):468–71. doi: 10.1016/s0140-6736(85)90401-5. [DOI] [PubMed] [Google Scholar]
- [46].Rosen C, Christensen P, Hovelius B, Prellner K. Effect of pneumococcal vaccination on upper respiratory tract infections in children. Design of a follow-up study. Scand J Infect Dis Suppl. 1983;39:39–44. [PubMed] [Google Scholar]
- [47].Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. J Infect Dis. 1984 Jun;149(6):861–9. doi: 10.1093/infdis/149.6.861. [DOI] [PubMed] [Google Scholar]
- [48].Riley ID, Lehmann D, Alpers MP, Marshall TF, Gratten H, Smith D. Pneumococcal vaccine prevents death from acute lower-respiratory-tract infections in Papua New Guinean children. Lancet. 1986 Oct 18;2(8512):877–81. doi: 10.1016/s0140-6736(86)90409-5. [DOI] [PubMed] [Google Scholar]