Abstract
Rat neonatal methamphetamine exposure results in corticosterone release and learning and memory impairments in later life; effects also observed after neonatal stress. Previous attempts to test the role of corticosterone release after methamphetamine using corticosterone inhibitors were unsuccessful and adrenalectomy caused reductions in hippocampal serotonin greater than those caused by methamphetamine alone. Here we tested whether adrenal autotransplantation could be used to attenuate methamphetamine-induced corticosterone release without also altering the effects of the drug on serotonin. Adrenal autotransplantation surgery occurred on postnatal day 9 followed by methamphetamine or saline treatment from postnatal day 11–20 (10 mg/kg/dose x 4/day). Plasma corticosterone and hippocampal serotonin and 5-hydroxyindoleacetic acid were determined 30 min following the first treatment on each day between postnatal days 11–20. Adrenal autotransplantation attenuated neonatal methamphetamine-induced corticosterone release by ~70% initially, ~55% midway through treatment, and ~25% by the end of treatment. Methamphetamine reduced serotonin and 5-hydroxyindoleacetic acid in the hippocampus to the same degree as in sham-surgery rats. The data show that neonatal adrenal autotransplantation is an effective method for partially reducing treatment-induce corticosterone release while providing sufficient corticosterone to sustain normal growth and development. The method should is applicable to other models of developmental stress/corticosterone release.
Keywords: methamphetamine, corticosterone, serotonin, adrenal autotransplantation
1. Introduction
Methamphetamine (MA) is one of the most widespread drugs of abuse [20,21]. Recent data show that 24% of pregnant women entering drug treatment programs report MA as their primary drug of abuse [42]. Prospectively ascertained data in humans suggest that ~40% of pregnant MA users continue to use throughout pregnancy [7,37], and since MA readily crosses the placenta [4,15] there is passive exposure of the fetus. Infants born to women who used MA during pregnancy are reported to have reduced birth weight, length, and head circumference and increased rates of anemia and hemorrhage [7,12,13,26,32,38]. Children exposed to MA in utero also show deficits in visual motor integration, attention, psychomotor speed, spatial and verbal memory [5,6], novel object recognition memory on the Fagan Test of Infant Intelligence [41], as well as reduced arousal and quality of movement in newborns [38]. Magnetic resonance imaging (MRI) studies of in utero MA-exposed children reveal decreased volume of the hippocampus, putamen, and globus pallidus [6], and changes in white matter diffusivity using diffusion tensor imaging (DTI-MRI) with no changes in fractional anisotropy [10]. Magnetic resonance spectroscopy (MRS) data show higher total creatine, N-acetyl aspartate, and glutamate/glutamine in frontal white matter [5].
Algorithms that compare brain development across species reveal that P11 brain development in rats is comparable to humans at 26 weeks of gestation for cortex and 19 weeks of gestation for limbic structures [8,9]. Rats treated with MA neonatally exhibit later deficits in spatial learning and memory, egocentric learning, have augmented acoustic startle reactivity, and other effects [45,46,47,48,49,52,55,56,58] as well as decreased spine density in the dentate gyrus and nucleus accumbens and increases in apical dendritic branching in the parietal cortex [53]. These animals also show reductions in 5-HT levels in the hippocampus and neostriatum during and immediately following drug exposure and at P90, however dopamine (DA) levels are unaffected during dosing, but depletions emerge by P90 [11,35]. Neonatal MA treatment also causes increased release of ACTH and corticosterone [1,36,54,57] lasting for at least 24 h [34,35]. This effect of MA is more potent than corticosterone released in response to stressors such as forced swim or isolation at the same age [16]. The increase in neonatal MA-induced corticosterone release occurs during a period of normal hypothalamic-pituitary-adrenal quiescence referred to as the stress hyporesponsive period (SHRP) (approximately P4–14) [33] when despite dampened responsiveness, exposure to stressors can have long-lasting affects, an observation that may be important in understanding how neonatal MA leads to long-term effects. For example, prolonged stress that triggers increases in corticosterone during the SHRP sometimes lead to long-term alterations in hypothalamic-pituitary-adrenal (HPA) axis reactivity, increased startle reactivity, and spatial learning deficits in the Morris water maze [2,14,18,22,23,52]; effects similar to those caused by neonatal MA treatment as described above.
Previous experiments using bilateral adrenalectomy (ADX) effectively prevented P11 MA-induced corticosterone release but caused secondary effects on 5-HT in which hippocampal 5-HT levels in ADX-MA treated animals were reduced more than those in SHAM-MA treated animals (unpublished observations). This is a potential confound since hippocampal 5-HT changes may be involved in the MA-induced learning deficits [27]. In order to avoid this we sought an alternative to ADX.
Here we describe a method of attenuating MA-induced neonatal corticosterone release that may be useful for testing hypotheses concerning the role of adrenal responses to neonatal MA treatment or other drugs/stressors. We chose adrenal autotransplantation (ADXA) because experiments using corticosterone synthesis inhibitors (ketoconazole or metyrapone), while initially blocking MA-induced corticosterone release, exhibited later corticosterone rebound 24 h later (unpublished observations). Partial restoration of the adrenal cortex function following ADXA has the advantage of attenuating MA-induced corticosterone release while still allowing sufficient corticosterone for normal growth and develop and reducing the compensatory mechanisms (increased release of CRF and ACTH) known to accompany ADX [50,51].
2. Materials and Methods
2.1 Subjects and Conditions
Male (251–275 g) and nulliparous female (151–175 g) Sprague-Dawley CD IGS rats (Charles River Laboratories, Raleigh, NC) were acclimated to the vivarium for at least one week prior to breeding. The offspring were the subjects of this experiment and a total of 34 litters were used. Environmentally-enriching stimuli (stainless steel enclosures) [46] were placed in cages animals throughout the experiment. Food and water were provided ad libitum and the housing room was maintained on a 14:10 h light-dark cycle (lights on at 600 h). Litters were culled to 12 with 4 animals removed from each litter for tissue collection at each assessment age (i.e., each day between P11 to P21) with each litter contributing to 3 time points. The offspring removed at each sampling were randomly assigned as follows: sham surgery (SHAM)-saline (SAL), SHAM-MA, ADXA-SAL, or ADXA-MA. Therefore, half of the litter received SHAM surgery or ADXA and half MA or SAL so that the 4 surgery/treatment groups were represented in each sampling per litter. Allocation of pups to time points for sacrifice was as follows: The 3 sampling periods were pseudo-randomized in clusters so that times of sacrifice within a litter would occur on successive days. For example, litters were sacrificed on P11, 12, and 13 or P12, 13 and 14, or P18, 19, and 20, etc. until all time points were filled. Cluster order was also pseudo-randomized for each litter. We have previously shown that MA causes equal corticosterone release in male and female pups at these ages [54], therefore offspring were sampled randomly. Litters with <12 pups at birth had up to two pups of equivalent age fostered from litters of the same age. Protocols were approved by the Institutional Animal Care and Use Committee, and the vivarium is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care.
2.2 Treatments
(+)-Methamphetamine HCl (expressed as the freebase and > 95% pure, National Institute on Drug Abuse, Bethesda, MD) was administered subcutaneously at a dose of 10 mg/kg in a volume of 3 ml/kg or an equal volume of SAL to controls 4 times each day at 2 h intervals except on the last day when they received one dose 30 min prior to tissue collection. The 10 mg/kg dose was based on previous experiments using allometric scaling [29]. A recent study estimated that among a group of MA users in treatment rearrested for drug use relapse had plasma or urine blood concentrations at the time of testing that pharmacokinetic modeling showed intake values of the median users to be 52 mg/dose and the heavy user 350–600 mg/dose or for a 60 kg human, ~1 mg/kg for the median users and 5.8–10.0 mg/kg for the heavy MA users [16,28]. Since T1/2 in humans is 10–12 h but in rats is ~1–1.5 h, rats must be dosed more frequently to compensate for their more rapid rate of clearance. Thus, the dose of MA used in the present experiment (10 mg/kg x 4 doses/day) represents a model for a heavy MA user based on interspecies scaling. Body weights were recorded prior to each dose.
2.3 Surgical Procedures
Adrenal autotransplantation or sham surgery was performed on P9. Half of each litter had ADXA surgery and the other half sham surgery. The incision site was swabbed with 70% ethanol and betadine and animals anesthetized with isoflurane. For the ADXA animals, a bilateral approach was used to excise the adrenals, after which the adrenals were placed back into the peritoneum. The sham operation involved the primary incision, locating the adrenal glands, but leaving them intact. After surgery, the body cavity was sutured, the dermis stapled, and the site swabbed with warm saline and betadine to prevent infection.
2.4 Plasma and Tissue Collection
On the day of sacrifice, animals were taken to an adjacent room and decapitated; blood was collected in polyethylene tubes containing 2% EDTA (0.05 ml), and stored on ice until centrifuged. Plasma was isolated from whole blood by centrifugation at 1300 RCF for 25 min and the supernatant collected and stored at −80 °C until assayed. Brains were removed and the hippocampus dissected over ice with the aid of a brain block (Zivic-Miller, Pittsburgh, PA). The brain was sliced coronally at the optic chiasm and immediately caudal to the mammillary body and the hippocampi were dissected bilaterally from this section. Hippocampal tissues were frozen on dry ice and stored at −80 °C until assayed.
2.5 Corticosterone Assay
Plasma samples were thawed and assayed with Octeia Corticosterone ELISA kits (IDS, Fountain Hills, AZ). Each sample was diluted 1:5 and assayed according to the manufacturer’s protocol. The ELISAs were measured and quantified on a SpectraMax Plus microtiter plate reader (Molecular Devices, Sunnyvale, CA).
2.6 High Performance Liquid Chromatography (HPLC)
Hippocampi were weighed and homogenized using a hand-held glass homogenizer in a volume of 0.2 N perchloric acid 50 times that of the tissue. The homogenate was centrifuged for 5 min at 12,000 × g, the supernatant collected and stored on ice, and 20 µl aliquots were injected into a C18-column (MD-150, 3×150mm; ESA, Chelmsford, MA). The column was connected to a Coulochem electrochemical detector (25 A, Chelmsford, MA) and an integrator recorded the heights of each peak. The mobile phase consisted of 35 mM citric acid, 54 mM sodium acetate, 50 mg/L of disodium ethylenedeamine tetraacetate, 70 mg/l of octanesulfonic acid sodium salt, 6% v/v methanol, and 6% v/v acetonitrile, with a final pH of 4.0. The flow rate was 0.4 ml/min and quantities of each sample were calculated from standard curves for 5-HT and 5-HIAA concentrations.
2.7 Statistics
Data were analyzed using mixed linear analyses of variance (ANOVA) (SAS Proc Mixed, SAS Institute 9.1, Cary, NC) unless otherwise specified. The covariance matrix for each data set was examined using best fit statistics and in most cases the best fit was to an autoregressive-1 (AR(1)) covariance structure. Mixed model ANOVAs used Kenward-Roger adjusted degrees of freedom; these do not match those obtained from general linear model ANOVA and can be fractional. Measures taken repetitively on the same animal, such as day, were repeated measure factors. If significant interactions were observed, analyses at each level of the repeated measure factor were performed using slice effect ANOVAs. Since different animals were sacrificed each day, body weight data were analyzed using separate ANOVAs for each day. Mortality data were analyzed using Fisher tests of uncorrelated proportions.
3. Results
3.1 Body Weight
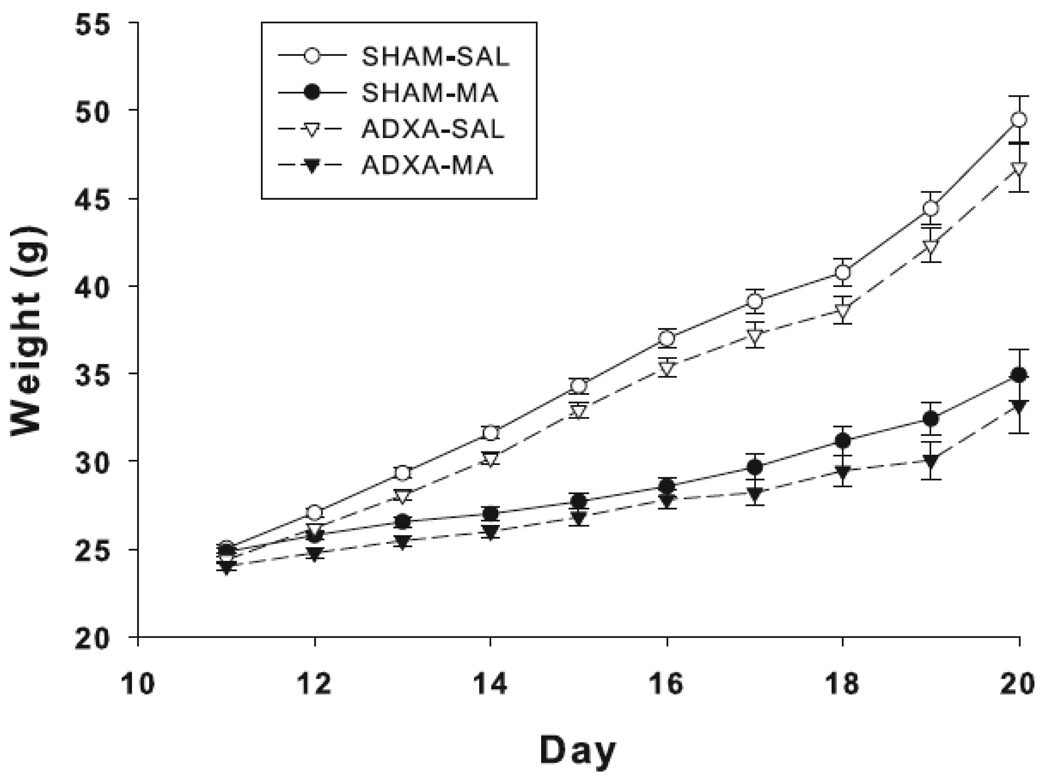
In the P11 group, there was an effect of surgery, F(1,363) = 10.5, p<0.001; the ADXA groups had reduced weight compared to the SHAM groups. This effect was also significant for the P12 through P19 groups (p-values from p< 0.001 to p < 0.03). No effect of surgery was observed on P20. There were also effects of drug and these began on P12, F(1,335) = 24.8, p<0.0001, and were significant on all days through P20, e.g., on P20 the treatment main effect was F(1,41) = 89.8, p<0.0001. Regardless of surgery, MA-treated groups had reduced body weight compared to the SAL-treated groups (Fig. 1). There were no surgery x treatment interactions.
Fig. 1.
Body weight of animals treated with MA or SAL following ADXA or SHAM operation on P9. ADXA significantly reduces weight gain from P11–19 and MA significantly reduces weight gain from P12–20. Data are represented by surgery condition and treatment group. N = 8–13 per surgery treatment pair, per day.
3.2 Mortality
Offspring mortality is shown in Table 1. Only the ADXA-MA groups treated from P11–20 showed a significant increase in mortality.
Table 1.
Mortality data (number deceased/total) for surgery/treatment pairs for each exposure period
| Exposure Period (postnatal age in days) |
SHAM-SAL | ADXA-SAL | SHAM-MA | ADXA-MA |
|---|---|---|---|---|
| 11–11 | 0/7 | 0/7 | 0/7 | 0/7 |
| 11–12 | 0/8 | 0/8 | 0/8 | 0/8 |
| 11–13 | 0/8 | 0/8 | 0/8 | 0/8 |
| 11–14 | 0/12 | 0/12 | 1/12 | 2/12 |
| 11–15 | 0/10 | 1/10 | 0/10 | 2/10 |
| 11–16 | 0/11 | 0/11 | 1/11 | 2/11 |
| 11–17 | 2/10 | 0/10 | 0/10 | 1/10 |
| 11–18 | 0/9 | 0/9 | 1/9 | 0/9 |
| 11–19 | 0/9 | 0/9 | 0/9 | 1/9 |
| 11–20 | 0/18 | 1/18 | 2/18 | 6/18** |
p< 0.01 compared to SHAM-SAL
3.3 Corticosterone
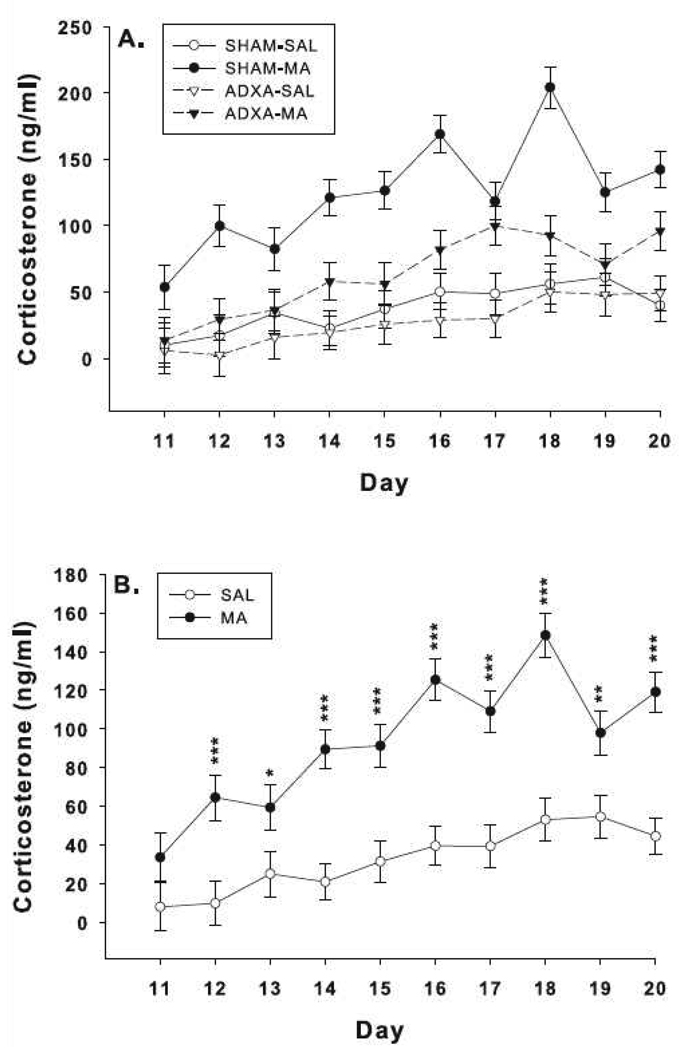
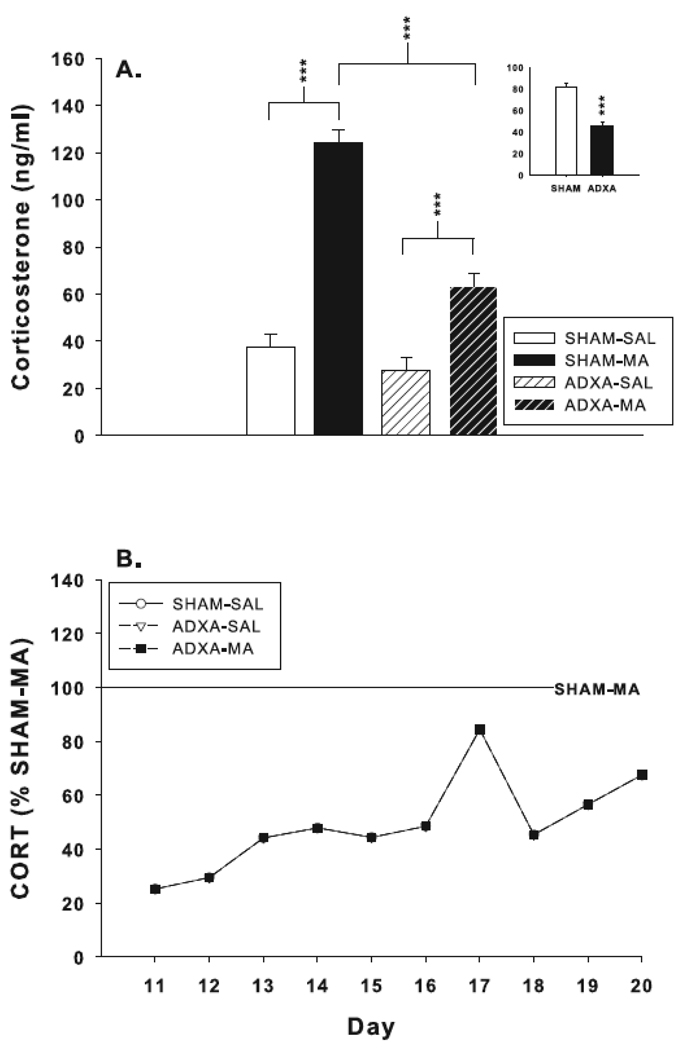
There were significant main effects of drug, F(1,294) = 184.8, p< 0.0001, surgery, F(1,294) = 62.1, p<0.0001, day, F(9,294) = 6.1, p< 0.0001, and the interactions of surgery x drug, F(1,294) = 31.5, p< 0.0001, and drug x day, F(9,294) = 2.3, p< 0.02. There were no drug x surgery x day effects (Fig. 2A). The drug x day interaction revealed that beginning on P12, the MA-treated groups had increased levels of corticosterone compared to the SAL-treated animals regardless of surgical condition (Fig. 2B). Analysis of the surgery x drug interaction revealed that SHAM-MA animals had increased corticosterone compared to SHAM-SAL; ADXA-MA had increased corticosterone compared to ADXA-SAL; and ADXA-MA corticosterone levels were reduced compared to SHAM-MA (Fig. 3A). No differences were observed between the SHAM-SAL and ADXA-SAL groups. On P11, ADXA-MA animals had corticosterone levels that were ~30% of SHAM-MA levels; the difference between these groups gradually diminished over the course of treatment reaching ~75% of SHAM-MA levels by P20 (Fig 3B). The combined ADXA groups had reduced corticosterone levels compared to the combined SHAM groups (Fig. 3 inset); in addition, corticosterone levels increased in all groups over time.
Fig. 2.
Corticosterone levels in rats exposed to MA or SAL from P11–20 following ADXA or SHAM surgery. (A) Representation of each surgery/treatment group. Groups sizes SHAM-SAL = = 7–13; SHAM-MA = 7–11; ADXA-SAL = 7–12; ADXA-MA = 7–10 per day. (B) Same data as in (A) except with the two MA-treated and two SAL-treated groups combined across surgical condition. SAL = 14–25; MA = 14–21 per day. ***p < 0.001, **p < 0.01, *p < 0.05 compared to SAL.
Fig. 3.
(A) Corticosterone levels averaged across treatment age. CORT levels are increased in SHAM-MA and ADXA-MA animals compared to their respective controls. ADXA significantly attenuated MA-induced CORT levels compared to SHAM-MA animals. Group sizes SHAM-SAL = 96; SHAM-MA = 92; ADXA-SAL = 94; ADXA-MA = 85. Inset: effect of treatment without regard to surgical condition. (B) CORT levels as a percent SHAM-MA values. ***p < 0.001.
3.4 Hippocampal 5-HT and 5-HIAA
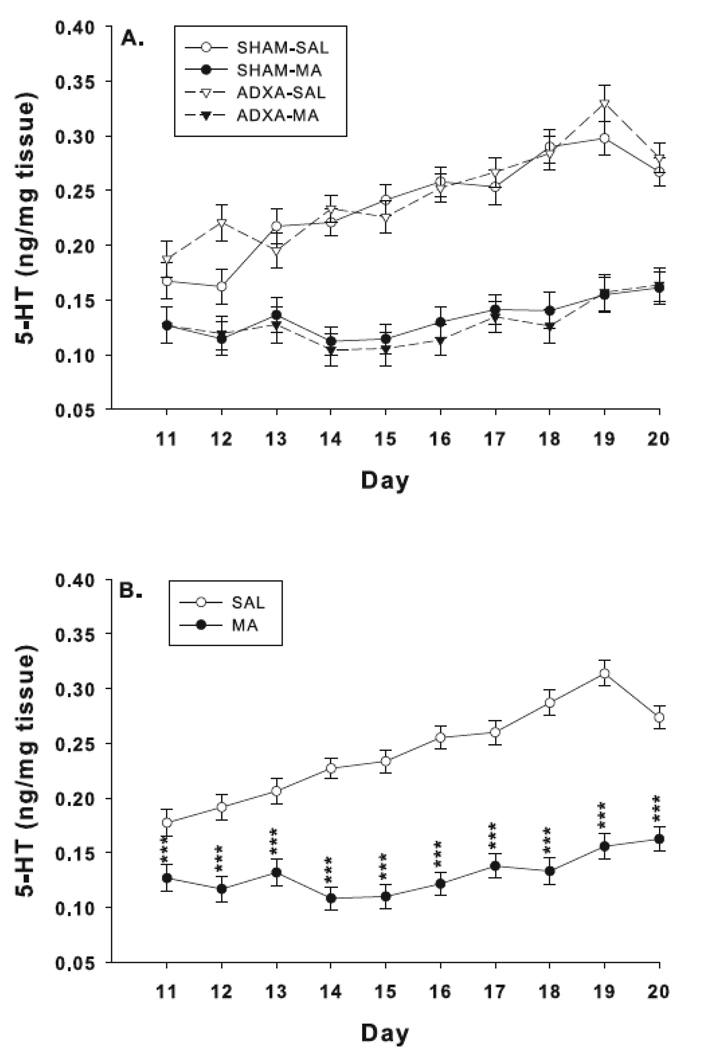
5-HT levels by drug and surgical condition are shown in Fig. 4A. For 5-HT there were significant main effects of drug, F(1,276) = 641.5, p< 0.0001, and day, F(9,276) = 7.4, p< 0.0001, but not surgery. The combined MA-treated groups had reduced 5-HT levels compared to the combined SAL-treated groups (Fig. 4B). There was also a significant interaction of drug x day, F(9,276) = 5.7, p< 0.0001, in which MA-treated groups had reduced 5-HT levels that varied in intensity as treatment progressed. There were no other significant effects or interactions for 5-HT.
Fig. 4.
Hippocampal 5-HT levels: 5-HT was measured 30 min following the first dose of the last day of treatment. (A) Effects on 5-HT for each surgery/treatment group. MA reduced 5-HT on all treatment days. Group sizes SHAM-SAL = 8–12; SHAM-MA = 7–11; ADXA-SAL = 7–12; ADXA-MA = 7–9 per day. (B) Same data as in (A) except with the two MA-treated and two SAL-treated groups averaged together to show the main effect of drug treatment. SAL = 15–24; MA = 14–20 per day. ***p < 0.0001 compared to SAL.
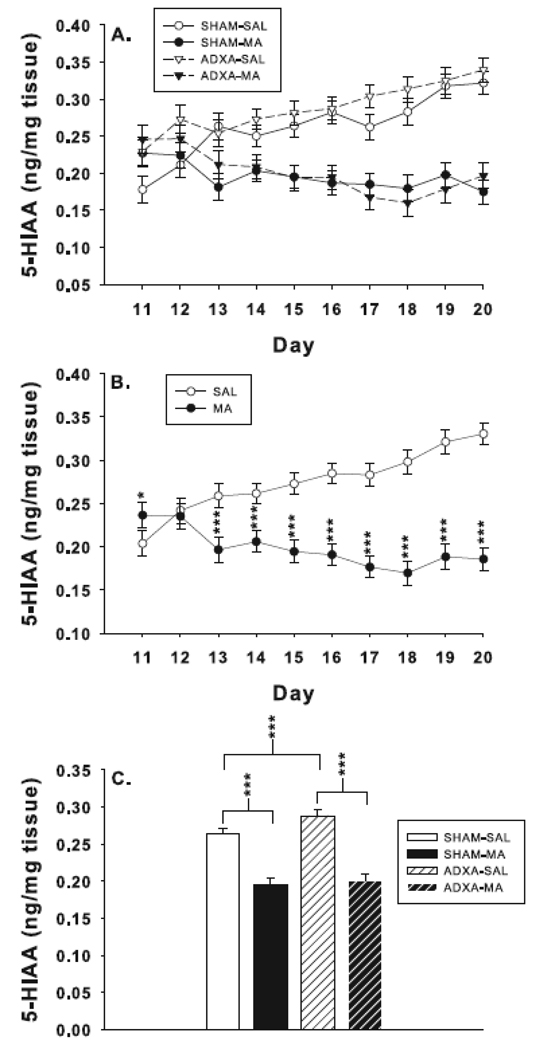
For 5-HIAA (Fig. 5A), there were main effects of surgery, F(1,277) = 10.5, p< 0.001, and drug, F(1,277) = 291.0, p< 0.0001. The combined ADXA groups had increased levels of 5-HIAA compared to the combined SHAM groups. The drug main effect was attributable to the fact that the combined MA-treated groups had reduced 5-HIAA levels compared to combined SAL-treated groups. There were interactions of surgery x drug, F(1,277) = 4.6, p< 0.03, and drug x day, F(9,277) = 14.4, p<0.0001. The drug x day interaction revealed that 5-HIAA was increased on P11 and decreased from P13–20 in the combined MA-treated groups compared to combined SAL-treated groups (Fig. 5B). The surgery x drug interaction is shown in Fig. 5C. The two MA-treated groups have nearly identical averages however the ADXA-SAL had increased 5-HIAA levels compared to SHAM-SAL. There was no surgery x drug x day interaction.
Fig. 5.
Hippocampal 5-HIAA levels: (A) Effects on 5-HIAA for each treatment/surgery group. Group sizes SHAM-SAL = 8–12; SHAM-MA = 7–11; ADXA-SAL = 7–12; ADXA-MA = 7–9 per day. (B) Same data as in (A) except with the two MA-treated and two SAL-treated groups averaged together to show the main effect of drug treatment. SAL = 15–24; MA = 14–20 per day. (C) Surgery x drug interaction revealed that 5-HIAA was reduced in SHAM-MA compared to SHAM-SAL and ADXA-MA compared to ADXA-SAL animals. 5-HIAA levels were also increased in ADXA-SAL compared to SHAM-SAL animals. SHAM-SAL = 91; SHAM-MA = 88: ADXA-SAL = 89; ADXA-MA = 80. ***p < 0.001, *p < 0.05 vs. SAL.
4. Discussion
MA significantly increases corticosterone from P12–20 (Fig. 2B). ADXA effectively attenuated this effect, reducing the increases in corticosterone to ~51% of SHAM-MA levels averaged across the 10 days of treatment (Fig. 3A). However, the degree of corticosterone release inhibition varied. Corticosterone levels in ADXA-MA animals were ~30% of SHAM-MA levels on P11 and rose to ~75% by P20. These data suggest that neonatal adrenal engraftment occurs more rapidly than in adults. In adult rats, adrenal autotransplantation has been reported to reduce plasma corticosterone levels beginning 1–5 weeks post-surgery, with progressively rising levels for 6–9 weeks [30,31,39,40,44]. In neonatal rats, the ADXA method was partially effective at blocking MA-induced corticosterone increases in the present experiment. Accordingly, this method appears useful for testing hypotheses concerning the role of corticosterone release in mediating or contributing to a number of the long-term effects of early MA exposure. Mortality from the ADXA procedure itself as reflected in the ADXA-SAL groups was not significantly above SHAM-SAL. Moreover, the combination of ADXA and MA-treatment did not increase mortality at any age from P11–19 but an increase was seen on P20. This increase was unexpected and given that the P11–19 groups had only one less day of treatment, it seems unlikely that the P11–20 increase is reliable.
The method uses adrenal autotransplantation to temporarily interrupt and gradually reinstate adrenal function. The regenerative properties of the adrenal gland have been investigated previously. For example in adult rats, adrenal cortex grafts, regardless of size, exhibit regeneration [3,30,31,39,44]. Histological evidence shows initial necrosis of the adrenal cortex and medulla, followed by proliferation and differentiation of the cortex and formation of an adrenal capsule [3,30,31,39,44] resembling normal morphology by 180 days [44]. There is also evidence that adrenal autografts become reinnervated [43], but the adrenal medulla degenerates entirely [39].
MA exposure from P11–20 depletes hippocampal 5-HT levels regardless of surgery, replicating and extending previous findings [34]. 5-HT levels were also significantly reduced on P11, which was not observed previously [34]. It is possible that such reductions, especially in a brain region important in spatial learning, may contribute to the long-term cognitive changes in MA-treated offspring. Future experiments will be needed to address this. Hippocampal 5-HIAA levels were also reduced in MA-treated animals regardless of surgery on most days of treatment (P13–20). This is likely due to the reduction in 5-HT presumably resulting from MA inhibition of 5-HT synthesis since MA is an established tryptophan hydroxylase inhibitor [17,19,24,25]. It is less clear why 5-HIAA levels were initially increased in MA animals on P11, but may be related to the fact that MA initially causes a large release of monoamine followed by depletion, a pattern consistent with this finding (Fig. 5C). More importantly, both 5-HT and 5-HIAA levels were similar between ADXA-MA and SHAM-MA groups demonstrating that ADXA eliminated the discrepancy previously observed between ADX-MA and SHAM-MA groups in 5-HT levels (unpublished observations).
5. Conclusions
Adrenal autotransplantation provides an effective method of attenuating corticosterone release in neonatal rats. This model could be utilized for examining the effects of early exposure to stress or other drugs on brain development and function. Of particular interest in the present context is determining whether the MA-induced corticosterone release in neonates contributes to later learning and other behavioral effects.
Acknowledgment
The authors express their appreciation to Mary Moran for assistance with the statistical analyses of the data. This research was supported by NIH project grant RO1 DA006733 and training grant T32 ES007031 (CEG; TLS). There are no conflicts of interest for this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors’ declare no conflict of interest concerning the research reported herein.
References
- 1.Acevedo SF, Pfankuch T, van MP, Raber J. Role of histamine in short-and long-term effects of methamphetamine on the developing mouse brain. J. Neurochem. 2008;107:976–986. doi: 10.1111/j.1471-4159.2008.05673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisa B, Tordera R, Lasheras B, Del RJ, Ramirez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–266. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Belloni AS, Vassanelli P, Robba C, Rebuffat P, Mazzocchi G, Nussdorfer GG. Ultrastructural observations on the regeneration of adrenocortical autotransplants in the rat spleen. J. Anat. 1982;135:245–253. [PMC free article] [PubMed] [Google Scholar]
- 4.Burchfield DJ, Lucas VW, Abrams RM, Miller RL, DeVane CL. Disposition and pharmacodynamics of methamphetamine in pregnant sheep. JAMA. 1991;265:1968–1973. [PubMed] [Google Scholar]
- 5.Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchthal S, Hedemark B, Smith LM, Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang L, Smith LM, LoPresti C, Yonekura ML, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Chomchai C, Na MN, Watanarungsan P, Yossuck P, Chomchai S. Methamphetamine abuse during pregnancy and its health impact on neonates born at Siriraj Hospital, Bangkok, Thailand, Southeast Asian. J. Trop. Med. Public Health. 2004;35:228–231. [PubMed] [Google Scholar]
- 8.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy B, Kersh B, Darlington RB, Anand KJ, Finlay BL. Web-based method for translating neurodevelopment from laboratory species to humans. Neuroinformatics. 2006;5:79–94. doi: 10.1385/ni:5:1:79. [DOI] [PubMed] [Google Scholar]
- 10.Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crawford CA, Williams MT, Newman ER, McDougall SA, Vorhees CV. Vorhees, Methamphetamine exposure during the preweanling period causes prolonged changes in dorsal striatal protein kinase A activity, dopamine D2-like binding sites, and dopamine content. Synapse. 2003;48:131–137. doi: 10.1002/syn.10197. [DOI] [PubMed] [Google Scholar]
- 12.Dixon SD. Effects of transplacental exposure to cocaine and methamphetamine on the neonate. West J. Med. 1989;150:436–442. [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon SD, Bejar R. Echoencephalographic findings in neonates associated with maternal cocaine and methamphetamine use: incidence and clinical correlates. J. Pediatr. 1989;115:770–778. doi: 10.1016/s0022-3476(89)80661-4. [DOI] [PubMed] [Google Scholar]
- 14.Felszeghy K, Bagdy G, Nyakas C. Blunted pituitary-adrenocortical stress response in adult rats following neonatal dexamethasone treatment. J. Neuroendocrinol. 2000;12:1014–1021. doi: 10.1046/j.1365-2826.2000.00551.x. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Bournissen F, Rokach B, Karaskov T, Koren G. Methamphetamine detection in maternal and neonatal hair: implications for fetal safety. Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F351–F355. doi: 10.1136/adc.2006.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace CE, Schaefer TL, Herring NR, Skelton MR, McCrea AE, Vorhees CV, Williams MT. (+)-Methamphetamine increases corticosterone in plasma and BDNF in brain more than forced swim or isolation in neonatal rats. Synapse. 2008;62:110–121. doi: 10.1002/syn.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J. Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- 18.Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr. Res. 2001;50:750–755. doi: 10.1203/00006450-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J. Pharmacol. Exp. Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- 20.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2008. Monitoring the Future national survey results on drug use, 1975–2007; pp. 1–738. [Google Scholar]
- 21.Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Volume II: College students and adults ages 19–45. Bethesda, MD: National Institute on Drug Abuse; 2008. Monitoring the Future national survey results on drug use, 1975–2007; pp. 1–342. [Google Scholar]
- 22.Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol. Biochem. Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- 23.Kamphuis PJ, Bakker JM, Broekhoven MH, Kunne C, Croiset G, Lentjes EG, Tilders FJ, van BF, Wiegant VM. Enhanced glucocorticoid feedback inhibition of hypothalamo-pituitary-adrenal responses to stress in adult rats neonatally treated with dexamethasone. Neuroendocrinology. 2002;76:158–169. doi: 10.1159/000064526. [DOI] [PubMed] [Google Scholar]
- 24.Kokoshka JM, Fleckenstein AE, Wilkins DG, Hanson GR. Age-dependent differential responses of monoaminergic systems to high doses of methamphetamine. J. Neurochem. 2000;75:2095–2102. doi: 10.1046/j.1471-4159.2000.0752095.x. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn DM. Tryptophan hydroxylase regulation. Drug-induced modifications that alter serotonin neuronal function. Adv. Exp. Med. Biol. 1999;467:19–27. doi: 10.1007/978-1-4615-4709-9_3. [DOI] [PubMed] [Google Scholar]
- 26.Little BB, Snell LM, Gilstrap LC., III Methamphetamine abuse during pregnancy: outcome and fetal effects. Obstet. Gynecol. 1988;72:541–544. [PubMed] [Google Scholar]
- 27.Mazer C, Muneyyirci J, Taheny K, Raio N, Borella A, Whitaker-Azmitia P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: a possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997;760:68–73. doi: 10.1016/s0006-8993(97)00297-7. [DOI] [PubMed] [Google Scholar]
- 28.Melega WP, Cho AK, Harvey D, Lacan G. Methamphetamine blood concentrations in human abusers: application to pharmacokinetic modeling. Synapse. 2007;61:216–220. doi: 10.1002/syn.20365. [DOI] [PubMed] [Google Scholar]
- 29.Mordenti J, Chapell W. Toxicokinetics in New Drug Development. New York: Pergamon Press; 1989. The use of interspecies scaling in toxicokinetics; pp. 42–96. [Google Scholar]
- 30.Nabishah BM, Khalid BA, Morat PB, Zanariyah A. Regeneration of adrenal cortical tissue after adrenal autotransplantation. Exp. Clin. Endocrinol. Diabetes. 1998;106:419–424. doi: 10.1055/s-0029-1212009. [DOI] [PubMed] [Google Scholar]
- 31.Okamoto T, Fujimoto Y, Obara T, Ito Y, Kodama T. Experimental study on adrenal autografts in rats to preserve normal adrenocortical function after bilateral adrenalectomy. Eur. Surg. Res. 1992;24:112–118. doi: 10.1159/000129196. [DOI] [PubMed] [Google Scholar]
- 32.Oro AS, Dixon SD. Perinatal cocaine and methamphetamine exposure: maternal and neonatal correlates. J. Pediatr. 1987;111:571–578. doi: 10.1016/s0022-3476(87)80125-7. [DOI] [PubMed] [Google Scholar]
- 33.Sapolsky RM, Meaney MJ. Maturation of the adrenocortical stress response: neuroendocrine control mechanisms and the stress hyporesponsive period. Brain Res. 1986;396:64–76. doi: 10.1016/s0006-8993(86)80190-1. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer TL, Ehrman LA, Gudelsky GA, Vorhees CV, Williams MT. Comparison of monoamine and corticosterone levels 24 h following (+)methamphetamine, (+/−)3,4-methylenedioxymethamphetamine, cocaine, (+)fenfluramine or (+/−)methylphenidate administration in the neonatal rat. J. Neurochem. 2006;98:1369–1378. doi: 10.1111/j.1471-4159.2006.04034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaefer TL, Skelton MR, Herring NR, Gudelsky GA, Vorhees CV, Williams MT. Short- and long-term effects of (+)-methamphetamine and (+/−)-3,4-methylenedioxymethamphetamine on monoamine and corticosterone levels in the neonatal rat following multiple days of treatment. J. Neurochem. 2008;104:1674–1685. doi: 10.1111/j.1471-4159.2007.05112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skelton MR, Williams MT, Schaefer TL, Vorhees CV. Neonatal (+)-methamphetamine increases brain derived neurotrophic factor, but not nerve growth factor, during treatment and results in long-term spatial learning deficits. Psychoneuroendocrinology. 2007;32:734–745. doi: 10.1016/j.psyneuen.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith L, Yonekura ML, Wallace T, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. J. Dev. Behav. Pediatr. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, Arria A, Huestis M, Haning W, Strauss A, Grotta SD, Fallone M, Liu J, Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicol. Teratol. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srougi M, Gittes RF. Adrenal autotransplantation. Urol. Surv. 1978;28:41–48. [PubMed] [Google Scholar]
- 40.Srougi M, Gittes RF, Underwood R. Influence of exogenous glucocorticoids and adrenocorticotropic hormone on experimental adrenal autografts. Surg. Forum. 1978;29:109–111. [PubMed] [Google Scholar]
- 41.Struthers JM, Hansen RL. Visual recognition memory in drug-exposed infants. J. Dev. Behav. Pediatr. 1992;13:108–111. doi: 10.1097/00004703-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstet. Gynecol. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- 43.Ulrich-Lai YM, Engeland WC. Rat adrenal transplants are reinnervated: an invalid model of denervated adrenal cortical tissue. J. Neuroendocrinol. 2000;12:881–893. doi: 10.1046/j.1365-2826.2000.00534.x. [DOI] [PubMed] [Google Scholar]
- 44.Vendeira P, Magalhaes MM, Magalhaes MC. Autotransplantation of the adrenal cortex: a morphological and autoradiographic study. Anat. Rec. 1992;232:262–272. doi: 10.1002/ar.1092320211. [DOI] [PubMed] [Google Scholar]
- 45.Vorhees CV, Ahrens KG, Acuff-Smith KD, Schilling MA, Fisher JE. Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology (Berl) 1994;114:392–401. doi: 10.1007/BF02249328. [DOI] [PubMed] [Google Scholar]
- 46.Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int. J. Dev. Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vorhees CV, Inman-Wood SL, Morford LL, Broening HW, Fukumura M, Moran MS. Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J. Neurosci. 2000;20:4732–4739. doi: 10.1523/JNEUROSCI.20-12-04732.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vorhees CV, Reed TM, Schilling MS, Acuff-Smith KD, Fisher JE, Moran MS. Neonatal methamphetamine-induced long-term acoustic startle facilitation in rats as a function of prepulse stimulus intensity. Neurotoxicol. Teratol. 1996;18:135–139. doi: 10.1016/0892-0362(95)02051-9. [DOI] [PubMed] [Google Scholar]
- 49.Vorhees CV, Skelton MR, Williams MT. Age-dependent effects of neonatal methamphetamine exposure on spatial learning. Behav. Pharmacol. 2007;18:549–562. doi: 10.1097/FBP.0b013e3282ee2abe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walker CD, Akana SF, Cascio CS, Dallman MF. Adrenalectomy in the neonate: adult-like adrenocortical system responses to both removal and replacement of corticosterone. Endocrinology. 1990;127:832–842. doi: 10.1210/endo-127-2-832. [DOI] [PubMed] [Google Scholar]
- 51.Walker CD, Sapolsky RM, Meaney MJ, Vale WW, Rivier CL. Increased pituitary sensitivity to glucocorticoid feedback during the stress nonresponsive period in the neonatal rat. Endocrinology. 1986;119:1816–1821. doi: 10.1210/endo-119-4-1816. [DOI] [PubMed] [Google Scholar]
- 52.Williams MT, Blankenmeyer TL, Schaefer TL, Brown CA, Gudelsky GA, Vorhees CV. Long-term effects of neonatal methamphetamine exposure in rats on spatial learning in the Barnes maze and on cliff avoidance, corticosterone release, and neurotoxicity in adulthood. Brain Res. Dev. Brain Res. 2003;147:163–175. doi: 10.1016/j.devbrainres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Williams MT, Brown RW, Vorhees CV. Neonatal methamphetamine administration induces region-specific long-term neuronal morphological changes in the rat hippocampus, nucleus accumbens and parietal cortex. Eur. J. Neurosci. 2004;19:3165–3170. doi: 10.1111/j.0953-816X.2004.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams MT, Inman-Wood SL, Morford LL, McCrea AE, Ruttle AM, Moran MS, Rock SL, Vorhees CV. Preweaning treatment with methamphetamine induces increases in both corticosterone and ACTH in rats. Neurotoxicol. Teratol. 2000;22:751–759. doi: 10.1016/s0892-0362(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 55.Williams MT, Moran MS, Vorhees CV. Refining the critical period for methamphetamine-induced spatial deficits in the Morris water maze. Psychopharmacology (Berl) 2003;168:329–338. doi: 10.1007/s00213-003-1433-y. [DOI] [PubMed] [Google Scholar]
- 56.Williams MT, Morford LL, Wood SL, Wallace TL, Fukumura M, Broening HW, Vorhees CV. Developmental D-methamphetamine treatment selectively induces spatial navigation impairments in reference memory in the Morris water maze while sparing working memory. Synapse. 2003;48:138–148. doi: 10.1002/syn.10159. [DOI] [PubMed] [Google Scholar]
- 57.Williams MT, Schaefer TL, Furay AR, Ehrman LA, Vorhees CV. Ontogeny of the adrenal response to (+)-methamphetamine in neonatal rats: The effect of prior drug exposure. Stress. 2006;9:153–163. doi: 10.1080/10253890600902842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams MT, Vorhees CV, Boon F, Saber AJ, Cain DP. Methamphetamine exposure from postnatal day 11 to 20 causes impairments in both behavioral strategies and spatial learning in adult rats. Brain Res. 2002;958:312–321. doi: 10.1016/s0006-8993(02)03620-x. [DOI] [PubMed] [Google Scholar]