Abstract
Background
Growing evidence supports a role of metabotropic glutamate receptors (mGluRs) in ethanol reinforcement, ethanol-seeking, and ethanol withdrawal. To extend the understanding of the role of mGluRs in the addiction-relevant effects of ethanol as well as of the treatment target potential of these receptors for alcohol abuse, the effects of a selective mGlu2/3 agonist (LY379268) and a selective mGlu5 antagonist (MTEP) were tested on two processes central to alcohol addiction: ethanol reinforcement and stress-induced reinstatement of ethanol-seeking in rats with a history of ethanol dependence.
Methods
Following operant ethanol self-administration training, male Wistar rats were made dependent by intragastric ethanol intubations. Ethanol dependence was confirmed by the presence of somatic withdrawal signs. Following 2 weeks of withdrawal, stable ethanol self-administration was re-established, and the effects of LY379268 (0-3 mg/kg, SC) and MTEP (0-3 mg/kg, IP) on ethanol self-administration were determined in both non-dependent and post-dependent rats. A second set of rats underwent extinction training and then was tested for the effects of LY379268 or MTEP on reinstatement of ethanol-seeking induced by footshock stress.
Results
LY379268 and MTEP dose-dependently reduced both ethanol self-administration and reinstatement of ethanol-seeking induced by footshock stress. Additionally, LY379268 was more effective than MTEP in inhibiting both behaviors in post-dependent than in non-dependent animals.
Conclusions
These findings suggest that neuroadaptation associated with chronic ethanol exposure or withdrawal alters the sensitivity of mGlu2/3 receptors, with implications for the understanding of the neural basis of alcohol dependence and the treatment target potential of these receptors.
Keywords: ethanol dependence, LY379268, MTEP, metabotropic glutamate receptor, self-administration, relapse
Introduction
Alcoholism is a chronic relapsing condition characterized by impaired ability to control intake and intense desire for alcohol, despite related adverse consequences (1, 2). Alcohol dependence is associated with dysregulation of glutamate transmission in the brain (3-5). Glutamate acts through ligand-gated ionotropic receptors and G-protein-coupled metabotropic receptors (mGluRs). MGluRs have received much recent interest in the addiction field because of accumulating evidence implicating these receptors in the reinforcing and addictive actions of drugs of abuse, including alcohol, and in withdrawal states (e.g., 6-11). Because of their location on specific cellular and synaptic compartments, mGluRs have been implicated in the fine-tuning of synaptic efficiency and control of the “sharpness” of glutamatergic transmission (12). Abnormalities in mGluR function are thought to be a factor in psychiatric disorders, including drug addiction, and may represent promising targets for the pharmacotherapeutic treatment of these disorders (12-16).
Among the eight mGluR subtypes identified to date (17), mGlu5 (Group I) and mGlu2/3 (Group II) receptors have received particular attention with respect to their role in the neurobehavioral actions of addictive drugs, and drug-seeking associated with stress and anxiety (14). MGlu5 receptors, located predominantly postsynaptically, positively modulate glutamate-induced neural excitability (18). Evidence implicates mGlu5 receptors in the reinforcing effects of ethanol (10, 19-23) and several drugs of abuse, including cocaine (6, 24-26) and nicotine (8, 24, 25, 27). Recent findings have also revealed a role for mGlu5 receptors in the conditioned reinforcing effects of drugs of abuse. For example, the mGlu5 antagonist MPEP attenuated the expression of morphine-induced conditioned place preference (28), conditioned reinforcement by a stimulus paired with cocaine on a second-order schedule of reinforcement (29), conditioned reinstatement of nicotine-seeking (30), and ethanol-seeking induced by concurrent ethanol priming and cue manipulations (31). MGlu2/3 receptors, located at the presynaptic and perisynaptic level, provide negative feedback to decrease glutamate release or synaptic availability of glutamate (18, 32, 33). These receptors have been implicated in the neurobehavioral effects of several drugs of abuse (e.g., 7, 34-40), but information on their role in ethanol-seeking and reinforcement is more limited. However, evidence exists that activation of mGlu2/3 receptors by the selective agonist LY379268 decreases both stress- and cue-induced ethanol-seeking in reinstatement models of relapse (41) and reverses ethanol-seeking induced by ethanol cue exposure paired with noncontingent ethanol administration (42).
The present experiments were designed to extend the understanding of the role of mGlu5 and mGlu2/3 receptors specifically in behavior linked to alcohol addiction and the treatment target potential of these receptors for alcohol abuse. The objective of these studies was to establish the effects of a selective mGlu5 antagonist (MTEP) and a selective mGlu2/3 agonist (LY379268), compounds that reduce glutamate-mediated neural excitability (albeit via different mechanisms of action), on two processes relevant to alcohol addiction: ethanol-maintained reinforcement and stress-induced reinstatement of ethanol-seeking (43). MTEP and LY379268 exert potent anxiolytic effects in animal models of anxiety (12, 44), thus implicating mGlu5 and mGlu2/3 receptors in the regulation of drug-seeking responses to anxiety and stress that represent a major factor for relapse to alcohol use. Moreover, mGluRs are susceptible to drug-induced neuroadaptation (11) and, as a possible consequence, long-lasting dysregulation of glutamatergic transmission, a likely factor in susceptibility to relapse. Therefore, emphasis in these studies was given to a comparison of the effects of mGlu2/3 and mGlu5 manipulation in alcohol non-dependent vs. post-dependent rats to determine whether neuroadaptive changes associated with chronic ethanol intoxication alter the pharmacological effects of MTEP and LY379268 on ethanol reinforcement and stress-induced reinstatement of ethanol-seeking.
Methods and Materials
Male Wistar rats were trained to orally self-administer 10% ethanol (w/v). Upon stable self-administration, one group (post-dependent) of rats was made ethanol dependent via intragastric ethanol intubations. Another group (non-dependent) received vehicle (for details, see Supplement 1). Separate subgroups of non-dependent and post-dependent rats then were tested for the effects of LY379268 or MTEP on ethanol self-administration and stress-induced reinstatement of ethanol-seeking.
Drugs
(-)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid (LY379268; Eli Lilly Research Laboratories, Indianapolis, IN) was dissolved in sterile water and administered subcutaneously (SC, 1 ml/kg) 30 min before behavioral testing. 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine (MTEP; Merck, Rahway, NJ) was dissolved in 10% (v/v) Tween 80 and administered intraperitoneally (IP, 1 ml/kg) 60 min before behavioral testing.
Effects of LY379268 or MTEP on Ethanol Self-Administration
Separate groups of non-dependent and post-dependent rats were used for testing the effects of LY379268 (0, 0.3, 1.0, 3.0 mg/kg, SC; non-dependent, n=11; post-dependent, n=12) or MTEP (0, 0.3, 1.0, 3.0 mg/kg, IP; non-dependent, n=9; post-dependent, n=9) on ethanol self-administration. Each animal was tested once with each LY379268 or MTEP dose according to Latin square design. Between drug tests, regular self-administration sessions continued until stable ethanol self-administration was reconfirmed. Drug testing began 2 weeks following completion of ethanol dependence induction and was conducted over a 4-week period.
Effects of LY379268 or MTEP on Stress-Induced Reinstatement
One day after the final extinction session, non-dependent and post-dependent rats were divided into two groups for testing the effects of LY379268 (0, 0.3, 1.0, 3.0 mg/kg, SC; non-dependent, n=36; post-dependent, n=39) or MTEP (0, 0.3, 1.0, 3.0 mg/kg, IP; non-dependent, n=41; post-dependent, n=42) on stress-induced reinstatement of ethanol-seeking. Using a between-subjects design, each rat received a single dose of either LY379268 or MTEP before testing. Reinstatement tests were conducted under extinction conditions 5 weeks following completion of ethanol dependence induction. Stress consisted of a 15-min variable intermittent electric footshock (0.5 mA; duration, 0.5 s; mean shock interval, 40 s; range, 10-70 s) administered through the grid floor of the operant conditioning chambers (45, 46). Two minutes following termination of footshock, levers were extended, and responses were recorded for 30 min.
Statistical Analysis
Effects of LY379268 or MTEP on ethanol self-administration were analyzed by mixed-factorial ANOVA with “dependence history” (non-dependent/post-dependent) as the between-subjects factor and “drug dose” as the within-subjects factor. Effects of LY379268 or MTEP on reinstatement were analyzed by two-way between-subjects ANOVA. Significant main effects or interactions were confirmed by Newman-Keuls post hoc tests. Differences in responding between the final extinction session and the reinstatement test conducted under vehicle conditions were analyzed by paired t-tests. Following confirmation of significant main effects or interactions in the overall ANOVA, the magnitude of the inhibition of responding (percent inhibition) was analyzed by mixed factorial ANOVA with “dependence history” as the between-subjects factor and “drug dose” as the within-subjects factor (self-administration tests), or by two-way between-subjects ANOVA (reinstatement tests), followed by Simple Effects analysis.
Results
Blood Alcohol Levels and Withdrawal Ratings
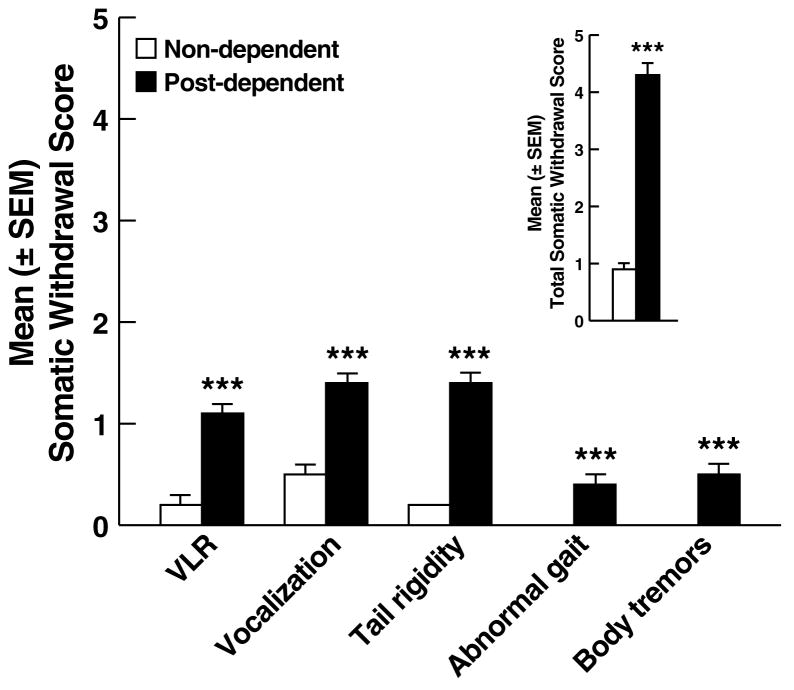
Mean (± SEM) BALs measured on day 5 of dependence induction were significantly elevated (bleed 1: 161.2 ± 4.9 mg%, bleed 2: 240.5 ± 7.3 mg%) with main effects of treatment (F1,196 = 1098.7, p < 0.001), bleeds (F1,196 = 210.1, p < 0.001), and a treatment x bleeds interaction (F1,196 = 186.6, p < 0.001). Twelve hours after the final ethanol administration, post-dependent rats showed overt withdrawal signs (Fig. 1) with significant increases in ventromedial limb retraction (VLR; Mann-Whitney, U = 2131.0, p < 0.001), vocalization (U = 2087.5, p < 0.001), tail rigidity (U = 832.5, p < 0.001), abnormal gait (U = 3342.5, p < 0.001), and body tremors (U = 2955.5, p < 0.001). The sum of the five rating scores revealed the presence of significant overall withdrawal severity (U = 861.5, p < 0.001; Fig. 1 Inset).
Figure 1.
Somatic withdrawal signs measured 12 h after the final ethanol administration. Inset: Overall withdrawal severity (sum of the somatic withdrawal scores across the five behavioral signs of ethanol withdrawal). ***p < 0.001 vs. non-dependent. VLR, ventromedial limb retraction.
Effects of LY379268 or MTEP on Ethanol Self-Administration
The mean (± SEM) number of ethanol-reinforced responses before drug testing remained within ±10% over three consecutive sessions and was not significantly different (F1,40 = 3.1, NS) in non-dependent (34.5 ± 3.7) vs. post-dependent (43.7 ± 3.8) rats.
LY379268
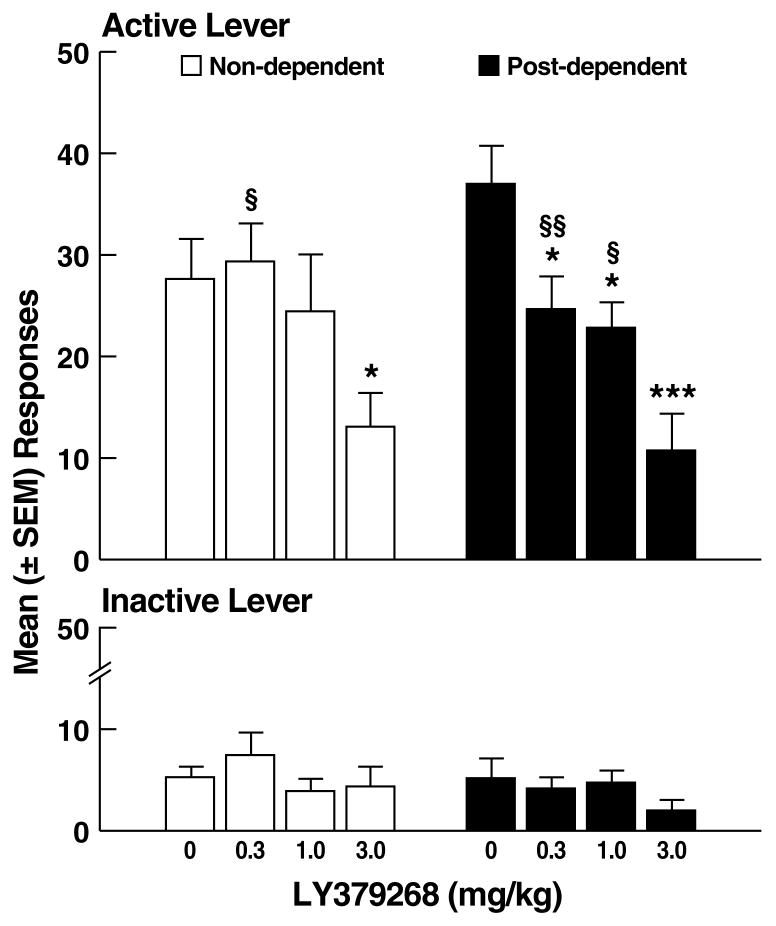
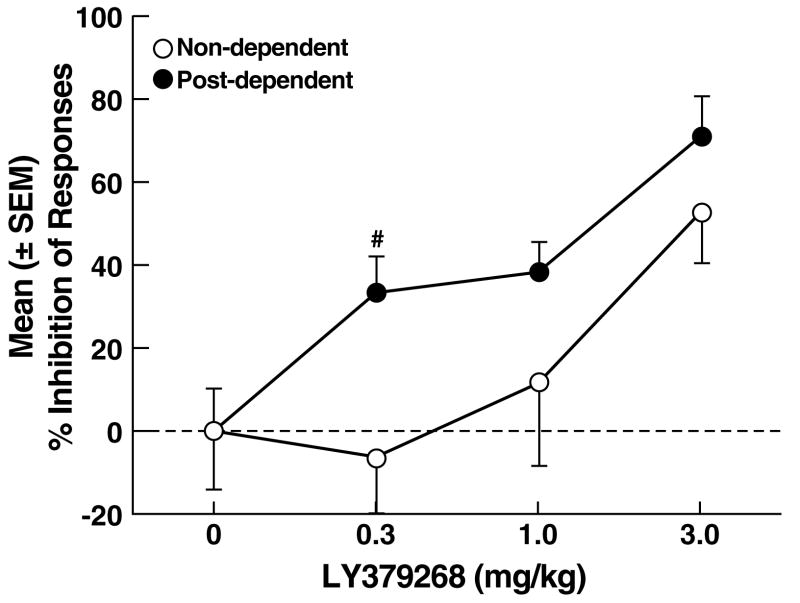
Following vehicle, the mean (± SEM) number of ethanol-reinforced responses was similar in non-dependent (27.6 ± 3.9) and post-dependent (37.0 ± 3.8) rats. LY379268 reduced ethanol self-administration in both non-dependent and post-dependent rats, but was more effective in post-dependent rats (Fig. 2A). This pattern of effects was confirmed by mixed-factorial ANOVA with significant main effects of ethanol dependence history (F1,21 = 4.6, p < 0.05), dose (F3,63 = 197.4, p < 0.001) and an ethanol dependence history x dose interaction (F3,63 = 38.7, p < 0.001). Post hoc tests (Newman Keuls) revealed that LY379268 significantly reduced ethanol self-administration in non-dependent rats at the 3.0 mg/kg dose (p < 0.05; Fig. 2A left panel), but produced significant effects at all doses in post-dependent rats (p < 0.05; Fig. 2A right panel). Scrutiny of the LY379268 dose-effect curve, expressed as the percentage of inhibition of responding (Fig. 2B), confirmed that LY379268 inhibited ethanol self-administration in both non-dependent and post-dependent rats (main effect of dose: F3,63 = 36.9, p < 0.001), and that LY379268 was more effective at suppressing ethanol self-administration in post-dependent rats (p < 0.05) at the lowest (0.3 mg/kg) dose (Simple Effects following main effect of ethanol dependence history: F1,21 = 17.9, p < 0.001 and ethanol dependence history x dose interaction: F3,63 = 3.8, p < 0.05). Inactive lever responses were negligible and unaltered by LY379268, with no main effects of ethanol dependence history (F1,21 = 0.9, NS), dose (F3,63 = 1.5, NS) or ethanol dependence history x dose interaction (F3,63 = 1.0, NS).
Figure 2.
(A) Effects of LY379268 on ethanol self-administration in non-dependent and post-dependent rats. (B) Percent inhibition of ethanol-reinforced responses by LY379268. *p < 0.05, ***p < 0.001 vs. 0 mg/kg (vehicle); §p < 0.05, §§p < 0.01 vs. 3.0 mg/kg; #p < 0.05 vs. 0.3 mg/kg non-dependent.
MTEP
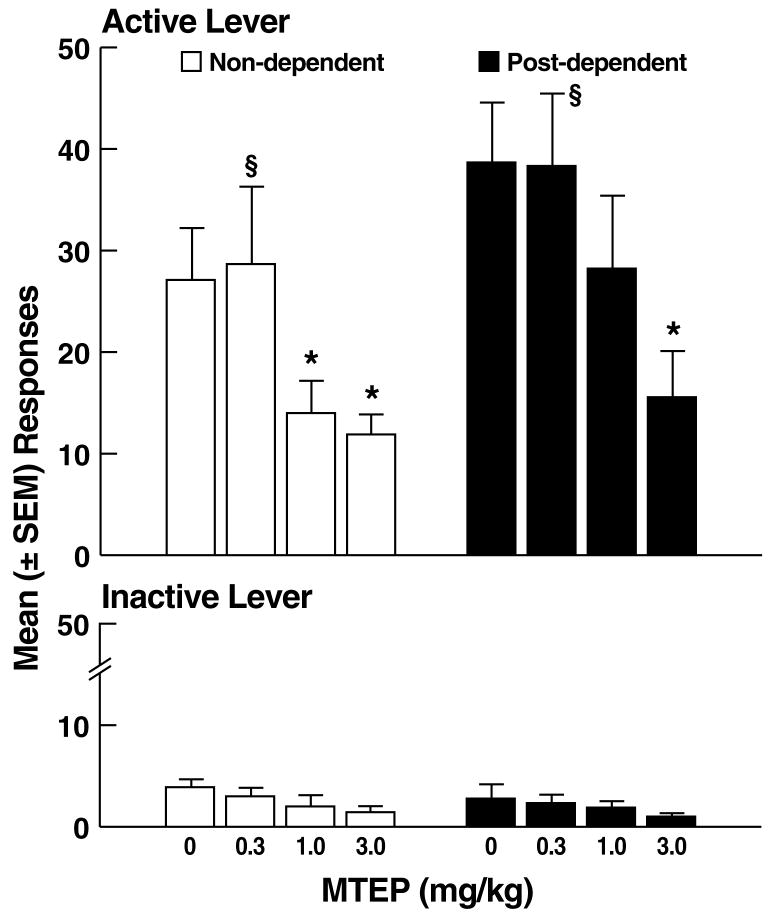
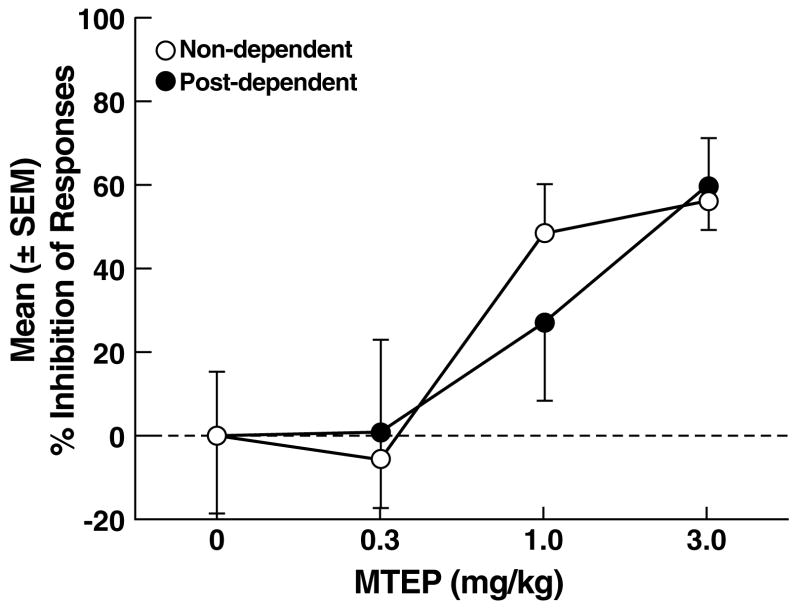
Following vehicle, the mean (± SEM) number of ethanol-reinforced responses was similar in non-dependent (27.1 ± 5.1) and post-dependent (38.7 ± 5.9) rats (Fig. 3A). MTEP reduced ethanol self-administration in both non-dependent and post-dependent rats, but was less effective in post-dependent rats. This pattern of effects was confirmed by mixed-factorial ANOVA, which revealed significant main effects of ethanol dependence history (F1,16 = 4.6, p < 0.05) and dose (F3,48 = 10.4, p < 0.001), but did not confirm an ethanol dependence history x dose interaction (F3,48 = 0.6, NS). Post hoc tests (Newman Keuls) revealed that MTEP significantly reduced ethanol self-administration in non-dependent rats at the 1.0 and 3.0 mg/kg doses (p < 0.05; Fig. 3A left panel), but only at the 3.0 mg/kg dose (p < 0.05) in post-dependent rats (Fig. 3A right panel). Analysis of the “percent inhibition” profiles produced by MTEP showed that although MTEP inhibited ethanol self-administration in both non-dependent and post-dependent rats (main effect of dose: F3,48 = 10.8, p < 0.001), neither a significant main effect of ethanol dependence history (F1,16 = 0.0, NS) nor an ethanol dependence history x dose interaction (F3,48 = 0.5, NS) were evident. Additionally, no statistical differences were detected in the effects of MTEP on ethanol self-administration in non-dependent vs. post-dependent rats at any dose (F1,16 = 0.0, NS; Fig. 3B). Inactive lever responses were negligible and unaltered by MTEP, with no main effects of ethanol dependence history (F1,16 = 0.6, NS), dose (F3,48 = 2.6, NS) or ethanol dependence history x dose interaction (F3,48 = 0.1, NS).
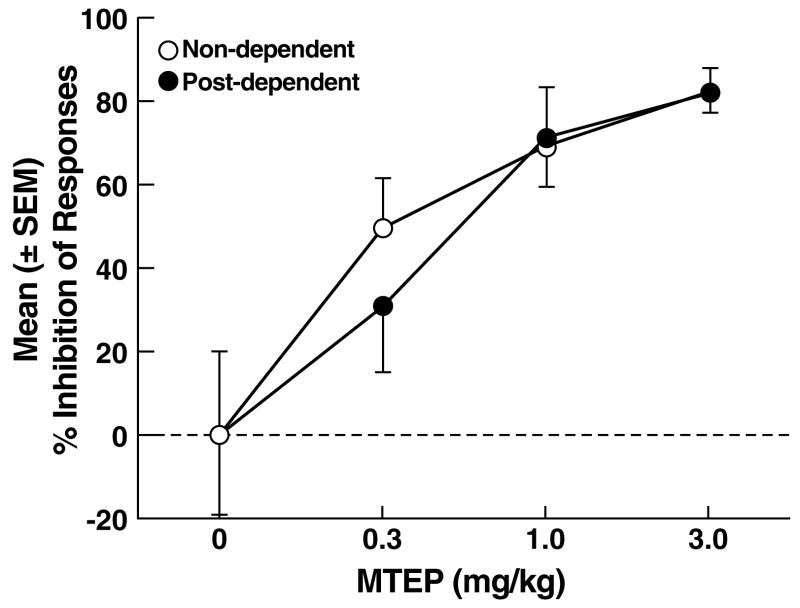
Figure 3.
(A) Effects of MTEP on ethanol self-administration in non-dependent and post-dependent rats. (B) Percent inhibition of ethanol-reinforced responses by MTEP. *p < 0.05 vs. 0 mg/kg (vehicle); §p < 0.05 vs. 3.0 mg/kg.
Effects of LY379268 or MTEP on Stress-Induced Reinstatement
Before extinction training, non-dependent and post-dependent rats maintained stable ethanol self-administration (±10% over three consecutive sessions) with 33.5 ± 2.1 and 33.8 ± 1.9 (mean ± SEM) responses (F1,157 = 0.0, NS), respectively. After 20 extinction sessions, responding decreased to 11.0 ± 0.9 (non-dependent) and 10.3 ± 0.8 (post-dependent) responses (Fig. 4A, 5A), with no differences between groups (F1,157 = 0.2, NS; for details, see Supplement 1).
Figure 4.
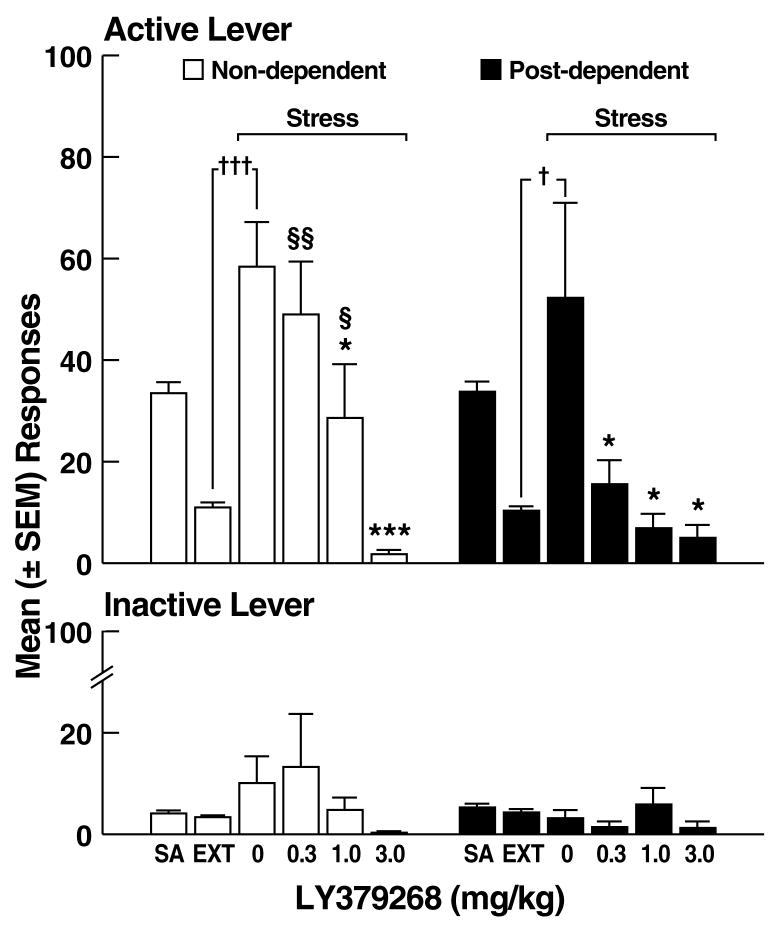
Effects of LY379268 on stress-induced reinstatement of ethanol-seeking. (A) Active and inactive lever responses during self-administration (SA), extinction (EXT), and footshock stress in non-dependent (left panel) and post-dependent (right panel) rats. (B) Percent inhibition of stress-induced reinstatement by LY379268. *p < 0.05, ***p < 0.001 vs. 0 mg/kg (vehicle); §p < 0.05, §§p < 0.01 vs. 3.0 mg/kg; †p < 0.05, †††p < 0.001 vs. EXT; #p < 0.05 vs. 0.3 mg/kg non-dependent; 0.1 > ‡p > 0.05 vs. 1.0 mg/kg non-dependent.
Figure 5.
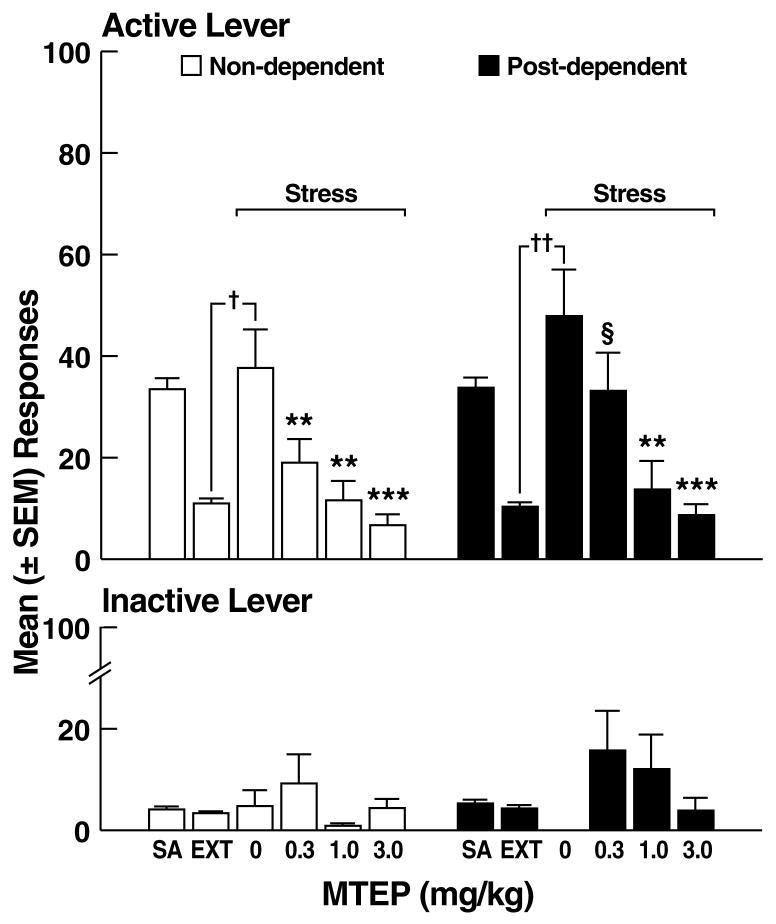
Effects of MTEP on stress-induced reinstatement of ethanol-seeking. (A) Active and inactive lever responses during self-administration (SA), extinction (EXT), and footshock stress in non-dependent (left panel) and post-dependent (right panel) rats. (B) Percent inhibition of stress-induced reinstatement by MTEP. **p < 0.01, ***p < 0.001 vs. 0 mg/kg (vehicle); §p < 0.05 vs. 3.0 mg/kg; †p < 0.05, ††p < 0.01 vs. EXT.
LY379268
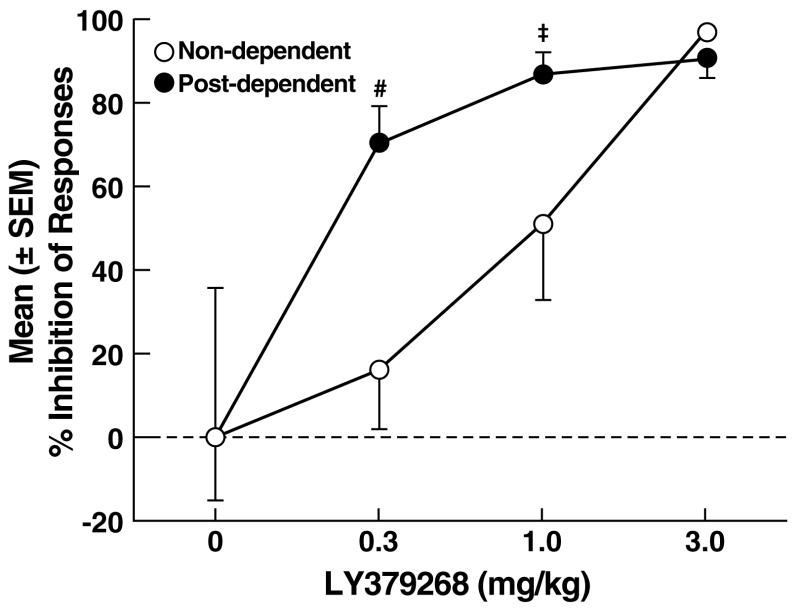
Footshock elicited significant response reinstatement in vehicle-treated rats of both groups (non-dependent, paired t-test: t19 = 19.9, p < 0.001; post-dependent, paired t-test: t21 = 4.6, p < 0.05; Fig. 4A), with similar mean (± SEM) number of responses in non-dependent (58.4 ± 8.9) and post-dependent (52.3 ± 18.7) rats. LY379268 reduced footshock-induced reinstatement of ethanol-seeking in both non-dependent and post-dependent rats, but with greater efficacy in the latter group. This profile of effects was confirmed by two-way ANOVA with significant main effects of ethanol dependence history (F1,75 = 12.6, p < 0.01), dose (F3,75 = 30.9, p < 0.001) and an ethanol dependence history x dose interaction (F3,75 = 3.6, p < 0.05). Post hoc analysis (Newman Keuls) revealed that LY379268 significantly reduced reinstatement in non-dependent rats at the 1.0 and 3.0 mg/kg doses (p < 0.05; Fig. 4A left panel), but significantly reduced reinstatement at all doses in post-dependent rats (p < 0.05; Fig. 4A right panel). Analysis of the “percent inhibition” profiles produced by LY379268 confirmed that LY379268 inhibited reinstatement in both non-dependent and post-dependent rats (main effect of dose: F3,75 = 44.2, p < 0.05), and that LY379268 was more effective at suppressing ethanol-seeking in post-dependent rats (p < 0.05) at the lowest (0.3 mg/kg) dose (Fig. 4B), with effects approaching statistical significance (0.1 > p > 0.05) at the 1.0 mg/kg dose (Simple Effects following main effect of ethanol dependence history: F1,75 = 10.2, p < 0.01 and significant ethanol dependence history x dose interaction: F3,75 = 4.7, p < 0.01). Inactive lever responses were negligible and unaltered by LY379268, with no main effects of ethanol dependence history (F1,75 = 2.4, NS), dose (F3,75 = 1.2, NS) or ethanol dependence history x dose interaction (F3,75 = 1.3, NS).
MTEP
Footshock elicited significant response reinstatement in vehicle-treated non-dependent (t17 = 8.4, p < 0.05) and post-dependent (t15 = 11.5, p < 0.01) rats (Fig. 5A), with similar mean (± SEM) number of responses in the non-dependent (37.7 ± 7.6) and post-dependent (47.9 ± 9.3) groups. MTEP reduced reinstatement of ethanol-seeking in both non-dependent and post-dependent rats, but with reduced efficacy in the post-dependent group. Specifically, two-way ANOVA revealed significant main effects of ethanol dependence history (F1,83 = 4.1, p < 0.05) and dose (F3,83 = 19.8, p < 0.001), but did not confirm an ethanol dependence history x dose interaction (F3,83 = 0.8, NS). Post hoc tests (Newman Keuls) revealed that MTEP significantly reduced reinstatement in non-dependent rats at all doses (p < 0.05; Fig. 5A left panel), but only at the 1.0 and 3.0 mg/kg doses (p < 0.05) in post-dependent rats (Fig. 5A right panel). Analysis of the “percent inhibition” profiles produced by MTEP in non-dependent and post-dependent rats showed that although MTEP inhibited reinstatement in both groups (main effect of dose: F3,83 = 13.9, p < 0.001), neither a significant main effect of ethanol dependence history (F1,83 = 0.2, NS) nor an ethanol dependence history x dose interaction (F3,83 = 0.3, NS) were evident. Additionally, there were no statistical differences in the effects of MTEP on ethanol-seeking in non-dependent vs. post-dependent rats (F1,83 = 0.2, NS; Fig. 5B). Inactive lever responses were negligible and unaltered by MTEP, with no main effects of ethanol dependence history (F1,83 = 0.7, NS), dose (F3,83 = 1.6, NS) or ethanol dependence history x dose interaction (F3,83 = 0.9, NS).
Discussion
An mGlu2/3 agonist (LY379268) and an mGlu5 antagonist (MTEP) reduced ethanol self-administration and footshock-induced reinstatement of ethanol-seeking. However, LY379268 was more effective at reducing both behaviors in rats with a history of ethanol dependence than in non-dependent rats. These findings confirm that mGlu2/3 and mGlu5 receptors participate in mediating ethanol-maintained reinforcement and stress-induced reinstatement of ethanol-seeking, supporting the hypothesis that these receptors provide potentially effective treatment targets for alcohol abuse and relapse prevention to alcohol use associated with stress exposure. Additionally, the results suggest that neuroadaptive changes associated with chronic ethanol exposure or ethanol withdrawal alter the sensitivity of mGlu2/3 receptors, with implications for the neural basis of alcohol dependence and the treatment target potential of these receptors.
The modification of ethanol self-administration and stress-induced reinstatement of ethanol-seeking by LY379268 and MTEP cannot be attributed to nonspecific motor impairment. At the dose range used here, LY379268 and MTEP do not modify either operant responding maintained by non-drug reinforcer (37, 41, 47) or motor coordination and activity (48-51).
Ethanol self-administration and stress-induced reinstatement were identical in vehicle-treated rats in the post-dependent and non-dependent groups. Other studies have demonstrated exacerbated ethanol consumption in post-dependent rats. However, in these studies, animals had been given the opportunity to self-administer ethanol during withdrawal (52, 53). Self-administration of ethanol during withdrawal modifies a subject's ethanol reinforcement history to include learning about negative reinforcement as an important aspect of ethanol's subjective effects. The present experimental design did not provide for such learning to occur. Thus, the lack of increased ethanol self-administration or stress-induced reinstatement in post-dependent rats is a likely consequence of the absence of ethanol availability during withdrawal. For the present purposes, this was a desired outcome permitting to study changes in mGlu2/3 and mGlu5 receptor sensitivity independent of learning factors.
Modification of ethanol reinforcement and stress-induced reinstatement by LY379268 and MTEP
LY379268 and MTEP reduced ethanol self-administration and footshock-induced reinstatement of ethanol-seeking in rats with and without a history of ethanol dependence. In non-dependent rats, reduction of glutamatergic transmission by mGlu2/3 agonists and mGlu5 antagonists is known to decrease ethanol-reinforced responding (10, 19-23, 42) and reinstatement of ethanol-seeking (31, 41, 42, 54). The reduction of footshock-induced reinstatement and ethanol self-administration by LY379268 is consistent with earlier findings in non-dependent rats (41, 42) and confirms a role for mGlu2/3 receptors in ethanol reinforcement and ethanol-seeking associated with stress. The present effects of LY379268 on ethanol reinforcement differ from a single earlier finding that LY404039, an mGlu2/3 agonist structurally related to LY379268, only minimally reduced ethanol self-administration in female alcohol-preferring (P) rats (54). The reasons for this discrepancy remain unclear, but may be related to several factors. The design of the earlier study included multiple tests for each animal in self-administration, extinction and reinstatement procedures, paired with repeated mGlu2/3 agonist pretreatments. Thus, differences in experimental design, drug treatment regimens, and the animals' history of reinforcement may have contributed to the divergence of findings. It can also not be ruled out that Wistar and P rats differ in neuroadaptive changes produced by even limited ethanol exposure (e.g., 55) that may be relevant for sensitivity to mGlu2/3 agonist effects. Indeed, repeated ethanol administration produces differential alterations in the expression of proteins associated with G-protein signaling in the nucleus accumbens in P and Wistar rats (56). Given that mGluRs are G-protein-coupled receptors these changes may be relevant for mGlu2/3 receptor-mediated effects and thereby differentially modify the agonist sensitivity of these receptors in P and Wistar rats.
The finding that MTEP decreased footshock-induced reinstatement of ethanol-seeking in rats is novel although not unexpected because LY379268 is known to attenuate the effects of stress on ethanol-seeking (41) and the net effect of both LY379268 and MTEP is to dampen glutamate-mediated neural excitability, albeit by acting at different synaptic sites. Nonetheless, this observation confirms a role for mGlu5 in addition to mGlu2/3 receptors in regulating ethanol-seeking responses associated with stress.
MTEP reduced ethanol self-administration in non-dependent rats, consistent with previous observations in mice and alcohol preferring P rats (19, 57), with findings using a less selective earlier generation mGlu5 antagonist, MPEP (10, 20-23), and recent reports suggestive of a role of mGlu5 receptors or its intracellular signaling cascades in excessive alcohol intake as determined in models of alcohol binge drinking (58) and genetic ethanol preference (i.e., in P rats; 59). The present data extend this evidence by establishing a role for mGlu5 receptors in the acute reinforcing actions of ethanol in rats not selected for ethanol preference (i.e., genetically heterogeneous Wistar rats) and in ethanol reinforcement under limited-access conditions. Reduction of glutamatergic transmission by either mGlu5 blockade or mGlu2/3 activation within the mesocorticolimbic dopamine circuitry is a likely factor in the reduction of ethanol-reinforced responding, given that decreases in extracellular glutamate are associated with reductions in ethanol reward-related dopamine transmission (60).
Effects of LY379268 and MTEP in ethanol post-dependent rats: Neuroadaptation
A central objective of this study was to establish whether a history of ethanol dependence is associated with alterations in the effects of LY379268 and MTEP on ethanol-seeking and reinforcement. Analysis of the “inhibition profiles” of LY379268's effects on ethanol self-administration and stress-induced reinstatement revealed a significantly greater reduction in both behaviors at the lowest dose in post-dependent vs. non-dependent rats, suggesting a leftward shift in the dose-effect curve. For MTEP, however, analysis of the inhibition profiles failed to confirm statistically significant differences between non-dependent and post-dependent animals. Thus, the results provide evidence of differential effects of mGlu2/3 (but not mGlu5) manipulation on ethanol self-administration and stress-induced reinstatement as a function of dependence history. The increased efficacy of LY379268 in post-dependent rats is likely to result from changes in mGlu2/3 functional activity that serves to reduce hyperglutamatergic tone associated with ethanol dependence (3, 16). Although this hypothesis remains to be tested directly in ethanol post-dependent animals, evidence from studies with chronic psychostimulant exposure supports this possibility (61). Rats with a history of escalated cocaine self-administration show increased sensitivity to the anxiolytic-like effects of LY379268 (62), and increased mGlu2/3 functional activity (Hao, Martin-Fardon and Weiss, unpublished), consistent with the present results. Additionally, stimulation of mGlu2/3 receptors by LY379268 decreases amphetamine-sensitized glutamate overflow in the rat nucleus accumbens and blocks amphetamine-induced locomotor sensitization (63). These observations suggest that mGlu2/3 receptors are functionally upregulated by chronic drug exposure, which would explain the increased efficacy of LY379268 on ethanol-motivated behavior in post-dependent rats.
In contrast to the findings with LY379268, the effects of MTEP remained unaltered in post-dependent rats. It is unclear at present whether ethanol exposure at dependence-inducing levels does not alter mGlu5 receptor sensitivity or whether longer periods or higher levels of ethanol intoxication than used here are necessary to alter mGlu5 signaling. Tentatively consistent with the latter possibility are findings that two months of ethanol liquid-diet exposure induced significant decreases in mGlu5 expression in the hippocampus of male Sprague Dawley rats (64), although the behavioral significance of these changes remains to be explored.
Implications for the Treatment Target Potential of mGluRs
The results confirm that attenuation of glutamatergic transmission by mGlu2/3 agonists or mGlu5 antagonists decreases the reinforcing effects of ethanol and attenuates the effects of stress on ethanol-seeking. In conjunction with previous findings, these results are significant from a treatment target potential perspective because they not only identify both mGlu2/3 and mGlu5 receptors as substrates mediating ethanol-maintained reinforcement and stress-induced reinstatement of ethanol-seeking but also implicate mGlu2/3 receptors as putative targets to counteract neuroadaptive changes in glutamate neurotransmission associated with ethanol dependence. Moreover, the effects of pharmacological manipulation of these receptors extend to attenuation of cue-induced reinstatement of ethanol-seeking (31, 42, 54, 65) and drug-seeking and reinforcement associated with other drugs of abuse (25, 29, 30, 37, 47, 66-69). Specifically, the mGlu2/3 agonist LY379268 has been shown to interfere with reinstatement of drug-seeking induced by cocaine “priming” doses (39, 70) and by cocaine- (37), heroin- (66), or nicotine-associated environmental stimuli (67). Similarly, the mGlu5 antagonists MTEP and MPEP reduce cocaine prime-induced reinstatement (69) and conditioned reinstatement of ethanol- (31, 65), cocaine- (29, 47, 69), methamphetamine- (68), and nicotine-seeking (25, 30). Additionally, mGlu5 blockade attenuates cocaine (25, 71) and nicotine (25, 27) reinforcement. These actions are indicative of potential “therapeutic” actions for a wide array of addiction-related behaviors across several drugs of abuse and implicate mGlu2/3 and mGlu5 receptors as promising targets for the treatment of drug and alcohol abuse and relapse prevention. The present finding that LY379268 and MTEP remain fully effective at reducing ethanol self-administration and stress-induced reinstatement following dependence-inducing ethanol exposure further imply strong treatment target potential for mGlu2/3 and mGlu5 receptors for alcohol addiction. Moreover, in the case of LY379268, efficacy at reducing ethanol self-administration and seeking was enhanced in post-dependent rats with significant suppression of alcohol-directed behavior at a low dose that is well outside the range of undesirable side effects, such as ataxia or locomotor impairment (e.g., 48, 49). Lastly, both LY379268 and MTEP (or MPEP) do not interfere with behavior reinforced by potent natural rewards (37, 72), and this selective interference with drug-seeking is likely to be further indicative of treatment target promise.
In conclusion, the results implicate mGlu2/3- and mGlu5-regulated glutamatergic transmission as mechanisms mediating alcohol-maintained reinforcement and stress-induced reinstatement of alcohol-seeking. Moreover, neuroadaptive changes associated with an alcohol dependence history modify mGlu2/3 receptor-mediated effects, thereby identifying these mGluRs as relevant for the understanding of the neurobiology of alcohol addiction and its pharmacotherapy.
Supplementary Material
Acknowledgments
Research was supported by National Institutes of Health grant AA010531 (FW) from the National Institute on Alcohol Abuse and Alcoholism. The authors thank Drs. James A. Monn and David McKinzie of Eli Lilly Research Laboratories (Indianapolis, IN) for providing LY379268 and Merck (Rahway, NJ) for providing MTEP. The authors also thank Y Zhao, JR Lewis, and E Gradillas for technical support and M Arends for editorial assistance. This is publication 20095-MIND from The Scripps Research Institute.
Abbreviations
- LY379268
(-)-2-oxa-4-aminobicylco[3.1.0]hexane-4,6-dicarboxylic acid
- MTEP
3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine
- MPEP
2-methyl-6-(phenyl-ethynyl)-pyridine
- mGluR
metabotropic glutamate receptor
Footnotes
Financial Disclosures: The authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 2.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 3.Tsai G, Coyle JT. The role of glutamatergic neurotransmission in the pathophysiology of alcoholism. Annu Rev Med. 1998;49:173–184. doi: 10.1146/annurev.med.49.1.173. [DOI] [PubMed] [Google Scholar]
- 4.Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- 5.Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, et al. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- 7.Kenny PJ, Gasparini F, Markou A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. J Pharmacol Exp Ther. 2003;306:1068–1076. doi: 10.1124/jpet.103.052027. [DOI] [PubMed] [Google Scholar]
- 8.Paterson NE, Semenova S, Gasparini F, Markou A. The mGluR5 antagonist MPEP decreased nicotine self-administration in rats and mice. Psychopharmacology (Berl) 2003;167:257–264. doi: 10.1007/s00213-003-1432-z. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen K, Martin H, Berger JE, Seager MA. The mGlu5 receptor antagonists MPEP and MTEP attenuate behavioral signs of morphine withdrawal and morphine-withdrawal-induced activation of locus coeruleus neurons in rats. Neuropharmacology. 2005;48:173–180. doi: 10.1016/j.neuropharm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 10.Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between Group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Knackstedt LA, Kalivas PW. Glutamate and reinstatement. Curr Opin Pharmacol. 2009;9:59–64. doi: 10.1016/j.coph.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R. Insight into the function of Group I and Group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol. 2003;14:257–277. doi: 10.1097/01.fbp.0000081783.35927.8f. [DOI] [PubMed] [Google Scholar]
- 13.Schoepp DD, Conn PJ. Metabotropic glutamate receptors in brain function and pathology. Trends Pharmacol Sci. 1993;14:13–20. doi: 10.1016/0165-6147(93)90107-u. [DOI] [PubMed] [Google Scholar]
- 14.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Heilig M, Egli M. Pharmacological treatment of alcohol dependence: target symptoms and target mechanisms. Pharmacol Ther. 2006;111:855–876. doi: 10.1016/j.pharmthera.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 18.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 19.Cowen MS, Djouma E, Lawrence AJ. The metabotropic glutamate 5 receptor antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]-pyridine reduces ethanol self-administration in multiple strains of alcohol-preferring rats and regulates olfactory glutamatergic systems. J Pharmacol Exp Ther. 2005;315:590–600. doi: 10.1124/jpet.105.090449. [DOI] [PubMed] [Google Scholar]
- 20.Olive MF, McGeehan AJ, Kinder JR, McMahon T, Hodge CW, Janak PH, et al. The mGluR5 antagonist 6-methyl-2-(phenylethynyl)pyridine decreases ethanol consumption via a protein kinase C epsilon-dependent mechanism. Mol Pharmacol. 2005;67:349–355. doi: 10.1124/mol.104.003319. [DOI] [PubMed] [Google Scholar]
- 21.Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PubMed] [Google Scholar]
- 22.Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, et al. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Besheer J, Faccidomo S, Grondin JJ, Hodge CW. Regulation of motivation to self-administer ethanol by mGluR5 in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008;32:209–221. doi: 10.1111/j.1530-0277.2007.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenny PJ, Paterson NE, Boutrel B, Semenova S, Harrison AA, Gasparini F, et al. Metabotropic glutamate 5 receptor antagonist MPEP decreased nicotine and cocaine self-administration but not nicotine and cocaine-induced facilitation of brain reward function in rats. Ann N Y Acad Sci. 2003;1003:415–418. doi: 10.1196/annals.1300.040. [DOI] [PubMed] [Google Scholar]
- 25.Tessari M, Pilla M, Andreoli M, Hutcheson DM, Heidbreder CA. Antagonism at metabotropic glutamate 5 receptors inhibits nicotine- and cocaine-taking behaviours and prevents nicotine-triggered relapse to nicotine-seeking. Eur J Pharmacol. 2004;499:121–133. doi: 10.1016/j.ejphar.2004.07.056. [DOI] [PubMed] [Google Scholar]
- 26.Lee B, Platt DM, Rowlett JK, Adewale AS, Spealman RD. Attenuation of behavioral effects of cocaine by the metabotropic glutamate receptor 5 antagonist 2-methyl-6-(phenylethynyl)-pyridine in squirrel monkeys: comparison with dizocilpine. J Pharmacol Exp Ther. 2005;312:1232–1240. doi: 10.1124/jpet.104.078733. [DOI] [PubMed] [Google Scholar]
- 27.Liechti ME, Markou A. Interactive effects of the mGlu5 receptor antagonist MPEP and the mGlu2/3 receptor antagonist LY341495 on nicotine self-administration and reward deficits associated with nicotine withdrawal in rats. Eur J Pharmacol. 2007;554:164–174. doi: 10.1016/j.ejphar.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Popik P, Wrobel M. Morphine conditioned reward is inhibited by MPEP, the mGluR5 antagonist. Neuropharmacology. 2002;43:1210–1217. doi: 10.1016/s0028-3908(02)00309-x. [DOI] [PubMed] [Google Scholar]
- 29.Backstrom P, Hyytia P. Ionotropic and metabotropic glutamate receptor antagonism attenuates cue-induced cocaine seeking. Neuropsychopharmacology. 2006;31:778–786. doi: 10.1038/sj.npp.1300845. [DOI] [PubMed] [Google Scholar]
- 30.Bespalov AY, Dravolina OA, Sukhanov I, Zakharova E, Blokhina E, Zvartau E, et al. Metabotropic glutamate receptor (mGluR5) antagonist MPEP attenuated cue- and schedule-induced reinstatement of nicotine self-administration behavior in rats. Neuropharmacology. 2005;49 1:167–178. doi: 10.1016/j.neuropharm.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Backstrom P, Bachteler D, Koch S, Hyytia P, Spanagel R. mGluR5 antagonist MPEP reduces ethanol-seeking and relapse behavior. Neuropsychopharmacology. 2004;29:921–928. doi: 10.1038/sj.npp.1300381. [DOI] [PubMed] [Google Scholar]
- 32.Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro PS, Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci. 2008;9:423–436. doi: 10.1038/nrn2379. [DOI] [PubMed] [Google Scholar]
- 34.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 35.Vandergriff J, Rasmussen K. The selective mGlu2/3 receptor agonist LY354740 attenuates morphine-withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal. Neuropharmacology. 1999;38:217–222. doi: 10.1016/s0028-3908(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 36.Cartmell J, Monn JA, Schoepp DD. The mGlu(2/3) receptor agonist LY379268 selectively blocks amphetamine ambulations and rearing. Eur J Pharmacol. 2000;400:221–224. doi: 10.1016/s0014-2999(00)00423-4. [DOI] [PubMed] [Google Scholar]
- 37.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group II metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y, Dayas CV, Aujla H, Baptista MA, Martin-Fardon R, Weiss F. Activation of group II metabotropic glutamate receptors attenuates both stress and cue-induced ethanol-seeking and modulates c-fos expression in the hippocampus and amygdala. J Neurosci. 2006;26:9967–9974. doi: 10.1523/JNEUROSCI.2384-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Backstrom P, Hyytia P. Suppression of alcohol self-administration and cue-induced reinstatement of alcohol seeking by the mGlu2/3 receptor agonist LY379268 and the mGlu8 receptor agonist (S)-3,4-DCPG. Eur J Pharmacol. 2005;528:110–118. doi: 10.1016/j.ejphar.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 43.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 44.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat Rev Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- 46.Ciccocioppo R, Martin-Fardon R, Weiss F, Massi M. Nociceptin/orphanin FQ inhibits stress- and CRF-induced anorexia in rats. Neuroreport. 2001;12:1145–1149. doi: 10.1097/00001756-200105080-00019. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Fardon R, Baptista MA, Dayas CV, Weiss F. Dissociation of the effects of MTEP [3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]piperidine] on conditioned reinstatement and reinforcement: comparison between cocaine and a conventional reinforcer. J Pharmacol Exp Ther. 2009;329:1084–1090. doi: 10.1124/jpet.109.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J Pharmacol Exp Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- 49.Cartmell J, Monn JA, Schoepp DD. Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine. Psychopharmacology (Berl) 2000;148:423–429. doi: 10.1007/s002130050072. [DOI] [PubMed] [Google Scholar]
- 50.Varty GB, Grilli M, Forlani A, Fredduzzi S, Grzelak ME, Guthrie DH, et al. The antinociceptive and anxiolytic-like effects of the metabotropic glutamate receptor 5 (mGluR5) antagonists, MPEP and MTEP, and the mGluR1 antagonist, LY456236, in rodents: a comparison of efficacy and side-effect profiles. Psychopharmacology (Berl) 2005;179:207–217. doi: 10.1007/s00213-005-2143-4. [DOI] [PubMed] [Google Scholar]
- 51.Belozertseva IV, Kos T, Popik P, Danysz W, Bespalov AY. Antidepressant-like effects of mGluR1 and mGluR5 antagonists in the rat forced swim and the mouse tail suspension tests. Eur Neuropsychopharmacol. 2007;17:172–179. doi: 10.1016/j.euroneuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 52.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 53.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 54.Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 55.Smith AD, Weiss F. Ethanol exposure differentially alters central monoamine neurotransmission in alcohol-preferring versus -nonpreferring rats. J Pharmacol Exp Ther. 1999;288:1223–1228. [PubMed] [Google Scholar]
- 56.McBride WJ, Schultz JA, Kimpel MW, McClintick JN, Wang M, You J, et al. Differential effects of ethanol in the nucleus accumbens shell of alcohol-preferring (P), alcohol-non-preferring (NP) and Wistar rats: a proteomics study. Pharmacol Biochem Behav. 2009;92:304–313. doi: 10.1016/j.pbb.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cowen MS, Krstew E, Lawrence AJ. Assessing appetitive and consummatory phases of ethanol self-administration in C57BL/6J mice under operant conditions: regulation by mGlu5 receptor antagonism. Psychopharmacology (Berl) 2007;190:21–29. doi: 10.1007/s00213-006-0583-0. [DOI] [PubMed] [Google Scholar]
- 58.Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Besheer J, Grondin JJ, Cannady R, Sharko AC, Faccidomo S, Hodge CW. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wigmore MA, Lacey MG. Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br J Pharmacol. 1998;123:667–674. doi: 10.1038/sj.bjp.0701662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalivas PW, Lalumiere RT, Knackstedt L, Shen H. Glutamate transmission in addiction. Neuropharmacology. 2009;56 1:169–173. doi: 10.1016/j.neuropharm.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology. 2008;33:1818–1826. doi: 10.1038/sj.npp.1301588. [DOI] [PubMed] [Google Scholar]
- 63.Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]; erratum. :225–908. [Google Scholar]
- 64.Simonyi A, Christian MR, Sun AY, Sun GY. Chronic ethanol-induced subtype- and subregion-specific decrease in the mRNA expression of metabotropic glutamate receptors in rat hippocampus. Alcohol Clin Exp Res. 2004;28:1419–1423. doi: 10.1097/01.alc.0000139825.35438.a4. [DOI] [PubMed] [Google Scholar]
- 65.Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP. Neuropharmacology. 2008;55:546–554. doi: 10.1016/j.neuropharm.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bossert JM, Gray SM, Lu L, Shaham Y. Activation of group II metabotropic glutamate receptors in the nucleus accumbens shell attenuates context-induced relapse to heroin seeking. Neuropsychopharmacology. 2006;31:2197–2209. doi: 10.1038/sj.npp.1300977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gass JT, Osborne MP, Watson NL, Brown JL, Olive MF. mGluR5 antagonism attenuates methamphetamine reinforcement and prevents reinstatement of methamphetamine-seeking behavior in rats. Neuropsychopharmacology. 2009;34:820–833. doi: 10.1038/npp.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kumaresan V, Yuan M, Yee J, Famous KR, Anderson SM, Schmidt HD, et al. Metabotropic glutamate receptor 5 (mGluR5) antagonists attenuate cocaine priming- and cue-induced reinstatement of cocaine seeking. Behav Brain Res. 2009;202:238–244. doi: 10.1016/j.bbr.2009.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology (Berl) 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 71.Kenny PJ, Boutrel B, Gasparini F, Koob GF, Markou A. Metabotropic glutamate 5 receptor blockade may attenuate cocaine self-administration by decreasing brain reward function in rats. Psychopharmacology (Berl) 2005;179:247–254. doi: 10.1007/s00213-004-2069-2. [DOI] [PubMed] [Google Scholar]
- 72.Herzig V, Capuani EM, Kovar KA, Schmidt WJ. Effects of MPEP on expression of food-, MDMA- or amphetamine-conditioned place preference in rats. Addict Biol. 2005;10:243–249. doi: 10.1080/13556210500223272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.