Abstract
Rationale
In rats, neurotoxic doses of methamphetamine (MA) induce astrogliosis, long lasting monoamine reductions, reuptake transporter down-regulation, and learning impairments.
Objective
We tested whether comparable effects occur in C57BL/6 mice.
Method
C57BL/6 mice were treated with 10 mg/kg s.c. × 4 MA on a single day and evaluated at various intervals thereafter.
Results
The neurotoxic dose regimen of MA caused the predicted acute hyperthermia and increased striatal glial fibrillary acidic protein and reduced neostriatal dopamine. The MA-treated mice were hypoactive 24 h later but not 48 h later. MA-treated mice also showed exaggerated initial hyperactivity after a pharmacological dose of MA used to stimulate locomotion followed by a later phase of hypoactivity compared to saline-treated mice. No differences were observed on learning or memory tests (novel object recognition, egocentric, or spatial learning/memory). MA-treated mice showed a trend toward increased prepulse inhibition but not baseline acoustic startle reactivity. After testing, MA-treated mice showed reduced neostriatal dopamine and increased basal plasma corticosterone.
Conclusions
A neurotoxic/binge regimen of MA in mice that produces the typical pattern of neurotoxic changes to those seen in rats, results in few behavioral changes. This may limit the utility of C57BL/6 mice for modeling the cognitive and behavioral effects described in human MA users who show such changes even after prolonged abstinence.
Keywords: dopamine, serotonin, neostriatum, hyperthermia, locomotor activity, spatial learning, allocentric learning, acoustic startle, egocentric learning, neurotoxicity, GFAP, novel object recognition
1. Introduction
Chronic methamphetamine (MA) abuse results in evidence of neurotoxicity and compromised cognition [16]. Magnetic resonance imaging of chronic MA users shows increased globus pallidus and putamen and decreased hippocampal volume [17,71]. [1H]-Magnetic resonance spectroscopy of MA users reveal reduced N-acetylaspartate/creatine ratios in the anterior cingulate and reduced creatine in the basal ganglia [53,62,66]. Positron emission tomography and autopsy studies show reduced levels of striatal dopamine (DA), tyrosine hydroxylase (TH), dopamine transporter (DAT), and vesicular monoamine transporter type 2 (VMAT-2) [36,39,43,72,77]. Serotonin transporter (SERT) density is also reduced [67]. In chronic MA users, monoamine transporter changes correlate with cognitive/memory impairments [36,72], including impairments of recall, manipulation of information, verbal and non-verbal fluency, attention, and executive function [27,28,32,37,50,68].
Acute high-dose treatment of rats with MA results in a pattern of neurotoxicity resembling that described in human chronic MA abuse. For example, a so-called neurotoxic/binge dosing paradigm in rats results in hyperthermia [12,14,25,30], neostriatal reactive gliosis based on increased expression of glial fibrillary acidic protein (GFAP) [12,25,30], and microgliosis [41] based on increased expression of antibodies against the CD11b receptor. Hyperthermia, increased GFAP, argyrophilia by silver staining, and microgliosis are also seen in MA-treated mice given a neurotoxic dosing regimen [23,54,55,56,69,70]. Cell death (via increased TUNEL staining) in the striatum and hippocampus of mice following high dose MA has also been reported [19].
In rats, a neurotoxic MA treatment regimen also causes DA and 5-HT reductions in the striatum and 5-HT reductions in the hippocampus [11,12,15,25,30,57,75] with partial recovery over time [24]. Reductions have been observed in striatal DAT [63], TH activity [40], and VMAT-2 [22], as well as hippocampal reductions in SERT [63] and tryptophan hydroxylase activity [40]. Similarly in mice, a neurotoxic dosing regimen of MA causes reductions in TH, DA, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), and DAT binding in striatum [35,46] and 5-HT, DA, and HVA reductions in forebrain regions [23].
Binge/neurotoxic MA treatment regimens in rats are reported to also affect behavior. For example, a MA dosing regimen that caused DA, 5-HT, and/or DAT reductions and/or GFAP increases resulted in impaired novel object recognition and egocentric learning, but only minor reductions in locomotor activity [30,42,75]. In terms of spatial learning, either no [30,63] or very small transient deficits [24] are reported.
Much less is known about behavioral effects in mice following a binge/neurotoxic regimen of MA. MA-induced dopaminergic reductions are associated with impaired conditioned place preference to cocaine and MA in Swiss Webster mice; responses which were ameliorated by N-acetylcysteine treatment [1,2]. Otherwise, there are no studies in mice of the behavioral consequences of neurotoxic/monoamine-depleting dosing regimens of MA. For example, there are no experiments examining the effects of a binge/neurotoxic dosing regimen on spatial, egocentric, or novel object learning in mice, or on the acoustic startle response (ASR), and/or sensory gating using prepulse inhibition (PPI). Nor could experiments be identified of this kind in mice on locomotor activity in response to an acute locomotor-stimulating challenge dose of MA after prior treatment with a neurotoxic dosing regimen of MA.
The objective of the present experiment was to determine whether a binge/neurotoxic regimen of MA given to C57BL/6 mice produces patterns of behavioral changes similar to those in rats, since mice offer advantages in terms of genetic manipulations that might be useful in testing future mechanistic hypotheses if the mouse is similarly sensitive. Our model was the well-established mouse model of O’Callaghan and Miller [55,56,69,70].
2. Materials and Methods
2.1 General Methods
2.1.1 Subjects and Conditions
Adult male C57BL/6N (Crl) mice (~60 days of age) were obtained from Charles River Laboratories (Raleigh, NC) and singly housed in polycarbonate cages for 6 days prior to experimentation. Mice were maintained on a 14 h light:10 h dark (lights on at 600 h) schedule in a vivarium with food and water freely available (except during drug treatment). Protocols were approved by the Institutional Animal Care and Use Committee. The vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.1.2 Treatments
It is well established that binge/neurotoxic MA treatment regimens induce hyperthermia and may cause death if body temperature is not controlled appropriately, as MA-induced neurotoxicity occurs only in a narrow range of doses in rodent models [44,45]. Accordingly, three days prior to treatment, mice were briefly anesthetized using isoflurane and were implanted with subcutaneous temperature transponders (IPTT-300: Bio Medic Data Systems, Seaford, DE) in the dorsum. Temperature was recorded every 30 min during treatment until 2 h following the last dose and again 12 h later. Body weights were recorded prior to dosing, 12 h, 3 days, and 1, 2, 3, 4 and 5 weeks post-treatment. Mice were randomly assigned to two groups: methamphetamine or saline (SAL) control. Animals were administered (+)-methamphetamine HCl (expressed as the freebase and > 95% pure, National Institute on Drug Abuse, Bethesda, MD) at a dose of 10 mg/kg in a volume of 10 ml/kg or an equal volume of saline. All animals were treated 4 times during a 6 h dosing period with 2 h intervals between doses. This dosing regimen has been widely used in mice (and rats) and shown to induce GFAP increases and dopamine reductions in the striatum [e.g.,[46,55,56]]. Injections were administered subcutaneously. During treatment, animals were maintained in separate cages at an ambient room temperature of 23 ± 1 °C in a room separate from the home room. To ensure that MA-induced hyperthermia did not exceed life-threatening levels, body temperatures were monitored frequently and the experimenter was prepared to cool any animal in a shallow water bath whose temperature exceeded 40.0°C. However, in this experiment with mice (unlike with rats) no animal’s body temperature exceeded this level. After treatment, animals were placed in one of three tracks described below.
2.2 Experiment 1
2.2.1 GFAP
GFAP in neostriatum was analyzed by ELISA as described previously with modification [55] as follows: neostriatal tissue was frozen on dry ice and stored at −80°C until assayed; tissue was homogenized in 250 µl NP-40 buffer (50mM Tris, 150mM NaCl, 1% NP-40) using a Power Gen 125 homogenizer (Fisher Scientific). Samples were centrifuged for 5 min at 12,000 RCF at 4°C and supernatant was collected. The block solution used was 100 µl/well 1× PBS, 1× casein, 0.1% Triton X-100. Mouse monoclonal anti-GFAP (IgG fraction) was 1:1 in blocking buffer (Abcam, Cambridge, MA). A group of animals consisting of 21 mice (8 SAL and 13 MA) were treated as above and sacrificed 72 h later. Mice were taken to an adjacent suite, decapitated and neostriata dissected over ice with the aid of a brain block (Zivic-Miller, Pittsburgh, PA). The brain was sliced coronally at the optic chiasm and again 2 mm rostral and the neostriatum bilaterally dissected from this section. Protein concentrations were determined using the Bradford method (Pierce Biotechnology, Rockford, IL) and 250 µg of total protein was used for each sample in the GFAP ELISA. The standard curve was developed using serial dilutions of GFAP protein (American Research Products, Inc., Belmont, MA). Samples were analyzed at 405 nm on a Spectramax microtiter plate reader.
2.3 Experiment 2-Behavior, Monoamines and Corticosterone
2.3.1 Locomotor Activity
A separate group of identically treated animals was used for experiment 2. On the first and second days following treatment animals were placed in locomotor chambers (Accuscan Instruments, Columbus, OH) and movement recorded. Subjects remained in the chamber for 1 h and data were collected every 5 min. The numbers of vertical and horizontal movements as well as the amount of time spent in the center or periphery of the chamber were analyzed. Chambers were cleaned with 70% ethanol between animals.
2.3.2 Novel Object Recognition
This task was performed 7–11 days post treatment. Subjects were placed in circular, polyethylene arenas (91 cm in diameter with 51 cm high walls) and allowed to habituate to the chamber for 10 min/day for 4 days prior to testing; chambers were cleaned with 70% ethanol between trials. On the fifth day, object recognition was tested in two phases. In the familiarization phase, two identical objects were placed along a line bisecting the diameter, 41 cm apart and 25 cm from the sides of the arena. Mice were placed in the center of the arena and allowed to explore until 30 s of combined object exploration time was accumulated. Exploration of objects was scored using a video camera above the arena. If an animal did not accumulate 30 s of exploration time within 10 min, it was removed and tested again using a different set of objects the following day. Twelve of 38 tested mice needed to be retested of which 6 failed the retest and were not included in the data analyses. Object exploration was scored when the animal was oriented toward and within 1 cm of the object but not climbing on the object. Following familiarization, animals were removed from the arena and placed back in their home cage for 1 h. In the retention phase, animals were placed back in the arena and presented with a new object and an identical copy of the original object. As before, animals were allowed to accumulate 30 s of combined object time. Arenas and objects were cleaned with 70% ethanol between each trial.
2.3.3 Acoustic Startle/Pre-pulse Inhibition
ASR/PPI was tested 1–2 days after novel object (day 12–13 post drug administration) during the same time each day and was measured in an SR Lab apparatus (San Diego Instruments, San Diego, CA). Animals were placed in an acrylic cylindrical test chamber mounted on a platform with a piezoelectric force transducer attached to the underside of the platform. The platform was located inside a sound-attenuated chamber and a 5 min acclimation period preceded test trials. Animals were tested for a total of 12 min using a 4 × 4 Latin square sequence of trials that were of 4 types: no stimulus, startle stimulus with no pre-pulse, 74 dB pre-pulse, or 76 dB pre-pulse (background noise level was 64 dB). Each set of 16 trials was repeated 3 times for a total of 48 trials. Trials of the same type were averaged together and the intertrial interval (ITI) was 8 s. The startle signal was a 20 ms 110 dB SPL mixed frequency white noise burst and the recording window was 100 ms after signal onset. Prepulses preceded the startle-eliciting stimulus by 70 ms (from pre-pulse onset to startle signal onset). The apparatus was cleaned with 70% ethanol between animals.
2.3.4 Morris Water Maze (Cued Platform)
Morris water maze (MWM) cued trials began the day following ASR/PPI and were conducted for 6 days (14–19 days post-treatment). Subjects were placed in a 122 cm diameter tank of water (21 ± 1 °C) and tested for latency to reach a 10 cm diameter platform. The platform was submerged 1–1.5 cm below the water and had an orange ball mounted on a brass rod above the surface to mark its location. Curtains were closed around the tank to minimize distal cues. On the first day of testing, subjects were administered 6 trials from a fixed starting position to a fixed platform position (i.e., start = west; platform = east) with a 15 s ITI. On the remaining days, 2 trials/day were given and platform and starting positions were randomized. The ITI was 15–30 s in the home cage. Animals were allowed 1 min to find the platform and were placed on it for 5 s if they failed to find it before being placed in their home cage during the ITI. White tempura paint was added to the water to make it opaque and latencies were recorded. All mice performed the task.
2.3.5 Morris Water Maze (Hidden Platform)
There were three phases of the MWM hidden platform test (with curtains open). Acquisition: initial learning to find the hidden platform was conducted for 7 days (20–26 days post treatment). A 10 cm diameter platform was used and the platform was placed in the southwest quadrant and starting positions were pseudo-randomized. Animals were given 4 trials/day for 6 consecutive days with a maximum of 1 min to reach the platform with an ITI of 15 s on the platform. If an animal did not find the platform within 1 min, it was placed on it. Data were collected using video tracking software (Smart® software, San Diego Instruments). The platform was submerged 1–1.5 cm below the water (21 ± 1°C). The day after acquisition, mice were tested in reversal with a 7 cm diameter platform in the northeast quadrant (27–33 days post treatment). During the third, shift phase (34–40 days post treatment), the platform (5 cm in diameter) was moved to the northwest quadrant and the same procedure as for acquisition and reversal was followed. On day 7 of each phase, animals were given a single probe trial with the platform removed for 30 s.
2.3.6 Locomotor Activity with MA Challenge
Animals were placed in the previously described locomotor chambers 2 days following MWM testing (42–44 days post-treatment) and baseline activity was reestablished for 30 min. After 30 min, animals were removed, administered a single s.c. dose of 1 mg/kg (+)-MA (expressed as the freebase), and placed back in the chambers for an additional 120 min. Chambers were cleaned with 70% ethanol between animals.
2.3.7 Plasma and Tissue Collection
Animals were sacrificed by decapitation 3 days after behavioral testing (47 days post-treatment). Brains were removed and blood collected in polyethylene tubes containing 2% EDTA (0.05 ml), and stored on ice until centrifuged. Plasma was isolated from whole blood by centrifugation at 1300 × g for 25 min and the supernatant collected and stored at −80°C until assayed. Brains were removed and neostriata dissected over ice as described in Experiment 1. Brain tissue was frozen on dry ice and stored at −80°C until assayed.
2.3.8 Corticosterone Assessment
Corticosterone concentrations in plasma (taken 3 days following behavioral testing) were assayed with Octeia Corticosterone ELISA kits (IDS, Fountain Hills, AZ). Each sample was diluted 1:5 and assayed in duplicate according to the manufacturer’s protocol. The samples were measured on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA).
2.3.9 High Performance Liquid Chromatography
Neostriata were weighed and homogenized using a hand-held glass homogenizer in 50 volumes of 0.2 N perchloric acid. The homogenate was centrifuged for 5 min at 12000 × g, the supernatant collected and stored on ice, and 20 µl aliquots were injected into a C18-column (MD-150, 3×150mm; ESA, Chelmsford, MA). The column was connected to a Coulochem II (25 A, Chelmsford, MA) detector and an integrator recorded the heights of each peak after injection. The mobile phase consisted of 35 mM citric acid, 54 mM sodium acetate, 50 mg/L of disodium ethylenedeamine tetraacetate, 70 mg/l of octanesulfonic acid sodium salt, 6% v/v methanol, and 6% v/v acetonitrile, with a final pH of 4.0. The flow rate was 0.4 ml/min and quantities of each sample were calculated from standard curves. DOPAC, DA, 5-HIAA, and 5-HT had retention times of approximately 6, 8, 11, and 17 min.
2.4 Experiment 3
2.4.1 Egocentric Learning
A separate group of mice identically treated to those in Experiments 1 and 2 was used for Experiment 3. The Cincinnati water maze (CWM) is a multiple T-maze used to test egocentric learning. Testing began 20 days post-treatment (similar to the start of hidden platform testing in the MWM in Experiment 2) and lasted for 15 days. Mice were tested in the cued version of the Morris water maze as above prior to CMW testing (days 14–19 post treatment) in order to acclimate the mice to swimming, teach them that escape was possible, and obtain a measure of swimming speed. The CWM consists of a series of nine black Ts that branch from a central circuitous channel. For mice, the maze was scaled-down from the previously described rat version [73] such that the width of the Ts and channel were ~50% narrower, i.e., 8 cm for mice, rather than 15 cm as used for rats, with walls 25 cm high filled half-way with water. The maze was fabricated of high density 0.6 cm black polyethylene (AB Plastics, Cincinnati, OH). Water was drained and refilled daily and allowed to equilibrate overnight to room temperature (21 ± 1 °C). The task was performed under infrared light with a CCD camera mounted above the maze and attached to a closed circuit TV monitor situated in an adjacent room. Groups of 8–10 mice were brought in the maze room and allowed at least 5 min of dark adaptation before testing. Two trials were given per day for 15 consecutive days with a maximum of 5 min/trial. All animals completed trial 1 before beginning trial 2; hence the ITI was15–120 min depending on how quickly the animals completed the first trial of the day. Latency to reach the escape platform and number of errors were recorded. An error was committed upon entry into a cul-de-sac and was scored whenever an animal crossed into either arm of a “T”. Animals that did not complete the task within 5 min were given an error score equal to the maximum number of errors committed by the worst performing animal + 1 in order to correct for animals that stopped searching and treaded water. The maximum error score was 47.
2.4.2 Statistics
Data were analyzed using SAS statistical analysis software (SAS Institute, Cary, NC). Weekly body weights were analyzed using general linear model analysis of variance (ANOVA) with week as a repeated measure factor. Locomotor activity was similarly analyzed (treatment × day × interval). T-tests for independent samples (2-tailed) were used to analyze corticosterone concentrations. Mixed linear model ANOVAs were used to analyze acoustic startle with a supplemental analysis for changes in PPI by analysis of covariance (ANCOVA) with no-prepulse trials as the covariate. For novel object recognition, t-tests for independent samples were used to analyze percent time spent with the novel object. Mixed linear ANOVA was used to analyze locomotor activity after MA challenge and ANCOVA to adjust for pre-challenge baseline activity using the last 10 min of the baseline as the covariate. Mixed linear ANOVAs were also used to analyze Morris and Cincinnati water maze data (treatment × day). Kenward-Roger adjusted degrees of freedom were used in these analyses; these do not match those used in standard ANOVAs and can be fractional. Significant effects were set at p ≤ 0.05.
3 Results
3.1 Body Weight and Temperature
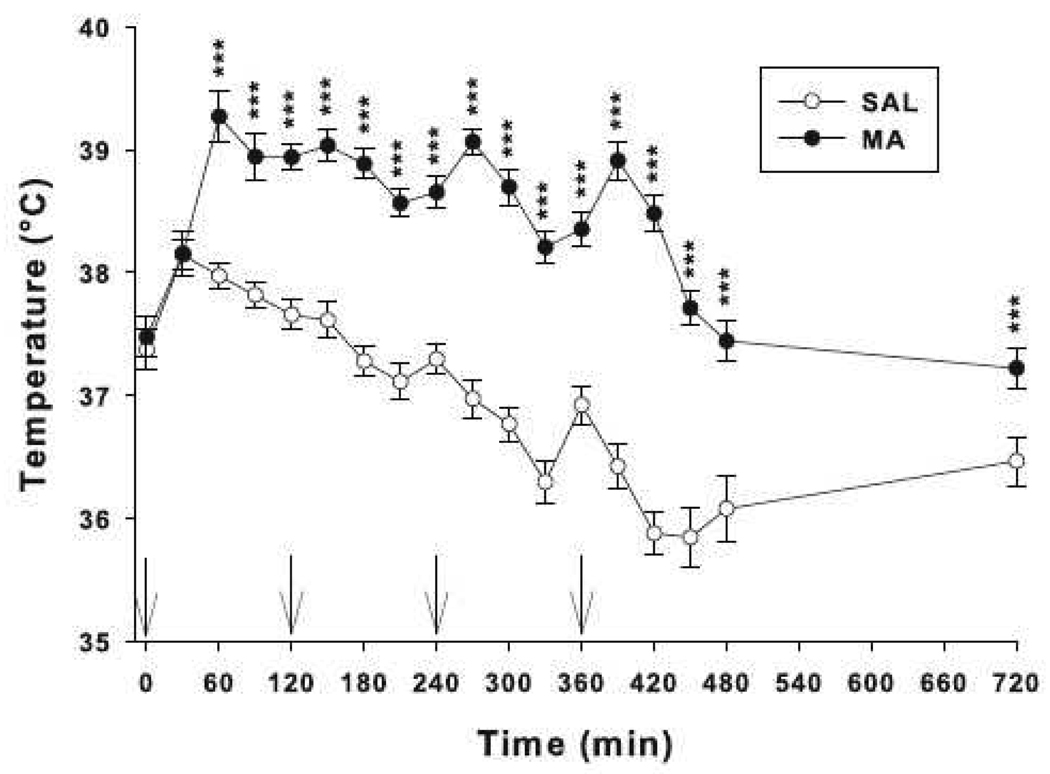
There were no significant effects of treatment on body weight between SAL and MA-treated animals during or after dosing (not shown). For body temperature, there were significant effects of treatment and treatment × time. The treatment × time interaction showed that animals treated with MA had higher body temperatures beginning 60 min after the first dose and continuing throughout the treatment period and lasting until 12 h later compared to SAL controls (Fig. 1).
Fig. 1.
Core body temperatures of mice treated with MA (10 mg/kg) or SAL 4 times on a single day. Beginning 60 min and lasting for at least 12 h after the first dose, animals treated with MA showed increased core body temperatures compared to SAL controls. Arrows indicate when injections were given. There were main effects of treatment, F(1, 114) = 60.01, p<0.0001, time, F(16, 1824) = 87.01, p<0.0001, and treatment × time, F(16, 1824) = 40.49, p<0.0001. ***p < 0.001
3.2 Experiment 1
3.2.1 GFAP
Neostriatal astrogliosis 72 h post drug-treatment was significantly increased in MA-treated animals as measured by GFAP compared to SAL controls, t(19) = 6.66, p< 0.0001. Expressed as percent control, MA animals had increased GFAP levels that were 538% of SAL animals. This was higher than that previously observed (~ 300 %) [55] with the difference likely attributable to the difference in antibody used and other assay details.
3.3 Experiment 2
3.3.1 Locomotor Activity
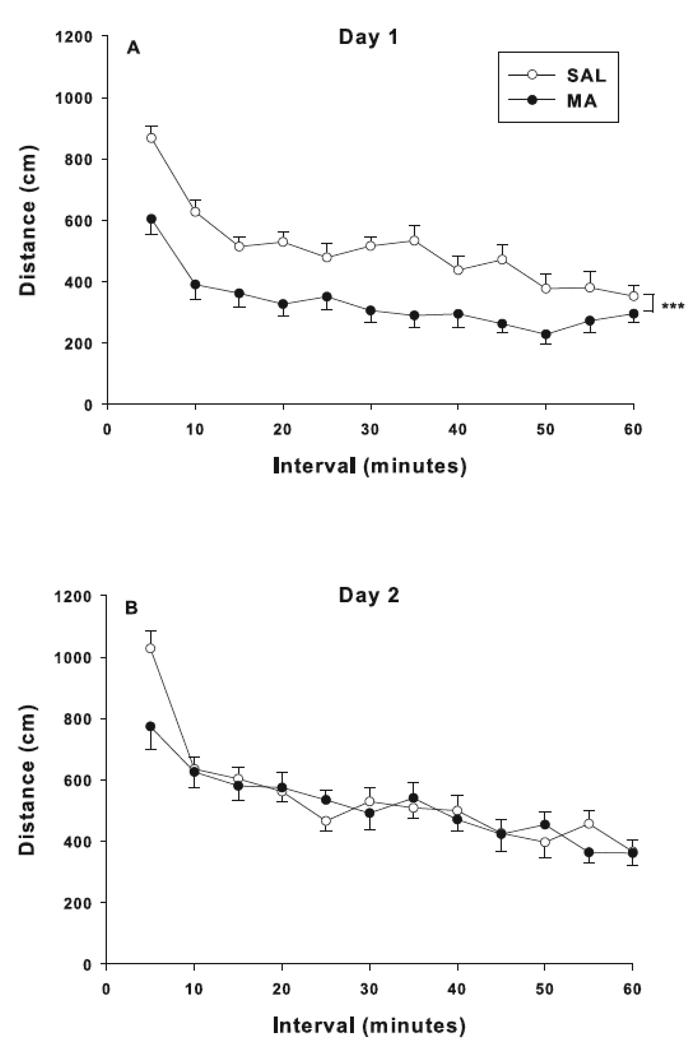
Measures analyzed for locomotor activity were distance traveled (total, center, and peripheral), vertical (rears), and repetitive-beam breaks (focused activity). For total distance, overall effects of treatment and treatment × day were observed (Fig. 2). The treatment × day interaction was attributable to hypoactivity in MA animals on day 1 (Fig. 2A, p<0.0001) with no effect on day 2 (Fig. 2B) compared to SAL animals. For center distance on day-1, there was an effect of treatment, interval, and treatment × interval. The treatment × interval effect was attributable to decreased center distance in MA-treated animals compared to SAL-treated animals on day-1, similar to what was found for total distance (not shown). On the second day of testing, there was a main effect of interval for center distance only. For peripheral distance, there were effects of treatment and treatment × day, F(1, 36) = 12.88, p<0.001. The treatment × day interaction showed that MA-treated animals on day-1 were less active than SAL-treated animals (p<0.0001; not shown). No other effects were observed for peripheral distance. There were no effects obtained for rearing or focused activity.
Fig. 2.
Locomotor activity: Locomotion was measured as distance traveled on day 1 (A) and day 2 (B) after treatment. Main effects of treatment, F(1, 36) = 7.00, p<0.05, day, F(1, 36) = 29.58, p<0.0001, and treatment × day, F(1, 36) = 13.35, p<0.001, were observed for total distance. The treatment × day interaction showed that animals treated with MA were hypoactive on day 1. Effects of treatment, F(1, 36) = 4.08, p<0.05, interval, F(11, 396) = 8.12, p<0.0001, and treatment × interval, F(11, 396) = 3.32, p< 0.001, were observed for center distance on day 1. For the treatment × interval interaction, decreased center distance was observed in MA-treated animals compared to SAL-treated animals (not shown). There was a main effect of interval for center distance on day 2, F(11, 396) = 6.48, p< 0.0001, only. Effects of treatment, F(1, 36) = 10.21, p<0.01, day, F(1, 36) = 56.26, p<0.0001, and treatment × day, F(1, 36) = 12.88, p<0.001, were observed for peripheral distance. The treatment × day interaction showed that MA-treated animals on day-1 were less active than SAL-treated animals (p<0.0001; not shown). ***p< 0.001
3.3.2 Novel Object Recognition
There were no differences in time spent investigating the two identical objects during familiarization. During retention, both SAL and MA-treated animals showed preference for the novel object. There were no significant differences observed for novel object preference as a function of treatment expressed as percent time observing the novel object (mean ± SEM; SAL = 74.5 ± 4.6% and MA = 73.1 ± 2.6%).
3.3.3 Acoustic Startle Reactivity/PPI
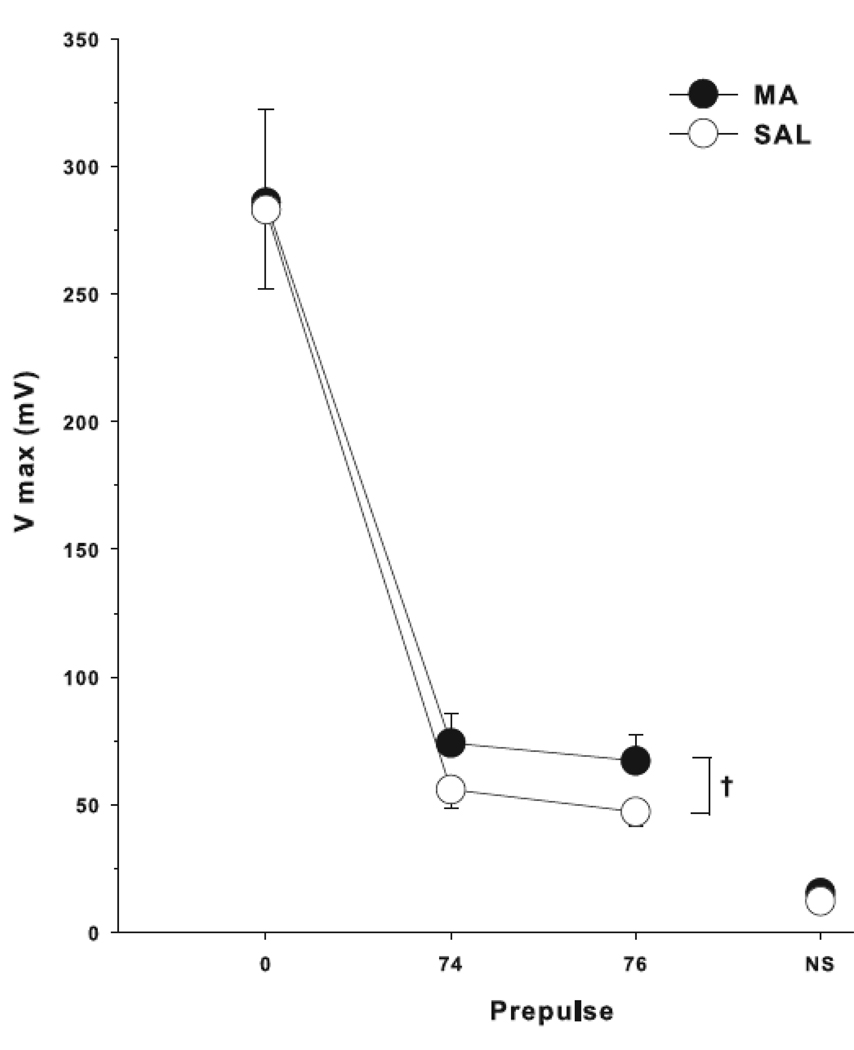
An ANOVA showed that both groups displayed prepulse inhibition (main effect of prepulse), but there was no significant main effect of drug treatment or interaction of drug treatment × prepulse. An ANCOVA with baseline startle as the covariate showed a trend toward an interaction between treatment group and prepulse (p < 0.06), with decreased PPI in the MA group compared to SAL (Fig. 3),
Fig. 3.
Acoustic startle/pre-pulse inhibition: Mice treated with 10 mg/kg MA or SAL all showed prepulse inhibition as a function of prepulse intensity but no significant group differences were obtained. A trend toward a treatment × prepulse interaction was observed, F(1, 35) = 3.94, p< 0.06. NS = no stimulus, †p< 0.06
3.3.4 Morris Water Maze
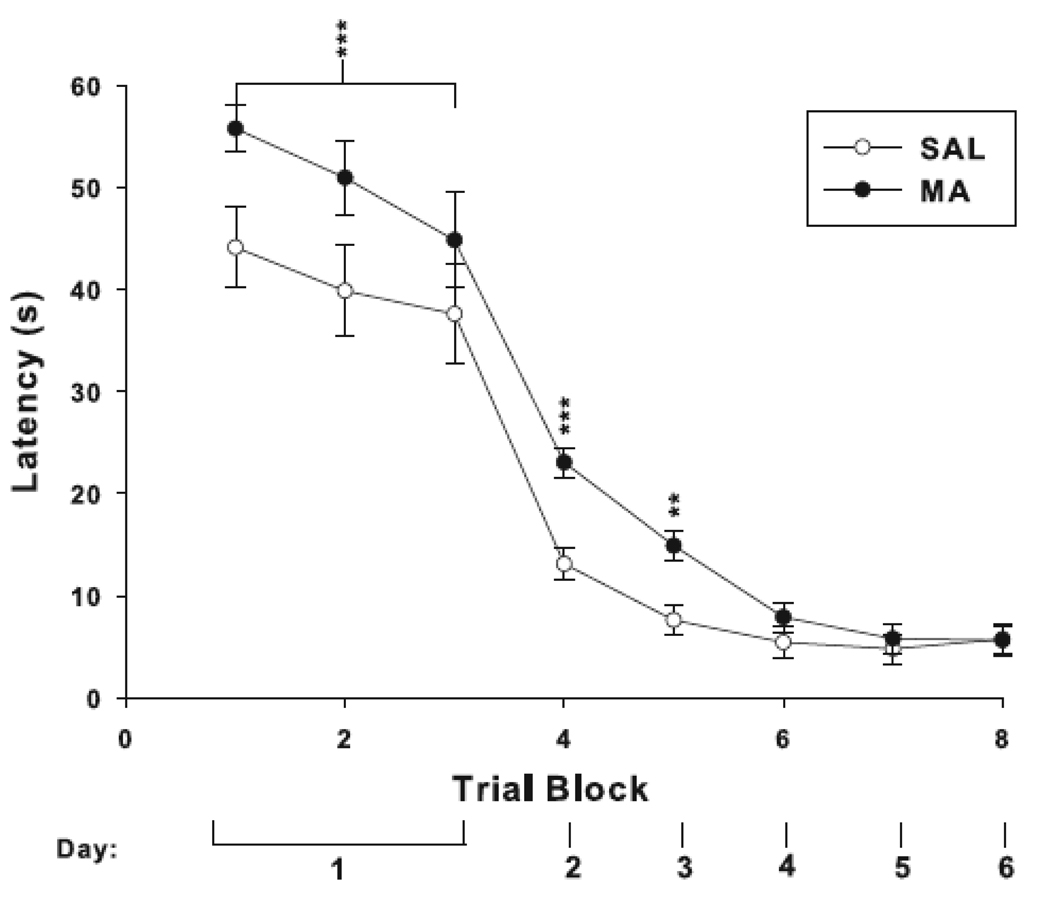
The six trials of cued with the platform and start in a constant location were analyzed in 2-trial blocks. There was an effect of treatment on latency, in which MA-treated animals took longer to reach the platform than SAL-treated controls. Days 2–6 (2 trials/day with the start and platform positions randomized) also showed a treatment effect for latency, day, and treatment × day. Similar to day-1 performance, the treatment × day interaction was attributable to increased latency to reach the platform in MA-treated animals on day 2 and 3 with no differences from SAL animals on days 4–6 (Fig. 4).
Fig. 4.
Morris water maze cued platform trials. Animals administered MA had increased latencies to reach the platform on day 1, F(1, 36) = 4.26, p<0.05. On the remaining days, there were main effects of treatment, F(1, 36) = 12.27, p<0.01, day, F(4, 144) = 31.80, p<0.0001, and treatment × day F(4, 144) = 5.05, p<0.001, for latency. The treatment × day interaction was due to increased latency in MA-treated animals on day 2 (p<0.0001) and 3 (p< 0.001). On days 4–6, latencies were similar to controls. **p< 0.01, ***p< 0.001
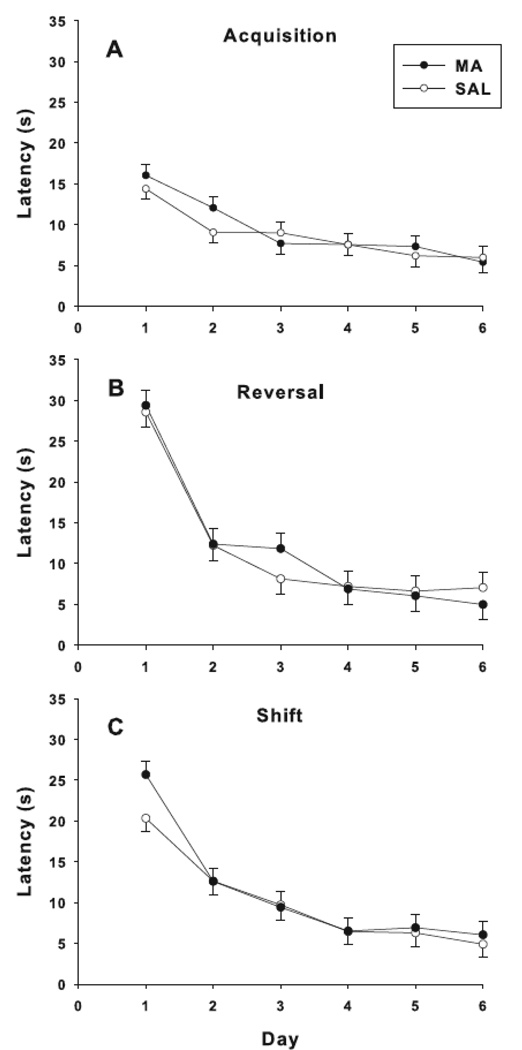
In all three phases of hidden platform testing (acquisition, reversal, and shift), no treatment main effects or treatment-related interactions were found on latency, path length, or cumulative distance. Learning was demonstrated during all phases (Fig. 5). Similarly, no treatment effects were obtained on probe trials given after acquisition, reversal, or shift. Analyses of swim speed during all phases showed no treatment or interaction effects.
Fig. 5.
Morris water maze hidden platform trials. Learning curves for latency to reach the platform during each phase of testing: acquisition (A), reversal (B), and shift (C). No differences were observed between treatment groups in any phase of the test.
3.3.5 Locomotor Activity with Methamphetamine Challenge
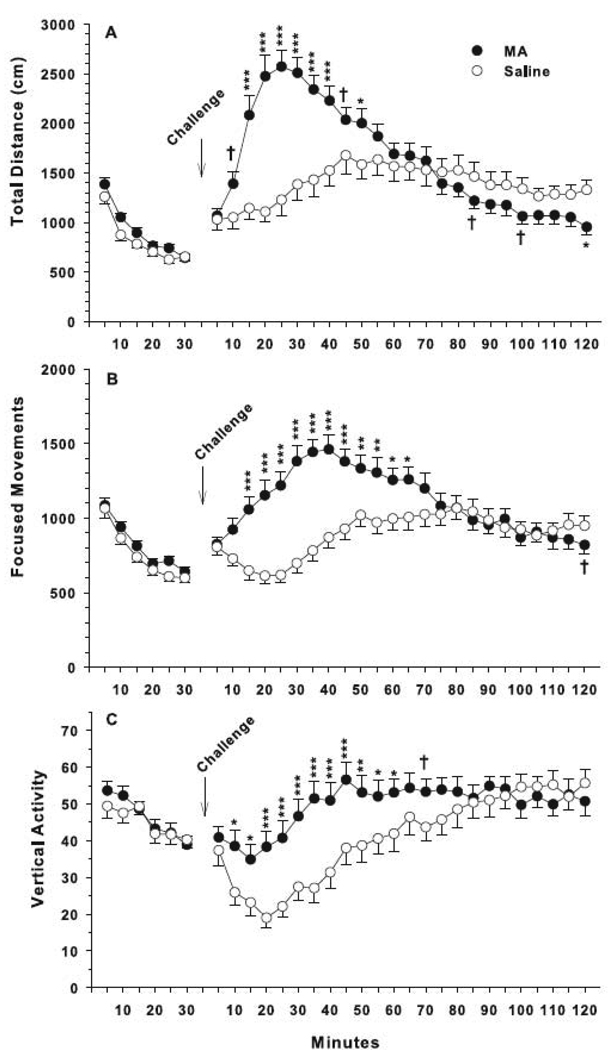
For locomotor activity with MA challenge, total distance showed a main effect of interval and treatment × interval, but no main effect of treatment. The interaction showed greater hyperactivity following MA-challenge in the MA-treated group compared to that seen in SAL-treated animals from 15 to 50 min post-challenge and reduced activity compared to SAL-treated animals 120 min post-challenge with trends at 85 and 100 min (Fig. 6A). For focused movements, there were effects of treatment, interval, and treatment × interval. The treatment × interval interaction was attributable to increased repetitive beam breaks in MA-treated animals challenged with MA 20–65 min post-challenge (Fig. 6B) compared to SAL-treated animals challenged with MA. For vertical activity, there were main effects of treatment, interval, and treatment × interval. The treatment × interval interaction showed that MA-treated animals responded to the MA challenge with a larger increase in vertical beam breaks from 10–60 min post-challenge compared to SAL-treated MA challenged animals (Fig. 6C).
Fig. 6.
Locomotor activity with MA challenge: The challenge (1 mg/kg MA) was administered following 30 min of baseline activity. Total distance showed a main effect of interval, F(23, 727) = 8.31, p<0.0001, and treatment × interval, F(23, 727) = 6.74, p<0.0001. The interaction showed both groups were initially hyperactive following drug challenge and then gradually showed reduced locomotion 85 min later (distance traveled (A)), although the MA-treated animals were even less active at 120 min compared to SAL-treated animals. Focused movements showed effects of treatment, F(1, 35) = 4.24, p<0.05, interval, F(23, 828) = 11.44, p<0.0001, and treatment × interval, F(23, 828) = 13.73, p<0.0001. The treatment × interval interaction showed MA-treated groups had increased focused movements (B) compared to controls that lasted approximately 60 min after acute MA challenge. For vertical activity (C), there were main effects of treatment, F(1, 35.2) = 4.30, p<0.05, interval, F(23, 748) = 7.53, p<0.0001, and treatment × interval, F(23, 748) = 2.73, p<0.0001. MA animals had increased vertical activity compared to controls. ***p< 0.001, **p< 0.01, *p< 0.05, †p< 0.06
3.3.6 Corticosterone
Basal corticosterone levels 3 days following behavioral testing (47 days following MA treatment) were found to be increased in MA-treated animals compared to SAL-treated animals, t(17) = −2.44, p< 0.03. The mean CORT values (ng/ml) were SAL = 20.0 ± 3.9 and MA = 37.0 ± 5.6.
3.3.7 Monoamines
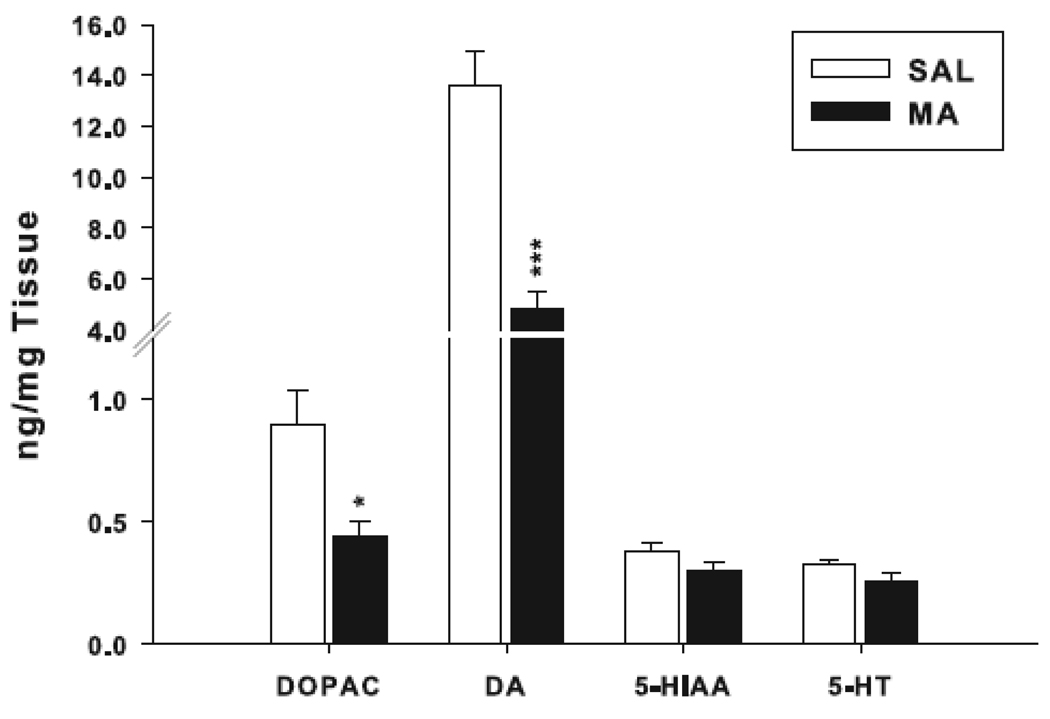
The same animals from which blood was taken for CORT were assayed for neostriatal monoamines. Reductions in DA and DOPAC were observed in MA-treated mice compared to SAL-treated mice (Fig. 7). Levels of 5-HT and 5-HIAA did not differ significantly (Fig. 7).
Fig. 7.
Neostriatal monoamine levels: Monoamine concentrations in SAL- and MA-treated animals 3 days following behavioral experiments (day 47). MA caused reductions in DA (t(18) = 6.08, p< 0.0001) and its primary metabolite, DOPAC, (t(18) = 2.97, p< 0.01), compared to SAL controls. 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) were unaffected. *p< 0.01, ***p< 0.001
3.4 Experiment 3
3.4.1 Egocentric Learning
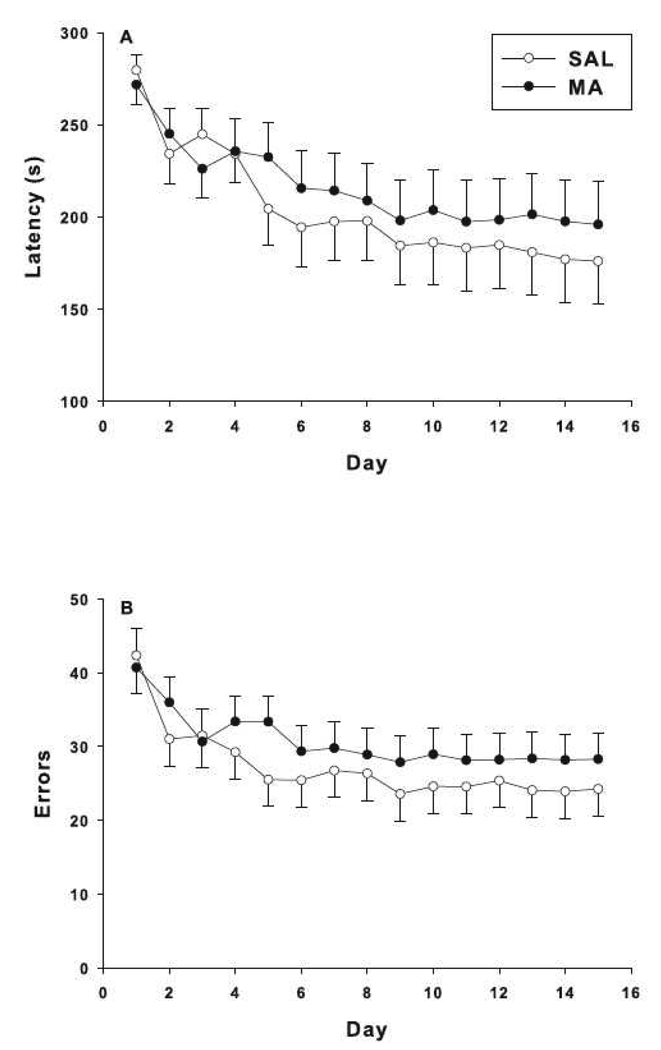
Latency to escape from the CWM showed a main effect of day, but no effect of treatment or treatment × day interaction (Fig. 8A). For errors, there was a main effect of day and a treatment × day interaction, but no main effect of treatment. Further analysis of the treatment × day interaction showed the interaction did not reach significance by slice ANOVA for any day (Fig. 8B).
Fig. 8.
Cincinnati water maze: Egocentric learning was assessed by latency to escape (A) and number of errors (B) in animals treated with MA or SAL. MA animals had similar latencies and errors as SAL controls. There was only an effect of day for latency to the platform, F(14, 597) = 3.92, p< 0.0001. There was a main effect of day, F(14, 666) = 5.89, p< 0.0001, and treatment × day interaction, F(14, 666) = 1.77, p< 0.04 for errors. The treatment × day interaction showed no significant effect of treatment by slice ANOVA for any day. *p< 0.05
4 Discussion
The data provided in the present experiments demonstrate that similar neurochemical and physiological effects following MA treatment may be observed in mice as are seen in rats after a neurotoxic dosing regimen [8,9,11,29,30,31,63,74]. Both rats and mice show increased body temperature and GFAP levels following MA treatment, along with reductions in brain monoamines. However, in terms of behavioral effects, rats and mice differ (at least in so far as egocentric and object recognition learning are concerned) despite similar changes in markers of neurotoxicity. MA causes substantial deficits in egocentric learning in the CWM in rats [30]whereas this effect was absent in mice. Neither rats nor mice showed differences in spatial learning in the MWM following MA, which is consistent across species. Rats show hypolocomotion for ~3 days after treatment and exhibit a modest differential response to a pharmacological challenge dose of MA [30], compared to mice that showed recovery of locomotor levels to those of SAL-treated controls after one day and showed an exaggerated hyperlocomotion following a pharmacological challenge dose of MA. Hence, despite neurochemical similarities between rats and mice following a binge/neurotoxic regimen of MA, functionally there are more differences than similarities.
We verified that mice given MA (10 mg/kg) at 2 h intervals 4 times on a single day had increased neostriatal GFAP protein levels 72 h post-treatment, demonstrating increased reactive gliosis as a marker of neurotoxicity as seen in rats. This marker has reliably been shown in rats and mice to reflect MA-induced neurotoxicity [30,31,45,55].
As in rats, MA-induced hyperthermia is seen in mice, along with monoamine reductions. Furthermore, the degree of hyperthermia is sensitive to ambient temperature; i.e., increases in ambient temperature heighten neurotoxicity and decreased temperature reduces neurotoxicity [3,44,55]. We observed increased core body temperatures in MA-treated C57BL/6 mice compared to controls, which, combined with increased GFAP levels and previous data [44,55,56], demonstrate that the dose regimen used here was neurotoxic. Higher doses typically cause sharp increases in mortality, placing practical limits on testing higher doses.
Despite evidence of neurotoxicity, MA-treated mice showed no impairments in recognition memory, spatial learning, reference memory, or in egocentric learning. We previously observed deficits in rats in egocentric learning in the CWM [30]. There was also impairment in novel object recognition memory in rats [30] that was not observed here, although this effect was not replicated in another study conducted in rats (Herring et al., unpublished). Other groups have observed deficits in novel object learning in mice following MA treatment [5,33,38,48]. However, these studies used a single, low (1 mg/kg) dose given on multiple days (7 days). These are not neurotoxic doses and are not comparable to the model used here. In addition to differences in dosing regimens, we used a different mouse strain.
In rats, novel object recognition deficits have been reported after binge/neurotoxic MA treatment [7,8,10,11,29,30,42,63] however, despite the number of reports, this effect has proven difficult to reliably replicate. No novel object recognition deficits were observed here in C57BL/6 mice, perhaps indicating a species difference or perhaps because the effect on novel object recognition is itself variable. It has also been demonstrated that rats treated with MA using an escalating dose + binge paradigm (14 days) or a single 1 mg/kg MA dose do not demonstrate object recognition deficits [7].
There is mounting evidence that recognition memory involves a multi-component system, consisting of contributions from the hippocampus (allocentric) and perirhinal cortex (discrimination of object familiarity and recency)[13] as well as the prefrontal cortex [52]. The glutamatergic system in the perirhinal cortex [6,78] and the dopaminergic system in prefrontal cortex (D1 receptors) [52] are important for encoding and/or retrieval in recognition memory tasks. Neither perirhinal nor prefrontal cortices were examined in this experiment and it may be that the current dose did not significantly affect these regions, although the doses used here would be predicted to affect these regions. Another consideration is time since treatment. Perhaps the extended period between treatment and the later tests allowed neurotransmitters to partially recover thereby eliminating behavioral differences on these tests. Prior behavioral testing may also have contributed to the absence of differences on tasks given later in the testing sequence.
To date, little data exist examining the effects of neurotoxic MA doses on spatial learning in the MWM in mice. One study found that mice given 10 mg/kg MA i.p. on a single day demonstrated increased latencies in MWM when examined one week after treatment and these deficits could be attenuated by pseudoginsenoside-F11+ treatment. Pseudoginsenoside-F11+ is a saponin-like compound found in ginseng [79]. Unfortunately, this study did not show whether the single MA dose was neurotoxic or not. No similar MWM deficits were observed in the current study; however the current experiment differed in that an increased interval (2 weeks vs. 1) was imposed between the time of treatment and MWM testing. The reason for our experimental design was to match the approach typically used with rats following a neurotoxic dosing regimen [30,63].
Egocentric learning is the ability of an animal to navigate to a destination using cues based on self-motion, without relying on distal landmarks. This form of learning is used by vertebrates and invertebrates to find their way in their environment and back again [20,21]. In our experiment, we did not observe egocentric deficits in MA-treated animals in the CWM. An analysis of escape latencies also showed no effect, suggesting that egocentric learning was not affected in MA-treated animals given neurotoxic doses. While we did not measure swimming speed in the CWM, it was captured by the tracking software during MWM testing and no differences in swimming speed were detected. We have noted that mice vary widely in how they perform in the CWM. For example, some mice search actively to escape, some swim rapidly but enter few cul-de-sacs, others swim slowly, and still others spend intervals not searching. This creates larger variations in performance than are seen when rats are tested in this maze and may result in the test being less sensitive in mice than in rats.
We observed hypoactivity 1 day after MA treatment, but activity levels in MA-treated mice returned to those of SAL-treated controls on the second day. It has been established that mice, as well as other species, become hyperactive shortly following MA treatment [34], but little is known about the effects on locomotor activity days following treatment with a neurotoxic regimen. We have previously demonstrated hypoactivity 1–3 [30] and 7 days [74] following a neurotoxic regimen of MA treatment in rats. The decreased initial locomotion observed in the MA-treated mice in the present experiment may be caused by drug-induced DA reductions in the neostriatum. Neostriatal monoamine levels measured following behavioral testing and those from published studies suggest that DA reductions were likely present shortly after drug treatment. Such monoamine reductions are also evident in rats treated with a neurotoxic MA regimen measured 3 days later; and have been shown to remain reduced several weeks later [30]. Although hypoactivity was observed in MA-treated mice, swimming ability/speed, assessed by measuring swim velocity in the MWM, was not affected.
Locomotor activity following a 1 mg/kg MA challenge produced a biphasic response in the MA-treated neurotoxic group. MA-treated animals were initially more hyperactive than SAL-treated animals after challenge, but later they became hypoactive. We previously observed this biphasic response in rats [30], however in mice the duration of the hyperactive phase was longer and the hypoactive phase less pronounced. It is unclear why such a biphasic response occurs. Aside from the reductions in neostriatal DA, it is possible that alterations in DA receptors are involved, but further testing will be needed to test this possibility.
Other neurotoxins such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA) have also shown inconsistencies in behavioral effects between rats and mice despite robust depletions of DA. One group demonstrated that MPTP produced increased akinesia and catalepsy [47,49,51], whereas others have not [76]. Similar observations have been made for spontaneous locomotion in the open field in MPTP models (see [65] for review). Reduced [60,61] and no differences [64] have been observed in mice on the rotorod test following MPTP. In 6-OHDA-treated mice, deficits in rotorod performance are partially restored by postnatal (P) day 28 [4]. Consistent MPTP-induced deficits in rotorod performance can only be achieved by chronic MPTP combined with co-administration of an adjuvant such as probenecid [58]. In the MPTP/probenecid model, motor deficits persist [58] while in other MPTP models they are transient [26] if seen at all [58]. Additionally, MPTP/probenecid mice have altered gait, MWM cued learning, motor deficits, and some of these (gait, balance, and movement deficits) are reversed by exercise [59]. Inconsistencies have also been reported in the spatial version of MWM following MPTP [18,59]. Therefore, it may not be surprising that we find differing effects of MA-induced DA depletions compared to what are seen in rats.
C57BL/6 mice are widely used in genetic studies and the present data demonstrate some similarities in response to a neurotoxic dose regimen of MA between rats and mice, but also differences. The C57BL/6 mouse does not appear to be an appropriate species for examining egocentric learning despite similar neostriatal DA and DOPAC reductions, increased basal corticosterone levels, and increased GFAP as in the rat, and this is congruent with differences between mice and rats in response to other dopaminergic depleting treatments (e.g. MPTP and 6-OHDA).
5. Conclusion
C57BL/6 mice, while suitable for investigating the neurochemical/neurotoxic effects of a neurotoxic regimen of MA exposure, are less well suited for studying the behavioral consequences. Mice do not show the same types of behavioral effects as seen in rats even following similar doses and comparable reductions in monoamine levels.
Acknowledgments
Support
This research was funded by NIH grant DA006733, a Scottish Right Fellowship and training grant ES07051. Sources were not involved in study design, collection, analysis, and interpretation of data or in the writing of the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no conflicts of interest for these data.
References
- 1.Achat-Mendes C, Ali SF, Itzhak Y. Differential effects of amphetamines-induced neurotoxicity on appetitive and aversive Pavlovian conditioning in mice. Neuropsychopharmacology. 2005;6:1128–1137. doi: 10.1038/sj.npp.1300675. [DOI] [PubMed] [Google Scholar]
- 2.Achat-Mendes C, Anderson KL, Itzhak Y. Impairment in consolidation of learned place preference following dopaminergic neurotoxicity in mice is ameliorated by N-acetylcysteine but not D1 and D2 dopamine receptor agonists. Neuropsychopharmacology. 2007;3:531–541. doi: 10.1038/sj.npp.1301119. [DOI] [PubMed] [Google Scholar]
- 3.Ali SF, Newport GD, Slikker W., Jr Methamphetamine-induced dopaminergic toxicity in mice. Role of environmental temperature and pharmacological agents. Ann. N. Y. Acad. Sci. 1996:187–198. doi: 10.1111/j.1749-6632.1996.tb17441.x. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Fischer D, Henze C, Strenzke C, Westrich J, Ferger B, Hoglinger GU, Oertel WH, Hartmann A. Characterization of the striatal 6-OHDA model of Parkinson's disease in wild type and alpha-synuclein-deleted mice. Exp. Neurol. 2008;1:182–193. doi: 10.1016/j.expneurol.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Arai S, Takuma K, Mizoguchi H, Ibi D, Nagai T, Kamei H, Kim HC, Yamada K. GABA(B) receptor agonist baclofen improves methamphetamine-induced cognitive deficit in mice. Eur. J. Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 6.Barker GR, Warburton EC, Koder T, Dolman NP, More JC, Aggleton JP, Bashir ZI, Auberson YP, Jane DE, Brown MW. The different effects on recognition memory of perirhinal kainate and NMDA glutamate receptor antagonism: implications for underlying plasticity mechanisms. J. Neurosci. 2006;13:3561–3566. doi: 10.1523/JNEUROSCI.3154-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belcher AM, Feinstein EM, O'Dell SJ, Marshall JF. Methamphetamine influences on recognition memory: comparison of escalating and single-day dosing regimens. Neuropsychopharmacology. 2008;6:1453–1463. doi: 10.1038/sj.npp.1301510. [DOI] [PubMed] [Google Scholar]
- 8.Belcher AM, O'Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav. Brain Res. 2006;1:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;11:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 10.Belcher AM, O'Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;11:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- 11.Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;1–2:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- 12.Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens core while sparing the shell. Synapse. 1997;2:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 13.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat. Rev. Neurosci. 2001;1:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 14.Brown PL, Wise RA, Kiyatkin EA. Brain hyperthermia is induced by methamphetamine and exacerbated by social interaction. J. Neurosci. 2003;9:3924–3929. doi: 10.1523/JNEUROSCI.23-09-03924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cappon GD, Pu C, Vorhees CV. Time-course of methamphetamine-induced neurotoxicity in rat caudate-putamen after single-dose treatment. Brain Res. 2000;1–2:106–111. doi: 10.1016/s0006-8993(00)02107-7. [DOI] [PubMed] [Google Scholar]
- 16.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 17.Chang L, Cloak C, Patterson K, Grob C, Miller EN, Ernst T. Enlarged striatum in abstinent methamphetamine abusers: a possible compensatory response. Biol. Psychiatry. 2005;9:967–974. doi: 10.1016/j.biopsych.2005.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deguil J, Chavant F, Lafay-Chebassier C, Perault-Pochat MC, Fauconneau B, Pain S. Neuroprotective Effect of PACAP on Translational Control Alteration and Cognitive Decline in MPTP Parkinsonian Mice. Neurotox. Res. 2009 doi: 10.1007/s12640-009-9091-4. [DOI] [PubMed] [Google Scholar]
- 19.Deng X, Wang Y, Chou J, Cadet JL. Methamphetamine causes widespread apoptosis in the mouse brain: evidence from using an improved TUNEL histochemical method. Brain Res. Mol. Brain Res. 2001;1:64–69. doi: 10.1016/s0169-328x(01)00184-x. [DOI] [PubMed] [Google Scholar]
- 20.Etienne AS, Jeffery KJ. Path integration in mammals. Hippocampus. 2004;2:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- 21.Etienne AS, Maurer R, Boulens V, Levy A, Rowe T. Resetting the path integrator: a basic condition for route-based navigation. J. Exp. Biol. Pt. 2004;9:1491–1508. doi: 10.1242/jeb.00906. [DOI] [PubMed] [Google Scholar]
- 22.Eyerman DJ, Yamamoto BK. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J. Neurochem. 2007;3:1219–1227. doi: 10.1111/j.1471-4159.2007.04837.x. [DOI] [PubMed] [Google Scholar]
- 23.Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La GR, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;2:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman SD, Castaneda E, Hodge GK. Long-term monoamine depletion, differential recovery, and subtle behavioral impairment following methamphetamine-induced neurotoxicity. Pharmacol. Biochem. Behav. 1998;1:35–44. doi: 10.1016/s0091-3057(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 25.Fukumura M, Cappon GD, Pu C, Broening HW, Vorhees CV. A single dose model of methamphetamine-induced neurotoxicity in rats: effects on neostriatal monoamines and glial fibrillary acidic protein. Brain Res. 1998;1:1–7. doi: 10.1016/s0006-8993(98)00656-8. [DOI] [PubMed] [Google Scholar]
- 26.Gerlach M, Riederer P. Animal models of Parkinson's disease: an empirical comparison with the phenomenology of the disease in man. J. Neural Transm. 1996;8–9:987–1041. doi: 10.1007/BF01291788. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez R, Bechara A, Martin EM. Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J. Clin. Exp. Neuropsychol. 2007;2:155–159. doi: 10.1080/13803390600582446. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, Cherner M, Grant I. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug Alcohol Depend. 2004;2:181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 29.He J, Yang Y, Yu Y, Li X, Li XM. The effects of chronic administration of quetiapine on the methamphetamine-induced recognition memory impairment and dopaminergic terminal deficit in rats. Behav. Brain Res. 2006;1:39–45. doi: 10.1016/j.bbr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of (+)-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008 doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herring NR, Schaefer TL, Tang PH, Skelton MR, Lucot JP, Gudelsky GA, Vorhees CV, Williams MT. Comparison of time-dependent effects of (+)-methamphetamine or forced swim on monoamines, corticosterone, glucose, creatine, and creatinine in rats. BMC. Neurosci. 2008:49. doi: 10.1186/1471-2202-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;2:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- 33.Ito Y, Takuma K, Mizoguchi H, Nagai T, Yamada K. A novel azaindolizinone derivative ZSET1446 (spiro[imidazo[1,2-a]pyridine-3,2-indan]-2(3H)-one) improves methamphetamine-induced impairment of recognition memory in mice by activating extracellular signal-regulated kinase 1/2. J. Pharmacol. Exp. Ther. 2007;2:819–827. doi: 10.1124/jpet.106.114108. [DOI] [PubMed] [Google Scholar]
- 34.Itoh T, Murai S, Yoshida H, Masuda Y, Saito H, Chen CH. Effects of methamphetamine and morphine on the vertical and horizontal motor activities in mice. Pharmacol. Biochem. Behav. 1987;1:193–197. doi: 10.1016/0091-3057(87)90496-5. [DOI] [PubMed] [Google Scholar]
- 35.Itzhak Y, Martin JL, Ali SF. Methamphetamine-induced dopaminergic neurotoxicity in mice: long-lasting sensitization to the locomotor stimulation and desensitization to the rewarding effects of methamphetamine. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;6:1177–1183. doi: 10.1016/s0278-5846(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 36.Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006 doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 37.Kalechstein AD, Newton TF, Green M. Methamphetamine dependence is associated with neurocognitive impairment in the initial phases of abstinence. J. Neuropsychiatry Clin. Neurosci. 2003;2:215–220. doi: 10.1176/jnp.15.2.215. [DOI] [PubMed] [Google Scholar]
- 38.Kamei H, Nagai T, Nakano H, Togan Y, Takayanagi M, Takahashi K, Kobayashi K, Yoshida S, Maeda K, Takuma K, Nabeshima T, Yamada K. Repeated methamphetamine treatment impairs recognition memory through a failure of novelty-induced ERK1/2 activation in the prefrontal cortex of mice. Biol. Psychiatry. 2006;1:75–84. doi: 10.1016/j.biopsych.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Kitamura O, Tokunaga I, Gotohda T, Kubo S. Immunohistochemical investigation of dopaminergic terminal markers and caspase-3 activation in the striatum of human methamphetamine users. Int. J. Legal Med. 2007;3:163–168. doi: 10.1007/s00414-006-0087-9. [DOI] [PubMed] [Google Scholar]
- 40.Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J. Neurochem. 2000;5:2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- 41.LaVoie MJ, Card JP, Hastings TG. Microglial activation precedes dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp. Neurol. 2004;1:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Marshall JF, Belcher AM, Feinstein EM, O'Dell SJ. Methamphetamine-induced neural and cognitive changes in rodents. Addiction. 2007:61–69. doi: 10.1111/j.1360-0443.2006.01780.x. [DOI] [PubMed] [Google Scholar]
- 43.McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;2:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 44.Miller DB, O'Callaghan JP. Elevated environmental temperature and methamphetamine neurotoxicity. Environ. Res. 2003;1:48–53. doi: 10.1016/s0013-9351(02)00051-8. [DOI] [PubMed] [Google Scholar]
- 45.Miller DB, O'Callaghan JP. Environment-,drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;2:752–760. [PubMed] [Google Scholar]
- 46.Miller DB, O'Callaghan JP, Ali SF. Age as a susceptibility factor in the striatal dopaminergic neurotoxicity observed in the mouse following substituted amphetamine exposure. Ann. N. Y. Acad. Sci. 2000:194–207. doi: 10.1111/j.1749-6632.2000.tb05196.x. [DOI] [PubMed] [Google Scholar]
- 47.Mitra N, Mohanakumar KP, Ganguly DK. Dissociation of serotoninergic and dopaminergic components in acute effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mice. Brain Res. Bull. 1992;3:355–364. doi: 10.1016/0361-9230(92)90035-v. [DOI] [PubMed] [Google Scholar]
- 48.Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2008;2:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- 49.Mohanakumar KP, Muralikrishnan D, Thomas B. Neuroprotection by sodium salicylate against 1-methyl-4-phenyl-1,2,36-tetrahydropyridine-induced neurotoxicity. Brain Res. 2000;2:281–290. doi: 10.1016/s0006-8993(00)02189-2. [DOI] [PubMed] [Google Scholar]
- 50.Moon M, Do KS, Park J, Kim D. Memory impairment in methamphetamine dependent patients. Int. J. Neurosci. 2007;1:1–9. doi: 10.1080/00207450500535503. [DOI] [PubMed] [Google Scholar]
- 51.Muralikrishnan D, Mohanakumar KP. Neuroprotection by bromocriptine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in mice. FASEB J. 1998;10:905–912. doi: 10.1096/fasebj.12.10.905. [DOI] [PubMed] [Google Scholar]
- 52.Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn. Mem. 2007;3:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch. Gen. Psychiatry. 2005;4:444–452. doi: 10.1001/archpsyc.62.4.444. [DOI] [PubMed] [Google Scholar]
- 54.O'Callaghan JP, Miller DB. Neurotoxic Effects of Substituted Amphetamines in Rats and Mice: Challenges to the Current Dogma. 2002:269–301. [Google Scholar]
- 55.O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;2:741–751. [PubMed] [Google Scholar]
- 56.O'Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Res. Monogr. 1993:188–212. [PubMed] [Google Scholar]
- 57.Ott T, Schmitt M, Pohle W, Matthies H. The effect of methamphetamine on the norepinephrine and 5-hydroxytryptamine contents in eleven rat brain regions. Brain Res. 1971;1:171–178. doi: 10.1016/0006-8993(71)90576-2. [DOI] [PubMed] [Google Scholar]
- 58.Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;3:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- 59.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson's disease with severe neurodegeneration. BMC. Neurosci. 2009:6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL. Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci. Lett. 1998;3:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 61.Rozas G, Lopez-Martin E, Guerra MJ, Labandeira-Garcia JL. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods. 1998;2:165–175. doi: 10.1016/s0165-0270(98)00078-8. [DOI] [PubMed] [Google Scholar]
- 62.Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH. Attentional control and brain metabolite levels in methamphetamine abusers. Biol. Psychiatry. 2007;11:1272–1280. doi: 10.1016/j.biopsych.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 63.Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;2:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- 64.Sedelis M, Hofele K, Auburger GW, Morgan S, Huston JP, Schwarting RK. MPTP susceptibility in the mouse: behavioral, neurochemical, and histological analysis of gender and strain differences. Behav. Genet. 2000;3:171–182. doi: 10.1023/a:1001958023096. [DOI] [PubMed] [Google Scholar]
- 65.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav. Brain Res. 2001;1–2:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 66.Sekine Y, Minabe Y, Kawai M, Suzuki K, Iyo M, Isoda H, Sakahara H, Ashby CR, Jr, Takei N, Mori N. Metabolite alterations in basal ganglia associated with methamphetamine-related psychiatric symptoms. A proton MRS study. Neuropsychopharmacology. 2002;3:453–461. doi: 10.1016/S0893-133X(02)00321-4. [DOI] [PubMed] [Google Scholar]
- 67.Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch. Gen. Psychiatry. 2006;1:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- 68.Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am. J Addict. 2000;3:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- 69.Thomas DM, Dowgiert J, Geddes TJ, Francescutti-Verbeem D, Liu X, Kuhn DM. Microglial activation is a pharmacologically specific marker for the neurotoxic amphetamines. Neurosci. Lett. 2004;3:349–354. doi: 10.1016/j.neulet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 70.Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J. Pharmacol. Exp. Ther. 2004;1:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- 71.Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J. Neurosci. 2004;26:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J Psychiatry. 2001;3:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- 73.Vorhees CV. Maze learning in rats: a comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol. Teratol. 1987;3:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 74.Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J. Neurosci. 1999;20:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine produces evidence of behavioral sensitization in the rat. Synapse. 2001;1:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 76.Willis GL, Donnan GA. Histochemical biochemical and behavioural consequences of MPTP treatment in C-57 black mice. Brain Res. 1987;2:269–274. doi: 10.1016/0006-8993(87)90033-3. [DOI] [PubMed] [Google Scholar]
- 77.Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human chronic methamphetamine users. Nat. Med. 1996;6:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 78.Winters BD, Bussey TJ. Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. J. Neurosci. 2005;17:4243–4251. doi: 10.1523/JNEUROSCI.0480-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu CF, Liu YL, Song M, Liu W, Wang JH, Li X, Yang JY. Protective effects of pseudoginsenoside-F11 on methamphetamine-induced neurotoxicity in mice. Pharmacol. Biochem. Behav. 2003;1:103–109. doi: 10.1016/s0091-3057(03)00215-6. [DOI] [PubMed] [Google Scholar]