Summary
The family of death receptors plays a critical role in regulating cell number and eliminating harmful or virally infected cells. Agonistic stimulation of death receptors is known to lead two alternative cell fates by either activating NF-κB to promote cell survival or inducing apoptosis to lead to cell death; and now a third pathway, termed necroptosis or programmed necrosis has been identified. Interestingly, a death-domain containing kinase, RIP1, is involved in mediating all 3 pathways, with its kinase activity specifically involved in regulating necroptosis. The availability of necrostatin-1, a specific inhibitor of RIP1 kinase, made it possible to dissect the distinct functional domains of RIP1. Recent genome-wide siRNA screens have identified multiple players of necroptosis that may interact with and/or regulate RIP1 kinase and mediate the signaling pathway and execution of necroptosis. Necroptosis and necrostatins provide an exciting new opportunity for developing new treatments for multiple human diseases involving necrosis and inflammation.
Introduction
How cells control their own demise is a fundamental question in biology. Although the notion of cellular suicide was still a novel idea two decades ago, it has now been well-accepted that cells may choose to die as a normal developmental cell fate or when damaged or infected with virus [1]. Caspases are a family of cysteine proteases that can be activated to mediate apoptotic cell death in response to agonistic ligands of death receptors, such as TNFα, FasL and Trail, or mitochondrial damage as a result of inducing BH3-only members of pro-apoptotic Bcl-2 family. Apoptosis has been established as an evolutionarily conserved pathway regulating programmed cell death. However, it has become increasingly clear that apoptosis is not the only cellular mechanism that regulates cell death. At least a part of necrotic cell death, which has been viewed traditionally as a form of passive cell death, may be executed through a mechanism termed necroptosis or programmed necrosis [2]. Interestingly, necroptosis may be activated upon stimulation by TNFα, FasL and Trail, the same ligands that can activate apoptosis. Thus, cell death induced by the activation of death receptor may be executed through alternative cell death pathways, apoptosis or necroptosis. The mechanism that dictates the cellular decision to undergo apoptosis or necroptosis and the mechanism that mediates the execution of necroptosis are under intensive investigation.
Receptor interacting protein 1 (RIP1)
Best known for its role in NF-κB activation, receptor interacting protein 1 (RIP1) plays a critical role in necroptosis. Its serine/threonine kinase activity is essential for this necrotic death pathway but dispensable for both NF-κB activation and apoptosis, which rely on the intermediate and death domains of the protein [3]. The small molecule inhibitor of necroptosis, necrostatin-1 (Nec-1), is a potent inhibitor of RIP1 kinase activity [4].
RIP1 is autophosphorylated on Ser 161 within the activation loop, or T-loop of the kinase [4]. This loop, modeled after the closely related kinase B-RAF, has two conformations: open and closed. In the closed form, the loop blocks access to the catalytic cleft, but swings away from the cleft when open, allowing the kinase access to its substrates. Autophosphorylation on Ser161 is predicted to move the loop into its open form and activate the kinase [4]. Interestingly, reconstitution of a phosphomimetic S161E RIP1 mutant into RIP1-deficient cells does not induce necroptosis by itself, indicating that conformation shift of the activation loop is not sufficient for its activation and other inputs are necessary. Dimerization of RIP1, however, is sufficient to induce necroptosis [2]. This suggests that under normal conditions a RIP1 inhibitor blocks kinase activity until upstream signaling, presumably from the TNFα receptor, inactivates this inhibitor. The forced dimerization constructs may not be subject to this negative regulation. Alternatively, the dimerization and activation of RIP1 may recruit additional activating proteins necessary for downstream signaling. Identification of RIP1 substrates is crucial to determining which pathways or targets are activated downstream. To date no substrates of RIP1 have been identified that are involved in necroptosis.
RIP3
A RIP1 family member, RIP3, has recently been implicated in necroptosis [5–7]. Expression of RIP3 has been proposed to sensitize necroptosis-insensitive cell lines to necroptosis. RIP1 and RIP3 interact via their RIP homotypic interaction motifs (RHIM) domains [8]. The two proteins share 33% similarity in the kinase domain. Despite this similarity in the kinase domain, Nec-1 is specific to RIP1 and does not inhibit RIP3 [4,5].
The kinase activity of RIP3 has also been proposed to be required for necroptosis. Reconstitution of RIP3 deficient cells with a kinase dead K50A mutant is unable to restore sensitivity to necroptosis [5,6]. RIP3 is phosphorylated on Ser 199 after necroptotic stimulation and interestingly, although it is not yet clear if RIP1 may directly phosphorylate RIP3, this phosphorylation event is inhibited by treatment with Nec-1 [6]. The effect of this RIP3 phosphorylation on necroptosis has not been analyzed. The ability of Nec-1 to inhibit this phosphorylation event raised the possibility that it is RIP1, rather than RIP3 itself, that mediates the phosphorylation of Ser199 on RIP3. Alternatively, since RIP1 is unable to phosphorylate RIP3 in vitro, it is possible that the kinase activity of RIP1 is required for interacting with RIP3 and/or activating RIP3 phosphorylation. Consistent with this possibility, Nec-1 is able to inhibit the interaction between RIP1 and RIP3 upon activation of necroptosis [5,6]. RIP3 phosphorylates RIP1 in vitro [5], although the physiological significance of this phosphorylation and the phosphorylation site have not yet been determined.
Interaction between RIP1 and RIP3 via the RHIM domain is required for necroptosis [5,6]. The interaction of RIP1 and RIP3 is a specific necroptotic signaling event as it can only be detected after treatment with necroptotic stimuli [5,6]. Since this interaction is inhibited by treatment with the RIP1 kinase inhibitor, Nec-1, RIP3 appears to be activated by interaction with RIP1. However, it is not clear if RIP1 requires RIP3 for its activity. Dimerization of the RIP1 kinase domain, which lacks the RHIM domain, is sufficient to induce necroptosis, arguing that perhaps RIP1 is able to induce necroptosis independently of RIP3 [2].
Necroptotic complex IIb
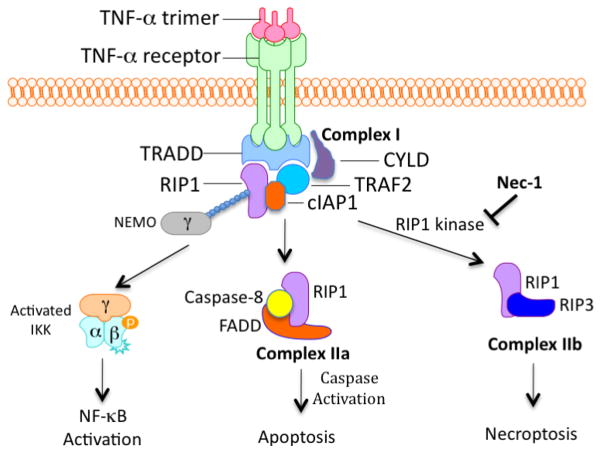
Under apoptotic competent condition, TNFα stimulation induces the formation of two sequential protein complexes, complex I and complex IIa, that lead to the activation of NF-κB and apoptosis, respectively [9] [10] (Figure 1). TNFα binding to TNFR1 induces the recruitment of RIP1 and other proteins to the receptor to form complex I. Subsequently, these components dissociate from TNFR1 and RIP1 can be found in the cytosol in complex IIa, which includes RIP1, caspase-8 and FADD. The signal leads to the transition from the complex I to complex IIa is currently unclear. There is evidence that a second complex also forms in necroptotic cells and is named complex IIb. Complex IIb contains at least one additional component, RIP3 (Figure 1). Treatment with the RIP1 kinase inhibitor Nec-1 prevents both RIP1 and RIP3 recruitment to complex IIb, suggesting RIP1 kinase activity is important for this complex formation in necroptotic cells [5,6]. The involvement of FADD and caspase-8 in the complex IIb is not clear as the loss of FADD or caspase-8 sensitizes cells to necroptosis.
Figure 1.
The signaling complexes induced by TNFα to mediate NF-κB activation, apoptosis and necroptosis. Stimulation of TNFR1 by TNFα leads to the formation of an intracellular complex at the cytoplasmic membrane (complex I) that includes TRADD, TRAF2, RIP1 and cIAP1. Ubiquitination of RIP1 at K377 by cIAP1 leads to the recruitment of NEMO, a regulatory subunit of IKK complex that in turn activates NF-κB pathway. RIP1 is also involved in the formation of complex IIa including FADD and caspase-8 to activate a caspase cascade to mediate apoptosis. Under apoptosis deficient conditions or when cells are infected by certain virus, RIP1 interacts with RIP3 to form complex IIb which is involved in mediating necroptosis. The formation complex IIb requires the kinase activity of RIP1 that is inhibited by Nec-1.
Regulation of RIP1 kinase by ubiquitination
RIP1 kinase is specifically required for necroptosis except when cells are stimulated with IAP antagonists (Smac mimetics), which leads to rapid degradation of cIAP1/cIAP2 [11]. For example, RIP1 kinase activity is required for mediating apoptosis of Panc-1 cells treated with TNFα and Smac mimetic. RIP1 is found in a complex containing FADD and caspase-8 upon treatment with TNFα and Smac mimetic (which is enhanced by treatment with zVAD.fmk), but not when treated with TNFα and zVAD.fmk only. This complex may be different from the other variations on complex II described above as it is formed in the presence of Smac mimetic to mediate apoptosis. However, we still do not understand why the loss of cIAP1/2 leads to the requirement of RIP1 kinase in the formation of the second complex to mediate apoptosis. In cells expressing RIP3, cells treated with TNFα and Smac mimetic may also undergo necroptosis which is inhibited by Nec-1 [6]. Since cIAP1/2 mediates RIP1 ubiquitination, one possibility is that the state of RIP1 ubiquitination is critical for the second complex. Consistently, knockdown of the deubiquitinase CYLD can protect against apoptosis induced by TNFα and Smac as well as necroptosis [11,12]. In addition, a number of E3 ligases have been implicated in RIP1 ubiquitination including cIAP1/2, TRAF2, TRAF6, and A20 [13–15].
Ubiquitination of K377 of RIP1 is necessary for NF-κB induction [16]. In addition, a mass spectrometry study identified four basally ubiquitinated lysines on RIP1 including K115 in the kinase domain, K570 in the intermediate domain, K603 and K626 in the death domain; however, there has been no further characterization of these sites [17]. Some work has also been done to examine the ubiquitin chain linkages on RIP1 after TNF stimulation. Immediately after TNFα treatment, K63-linked chains are found on RIP1, which is required for binding NEMO, a cofactor in the IKK complex, and involved in activating NF-κB [16,18]. RIP1 ubiquitination status is dynamic after TNFα stimulation as over time these are changed to K48 linkages through the action of A20 [15,19].
A study of the ubiquitination sites and enzymes involved in necroptosis will be important to understand RIP1 complex formation in necroptosis. It is likely that the ubiquitination state of RIP1 may influence its binding to interaction partners and therefore downstream effects in necroptosis, much as it does in NF-κB signaling. The critical role of CYLD is direct evidence of this. The expression of modifying enzymes, or lack thereof, may confer necroptosis sensitivity or resistance to cells.
Downstream components of necroptosis
While the upstream signaling and modification of RIP1 are important to determine how necroptosis is initiated, the identification of downstream components is necessary to show how necroptosis is executed. Caspases are the executioners of apoptosis; however, these proteases have no positive role in necroptosis. Thus far, an analogous class of executioner proteins has not been identified for necroptosis. Below we discuss a number of events that have been proposed to contribute to the downstream events in necroptosis.
Reactive oxygen species
Reactive oxygen species (ROS) production is proposed to be an executioner of necroptosis. Some cell types, such as L929 and MEFs, produce ROS in response to TNFα stimulation [20,21]. ROS are thought to act by oxidizing, in addition to other cellular proteins, MAP kinase phosphatases (MKPs) whose normal function is to downregulate JNK pathway signaling [22]. This results in prolonged JNK activation and subsequently, cell death. However, reports on the effect of JNK inhibition on necroptosis are conflicting [21,23]. Treatment with an antioxidant such as butylated hydroxyanisole (BHA) is able to reduce ROS levels and also inhibit cell death in some cell types [21]. However, antioxidant treatment is unable to protect all cell lines from necroptosis [2], suggesting that ROS as an executioner of necroptosis is likely a cell-type dependent phenomenon.
RIP3 has been reported to interact with several metabolic enzymes including glycogen phosphorylase (PYGL), glutamate-ammonia ligase (GLUL), and glutamate dehydrogenase 1 (GLUD1) [7]. These enzymes increase substrates for use in oxidative phosphorylation, which is a major source of ROS in the cell. RIP3-deficient cells have reduced ROS production downstream of TNFα signaling [5]. However, ROS has not been shown to directly contribute to necroptotic cell death in these systems.
An alternative, mitochondrial-independent pathway for ROS activation downstream of TNFα receptor, TNFR1, has recently been described. Riboflavin kinase (RFK) interacts with TNFR1 and TRADD after TNFα treatment. RFK in turn recruits the NADPH oxidases Nox1, Nox2, and p22phox to the receptor [24]. RFK, Nox1 and Nox2 are crucial for downstream ROS production [23,24]. RIP1 is not directly involved in this pathway. Nox1 knockdown partially protects L929 cells from TNFα-induced necroptosis [23]. However, Nox1 knockdown does not protect other cell types such as Jurkat cells from TNFα-induced necroptosis [4]. Thus, the contribution of ROS to the execution of necroptosis must be evaluated by further studies.
Mitochondria
The mitochondrion is well known for its function in apoptotic cell death and has also been implicated downstream of RIP1. A role for adenine nucleotide translocase (ANT) and cyclophilin D (CypD) in the mitochondrial permeability transition has been proposed in necroptosis. In a cardiac ischemia and reperfusion injury, CypD-deficient mice had decreased infarct size and reduced levels of necrotic cell death compared to control animals [25]. CypD is involved in necrotic cell death although its role in necroptosis has not been specifically tested. TNFα and zVAD.fmk treatment results in a mitochondrial defect in ADP transport through ANT, which is dependent on RIP1 [26]. However, there has been no further analysis of mitochondrial function in necroptotic cells.
A BH3-only protein, Bmf, was identified in a siRNA screen for genes involved in necroptosis [12]. Bmf-deficient thymocytes are still sensitive to death induced by most apoptotic stimuli [27]. Upon addition of specific apoptotic stimuli, Bmf is believed to dissociate from the cytoskeleton and translocate to the mitochondria and inhibit Bcl-2 cytoprotective proteins [28]. Bmf appears to induce cell death in a manner dependent on its BH3 domain. It remains to be shown what role Bmf plays in necroptosis.
Autophagy
Autophagic vesicles are commonly observed in necroptotic cells, leading some to propose that autophagy is an execution mechanism for necroptosis. In L929 cells, treatment with autophagy inhibitors 3-MA and wortmannin are able to partially inhibit zVAD-induced cell death. Knockdown of autophagy related genes such as beclin1 and Atg7 were shown to be able to inhibit necroptosis in L929 cells [29]. However, in other cell types this finding has not held up. Jurkat cells, MEFs, and Balb c/3T3 cells were extensively tested for an effect of autophagy inhibitors or knockdown of beclin1 and Atg5. None of these inhibitors of autophagy were able to reduce cell death [2].
Necroptosis signaling may in fact activate autophagy, perhaps as a clean-up mechanism for cell death. Experiments using proliferating T cells have shown that loss of FADD or caspase-8 increases cell death, which limits cell proliferation [30,31]. Addition of Nec-1 inhibits this phenotype, suggesting that RIP1 kinase is activated and the cells die, presumably by necroptosis [31]. In this case, it seems that what was thought to be type II autophagic cell death is actually necroptosis and induced by RIP1 kinase activation.
It remains to be determined if autophagy is actually a response to necroptotic cell death. ROS production is cell type dependent and unlikely to be a general execution mechanism. The involvement of mitochondria is potentially very interesting, but has not yet been thoroughly investigated.
Physiological Role of Necroptosis
Apoptosis plays a major role in physiological cell death, however, under pathological conditions, necrosis is very common [32]. Necroptosis has been implicated in mediating neuronal excitotoxicity. The RIP1 kinase inhibitor Nec-1 protects against glutamate-induced oxytosis in hippocampal HT-22 cells [33]. Furthermore, necroptosis is involved in NMDA-induced excitotoxicity in rat cortical neurons [34]. Excitotoxicity is involved in the pathologies associated with chronic neurodegeneration such as Alzheimer’s disease and Parkinson’s disease, and also in acute neurodegeneration such as that resulting from stroke [32]. Consistently, Nec-1 significantly decreases infarct size in a middle cerebral artery occlusion mouse stroke model [2].
Additional evidence suggests that necroptosis can occur in both the neuronal and immune systems. A murine genome wide siRNA screen for genes involved in necroptosis found clusters of hits that were upregulated in neuronal tissue and immune cells [12]. Activation of innate immune signaling through Toll-like receptor 3 (TLR3) in conjunction with IFNγ treatment can induce necroptosis. Importantly, necroptosis may act as a second line of defense against viral infections. Viruses encode caspase inhibitors like CrmA to block apoptosis, and a viral protein inhibitor of RIP1, M45, has been discovered that can block RIP1 signaling [35], suggesting regulation of necroptosis may be involved in virus-host interaction. RIP3−/− cells are protected against cell death after viral infection; however, these mice have a reduced survival rate compared to WT mice [5]. Necroptosis may function to stimulate the immune system in response to infection.
Conclusions
Necroptosis is an important cellular death mechanism likely to be involved in many human pathologies from viral infections to neurodegenerative diseases. Understanding the modifications and regulation of RIP1 is critical for us to understand how cells make the critical decision either to survive by activating NF-κB or to die through apoptosis or necroptosis. The availability of Nec-1 as a specific inhibitor of RIP1 kinase has made it possible to dissect the multiple functions of RIP1 in mediating the distinct downstream signaling pathway of cell survivals through NF-κB activation, and cell death through apoptosis and necroptosis. The recent genome-wide studies for genes involved in regulating necroptosis and discovery of RIP3 in necroptosis open exciting new avenues for research [5–7,12]. Compared to what is known about upstream signaling in necroptosis, the downstream mechanism of cell death is largely open to future exploration. We expect necroptosis will be an exciting area of rapid research development in the foreseeable future. Furthermore, neurostatins may provide an exciting opportunity for developing new treatments for multiple human diseases involving necrosis and inflammation.
Acknowledgments
We thank Ying Li for comments on the manuscript. This work was supported in part a NIH Director’s Pioneer Award and a grant from the National Institute on Aging (R37-AG012859) to J.Y.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Degterev A, Yuan J. Expansion and evolution of cell death programmes. Nat Rev Mol Cell Biol. 2008;9:378–390. doi: 10.1038/nrm2393. [DOI] [PubMed] [Google Scholar]
- 2*.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. Using a cell-based screen, the authors identified necrostatin-1 (Nec-1), a highly specific and potent inhibitor of necroptosis and demonstrated the role of necroptosis in mouse model of stroke. [DOI] [PubMed] [Google Scholar]
- 3*.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. The authors demonstrated the requirement of RIP1 kinase activity in mediating this alternative necrotic cell death activated by death receptors. [DOI] [PubMed] [Google Scholar]
- 4*.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. The authors demonstrated that necrostatins directly target RIP1 kinase activity to inhibit necroptosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5*.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. The authors demonstrated the role of RIP3 in mediating necroptosis and the interaction of RIP1 and RIP3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. The authors demonstrated the role of RIP3 in mediating necroptosis induced by Smac mimetic and TNFα. [DOI] [PubMed] [Google Scholar]
- 7*.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, Dong MQ, Han J. RIP3, an Energy Metabolism Regulator that Switches TNF-Induced Cell Death from Apoptosis to Necrosis. Science. 2009 doi: 10.1126/science.1172308. The authors demonstrated the role of RIP3 in mediating necroptosis and the interaction of RIP3 with metabolic regulators. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptor-interacting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 9*.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. The authors defined two sequential complexes, complex I and complex II, after activating TNFR1 to mediate apoptosis. [DOI] [PubMed] [Google Scholar]
- 10.Declercq W, Vanden Berghe T, Vandenabeele P. RIP kinases at the crossroads of cell death and survival. Cell. 2009;138:229–232. doi: 10.1016/j.cell.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 12.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Festjens N, Vanden Berghe T, Cornelis S, Vandenabeele P. RIP1, a kinase on the crossroads of a cell’s decision to live or die. Cell Death Differ. 2007;14:400–410. doi: 10.1038/sj.cdd.4402085. [DOI] [PubMed] [Google Scholar]
- 14.Park SM, Yoon JB, Lee TH. Receptor interacting protein is ubiquitinated by cellular inhibitor of apoptosis proteins (c-IAP1 and c-IAP2) in vitro. FEBS Lett. 2004;566:151–156. doi: 10.1016/j.febslet.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NF-kappaB activation. J Biol Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 17.Mollah S, Wertz IE, Phung Q, Arnott D, Dixit VM, Lill JR. Targeted mass spectrometric strategy for global mapping of ubiquitination on proteins. Rapid Commun Mass Spectrom. 2007;21:3357–3364. doi: 10.1002/rcm.3227. [DOI] [PubMed] [Google Scholar]
- 18*.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. The authors demonstrated the mechanism by which ubiquitination of K377 residue of RIP1 in mediating the activation of NF-κB by binding to NEMO. [DOI] [PubMed] [Google Scholar]
- 19*.Newton K, Matsumoto ML, Wertz IE, Kirkpatrick DS, Lill JR, Tan J, Dugger D, Gordon N, Sidhu SS, Fellouse FA, et al. Ubiquitin chain editing revealed by polyubiquitin linkage-specific antibodies. Cell. 2008;134:668–678. doi: 10.1016/j.cell.2008.07.039. The authors generated anti-K63 ubiquitin and anti-K48 ubiquitin specific antibodies to demonstrate the switch of ubiquitin chains during TNFR1 signaling. [DOI] [PubMed] [Google Scholar]
- 20.Goossens V, De Vos K, Vercammen D, Steemans M, Vancompernolle K, Fiers W, Vandenabeele P, Grooten J. Redox regulation of TNF signaling. Biofactors. 1999;10:145–156. doi: 10.1002/biof.5520100210. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 22.Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 23.Kim YS, Morgan MJ, Choksi S, Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell. 2007;26:675–687. doi: 10.1016/j.molcel.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Yazdanpanah B, Wiegmann K, Tchikov V, Krut O, Pongratz C, Schramm M, Kleinridders A, Wunderlich T, Kashkar H, Utermohlen O, et al. Riboflavin kinase couples TNF receptor 1 to NADPH oxidase. Nature. 2009;460:1159–1163. doi: 10.1038/nature08206. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Shimizu S, Watanabe T, Yamaguchi O, Otsu K, Yamagata H, Inohara H, Kubo T, Tsujimoto Y. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 26.Temkin V, Huang Q, Liu H, Osada H, Pope RM. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol Cell Biol. 2006;26:2215–2225. doi: 10.1128/MCB.26.6.2215-2225.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Labi V, Erlacher M, Kiessling S, Manzl C, Frenzel A, O’Reilly L, Strasser A, Villunger A. Loss of the BH3-only protein Bmf impairs B cell homeostasis and accelerates gamma irradiation-induced thymic lymphoma development. J Exp Med. 2008;205:641–655. doi: 10.1084/jem.20071658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puthalakath H, Villunger A, O’Reilly LA, Beaumont JG, Coultas L, Cheney RE, Huang DC, Strasser A. Bmf: a proapoptotic BH3-only protein regulated by interaction with the myosin V actin motor complex, activated by anoikis. Science. 2001;293:1829–1832. doi: 10.1126/science.1062257. [DOI] [PubMed] [Google Scholar]
- 29.Yu L, Alva A, Su H, Dutt P, Freundt E, Welsh S, Baehrecke EH, Lenardo MJ. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 30*.Ch’en IL, Beisner DR, Degterev A, Lynch C, Yuan J, Hoffmann A, Hedrick SM. Antigen-mediated T cell expansion regulated by parallel pathways of death. Proc Natl Acad Sci U S A. 2008;105:17463–17468. doi: 10.1073/pnas.0808043105. The authors demonstrated that antigen or mitogen activated Casp8-deficient T cells undergo necroptosis that can be inhibited by Nec-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell BD, Leverrier S, Weist BM, Newton RH, Arechiga AF, Luhrs KA, Morrissette NS, Walsh CM. FADD and caspase-8 control the outcome of autophagic signaling in proliferating T cells. Proc Natl Acad Sci U S A. 2008;105:16677–16682. doi: 10.1073/pnas.0808597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Artal-Sanz M, Tavernarakis N. Proteolytic mechanisms in necrotic cell death and neurodegeneration. FEBS Lett. 2005;579:3287–3296. doi: 10.1016/j.febslet.2005.03.052. [DOI] [PubMed] [Google Scholar]
- 33.Xu X, Chua CC, Kong J, Kostrzewa RM, Kumaraguru U, Hamdy RC, Chua BH. Necrostatin-1 protects against glutamate-induced glutathione depletion and caspase-independent cell death in HT-22 cells. J Neurochem. 2007;103:2004–2014. doi: 10.1111/j.1471-4159.2007.04884.x. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, Yang X, Ma C, Qiao J, Zhang C. Necroptosis contributes to the NMDA-induced excitotoxicity in rat’s cultured cortical neurons. Neurosci Lett. 2008;447:120–123. doi: 10.1016/j.neulet.2008.08.037. [DOI] [PubMed] [Google Scholar]
- 35*.Mack C, Sickmann A, Lembo D, Brune W. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc Natl Acad Sci U S A. 2008;105:3094–3099. doi: 10.1073/pnas.0800168105. The authors demonstrated that murine CMV virus encodes a protein M45 that binds to RIP1 and inhibits TNFα–induced activation of NF-κB, p38 MAPK, and caspase-independent cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]