Abstract
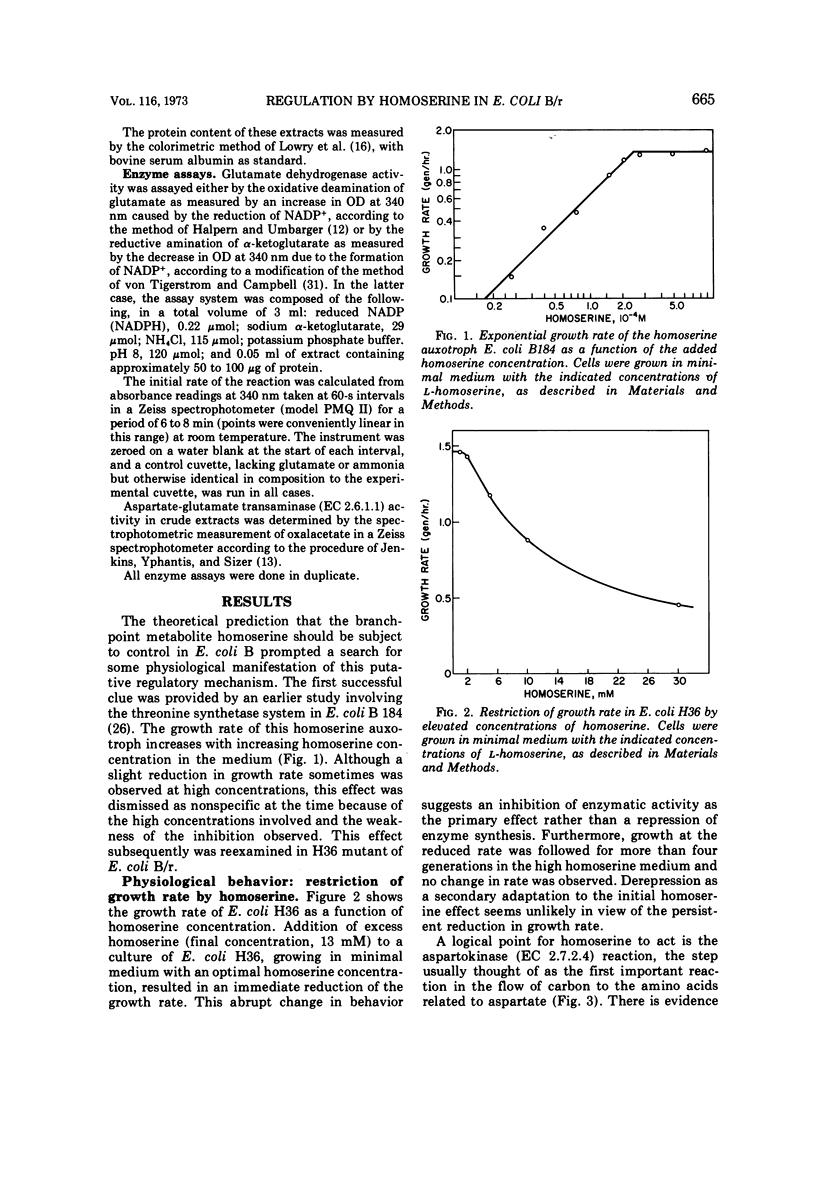
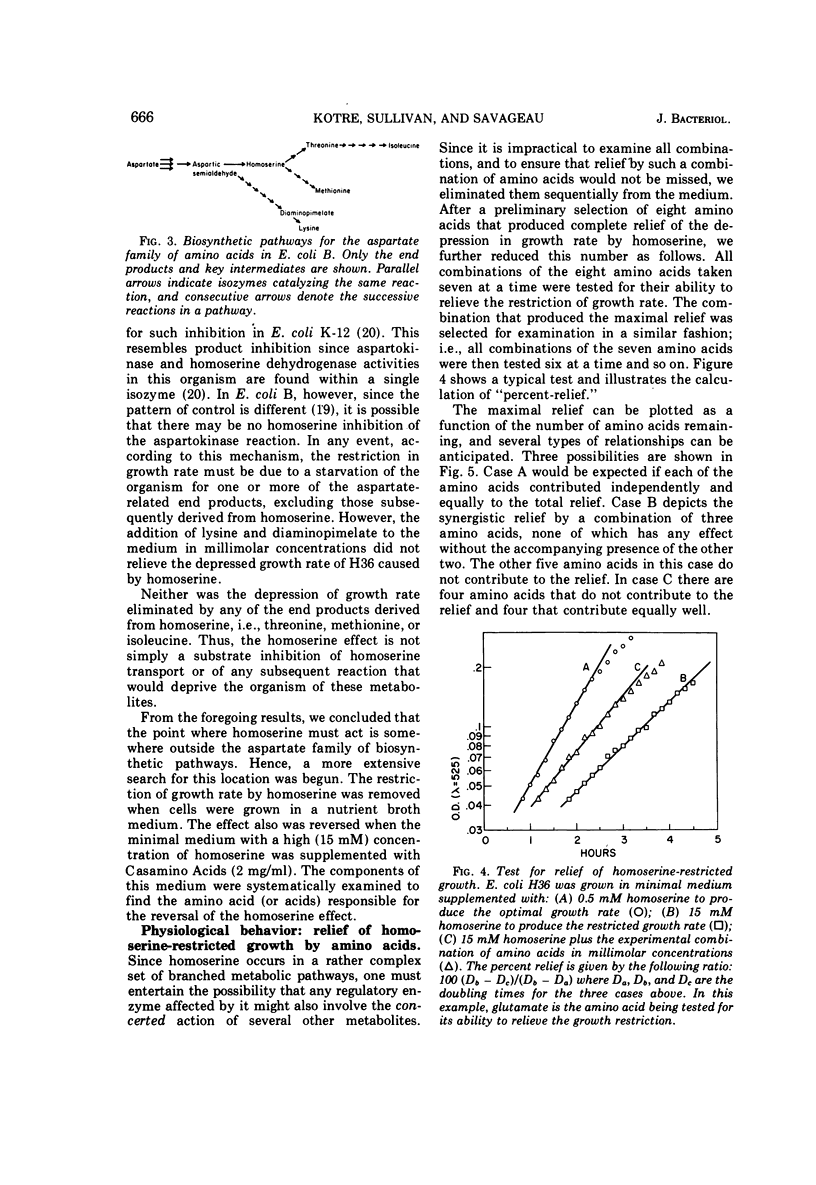
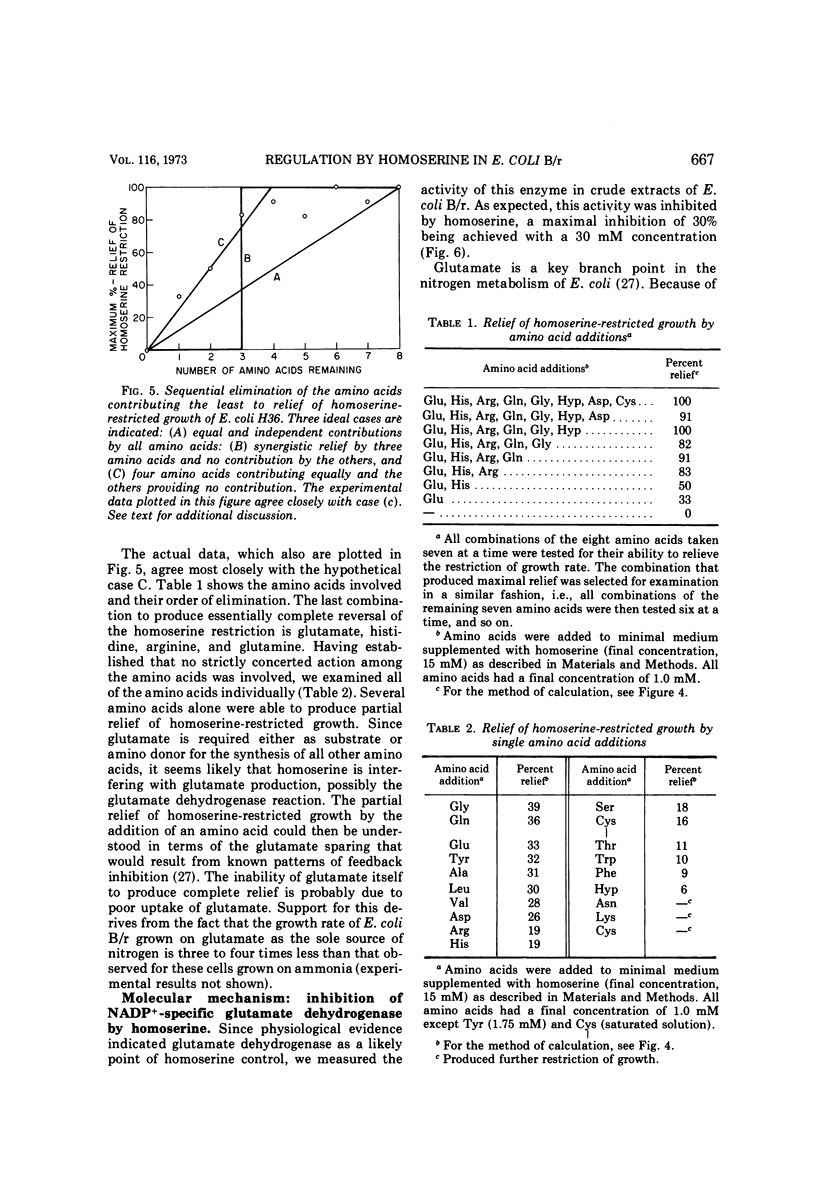
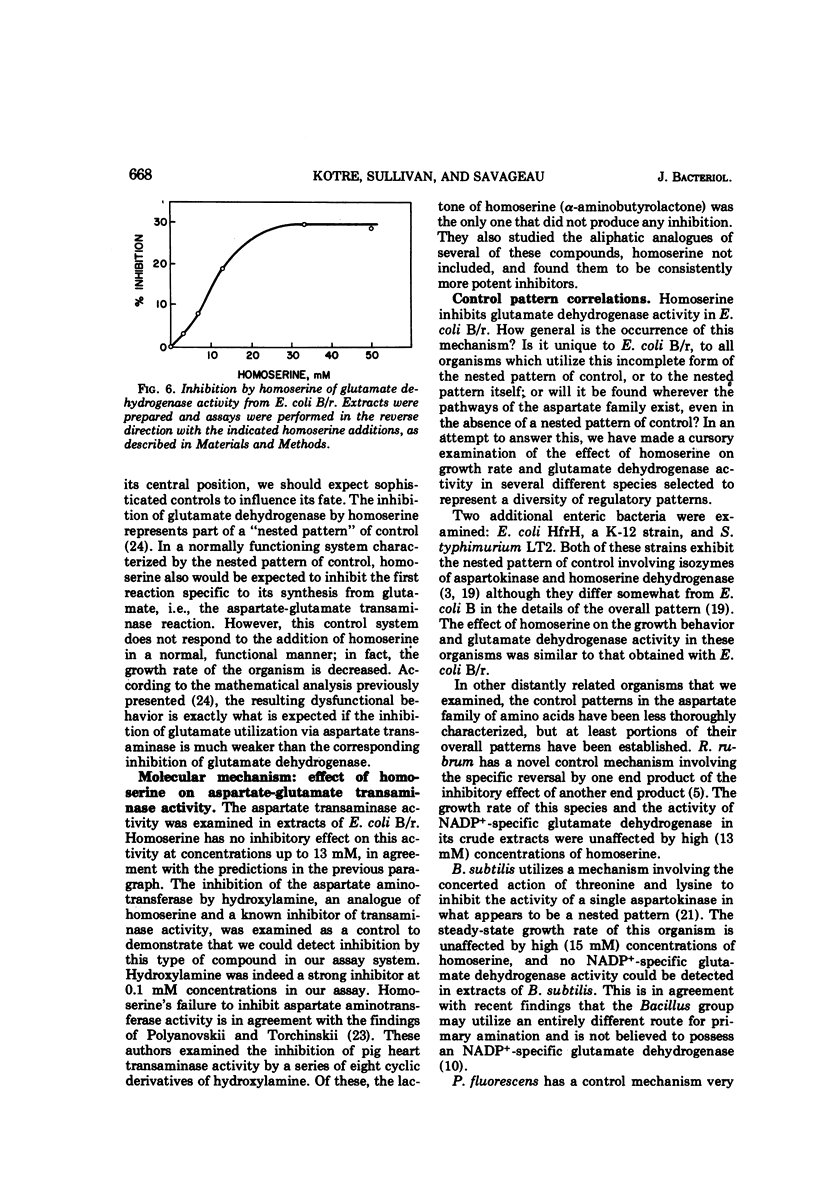
A mathematical analysis of branched pathway regulation has led to the prediction of a novel homoserine control in Escherichia coli B. Experimental support for such control is presented in this paper. Homoserine, the precursor of both threonine and methionine, inhibits nicotinamide adenine dinucleotide phosphate (NADP+)-specific glutamate dehydrogenase (EC 1.4.1.4), the enzyme catalyzing the first reaction in ammonia assimilation. Physiological and biochemical evidence for this effect are offered. Homoserine depresses the growth rate of the organism, and glutamate, the product of the inhibited reaction, reverses this effect. The NADP+-specific glutamate dehydrogenase activity in cell-free extracts is inhibited by homoserine, and this inhibition parallels the restriction of growth rate. These effects are found in other enteric bacteria which share a similar overall pattern of control for the amino acids derived from aspartate. On the other hand, a sampling of more distantly related species which have different pathways and/or regulatory patterns provides no evidence for homoserine inhibition of the glutamate dehydrogenase reaction.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRAUN W., GOODLOW R. J., KRAFT M., ALTENBERN R., MEAD D. The effects of metabolites upon interactions between variants in mixed Brucella abortus populations. J Bacteriol. 1951 Jul;62(1):45–52. doi: 10.1128/jb.62.1.45-52.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferata R. L., Freundlich M. Evidence for a methionine-controlled homoserine dehydrogenase in Salmonella typhimurium. J Bacteriol. 1969 Jan;97(1):193–198. doi: 10.1128/jb.97.1.193-198.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. N., Stanier R. Y., Le Bras G. Regulation of the biosynthesis of amino acids of the aspartate family in Coliform bacteria and Pseudomonads. J Bacteriol. 1969 Sep;99(3):791–801. doi: 10.1128/jb.99.3.791-801.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DATTA P., GEST H. HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. PURIFICATION, PROPERTIES, AND FEEDBACK CONTROL OF ACTIVITY. J Biol Chem. 1965 Jul;240:3023–3033. [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Prakash L. Aspartokinase of Rhodopseudomonas spheroides. Regulation of enzyme activity by aspartate beta-semialdehyde. J Biol Chem. 1966 Dec 25;241(24):5827–5835. [PubMed] [Google Scholar]
- Elmerich C., Aubert J. P. Synthesis of glutamate by a glutamine: 2-oxo-glutarate amidotransferase (NADP oxidoreductase) in Bacillus megaterium. Biochem Biophys Res Commun. 1971 Feb 5;42(3):371–376. doi: 10.1016/0006-291x(71)90380-9. [DOI] [PubMed] [Google Scholar]
- HALPERN Y. S., UMBARGER H. E. Conversion of ammonia to amino groups in Escherichia coli. J Bacteriol. 1960 Sep;80:285–288. doi: 10.1128/jb.80.3.285-288.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINS W. T., YPHANTIS D. A., SIZER I. W. Glutamic aspartic transaminase. I. Assay, purification, and general properties. J Biol Chem. 1959 Jan;234(1):51–57. [PubMed] [Google Scholar]
- Jensen R. A. Metabolic interlock. Regulatory interactions exerted between biochemical pathways. J Biol Chem. 1969 Jun 10;244(11):2816–2823. [PubMed] [Google Scholar]
- Kuramitsu H. K. Concerted feedback inhibition of aspartokinase from Bacillus stearothermophillus. Biochim Biophys Acta. 1968 Nov 19;167(3):643–645. doi: 10.1016/0005-2744(68)90064-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nester E. W. Cross pathway regulation: effect of histidine on the synthesis and activity of enzymes of aromatic acid biosynthesis in Bacillus subtilis. J Bacteriol. 1968 Nov;96(5):1649–1657. doi: 10.1128/jb.96.5.1649-1657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMEROD J. G., ORMEROD K. S., GEST H. Light-dependent utilization of organic compounds and photoproduction of molecular hydrogen by photosynthetic bacteria; relationships with nitrogen metabolism. Arch Biochem Biophys. 1961 Sep;94:449–463. doi: 10.1016/0003-9861(61)90073-x. [DOI] [PubMed] [Google Scholar]
- Patte J. C., Le Bras G., Cohen G. N. Regulation by methionine of the synthesis of a third aspartokinase and of a second homoserine dehydrogenase in Escherichia coli K 12. Biochim Biophys Acta. 1967 Mar 22;136(2):245–247. doi: 10.1016/0304-4165(67)90069-4. [DOI] [PubMed] [Google Scholar]
- Paulus H., Gray E. Multivalent feedback inhibition of aspartokinase in Bacillus polymyxa. I. Kinetic studies. J Biol Chem. 1967 Nov 10;242(21):4980–4986. [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Bernlohr R. W. Purification, properties, and regulation of glutamic dehydrogenase of Bacillus licheniformis. J Bacteriol. 1971 May;106(2):375–385. doi: 10.1128/jb.106.2.375-385.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savageau M. A., Kotre A. M., Sakamoto N. A possible role in the regulation of primary animation for a complex of glutamine: -ketoglutarate amidotransferase and glutamate dehydrogenase in Escherichia coli. Biochem Biophys Res Commun. 1972 Jul 11;48(1):41–47. doi: 10.1016/0006-291x(72)90341-5. [DOI] [PubMed] [Google Scholar]
- Savageau M. A., Steward J. P. Repression of the threonine synthetase system in Escherichia coli. Arch Biochem Biophys. 1970 Mar;137(1):181–184. doi: 10.1016/0003-9861(70)90425-x. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Shapiro B. M., Kingdon H. S., Woolfolk C. A., Hubbard J. S. Cellular regulation of glutamine synthetase activity in Escherichia coli. Adv Enzyme Regul. 1968;6:257–289. doi: 10.1016/0065-2571(68)90017-4. [DOI] [PubMed] [Google Scholar]
- Stahly D. P., Bernlohr R. W. Control of aspartokinase during development of Bacillus licheniformis. Biochim Biophys Acta. 1967;146(2):467–476. doi: 10.1016/0005-2744(67)90230-6. [DOI] [PubMed] [Google Scholar]
- Tempest D. W., Meers J. L., Brown C. M. Synthesis of glutamate in Aerobacter aerogenes by a hitherto unknown route. Biochem J. 1970 Apr;117(2):405–407. doi: 10.1042/bj1170405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varricchio F. Control of glutamate dehydrogenase synthesis in Escherichia coli. Biochim Biophys Acta. 1969 May 6;177(3):560–564. doi: 10.1016/0304-4165(69)90319-5. [DOI] [PubMed] [Google Scholar]
- Von Tigerstrom M., Campbell J. J. The accumulation of alpha-ketoglutarate by suspensions of Pseudomonas aeruginosa. Can J Microbiol. 1966 Oct;12(5):1005–1013. doi: 10.1139/m66-135. [DOI] [PubMed] [Google Scholar]


