Abstract
Temporomandibular joint (TMJ) pain has been reported to last for prolonged periods in humans. In rodents a variety of methods have been used to measure TMJ nociception, but for most of these methods the period of measurement has been minutes to a couple of hours. In addition, most measurement protocols required restraint or training of the animal. Previous studies from our laboratory demonstrated that feeding behavior, particularly meal duration, was an indicator of TMJ nociception in unrestrained and untrained male and female Sprague Dawley rats for up to two days. In this study, we first found that injection of complete Freund's adjuvant (CFA) into the TMJ of rats significantly lengthened meal duration for 19 days and also decreased meal frequency for 42 days. Interestingly, the meal duration varied significantly from day to day within the 19 day period. TMJ interleukin-1β (IL-1β) and calcitonin gene-related peptide (CGRP) were significantly elevated in the TMJ tissues of CFA injected animals and the level of these markers was attenuated as the meal duration decreased with time. Control animals injected with saline into the TMJ or CFA into the knee did not show a significant lengthening in meal duration but did show a decrease in meal frequency. In a second study, DBA/1LacJ mice given TMJ CFA injections showed a significantly lengthened meal duration on four of the seven days measured using end-of-the meal definition of 5 or 10 minutes. No other meal pattern changed significantly. Two days post-CFA injection, the DBA/1LacJ mice showed significantly elevated interleukin-6 (IL-6), but not elevated IL-1β. Seven days post-injection, both IL-6 and IL-1β were significantly elevated. No change in CGRP was detected. In this study C57BL/6 mice also received TMJ CFA injections, but they did not show a lengthening in any meal pattern or significant increases in IL-1β, IL-6 or CGRP. Our data show, for the first time, that meal duration can be used to measure CFA-induced nociception in the TMJ over the course of several weeks in unrestrained rats and for up to seven days in the DBA/1LacJ mouse strain. In addition, C57BL/6 mice are resistant to CFA-induced TMJ nociception at the same dose used in the DBA/1LacJ mice.
Introduction
Temporomandibular joint disorders (TMD) are often characterized by pain and dysfunction of the temporomandibular joint (TMJ) and surrounding muscles. A portion of the individuals with TMJ pain show an accompanying inflammatory component of the joint. For example, patients with cartilage degradation are often observed to have some level of synovitis [1].
In male and female rats, meal duration was a measure of nociception resulting from injection of complete Freund's adjuvant (CFA) into the TMJ but our previous studies were only for 2 days [2-6]. Injection of CFA into the TMJ significantly lengthened meal duration in rats for two days, while the same amount of CFA in the knee did not lengthen meal duration [5]. Food intake and meal frequency were reduced in animals after injecting CFA into the TMJ but these meal patterns were also reduced after injecting the knee suggesting these meal patterns were not specific for orofacial pain [5]. Another meal pattern, meal size, remained unchanged by administration of CFA. For these reasons, meal duration was a focus of study in our lab. When the rats received prior treatment with ibuprofen meal duration was normal after CFA injection in both male and female rats [6]. Interestingly IL-1β remained significantly elevated in the TMJ of the ibuprofen treated animals injected with CFA [6], suggesting that some inflammation from the CFA injection remained, but because the ibuprofen attenuated the nociception meal duration normalized as the nociception subsided. In another study, cyclooxgenase-II (COX-2) inhibitors normalized meal duration in rats after CFA injection [2]. In this study the COX-2 inhibitor also attenuated the inflammation, i.e., TMJ tissue IL-1β normalized [2]. In still another study, rats were given capsaicin or vehicle at 2 and 10 days of age; capsaicin permanently destroyed afferent nociceptive fibers in these animals [7]. When these male rats reached adulthood saline or CFA was injected into the TMJ and their meal duration was measured. Capsaicin treatment alone had no effect on meal duration, because saline injected, non-capsaicin treated rats had the same meal duration as saline injected, capsaicin treated rats. Non-capsaicin treated rats injected with CFA had longer meal durations than rats that were pre-treated with capsaicin, which demonstrated meal duration after CFA injection was normalized due to a capsaicin-induced loss of afferent nociception neuronal fibers [7]. The lack of change in meal duration in these capsaicin treated male rats occurred despite CFA inducing greater TMJ swelling, which demonstrated that the physical and mechanical changes in the inflamed TMJ synovial joint did not affect meal duration measurements. Another rationale for suggesting that meal duration is a measure of nociception stems from the finding that eating is impaired in patients with TMD [8] and from a clinical study of juvenile rheumatoid arthritic children [9] that examined chewing performance as an objective measure of masticatory function. It showed that the juvenile rheumatoid arthritic children with TMD symptoms changed their chewing habits presumably to “guard” against pain. Most recently, meal duration in the rat was shown to be increased over the course of a week following pulp exposures demonstrating meal duration can also be used as a measure of tooth nociception [10].
Using meal duration to measure TMJ nociception in rats offers several advantages over other methods used today: 1) the animal does not have to be trained prior to testing as compared to a previous thermal sensitivity test [11, 12]; 2) the animal is no longer handled once the TMJ inflammation is induced, thus reducing further compounding stresses that might arise in bite force and Von Frey filament tests [13, 14]; 3) no artificial test-induced competing behaviors are generated [11, 12]; 4) testing continues 24 hours a day, thereby eliminating any artifacts of testing in the light phase, as has occurred in many previous studies, when the rodent normally sleeps [13-15]; and 5) testing can continue in the undisturbed animal for days in contrast to a test for scratching behavior [15]. All of these factors make the TMJ meal duration measurement a powerful tool for studying the mechanisms of TMD nociception.
As mentioned above, CFA induced changes in TMJ nociception, as measured by meal duration, lasted only a few days. For the first time we investigated the effect for over three weeks. Also, we measured inflammatory mediators interleukin 1β (IL-1β) and interleukin-6 (IL-6) and calcitonin gene-related peptide (CGRP) in the TMJ and CGRP in the trigeminal ganglia (TG) to determine if there was an association between expression of one of these molecules and nociception.
The second study was based on the fact that mice have been used to study arthritis and inflammatory nociception and that differences in species have been shown to impact the incidence and severity of these measurements. The mouse strain DBA/1 has the major histocompatibility complex H-2q resulting in the animal being highly sensitive to heterologous and homologous collagen-induced arthritis [16, 17]. In addition to collagen-induced arthritis, CFA can induce an arthritic response in certain mouse strains [18]. Because the DBA/1 strain has H-2q injection of the adjuvant CFA into the TMJ was expected to elicit a robust nociceptive response. In contrast, the C57BL/6 mouse strain does not carry this allele and may be more resistant to artificially induced TMJ nociception. C57Bl/6 mice are important because many knockout mice have this background and future studies using feeding behavior to test for a gene's role in nociception would use a knockout having a C57Bl6 background. In our studies, we wanted to determine if the DBA/1 mice were more sensitive to TMJ CFA-induced nociception than the C57Bl/6 strain.
The first goal of the following experiments was to show for the first time that a meal pattern can measure a persistent increase in TMJ nociception in rats and a second goal was to show that a meal pattern is a measure of nociception in the TMJ of mice, particularly DBA/1 mice. A third primary goal was to determine the protein concentration of inflammatory molecules in the joint during which the nociceptive measurements were being completed.
Materials and Methods
These studies were approved by the Baylor College of Dentistry Institutional Animal Care and Use Committee in accordance of the guidelines of the USDA and National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985). In the first study, male Sprague Dawley rats (250 grams) were purchased from Harlan Industries (Houston, TX) and in the second study, six-week-old male C57BL/6 mice (22 grams) and DBA/1LacJ mice (18 grams) were purchased from Jackson Laboratory (Bar Harbor, ME). The rodents were housed individually in a constant-temperature room at 23°C with standard food pellets (Harlan Industries) and water available ad libitum for at least three days. All the animals were maintained on a 12:12 h light/dark cycle with the lights on at 06:00. The animals were transferred to the feeding modules and left undisturbed for five days before experimentation. Their body weights were recorded daily.
Meal Pattern Analysis
The computer record of pellets dropped over time establishes the meal patterns (meal duration, meal frequency, food intake and meal size). Meal patterns were characterized using data acquired from 32 feeding modules that were situated within sound-attenuated chambers equipped with photobeam computer-activated pellet feeders (Med Assoc. Inc., East Fairfield, VT). The rats were given 45 mg rodent chow pellets, and the mice were fed 20 mg rodent chow pellets (Bioserv, Frenchtown, NJ). When the animal removed a pellet from the feeder trough, a photobeam placed at the bottom of the trough would no longer be blocked, signaling the computer to drop another pellet, record the date and time, and keep a running tally of the total daily food consumption. The record of pellets dropped over time was computer-analyzed with a proprietary computer program to establish the meal patterns [19]. In the meal pattern calculations for the rat, the end-of-the-meal was defined as when no pellets were removed from the feeder for ten minutes [20]. In the mice the end-of-the-meal has not been defined and thus, three end-of-the-meal definitions were used in our meal pattern calculations; 5, 10 or 15 minutes. The minimum meal size needed to be at least three pellets.
CFA injection
At 08:00 the Sprague Dawley rats or DBA/1LacJ or C57Bl/6 mice were anesthetized with isoflurane (5% flow), and given bilaterally either saline or CFA injections into the TMJ or knee. The injections were made using a 29-gauge, one-half-inch needle (Becton Dickinson, Franklin Lakes, NJ). The TMJ injections were completed by guiding the needle, angled at 30-40 degrees to the sagittal plane, under the zygomatic arch, and inserting it 2-3 mm, followed by injection into the peri-articular space of the TMJ within 5 seconds. For the knee the needle was inserted anteriorly for an intracapsular injection.
The rats received 250 μg of CFA (Chondrex, Redmond, WA) or 0.9% saline in a 50 μl volume into the TMJ or the knee. Previously we observed [21, 22] that a similar injection caused a persistent TMJ inflammation for up to 6 weeks; in that study meal duration was not measured.
The mice of each strain were injected bilaterally into the TMJ with 30 μg of CFA or 0.9% saline in a 6 μl volume. An additional group of DBA/1LacJ mice also received a bilateral CFA injection (30 μg/6 μl) into the knee. The dosage of CFA injected into the mice was based on a dose-response experiment indicating that this dose produced a significant decrease in food intake compared to the controls one day after injection (Table 1). A saline knee injection was not performed since previous studies showed that saline knee injections did not affect meal patterns [5].
Table 1. 24-hour food intake after injection of varying doses of CFA into C57Bl/6 mice.
| CFA Dose per TMJ | Day of Measurement | Control | CFA |
|---|---|---|---|
| 6 μg | 0 | 3.9±0.3 | 4.2±0.5 |
| 1 | 3.9±0.1 | 3.1±0.4 | |
| 12 μg | 0 | 3.9±0.3 | 4.2±0.4 |
| 1 | 3.9±0.1 | 3.5±0.1 | |
| 30 μg | 0 | 3.8±0.2 | 3.4±0.2 |
| 1 | 3.6±0.3 | 2.5±0.3a |
Food intake values (grams) one day before (0) and one day after (1) TMJ injection of varying CFA doses. Values are given as the means ± SEM. A total of at least four animals were included for each treatment and control group.
P<0.05, comparing the TMJ CFA-injected group to the control group.
Following injection all the animals were mobile within five minutes or less after the removal of anesthesia. After injection, a portion of the Sprague Dawley rats or the DBA/1LacJ or C57Bl/6 mice were placed into the feeding monitors for pattern measurement and analysis.
In the rat study, the meal patterns were recorded and calculated from one day before injection up to 42 days after TMJ injection. In the saline injected animals 13 were in the group; in the CFA injected animals 14 were in the group and for the 21 day measurement after CFA knee injection 7 animals were in the group.
In the mouse study, the DBA/1LacJ mice had their TMJ injected with saline (n = 12 animals/group) or CFA (n= 11 per group) or they had their knee injected with CFA (n = 5 per group). The C57Bl/6 mice had their meal patterns measured for one day before and seven days after TMJ injection (saline n = 7; CFA n = 5).
Inflammatory marker analysis
Additional Sprague Dawley rats, DBA/1LacJ or C57Bl/6 mice were placed in hanging cages; the animals were fed the pellet diets and their TMJs injected with saline or CFA. TMJ retrodiscal, synovium and disc tissue samples were collected from the rats 7, 21 and 42 days after TMJ saline or CFA injection (n = 7-8 per group) and in the mice two and seven days after the injection (n = 9 per group) for later assay of IL-β, IL-6 and CGRP. On the day of collection, the animals were removed from their cages and sacrificed within 20 seconds by decapitation to minimize stress, and the samples were placed in plastic bags and submerged in ice water. Removal of TMJ tissue was initiated by performing a horizontal skin incision parallel to and just inferior to the zygomatic arch. The masseter and temporalis muscles were cut away from the arch and the arch removed with ronjeurs. After freeing the soft tissue from its attachments to the temporal and zygomatic bones, the soft tissue were cut in a coronal plane just anterior to the ear canal and anterior to the condyle followed by two horizontal cuts at the base of the condylar head medially and laterally. The soft tissue, which includes the synovial membrane, joint capsule, retrodiscal tissue, articular disc, and a small amount of the lateral pterygoid muscle, were then excised. After dissection, the tissues were placed in liquid nitrogen and stored at −80°C until assayed for cytokine and CGRP expression.
At the time of the analysis, the soft tissue from one side was ground with a tissue homogenizer (Ultra-Turrax, Jankel & Kunkel, Germany) in a lysis buffer (75 mM potassium acetate pH 7.4, 300 mM NaCl, 10 mM EDTA, .25% Triton X-100, protease inhibitors) and evaluated for IL-1β and IL-6 in both rats and mice by ELISA assays (R & D Systems, Minneapolis, MN) following the manufacturer's directions. For the CGRP assay, the soft tissue from the opposite side and from the TG was ground in 1M acetic acid followed by centrifugation of the sample [23]. The supernates were lyophylized and re-suspended in the appropriate RIA buffer supplied with the CGRP kit [7] (Phoenix Pharmaceuticals, Burlingame, CA). The total protein in the ground TMJ and TG samples was determined using the BCA Protein Assay (Pierce, Rockford, IL) following the manufacturer's directions. The concentrations of the cytokines and CGRP are expressed as the amount of cytokine or CGRP per mg of total protein.
Statistical analysis
Two-way ANOVA with repeated measures was used to analyze the rat meal patterns (i.e., meal duration, meal frequency, food intake and meal size), cytokine and CGRP data. The independent variables were treatment (saline and CFA) and time. The dependent variable was either a meal pattern or the amount of cytokine or CGRP. Visual inspection of the dark phase meal duration data indicated that treatment had no effect from day 31 through day 42. Therefore, we performed an ANOVA for the first 30 days and reported those results.
Meal duration values for each rat were plotted over the course of the 42 day measurement period. Inspection of the data showed that individual rats had an atypical lengthening of their meal duration on certain days. To determine if a significant change in meal duration occurred between days for a given rat, we utilized the fact that each rat ate 5-15 meals a day and compared the average meal duration per day between two different days using a paired t-test. For the rats, Spearman's correlations were run to test for associations between meal duration and cytokine or CGRP concentration over time (SPSS v. 17.0, SPSS, Inc, Chicago, IL.).
Three-way ANOVA with repeated measures was used to analyze the mouse meal patterns, cytokine and CGRP data. The independent variables were treatment (saline and CFA), strain (DBA/1LacJ and C57Bl/6) and time. The dependent variable was either a meal pattern or the amount of cytokine or CGRP.
The meal pattern data in the rats and DBA/1LacJ mice given knee injections were analyzed using a two-way ANOVA with repeated measures. The independent variables were treatment (saline or CFA) and time. A meal pattern was the dependent variable.
Total protein was analyzed using a two-way ANOVA with repeated measures. The independent variables were treatment and time. Total protein (mg/ml) was the dependent variable.
Tests having significant main effects were further analyzed using Duncan's post-hoc test. P values less than 0.05 were deemed to be statistically significant.
Results
Rat meal patterns
Rats and mice take 85-95% of their meals during the dark phase [24, 25] and because most feeding activity is when the animal is in a dark environment all meal pattern data is reported for the dark phase. One meal pattern for the rat, meal duration, showed a significant main effect for treatment [(SALINE/TMJ, CFA/TMJ), F(1, 26) = 5.61, P<0.05] and time [(DAYS), F(29, 754) = 14.14, P<0.001]. Meal frequency also showed a significant main effect for treatment [(SALINE/TMJ, CFA/TMJ), F(1, 26) = 16.18, P<0.001] and time [(DAYS), F(29, 754) = 13.22, P<0.001]. In the rat no significant main effects for treatment were noted for the dark phase food intake or meal size.
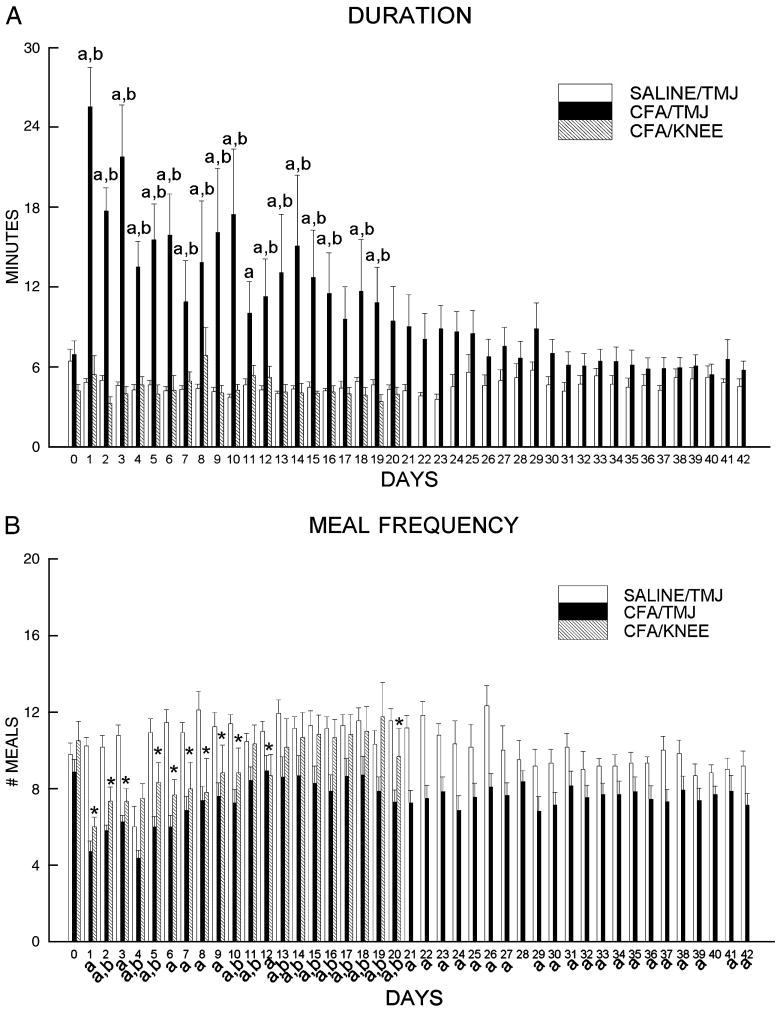
A post-hoc test showed that meal duration was significantly lengthened by CFA injection (SALINE/TMJ versus CFA/TMJ) for 19 days (Fig. 1A) and meal frequency was significantly reduced by CFA injection for 42 days (Fig. 1B), with the greatest difference just after injection. The difference in meal duration slowly declined until it was no longer significant at day 20 and meal frequency increased in the CFA injected rats during the first week but the difference remained significant throughout the measurement period (Fig. 1). For 17 of the 19 days in which knee data was collected, animals that received injection of CFA in the TMJ had a significantly longer meal duration in comparison to rats that had CFA injected into their knee (Fig. 1A). For 13 of the 19 days the rats that received a CFA injection in the TMJ showed a significant reduction in meal frequency versus rats receiving CFA injection in the knee (Fig. 1B). The meal duration of the knee injected rats did not differ significantly from TMJ saline injected animals over the same measurement period. In contrast, meal frequency was significantly reduced 11 of the 19 days when comparing the CFA knee injected rats versus the rats that received a saline TMJ injection (Fig. 1B)
Figure 1.
A) Dark phase meal duration and B) dark phase meal frequency are shown for Sprague-Dawley rats after bilateral injection of 50 μl saline (SALINE) into the temporomandibular joint (TMJ) (SALINE/TMJ, n = 13) or 250 μg of complete Freund's adjuvant (CFA) into the TMJ (CFA/TMJ, n = 14) or knee (KNEE/CFA, n = 7). Data include pre-day (0) to TMJ injection through 42 days after injection. A 10 minute end-of-the-meal definition was used in the calculations for these graphs. Values are given as the means ± SEM. SALINE/TMJ group compared to the CFA/TMJ group, a = P<0.05 and CFA/TMJ group as compared to the CFA/KNEE group, b = P<0.05. SALINE/TMJ group compared to the CFA/KNEE group, *=<0.05.
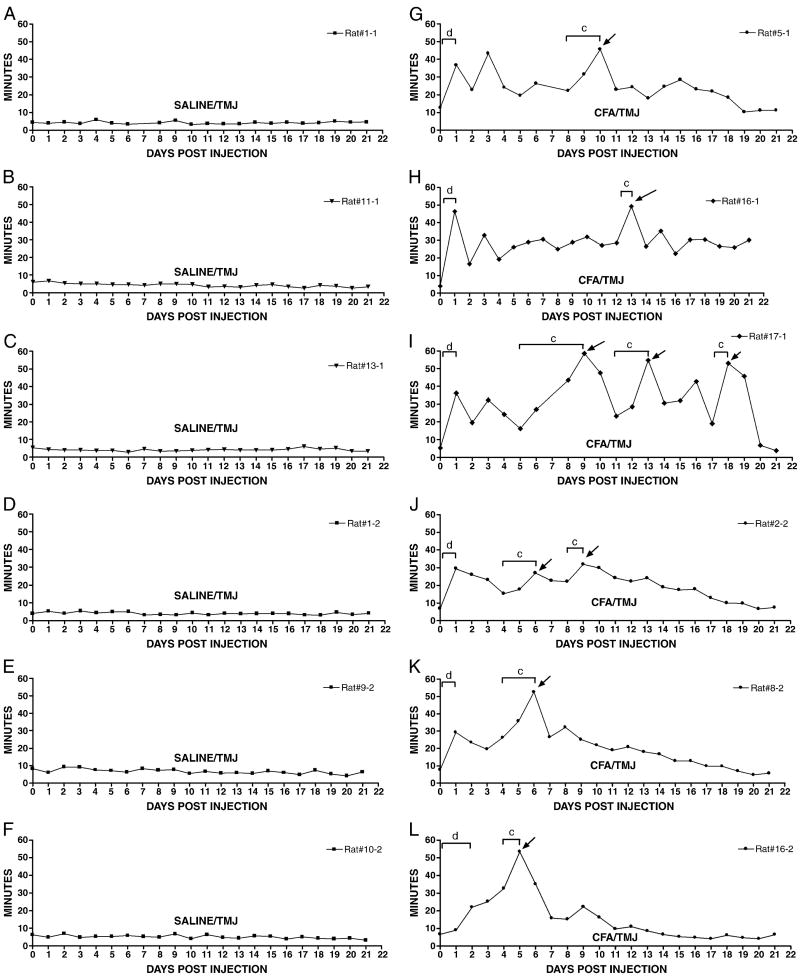
The meal durations of six SALINE/TMJ (Fig. 2A-F) and six CFA/TMJ treated rats (Fig. 2G-L) were selected from the main group and individually plotted for 21 days. The meal durations of the control animals remained consistently low with no large fluctuations over the 21 days of measurement (panels A-F). The meal duration of the six CFA injected animals remained significantly higher than the controls over most of this measurement period (panels G-L). During this 21 day measurement period the six CFA injected rats showed significant day to day variation in meal duration (panels G-L). For example, on day 10, the meal durations of the CFA-injected rat (Fig. 2, panel G) was significantly longer than on day 8.
Figure 2.
Meal duration measurements are shown for representative Sprague-Dawley rats over a three week period. Panels A-F show 24-hour meal duration values for six different rats over a 21 day period in the SALINE/TMJ group. Panels G-L shows 24-hour meal duration values for six different rats in the CFA/TMJ group. Meal duration was recorded by computer for 24 hours prior to CFA injection, pre-day (0) and 22 days thereafter, post-days (1, 2, 3, etc.). About half of the rats treated with CFA showed additional lengthening in meal durations. Statistical analysis showed that meal duration values (arrow) were significantly longer than the previous day's meal duration. Note brackets comparing meal duration values for specific days where c = P<0.05, d = P<0.001. See the legend of Figure 1 for explanations and abbreviations.
Rat molecular response
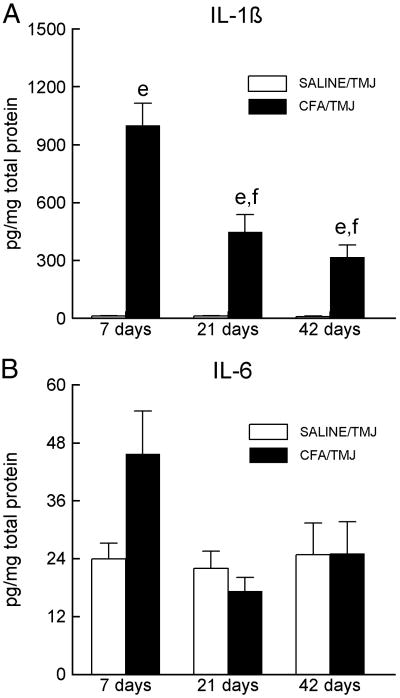
Statistical analysis of the IL-1β data indicated a significant main effect for treatment [(SALINE/TMJ vs. CFA/TMJ), F(1, 14) = 365.0, P<0.001] and time [F(2, 28) = 10.52, P<0.001]. Post-hoc analysis showed that the concentration of IL-1β was significantly higher in the CFA treated animals as compared to the saline injected animals on days seven, 21 and 42 (Fig. 3A). In the CFA injected animals, the IL-1β concentration decreased significantly on days 21 and 42 compared to day seven (Fig. 3A).
Figure 3.
Interleukin 1β (IL-1β) and IL-6 levels in the TMJ tissues of Sprague-Dawley rats. (A) IL-1β or (B) IL-6 levels within the synovial membrane, joint capsule, retrodiscal tissue, articular disc and a small portion of the lateral pterygoid muscle 7, 21 and 42 days after saline or CFA injection. Within a particular day, the SALINE/TMJ group was compared to the CFA/TMJ group and is indicated by e = P<0.01. The letter “f” indicates that the cytokine levels in the CFA/TMJ-treated groups on days 21 and 42 were significantly (P<0.01) reduced compared to the values of the CFA/TMJ-treated group on day 7. See the legend of Figure 1 for explanations and abbreviations.
Although the concentration of IL-6 was higher in the CFA group than in the saline injected group on day 7, the difference was not significant (Fig. 3B).
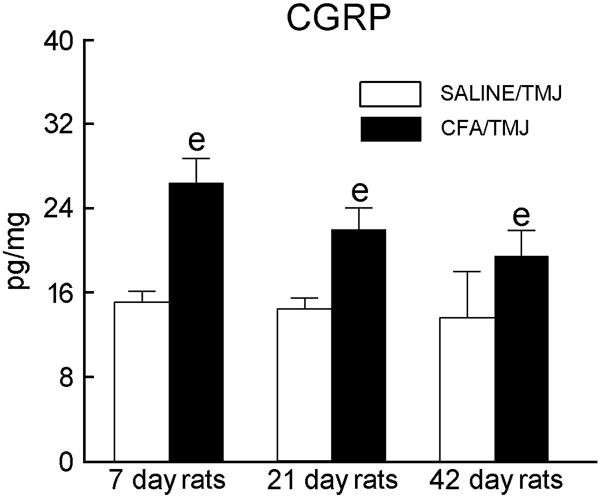
The concentration of CGRP was measured in the rat TMJ, treatment [(SALINE/TMJ vs. CFA/TMJ), F(1, 15) = 17.5, P<0.001] but not time [F(2, 28) = 10.52, P<0.10] showed a significant main effect. The concentration of CGRP in the TMJ was significantly higher in the CFA injected animals on days seven, 21 and 42 when compared to the saline-injected animals (Fig. 4). CGRP in the TG was much higher (ranging from 284.7 to 403.4 pg/mg protein) than that found in the TMJ, but in the TG treatment and time showed no significant main effect.
Figure 4.
Calcitonin gene-related peptide (CGRP) levels in the retrodiscal, synovial and disc tissue of the TMJ of Sprague-Dawley rats 7, 21 and 42 days after saline or CFA injection. Within a particular day, the SALINE/TMJ group was compared to the CFA/TMJ group, as indicated by e = P<0.01. See the legend of Figure 1 for explanations and abbreviations.
In rats injected with CFA, there were significant positive associations between meal duration and time (i.e. 7, 21 and 42 days), meal duration and IL-1β concentration (r = 0.99, P<0.01) and meal duration and CGRP concentration in the TMJ (r = 0.99, P<0.01) (Fig. 1S). No association was detected between meal duration the concentration of TMJ IL-6 (r = 0.50, P=0.68).
Mice meal patterns
When analyzing the meal patterns for the mice the three end-of-the-meal definitions (i.e., 5, 10 and 15 minutes) were applied because a end-of-the-meal definition has not been characterized in the mouse as in the rat [20]. When the end-of-the-meal definition was 5 minutes the main effect for dark meal duration was significant for treatment [(SALINE/TMJ vs. CFA/TMJ), F(1,31) = 8.97, P<0.01], strain [(DBA/1LacJ vs. C57Bl/6), F(1,31) = 10.33, P<0.01] and time [(DAYS), F(6,186) = 3.22, P<0.01]. Similarly, when the end-of-the-meal definition was 10 minutes the main effect for dark meal duration was significant for treatment [F(1,31) = 5.26, P<0.05], strain [F(1,31) = 4.18, P<0.05] and time [F(6,186) = 3.55, P<0.01]. But when the end-of-the-meal definition was 15 minutes the main effect for dark meal duration was significant only for strain [F(1,31) = 4.68, P<0.05] but not treatment [F(1,31) = 3.09, P=0.08] or time [F(1,31) = 1.92, P=0.08]. Importantly no significant main effects for treatment were noted for the dark phase food intake, meal size or meal frequency when a 5, 10 or 15 minute end-of-the-meal definition was applied.
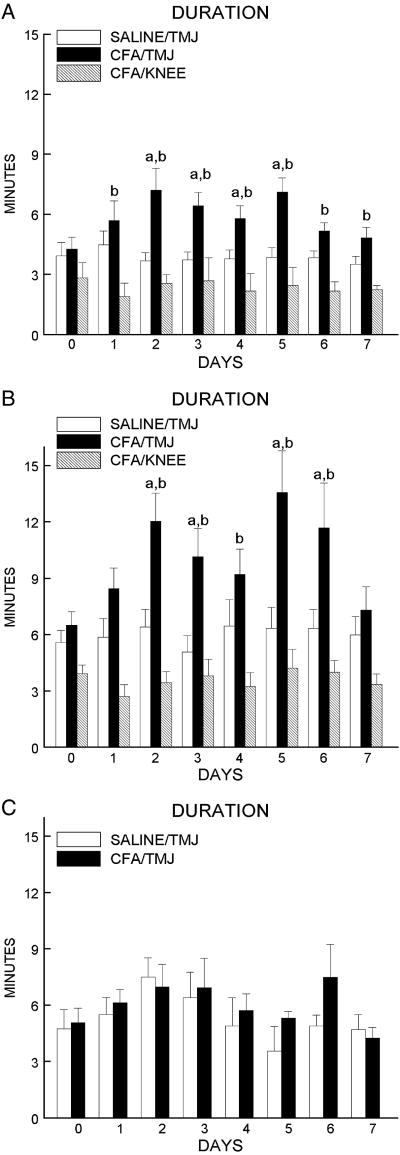
Post-hoc tests indicated the dark phase meal duration for the DBA/1LacJ mice was significantly longer on days 2, 3, 4 and 5 post CFA injection when using an end-of-the-meal definition of 5 minutes (Fig. 5A) and was longer on days 2, 3, 5 and 6 when using an end-of-the definition of 10 minutes (Fig. 5B). In DBA/1LacJ mice, meal duration was significantly higher in the CFA/TMJ group versus the CFA/KNEE group for days 1 through 7 when using an end-of-the definition of 5 minutes (Fig. 5A) and for days 2, 3, 4, 5 and 6 when using an end-of-the definition of 10 minutes (Fig. 5B).
Figure 5.
DBA/1LacJ mice received a bilateral injection of 6 μl saline (n = 12) into the TMJ (SALINE/TMJ) or 30 μg of CFA (n = 11) into the TMJ (CFA/TMJ) or 30 μg of CFA (n = 5) was injected into the knee (CFA/KNEE). A) Dark phase meal duration was calculated for the DBA/1LacJ mice using a 5 minute end-of-the meal definition and a B) 10 minute end-of-the meal definition. C) C57BL/6 mice received bilateral injection of 6 μl saline (n = 7) into the TMJ (SALINE/TMJ) or 30 μg of CFA (n = 5) into the TMJ (CFA/TMJ) and the dark phase meal duration was calculated using an end-of-the-meal definition of 10 minutes. Meal duration was recorded on the day before injection, pre-day (0), and seven days after injection. Values are given as the means ± SEM. A comparison of the SALINE/TMJ group to the CFA/TMJ group is indicated by a = P<0.05 and a comparison of the CFA/TMJ group to the CFA/KNEE group is indicated by b = P<0.05.
In the C57B1/6 mice, the dark phase meal duration, meal frequency, food intake and meal size did not differ significantly between the SALINE/TMJ and CFA/TMJ group on any of the seven days of measurement after applying an end-of-the-meal definition of 5, 10 (Fig. 5C) or 15 minutes. Non-injected DBA/1LacJ control mice of the same weight had an average daily meal duration of 6.6 ± 1.5 minutes and the non-injected C57B1/6 mice had a average daily meal duration of 5.9 ± 1.2 minutes.
Mice molecular response
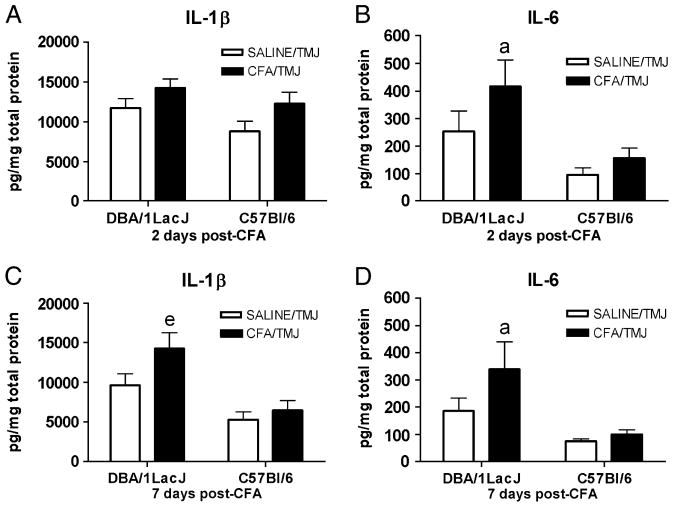
In the mice, the TMJ IL-1β concentration was significantly affected by treatment [(SALINE/TMJ vs. CFA/TMJ), F(1,28) = 7.64, P<0.01]; strain [(DBA/1LacJ vs. C57Bl/6), F(1,28) = 16.03, P<0.01]; and time [F(1,28) = 12.94, P<0.01]. The DBA/1LacJ CFA/TMJ mice had a higher IL-1β concentration than the SALINE/TMJ group on day seven (Fig. 6C), but not on day two (Fig. 6A). Interestingly, the TMJ IL-1β concentration of the C57Bl/6 mice (Fig. 6) did not differ significantly between treatment groups (SALINE/TMJ vs. CFA/TMJ) on either measurement days two or seven. Note: in non-injected DBA/1LacJ mice the IL-1β concentration in the TMJ was 8793 ± 1850 pg/mg total protein and the IL-1β concentration in the TMJ of non-injected (SALINE/TMJ) C57Bl/6 mice was 8775 ± 1281 pg/mg total protein (n = 9 per group).
Figure 6.
IL-1β and IL-6 cytokine levels in the TMJ tissues of DBA/1LacJ and C57Bl/6 mice after CFA injection. IL-1β levels per mg of total protein within the retrodiscal, synovial and disc tissue of the TMJ two days (A) and seven days (C) after CFA injection. IL-6 levels per mg of total protein within the TMJ tissues two days (B) and seven days (D) after CFA injection. Within a particular day when comparing the SALINE/TMJ group to the CFA/TMJ group a significance of P<0.05 is indicated by the letter “a” and a significance of P<0.01 is indicated by the “e”. See the legend of Figure 5 for explanations and abbreviations.
ANOVA revealed that the TMJ IL-6 concentration was significantly affected by treatment [(SALINE/TMJ vs. CFA/TMJ), F(1,28) = 6.59, P<0.01]; strain [(DBA/1LacJ vs. C57Bl/6), F(1,28) = 24.08, P<0.01], but not time. The DBA/1LacJ CFA/TMJ mice had a higher IL-6 concentration than the SALINE/TMJ group on both days two (Fig. 6B) and seven (Fig. 6D). In contrast, the TMJ IL-6 concentration of the C57Bl/6 mice (Fig. 6) did not differ significantly between treatment groups (SALINE/TMJ vs. CFA/TMJ) on either measurement days two or seven.
Mice receiving TMJ CFA injections showed no significant increase in TMJ or trigeminal ganglia levels of CGRP two or seven days after injection (data not shown).
Discussion
This study tested the feasibility of using a meal pattern to measure persistent TMJ nociception and this study measured gene expression in the joint during the nociceptive response. In this study we demonstrate that meal duration was a measure of TMJ nociception for up to 19 days in Sprague Dawley rats. Meal duration was not affected by injections of CFA into the knee. Meal frequency was a measure of TMJ nociception for 42 days but meal frequency was also affected by injecting CFA into the knee. Measurement of meal patterns in mice indicated meal duration but not meal frequency was affected by CFA injection in the DBA/1LacJ mouse strain for nearly a week. The strain of mouse was important because the CFA-treated C57Bl/6 mice did not show a nociceptive or inflammatory response to the same dose of CFA used in the DBA/1LacJ mice. Cytokines or CGRP increased in the rat and mouse after injection of CFA into the TMJ but the type and amount differed between species. Together these results suggest that meal frequency can measure TMJ nociception for a greater length of time than meal duration but that meal duration is a specific measure of orofacial nociception. We also have demonstrated that meal duration measurements have the sensitivity necessary to determine the differences in the nociception between the arthritis-prone DBA/1LacJ and the C57Bl/6 strains of mice. Species and strain differences not only affect the nociceptive response but also the molecular response because expression of IL-1β, IL-6 and/or CGRP in the TMJ was different between the rats and mice.
Meal pattern changes after injecting the TMJ with CFA
To date, all studies using meal patterns to measure TMJ nociception have lasted only two days [2, 4-6]. Therefore, it was important to determine a meal pattern that can detect TMJ nociception for longer than two days. In previous reports meal size remained unchanged after CFA injection; food intake and meal frequency were shown to measure TMJ nociception knee nociception [5]. In the present study, meal duration was lengthened by approximately three-fold after injecting CFA into the TMJ, as compared to the saline-injected rats, and meal duration remained significantly elevated for 19 days post-injection. Meal frequency decreased for all 42 days but injection of CFA into the knee also altered meal frequency consistent with previous two day studies [5]. These findings suggested that meal duration was a more specific measure of persistent TMJ nociception in rats but that greater sensitivity can be achieved by calculating the meal frequency.
About half of the rats injected with CFA showed a significant change in their meal duration when comparing one day to the next; in contrast, none of the TMJ saline-injected controls showed this day to day variation. This phenomenon may be due to a lack of “guarding” by the rat, whereby the rat bit on a pellet or part of the cage and this brief event activated TMJ neurons to respond with an increase in bite-induced mechanical hyperalgesia over the next 12 to 24 hours. This response would not result from a newly acquired injury, but rather from an aggravation of the original CFA induced injury. These heightened periods of nociception are similar to that experienced by people with persistent TMJ pain who aggravate their ongoing TMJ pain during mastication, which produces pain leading to a return of guarding behavior during mastication [9, 26]. Importantly, meal duration measurements allow for individuals to be singled out during the heightened nociception, thus making it possible to test single animals instead of only groups for molecular changes in the TMJ, TG or elsewhere, e.g., centrally.
The dose of CFA used in this study maintained nociception over several days in the DBA/1LacJ mice but did not produce as robust a response as that observed in the rats. The injected dosages of CFA were based on changes in the food intake and the size difference between rats and mice. In rats, the tested dose lengthened the meal duration three-fold, whereas the change in the meal duration of the DBA/1LacJ mice was only two-fold after CFA injection; the results suggest that DBA/1LacJ mice are less susceptible to TMJ CFA-induced nociception than rats. In support of this idea no significant difference in meal frequency was observed in the mouse after CFA injection (power = 40%). Other than susceptibility, one factor that can effect meal duration is the size of the food pellets. In a rat with an inflamed TMJ a larger pellet will result in a longer time between pellet consumption [4]. Mice are 10 times smaller than a rat and the 20 mg pellet is large for a mouse in comparison to a 45 mg pellet for a rat. Thus, meal duration in mice should be longer than a rat if pellet size were an important factor. But, meal duration in mice was shorter after CFA injection suggesting that pellet size was not a major factor effecting meal duration. A second factor that could affect the meal duration response in mice would be injection location. Mice have a much smaller joint than a rat which makes injection of the exact same site more difficult. In the event mice received an injection in a periarticular site that did not respond to CFA a lower mean response (shorter meal duration) versus the rat would be observed.
Molecular changes in the TMJ after CFA injection
Previous studies support the idea that elevated cytokine levels are present in the TMJ of humans with TMJ disorders [27-37]. The increase in such cytokines after injecting CFA into the TMJ potentially contributes to joint inflammation [2, 6, 38], including the amount of fluid buildup in the joint [39]. In the present study, the concentration of the pro-inflammatory cytokine IL-1β increased following TMJ CFA injection. The highest concentration of IL-1β was observed at the first measurement point (day seven), and then it declined significantly on days 21 and 42. Even after the TMJ concentration of IL-1β had dropped sharply by day 42, the IL-1β levels in the TMJ of CFA-treated rats were still significantly higher than in the saline-injected controls.
Proteins expressed in the TMJ that can be involved in inflammation and nociception have been studied in rats following CFA injection; however, few similar studies have been conducted in mice [21, 40-42]. Two days after the DBA/1LacJ or C57/Bl6 mice were injected in the TMJ with CFA, IL-1β did not significantly increase in either group. These results contrasted with the significant increase in IL-1β found in rats during the early stages of a hypersensitive response, two days after TMJ CFA injection [2, 5, 6, 21, 40]. By the seventh day, TMJ IL-1β was significantly increased in the TMJ of the CFA-injected DBA/1LacJ mice, but not in the C57/Bl6 mice. Two days after the TMJ injection with CFA, the amount of IL-6 was significantly higher in the DBA/1LacJ group, but not in the C57/Bl6 group. By the seventh day, IL-6 was still significantly greater in the TMJ CFA-injected DBA/1LacJ mice, but not in the C57/Bl6 mice. These results were consistent with the finding that DBA/1LacJ mice are more prone to arthritis than many other strains [16, 17], whereas the C57BL/6 mouse strain is not [43].
The cytokine response or the expression of cytokines can also differ between species [44-46] and previous reports have shown rats and mice differ in their response to IL-1 [45] consistent with the more robust cytokine response we observed in the rat in comparison to the mouse. Sprague Dawley rats used in our studies have been shown to be a model for human arthritis[47] and might be a better model of arthritis because a recent report suggests adjuvant induced arthritis in rat models are representative of human arthritis [48]. In support of this idea, the response to TGF-beta 1 was similar in rats and humans but differed from that in mice [49]. Although greater similarities between rat and human arthritis have been noted clear differences exist, for example neutrophil migration and macrophage activation are quite different between these two species after exposure to cytokines or antigen [46, 50].
In the present study injecting CFA into the TMJ increased the CGRP concentration in the rat joint potentially contributing to the increase in nociception. The highest level of CGRP was observed at the first measurement point (day seven), and then it declined by day 42. Even after this decline, the concentration within the joint was still significantly higher than that found in the saline-injected controls. Consistent with this result, CGRP has been shown to be elevated in the TMJ for at least six weeks following CFA injection in the rat TMJ [40, 51]. Previous studies have shown the staining pattern for CGRP in efferent nerve fibers differed between species [52] and we observed CGRP expression increased after adjuvant injection in the rat but no change was seen in the mouse. Substance-P and nerve growth factor increased in the TMJ following TMJ CFA injection and can potentially contribute to the TMJ nociception in the rat [40]. Interestingly, species differences in substance-P expression have not been reported [52]. Future work will be directed at measuring changes in Substance P and nerve growth factor in the TMJ of CFA-treated mice.
The species differences we observed in gene expression may be the result, in part, of a change in total protein content after CFA injection. Cytokine and CGRP concentrations were reported as a ratio (cytokine/total protein). A significant increase (p<0.05) in total protein was measured in the tissue as a result of injecting CFA. This increase in protein content may result from plasma proteins leaking from the vasculature into the interstitial space, as well as, from increased cellular activities. The increase in total protein reduced the reported cytokine and CGRP ratio in both rats and mice. A change in total protein could partly explain the differences in gene expression we observed between the species and strains but the total protein was the same for IL-1β and IL-6 because these cytokines were measured from the same sample and thus, changes in total protein would not affect the species/strain differences indicated between these two cytokines.
Association of molecular changes to TMJ nociception
The meal duration was no longer significantly elevated in the CFA injected rats after day 19, but the meal frequency remained depressed through day 42 consistent with the concentration of TMJ IL-1β and CGRP which remained elevated through day 42. Although meal frequency remained depressed for 42 days, meal duration and not meal frequency correlated with the expression of IL-1β and CGRP. While cytokines and CGRP are important in continued TMJ nociception [53, 54] there are several possible explanations why meal duration normalized in the face of continued TMJ inflammation. The first possibility is that a certain threshold level of inflammation was necessary to evoke pain. It is a common finding in humans that some injuries involve pain and inflammation and after time the swelling may still be present, but the pain subsides. A second possibility is that the cytokines and CGRP measured were not the primary genes involved in causing the nociception, and anti-inflammatory cytokines (IL-1ra) and cytokine receptors (i.e., IL-1sRII, TNFsRII), not measured in this study, correlate better with the TMJ nociception following the CFA injection. A third possibility is that the meal duration measurement was not sensitive enough to detect the level of nociception after day 19; although we have observed a lengthening in meal duration in rats injected with as little as 1 μg of CFA into the TMJ suggesting the meal duration measurement was very sensitive to changes in nociception [55]. A fourth possibility is that over time, the animals accommodated for the TMJ nociception, and their threshold for detecting such nociception increased. A fifth possibility is that the role of the inflammatory mediators changed such that, after a period of time their influence on nociception diminished. Finally, a sixth possibility is that actual level of cytokine or CGRP expression remained high and resulted in an increased nociceptive response but the ratio reported here was low due to an increase in total protein content of the tissue.
In summary, we have demonstrated for the first time that meal duration and meal frequency can measure persistent nociception resulting from inflammation in the TMJ. Importantly, a lengthening of meal duration and a reduction in meal frequency were a continuous non-invasive biological marker of TMJ nociception (surface and deep) in the undisturbed rat. We also demonstrated that meal duration measurements have the sensitivity necessary to determine the differences in the nociception between the arthritis-prone DBA/1LacJ and the C57Bl/6 strains of mice. Species and strain differences were observed after injection of CFA in the TMJ for markers IL-1β, IL-6 and/or CGRP.
Supplementary Material
Scatter plot of the meal duration and cytokines or CGRP in a CFA (250 μg) injected rat. Data from day 7, day 21 and day 42 post-CFA TMJ injection are shown. The Spearman correlations for panel A and C were r = 0.99, P<0.01. No association was detected in panel C (r = 0.50, P=0.68).
Acknowledgments
The authors wish to thank Connie Tillberg, Vanessa Winger, Priscilla Gillaspie and Gerald Hill for their excellent technical assistance. This study was supported by R01 DE016059 to LLB and R01 DE015372 to PRK from the National Institute of Dental and Craniofacial Research (NIDCR) and the Office of Research on Women's Health (ORWH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Chang H, Israel H. Analysis of inflammatory mediators in temporomandibular joint synovial fluid lavage samples of symptomatic patients and asymptomatic controls. J Oral Maxillofac Surg. 2005;63:761–765. doi: 10.1016/j.joms.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Kerins C, Carlson D, McIntosh J, Bellinger L. A role for cyclooxygenase II inhibitors in modulating temporomandibular joint inflammation from a meal pattern analysis perspective. J Oral Maxillofac Surg. 2004;62:989–995. doi: 10.1016/j.joms.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Kramer PR, Bellinger LL. The effects of cycling levels of 17beta-estradiol and progesterone on the magnitude of temporomandibular joint-induced nociception. Endocrinology. 2009;150:3680–3689. doi: 10.1210/en.2008-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thut PD, Hermanstyne TO, Flake NM, Gold MS. An operant conditioning model to assess changes in feeding behavior associated with temporomandibular joint inflammation in the rat. J Orofac Pain. 2007;21:7–18. [PubMed] [Google Scholar]
- 5.Kerins CA, Carlson DS, Hinton RJ, Grogan DM, Marr K, Kramer PR, Spears RD, Bellinger LL. Specificity of meal pattern analysis as an animal model of dermining temporomandibular joint inflammation/pain. International Journal of Oral Maxiollofacial Surgery. 2005;34:425–431. doi: 10.1016/j.ijom.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Kerins CA, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern changes associated with temporomandibular joint inflammation/pain in rats; analgesic effects. Pharmacol Biochem Behav. 2003;75:181–189. doi: 10.1016/s0091-3057(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 7.Bellinger LL, Spears R, King CM, Dahm F, Hutchins B, Kerins CA, Kramer PR. Capsaicin sensitive neurons role in the inflamed TMJ acute nociceptive response of female and male rats. Physiol Behav. 2007;90:782–789. doi: 10.1016/j.physbeh.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Haketa T, Kino K, Sugisaki M, Amemori Y, Ishikawa T, Shibuya T, Sato F, Yoshida N. Difficulty of food intake in patients with temporomandibular disorders. Int J Prosthodont. 2006;19:266–270. [PubMed] [Google Scholar]
- 9.Harper RP, Brown CM, Triplett MM, Villasenor A, Gatchel RJ. Masticatory function in patients with juvenile rheumatoid arthritis. Pediatr Dent. 2000;22:200–206. [PubMed] [Google Scholar]
- 10.Bellinger LL, He L, Kramer PR. 2010:p1267.5/AA31. Washington, DC: Meal duration; a measure of tooth and temporomadibular joint nociception. [Google Scholar]
- 11.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Mol Pain. 2008;4:43. doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan J, Benoliel R, Herzberg U, Mannes AJ, Caudle RM, Young A, Eliav E. Bite force and pattern measurements for dental pain assessment in the rat. Neurosci Lett. 2008;447:175–178. doi: 10.1016/j.neulet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–716. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 15.Roveroni RC, Parada CA, Cecilia M, Veiga FA, Tambeli CH. Development of a behavioral model of TMJ pain in rats: the TMJ formalin test. Pain. 2001;94:185–191. doi: 10.1016/S0304-3959(01)00357-8. [DOI] [PubMed] [Google Scholar]
- 16.Courtenay JS, Dallman MJ, Dayan AD, Martin A, Mosedale B. Immunisation against heterologous type II collagen induces arthritis in mice. Nature. 1980;283:666–668. doi: 10.1038/283666a0. [DOI] [PubMed] [Google Scholar]
- 17.Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- 18.Ratkay LG, Tait B, Tonzetich J, Waterfield JD. Lpr and MRL background gene involvement in the control of adjuvant enhanced arthritis in MRL-lpr mice. J Autoimmun. 1994;7:561–573. doi: 10.1006/jaut.1994.1041. [DOI] [PubMed] [Google Scholar]
- 19.Bellinger LL, Fabia R, Husberg BS. Meal patterns prior to and following liver transplantation in rats. Physiol Behav. 1997;62:525–529. doi: 10.1016/s0031-9384(97)80329-0. [DOI] [PubMed] [Google Scholar]
- 20.Castonguay TW, Kaiser LL, Stern JS. Meal pattern analysis: artifacts, assumptions and implications. Brain Res Bull. 1986;17:439–443. doi: 10.1016/0361-9230(86)90252-2. [DOI] [PubMed] [Google Scholar]
- 21.Harper RP, Kerins CA, McIntosh JE, Spears R, Bellinger LL. Modulation of the inflammatory response in the rat TMJ with increasing doses of complete Freund's adjuvant. Osteoarthritis Cartilage. 2001;9:619–624. doi: 10.1053/joca.2001.0461. [DOI] [PubMed] [Google Scholar]
- 22.Harper RP, Kerins CA, Talwar R, Spears R, Hutchins B, Carlson DS, McIntosh JE, Bellinger LL. Meal pattern analysis in response to temporomandibular joint inflammation in the rat. J Dent Res. 2000;79:1704–1711. doi: 10.1177/00220345000790091101. [DOI] [PubMed] [Google Scholar]
- 23.Kraiczi H, Karlsson G, Ekman R. Analytical Extraction of Regulatory Peptides from Rat Lung Tissue. Peptides. 1997;18:1597–1601. doi: 10.1016/s0196-9781(97)00248-9. [DOI] [PubMed] [Google Scholar]
- 24.Ho A, Chin A. Circadian feeding and drinking patterns of genetically obese mice fed solid chow diet. Physiology & Behavior. 1988;43:651–656. doi: 10.1016/0031-9384(88)90221-1. [DOI] [PubMed] [Google Scholar]
- 25.Bellinger LL, Mendel VE. Hypophysectomy alters the diurnal food intake patterns in rats. Proc Soc Exp Biol Med. 1978;159:80–83. doi: 10.3181/00379727-159-40288. [DOI] [PubMed] [Google Scholar]
- 26.Stohler CS, Ashton-Miller JA, Carlson DS. The effects of pain from the mandibular joint and muscles on masticatory motor behaviour in man. Arch Oral Biol. 1988;33:175–182. doi: 10.1016/0003-9969(88)90042-8. [DOI] [PubMed] [Google Scholar]
- 27.Alstergren P, Kopp S. Insufficient endogenous control of tumor necrosis factor-alpha contributes to temporomandibular joint pain and tissue destruction in rheumatoid arthritis. J Rheumatol. 2006;33:1734–1739. [PubMed] [Google Scholar]
- 28.Hamada Y, Kondoh T, Holmlund AB, Yamamoto M, Horie A, Saito T, Ito K, Seto K, Sekiya H. Inflammatory cytokines correlated with clinical outcome of temporomandibular joint irrigation in patients with chronic closed lock. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:596–601. doi: 10.1016/j.tripleo.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Kardel R, Ulfgren AK, Reinholt F, Hamada Y, Holmlund A. Inflammatory cell and cytokine patterns in patients with chronic polyarthritis and temporomandibular joint involvement. Acta Odontol Scand. 2006;64:221–226. doi: 10.1080/00016350600573183. [DOI] [PubMed] [Google Scholar]
- 30.Voog U, Alstergren P, Eliasson S, Leibur E, Kallikorm R, Kopp S. Progression of radiographic changes in the temporomandibular joints of patients with rheumatoid arthritis in relation to inflammatory markers and mediators in the blood. Acta Odontol Scand. 2004;62:7–13. doi: 10.1080/00016350310007860. [DOI] [PubMed] [Google Scholar]
- 31.Tominaga K, Habu M, Sukedai M, Hirota Y, Takahashi T, Fukuda J. IL-1 beta, IL-1 receptor antagonist and soluble type II IL-1 receptor in synovial fluid of patients with temporomandibular disorders. Arch Oral Biol. 2004;49:493–499. doi: 10.1016/j.archoralbio.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Kardel R, Ulfgren AK, Reinholt FP, Holmlund A. Inflammatory cell and cytokine patterns in patients with painful clicking and osteoarthritis in the temporomandibular joint. Int J Oral Maxillofac Surg. 2003;32:390–396. doi: 10.1054/ijom.2002.0357. [DOI] [PubMed] [Google Scholar]
- 33.Sato J, Segami N, Nishimura M, Demura N, Yoshimura H, Yoshitake Y, Nishikawa K. Expression of interleukin 6 in synovial tissues in patients with internal derangement of the temporomandibular joint. Br J Oral Maxillofac Surg. 2003;41:95–101. doi: 10.1016/s0266-4356(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki T, Segami N, Nishimura M, Nojima T. Co-expression of interleukin-1beta and tumor necrosis factor alpha in synovial tissues and synovial fluids of temporomandibular joint with internal derangement: comparison with histological grading of synovial inflammation. J Oral Pathol Med. 2002;31:549–557. doi: 10.1034/j.1600-0714.2002.00022.x. [DOI] [PubMed] [Google Scholar]
- 35.Ogura N, Tobe M, Sakamaki H, Kujiraoka H, Akiba M, Abiko Y, Nagura H. Interleukin-1 beta induces interleukin-6 mRNA expression and protein production in synovial cells from human temporomandibular joint. J Oral Pathol Med. 2002;31:353–360. doi: 10.1034/j.1600-0714.2002.310606.x. [DOI] [PubMed] [Google Scholar]
- 36.Shinoda C, Takaku S. Interleukin-1 beta, interleukin-6, and tissue inhibitor of metalloproteinase-1 in the synovial fluid of the temporomandibular joint with respect to cartilage destruction. Oral Dis. 2000;6:383–390. doi: 10.1111/j.1601-0825.2000.tb00131.x. [DOI] [PubMed] [Google Scholar]
- 37.Alstergren P. Cytokines in temporomandibular joint arthritis. Oral Dis. 2000;6:331–334. doi: 10.1111/j.1601-0825.2000.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 38.Kerins CA, Spears R, Bellinger LL, Hutchins B. The prospective use of COX-2 inhibitors for the treatment of temporomandibular joint inflammatory disorders. Int J Immunopathol Pharmacol. 2003;16:1–9. [PubMed] [Google Scholar]
- 39.Sato J, Segami N, Nishimura M, Demura N, Yoshimura H, Yoshitake Y, Nishikawa K. Expression of interleukin 6 in synovial tissues in patients with internal derangement of the temporomandibular joint. Br J Oral Maxillofac Surg. 2003;41:95–101. doi: 10.1016/s0266-4356(02)00294-2. [DOI] [PubMed] [Google Scholar]
- 40.Spears R, Dees LA, Sapozhnikov M, Bellinger LL, Hutchins B. Temporal changes in inflammatory mediator concentrations in an adjuvant model of temporomandibular joint inflammation. J Orofac Pain. 2005;19:34–40. [PubMed] [Google Scholar]
- 41.Spears R, Oakes R, Bellinger LL, Hutchins B. Tumour necrosis factor-alpha and apoptosis in the rat temporomandibular joint. Arch Oral Biol. 2003;48:825–834. doi: 10.1016/s0003-9969(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 42.Spears R, Oakes R, Moore C, Bellinger LL, Hutchins B. A determination of tumor necrosis factor expression in TMJ inflammation with the use of microarray analysis. J Dent Res. 2003;82:807–813. doi: 10.1177/154405910308201009. [DOI] [PubMed] [Google Scholar]
- 43.Wooley PH, Luthra HS, Griffiths MM, Stuart JM, Huse A, David CS. Type II collagen-induced arthritis in mice. IV. Variations in immunogenetic regulation provide evidence for multiple arthritogenic epitopes on the collagen molecule. J Immunol. 1985;135:2443–2451. [PubMed] [Google Scholar]
- 44.Faas MM, Bouman A, Veenstra van Nieuwenhoven AL, van der SG, Moes H, Heineman MJ, de VP. Species differences in the effect of pregnancy on lymphocyte cytokine production between human and rat. J Leukoc Biol. 2005;78:946–953. doi: 10.1189/jlb.0405186. [DOI] [PubMed] [Google Scholar]
- 45.Takao T, Newton RC, De Souza EB. Species differences in [125I]interleukin-1 binding in brain, endocrine and immune tissues. Brain Res. 1993;623:172–176. doi: 10.1016/0006-8993(93)90026-j. [DOI] [PubMed] [Google Scholar]
- 46.Upham JW, Strickland DH, Bilyk N, Robinson BW, Holt PG. Alveolar macrophages from humans and rodents selectively inhibit T-cell proliferation but permit T-cell activation and cytokine secretion. Immunology. 1995;84:142–147. [PMC free article] [PubMed] [Google Scholar]
- 47.Cai X, Wong YF, Zhou H, Liu ZQ, Xie Y, Jiang ZH, Bian ZX, Xu HX, Liu L. Manipulation of the induction of adjuvant arthritis in Sprague-Dawley rats. Inflamm Res. 2006;55:368–377. doi: 10.1007/s00011-006-6026-x. [DOI] [PubMed] [Google Scholar]
- 48.Kim EY, Moudgil KD. The determinants of susceptibility/resistance to adjuvant arthritis in rats. Arthritis Res Ther. 2009;11:239. doi: 10.1186/ar2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petersen B, Yee CJ, Bowen W, Zarnegar R, Michalopoulos GK. Distinct morphological and mito-inhibitory effects induced by TGF-beta 1, HGF and EGF on mouse, rat and human hepatocytes. Cell Biol Toxicol. 1994;10:219–230. doi: 10.1007/BF00756762. [DOI] [PubMed] [Google Scholar]
- 50.Sugawara T, Miyamoto M, Takayama S, Kato M. Separation of neutrophils from blood in human and laboratory animals and comparison of the chemotaxis. J Pharmacol Toxicol Methods. 1995;33:91–100. doi: 10.1016/1056-8719(94)00062-9. [DOI] [PubMed] [Google Scholar]
- 51.Carleson J, Alstergren P, Appelgren A, Appelgren B, Kopp S, Srinivasan GR, Theodorsson E, Lundeberg T. Effects of adjuvant on neuropeptide-like immunoreactivity in experimentally induced temporomandibular arthritis in rats. Arch Oral Biol. 1996;41:705–712. doi: 10.1016/s0003-9969(96)00027-1. [DOI] [PubMed] [Google Scholar]
- 52.Matsubara A, Usami S, Fujita S, Shinkawa H. Expression of substance P, CGRP, and GABA in the vestibular periphery, with special reference to species differences. Acta Otolaryngol Suppl. 1995;519:248–252. doi: 10.3109/00016489509121916. [DOI] [PubMed] [Google Scholar]
- 53.Ahn DK, Chae JM, Choi HS, Kyung HM, Kwon OW, Park HS, Youn DH, Bae YC. Central cyclooxygenase inhibitors reduced IL-1beta-induced hyperalgesia in temporomandibular joint of freely moving rats. Pain. 2005;117:204–213. doi: 10.1016/j.pain.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kramer PR, Bereiter DA, Bellinger LL. Forced swim stress enhances meal duration in an inflammatory model of TMJ injury in male rats. 2008 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatter plot of the meal duration and cytokines or CGRP in a CFA (250 μg) injected rat. Data from day 7, day 21 and day 42 post-CFA TMJ injection are shown. The Spearman correlations for panel A and C were r = 0.99, P<0.01. No association was detected in panel C (r = 0.50, P=0.68).