Abstract
The mechanisms that govern human neural specification are not completely characterized. Here we used human embryonic stem cells (hESCs) to study the role of fibroblast growth factor (FGF)-signaling in early human neural specification. Differentiation was obtained by culturing clusters of hESCs in chemically-defined medium. We show that FGF-signaling, which is endogenously active during early differentiation of hESCs, induces early neural specification, while its blockage inhibits neuralization. The early neuralization effect of FGF-signaling is not mediated by promoting the proliferation of existing neural precursors (NPs) or prevention of their apoptosis. The neural instructive effect of FGF-signaling occurs after an initial FGF-independent differentiation into primitive ectoderm-like fate. We further show that FGF-signaling can induce neuralization by a mechanism which is independent of modulating bone morphogenic protein (BMP)-signaling. Still, FGF-signaling is not essential for hESC neuralization which can occur in the absence of FGF and BMP-signaling. Collectively, our data suggest that human neural induction is instructed by FGF-signaling, though neuralization of hESCs can occur in its absence.
Keywords: human embryonic stem cells, neural induction, FGF-signaling, Erk1/2 signaling, primitive ectoderm
Introduction
The induction of neural tissue represents a fundamental step in the generation of the vertebrate nervous system. Early studies in Xenopus embryos suggested that the nascent ectoderm receives an instructive signal from the organizer, a specialized group of dorsal mesodermal cells, which directs it to adopt a neural fate (Spemann and Mangold, 1924). These data set the foundation for the classical model of neuralization, suggesting that organizer’s signals direct the ectoderm towards neuralization, while in their absence, an epidermal fate is adopted.
Subsequent studies have indicated that the signals of the organizer do not directly instruct neuralization, and that the organizer-secreted molecules Noggin, Chordin, and Follistatin exert potent neuralizing effects (Hemmati-Brivanlou et al., 1994; Lamb et al., 1993; Sasai et al., 1995; Smith and Harland, 1992) by binding BMPs, which inhibit neural and promote epidermal differentiation (Fainsod et al., 1997; Piccolo et al., 1996; Zimmerman et al., 1996). These findings led to the ‘default model’ of neural induction, which proposes that ectodermal cells have an autonomous tendency to differentiate into neural tissue (Hemmati-Brivanlou and Melton, 1997).
A variety of studies in chick embryos have challenged the idea that the activity of BMP-antagonists is sufficient to induce neuralization, and suggested that FGF-signaling has a key role in early neural differentiation (reviewed in Stern, 2005; Wilson and Edlund, 2001). It was found that FGFs can induce acquisition of neural identity when they are ectopically expressed in vivo. In addition, in some experimental systems, neural induction is inhibited when FGF activity is disrupted (Bertrand et al., 2003; Hongo et al., 1999; Kengaku and Okamoto, 1993; Koshida et al., 2002; Lamb and Harland, 1995; Launay et al., 1996; Streit et al., 2000; Wilson et al., 2000).
FGFs were suggested to exert their neuralizing-effect through inhibition of BMP-signaling either by phosphorylation of Smad1, (Kuroda et al., 2005) or by repressing the expression of genes involved in BMP-signaling (Koshida et al., 2002; Wilson et al., 2000). Alternatively, recent experiments in various animal-models suggested that FGF-signaling directs neural differentiation via a BMP-independent mechanism (Delaune et al., 2005; Kudoh et al., 2004; Wilson et al., 2001).
In mammals, neuralization of mouse single ESCs was suggested to be independent of FGF-signaling (Smukler et al., 2006; Tropepe et al., 2001). However, others suggested that neural induction of mouse ESCs (mESCs) is not a simple default pathway but depends on autocrine FGF-signaling (Kunath et al., 2007; Pollard et al., 2008; Ying et al., 2003b). Furthermore, it was shown that extracellularly-regulated kinases 1 and 2 (Erk1/2) activity mediates the neuralizing effect of FGF-signaling via a BMP-independent mechanism (Stavridis et al., 2007).
Human ESCs may serve as a model to address these mechanisms during human neurogenesis. For this purpose, we induce differentiation of hESC clusters in chemically-defined suspension culture-conditions (Cohen et al., 2007). We have previously shown in this system that noggin significantly enhances the differentiation into NPs, probably by suppressing the differentiation into lineages other than the neural fate (Itsykson et al., 2005). Similar results were shown in other differentiation culture systems of hESCs (Chambers et al., 2009; Gerrard et al., 2005). Moreover, a recent study reported that hESCs have an intrinsic capability to inhibit BMP-signaling at multiple levels of the BMP cascade during neutralization (Lavaute et al., 2009).
Here, we further use our differentiation system to elucidate the role of FGF-signaling in early neural commitment of hESCs. We show that FGF-signaling has a role in inducing early neural specification. Moreover, the effect of FGF is probably mediated, at least in part, independent of modulating BMP-signaling. Still, in line with the ‘default’ model, FGFs are not essential for neuralization of hESCs, which can occur in the absence of FGF and BMP-signaling. In summary, our data support an instructive role of FGF-signaling in human neural specification.
Material and Methods
Cell culture
Cell culture, immunostaining, and fluorescence activated cell sorting (FACS) analysis were performed as previously described (Cohen et al., 2007). Briefly, hESCs with normal karyotypes (46XX) were cultured up to 10 passages on human foreskin feeders in KnockOut DMEM supplemented with 14% KnockOut Serum Replacement, 1 mM L-glutamine, 1% nonessential amino acids, 50 U/ml penicillin and 50 μg/ml streptomycin (all from Invitrogen Corp., Carlsbad, CA). Colonies of hESCs were dislodged 7 days after passage using Collagenase IV (Invitrogen) into small clusters that were randomly allocated to suspension culture in CDM comprised of DMEM/F12 (1:1), 2% B27, 2 mM L-glutamine, 50 U/ml penicillin, 50 μg/ml streptomycin (Invitrogen), with or without rh-basic-FGF (bFGF; FGF-2; 20 ng/ml), rm-noggin (500 ng/ml; R&D Systems Inc., Minneapolis, MN), SU5402 (5 μM), U0126 (10 μM; Calbiochem, Darmstadt, Germany). SU5402 and U0126 were dissolved in DMSO (Sigma, St. Louis, MO), and the final concentration of DMSO in CDM was 0.01%. DMSO at this concentration did not have an effect on neural differentiation of hESC cluster (Supplementary Fig. S1).
Immunofluorescence staining
Cell clusters were dissociated, plated on laminin (1–24 hour), and fixed in 4% PFA. 0.2% Triton X-100 or 0.1% saponin (Sigma) were used for membrane permeabilization. Cells were incubated with anti-human-Oct-4 (1:50, IgG2b; Santa-Cruz Biotechnology, Inc., Santa-Cruz, CA), human-SSEA-4 (1:100, IgG3; Chemicon, Temecula, CA), polysialic acid (PSA) neural cell adhesion molecule (NCAM; 1:200; IgM; Chemicon), Cytokeratin-8 (1:1; IgG: Becton-Dickinson, San-Jose, CA), human-FGFR1 (Flg; 1:100; Santa-Cruz), human-Nestin (1:200, Chemicon), Musashi1 (1:100, Chemicon), β-tubulinIII (1:2000, IgG2b; Sigma), and PCNA (Proliferating Cell Nuclear Antigen; Rb IgG, 1:200, Bethyl Laboratories, Montgomery, TX). Secondary antibodies: fluorescein-isothiocyanate (FITC)-conjugated goat anti-mouse (1:20; Dako, Glostrup, Denmark) and donkey anti-mouse IgM (1:100; Jackson Immunoresearch Laboratories, Inc., West Grove, PA) antibodies, Texas Red-conjugated goat anti-rabbit or Rhodamine Red-conjugated donkey anti-rabbit (1:200; Jackson) antibodies. Specimens were mounted with medium containing 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories Inc., Burlingame, CA) and visualized with fluorescent (E600; Nikon, Kanagawa, Japan) or confocal microscopes (Fluoview; Olympus, Melville, NY). For quantitative analysis, 200 cells were scored in 4 random fields.
FACS analysis
Cells were dissociated, incubated with anti-SSEA-3 (1:100, rat IgM; Chemicon), or anti-SSEA-4 or anti-PSA-NCAM antibodies. For Oct-4 detection, hESCs were fixed with 100% ethanol (−20°C, 15 minutes) and incubated with anti-Oct-4 (1:20). Secondary antibodies: FITC-conjugated goat anti-mouse (1:100; Dako), allophycocyanin (APC)-conjugated goat anti-mouse (1:500) or R-phycoerythrin (R-PE)-conjugated mouse anti-rat IgM (1:50; Southern Biotech Associates, Inc. Birmingham, AL). For survival assays, following incubation with anti-PSA-NCAM and APC-conjugated goat anti-mouse, cells were further stained for AnnexinV using the MEBCYTO kit (MBL, Woburn, MA). 20,000 live cells were acquired with the FACSCalibur system (Becton-Dickinson) and analyzed with FCS-Expresses software (De-Novo software, LA, CA).
Western blotting
Whole cell lysates (50 μg) were electrophoresed on 3–10% gradient SDS-PAGE gels and transferred to polyvinylidene-fluoride (PVDF) membranes (Bio-Rad Laboratories, Hercules, CA). The blots were probed with anti-Erk2 (1:1,000, Santa-Cruz), anti-double phosphorylated (dp)-Erk1/2 (1:4,000, Sigma), and anti-β-actin (1:10,000, Sigma) antibodies. Membranes were then probed with peroxidase-conjugated secondary antibody (1:5000; Jackson Immunoresearch Laboratories, Inc) and processed with ECL (Thermo-Scientific, Rockford, IL).
Reverse transcriptase (RT) and quantitative polymerase chain reaction (qPCR) analysis
Total RNA was isolated using TRIzol reagent (Invitrogen) and reverse transcribed by M-MLV-RT with random primers (Promega, Madison, WI). Each sample was treated with DNA-free DNase (Applied Biosystems, Foster City, CA). TaqMan qPCR assays were used on a 7900HT cycler (Applied Biosystems). Gene expressions threshold-cycle values were normalized to endogenous levels of GusB.
Population doubling time
hESC clusters were cultured in suspension in 12-well plates. Cell clusters from each well were collected, dissociated, the number of cells per well was counted and the average number of cells per well was calculated in day 0, 4 and 7 of differentiation. The average count numbers were plotted as cells/well against time (days). Population doubling time was analyzed by regression curve analysis using an exponential series.
Statistical analysis
Data are presented as mean ± SEM. Statistical significance was determined by Student’s t-test.
Results
hESCs (HES1 and HES2 lines (Reubinoff et al., 2000)) were cultured on mitotically-inactivated human foreskin-fibroblasts. Since foreskin-fibroblasts secrete bFGF (Eiselleova et al., 2008), supplementation of bFGF was not required to sustain undifferentiated proliferation of the hESCs. To ensure that the cells maintained a pluripotent phenotype under these culture conditions, we analyzed the expression of markers of undifferentiated stem cells after at least 5 passages. FACS analysis and immunostaining demonstrated that above 94.9% of the cells expressed markers of undifferentiated pluripotent stem cells, including SSEA-3, SSEA-4, and Oct-4; 89±3.1% expressed the epithelial marker cytokeratin-8 (CK-8), while only 1.6±0.4% PSA-NCAM (CD56), which is a marker of early NPs (Fig. 1A–C and Supplementary Fig. S2A) (Carpenter et al., 2001; Perrier et al., 2004). Thus, the starting cell populations that were used to study neural differentiation were relatively homogeneous and were comprised mainly of undifferentiated hESCs.
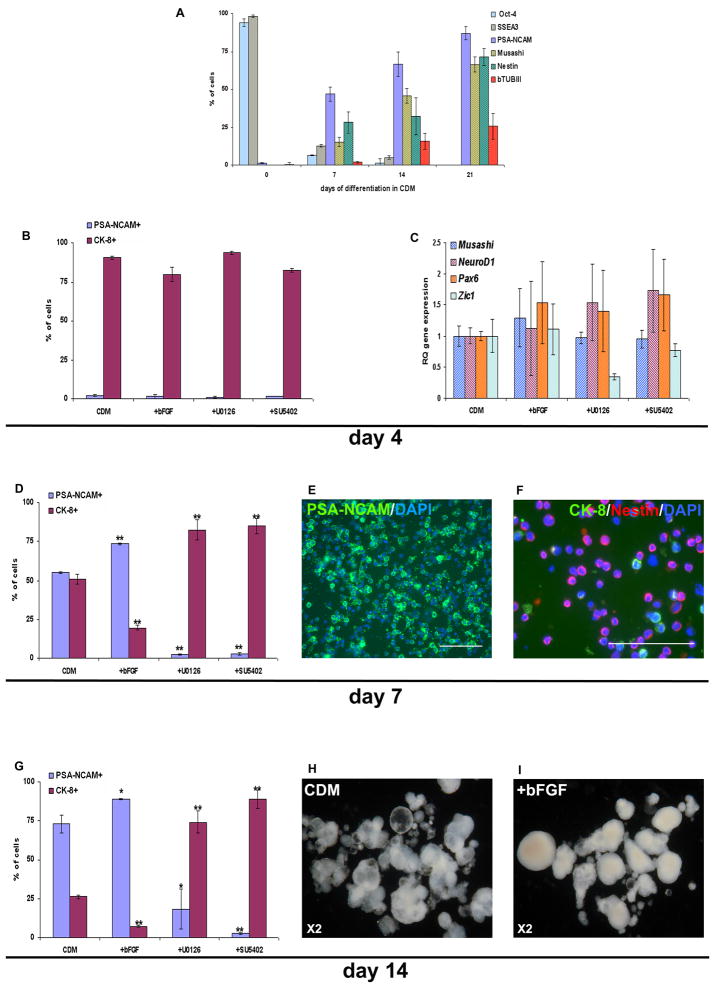
Figure 1. The phenotype of hESCs and modulation of FGF-signaling in their differentiated progeny.
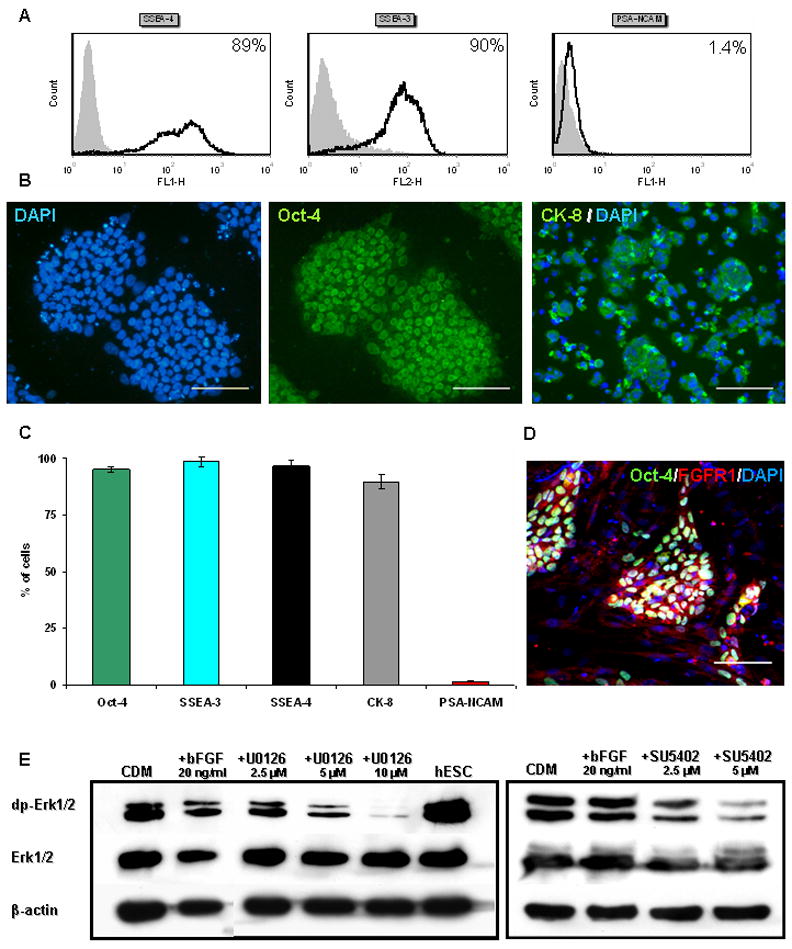
Representative FACS analysis (A) and immunostaining (B) showing that the majority of HES-1 cells express SSEA-4, SSEA-3, Oct-4 and CK-8, while minute percentage express PSA-NCAM. (C) A histogram summarizing the results from four independent experiments. (D) Immuno-fluorescence staining of undifferentiated hESCs co-expressing Oct-4 and FGFR1. (E) Western Blot analysis of the level of dp-Erk1/2 in hESCs, and in hESC-clusters after 7 days’ differentiation in CDM. After 3 hours’ treatment with the inhibitors U0126 and SU5402, a dose-dependent decrease in the levels of dp-Erk1/2 is evident.
Scale bars 100μM
To induce differentiation, small hESC colonies were removed from the feeders and cultured 14 days as free-floating clusters in a chemically-defined medium (CDM). The clusters gradually acquired a morphology characteristic of cystic embryoid-bodies (Fig. 2H). 73±5.6% of the cells within the clusters expressed PSA-NCAM while 26.3±1.1% expressed CK-8, which is not expressed by neural cells (Fig. 2G and Supplementary Fig. S2D). Among various markers of early NPs, PSA-NCAM was the first to appear (Fig. 2A), as shown in hESCs by others (Carpenter et al., 2001; Perrier et al., 2004) and therefore PSA-NCAM was further used to identify neural differentiation.
Figure 2. FGF-signaling promotes the neuralization of hESCs.
Histogram presentation of the analysis of markers of undifferentiated HES-1 hESCs and NPs derived from them by FACS (Oct-4, SSEA-3 and PSA-NCAM) and immunostaining (Musashi, Nestin and β-tubulin-III) during 3 weeks’ differentiation in CDM is presented in (A). Histogram presentation of the percentage of HES-1 cells expressing PSA-NCAM and CK-8 after 4 (B), 7 (D) and 14 (G) days’ differentiation in CDM, CDM supplemented with bFGF, U0126 or SU5402. qPCR analysis of the expression of neural markers at 4 days’ differentiation in the various conditions (C). Immunostaining with anti-PSA-NCAM (E), CK-8 and Nestin (F) of dissociated cells from clusters after 7 days’ differentiation in the presence of bFGF. Dark-field stereomicroscopic images of clusters after 14 days’ differentiation in the absence (H) or presence (I) of bFGF. * P<0.05; ** P<0.01; Scale bars 100μM
We initially confirmed that under our culture conditions, undifferentiated as well as differentiating hESCs express molecules along the FGF signal-transduction pathway. Immunostaining showed that Oct-4+ undifferentiated hESCs co-expressed FGF-receptor-1 (FGFR1; Fig. 1D). In addition, western blot analysis showed high levels of dp-Erk1/2, suggesting active FGF-signaling within undifferentiated hESCs, as reported by others (Kunath et al., 2007; Stavridis et al., 2007). Furthermore, double phosphorylation of Erk1/2 was also demonstrated during hESCs differentiation, within hESC clusters after 1 week of suspension culture (Fig. 1E).
To characterize the role of FGFs, we modulated their signaling by supplementing the medium either with bFGF or two inhibitors of FGF-signaling: the FGFR1-TK-activity inhibitor, SU5402 (Mohammadi et al., 1997) and the mitogen activated protein kinase kinases 1 and 2 (MEK1/2) inhibitor, U0126 (Favata et al., 1998). We studied the effect of U0126, which blocks the Erk1/2 pathway, since it was shown in the mESC system that FGF-induced Erk1/2 signaling is required for neural specification (Stavridis et al., 2007). We determined the minimal concentration of the inhibitors that effectively blocked FGF-signaling, as monitored by the level of dp-Erk1/2 (Fig. 1E). These concentrations were further used along the study.
Profound differences in cluster-morphology were observed when differentiation was induced in the presence of exogenous bFGF, as compared to its absence. In the presence of bFGF, clusters were denser, round, and opaque, did not develop cystic structures, and resembled neural spheres (Fig. 2H–I). After 14 days’ differentiation in the presence of bFGF, the percentage of PSA-NCAM+ NPs was increased to 89.1±0.5% while only 7.3±0.4% expressed CK-8, suggesting that bFGF significantly (p<0.05) augmented the differentiation to NPs (Fig. 2G). When we blocked FGF-signaling by U0126 and SU5402, the percentage of cells expressing PSA-NCAM was significantly (p<0.05, p<0.01; respectively) reduced compared to control cultures (Fig. 2G and Supplementary Fig. S2D). Under these conditions, the majority of cells expressed CK-8 (Fig. 2G and Supplementary Fig. S2D), while the expression of markers of pluripotency was down regulated, (Supplementary Fig. S3) suggesting differentiation towards non-neural lineages. Thus, in the absence of exogenous and endogenous FGF activity, neural differentiation was minimal.
The high percentage of NPs at 14 days, in the presence of bFGF, could be related to a neural inductive-effect of FGFs, a mitogenic effect (Itsykson et al., 2005), or to the promotion of survival of NPs. To further analyze the effect of FGFs and to determine its timing, we characterized the process of neural differentiation at 4 and 7 days. At 4 days of differentiation, the percentage of PSA-NCAM+ NPs was minimal (<1.9%) and not affected by bFGF or FGF-inhibitors supplementation (Fig. 2B and Supplementary Fig. S2B). Furthermore, qPCR analysis showed that exogenous bFGF and blockage of FGF-signaling did not significantly alter the expression of transcripts of early neural genes (Fig. 2C). Regarding proliferation, the percentage of PCNA expressing cells within the population of PSA-NCAM+ NPs was not altered by modulating FGF-signaling (Fig. 3A). These data suggested that at 4 days of differentiation, FGFs had neither a significant mitogenic-effect nor a neural inductive one.
Figure 3. The effect of FGF-signaling on proliferation and apoptosis.
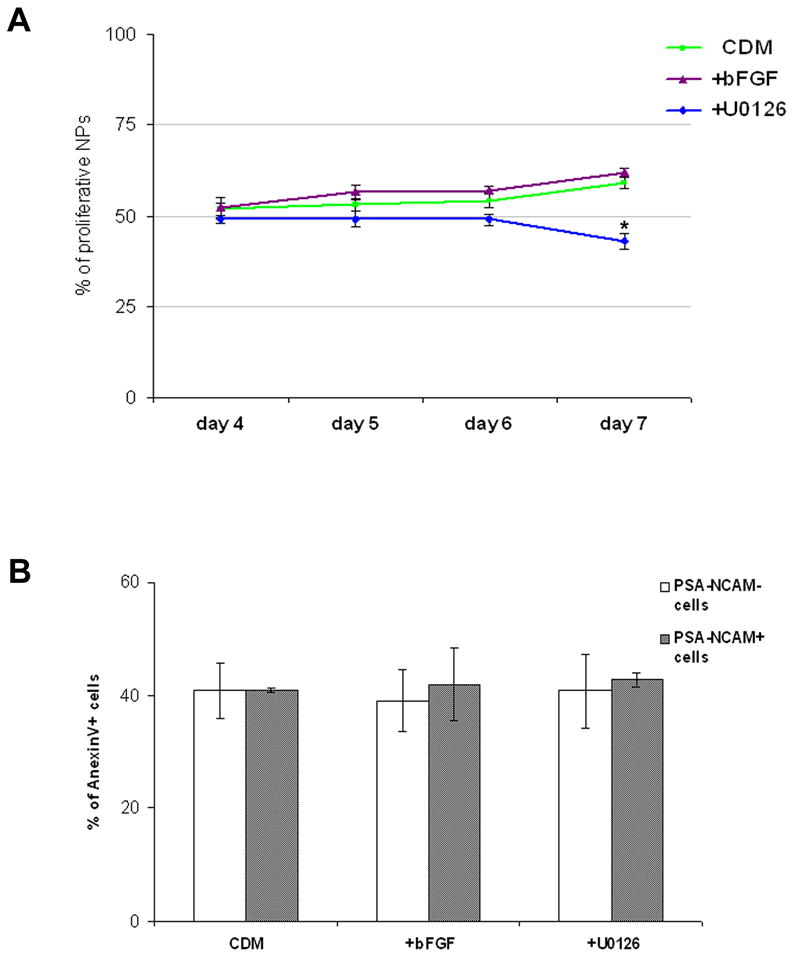
The percentage of proliferating NPs co-expressing PSA-NCAM and PCNA at days 4, 5, 6 and 7 of differentiation in CDM, CDM supplemented with bFGF or U0126 (A). FACS analysis of the percentage of AnexinV+ cells after 7 days of differentiation in the various culture conditions is presented in (B). The percentage of AnexinV+ cells was analyzed in the fraction of PSA-NCAM+ neural and the PSA-NCAM− non-neural cells.
* P<0.05
At 7 days of differentiation, the percentage of NPs was profoundly increased to 73.6±0.6% in the presence of bFGF supplementation. It was minimal in the presence of FGF-signaling inhibitors (2.2±0.5% and 2.6±0.8% with U0126 and SU5402, respectively), while 55±0.8% of the cells differentiated into NPs in CDM, in the absence of exogenous bFGF reflecting the neural inducing effect of endogenous FGF-signaling (Fig. 2D–F and Supplementary Fig. S2C). We sought to determine whether the profound increase in the percentage of NPs from days 4 to 7 was related to an inductive or mitogenic effect of FGFs.
To address this issue we determined the doubling time during days 4–7 in the presence and absence of FGF-signaling. The doubling time was 2.35±0.15 days in the presence of bFGF, 2.58±0.36 days in CDM and was not significantly different with U0126 supplementation (2.71±0.37 days). The similar doubling time between days 4–7, in the presence and absence of FGF-signaling suggested that the profound neuralizing effect of FGF-signaling during this time period could not be attributed to a mitogenic effect. To further address this issue we evaluated the percentage of proliferating NPs, as determined by analyzing the expression of PCNA within PSA-NCAM expressing cells, between days 4–7. The percentages of proliferating NPs were similar in days 4, 5 and 6 when differentiation occurred in the presence of exogenous bFGF, U0126 or in CDM, suggesting that FGF-signaling did not have a mitogenic effect on the NPs during this time period. At day 7, a mild but still significant increase in the percentage of proliferating NPs was observed in the presence of FGF-signaling (61.7±1.3% in CDM supplemented with bFGF and 59.1±1.4% in CDM) compared to its absence (42.9±2.1% in the presence of U0126: Fig 3A). Given the 2.4 days doubling time of the cells between days 4–7 in the presence of exogenous FGF, the mitogenic effect of FGF-signaling at day 7 could only have a marginal contribution to the profound neuralization at this time period.
We further determined whether FGF-signaling augments neuralization by promoting the survival of NPs. At 7 days, the percentage of AnexinV+ apoptotic cells among PSA-NCAM+ NPs was similar to the percentage among non-neural cells (PSA-NCAM−) and both were not altered by modulating FGF-signaling (Fig. 3B). Thus FGFs did not promote the survival of NPs. Since the profound neuralization between days 4–7 could not be related to promotion of proliferation or survival, our data suggest that FGF-signaling have a neural inductive effect, which is initiated after 4 days’ differentiation of free-floating hESC clusters.
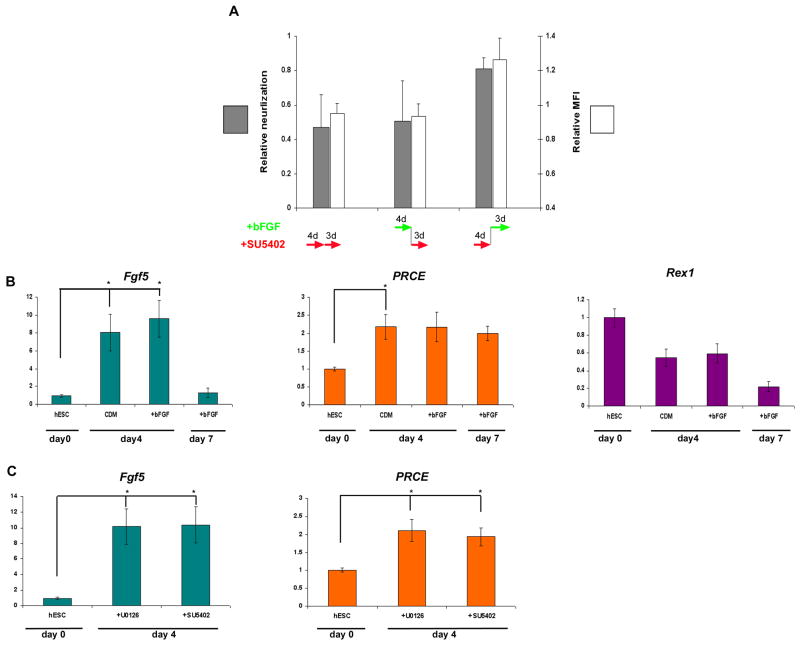
To further support the role of FGFs in inducing neuralization between days 4–7 and the lack of their effect during the first 4 days of differentiation, we performed perturbation studies. The differentiating hESC clusters were treated either for 4 days with SU5402 followed by 3 days of bFGF, or the opposite: first 4 days with bFGF, followed by 3 days of SU5402. Control clusters were treated 7 days with either SU5402 or bFGF. FACS analysis showed that the percentage of NPs expressing PSA-NCAM, and its mean fluorescence intensity (MFI) was low in hESC clusters treated during days 5–7 with SU5402, and was similar to the measurements in clusters after 7 days’ treatment with the inhibitor. Moreover, the FACS parameters were similar in hESC clusters that were treated 7 days with bFGF, and those treated by SU5402 during the first 4 days followed by bFGF during days 5–7. These results further support our finding that FGF-signaling induces neuralization during days 4–7 of differentiation (Fig. 4A).
Figure 4. hESC clusters differentiate initially into primitive ectoderm in an FGF-independent process, which is followed by FGF-directed neuralization.
(A) FACS analysis of the fraction of PSA-NCAM+ cells (gray) and their mean fluorescence intensity (MFI; white) after 7 days’ differentiation in the presence of various combinations of bFGF and SU5402 as indicated. The data was normalized against the same measurements in HES1-clusters after 7 days’ differentiation in the presence of bFGF. (B) qPCR analysis of the expression of markers of primitive ectoderm (Fgf5 and PRCE) and ICM (Rex1) in undifferentiated HES-1 cells, after 4 days’ differentiation in the absence (CDM) and presence of bFGF, and after 7 days’ differentiation in the presence of bFGF. (C) qPCR analysis of the expression of primitive ectoderm markers after 4 days’ differentiation in the presence of FGF-inhibitors.
* P<0.05
Accumulating data support the role of FGF-signaling in promoting pluripotency of hESCs (Xu et al., 2008), while here we demonstrate its role in inducing neuralization between days 4–7. To further study this shift from promoting pluripotency to inducing neuralization, we characterized the differentiation process and its dependence on FGF-signaling during the initial 4 days of suspension culture. qPCR analysis showed that the expression of markers of primitive ectoderm (Rathjen and Rathjen, 2003; Rodda et al., 2002) was up-regulated after 4 days of differentiation. Concomitantly, the expression of the inner cell mass (ICM) marker Rex1 (Rodda et al., 2002; Toyooka et al., 2008) was down-regulated, suggesting that differentiation towards primitive ectoderm-like fate occurred at day 4 (Fig. 4B). Moreover, the expression of markers of primitive ectoderm at day 4 was not affected by inhibiting FGF-signaling (Fig. 4C). These results suggest that under our suspension culture conditions, FGF-signaling can not prevent and does not have a significant role in the early process of differentiation into primitive ectoderm.
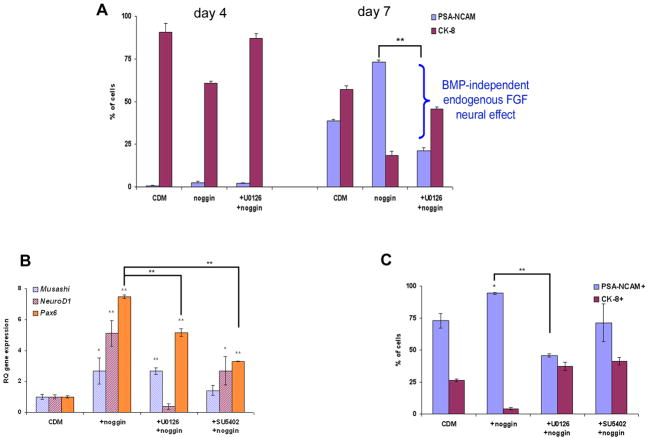
Recent reports in animal models suggest that FGF-signaling may direct neural differentiation via a BMP-independent mechanism (Delaune et al., 2005; Kudoh et al., 2004; Stavridis et al., 2007; Wilson et al., 2001). We therefore determined whether a similar mechanism may mediate, at least in part, the neural inducing effect of FGF-signaling on hESCs. To study a potential BMP-independent effect of FGFs, we blocked BMP-signaling by noggin. In the presence of BMP-signaling blockage, the percentage of NPs was minimal and unchanged at 4 days of differentiation and was significantly augmented at 7 days (p<0.01; Fig. 5A) (Itsykson et al., 2005). When we further inhibited FGF-signaling in addition to the block in BMP-signaling, an increase in the percentage of PSA-NCAM+ NPs was still observed at 7 days. However, the percentage of NPs at day 7 was significantly lower in the presence of noggin plus U0126 compared to noggin alone. This reduction in the percentage of NPs upon the addition of U0126 to noggin reflected the inhibition of neuralizing effect of endogenous FGF-signaling which occurred when BMP-signaling was blocked (marked in Fig. 5A and Supplementary Fig. S4A). These results further support the neural instructive effect of FGF-signaling and suggest that FGF-signaling can induce neuralization of hESCs through a mechanism which is independent of modulation of BMP-signaling.
Figure 5. Neural specification of hESCs occurs in the absence of FGF- and BMP- signaling.
(A) Histogram of the percentage of PSA-NCAM+ and CK-8+ cells after 4 and 7 days’ differentiation of HES-1 clusters in CDM, CDM supplemented with noggin or noggin plus U0126. (B) qPCR analysis of the expression of neural markers after 4 days’ differentiation in the presence of noggin, and noggin plus FGF-inhibitors. (C) The percentages of PSA-NCAM+ and CK-8+ cells after 14 days’ differentiation in the same conditions as in (B).
* P<0.05; ** P<0.01
Using the same experimental approach, we next determined whether FGF-signaling is essential for neuralization. After 4 days’ differentiation in the presence of noggin, the expression levels of transcripts of three early neural markers were significantly up-regulated compared to the levels in control clusters differentiating in CDM. Upon further blockage of FGF in addition to BMP-signaling, the expression levels of the transcripts of early neural markers were still up-regulated, but to a lesser extent (the augmentation of Pax6 expression was significantly lower, NeuroD1 and Musashi were not upregulated in the presence of U0126 and SU5402, respectively; Fig. 5B). Along the same line, 21.35±1.55% and more than 45.8±2.3% of the cells expressed PSA-NCAM after 7 and 14 days, respectively, of differentiation in the presence of noggin and U0126 (Fig. 5A, C and Supplementary Fig. S4), suggesting that FGF-signaling was not required for early neuralization when BMP-signaling was blocked. Still, as noted above, endogenous FGF-signaling profoundly augmented the process of neuralization in the presence of noggin (Fig. 5 and Supplementary Fig. S4). Thus, when BMP-signaling was blocked, FGF-signaling was not essential for neuralization, though it still promoted the tendency for neuralization (Fig. 6).
Figure 6. Early neural differentiation events in hESC clusters – the role of FGF.
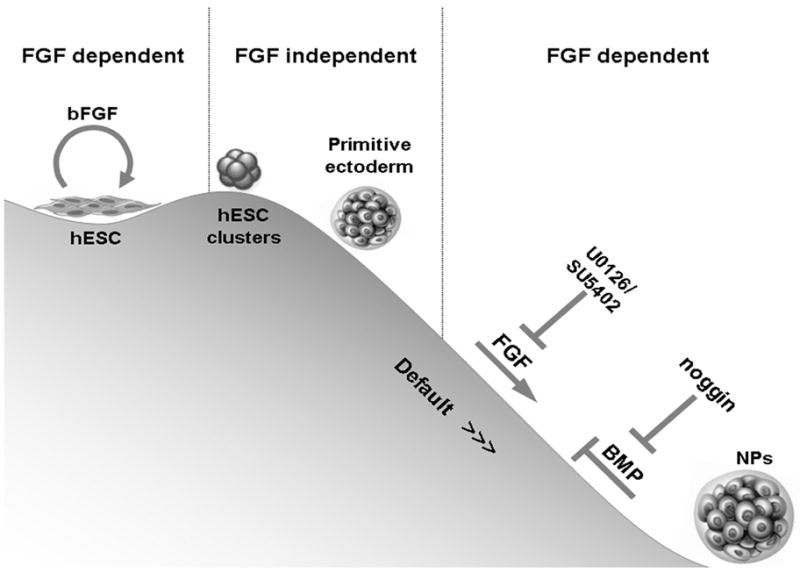
Floating hESC clusters differentiate towards the primitive ectoderm lineage independent of FGF signaling. Further neuralization is instructed by FGF signaling. FGF-signaling induces neuralization, at least in part, through a mechanism which is independent of inhibition of BMP signaling. In line with the default model, inhibition of BMP signaling promotes neuralization of hESCs. FGF signaling encourages the neuralization tendency in the presence of noggin, though it is not essential, since neuralization still occurs when both BMP and FGF signaling are blocked.
Discussion
In this study we show that FGFs induce early neural specification of hESCs, after an initial FGF-independent differentiation into primitive ectoderm-like fate. The neural inductive effect of FGF-signaling is mediated at least in part by a mechanism which is independent of modulating BMP-signaling. Still, neuralization occurs in the absence of FGF and BMP-signaling, suggesting that FGFs are not essential for neural differentiation of hESCs.
To allow the dissection of the events that occur during early human neuralization, we used a simple single-step differentiation approach within a defined serum- and feeder-free suspension culture system. Cultures of undifferentiated hESCs show various levels of background differentiation under standard feeder-dependent culture conditions (Adewumi et al., 2007). To avoid bias of the results due to pre-existing differentiated cells, we confirmed that the starting population of cells was homogenous and mainly comprised of undifferentiated hESCs.
In our differentiation system, after 4 days, hESCs initially differentiated into a primitive ectoderm-like fate as indicated by down-regulation of the expression of the ICM marker Rex1 and up-regulation of Fgf5 and PRCE (Espl1) (Rathjen and Rathjen, 2003; Rodda et al., 2002; Toyooka et al., 2008). A similar initial step of differentiation of hESCs into primitive ectoderm within 3–6 days was also reported by others using different differentiation conditions (Bajpai et al., 2009; Chambers et al., 2009; Pankratz et al., 2007). This initial step was unaffected by modulating FGF-signaling, suggesting that the initial differentiation of hESCs into primitive ectoderm was independent of FGFs.
A similar initial step of differentiation into a primitive ectoderm-like fate was also demonstrated in mESCs (Stavridis et al., 2007). There are significant differences between human and mouse ESCs with regard to marker expression and signaling pathways that promote pluripotency and differentiation. While BMP and LIF signaling promote pluripotency in the mouse system (Ying et al., 2003a; Ying et al., 2008), TGF-β and FGF-signaling play a major role in hESCs (Vallier et al., 2005; Xu et al., 2008). Interestingly, both in the human and mouse systems (Stavridis et al., 2007), differentiation into a primitive ectoderm-like state occur when FGF-signaling is blocked.
Our data suggest that FGF-signaling promoted the differentiation form the stage of primitive ectoderm to the early NP stage. We showed that promotion of proliferation of pre-existing NPs or prevention of their apoptosis could not mediate the neuralizing effect of FGF. The neural inductive effect of FGF-signaling in hESCs is in line with its proposed neuralizing effect in early development of other species (Bertrand et al., 2003; Hongo et al., 1999; Kengaku and Okamoto, 1993; Koshida et al., 2002; Lamb and Harland, 1995; Launay et al., 1996; Stern, 2005; Streit et al., 2000; Wilson and Edlund, 2001; Wilson et al., 2000). FGF-induced neuralization was blocked when we inhibited MEK1/2 suggesting that active Erk1/2 signaling pathway was essential for the FGF neuralization effect. These results are in line with the reported requirement for active Erk1/2 signaling for FGF-induced neural specification in mESCs (Stavridis et al., 2007).
FGF-signaling has diverse roles during development depending on the temporal and spatial states (Mason, 2007). While FGF-signaling was shown to promote the maintenance of undifferentiated hESCs (Vallier et al., 2005; Xu et al., 2008), we demonstrate its role in promoting neural differentiation of hESCs. This dual effect can probably occur, given our results showing FGF-independent differentiation from hESCs into a primitive ectoderm fate, clarifying the potential of FGFs to promote hESC pluripotency on one hand and to promote neural differentiation at a subsequent stage of primitive ectoderm differentiation on the other hand (Fig. 6). In mESCs and chick embryos FGF was also shown to exert its neuralizing effect at the primitive ectoderm stage (Stavridis et al., 2007).
In the presence of noggin, which blocks BMP-signaling, we showed that inhibition of Erk1/2 signaling reduced the percentage of NPs after 7 days of differentiation. This reduction probably reflected the inhibition of a BMP-independent neuralizing effect of endogenous Erk1/2 signaling. Reports in various animal models suggest that FGF-signaling directs neural differentiation via a BMP-independent mechanism (Delaune et al., 2005; Kudoh et al., 2004; Stavridis et al., 2007; Wilson et al., 2001). In line with this notion, a recent study in hESCs revoked the suggested neuralizing-effect of FGFs through the inhibition of BMP-signaling by phosphorylation of Smad1 (Lavaute et al., 2009). Similarly, our results indicate that the effect of FGF on the neuralization of hESCs, at least in part, was not mediated through modulation of BMP-signaling. While our data support the inductive role of FGF-signaling in neural specification of hESCs, both in the presence and block of BMP-signaling, we also show that FGFs are not essential for neuralization of hESCs. In the presence of inhibition of BMP and FGF-signaling, neural differentiation still occurred. Similar results were reported with single mESCs in suspension, which also gave rise to neural cells in the absence of FGF-signaling (Smukler et al., 2006). These findings are in line with the ‘default’ model of neuralization in amphibians (Hemmati-Brivanlou and Melton, 1997).
bFGF is used in protocols for neural induction of hESCs (Bajpai et al., 2009; Cohen et al., 2007; Li and Zhang, 2006). Here we characterized, for the first time, its role and time of action during early neural differentiation of hESCs. Furthermore, in our simple, defined single-step differentiation approach, by using bFGF as a single differentiation inducing factor, 90% of the cells expressed the early neural marker PSA-NCAM within 2 weeks of differentiation. Thus, we show that bFGF can efficiently induce neural differentiation of hESCs and may be used to augment the derivation of NPs from hESCs.
In conclusion, our data suggest that hESCs differentiate into a primitive ectoderm-like fate independent of FGF-signaling, while further neural differentiation is instructed by FGF-signaling. Still, in the presence of BMP antagonism there is a tendency for neuralization which is independent of FGFs but is promoted by their signaling (Fig. 6).
Supplementary Material
Acknowledgments
We thank Michal Gropp, Nurit Yachimovich-Cohen and Michal Aharonowiz for their advice and technical assistance. We acknowledge Shelly Tannenbaum for editing the manuscript. This study was supported by grants from Legacy Heritage Fund, the National Institute of Neurological Diseases and Stroke and Sidney Swartz Chair in Human Embryonic Stem Cell Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewumi O, Aflatoonian B, Ahrlund-Richter L, Amit M, Andrews PW, Beighton G, Bello PA, Benvenisty N, Berry LS, Bevan S, Blum B, Brooking J, Chen KG, Choo AB, Churchill GA, Corbel M, Damjanov I, Draper JS, Dvorak P, Emanuelsson K, Fleck RA, Ford A, Gertow K, Gertsenstein M, Gokhale PJ, Hamilton RS, Hampl A, Healy LE, Hovatta O, Hyllner J, Imreh MP, Itskovitz-Eldor J, Jackson J, Johnson JL, Jones M, Kee K, King BL, Knowles BB, Lako M, Lebrin F, Mallon BS, Manning D, Mayshar Y, McKay RD, Michalska AE, Mikkola M, Mileikovsky M, Minger SL, Moore HD, Mummery CL, Nagy A, Nakatsuji N, O’Brien CM, Oh SK, Olsson C, Otonkoski T, Park KY, Passier R, Patel H, Patel M, Pedersen R, Pera MF, Piekarczyk MS, Pera RA, Reubinoff BE, Robins AJ, Rossant J, Rugg-Gunn P, Schulz TC, Semb H, Sherrer ES, Siemen H, Stacey GN, Stojkovic M, Suemori H, Szatkiewicz J, Turetsky T, Tuuri T, van den Brink S, Vintersten K, Vuoristo S, Ward D, Weaver TA, Young LA, Zhang W. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–816. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Coppola G, Kaul M, Talantova M, Cimadamore F, Nilbratt M, Geschwind DH, Lipton SA, Terskikh AV. Molecular stages of rapid and uniform neuralization of human embryonic stem cells. Cell Death Differ. 2009;16:807–825. doi: 10.1038/cdd.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand V, Hudson C, Caillol D, Popovici C, Lemaire P. Neural tissue in ascidian embryos is induced by FGF9/16/20, acting via a combination of maternal GATA and Ets transcription factors. Cell. 2003;115:615–627. doi: 10.1016/s0092-8674(03)00928-0. [DOI] [PubMed] [Google Scholar]
- Carpenter MK, Inokuma MS, Denham J, Mujtaba T, Chiu CP, Rao MS. Enrichment of neurons and neural precursors from human embryonic stem cells. Exp Neurol. 2001;172:383–397. doi: 10.1006/exnr.2001.7832. [DOI] [PubMed] [Google Scholar]
- Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MA, Itsykson P, Reubinoff BE. Neural differentiation of human ES cells. Curr Protoc Cell Biol. 2007;Chapter 23(Unit 23–27) doi: 10.1002/0471143030.cb2307s36. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Eiselleova L, Peterkova I, Neradil J, Slaninova I, Hampl A, Dvorak P. Comparative study of mouse and human feeder cells for human embryonic stem cells. Int J Dev Biol. 2008;52:353–363. doi: 10.1387/ijdb.082590le. [DOI] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, Blum M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, Copeland RA, Magolda RL, Scherle PA, Trzaskos JM. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Gerrard L, Rodgers L, Cui W. Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells. 2005;23:1234–1241. doi: 10.1634/stemcells.2005-0110. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Kelly OG, Melton DA. Follistatin, an antagonist of activin, is expressed in the Spemann organizer and displays direct neuralizing activity. Cell. 1994;77:283–295. doi: 10.1016/0092-8674(94)90320-4. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate embryonic cells will become nerve cells unless told otherwise. Cell. 1997;88:13–17. doi: 10.1016/s0092-8674(00)81853-x. [DOI] [PubMed] [Google Scholar]
- Hongo I, Kengaku M, Okamoto H. FGF signaling and the anterior neural induction in Xenopus. Dev Biol. 1999;216:561–581. doi: 10.1006/dbio.1999.9515. [DOI] [PubMed] [Google Scholar]
- Itsykson P, Ilouz N, Turetsky T, Goldstein RS, Pera MF, Fishbein I, Segal M, Reubinoff BE. Derivation of neural precursors from human embryonic stem cells in the presence of noggin. Mol Cell Neurosci. 2005;30:24–36. doi: 10.1016/j.mcn.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993;119:1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- Koshida S, Shinya M, Nikaido M, Ueno N, Schulte-Merker S, Kuroiwa A, Takeda H. Inhibition of BMP activity by the FGF signal promotes posterior neural development in zebrafish. Dev Biol. 2002;244:9–20. doi: 10.1006/dbio.2002.0581. [DOI] [PubMed] [Google Scholar]
- Kudoh T, Concha ML, Houart C, Dawid IB, Wilson SW. Combinatorial Fgf and Bmp signalling patterns the gastrula ectoderm into prospective neural and epidermal domains. Development. 2004;131:3581–3592. doi: 10.1242/dev.01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunath T, Saba-El-Leil MK, Almousailleakh M, Wray J, Meloche S, Smith A. FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development. 2007;134:2895–2902. doi: 10.1242/dev.02880. [DOI] [PubMed] [Google Scholar]
- Kuroda H, Fuentealba L, Ikeda A, Reversade B, De Robertis EM. Default neural induction: neuralization of dissociated Xenopus cells is mediated by Ras/MAPK activation. Genes Dev. 2005;19:1022–1027. doi: 10.1101/gad.1306605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb TM, Harland RM. Fibroblast growth factor is a direct neural inducer, which combined with noggin generates anterior-posterior neural pattern. Development. 1995;121:3627–3636. doi: 10.1242/dev.121.11.3627. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Launay C, Fromentoux V, Shi DL, Boucaut JC. A truncated FGF receptor blocks neural induction by endogenous Xenopus inducers. Development. 1996;122:869–880. doi: 10.1242/dev.122.3.869. [DOI] [PubMed] [Google Scholar]
- Lavaute TM, Yoo YD, Pankratz MT, Weick JP, Gerstner JR, Zhang SC. Regulation of Neural Specification from Human Embryonic Stem Cells by BMP and FGF. Stem Cells. 2009 doi: 10.1002/stem.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XJ, Zhang SC. In vitro differentiation of neural precursors from human embryonic stem cells. Methods Mol Biol. 2006;331:169–177. doi: 10.1385/1-59745-046-4:168. [DOI] [PubMed] [Google Scholar]
- Mason I. Initiation to end point: the multiple roles of fibroblast growth factors in neural development. Nat Rev Neurosci. 2007;8:583–596. doi: 10.1038/nrn2189. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, Hubbard SR, Schlessinger J. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Pankratz MT, Li XJ, Lavaute TM, Lyons EA, Chen X, Zhang SC. Directed neural differentiation of human embryonic stem cells via an obligated primitive anterior stage. Stem Cells. 2007;25:1511–1520. doi: 10.1634/stemcells.2006-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier AL, Tabar V, Barberi T, Rubio ME, Bruses J, Topf N, Harrison NL, Studer L. Derivation of midbrain dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci U S A. 2004;101:12543–12548. doi: 10.1073/pnas.0404700101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard SM, Wallbank R, Tomlinson S, Grotewold L, Smith A. Fibroblast growth factor induces a neural stem cell phenotype in foetal forebrain progenitors and during embryonic stem cell differentiation. Mol Cell Neurosci. 2008;38:393–403. doi: 10.1016/j.mcn.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Rathjen J, Rathjen PD. Lineage specific differentiation of mouse ES cells: formation and differentiation of early primitive ectoderm-like (EPL) cells. Methods Enzymol. 2003;365:3–25. doi: 10.1016/s0076-6879(03)65001-9. [DOI] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, Kavanagh SJ, Rathjen J, Rathjen PD. Embryonic stem cell differentiation and the analysis of mammalian development. Int J Dev Biol. 2002;46:449–458. [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smukler SR, Runciman SB, Xu S, van der Kooy D. Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol. 2006;172:79–90. doi: 10.1083/jcb.200508085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Uber Inductuion von Embryonalangen durch Implantation artfremder Organisatoren. Roux’s Arch EnteMech Org. 1924;100:599–638. [Google Scholar]
- Stavridis MP, Lunn JS, Collins BJ, Storey KG. A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development. 2007;134:2889–2894. doi: 10.1242/dev.02858. [DOI] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development. 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Toyooka Y, Shimosato D, Murakami K, Takahashi K, Niwa H. Identification and characterization of subpopulations in undifferentiated ES cell culture. Development. 2008;135:909–918. doi: 10.1242/dev.017400. [DOI] [PubMed] [Google Scholar]
- Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J, van der Kooy D. Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron. 2001;30:65–78. doi: 10.1016/s0896-6273(01)00263-x. [DOI] [PubMed] [Google Scholar]
- Vallier L, Alexander M, Pedersen RA. Activin/Nodal and FGF pathways cooperate to maintain pluripotency of human embryonic stem cells. J Cell Sci. 2005;118:4495–4509. doi: 10.1242/jcs.02553. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Edlund T. Neural induction: toward a unifying mechanism. Nat Neurosci. 2001;4(Suppl):1161–1168. doi: 10.1038/nn747. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Rydstrom A, Trimborn T, Willert K, Nusse R, Jessell TM, Edlund T. The status of Wnt signalling regulates neural and epidermal fates in the chick embryo. Nature. 2001;411:325–330. doi: 10.1038/35077115. [DOI] [PubMed] [Google Scholar]
- Xu RH, Sampsell-Barron TL, Gu F, Root S, Peck RM, Pan G, Yu J, Antosiewicz-Bourget J, Tian S, Stewart R, Thomson JA. NANOG is a direct target of TGFbeta/activin-mediated SMAD signaling in human ESCs. Cell Stem Cell. 2008;3:196–206. doi: 10.1016/j.stem.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell. 2003a;115:281–292. doi: 10.1016/s0092-8674(03)00847-x. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003b;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.