Summary
Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one), a synthetic analog of the endogenous neurosteroid allopregnanolone and a positive allosteric modulator of GABAA receptors, may represent a new treatment approach for epilepsy. Here we demonstrate that pretreatment with ganaxolone (1.25–20 mg/kg, s.c.) causes a dose-dependent suppression of behavioral and electrographic seizures in fully amygdala kindled female mice, with nearly complete seizure protection at the highest dose tested. The ED50 for suppression of behavioral seizures was 6.6 mg/kg. The seizure suppression produced by ganaxolone was comparable to that of clonazepam (ED50, 0.1 mg/kg, s.c.). To the extent that amygdala kindling represents a model of mesial temporal lobe epilepsy, this study supports the utility of ganaxolone in the treatment of patients with temporal lobe seizures.
Keywords: ganaxolone, neurosteroid, clonazepam, GABAA receptor, amygdala kindling, epilepsy, seizure, female mice
Introduction
Ganaxolone (3α-hydroxy-3β-methyl-5α-pregnan-20-one), the 3β-methyl analog of the progesterone metabolite allopregnanolone, is the first neurosteroid related agent to be evaluated in man for the drug treatment of epilepsy (Nohria and Giller, 2007). According to a widely held definition, neurosteroids are endogenous steroids that directly modulate neural excitability through non-genomic actions. The most studied and best accepted molecular target of neurosteroids is GABAA receptors (Belelli et al., 2005; Reddy, 2009). Ganaxolone, like allopregnanolone, acts as a powerful positive allosteric modulator of GABAA receptors, resulting in enhanced neural inhibition. As is the case for other agents that enhance GABAA receptor mediated inhibition, ganaxolone exhibits protective activity in diverse animal seizure models (Rogawski and Reddy, 2004; Liptáková et al., 2000; Gasior et al., 2000). Moreover, in phase 2 clinical trials, ganaxolone treatment has been found to reduce the frequency of partial seizures in adult patients (Laxer et al., 2000; Marinus Pharmaceuticals, unpublished). Neurosteroid-related anticonvulsants like ganaxolone have unique properties that distinguish them from other anticonvulsants that act on brain GABA systems. For example, GABAA receptor modulating neurosteroids preferentially act on extrasynaptic (δ-subunit-containing) GABAA receptors (Glykys and Mody, 2007) and tolerance to the anticonvulsant effects of neurosteroids generally does not occur when they are administered systemically (Reddy and Rogawski, 2000a). In comparison with endogenous 3α-hydroxy-pregnane neurosteroids such as allopregnanolone, ganaxolone has the advantage that it cannot be converted by 3α-hydroxysteroid oxidoreductase isoenzymes to the hormonally active 3-keto form (Rupprecht et al., 1993), and hence it is believed that patients taking ganaxolone would not be at risk of hormonal side effects (Monaghan et al., 1997; Reddy and Woodward, 2004).
Amygdala kindling has been proposed as a model of human temporal lobe epilepsy (Albright and Burnham, 1979). To elicit the kindled state, a mild focal electrical stimulus is applied unilaterally to the amygdala on a daily basis causing a local electrographic seizure known as the “afterdischarge.” With time, the initially behaviorally innocuous electrical stimulus elicits increasingly more robust limbic seizure-like behavioral events (Goddard et al., 1969). The kindled state is stable during the animal’s lifetime such that the kindling stimulus always elicits a full-blown behavioral and electrographic seizure. The kindling model has been widely used in the evaluation of drugs for antiseizure activity. Previous studies have indicated that neurosteroids with GABAA receptor modulatory activity have protective effects in the amygdala kindling model (Holmes and Weber, 1984; Reddy and Rogawski, 2002; Reddy et al., 2004; Lonsdale et al., 2006; Lonsdale and Burnham, 2007). Moreover, ganaxolone itself has been shown to protect against various forms of kindled seizures in mice and rats, including corneal kindling (Carter et al., 1997) and cocaine kindling (Gasior et al., 2000; Kaminski et al., 2003). However, ganaxolone has not been previously studied in the amygdala kindling model, which is the best established kindling model and shows pharmacological differences from other kindling models used for anticonvulsant drug evaluation (Potschka and Löscher, 1999). Since ganaxolone is under clinical development, we sought to characterize its activity in the amygdala kindling model in mice. For comparison, we conducted parallel experiments with clonazepam, which has previously been shown to be highly effective against amygdala kindled seizures (Ashton and Wauquier, 1979; Albertson et al., 1980; Löscher et al., 1986; Tietz et al., 1989; Fukinaga et al., 1998). Our results indicate that, in fully kindled mice, ganaxolone produces a marked suppression of stimulation-evoked behavioral seizures and electrographic seizure activity, supporting the potential utility of ganaxolone to treat complex partial seizures.
Materials and methods
Animals
Adult female mice of C57BL6 strain weighing approximately 25–30 g were used in the study. The mice were housed in an environmentally controlled animal facility under a normal 12 h/12 h light/dark cycle and allowed free access to food and water, except during the experimental sessions. The mice were randomly assigned for drug testing and did not check for ovarian cycle stages. Female mice were used the study because of targeted indication of ganaxolone for treating women with epilepsy. All procedures were performed in strict compliance with the NIH Guide for the Care and Use of Laboratory Animals under a protocol approved by the Institutional Animal Care and Use Committee.
Electrode implantation
Electrode implantation and stimulation procedures for mouse amygdala kindling were performed as described previously (Rogawski et al., 2001; Reddy and Rogawski, 2002). Briefly, mice were anesthetized by intraperitoneal injection of a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg). A twisted bipolar stainless steel wire electrode (model MS303/1; Plastic One, Roanoke, VA) was stereotaxically implanted in the right amygdala complex (1.3 mm posterior and 3.0 mm lateral to bregma, and 4.6 mm below the dorsal surface of the skull) (Franklin and Paxinos, 1997) and anchored with dental acrylic to three jeweler’s screws placed in the skull. A period of 7 to 10 days was allowed for recovery.
Kindling procedure
The stimulation paradigm consisted of 1 ms-duration, bipolar, square current pulses delivered at 60 Hz for 1 s using a kindling stimulator (A-M Systems, Sequim, WA). The afterdischarge threshold was determined by stimulating at 5 min intervals beginning with an intensity of 75 μA and increasing in steps of 50 μA until an afterdischarge of at least 5 s was obtained. Stimulation on subsequent days used a stimulation intensity 125% of the threshold value. Seizure activity following each stimulation was rated according to the criterion of Racine (1972) as modified for the mouse: stage 0, no response or behavior arrest; stage 1, chewing or head nodding; stage 2, chewing and head nodding; stage 3, forelimb clonus; stage 4, bilateral forelimb clonus and rearing; stage 5, falling. The afterdischarge was recorded from the amygdala electrode with a Grass CP511 AC electroencephalogram preamplifier (Astro-Med, West Warwick, RI) and stored in digital form using Axoscope 8.1 (Axon Instruments, Foster City, CA). Afterdischarge duration was the total duration of amygdala electrographic spike activity (amplitude > 2 × baseline) occurring in a rhythmic pattern at a frequency > 1Hz. The afterdischarge often exhibited two phases of waxing and waning amplitude; when this occurred, the afterdischarge duration was taken as the time to the end of the second phase. The day of afterdischarge threshold determination was considered day 1 of kindling. Kindling stimulation was delivered daily until stage 5 seizures were elicited on three consecutive days. Stimulation was continued on a five-day per week schedule each afternoon. Mice were used for drug testing when they consistently exhibited stage 5 seizures with stimulation, which is considered the “fully kindled” state.
Kindling seizure studies
To examine the ability of the test drugs (ganaxolone or clonazepam) to suppress the expression of kindled seizures, the kindled mice underwent a 3-day test protocol. On day 1, they were verified to exhibit a stimulation-induced stage 5 seizures. On day 2, a subcutaneous ganaxolone injection was administered 15 min prior to stimulation. On day 3, the animals were stimulated again without drug pretreatment. During each test session, the behavioral seizure score and the afterdischarge duration (recorded from the implanted amygdala stimulating electrode) were noted. The seizure scores and afterdischarge durations on days 1 and 3 were averaged and taken as the control values. At the end of the study, mice were anesthetized and perfused transcardially with paraformaldehyde for Nissl staining to verify the electrode placement.
Data analysis
Group data are expressed as the mean ± standard error of the mean (S.E.M.); differences in seizure stage between groups were compared with the nonparametric Kruskal-Wallis test followed by the Mann-Whitney U-test. Comparison of means of the afterdischarge duration between groups was made with one-way analysis of variance, followed by unpaired two-tailed Student’s t-test. Comparison of the mean percentage inhibition of seizure stage and afterdischarge duration in fully kindled animals was made by Wilcoxon signed ranks test and paired two-tailed Student’s t-test, respectively. ED50 (estimated dose resulting in 50% inhibition) values were determined by non-linear curve fitting using the Levenberg-Marquardt algorithm to a logistic equation where the maximum inhibition was assumed to be 100%. In all statistical tests, the criterion for statistical significance was p < 0.05.
Drugs
Stock solutions of ganaxolone for injection were made in 30% hydroxypropyl-β-cyclodextrin in water, and additional dilutions were made using normal saline. By itself, β-cyclodextrin at concentrations as high as 50% failed to affect kindled seizures. Drug solutions were administered s.c. or i.p. in a volume equaling 1% of the animal’s body weight. Ganaxolone and clonazepam were purchased from Sigma (St. Louis, MO).
Results
Amygdala kindling development
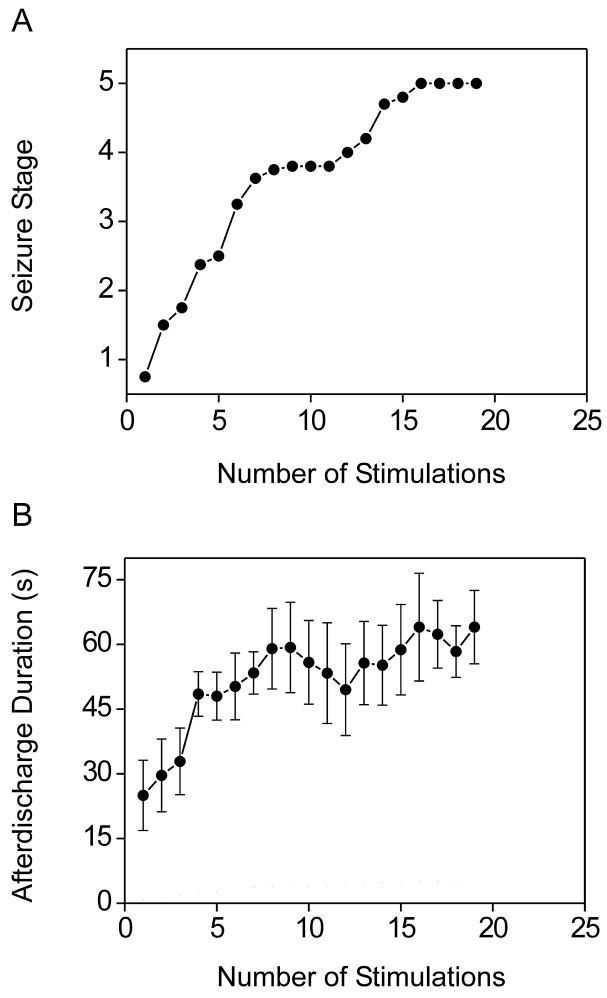
The initial kindling stimulation most commonly evoked no behavioral response; occasionally there was behavioral arrest or facial movement. The overall average value of the initial afterdischarge threshold was 373 ± 40 μA (n=8). Daily kindling stimulation was associated with an orderly progression to increasing behavioral seizure stage (Fig. 1A). The early phase of kindling was associated with a gradual prolongation in the afterdischarge duration after which the duration remained in the range of 45 to 80 s (Fig. 1B). Mice reached the fully kindled state in which they consistently exhibited stage 5 seizures after about 14 stimulations.
Figure 1.
Kindling development in adult female mice. (A) Progression of behavioral seizure stage with number of amygdala kindling stimulations. Animals were stimulated daily until stage 5 seizures were elicited on three consecutive days. Each data point represents the mean of the behavioral seizure stage of 6 to 8 animals. The overall average number of stimulations to achieve stage 5 kindling was 14.5. (B) Afterdischarge duration during the development of amygdala kindling in the same animals. Each data point represents the mean ± S.E.M. of the total afterdischarge duration obtained with each daily stimulation.
Ganaxolone protection against amygdala-kindled seizures
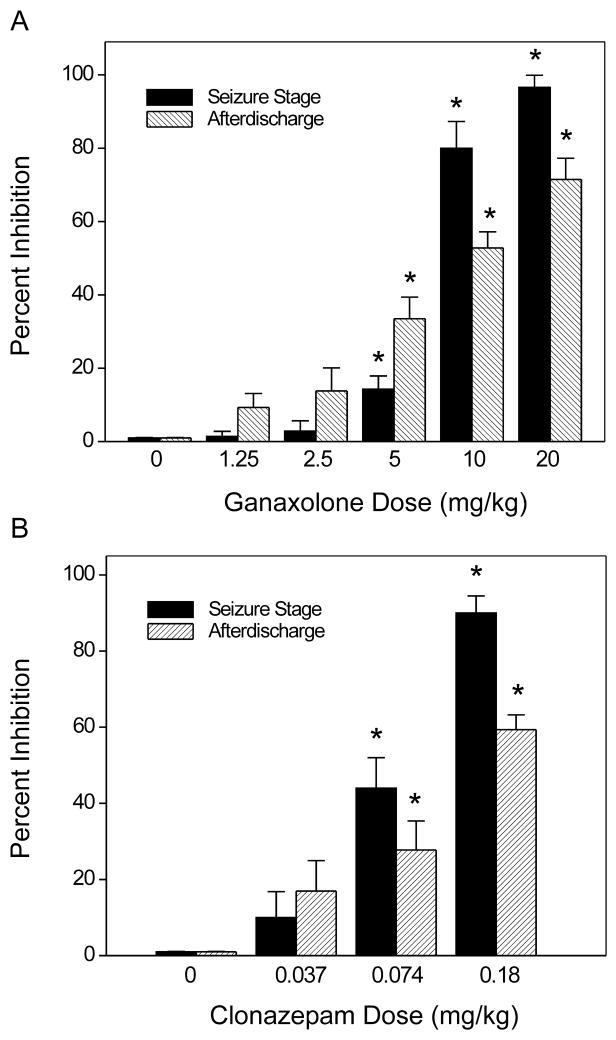
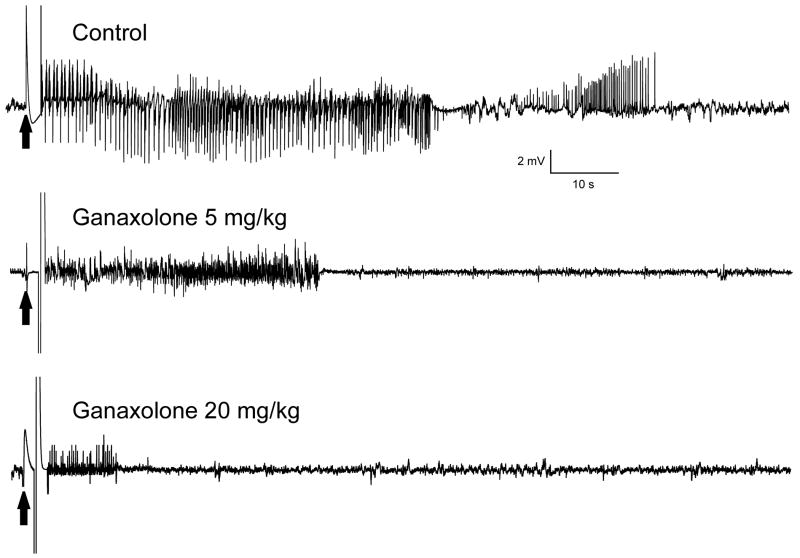
To evaluate the activity of ganaxolone in protecting against amygdala kindled seizures, fully kindled mice were treated with various doses of ganaxolone 15 min prior to stimulation. As shown in Fig. 2, ganaxolone produced a dose-dependent suppression of behavioral seizure activity (Fig. 2A) and afterdischarge duration (Fig. 2B) with significant effects on both at 5, 10 and 20 mg/kg. The percentage inhibition at the various doses is shown in Fig. 4A. At the highest dose tested, behavioral seizures were nearly completely suppressed. The estimated ED50 value for suppression of seizure stage and afterdischarge duration is 6.6 ± 1.1 mg/kg and 11 ± 1.6 mg/kg, respectively. Sample recordings of afterdischarges from one animal are shown in Fig. 3. Ganaxolone pretreatment markedly reduced the duration of the afterdischarge duration in a dose-dependent fashion. In addition, the overall amplitude was reduced by about 50% after 5 and 20 mg/kg ganaxolone. On the day after ganaxolone treatment, all mice exhibited full-blown stage 5 seizures with afterdischarge duration (51.0 ± 2.6 s) not significantly different from the control duration (55.5 ± 4.0 s), indicating that ganaxolone treatment suppresses the expression of kindled seizures but does not alter the kindled state.
Figure 2.
Inhibition of amygdala kindled seizures by ganaxolone (A,B) and clonazepam (C,D) in female mice. Dose-response curves for behavioral seizure stage (A) and afterdischarge duration (B) with varying doses of ganaxolone (1.25–20 mg/kg, sc). Dose-response curves for behavioral seizure stage (C) and afterdischarge duration (D) with varying doses of clonazepam (0.037–0.18 mg/kg, ip). Mice that were fully kindled as described in Fig. 1 were injected subcutaneously with vehicle or ganaxolone 15 min before stimulation. Each point represents the mean ± S.E.M. of data from 6 to 8 animals. *P < 0.05 vs. control. A minimum of two weeks was allowed to elapse between test doses.
Figure 4.
Percent inhibition of fully kindled seizures and afterdischarge duration by ganaxolone (A) and clonazepam (B). Percent inhibition of seizure stage was calculated as 100 × (1S/5), where S is the seizure stage following drug treatment. Percentage inhibition of afterdischarge was calculated as 100 × (1–D/Dc), where D is the afterdischarge duration after drug treatment and Dc is the average control afterdischarge duration without any drug treatment. The overall mean control afterdischarge duration was 55.5 ± 4.0 s. Each bar represents the mean ± S.E.M. of values from 6 to 8 animals. *P < 0.05 vs. control.
Figure 3.
Representative traces illustrating inhibition of afterdischarge in a fully kindled mouse by 5 and 20 mg/kg ganaxolone administered 15-min prior to stimulation. Traces show depth electroencephalographic recordings from a stimulating electrode in the right amygdala. Arrows indicate onset of the 1 s kindling stimulus, which is followed by the stimulus artifact (mostly blanked to prevent saturation of the amplifier circuitry). In each trace, a slow negative wave following stimulation was digitally subtracted. Ganaxolone was administered s.c. 15-min prior to the stimulation; control trace was obtained without drug treatment.
Clonazepam protection against amygdala-kindled seizures
For comparison, experiments were conducted in the same mice with clonazepam after a two week washout period. As shown in Fig. 2, clonazepam treatment produced a dose-dependent suppression of the behavioral seizure score (Fig. 2C) and afterdischarge duration (Fig. 2D). The percentage inhibition at the various doses is shown in Fig. 4B. The estimated ED50 values for suppression of seizure stage and afterdischarge duration is 0.10 ± 0.02 and 0.10 ± 0.03 mg/kg, respectively.
Discussion
In this study, the synthetic neurosteroid analog ganaxolone was found to have powerful antiseizure activity in the mouse amygdala kindling model of epilepsy. Animals were fully kindled and then tested with a supramaximal stimulus that was 125% of their individual afterdischarge threshold. Pretreatment with ganaxolone caused a dose-dependent reduction in behavioral seizure score and a corresponding suppression of the duration of electrographic discharges. The behavioral and electrographic features of kindled seizures have similarities to human limbic seizures and the neuropathology is comparable to that observed in human mesial temporal lobe epilepsy (Sutula, 1990). Therefore, kindling is considered to be a model of human complex partial epilepsy and it is often used in antiepileptic drug development (Albertson et al., 1980). Our results support the utility of ganaxolone in the treatment of complex partial seizures.
Ganaxolone has antiseizure potency in the mouse amygdala kindling model that is similar to its potency in other seizure models in which it is highly active. Table 1 compares the potency of ganaxolone against amygdala kindled seizures with its potencies in other mouse seizure models. As is the case for neurosteroids and other agents that act as positive modulators of GABAA receptors, ganaxolone is effective against seizures induced by chemoconvulsants that antagonize GABAA receptors including pentylenetetrazol, bicuculline, t-butylbicycloorthobenzoate and flourothyl (Reddy and Woodward, 2004; Krasowski, 2000). It is also active in the 6 Hz electroshock model, which has been shown to be highly sensitive to agents that positively modulate GABAA receptors (Kaminski et al., 2004). In addition, ganaxolone exhibits activity against cocaine kindled seizures (Gasior et al., 2000; Kaminski et al., 2003). At higher doses, ganaxolone protects mice against maximal electroshock (MES)-induced seizures. In general, GABAA receptor positive modulators, such as benzodiazepines and neurosteroids, have weak activity in the MES test (Kokate et al., 1994).
Table 1.
Antiseizure activity of ganaxolone in mouse models of epilepsy.
| Seizure model | ED50 value (mg/kg) | Literature citation |
|---|---|---|
| Pentylenetetrazol | 3.5 (2.1–5.8) | Reddy and Rogawski, 2000b |
| Pentylenetetrazol kindling | 4.1 (2.7–6.4) | Gasior et al., 2000 |
| Bicuculline | 4.6 (3.2–6.8) | Carter et al., 1997 |
| Flourothyl | 5.0 (ND) | Liptakova et al., 2000 |
| 6 Hz | 6.3 (4.0–9.8) | Kaminski et al., 2004 |
| Amygdala kindling | 6.6 (5.1–9.7) | This paper |
| t-Butylbicycloorthobenzoate | 11.7 (8.8–15.7) | Carter et al., 1997 |
| Aminophylline | 11.5 (8.1–16.3) | Carter et al., 1997 |
| Cocaine kindling | 17.0 (ND) | Kaminski et al., 2003 |
| Maximal electroshock | 29.7 (25.3–34.8) | Carter et al., 1997 |
| N-methyl-D-aspartate | > 30 (ND) | Carter et al., 1997 |
| Strychnine | > 40 (ND) | Carter et al., 1997 |
Numbers in parentheses are 95% confidence intervals. ND, not determined.
Electrophysiological studies in Xenopus oocytes expressing recombinant human GABAA receptors demonstrated that ganaxolone is a potent positive allosteric modulator of GABAA receptor function (Carter et al., 1997). The steroid is not known to interact with other molecular targets. Therefore, it is likely that the protective activity against kindled-seizures relates to the actions on GABAA receptors. The estimated plasma concentrations of ganaxolone at doses that protect in the kindling model (~3 μM at ED50, 6.6 mg/kg; Reddy and Rogawski, 2000a; Reddy et al., 2004) exceed the range of concentrations that modulate GABAA receptors in in vitro preparations (EC50, 94–213 nM; Carter et al., 1997). It seems likely that brain penetration is limiting so that target concentrations do not fully reflect the plasma concentrations. However, it is noteworthy that at relatively high (micromolar) concentrations, neurosteroids including ganaxolone directly activate GABAA receptor channels in the absence of GABA (Kokate et al., 1994; Carter et al., 1997; Reddy and Rogawski, 2002). Such a direct agonist action could contribute to the protective activity of ganaxolone in the kindling model.
Many previous studies of ganaxolone have utilized male animals to avoid the potentially confounding influence of ovarian hormone fluctuations associated with the estrous cycle that occurs in female rodents of reproductive age. In the present study, we utilized female mice so as to obtain information of utility in assessing the potential of ganaxolone to treat women with epilepsy. Our results demonstrate that the drug is as likely to be efficacious in women as in men. Changes in seizure susceptibility are not evident among phases of the estrous cycle in fully kindled animals (Wahnschaffe and Löscher, 1992). Nevertheless, had we timed our testing to the estrous cycle, it is possible that we might have detected differences in ganaxolone sensitivity between the estrous cycle stages. In addition, since we restricted our experiments to females, our results are not strictly generalizable to males although we do note that the potency of ganaxolone obtained in the present study is similar to that obtained in male mice in other models (Table 1).
To provide a validated positive control, in the present study we conducted a parallel series of experiments with clonazepam, which is well recognized to protect against amygdala kindled seizures (Ashton and Wauquier, 1979; Albertson et al., 1980; Löscher et al., 1986; Tietz et al., 1989; Fukinaga et al., 1998). As expected, pretreatment with clonazepam caused a dose-dependent inhibition of seizure stage and duration that was similar to that produced by ganaxolone, except that clonazepam was substantially more potent on a molar basis. The lower potency of ganaxolone is consistent with the relatively low binding affinity of neurosteroids for GABAA receptors (Chisari et al., 2009) but also likely relates to its restricted bioavailability. It is noteworthy that antiepileptic drugs for the most part have low binding affinities for their molecular targets and that the systemic potency of ganaxolone is typical of the potencies of common antiepileptic drugs. Although benzodiazepines such as clonazepam are effective antiseizure drugs, their clinical usefulness in the chronic treatment of epilepsy is limited because tolerance develops to their protective effects with long-term therapy. In a previous study comparing ganaxolone and the benzodiazepine diazepam in a repeated-dosing paradigm, we observed that tolerance for protection against pentylenetetrazol-induced seizures occurs with diazepam but not ganaxolone (Reddy and Rogawski, 2000a). Moreover, there was no evidence of tolerance in a recent clinical trial of ganaxolone as add-on therapy for adult partial seizures (Marinus Pharmaceuticals, unpublished). Therefore, lack of tolerance may confer ganaxolone with a substantial advantage in comparison with benzodiazepines for the chronic treatment of epilepsy. Ganaxolone also appears to have a favorable safety profile and there is no evidence that it is teratogenic or causes major systemic toxicity with chronic administration, although it does cause dose-related sedation, consistent with its action on GABAA receptors (Reddy, 2003).
In conclusion, our results demonstrate that ganaxolone has powerful antiseizure effects in the amygdala kindling model in mice, supporting the utility of ganaxolone in the treatment of temporal lobe epilepsy. The observation that ganaxolone is effective in female animals highlights its application to address the unique challenges faced in treating women with epilepsy, which include drug-resistant catamenial seizure exacerbations (Reddy, 2009; Reddy and Rogawski, 2000b, 2009).
Acknowledgments
This work was supported in part by National Institutes of Health grant NS051398 (to D.S.R.).
Footnotes
Disclosure
M.A.R. is a scientific founder of and has served as a consultant to Marinus Pharmaceuticals, Inc., Branford, CT.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albertson TE, Peterson SL, Stark LG. Anticonvulsant drugs and their antagonism of kindled amygdaloid seizures in rats. Neuropharmacology. 1980;19:643–652. doi: 10.1016/0028-3908(80)90038-6. [DOI] [PubMed] [Google Scholar]
- Albright PS, Burnham WM. Development of a new pharmacological seizure model: effects of anticonvulsants on cortical- and amygdala-kindled seizures in the rat. Epilepsia. 1980;21:681–689. doi: 10.1111/j.1528-1157.1980.tb04321.x. [DOI] [PubMed] [Google Scholar]
- Ashton D, Wauquier A. Behavioral analysis of the effects of 15 anticonvulsants in the amygdaloid kindled rat. Psychopharmacology (Berl) 1979;65:7–13. doi: 10.1007/BF00491971. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Carter RB, Wood PL, Wieland S, Hawkinson JE, Belelli D, Lambert JJ, White HS, Wolf HH, Mirsadeghi S, Tahir SH, Bolger MB, Lan NC, Gee KW. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther. 1997;280:1284–1295. [PubMed] [Google Scholar]
- Chisari M, Eisenman LN, Krishnan K, Bandyopadhyaya AK, Wang C, Taylor A, Benz A, Covey DF, Zorumski CF, Mennerick S. The influence of neuroactive steroid lipophilicity on GABAA receptor modulation: evidence for a low-affinity interaction. J Neurophysiol. 2009;102:1254–1264. doi: 10.1152/jn.00346.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. 1. Academic Press; 1997. [Google Scholar]
- Fukinaga M, Ishizawa K, Kamei C. Anticonvulsant properties of 1,4-benzodiazepine derivatives in amygdaloid-kindled seizures and their chemical structure-related anticonvulsant action. Pharmacology. 1998;57:233–241. doi: 10.1159/000028247. [DOI] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–1196. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–770. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Holmes GL, Weber DA. The effect of progesterone on kindling: a developmental study. Brain Res. 1984;318:45–53. doi: 10.1016/0165-3806(84)90061-0. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Gasior M, Carter RB, Witkin JM. Protective efficacy of neuroactive steroids against cocaine kindled-seizures in mice. Eur J Pharmacol. 2003;474:217–222. doi: 10.1016/s0014-2999(03)02086-7. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia. 2004;45:864–867. doi: 10.1111/j.0013-9580.2004.04504.x. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Krasowski MD. Differential modulatory actions of the volatile convulsant flurothyl and its anesthetic isomer at inhibitory ligand-gated ion channels. Neuropharmacology. 2000;39:1168–1183. doi: 10.1016/s0028-3908(99)00221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia. 2000;41:1187–1194. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]
- Liptáková S, Velísek L, Velísková J, Moshé SL. Effect of ganaxolone on flurothyl seizures in developing rats. Epilepsia. 2000;41:788–793. doi: 10.1111/j.1528-1157.2000.tb00244.x. [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Burnham WM. The anticonvulsant effects of allopregnanolone against amygdala-kindled seizures in female rats. Neurosci Lett. 2007;411:147–151. doi: 10.1016/j.neulet.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Lonsdale D, Nylen K, Burnham WM. The anticonvulsant effects of progesterone and its metabolites on amygdala-kindled seizures in male rats. Brain Res. 2006;1101:110–116. doi: 10.1016/j.brainres.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Löscher W, Jäckel R, Czuczwar SJ. Is amygdala kindling in rats a model for drug-resistant partial epilepsy? Exp Neurol. 1986;93:211–226. doi: 10.1016/0014-4886(86)90160-3. [DOI] [PubMed] [Google Scholar]
- Monaghan EP, Navalta LA, Shum L, Ashbrook DW, Lee DA. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia. 1997;38:1026–1031. doi: 10.1111/j.1528-1157.1997.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–105. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H, Löscher W. Corneal kindling in mice: behavioral and pharmacological differences to conventional kindling. Epilepsy Res. 1999;37:109–120. doi: 10.1016/s0920-1211(99)00062-5. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003;15:197–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000a;295:1241–1248. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000b;294:909–915. [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulates GABAA receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–3805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. Neurosteroid replacement therapy for catamenial epilepsy. Neurotherapeutics. 2009;6:392–401. doi: 10.1016/j.nurt.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy DS, Castaneda DC, O’Malley BW, Rogawski MA. Anticonvulsant activity of progesterone and neurosteroids in progesterone receptor knockout mice. J Pharmacol Exp Ther. 2004;310:230–239. doi: 10.1124/jpet.104.065268. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Woodward R. Ganaxolone: A prospective overview. Drugs Future. 2004;29:227–242. [Google Scholar]
- Rogawski MA, Reddy DS. Neurosteroids: endogenous modulators of seizure susceptibility. In: Rho JM, Sankar R, Cavazos JE, editors. Epilepsy: scientific foundations of clinical practice. New York: Marcel Dekker; 2004. pp. 319–355. [Google Scholar]
- Rogawski MA, Kurzman PS, Yamaguchi S, Li H. Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse. Neuropharmacology. 2001;40:28–35. doi: 10.1016/s0028-3908(00)00112-x. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgansberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–530. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- Sutula TP. Experimental models of temporal lobe epilepsy: new insights from the study of kindling and synaptic reorganization. Epilepsia. 1990;31(Suppl 3):S45–54. doi: 10.1111/j.1528-1157.1990.tb05859.x. [DOI] [PubMed] [Google Scholar]
- Tietz EI, Rosenberg HC, Chiu TH. A comparison of the anticonvulsant effects of 1,4- and 1,5-benzodiazepines in the amygdala-kindled rat and their effects on motor function. Epilepsy Res. 1989;3:31–40. doi: 10.1016/0920-1211(89)90065-x. [DOI] [PubMed] [Google Scholar]
- Wahnschaffe U, Löscher W. Lack of changes in seizure susceptibility during the estrous cycle in kindled rats. Epilepsy Res. 1992;13:199–204. doi: 10.1016/0920-1211(92)90053-v. [DOI] [PubMed] [Google Scholar]