Abstract
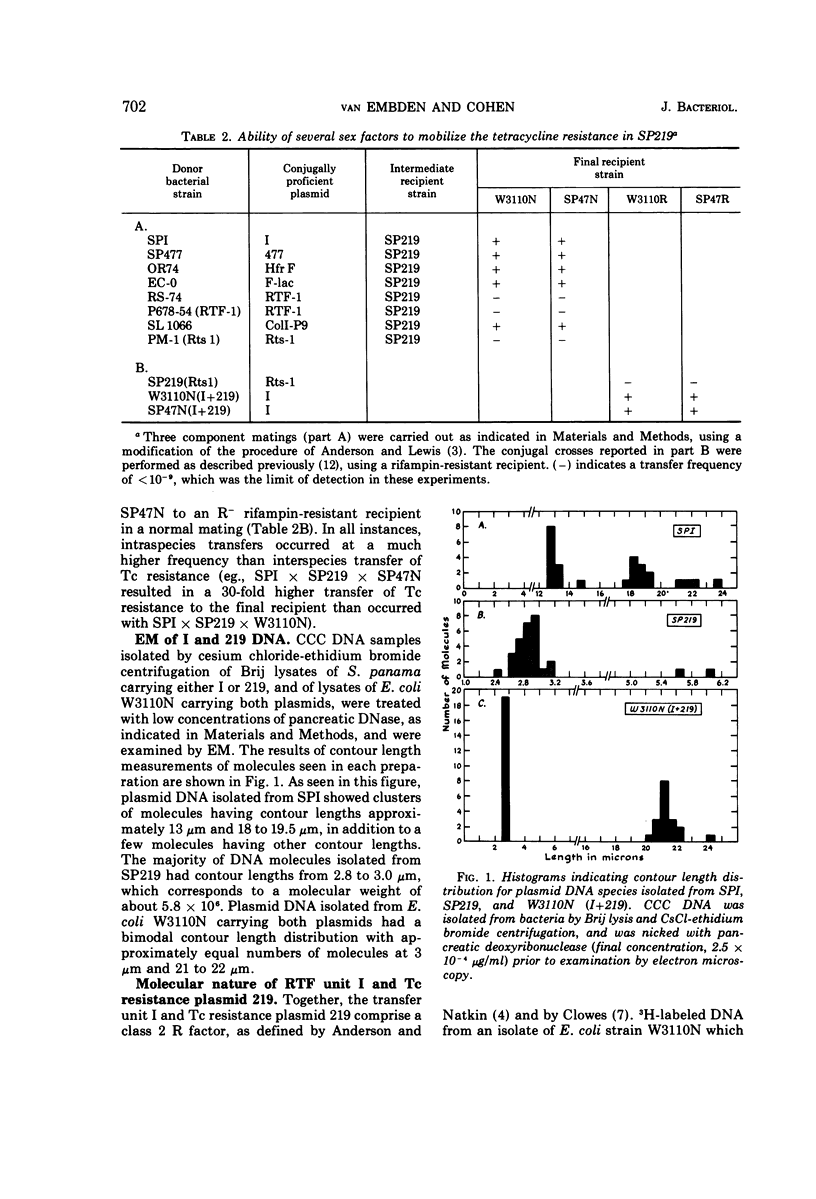
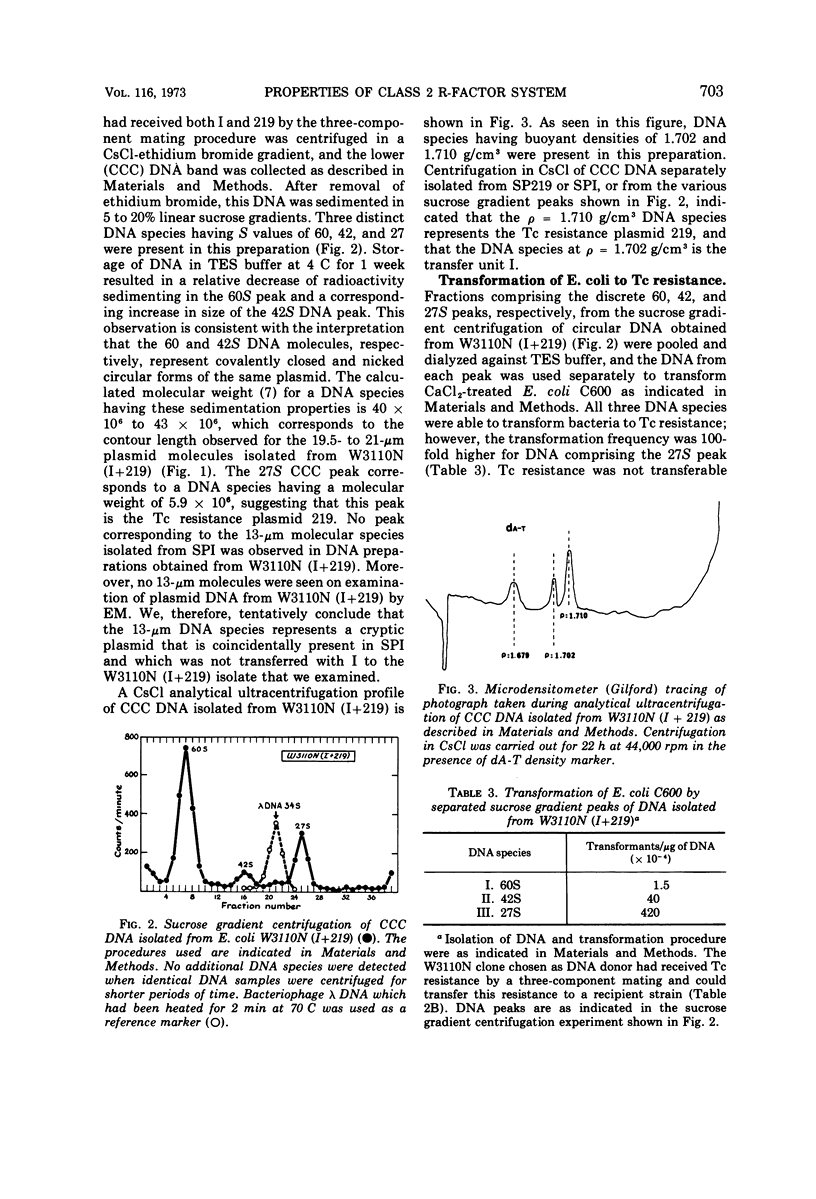
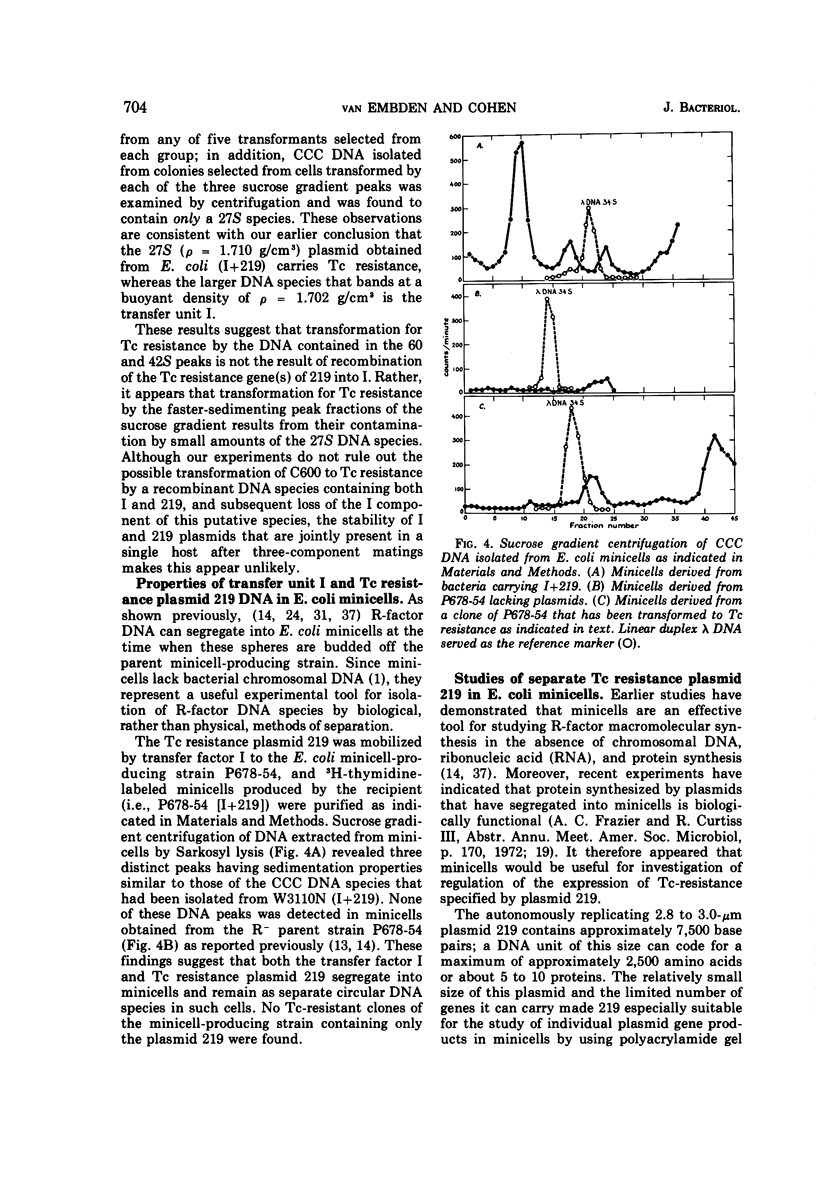
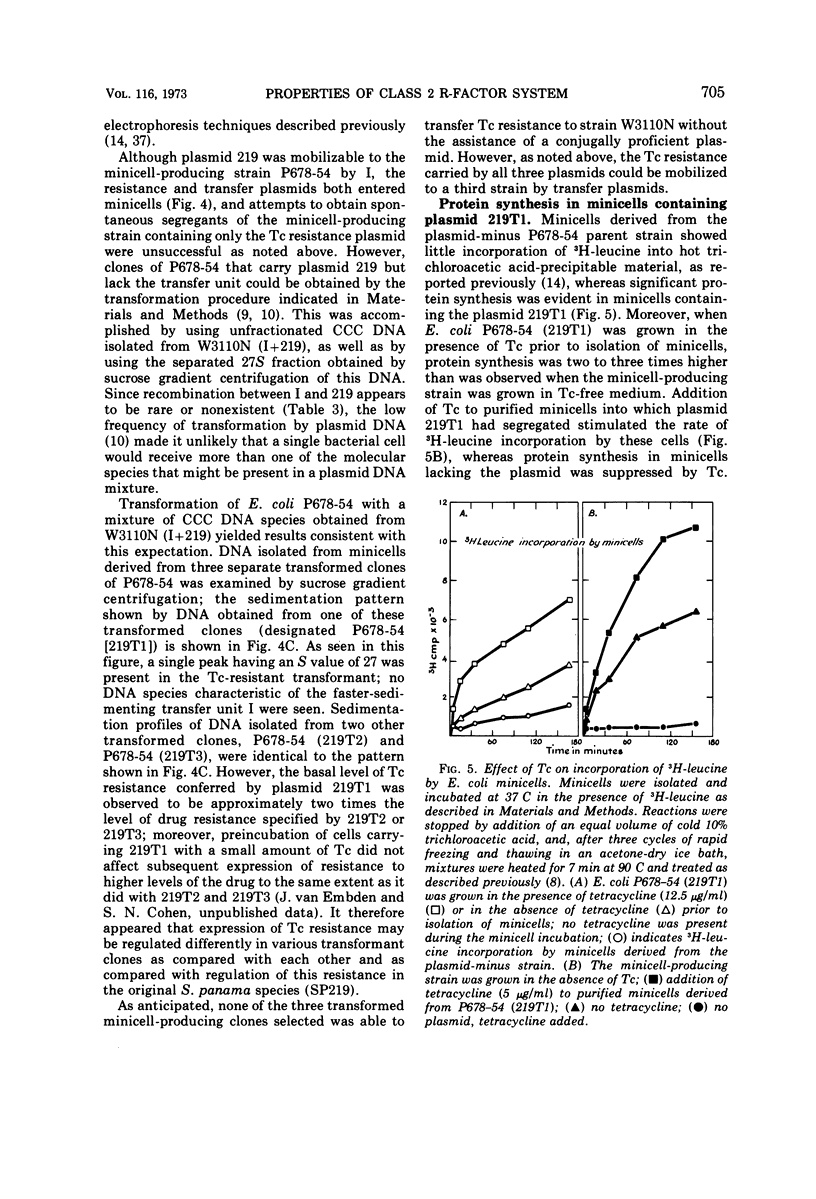
Certain genetic, structural, and biochemical properties of a class 2 R-factor system consisting of the conjugally proficient transfer plasmid I and the naturally occurring non-conjugative tetracycline (Tc) resistance plasmid 219 are reported. I and 219 exist as separate plasmid deoxyribonucleic acid (DNA) species in both Escherichia coli and Salmonella panama, having molecular weights of 42 × 106 and 5.8 × 106, respectively. The buoyant densities of I and 219 are 1.702 and 1.710 g/cm3, respectively, in neutral cesium chloride. Although the Tc resistance plasmid is not transmissible in a normal conjugal mating, it is mobilized in a three-component mating by plasmid I and by certain other conjugative plasmids of the fi+ or fi− phenotype. Mobilization does not appear to involve intermolecular recombination between plasmids, and no covalent linkage of resistance markers and fertility functions is observed. Transformation of CaCl2-treated E. coli by plasmid DNA is shown to be a useful procedure for studying the biological properties of different plasmid molecular species that have been fractionated in vitro, and for selectively inserting non-self-transmissible plasmids into specific bacterial strains. The effects of tetracycline on the rate of protein synthesis carried out by plasmid 219 were studied by using isolated E. coli minicells into which this plasmid had segregated. Consistent with the results of earlier investigations showing the inducibility of plasmid-mediated Tc resistance in E. coli, the antibiotic was observed to stimulate protein synthesis in minicells carrying the plasmid 219 and totally inhibit 3H-leucine incorporation by minicells lacking the Tc resistance marker. Five discrete polypeptide species were synthesized by minicells carrying plasmid 219; exposure of minicells or parent bacteria to Tc resulted in specific and reproducible changes in polypeptide synthesis patterns.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E. S., Lewis M. J. Characterization of a transfer factor associated with drug resistance in Salmonella typhimurium. Nature. 1965 Nov 27;208(5013):843–849. doi: 10.1038/208843a0. [DOI] [PubMed] [Google Scholar]
- Anderson E. S., Natkin E. Transduction of resistance determinants and R factors of the transfer systems by phage Plkc. Mol Gen Genet. 1972;114(3):261–265. doi: 10.1007/BF01788895. [DOI] [PubMed] [Google Scholar]
- Anderson E. S. The ecology of transferable drug resistance in the enterobacteria. Annu Rev Microbiol. 1968;22:131–180. doi: 10.1146/annurev.mi.22.100168.001023. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Replication of a bacterial plasmid and an episome in Escherichia coli. Biochemistry. 1970 Jan 20;9(2):399–406. doi: 10.1021/bi00804a029. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Genetic expression in bacteriophage lambda. 3. Inhibition of Escherichia coli nucleic acid and protein synthesis during lambda development. J Mol Biol. 1970 May 14;49(3):557–575. doi: 10.1016/0022-2836(70)90281-0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C. Recircularization and autonomous replication of a sheared R-factor DNA segment in Escherichia coli transformants. Proc Natl Acad Sci U S A. 1973 May;70(5):1293–1297. doi: 10.1073/pnas.70.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Multiple molecular species of circular R-factor DNA isolated from Escherichia coli. Nature. 1969 Dec 27;224(5226):1273–1277. doi: 10.1038/2241273a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., McCoubrey A. E. Isolation of catenated forms of R factor DNA from minicells. Nat New Biol. 1971 Jun 23;231(25):249–252. doi: 10.1038/newbio231249a0. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Silver R. P., Sharp P. A., McCoubrey A. E. The problems of drug-resistant pathogenic bacteria. Studies on the molecular nature of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:172–187. doi: 10.1111/j.1749-6632.1971.tb30655.x. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKLIN T. J., GODFREY A. RESISTANCE OF ESCHERICHIA COLI TO TETRACYCLINES. Biochem J. 1965 Jan;94:54–60. doi: 10.1042/bj0940054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T. J., Cook J. M. R factor with a mutation in the tetracycline resistance marker. Nature. 1971 Jan 22;229(5282):273–274. doi: 10.1038/229273a0. [DOI] [PubMed] [Google Scholar]
- Franklin T. J. Resistance of Escherichia coli to tetracyclines. Changes in permeability to tetracyclines in Escherichia coli bearing transferable resistance factors. Biochem J. 1967 Oct;105(1):371–378. doi: 10.1042/bj1050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Curtiss R., 3rd Derepression of anthranilate synthase in purified minicells of Escherichia coli containing the Col-trp plasmid. J Bacteriol. 1973 Aug;115(2):615–622. doi: 10.1128/jb.115.2.615-622.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. R., Freifelder D. Studies on Escherichia coli sex factors. I. Specific labeling of F'Lac DNA. J Mol Biol. 1968 Feb 28;32(1):15–23. doi: 10.1016/0022-2836(68)90141-1. [DOI] [PubMed] [Google Scholar]
- Haapala D. K., Falkow S. Physical studies of the drug-resistance transfer factor in Proteus. J Bacteriol. 1971 Apr;106(1):294–295. doi: 10.1128/jb.106.1.294-295.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M., Hashimoto H., Mitsuhashi S. Mechanism of tetracycline resistance in Staphylococcus aureus. I. Inducible resistance to tetracycline. J Antibiot (Tokyo) 1970 Feb;23(2):68–74. doi: 10.7164/antibiotics.23.68. [DOI] [PubMed] [Google Scholar]
- Inselburg J. R factor deoxyribonucleic acid in chromosomeless progeny of Escherichia coli. J Bacteriol. 1971 Feb;105(2):620–628. doi: 10.1128/jb.105.2.620-628.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Kiuchi K., Arima K. Specificity and mechanism of tetracycline resistance in a multiple drug resistant strain of Escherichia coli. J Bacteriol. 1966 Feb;91(2):628–633. doi: 10.1128/jb.91.2.628-633.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Kopecko D. J., Punch J. D. The problems of drug-resistant pathogenic bacteria. Regulation of R-factor replication in Proteus mirabilis. Ann N Y Acad Sci. 1971 Jun 11;182:201–216. doi: 10.1111/j.1749-6632.1971.tb30657.x. [DOI] [PubMed] [Google Scholar]
- Krcméry V., Fredericq P., Wiedemann B., Hurwitz C. Mobilization of extrachromosomal determinants for streptomycin resistance by transferable colicinogenic factors. Ann N Y Acad Sci. 1971 Jun 11;182:118–122. doi: 10.1111/j.1749-6632.1971.tb30650.x. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Levy S. B., Norman P. Segregation of transferable R factors into Escherichia coli minicells. Nature. 1970 Aug 8;227(5258):606–607. doi: 10.1038/227606a0. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Milliken C. E., Clowes R. C. Molecular structure of an R factor, its component drug-resistance determinants and transfer factor. J Bacteriol. 1973 Feb;113(2):1026–1033. doi: 10.1128/jb.113.2.1026-1033.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisioka T., Mitani M., Clowes R. Composite circular forms of R factor deoxyribonucleic acid molecules. J Bacteriol. 1969 Jan;97(1):376–385. doi: 10.1128/jb.97.1.376-385.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO S., MIZUNO D. MECHANISM OF CHLORAMPHENICOL AND TETRACYCLINE RESISTANCE IN ESCHERICHIA COLI. J Gen Microbiol. 1964 Apr;35:125–133. doi: 10.1099/00221287-35-1-125. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozen K. J., Fenwick R. G., Jr, Curtiss R., 3rd Synthesis of ribonucleic acid and protein in plasmid-containing minicells of Escherichia coli K-12. J Bacteriol. 1971 Jul;107(1):21–33. doi: 10.1128/jb.107.1.21-33.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rownd R., Mickel S. Dissociation and reassociation of RTF and r-determinants of the R-factor NR1 in Proteus mirabilis. Nat New Biol. 1971 Nov 10;234(45):40–43. doi: 10.1038/newbio234040a0. [DOI] [PubMed] [Google Scholar]
- Rownd R. Replication of a bacterial episome under relaxed control. J Mol Biol. 1969 Sep 28;44(3):387–402. doi: 10.1016/0022-2836(69)90368-4. [DOI] [PubMed] [Google Scholar]
- Silver R. P., Cohen S. N. Nonchromosomal antibiotic resistance in bacteria. V. Isolation and characterization of R factor mutants exhibiting temperature-sensitive repression of fertility. J Bacteriol. 1972 Jun;110(3):1082–1088. doi: 10.1128/jb.110.3.1082-1088.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver R. P., Falkow S. Specific labeling and physical characterization of R-factor deoxyribonucleic acid in Escherichia coli. J Bacteriol. 1970 Oct;104(1):331–339. doi: 10.1128/jb.104.1.331-339.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky D., Krawitz T., Zaidenzaig Y., Abramova N. Inducible resistance to tetracycline in Staphylococcus aureus. J Gen Microbiol. 1970 Aug;62(3):341–349. doi: 10.1099/00221287-62-3-341. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Kakizawa Y., Takayasu H., Yoshikawa M. Temperature sensitivity of cell growth in Escherichia coli associated with the temperature sensitive R(KM) factor. Nature. 1968 Jul 20;219(5151):284–285. doi: 10.1038/219284a0. [DOI] [PubMed] [Google Scholar]
- Watanabe T. The problems of drug-resistant pathogenic bacteria. The origin of R factors. Ann N Y Acad Sci. 1971 Jun 11;182:126–140. doi: 10.1111/j.1749-6632.1971.tb30652.x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


